Abstract
AIM: To assess the effect of different hypolipidemic treatment strategies on glycemic profile in mixed dyslipidemia patients.
METHODS: This is a prespecified analysis of a prospective, randomized, open-label, blinded end point (PROBE) study (ClinicalTrials.gov identifier: NCT01010516). Patients (n = 100) with mixed dyslipidemia on a standard statin dose who had not achieved lipid targets were randomized to switch to the highest dose of rosuvastatin (40 mg/d) or to add-on-statin extended release nicotinic acid (ER-NA)/laropiprant (LRPT) or to add-on-statin micronised fenofibrate for a total of 3 mo. Fasting plasma glucose (FPG), glycosylated hemoglobin (HbA1c), homeostasis model assessment of insulin resistance (HOMA-IR) index and lipid profile were evaluated at baseline and 3 mo after treatment intervention.
RESULTS: FPG increased in add-on ER-NA/LRPT and rosuvastatin monotherapy groups by 9.7% and 4.4%, respectively (P < 0.01 between the 2 groups and compared with baseline), while it did not significantly change in the add-on fenofibrate group. Similarly, HbA1c increased by 0.3% in add-on ER-NA/LRPT group and by 0.2% in the rosuvastatin monotherapy group (P < 0.01 for all comparisons vs baseline and for the comparison between the 2 groups), while no significant change was reported in the add-on fenofibrate group. HOMA-IR increased by 65% in add-on ER-NA/LRPT and by 14% in rosuvastatin monotherapy group, while it decreased by 6% in the add-on fenofibrate group (P < 0.01 vs baseline and for all comparisons among the groups). Non-HDL-C decreased in all groups (by 23.7%, 24.7% and 7% in the rosuvastatin, ER-NA/LRPT and fenofibrate group, respectively, P < 0.01 for all vs baseline and P < 0.01 for all vs with fenofibrate group).
CONCLUSION: Both addition of ER-NA/LRPT and switch to the highest dose of rosuvastatin deteriorated glycemic profile in patients with mixed dyslipidemia, while add-on fenofibrate seems to increase insulin sensitivity.
Keywords: Diabetes mellitus, Fasting plasma glucose, Fenofibrate, Insulin, Nicotinic acid/laropiprant, Rosuvastatin
Core tip: In this study both addition of extended release nicotinic acid/laropiprant and switch to the highest dose of rosuvastatin deteriorated glycemic profile in patients with mixed dyslipidemia who were inadequately controlled with a standard statin dose. Add-on fenofibrate, on the other hand, seems to increase insulin sensitivity. Larger prospective studies should address the effect of these treatment interventions on new onset diabetes incidence and cardiovascular disease risk.
INTRODUCTION
As diabetes mellitus (DM) is a worldwide health problem with epidemic proportions which may lead to functional disability, vascular complications and premature death, the prevention or delay of DM development is of major clinical importance[1,2]. Lipid-lowering drugs may affect glucose metabolism in different ways. Noteworthy, a potentially diabetogenic role for statins has been suggested both from large studies and meta-analyses[3-5]. Previously, we showed that rosuvastatin may increase insulin resistance and homeostasis model assessment of insulin resistance (HOMA-IR) levels in patients with impaired fasting glucose in a dose-dependent manner and may increase the risk for new onset DM[6,7]. Similarly, nicotinic acid (NA) has been associated with both deterioration of glycemic profile and new onset DM[8]. On the other hand, fenofibrate administration has been linked with increased insulin sensitivity[9,10].
Mixed dyslipidemia is characterized by both elevated triglyceride (TG) and low density lipoprotein cholesterol (LDL-C) levels and by reduced high-density lipoprotein cholesterol (HDL-C) levels[11]. For that, monotherapy with a conventional statin dose may not achieve all treatment targets. Currently, it remains unknown which is the best treatment strategy to address all lipid abnormalities in these patients. We recently showed that both switch to the highest dose of rosuvastatin monotherapy (40 mg) and add-on-current-statin extended release NA (ER-NA)/laropiprant (LRPT) were associated with marked reductions in non-HDL-C and LDL-C levels compared with add-on fenofibrate in patients with mixed dyslipidemia not on goal with a standard statin dose[12]. We now report the results of a prespecified analysis on the effect of these 3 treatment strategies on glycemic profile.
