Abstract
Diabetic nephropathy (DN) is one of the most important long-term complications of diabetes. Patients with diabetes and chronic kidney disease have an increased risk of all-cause mortality, cardiovascular mortality, and kidney failure. The clinical diagnosis of DN depends on the detection of microalbuminuria. This usually occurs after the first five years from the onset of diabetes, and predictors of DN development and progression are being studied but are not yet implemented into clinical practice. Diagnostic tests are useful tools to recognize onset, progression and response to therapeutic interventions. Microalbuminuria is an indicator of DN, and it is considered the only noninvasive marker of early onset. However, up to now there is no diagnostic tool that can predict which patients will develop DN before any damage is present. Pathological renal injury is hard to predict only with clinical and laboratory findings. An accurate estimate of damage in DN can only be achieved by the histological analysis of tissue samples. At the present time, renal biopsy is indicated on patients with diabetes under the suspicion of the presence of nephropathies other than DN. Results from renal biopsies in patients with diabetes had made possible the classification of renal biopsies in three major groups associated with different prognostic features: diabetic nephropathy, non-diabetic renal disease (NDRD), and a superimposed non-diabetic condition on underlying diabetic nephropathy. In patients with type 2 diabetes with a higher degree of suspicion for NDRD, it is granted the need of a renal biopsy. It is important to identify and differentiate these pathologies at an early stage in order to prevent progression and potential complications. Therefore, a more extensive use of biopsy is advisable.
Keywords: Diabetic nephropathy, Kidney biopsy, Non-diabetic renal disease
Core tip: Diagnostic tests are useful to predict onset, progression and response to therapeutic interventions in diabetic nephropathy (DN). Renal biopsies help to classify renal diseases in three major groups associated with different prognostic features: diabetic nephropathy, non-diabetic nephropathy (NDRD), and a superimposed non-diabetic condition on underlying DN. Pathological renal damage is hard to predict only with clinical and laboratory findings. In patients with a higher degree of suspicion for NDRD, it is granted the need of a renal biopsy. For this reason, more studies are required to assess the routine use of kidney biopsies as a gold standard for diagnosis of diabetic nephropathy.
INTRODUCTION
Chronic kidney disease (CKD) is a worldwide public health problem, and one of the major causes of mortality in the United States. It is characterized by kidney damage for more than 3 mo; defined by structural or functional abnormalities of the kidney, with or without decreased estimated glomerular filtration rate (GFR); or GFR < 60 mL/min per 1.73 m2 for more than 3 mo, with or without kidney damage[1]. Decreased GFR and albuminuria are indicators of major health outcomes of this condition, including end-stage renal disease (ESRD) and death[2].
There is no definitive cure for this condition; and many of the patients may develop complications even before they are able to receive renal replacement therapies, including long term dialysis and kidney transplants. In 2010, more than 117000 patients started therapy for ESRD; the prevalent population reached 594000, from those a 74% (439560) required dialysis and 3% (17778) received kidney transplants[3].
The most common cause of ESRD requiring dialysis is diabetes mellitus (DM). Up to 44% of patients with newly diagnosed ESRD cases also carry a diagnosis of diabetes[3]. According to the World Health Organization (WHO) in 2012, 347 million people suffered from DM; this is about 5% of the total population. Individuals with diabetes and CKD have an increased risk of all-cause mortality, cardiovascular mortality, and kidney failure[3-5]. WHO estimates that deaths related to diabetes will double from 2005 to 2030[4].
DEFINITION OF DIABETIC NEPHROPATHY AND WORLDWIDE IMPACT
Diabetic nephropathy (DN) is one of the most important long-term complications of diabetes. It is characterized by the development of proteinuria with a subsequent decline in glomerular filtration rate, which progresses over a long period of time, often for 10-20 years[3,6,7].
Over the past 20 years, the prevalence of DN in the United States has increased in direct proportion to the prevalence of diabetes[6]. Although DN cases vary largely among countries; in average it develops in 30% to 40% of patients with diabetes[7]. The clinical diagnosis of DN usually depends on the detection of microalbuminuria (albumin excretion of more than 30 mg/g of creatinine in 2 out of 3 random urine samples collected in within a six month period)[8]. A subset of patients with microalbuminuria will develop advanced DN; referred as overt nephropathy, clinical nephropathy, proteinuria, or macroalbuminuria[9]. However, progression to microalbuminuria usually occurs after five years from the onset of diabetes. Pathogenesis of the disease is multifactorial, e.g., smoking, hyperglycemia, hypertension, male, genetic predisposition, advance age, retinopathy, macrovascular disease were the risk factors of diabetic nephropathy; and it involves genetic and environmental factors that affect multiple metabolic pathways not necessarily activated by hyperglycemia[10].
STAGES OF DIABETIC NEPHROPATHY
Progression of the diabetic nephropathy is divided in clinical stages depending on the duration of the disease[11-13] (Table 1). The first stage starts prior to any renal damage. It is characterized by renal vasodilation and hyperfiltration that occur early in the onset of diabetes. Several factors may lead to this hyperfiltration: including hyperglycemia, prostaglandins secretion and increased sodium/glucose reabsorption in the proximal tubule[14]. It has also been associated to increased urinary albumin excretion related to physical activity[15].
Table 1.
Diabetic nephropathy stages
| Stage 1 Early hyperfunction and hypertrophy | ACR < 30 mg/g creatinine |
| Stage 2 Morphologic lesions without signs of clinical disease | ACR > 30 and < 300 mg/g creatinine |
| Stage 3 Microalbuminuria | ACR > 300 mg/g creatinine and/or persistent |
| proteinuria with serum concentration of creatinine 2.0 mg/dL | |
| Stage 4 Overt nephropathy | Serum concentration of creatinine 2.0 mg/dL with proteinuria |
| Stage 5 End-stage renal disease with uremia | On dialysis |
ACR: Albumin to creatinine ratio.
During the second stage, morphologic lesions develop without signs of clinical disease. The earliest structural abnormality in diabetes is glomerular basement membrane (GBM) thickening. The kidney with early diabetes suffers significant hypertrophy; characterized by enlargement of the organ with a combination of hyperplasia and hypertrophy[16]. This occurs in nearly all patients 1.5 to 2.5 years after the onset of type 1 DM (T1DM). Nonspecific vascular or interstitial changes are prevalent in these patients. Mesangial expansion and occlusion of glomerular capillaries lead to a loss of available surface area for filtration and to a decline in function[14].
Third stage is characterized by small amounts of albumin in the urine, not usually detected by conventional methods. This stage is also named incipient nephropathy[9]. A slow and gradual increase of albuminuria over the years is a prominent feature in this stage. According to the DCCT/EDIC Study, persistent microalbuminuria develops most frequently during the second decade after diagnosis of diabetes[6]. It reflects the existence of endothelial damage in the absence of specific renal lesions; and it is also associated with the beginning of advanced renal pathology[7]. Microalbuminuria could also represent podocytes loss; as podocyte number in patients with type 2 DM (T2DM) correlates with the change of albuminuria over time[17].
Although microalbuminuria has been considered a risk factor for macroalbuminuria, not all patients progress to this stage; some of them stay or even may regress to normoalbuminuria[18]. Microalbuminuria is considered to be predictive of progression to nephropathy in T2DM. However, that may not be the case in T1DM[14]. Normoalbuminuric patients with diabetes are extremely heterogeneous in renal function and structure[19]. Both, microalbuminuric and normoalbuminuric patients benefit from optimal glycemic control[20]; since it has been shown that about one third of the normoalbuminuric subjects develop diabetic nephropathy within few years after onset of diabetes[19,21-23]. The cause of albuminuria in patients without diabetic glomerulopathy is unclear. It might be related to early and very mild ultrastructural changes[24].
Overt nephropathy is characterized by persistent albuminuria (UAE > 300 mg/d or > 500 mg/d urinary protein excretion) that usually accompanies a decrease in GFR[25]. Macroalbuminuria has been associated to the presence of proliferative retinopathy, coronary heart disease, and foot ulcers[14]. The prevalence of hypertension increases with higher levels of albuminuria[15]. Other risk factors to develop overt nephropathy include uncontrolled diabetes, smoking, advanced age and high lipids levels[26,27]. ESRD is defined by the presence of signs and symptoms of kidney failure requiring replacement therapy, regardless of the GFR level[1]. It has been described as an important independent predictor of hospitalization and death in adults with heart failure. The deterioration rate from one stage to the next one is 2% to 3% per year[28].
DIAGNOSTIC TOOLS FOR DIABETIC NEPHROPATHY
One of the main goals concerning the timely diagnosis of DN is to delay and if possible interrupt the natural course of this disease; from the progression of renal damage to ESRD in patients with diabetes. Diagnostic tests are useful tools to determine onset, progression and to predict response to therapeutic interventions.
Current screening recommendations
Although not all patients with early renal involvement, such as microalbuminuria progress to macroalbuminuria and ESRD; the risk is higher among these patients. Some of them stay or even may regress to normoalbuminuria[18]. Ideally, it would be very useful to be able to predict which patients are at higher risk to develop ESRD, even before onset of DN. Unfortunately, at the present time, there is a lack of precise diagnostic tools that could definitively identify such patients[29].
The National Kidney Foundation (NKF) and the American Diabetes Association (ADA) recommend that patients with CKD and diabetes should be screened every year for DN. Screening should start 5 years after diagnosis in T1DM patients, and at the time of diagnosis in T2DM patients. This is done by measuring the urinary albumin/creatinine ratio in spot urine, serum creatinine and GFR[1]. Although UAE is routinely used to diagnose DN; in some cases, patients with diabetes have a decrease GFR with normal UAE. Both GFR and UAE, correlate with the severity of glomerular lesions, duration of diabetes, glycemic control and genetic factors[30,31].
Currently, microalbuminuria is considered the earlier noninvasive marker[32-34]. Patients with both elevated albuminuria and reduced GFR are at higher risk for a cardiovascular event[35]. This emphasizes the importance to detect microalbuminuria and a close follow up of especially in young patients with diabetes.
Renal biopsy
Once the presence of albumin in the urine is confirmed, patients should undergo complete evaluation; including work-up for other etiologies. Renal diseases other than DN have been reported in patients with diabetes. DN usually develops 10 years after onset of T1DM[18]; however, in T2DM this is variable[23].
An accurate estimate of damage in DN can only be achieved by the histological analysis of tissue samples[7]. Therefore, the kidney biopsy in patients with diabetes could represent a valuable procedure to establish the stage of the renal disease[36]. The relevance of this diagnostic tool is supported by the observation that when a renal biopsy is performed in patients with DM, results may vary from primary and secondary renal disease with changes unrelated to diabetes to changes of underlying DM[23].
Some of the earliest lesions are characterized by the thickening of the GMB visualized under electron microscopy, but with no findings under light microscopy. The morphologic lesions in T1DM predominantly affect the glomeruli, with thickening of the GBM and mesangial expansion; although the podocytes, renal tubules, interstitium, and arterioles also undergo substantial changes, especially at later stages of disease[37,38].
Nephropathy in patients with T2DM is associated with two distinctive patterns of glomerular pathology (nodular and non-nodular)[39]. Nodular type glomerulosclerosis (Kimmelstiel-Wilson nodules) was reported in 1936 by light microscopy. This lesion was initially identified as the only specific feature of DN[40]. It consists of nodular lesions containing areas of marked mesangial expansion forming large round fibrillar mesangial zones with palisading of mesangial nuclei around the periphery of the nodule and compression of the associated glomerular capillaries. Later on, diffuse type glomerulosclerosis was described as a different type of diabetic glomerular lesion[41]. All these diabetic glomerular changes are related to advanced or late DN associated to heavy proteinuria and/or decreased renal function. Arteriosclerosis is also frequently associated to diabetic glomureolopathy[42].
It has been shown that there is not substantial difference in the injury caused in patients with T1DM in comparison to T2DM; and damages are considered basically similar in both types[29]. For this reason, there is a consensus classification combining type 1 and type 2 DN. It is divided into four classes of glomerular lesions. Class I: GMB thickening, composed of isolated GMB thickening and only mild, nonspecific changes by light microscopy that do not meet the criteria of classes II through IV. Class II: mesangial expansion; mild (IIa) or severe (IIb), without nodular sclerosis or global glomerulosclerosis in more than 50% of glomeruli. Class III: nodular sclerosis (Kimmelstiel-Wilson lesions); at least one glomerulus with nodular increase in mesangial matrix (Kimmelstiel-Wilson) without changes described in class IV. Class IV: advanced diabetic glomerulosclerosis, more than 50% global glomerulosclerosis with other clinical or pathologic evidence that sclerosis is caused by diabetic nephropathy[42,43] (Figure 1).
Figure 1.
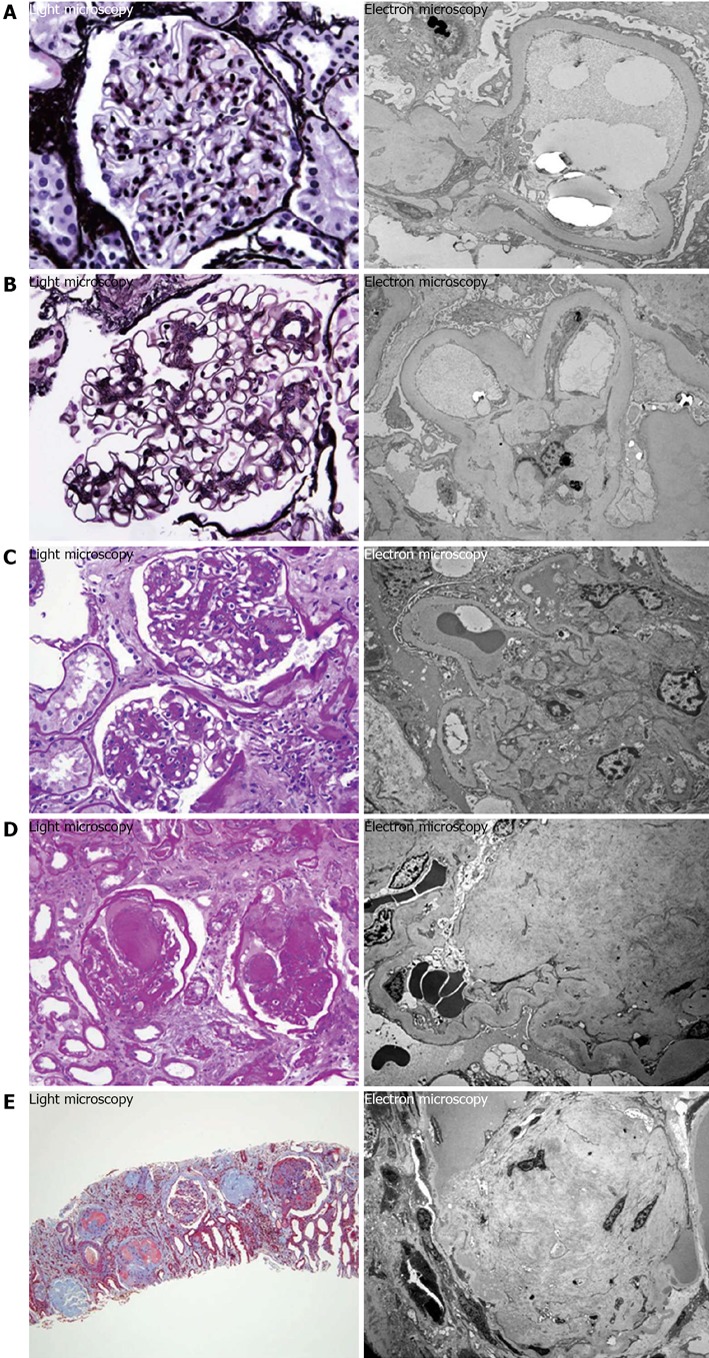
Diabetic nephropathy class. A: Glomerular basement membrane thickening; B: Mild mesangial expansion; C: Severe mesangial expansion; D: Nodular sclerosis (Kimmelstiel-Wilson lesions); E: Advanced diabetic glomerulosclerosis.
Podocyte injury is also an important feature of DN[16,44-49]; and podocyte loss (podocytopenia) is considered an independent predictor of DN progression in patients with T2DM[17].