MATERIALS AND METHODS
Study population
Study details have been previously described[12]. Briefly, consecutive subjects with primary hypercholesterolemia (n = 100) attending the Outpatient Lipid and Obesity Clinic of the University Hospital of Ioannina, Ioannina, Greece were recruited. Eligible patients were those treated for at least 3 mo with a conventional statin dose (10-40 mg simvastatin or 10-20 mg atorvastatin or 5-10 mg rosuvastatin) and their LDL-C or non-HDL-C levels were above those recommended by the National Cholesterol Education Program Adult Treatment Panel (NCEP-ATP) III based on each patient risk factors[13].
Subjects with TG > 500 mg/dL (5.65 mmol/L), renal disease (serum creatinine levels > 1.6 mg/dL; 141 μmol/L), hypothyroidism [thyroid stimulating hormone (TSH) > 5 IU/mL] and liver disease [alanine aminotransferase (ALT) and/or aspartate aminotransferase (AST) levels > 3-fold upper limit of normal in 2 consecutive measurements] were excluded from the study. Patients with hypertension and/or DM were considered eligible if they were on stable medication for at least 3 mo and their blood pressure and/or glycemic profile were adequately controlled (no change in their treatment was allowed during study period).
The study had a prospective, randomized, open-label, blinded end point (PROBE) design. Patients were randomly allocated (without a wash-out phase) to open-label the highest approved dose of rosuvastatin (40 mg/d) or to add-on-current-statin treatment with ER-NA/LRPT (1000/20 mg/d for the first 4 wk, followed by 2000/40 mg/d for the next 8 wk) or to add-on-statin micronised fenofibrate (200 mg/d) for a total of 3 mo (Figure 1).
Figure 1.
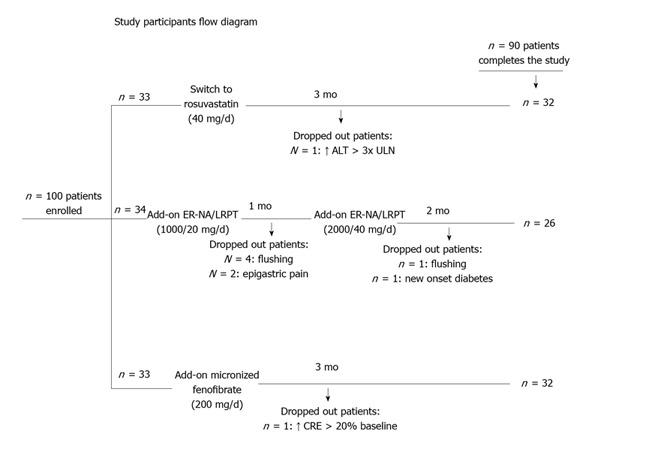
Study participants flow diagram. ER-NA/LRPT: Extended release nicotinic acid/laropiprant; ALT: Alanine aminotransferase; ULN: Upper normal limit; CRE: Serum creatinine.
All patients were given similar dietary advice. Compliance with treatment and lifestyle habits were assessed by questionnaire and tablet count. This trial has been carried out in accordance with the Declaration of Helsinki (2000) of the World Medical Association. All study participants gave their written informed consent prior to enrolment and the Ethics Committee of the University Hospital of Ioannina approved the study protocol. This study is registered at ClinicalTrials.gov (NCT01010516).
Laboratory measurements
Blood samples for laboratory tests were obtained at baseline and 12 wk after the start of treatment after a 12-h overnight fast. Serum levels of fasting glucose were determined enzymatically in the laboratory of the University Hospital of Ioannina using an Olympus AU 600 analyzer (Olympus Diagnostica GmbH, Hamburg, Germany). Intra-assay and total coefficient variations for glucose assay were 0.7% and 1.6%, respectively. The determination of glycosylated haemoglobin (HbA1c) (expressed as percentage of the total haemoglobin concentration) was based on a latex agglutination inhibition assay (Randox Laboratories Ltd., Crumlin, United Kingdom). HbA1c values are expressed as percentage of the total haemoglobin concentration. The sensitivity of the assay is 0.25 g/dL of HbA1c and the within- and between- run precision is < 6.67% and < 4.82%, respectively. Fasting serum insulin was measured by an AxSYM insulin assay microparticle enzyme immunoassay on an AzSYM analyzer (Abbott Diagnostics, Illinois, United States). Intra-assay and total coefficient variations for insulin assay were 4.1% and 5.3%, respectively. The HOMA-IR index was calculated as follows: HOMA-IR index = fasting insulin (mU/L) × FPG (mg/dL)/405.