Indications for kidney biopsy
There are no standardized criteria for kidney biopsy in patients with DM; therefore, currently the decision to perform one is made by the primary physician[50,51]. Nowadays, up to 25% of all renal biopsies are done in patients with DM[51].
Rapid onset of proteinuria (regardless of the progression from microalbuminuria to macroalbuminuria), absence of retinopathy, presence of hematuria, active urinary sediment, rapid decrease of renal function, and suspicion of other nephropathies secondary to systemic disease, are some of the indications for renal biopsy[50,52], e.g., Nephrotic syndrome, Urinary abnormalities, Isolated hematuria, Nephritic syndrome, Rapid onset of renal insufficiency, Unexplained renal failure at presentation, and No retinopathy are major indications for kidney biopsy. Some authors had considered retinopathy as a highly specific indicator for DN[53,54]. On the other hand, other studies have shown to be a poor predictor of DN in T2DM[55,56]. For this reason, kidney biopsy may have an opportunity to be proven as a gold standard for diagnosis of DN[57].
Biopsy findings in patients with diabetes
Results from renal biopsies in patients with diabetes have made possible the classification of renal biopsies in three major groups associated with different prognostic features: DN, non-diabetic renal disease (NDRD), and a superimposed non-diabetic condition on underlying DN. There has been described a more rapid deterioration of renal function on patients with DN than with NDRD[53]. Patients with non-diabetic nephropathies, including extracapillary glomerulonephritis, minimal change glomerulopathy, cryoglobulinemic nephritis, non-diabetic membranous glomerulopathy, focal glomerulosclerosis, and IgA nephropathy, among others, might be modified by therapy; and for this reason, it results important the detection of such histological patterns following an appropriate therapeutic management; as this could promote a better outcome in those patients[23]. The most common non-diabetic glomerulopathy found across reports in literature is IgA nephropathy[60-72].
Clinical presentation varies among age groups. It is known that chronic nephritic syndrome is usually more common in young patients, and nephrotic syndrome and CKD in the elderly[50]. The average time of onset of nephropathy in patients with diabetes is about 7-10 years[18].
It is important to emphasize that among patients with diabetes; especially in those with T2DM, renal complications may be frequently due to heterogeneous non-diabetic lesions. Reported NDRD varies in the literature from a range of 14%-82.9% (Table 2), regardless of geographic region or ethnicity. The wide range in these highly variable results could be explained by the heterogeneity of the populations in the studies done around the world. Nzerue et al[61], reported a prevalence of NDRD among African-Americans with T2DM based on renal biopsy. They found DN alone in 42%, while NDRD was seen in 19%; the rest of the patients had a superimposed NDRD with DN[62]. Similar results were reported in two different studies in Japan[68,73]. On the other hand; Wong et al[53], reported different results in the Chinese population which showed a predominance of NDRD. As the population sample used in some of these studies was larger (i.e., studies with population sample of more than 70 patients), the proportion of DN vs NDRD become more homogeneous; resulting in 30% prevalence for each of these groups. Although there is a male predominance for DN in general, this is not statistically significant among the studies[58,62,71,74].
Table 2.
Comparison of diabetic nephropathy and non-diabetic renal disease prevalence reported in the literature
| Ref. | Country | Population | Type 1 or 2 DM | % DN | % NDRD | % Mixed |
| Mazzucco et al[23] | Italy | 393 | 2 | 39.7 | 43 | 17.3 |
| Christensen et al[24] | Denmark | 51 | 2 | 68.6 | 13.8 | NR |
| Zhang et al[37] | China | 130 | 2 | 73.9 | 26.1 | NR |
| Zhuo et al[50] | China/Japan | 216 | 2 | 6.5 | 82.9 | 10.7 |
| Sharma et al[51] | United States | 620 | 2 | 37 | 36 | 27 |
| Hironaka et al[52] | Japan | 35 | 1 and 2 | 71.4 | 14.3 | 14.3 |
| Wong et al[53] | China | 68 | 2 | 35 | 46 | 19 |
| Prakash et al[55] | India | 23 | 2 | 56.5 | 30.5 | 13 |
| Mak et al[56] | China | 51 | 2 | 67 | 16 | 17 |
| Biensebach et al[57] | Austria | 84 | 2 | 78.5 | 21.5 | NR |
| Richards et al[58] | United Kingdom | 68 | 1 and 2 | 61 | 32 | 3 |
| Parving et al[59] | Denmark | 35 | 2 | 77.1 | 20 | 2.9 |
| Cordonnier et al[60] | United Kingdom | 26 | 2 | 85 | 15 | NR |
| Nzerue et al[61] | United States | 31 | 2 | 41.9 | 19.4 | 38.7 |
| Lee et al[62] | South Korea | 22 | 2 | 36.4 | 50 | 13.6 |
| Izzedine et al[63] | France | 21 | 1 and 2 | 62 | 38 | NR |
| Castellano et al[64] | Spain | 20 | 2 | 45 | 55 | NR |
| Serra et al[65] | Spain | 35 | 2 | 74.3 | 17.2 | 8.5 |
| Premalatha et al[66] | India | 18 | 2 | 50 | 50 | NR |
| Rychlík et al[67] | Czech Republic | 163 | 2 | 42.4 | 47.5 | 10.1 |
| Tone et al[68] | Japan | 97 | 2 | 36 | 16.5 | 47.5 |
| Moger et al[69] | India | 26 | 2 | 34.6 | 23.1 | 42.3 |
| Soni et al[70] | India | 160 | 2 | 42.5 | 27.5 | 30 |
| Pham et al[71] | United States | 233 | 2 | 27.5 | 53.2 | 19.3 |
| Kharrat et al[72] | Tunisia | 72 | 2 | 34.1 | 69.5 | NR |
| Akimoto et al[73] | Japan | 50 | 2 | 68 | 26 | 6 |
| Huang et al[74] | China | 52 | 2 | 55.7 | 38.5 | 5.8 |
| Lin et al[75] | Taiwan, China | 50 | 2 | 48 | 22 | 30 |
| Ghani et al[76] | Kuwait | 31 | 2 | 54.8 | NR | 45.2 |
| Arif et al[77] | Pakistan | 73 | 2 | 27.3 | 49.3 | NR |
| Hashim Al-Saedi et al[78] | Iraq | 80 | 1 and 2 | NR | NR | 100 |
| Mou et al[79] | China | 69 | 2 | 47.8 | 52.2 | NR |
| Haider et al[80] | Austria | 567 | 1 and 2 | 68 | 17.4 | NR |
| Chang et al[81] | South Korea | 119 | 2 | 36.2 | 53.8 | 10 |
| Bi et al[82] | China | 220 | 2 | 54.5 | NR | 45.5 |
| Chong et al[83] | Malaysia | 110 | 2 | 62.7 | 18.2 | 19.1 |
| Harada et al[84] | Japan | 55 | 2 | 54.5 | 34.5 | 10.9 |
| Oh et al[85] | South Korea | 126 | 2 | 39.7 | 51.6 | 8.7 |
| Yaqub et al[86] | Pakistan | 68 | 2 | 31 | 52 | 17 |
DN: Diabetic nephropathy; NDRD: Non-diabetic renal disease; DM: Diabetes mellitus.
Pathological renal damage is hard to predict only with clinical and laboratory findings[23,52,54]. In patients with T2DM with a higher degree of suspicion for NDRD, it is granted the need of a renal biopsy[53]. Therefore, a more extensive use of biopsy is advisable. There are other cases where is not routinely performed; for example in patients with T2DM and ESRD, especially in those who present with criteria for clinical diagnosis[57].
Complications of kidney biopsy
Kidneys are highly vascular organs; therefore the most common complications associated to kidney biopsies are those related to bleeding, including hematomas and gross hematuria[87]. Iversen and Brun, in 1951 reported the first large series of needle biopsies in kidneys[88]. Later on, Parrish et al[89] also engaged in the labor of performing renal biopsies. Initially, the position of the kidney was determined by abdominal X-ray. Later on, sonography became available. They reported the common complications encountered in a period of 37 years (1951-1988). Complications occurred in 7% of the total biopsies performed (> 1800), consisting mainly of gross hematuria lasting for more than 12 h and pain.