Statistical analysis
The analysis only included patients who completed the study as per protocol. Values are given as mean ± SD and median (range) for parametric and non-parametric data, respectively. Continuous variables were tested for lack of normality by the Kolmogorov-Smirnov test, and logarithmic transformations were accordingly performed for nonparametric variables. The paired-sample t-test was used for assessing the effect of treatment in each group. ANCOVA, adjusted for baseline values, was used for comparisons between groups. All reported P values are based on two-sided tests with a significance level of 5%. Because of multiple comparisons we used Bonferroni’s correction to account for the increase in type I error. Analyses were performed using the Statistical Package for the SPSS 15.0 (SPSS Inc, Chicago, IL).
RESULTS
Recruitment took place from October 2009 to September 2011 and follow-up ended in December 2011. Initially, 100 Caucasian patients were enrolled (n = 33, 34 and 33 in the switch to the highest dose of rosuvastatin, add-on ER-NA/LRPT and add-on fenofibrate group, respectively) (Figure 1). Ten patients dropped out due to side effects (see below). Eventually, ninety subjects (47 men, 59 ± 11 years) completed the study (n = 32, 26, 32 in the switch to highest-dose rosuvastatin monotherapy, add-on ER-NA/LRPT and add-on fenofibrate group, respectively) and included in the final analysis. No significant differences in baseline data were found across groups regarding demographic characteristics and serum metabolic parameters (Table 1). Compliance rate was > 90% in all participants who completed the study. No changes in body weight, dietary habits, antihypertensive or antidiabetic medications were reported during follow-up.
Table 1.
Baseline characteristics of patients who completed the study
| Switch to the highest dose of rosuvastatin | Add-on-statin ER-NA/LRPT | Add-on-statin micronised fenofibrate | P | |
| Sex (males/females) | 32 (17/15) | 26 (14/12) | 32 (16/16) | NS |
| Age (yr) | 62 ± 10 | 58 ± 14 | 59 ± 12 | NS |
| Hypertension | 17 (53) | 14 (54) | 16 (50) | NS |
| Diabetes mellitus | 6 (19) | 6 (23) | 6 (19) | NS |
| Metabolic syndrome1 | 17 (53) | 15 (58) | 18 (56) | NS |
| Smoking | 10 (31) | 9 (35) | 9 (28) | NS |
| BMI (kg/m2) | 29.1 ± 2.5 | 29.1 ± 3.1 | 28.8 ± 3.2 | NS |
| TC | NS | |||
| (mg/dL) | 205 ± 40 | 200 ± 42 | 200 ± 37 | |
| (mmol/L) | 5.5 ± 1 | 5.2 ± 1.1 | 5.2 ± 1 | |
| Triglycerides | NS | |||
| (mg/dL) | 190 (173-210) | 213 (190-254) | 218 (189-260) | |
| (mmol/L) | 2.2 (2.0-2.4) | 2.4 (2.2-2.9) | 2.5 (2.1-2.9) | |
| HDL-C | NS | |||
| (mg/dL) | 50 ± 10 | 47 ± 11 | 45 ± 9 | |
| (mmol/L) | 1.3 ± 0.3 | 1.2 ± 0.3 | 1.1 ± 0.2 | |
| LDL-C | NS | |||
| (mg/dL) | 116 ± 40 | 109 ± 35 | 112 ± 32 | |
| (mmol/L) | 3.2 ± 1.0 | 2.9 ± 0.9 | 2.9 ± 0.8 | |
| Non-HDL-C | NS | |||
| (mg/dL) | 155 ± 40 | 153 ± 37 | 155 ± 34 | |
| (mmol/L) | 4.0 ± 1.0 | 4.0 ± 1.0 | 4.0 ± 0.9 | |
| Fasting plasma glucose | NS | |||
| (mg/dL) | 91 ± 26 | 93 ± 17 | 94 ± 10 | |
| (mmol/L) | 5.1 ± 1.4 | 5.2 ± 0.9 | 5.2 ± 0.6 | |
| HbA1c (%) | 6.1 ± 0.5 | 6.3 ± 1.1 | 6.1 ± 0.8 | NS |
| HOMA-IR index | 1.4 1.2-2.1 | 1.5 1.4-2.1 | 1.7 1.5-2.3 | NS |
| Medications at baseline | ||||
| Aspirin | 9 (28) | 8 (31) | 7 (22) | NS |
| Beta blockers | 9 (28) | 8 (31) | 9 (28) | NS |
| HCTZ | 11 (34) | 10 (38) | 10 (31) | NS |
| ACEIs/ARBs | 13 (41) | 11 (42) | 12 (38) | NS |
| Calcium channel blockers | 8 (24) | 8 (31) | 10 (31) | NS |
| Metformin | 6 (19) | 6 (23) | 5 (16) | NS |
| Pioglitazone | 2 (7) | 1 (4) | 3 (9) | NS |
| Sulfonylurea | 4 (13) | 3 (12) | 2 (6) | NS |
| Atorvastatin 5-20 mg/d | 11 (34) | 9 (35) | 12 (38) | NS |
| Simvastatin 10-40 mg/d | 11 (34) | 9 (35) | 9 (28) | NS |
| Rosuvastatin 5-10 mg/d | 10 (31) | 8 (31) | 11 (34) | NS |
Values are expressed as absolute mean ± SD [except for triglycerides and HOMA-IR index which are expressed as median (range)] or numbers (percentage).
Participants who fulfilled 3 or more of the American Heart Association (AHA) criteria for the diagnosis of metabolic syndrome (waist circumference > 102 cm in men, > 88 cm in women, fasting serum triglycerides > 150 mg/dL, HDL-C < 40 mg/dL in men, < 50 mg/dL in women, blood pressure > 130/85 mm Hg, fasting serum glucose > 100 mg/dL). ACEIs: Angiotensin converting enzyme inhibitors; ApoB: Apolipoprotein B; ARBs: Angiotensin receptor blockers; BMI: Body mass index; ER-NA/LRPT: Extended release nicotinic acid/laropiprant; HCTZ: Hydrochlorothiazide; HDL-C: High-density lipoprotein cholesterol; HbA1c: Glycosylated hemoglobin; HOMA-IR IR index: Homeostasis model assessment of insulin resistance index; NS: Non significant; TC: Total cholesterol.
As previously reported, among study completers non-HDL-C decreased in all groups (by 23%, 24% and 7% in the rosuvastatin monotherapy, add-on ER-NA/LRPT and add-on fenofibrate group, respectively, P < 0.01 for all vs baseline and P < 0.01 for all vs fenofibrate group). LDL-C decreased by 23% and 19% in the rosuvastatin and ER-NA/LRPT group, respectively (P < 0.01 vs baseline), but not in the add-on fenofibrate group.
As shown in Table 2, FPG increased in add-on ER-NA/LRPT and rosuvastatin monotherapy groups by 9.7% (from 93 ± 17 to 102 ± 29 mg/dL) and 4.4% (from 91 ± 26 to 95 ± 12 mg/dL), respectively (P < 0.01 for all comparisons vs baseline and for the comparison between the 2 groups), while it did not significantly change in the add-on fenofibrate group (from 98 ± 11 to 98 ± 12 mg/dL, P > 0.05 vs baseline). Of note one case of new onset DM was reported in the add-on ER-NA/LRPT group. HbA1c increased by 0.3% (from 6.3% ± 0.7% to 6.6% ± 0.9%) in add-on ER-NA/LRPT group and by 0.2% (from 6.1% ± 0.5% to 6.3% ± 0.5%) in the rosuvastatin monotherapy group (P < 0.01 for all comparisons vs baseline and for the comparison between the 2 groups), while no significant change was reported in the add-on fenofibrate group (+ 0.1%, from 6.3% ± 0.8% to 6.4% ± 1.0%, P > 0.05 vs baseline). HOMA-IR increased by 65% [from 1.5% (1.4%-2.1%) to 2.5% (1.5%-2.8%)] in add-on ER-NA/LRPT and by 14% [from 1.4% (1.2%-2.1%) to 1.6% (1.5%-2.6%)] in the rosuvastatin monotherapy group (P < 0.01 vs baselin), while HOMA-IR level decreased in the add-on fenofibrate group [-6%, from 1.7% (1.5%-2.3%) to 1.6% (1.4%-2.2%), P < 0.01 vs h baseline and for the comparisons among the groups].