With the introduction of the ultrasound, renal biopsy has become easier and safer. Ultrasound-guided biopsy is the standard method to obtain kidney tissue for diagnosis[90]. Currently, complications are usually minor[37]. A recent meta-analysis that included more than 9400 renal biopsies showed a small risk of macroscopic hematuria of 3%, only requiring blood transfusion in 0.9% of the cases[91]. However, these events are not considered to represent serious medical problems; as they resolve within few hours after the procedure. Bleeding risks are also reduced by using smaller needle gauge, in order to obtain less tissue; but with adequate number of glomeruli per biopsy specimen for pathological diagnosis[90].
Major complications, such as embolization of the renal artery, surgical intervention or death are relatively low. Patients with higher serum creatinine levels, especially women, have shown higher complication rates[87,91].
Biopsy should be avoided in patients with bleeding problems, uncontrolled hypertension, or those unable to cooperate; as these cases have been associated with an increased risk for complications after renal biopsy[92].
Relative contraindications include: severe azotemia, anatomic abnormalities of the kidney such as arterial aneurysm, anticoagulant use, pregnancy, and urinary tract infection[93].
Ongoing development of minimally invasive diagnostic tools
Some useful clinical indicators for DN are the presence of diabetic retinopathy and longer duration of diabetes. In contrast; for NDRD, signs include the presence of acute renal failure and microscopic hematuria[83]. However, these clinical markers are not completely accurate and therefore, efforts have been directed to develop more modern technology in non-invasive diagnosis of DN to help clinicians to decide when a kidney biopsy should be warranted. These include the use of imaging techniques as well as the identification of serum and urinary biomarkers.
Nowadays, diagnostic imaging technology has evolved to help clinicians on their daily decision making regarding which patients to biopsy in order to confirm DN. Insalaco et al[94] had reported the use of eco-colour-Doppler sampling of interlobular renal arteries and determination of their intrarenal resistance indices (RI) to differentiate DN from NDRD.
RI helps to measure hemodynamic changes in the renal arteries. These are usually seen in patients with DN, due to alterations in the compliance of the vessels affecting the blood flow. Therefore, early changes in blood flow are detected by renal Doppler and they may reflect the progression of DN[95]. RI higher than 0.70 is a strong predictor of disease progression to renal failure, as well as RI lower than 0.70 is associated to a slow progression of renal disease[96]. Also, it has been shown that RI in patients with DN is significantly higher than those with NDRD. RI evaluation could help determine prognosis and guide therapy; as this could potentially help to predict which patients with diabetes presenting with proteinuria should undergo renal biopsy; consequently reducing the indications for this procedure.
However, there is still no general agreement for the routine use of Doppler ultrasonography in patients with DN. Results may vary due to other factors that also modify renal vascular resistance; such as age, vascular compliance, high blood pressure, elevated heart rate, and the use of ACE-Inhibitors[97].
Several serum circulating biomarkers may also help to identify patients that will develop DN in patients with diabetes and/or to identify those patients at risk to progress to ESRD in those with DN. Among them: uric acid[98], vitamin D[99], FGF23[100] and TNFR1 and TNFR2[101,102] are promising biomarkers.
Elevated serum concentrations of TNFR1 and TNFR2 are strongly associated with early renal function loss in patients with T1DM and T2DM[101,102]. In contrast, low complement levels (C3 and/or C4) and M-spike have been associated with NDRD (alone or with coexistent DN) in kidney biopsies[51]. Whether any of this biomarker could be causative of the disease initiation and progression remains to be proven through either experimental studies or through intervention studies. It would also be interesting to know if and how these biomarkers correlate to any given histological finding.
MicroRNA profiling has also been studied as a promising tool in the diagnosis of DN. Studies have been reported using this approach to determine different stages of diabetic nephropathy by analyzing urinary microRNA. This includes the potential benefit to distinguish early indicators of DN and to provide a tool for personalized medical therapy[103].
Metabolomics is an evolving field dedicated to identify new metabolites predicting DN in patients with diabetes[104]. Similarly, analysis of urinary proteomics and urinary exosomes have yield promising results[105]. More recently, we have reported that a cell based assay where normal human podocytes are cultured in the presence of the sera of patients with diabetes may help predict the progression to CKD[106].
Finally, it would be interesting to have an integrative approach, where clinical phenotype combines to findings on kidney biopsies. This biologic application would likely represent a very powerful individualized diagnostic and prognostic tool in DN[107].
CONCLUSION
Unfortunately, patients with NDRD are often designated as having DN because of the overlapping features of glomerulopathies[70]. It is important to identify and differentiate these pathologies at an early stage in order to prevent progression and potential complications.
There is an overwhelming number of cases where these diagnoses would lead to changes in treatment, ranging from the use of immunosuppression to titration of renin-angiotensin-aldosterone system blockade[108].
Common clinical practice is to biopsy patients with diabetes with a low pre-test probability for DN, such as patients presenting with AKI, low complements, and hematuria suggesting an increased likelihood of finding NDRD on biopsy[51]. Nevertheless, as loss of renal function correlates with increased mortality. Therefore, any intervention that would help to delay progression to ESRD should significantly increase survival. In view of the fact that histology is necessary to characterize different glomerular diseases originating several nonspecific clinical presentations, kidney biopsy would help to direct a better management[109].
Routine use of renal biopsy should be implemented, especially in those with atypical features[83], for several reasons: (1) it helps to characterize the epidemiological features of renal diseases in diabetic patients[110,111]; (2) it provides the opportunity to determine how histological and high-throughput profiling correlate with the clinical phenotype[111]; and (3) it set the basis for personalized management strategies[23,112].
The ability to differentiate between renal pathologies other than DN (that could be reversed with a specific treatment) and DN would be of extreme importance. We strongly support the recommendation that kidney biopsy should become a routine tool in specific patients at high risk of developing CKD, especially in those cases where this practice helps to reverse and/or prevent further kidney damage as it will help to direct assertive and aggressive treatment to many of the non-diabetic nephropathies to prevent or delay poor outcomes[81,108].
There is lack of studies in the literature regarding the universal use of kidney biopsy on patients with diabetes. As new studies have become available to demonstrate how quantitative histological features may predict the disease course earlier than albuminuria[113], our level of confidence to perform routine kidney biopsies in patients with diabetes should increase. New research studies are required, longitudinal observational clinical trials as well as interventional trials, where the implementation of routine kidney biopsy is evaluated for patients with diabetes at time of diagnosis to evidence improvement in outcomes. These findings on kidney biopsies may help select the population of patients at highest risk of disease progression and may offer a new hard outcome measure to study DN.