Table 2.
Laboratory parameters at baseline and 3 mo later
| Baseline | 3 mo | Percentage change | |
| Fasting plasma glucose, mg/dL (mmol/L) | |||
| Switch to the highest dose rosuvastatin | 91 ± 26 (5.1 ± 1.4) | 95 ± 19 (5.3 ± 1.1) | 4%b |
| Add-on-statin ER-NA/LRPT | 93 ± 17 (5.2 ± 0.9) | 102 ± 27 (5.7 ± 1.5) | 10%bdf |
| Add-on-statin fenofibrate | 94 ± 10 (5.2 ± 0.6) | 94 ± 11 (5.2 ± 0.6) | 0% |
| HbA1c, % | |||
| Switch to the highest dose rosuvastatin | 6.1 ± 0.5 | 6.3 ± 0.5 | 0.2%b |
| Add-on-statin ER-NA/LRPT | 6.3 ± 1.1 | 6.6 ± 1.2 | 0.3%bdf |
| Add-on-statin fenofibrate | 6.1 ± 0.8 | 6.2 ± 1.0 | 0.10% |
| HOMA-IR index | |||
| Switch to the highest dose rosuvastatin | 1.4 (1.2-2.1) | 1.6 (1.5-2.6) | 14%bf |
| Add-on-statin ER-NA/LRPT | 1.5 (1.4-2.1) | 2.5 (1.5-2.8) | 65%bdf |
| Add-on-statin fenofibrate | 1.7 (1.5-2.3) | 1.6 (1.4-2.2) | -6%b |
Values are expressed as mean ± SD [except for fasting plasma insulin and HOMA-IR index which are expressed as median (range)]. No significant differences in baseline data were found across groups.
P < 0.01 vs baseline;
P < 0.01 vs switch to the highest dose rosuvastatin group;
P < 0.01 vs add-on-statin fenofibrate group. ER-NA/LRPT: Extended release nicotinic acid/laropiprant; HOMA-IR index: Homeostasis model assessment of insulin resistance index; HbA1c: Glycosylated haemoglobin.
Safety
Of the 100 patients enrolled, 8 (24%) of the 34 initially randomized to the ER-NA/laropiprant group dropped out during the study due to flushing (n = 5), epigastric pain (n = 2) and new onset diabetes (n = 1). Also, 1 (3%) patient of the 33 initially randomized to the rosuvastatin group dropped out due to asymptomatic ALT elevation > 3-fold ULN and 1 (3%) patient of the 33 randomized in the fenofibrate group due to serum creatinine elevation (> 20% from baseline) (Figure 1).
DISCUSSION
We directly compared for the first time the switch to the highest dose of rosuvastatin versus add-on ER-NA/LRPT versus add-on micronised fenofibrate in patients with mixed dyslipidemia on a standard statin dose who had not achieved treatment goals. In the present prespecified analysis both add-on ER-NA/LRPT and switch to high-dose rosuvastatin were associated with glycemic profile deterioration, while add-on fenofibrate increased insulin sensitivity.
Concerns regarding the effects of statins on glucose metabolism rose by the Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) study. An increase in the incidence of physician reported DM with rosuvastatin 20 mg/d in apparently healthy subjects with LDL-C < 3.36 mmol/L (130 mg/dL) and high-sensitivity C-reactive protein (hsCRP) > 2 mg/L was reported in this study[3]. A meta-analysis demonstrated a small increase in DM risk with statins with no evidence of heterogeneity across trials[4]. However, this estimate was attenuated and became no longer significant when the West of Scotland Coronary Prevention Study (WOSCOPS) was included[4]. A subsequent meta-analysis demonstrated that statin therapy was associated with a 9% increased risk for incident diabetes with little heterogeneity between trials[5]. Of note, this effect of statins seems to be dose dependent[14]. In fact, previously, we showed that rosuvastatin may increase insulin resistance and HOMA-IR levels in patients with impaired FPG in a dose-dependent manner and may increase the risk for new onset DM[6,7]. The mechanisms by which statins may adversely affect glucose homeostasis are not fully understood. Statins by inhibiting mevalonate pathway block the synthesis not only of cholesterol, but also of several mevalonate products known as isoprenoids[15]. Isoprenoids, including farsenyl pyrophosphate, geranylgeranyl pyrophosphate and ubiquinone, are known to enhance glucose uptake by upregulating the membrane transporter protein glucose transporter 4, which plays a key role in glucose uptake by adipocytes[15]. Therefore, statin-induced inhibition of isoprenoid synthesis may increase insulin resistance in the adipose tissue and induce hyperinsulinemia. Most potent statins in terms of inhibiting mevalonate pathway, such as rosuvastatin, as well as higher doses of statin treatment may exert a more profound impact on insulin resistance compared with conventional statins at low dosage regimens. Last, statins may exert a deleterious effect on insulin secretion by pancreatic islets[15].
NA has been associated with modest, transient and reversible elevation of FPG (an increase of approximately 4%-5%) and HbA1c levels (an increase of ≤ 0.3%)[16-18]. Although the mechanism remains unclear, an increase in insulin resistance seems to be involved. NA inhibits lipolysis in adipose tissue and decreases circulating free fatty acids (FFA). Paradoxically, the initially decreased FFA levels rebound during long-term NA treatment resulting in insulin resistance as elevated plasma FFA levels have been associated with insulin resistance[8]. Moreover, NA was associated with decreased expression of phosphoenolopyruvate carboxykinase (PEPCK1) in adipose tissue. PEPCK1 is a key enzyme in adipose tissue gluconeogenesis and its deficit leads to increased FFA release, partly explaining the rebound phenomenon[8]. Another contributing mechanism to FFA rebound may be the NA-induced up-regulation of tumor necrosis factor-a transcription and the consequent increase of interleukin-6, as both of them comprise cytokines with lipolytic properties. Apart from the FFA rebound, NA was also demonstrated to decrease protein kinase B and FOXO1 transcription factor phosphorylation. Both protein kinase B and FOXO1 are present in insulin-sensitive tissues and are involved in lipid and glucose metabolism. Of note, their phosphorylation is induced by insulin, while NA’s opposite impact result in increased transcription of gluconeogenic enzymes and thus glucose overproduction and hepatic insulin resistance[8]. Of note, both body mass index and baseline FPG have been positively associated with the risk of new onset diabetes in non-diabetic patients receiving ER-NA[19]. In HPS-2 THRIVE (The Heart Protection Study 2-Treatment of HDL to Reduce the Incidence of Vascular Events) trial patients (n = 25673) with established vascular disease who were already treated with simvastatin (± ezetimibe) were randomized to addition of ER-NA/LRPT (2000/40 mg/d) or placebo. After nearly 4 years of follow-up ER-NA/LRPT did not significantly reduce coronary deaths, nonfatal myocardial infarctions, strokes, or coronary revascularizations compared with statin (± ezetimibe) monotherapy[20]. What is more, diabetic complications (typically hyperglycemia) were about twice as common as a reason for stopping randomized treatment in participants allocated to ER-NA/LRPT. Of note participants who developed DM were encouraged to continue their study treatment and this was rarely given as a reason for stopping[21]. As ER-NA/LRPT was associated with an excess of serious nonfatal side effects the drug was suspended worldwide[22].
On the other hand, fenofibrate has been associated with increased insulin sensitivity and improved glycemic profile in patients with metabolic syndrome and impaired FPG, even though fenofibrate does not seem to affect insulin sensitivity in normolipidemic subjects[9,10,23]. Fenofibrate is a known peroxisome proliferator-activated receptor a (PPAR-a) activator. Activated PPAR-a downregulates lipid accumulation in liver and skeletal muscle decreasing hepatic very low density lipoprotein (VLDL) particles and subsequently levels of circulating FFA leading to increased insulin sensitivity[24].
A major limitation of our study is its open-label design and the relatively small number of participants. On the other hand, it is an adequately powered randomized study with all laboratory determinations being performed blindly to treatment allocation. Also, study design is relevant to every day clinical practice.