Footnotes
P- Reviewers: Kumar KVS, Lehtonen SH, Lim AKH, Ido Y S- Editor: Wen LL L- Editor: A E- Editor: Liu XM
References
- 1.National Kidney Foundation. KDOQI Clinical Practice Guideline for Diabetes and CKD: 2012 Update. Am J Kidney Dis. 2012;60:850–886. doi: 10.1053/j.ajkd.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 2.Fox CS, Matsushita K, Woodward M, Bilo HJ, Chalmers J, Heerspink HJ, Lee BJ, Perkins RM, Rossing P, Sairenchi T, et al. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis. Lancet. 2012;380:1662–1673. doi: 10.1016/S0140-6736(12)61350-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collins AJ, Foley RN, Herzog C, Chavers B, Gilbertson D, Herzog C, Ishani A, Johansen K, Kasiske B, Kutner N, et al. US Renal Data System 2012 Annual Data Report. Am J Kidney Dis. 2013;61:A7, e1–476. doi: 10.1053/j.ajkd.2012.11.031. [DOI] [PubMed] [Google Scholar]
- 4.Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, Lin JK, Farzadfar F, Khang YH, Stevens GA, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2·7 million participants. Lancet. 2011;378:31–40. doi: 10.1016/S0140-6736(11)60679-X. [DOI] [PubMed] [Google Scholar]
- 5.Sowers JR, Epstein M. Diabetes mellitus and associated hypertension, vascular disease, and nephropathy. An update. Hypertension. 1995;26:869–879. doi: 10.1161/01.hyp.26.6.869. [DOI] [PubMed] [Google Scholar]
- 6.de Boer IH, Rue TC, Hall YN, Heagerty PJ, Weiss NS, Himmelfarb J. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA. 2011;305:2532–2539. doi: 10.1001/jama.2011.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Papale M, Di Paolo S, Magistroni R, Lamacchia O, Di Palma AM, De Mattia A, Rocchetti MT, Furci L, Pasquali S, De Cosmo S, et al. Urine proteome analysis may allow noninvasive differential diagnosis of diabetic nephropathy. Diabetes Care. 2010;33:2409–2415. doi: 10.2337/dc10-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Diabetes Association. Standards of medical care in diabetes--2013. Diabetes Care. 2013;36 Suppl 1:S11–S66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gross JL, de Azevedo MJ, Silveiro SP, Canani LH, Caramori ML, Zelmanovitz T. Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care. 2005;28:164–176. doi: 10.2337/diacare.28.1.164. [DOI] [PubMed] [Google Scholar]
- 10.Diez-Sampedro A, Lenz O, Fornoni A. Podocytopathy in diabetes: a metabolic and endocrine disorder. Am J Kidney Dis. 2011;58:637–646. doi: 10.1053/j.ajkd.2011.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rudberg S, Osterby R. Decreasing glomerular filtration rate--an indicator of more advanced diabetic glomerulopathy in the early course of microalbuminuria in IDDM adolescents? Nephrol Dial Transplant. 1997;12:1149–1154. doi: 10.1093/ndt/12.6.1149. [DOI] [PubMed] [Google Scholar]
- 12.Rabkin R. Diabetic nephropathy. Clin Cornerstone. 2003;5:1–11. doi: 10.1016/s1098-3597(03)90014-7. [DOI] [PubMed] [Google Scholar]
- 13.Nelson RG, Knowler WC, McCance DR, Sievers ML, Pettitt DJ, Charles MA, Hanson RL, Liu QZ, Bennett PH. Determinants of end-stage renal disease in Pima Indians with type 2 (non-insulin-dependent) diabetes mellitus and proteinuria. Diabetologia. 1993;36:1087–1093. doi: 10.1007/BF02374503. [DOI] [PubMed] [Google Scholar]
- 14.Ismail N, Becker B, Strzelczyk P, Ritz E. Renal disease and hypertension in non-insulin-dependent diabetes mellitus. Kidney Int. 1999;55:1–28. doi: 10.1046/j.1523-1755.1999.00232.x. [DOI] [PubMed] [Google Scholar]
- 15.Mogensen CE, Christensen CK, Vittinghus E. The stages in diabetic renal disease. With emphasis on the stage of incipient diabetic nephropathy. Diabetes. 1983;32 Suppl 2:64–78. doi: 10.2337/diab.32.2.s64. [DOI] [PubMed] [Google Scholar]
- 16.Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiol Rev. 2013;93:137–188. doi: 10.1152/physrev.00045.2011. [DOI] [PubMed] [Google Scholar]
- 17.Pagtalunan ME, Miller PL, Jumping-Eagle S, Nelson RG, Myers BD, Rennke HG, Coplon NS, Sun L, Meyer TW. Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Invest. 1997;99:342–348. doi: 10.1172/JCI119163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caramori ML, Kim Y, Huang C, Fish AJ, Rich SS, Miller ME, Russell G, Mauer M. Cellular basis of diabetic nephropathy: 1. Study design and renal structural-functional relationships in patients with long-standing type 1 diabetes. Diabetes. 2002;51:506–513. doi: 10.2337/diabetes.51.2.506. [DOI] [PubMed] [Google Scholar]
- 19.Fioretto P, Steffes MW, Mauer M. Glomerular structure in nonproteinuric IDDM patients with various levels of albuminuria. Diabetes. 1994;43:1358–1364. doi: 10.2337/diab.43.11.1358. [DOI] [PubMed] [Google Scholar]
- 20.Newman DJ, Mattock MB, Dawnay AB, Kerry S, McGuire A, Yaqoob M, Hitman GA, Hawke C. Systematic review on urine albumin testing for early detection of diabetic complications. Health Technol Assess. 2005;9:iii–vi, xiii-163. doi: 10.3310/hta9300. [DOI] [PubMed] [Google Scholar]
- 21.Berg UB, Torbjörnsdotter TB, Jaremko G, Thalme B. Kidney morphological changes in relation to long-term renal function and metabolic control in adolescents with IDDM. Diabetologia. 1998;41:1047–1056. doi: 10.1007/s001250051029. [DOI] [PubMed] [Google Scholar]
- 22.Nyumura I, Honda K, Tanabe K, Teraoka S, Iwamoto Y. Early histologic lesions and risk factors for recurrence of diabetic kidney disease after kidney transplantation. Transplantation. 2012;94:612–619. doi: 10.1097/TP.0b013e31825e4a5f. [DOI] [PubMed] [Google Scholar]
- 23.Mazzucco G, Bertani T, Fortunato M, Bernardi M, Leutner M, Boldorini R, Monga G. Different patterns of renal damage in type 2 diabetes mellitus: a multicentric study on 393 biopsies. Am J Kidney Dis. 2002;39:713–720. doi: 10.1053/ajkd.2002.31988. [DOI] [PubMed] [Google Scholar]
- 24.Christensen PK, Larsen S, Horn T, Olsen S, Parving HH. Causes of albuminuria in patients with type 2 diabetes without diabetic retinopathy. Kidney Int. 2000;58:1719–1731. doi: 10.1046/j.1523-1755.2000.00333.x. [DOI] [PubMed] [Google Scholar]
- 25.Packham DK, Ivory SE, Reutens AT, Wolfe R, Rohde R, Lambers Heerspink H, Dwyer JP, Atkins RC, Lewis J. Proteinuria in type 2 diabetic patients with renal impairment: the changing face of diabetic nephropathy. Nephron Clin Pract. 2011;118:c331–c338. doi: 10.1159/000323139. [DOI] [PubMed] [Google Scholar]
- 26.Bruno G, Merletti F, Biggeri A, Bargero G, Ferrero S, Pagano G, Cavallo Perin P. Progression to overt nephropathy in type 2 diabetes: the Casale Monferrato Study. Diabetes Care. 2003;26:2150–2155. doi: 10.2337/diacare.26.7.2150. [DOI] [PubMed] [Google Scholar]
- 27.Romero-Aroca P, Baget-Bernaldiz M, Reyes-Torres J, Fernandez-Ballart J, Plana-Gil N, Mendez-Marin I, Pareja-Rios A. Relationship between diabetic retinopathy, microalbuminuria and overt nephropathy, and twenty-year incidence follow-up of a sample of type 1 diabetic patients. J Diabetes Complications. 2012;26:506–512. doi: 10.1016/j.jdiacomp.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 28.Adler AI, Stevens RJ, Manley SE, Bilous RW, Cull CA, Holman RR. Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64) Kidney Int. 2003;63:225–232. doi: 10.1046/j.1523-1755.2003.00712.x. [DOI] [PubMed] [Google Scholar]