Both addition of ER-NA/LRPT and switch to the highest dose of rosuvastatin deteriorated glycemic profile in patients with mixed dyslipidemia who were inadequately controlled with a standard statin dose. Add-on fenofibrate, on the other hand, improved insulin sensitivity. Larger prospective studies should address the effect of these treatment interventions on new onset diabetes incidence and cardiovascular disease risk.
COMMENTS
Background
Both cardiovascular disease and diabetes mellitus comprise major worldwide health problems with epidemic proportions and their prevention or delay is of major clinical significance. However, hypolipidemic treatment has been associated with deterioration of glycemic profile and even cases of new onset diabetes.
Research frontiers
Lipid lowering treatments have been associated with controversial data regarding glucose metabolism. In this trial the authors demonstrated that both addition of extended release nicotinic acid/laropiprant and switch to the highest dose of rosuvastatin deteriorated glycemic profile in patients with mixed dyslipidemia who were inadequately controlled with a standard statin dose. Add-on fenofibrate, on the other hand, seems to increase insulin sensitivity.
Innovations and breakthroughs
A number of studies and meta-analyses have suggested a potential diabetogenic role for both statins and nicotinic acid. The authors directly compared for the first time the effect on glycemic profile of the switch to the highest-dose of rosuvastatin with add-on nicotinic acid/laropiprant or add-on micronised fenofibrate in patients with mixed dyslipidemia on standard statin dose who had not achieved treatment goals.
Applications
By knowing how lipid treatment may affect glucose metabolism in patients with mixed dyslipidemia inadequately controlled with a standard statin dose, the authors can choose on an individual patient basis the next therapeutic step taking under consideration both lipid and glycemic profile.
Peer review
The authors examined the effect of hypolipidemic treatment on glycemic profile in patients with mixed dyslipidemia. Both addition of extended release nicotinic acid/laropiprant and switch to the highest dose of rosuvastatin deteriorated glycemic profile, while add-on fenofibrate increased insulin sensitivity. Larger prospective studies should address the effect of these treatment interventions on new onset diabetes incidence and cardiovascular disease risk.
Footnotes
P- Reviewers: Adeghate E, Kumar KVS, Pastromas S S- Editor: Zhai HH L- Editor: A E- Editor: Liu XM
References
- 1.Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liberopoulos EN, Tsouli S, Mikhailidis DP, Elisaf MS. Preventing type 2 diabetes in high risk patients: an overview of lifestyle and pharmacological measures. Curr Drug Targets. 2006;7:211–228. doi: 10.2174/138945006775515419. [DOI] [PubMed] [Google Scholar]
- 3.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 4.Rajpathak SN, Kumbhani DJ, Crandall J, Barzilai N, Alderman M, Ridker PM. Statin therapy and risk of developing type 2 diabetes: a meta-analysis. Diabetes Care. 2009;32:1924–1929. doi: 10.2337/dc09-0738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sattar N, Preiss D, Murray HM, Welsh P, Buckley BM, de Craen AJ, Seshasai SR, McMurray JJ, Freeman DJ, Jukema JW, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375:735–742. doi: 10.1016/S0140-6736(09)61965-6. [DOI] [PubMed] [Google Scholar]
- 6.Kostapanos MS, Milionis HJ, Agouridis AD, Rizos CV, Elisaf MS. Rosuvastatin treatment is associated with an increase in insulin resistance in hyperlipidaemic patients with impaired fasting glucose. Int J Clin Pract. 2009;63:1308–1313. doi: 10.1111/j.1742-1241.2009.02101.x. [DOI] [PubMed] [Google Scholar]
- 7.Florentin M, Liberopoulos EN, Rizos CV, Kei AA, Liamis G, Kostapanos MS, Elisaf MS. Colesevelam plus rosuvastatin 5 mg/day versus rosuvastatin 10 mg/day alone on markers of insulin resistance in patients with hypercholesterolemia and impaired fasting glucose. Metab Syndr Relat Disord. 2013;11:152–156. doi: 10.1089/met.2012.0103. [DOI] [PubMed] [Google Scholar]