- 29.Fineberg D, Jandeleit-Dahm KA, Cooper ME. Diabetic nephropathy: diagnosis and treatment. Nat Rev Endocrinol. 2013;9:713–723. doi: 10.1038/nrendo.2013.184. [DOI] [PubMed] [Google Scholar]
- 30.White KE, Bilous RW. Type 2 diabetic patients with nephropathy show structural-functional relationships that are similar to type 1 disease. J Am Soc Nephrol. 2000;11:1667–1673. doi: 10.1681/ASN.V1191667. [DOI] [PubMed] [Google Scholar]
- 31.Mauer M, Drummond K. The early natural history of nephropathy in type 1 diabetes: I. Study design and baseline characteristics of the study participants. Diabetes. 2002;51:1572–1579. doi: 10.2337/diabetes.51.5.1572. [DOI] [PubMed] [Google Scholar]
- 32.Steffes MW, Bilous RW, Sutherland DE, Mauer SM. Cell and matrix components of the glomerular mesangium in type I diabetes. Diabetes. 1992;41:679–684. doi: 10.2337/diab.41.6.679. [DOI] [PubMed] [Google Scholar]
- 33.Bangstad HJ, Østerby R, Rudberg S, Hartmann A, Brabrand K, Hanssen KF. Kidney function and glomerulopathy over 8 years in young patients with Type I (insulin-dependent) diabetes mellitus and microalbuminuria. Diabetologia. 2002;45:253–261. doi: 10.1007/s00125-001-0744-y. [DOI] [PubMed] [Google Scholar]
- 34.Schoolwerth AC, Engelgau MM, Hostetter TH, Rufo KH, Chianchiano D, McClellan WM, Warnock DG, Vinicor F. Chronic kidney disease: a public health problem that needs a public health action plan. Prev Chronic Dis. 2006;3:A57. [PMC free article] [PubMed] [Google Scholar]
- 35.Ninomiya T, Perkovic V, de Galan BE, Zoungas S, Pillai A, Jardine M, Patel A, Cass A, Neal B, Poulter N, et al. Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J Am Soc Nephrol. 2009;20:1813–1821. doi: 10.1681/ASN.2008121270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Penescu M, Mandache E. The value of kidney biopsy in diabetes mellitus. Rom J Morphol Embryol. 2010;51:13–19. [PubMed] [Google Scholar]
- 37.Zhang PP, Ge YC, Li SJ, Xie HL, Li LS, Liu ZH. Renal biopsy in type 2 diabetes: timing of complications and evaluating of safety in Chinese patients. Nephrology (Carlton) 2011;16:100–105. doi: 10.1111/j.1440-1797.2010.01369.x. [DOI] [PubMed] [Google Scholar]
- 38.Kanwar YS, Sun L, Xie P, Liu FY, Chen S. A glimpse of various pathogenetic mechanisms of diabetic nephropathy. Annu Rev Pathol. 2011;6:395–423. doi: 10.1146/annurev.pathol.4.110807.092150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raparia K, Usman I, Kanwar YS. Renal morphologic lesions reminiscent of diabetic nephropathy. Arch Pathol Lab Med. 2013;137:351–359. doi: 10.5858/arpa.2012-0243-RA. [DOI] [PubMed] [Google Scholar]
- 40.Schwartz MM, Lewis EJ, Leonard-Martin T, Lewis JB, Batlle D. Renal pathology patterns in type II diabetes mellitus: relationship with retinopathy. The Collaborative Study Group. Nephrol Dial Transplant. 1998;13:2547–2552. doi: 10.1093/ndt/13.10.2547. [DOI] [PubMed] [Google Scholar]
- 41.Kimmelstiel P, Wilson C. Benign and Malignant Hypertension and Nephrosclerosis: A Clinical and Pathological Study. Am J Pathol. 1936;12:45–82.3. [PMC free article] [PubMed] [Google Scholar]
- 42.Fioretto P, Mauer M. Histopathology of diabetic nephropathy. Semin Nephrol. 2007;27:195–207. doi: 10.1016/j.semnephrol.2007.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tervaert TW, Mooyaart AL, Amann K, Cohen AH, Cook HT, Drachenberg CB, Ferrario F, Fogo AB, Haas M, de Heer E, et al. Pathologic classification of diabetic nephropathy. J Am Soc Nephrol. 2010;21:556–563. doi: 10.1681/ASN.2010010010. [DOI] [PubMed] [Google Scholar]
- 44.Meyer TW, Bennett PH, Nelson RG. Podocyte number predicts long-term urinary albumin excretion in Pima Indians with Type II diabetes and microalbuminuria. Diabetologia. 1999;42:1341–1344. doi: 10.1007/s001250051447. [DOI] [PubMed] [Google Scholar]
- 45.Steffes MW, Schmidt D, McCrery R, Basgen JM. Glomerular cell number in normal subjects and in type 1 diabetic patients. Kidney Int. 2001;59:2104–2113. doi: 10.1046/j.1523-1755.2001.00725.x. [DOI] [PubMed] [Google Scholar]
- 46.White KE, Bilous RW, Marshall SM, El Nahas M, Remuzzi G, Piras G, De Cosmo S, Viberti G. Podocyte number in normotensive type 1 diabetic patients with albuminuria. Diabetes. 2002;51:3083–3089. doi: 10.2337/diabetes.51.10.3083. [DOI] [PubMed] [Google Scholar]
- 47.White KE, Bilous RW. Structural alterations to the podocyte are related to proteinuria in type 2 diabetic patients. Nephrol Dial Transplant. 2004;19:1437–1440. doi: 10.1093/ndt/gfh129. [DOI] [PubMed] [Google Scholar]
- 48.Verzola D, Gandolfo MT, Ferrario F, Rastaldi MP, Villaggio B, Gianiorio F, Giannoni M, Rimoldi L, Lauria F, Miji M, et al. Apoptosis in the kidneys of patients with type II diabetic nephropathy. Kidney Int. 2007;72:1262–1272. doi: 10.1038/sj.ki.5002531. [DOI] [PubMed] [Google Scholar]
- 49.Nakamura T, Ushiyama C, Suzuki S, Hara M, Shimada N, Ebihara I, Koide H. Urinary excretion of podocytes in patients with diabetic nephropathy. Nephrol Dial Transplant. 2000;15:1379–1383. doi: 10.1093/ndt/15.9.1379. [DOI] [PubMed] [Google Scholar]
- 50.Zhuo L, Ren W, Li W, Zou G, Lu J. Evaluation of renal biopsies in type 2 diabetic patients with kidney disease: a clinicopathological study of 216 cases. Int Urol Nephrol. 2013;45:173–179. doi: 10.1007/s11255-012-0164-6. [DOI] [PubMed] [Google Scholar]
- 51.Sharma SG, Bomback AS, Radhakrishnan J, Herlitz LC, Stokes MB, Markowitz GS, D’Agati VD. The modern spectrum of renal biopsy findings in patients with diabetes. Clin J Am Soc Nephrol. 2013;8:1718–1724. doi: 10.2215/CJN.02510213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hironaka K, Makino H, Ikeda S, Haramoto T, Ota Z. Nondiabetic renal disease complicating diabetic nephropathy. J Diabet Complications. 1991;5:148–149. doi: 10.1016/0891-6632(91)90051-p. [DOI] [PubMed] [Google Scholar]
- 53.Wong TY, Choi PC, Szeto CC, To KF, Tang NL, Chan AW, Li PK, Lai FM. Renal outcome in type 2 diabetic patients with or without coexisting nondiabetic nephropathies. Diabetes Care. 2002;25:900–905. doi: 10.2337/diacare.25.5.900. [DOI] [PubMed] [Google Scholar]
- 54.He F, Xia X, Wu XF, Yu XQ, Huang FX. Diabetic retinopathy in predicting diabetic nephropathy in patients with type 2 diabetes and renal disease: a meta-analysis. Diabetologia. 2013;56:457–466. doi: 10.1007/s00125-012-2796-6. [DOI] [PubMed] [Google Scholar]
- 55.Prakash J, Lodha M, Singh SK, Vohra R, Raja R. Diabetic retinopathy is a poor predictor of type of nephropathy in proteinuric type 2 diabetic patients. J Assoc Physicians India. 2007;55:412–416. [PubMed] [Google Scholar]
- 56.Mak SK, Gwi E, Chan KW, Wong PN, Lo KY, Lee KF, Wong AK. Clinical predictors of non-diabetic renal disease in patients with non-insulin dependent diabetes mellitus. Nephrol Dial Transplant. 1997;12:2588–2591. doi: 10.1093/ndt/12.12.2588. [DOI] [PubMed] [Google Scholar]