- 8.Kei A, Liberopoulos EN, Elisaf MS. What restricts the clinical use of nicotinic acid? Curr Vasc Pharmacol. 2011;9:521–530. doi: 10.2174/157016111796197215. [DOI] [PubMed] [Google Scholar]
- 9.Wan Q, Wang F, Wang F, Guan Q, Liu Y, Wang C, Feng L, Gao G, Gao L, Zhao J. Regression to normoglycaemia by fenofibrate in pre-diabetic subjects complicated with hypertriglyceridaemia: a prospective randomized controlled trial. Diabet Med. 2010;27:1312–1317. doi: 10.1111/j.1464-5491.2010.03107.x. [DOI] [PubMed] [Google Scholar]
- 10.Krysiak R, Gdula-Dymek A, Bachowski R, Okopien B. Pleiotropic effects of atorvastatin and fenofibrate in metabolic syndrome and different types of pre-diabetes. Diabetes Care. 2010;33:2266–2270. doi: 10.2337/dc10-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Durrington P. Dyslipidaemia. Lancet. 2003;362:717–731. doi: 10.1016/S0140-6736(03)14234-1. [DOI] [PubMed] [Google Scholar]
- 12.Kei A, Liberopoulos EN, Mikhailidis DP, Elisaf M. Comparison of switch to the highest dose of rosuvastatin vs. add-on nicotinic acid vs. add-on fenofibrate for mixed dyslipidaemia. Int J Clin Pract. 2013;67:412–419. doi: 10.1111/ijcp.12098. [DOI] [PubMed] [Google Scholar]
- 13.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 14.Preiss D, Seshasai SR, Welsh P, Murphy SA, Ho JE, Waters DD, DeMicco DA, Barter P, Cannon CP, Sabatine MS, et al. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: a meta-analysis. JAMA. 2011;305:2556–2564. doi: 10.1001/jama.2011.860. [DOI] [PubMed] [Google Scholar]
- 15.Kostapanos MS, Liamis GL, Milionis HJ, Elisaf MS. Do statins beneficially or adversely affect glucose homeostasis? Curr Vasc Pharmacol. 2010;8:612–631. doi: 10.2174/157016110792006879. [DOI] [PubMed] [Google Scholar]
- 16.Goldberg RB, Jacobson TA. Effects of niacin on glucose control in patients with dyslipidemia. Mayo Clin Proc. 2008;83:470–478. doi: 10.4065/83.4.470. [DOI] [PubMed] [Google Scholar]
- 17.TREDAPTIVE [package insert] Merck Sharp & Dohme Ltd. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000889/WC500042216.pdf.
- 18.Guyton JR, Fazio S, Adewale AJ, Jensen E, Tomassini JE, Shah A, Tershakovec AM. Effect of extended-release niacin on new-onset diabetes among hyperlipidemic patients treated with ezetimibe/simvastatin in a randomized controlled trial. Diabetes Care. 2012;35:857–860. doi: 10.2337/dc11-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Libby A, Meier J, Lopez J, Swislocki AL, Siegel D. The effect of body mass index on fasting blood glucose and development of diabetes mellitus after initiation of extended-release niacin. Metab Syndr Relat Disord. 2010;8:79–84. doi: 10.1089/met.2009.0074. [DOI] [PubMed] [Google Scholar]
- 20. Available from: http://www.mercknewsroom.com/press-release/prescription-medicine-news/merck-announces-hps2-thrive-study-tredaptive-extended-relea.
- 21.HPS2-THRIVE Collaborative Group. HPS2-THRIVE randomized placebo-controlled trial in 25 673 high-risk patients of ER niacin/laropiprant: trial design, pre-specified muscle and liver outcomes, and reasons for stopping study treatment. Eur Heart J. 2013;34:1279–1291. doi: 10.1093/eurheartj/eht055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Available from: http://www.mercknewsroom.com/press-release/research-and-development-news/merck-provides-update-next-steps-tredaptive-extended-rel.
- 23.Perreault L, Bergman BC, Hunerdosse DM, Howard DJ, Eckel RH. Fenofibrate administration does not affect muscle triglyceride concentration or insulin sensitivity in humans. Metabolism. 2011;60:1107–1114. doi: 10.1016/j.metabol.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Azhar S. Peroxisome proliferator-activated receptors, metabolic syndrome and cardiovascular disease. Future Cardiol. 2010;6:657–691. doi: 10.2217/fca.10.86. [DOI] [PMC free article] [PubMed] [Google Scholar]