- 57.Biesenbach G, Bodlaj G, Pieringer H, Sedlak M. Clinical versus histological diagnosis of diabetic nephropathy--is renal biopsy required in type 2 diabetic patients with renal disease? QJM. 2011;104:771–774. doi: 10.1093/qjmed/hcr059. [DOI] [PubMed] [Google Scholar]
- 58.Richards NT, Greaves I, Lee SJ, Howie AJ, Adu D, Michael J. Increased prevalence of renal biopsy findings other than diabetic glomerulopathy in type II diabetes mellitus. Nephrol Dial Transplant. 1992;7:397–399. [PubMed] [Google Scholar]
- 59.Parving HH, Gall MA, Skøtt P, Jørgensen HE, Løkkegaard H, Jørgensen F, Nielsen B, Larsen S. Prevalence and causes of albuminuria in non-insulin-dependent diabetic patients. Kidney Int. 1992;41:758–762. doi: 10.1038/ki.1992.118. [DOI] [PubMed] [Google Scholar]
- 60.Cordonnier DJ, Pinel N, Barro C, Maynard M, Zaoui P, Halimi S, Hurault de Ligny B, Reznic Y, Simon D, Bilous RW. Expansion of cortical interstitium is limited by converting enzyme inhibition in type 2 diabetic patients with glomerulosclerosis. The Diabiopsies Group. J Am Soc Nephrol. 1999;10:1253–1263. doi: 10.1681/ASN.V1061253. [DOI] [PubMed] [Google Scholar]
- 61.Nzerue CM, Hewan-Lowe K, Harvey P, Mohammed D, Furlong B, Oster R. Prevalence of non-diabetic renal disease among African-American patients with type II diabetes mellitus. Scand J Urol Nephrol. 2000;34:331–335. doi: 10.1080/003655900750048378. [DOI] [PubMed] [Google Scholar]
- 62.Lee EY, Chung CH, Choi SO. Non-diabetic renal disease in patients with non-insulin dependent diabetes mellitus. Yonsei Med J. 1999;40:321–326. doi: 10.3349/ymj.1999.40.4.321. [DOI] [PubMed] [Google Scholar]
- 63.Izzedine H, Fongoro S, Pajot O, Beaufils H, Deray G. Retinopathy, hematuria, and diabetic nephropathy. Nephron. 2001;88:382–383. doi: 10.1159/000046025. [DOI] [PubMed] [Google Scholar]
- 64.Castellano I, Covarsí A, Novillo R, Gómez-Martino JR, Ferrando L. [Renal histological lesions in patients with type II diabetes mellitus] Nefrologia. 2002;22:162–169. [PubMed] [Google Scholar]
- 65.Serra A, Romero R, Bayés B, Lopez D, Bonet J. Is there a need for changes in renal biopsy criteria in proteinuria in type 2 diabetes? Diabetes Res Clin Pract. 2002;58:149–153. doi: 10.1016/s0168-8227(02)00131-6. [DOI] [PubMed] [Google Scholar]
- 66.Premalatha G, Vidhya K, Deepa R, Ravikumar R, Rema M, Mohan V. Prevalence of non-diabetic renal disease in type 2 diabetic patients in a diabetes centre in Southern India. J Assoc Physicians India. 2002;50:1135–1139. [PubMed] [Google Scholar]
- 67.Rychlík I, Jancová E, Tesar V, Kolsky A, Lácha J, Stejskal J, Stejskalová A, Dusek J, Herout V. The Czech registry of renal biopsies. Occurrence of renal diseases in the years 1994-2000. Nephrol Dial Transplant. 2004;19:3040–3049. doi: 10.1093/ndt/gfh521. [DOI] [PubMed] [Google Scholar]
- 68.Tone A, Shikata K, Matsuda M, Usui H, Okada S, Ogawa D, Wada J, Makino H. Clinical features of non-diabetic renal diseases in patients with type 2 diabetes. Diabetes Res Clin Pract. 2005;69:237–242. doi: 10.1016/j.diabres.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 69.Moger V, Kumar SK, Sakhuja V, Joshi K, Walker R, Kohli HS, Sud K, Gupta KL, Jha V. Rapidly progressive renal failure in type 2 diabetes in the tropical environment: a clinico-pathological study. Ren Fail. 2005;27:595–600. doi: 10.1080/08860220500200205. [DOI] [PubMed] [Google Scholar]
- 70.Soni SS, Gowrishankar S, Kishan AG, Raman A. Non diabetic renal disease in type 2 diabetes mellitus. Nephrology (Carlton) 2006;11:533–537. doi: 10.1111/j.1440-1797.2006.00681.x. [DOI] [PubMed] [Google Scholar]
- 71.Pham TT, Sim JJ, Kujubu DA, Liu IL, Kumar VA. Prevalence of nondiabetic renal disease in diabetic patients. Am J Nephrol. 2007;27:322–328. doi: 10.1159/000102598. [DOI] [PubMed] [Google Scholar]
- 72.Kharrat M, Kammoun K, Charfeddine K, Yaich S, Zaghdene S, Chaker H, Jarraya F, Ben Hmida M, Jlidi R, Hachicha J. [Renal biopsy findings in diabetes mellitus] Tunis Med. 2007;85:216–219. [PubMed] [Google Scholar]
- 73.Akimoto T, Ito C, Saito O, Takahashi H, Takeda S, Ando Y, Muto S, Kusano E. Microscopic hematuria and diabetic glomerulosclerosis--clinicopathological analysis of type 2 diabetic patients associated with overt proteinuria. Nephron Clin Pract. 2008;109:c119–c126. doi: 10.1159/000145454. [DOI] [PubMed] [Google Scholar]
- 74.Huang F, Yang Q, Chen L, Tang S, Liu W, Yu X. Renal pathological change in patients with type 2 diabetes is not always diabetic nephropathy: a report of 52 cases. Clin Nephrol. 2007;67:293–297. doi: 10.5414/cnp67293. [DOI] [PubMed] [Google Scholar]
- 75.Lin YL, Peng SJ, Ferng SH, Tzen CY, Yang CS. Clinical indicators which necessitate renal biopsy in type 2 diabetes mellitus patients with renal disease. Int J Clin Pract. 2009;63:1167–1176. doi: 10.1111/j.1742-1241.2008.01753.x. [DOI] [PubMed] [Google Scholar]
- 76.Ghani AA, Al Waheeb S, Al Sahow A, Hussain N. Renal biopsy in patients with type 2 diabetes mellitus: indications and nature of the lesions. Ann Saudi Med. 2009;29:450–453. doi: 10.4103/0256-4947.57167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Arif M, Arif MK, Arif MS. An evaluation of renal biopsy in type-II diabetic patients. J Coll Physicians Surg Pak. 2009;19:627–631. doi: 10.2009/JCPSP.627631. [DOI] [PubMed] [Google Scholar]
- 78.Hashim Al-Saedi AJ. Pathology of nondiabetic glomerular disease among adult Iraqi patients from a single center. Saudi J Kidney Dis Transpl. 2009;20:858–861. [PubMed] [Google Scholar]
- 79.Mou S, Wang Q, Liu J, Che X, Zhang M, Cao L, Zhou W, Ni Z. Prevalence of non-diabetic renal disease in patients with type 2 diabetes. Diabetes Res Clin Pract. 2010;87:354–359. doi: 10.1016/j.diabres.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 80.Haider DG, Peric S, Friedl A, Fuhrmann V, Wolzt M, Hörl WH, Soleiman A. Kidney biopsy in patients with diabetes mellitus. Clin Nephrol. 2011;76:180–185. doi: 10.5414/cn106955. [DOI] [PubMed] [Google Scholar]
- 81.Chang TI, Park JT, Kim JK, Kim SJ, Oh HJ, Yoo DE, Han SH, Yoo TH, Kang SW. Renal outcomes in patients with type 2 diabetes with or without coexisting non-diabetic renal disease. Diabetes Res Clin Pract. 2011;92:198–204. doi: 10.1016/j.diabres.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 82.Bi H, Chen N, Ling G, Yuan S, Huang G, Liu R. Nondiabetic renal disease in type 2 diabetic patients: a review of our experience in 220 cases. Ren Fail. 2011;33:26–30. doi: 10.3109/0886022X.2010.536292. [DOI] [PubMed] [Google Scholar]
- 83.Chong YB, Keng TC, Tan LP, Ng KP, Kong WY, Wong CM, Cheah PL, Looi LM, Tan SY. Clinical predictors of non-diabetic renal disease and role of renal biopsy in diabetic patients with renal involvement: a single centre review. Ren Fail. 2012;34:323–328. doi: 10.3109/0886022X.2011.647302. [DOI] [PubMed] [Google Scholar]
- 84.Harada K, Akai Y, Sumida K, Yoshikawa M, Takahashi H, Yamaguchi Y, Kubo A, Iwano M, Saito Y. Significance of renal biopsy in patients with presumed diabetic nephropathy. J Diabetes Investig. 2013;4:88–93. doi: 10.1111/j.2040-1124.2012.00233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Oh SW, Kim S, Na KY, Chae DW, Kim S, Jin DC, Chin HJ. Clinical implications of pathologic diagnosis and classification for diabetic nephropathy. Diabetes Res Clin Pract. 2012;97:418–424. doi: 10.1016/j.diabres.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 86.Yaqub S, Kashif W, Hussain SA. Non-diabetic renal disease in patients with type-2 diabetes mellitus. Saudi J Kidney Dis Transpl. 2012;23:1000–1007. doi: 10.4103/1319-2442.100882. [DOI] [PubMed] [Google Scholar]
- 87.Whittier WL. Complications of the percutaneous kidney biopsy. Adv Chronic Kidney Dis. 2012;19:179–187. doi: 10.1053/j.ackd.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 88.Cameron JS, Hicks J. The introduction of renal biopsy into nephrology from 1901 to 1961: a paradigm of the forming of nephrology by technology. Am J Nephrol. 1997;17:347–358. doi: 10.1159/000169122. [DOI] [PubMed] [Google Scholar]
- 89.Parrish AE. Complications of percutaneous renal biopsy: a review of 37 years’ experience. Clin Nephrol. 1992;38:135–141. [PubMed] [Google Scholar]
- 90.Hergesell O, Felten H, Andrassy K, Kühn K, Ritz E. Safety of ultrasound-guided percutaneous renal biopsy-retrospective analysis of 1090 consecutive cases. Nephrol Dial Transplant. 1998;13:975–977. doi: 10.1093/ndt/13.4.975. [DOI] [PubMed] [Google Scholar]
- 91.Corapi KM, Chen JL, Balk EM, Gordon CE. Bleeding complications of native kidney biopsy: a systematic review and meta-analysis. Am J Kidney Dis. 2012;60:62–73. doi: 10.1053/j.ajkd.2012.02.330. [DOI] [PubMed] [Google Scholar]
- 92.Sethi I, Brier M, Dwyer A. Predicting post renal biopsy complications. Semin Dial. 2013;26:633–635. doi: 10.1111/sdi.12076. [DOI] [PubMed] [Google Scholar]
- 93.Clinical competence in percutaneous renal biopsy. Health and Public Policy Committee. American College of Physicians. Ann Intern Med. 1988;108:301–303. [PubMed] [Google Scholar]
- 94.Insalaco M, Zamboli P, Floccari F, Marrocco F, Andrulli S, Logias F, Di Lullo L, Fiorini F, Granata A. Indication to renal biopsy in DM2 patients: potential role of intrarenal resistive index. Arch Ital Urol Androl. 2012;84:283–286. [PubMed] [Google Scholar]
- 95.Raut TP, Patil TB, Khot RS, Sargar K M, Patil MB, Bansod YV. Clinical Profile of Diabetic Nephropathy and Correlation With Intrarenal Resistivity Index by Duplex Ultrasonography. World J Nephrol Urol. 2012;1:107–114. [Google Scholar]
- 96.Parolini C, Noce A, Staffolani E, Giarrizzo GF, Costanzi S, Splendiani G. Renal resistive index and long-term outcome in chronic nephropathies. Radiology. 2009;252:888–896. doi: 10.1148/radiol.2523080351. [DOI] [PubMed] [Google Scholar]
- 97.Hanamura K, Tojo A, Kinugasa S, Asaba K, Fujita T. The resistive index is a marker of renal function, pathology, prognosis, and responsiveness to steroid therapy in chronic kidney disease patients. Int J Nephrol. 2012;2012:139565. doi: 10.1155/2012/139565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jalal DI, Maahs DM, Hovind P, Nakagawa T. Uric acid as a mediator of diabetic nephropathy. Semin Nephrol. 2011;31:459–465. doi: 10.1016/j.semnephrol.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Agarwal R. Vitamin D, proteinuria, diabetic nephropathy, and progression of CKD. Clin J Am Soc Nephrol. 2009;4:1523–1528. doi: 10.2215/CJN.02010309. [DOI] [PubMed] [Google Scholar]
- 100.Isakova T. Comparison of mineral metabolites as risk factors for adverse clinical outcomes in CKD. Semin Nephrol. 2013;33:106–117. doi: 10.1016/j.semnephrol.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 101.Gohda T, Niewczas MA, Ficociello LH, Walker WH, Skupien J, Rosetti F, Cullere X, Johnson AC, Crabtree G, Smiles AM, et al. Circulating TNF receptors 1 and 2 predict stage 3 CKD in type 1 diabetes. J Am Soc Nephrol. 2012;23:516–524. doi: 10.1681/ASN.2011060628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Niewczas MA, Gohda T, Skupien J, Smiles AM, Walker WH, Rosetti F, Cullere X, Eckfeldt JH, Doria A, Mayadas TN, et al. Circulating TNF receptors 1 and 2 predict ESRD in type 2 diabetes. J Am Soc Nephrol. 2012;23:507–515. doi: 10.1681/ASN.2011060627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Argyropoulos C, Wang K, McClarty S, Huang D, Bernardo J, Ellis D, Orchard T, Galas D, Johnson J. Urinary microRNA profiling in the nephropathy of type 1 diabetes. PLoS One. 2013;8:e54662. doi: 10.1371/journal.pone.0054662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hirayama A, Nakashima E, Sugimoto M, Akiyama S, Sato W, Maruyama S, Matsuo S, Tomita M, Yuzawa Y, Soga T. Metabolic profiling reveals new serum biomarkers for differentiating diabetic nephropathy. Anal Bioanal Chem. 2012;404:3101–3109. doi: 10.1007/s00216-012-6412-x. [DOI] [PubMed] [Google Scholar]
- 105.Zubiri I, Vivanco F, Alvarez-Llamas G. Proteomic analysis of urinary exosomes in cardiovascular and associated kidney diseases by two-dimensional electrophoresis and LC-MS/MS. Methods Mol Biol. 2013;1000:209–220. doi: 10.1007/978-1-62703-405-0_16. [DOI] [PubMed] [Google Scholar]
- 106.Merscher-Gomez S, Guzman J, Pedigo CE, Lehto M, Aguillon-Prada R, Mendez A, Lassenius MI, Forsblom C, Yoo T, Villarreal R, et al. Cyclodextrin protects podocytes in diabetic kidney disease. Diabetes. 2013;62:3817–3827. doi: 10.2337/db13-0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Komorowsky CV, Brosius FC, Pennathur S, Kretzler M. Perspectives on systems biology applications in diabetic kidney disease. J Cardiovasc Transl Res. 2012;5:491–508. doi: 10.1007/s12265-012-9382-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shimizu M, Furuichi K, Toyama T, Kitajima S, Hara A, Kitagawa K, Iwata Y, Sakai N, Takamura T, Yoshimura M, et al. Long-term outcomes of Japanese type 2 diabetic patients with biopsy-proven diabetic nephropathy. Diabetes Care. 2013;36:3655–3662. doi: 10.2337/dc13-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Di Palma AM, d’Apollo AM, Vendemia F, Stallone G, Infante B, Gesualdo L. Kidney biopsy in the elderly. J Nephrol. 2010;23 Suppl 15:S55–S60. [PubMed] [Google Scholar]
- 110.Li LS, Liu ZH. Epidemiologic data of renal diseases from a single unit in China: analysis based on 13,519 renal biopsies. Kidney Int. 2004;66:920–923. doi: 10.1111/j.1523-1755.2004.00837.x. [DOI] [PubMed] [Google Scholar]
- 111.Haas M, Spargo BH, Wit EJ, Meehan SM. Etiologies and outcome of acute renal insufficiency in older adults: a renal biopsy study of 259 cases. Am J Kidney Dis. 2000;35:433–447. doi: 10.1016/s0272-6386(00)70196-x. [DOI] [PubMed] [Google Scholar]
- 112.Stratta P, Canavese C, Marengo M, Mesiano P, Besso L, Quaglia M, Bergamo D, Monga G, Mazzucco G, Ciccone G. Risk management of renal biopsy: 1387 cases over 30 years in a single centre. Eur J Clin Invest. 2007;37:954–963. doi: 10.1111/j.1365-2362.2007.01885.x. [DOI] [PubMed] [Google Scholar]
- 113.Caramori ML, Parks A, Mauer M. Renal lesions predict progression of diabetic nephropathy in type 1 diabetes. J Am Soc Nephrol. 2013;24:1175–1181. doi: 10.1681/ASN.2012070739. [DOI] [PMC free article] [PubMed] [Google Scholar]


