Abstract
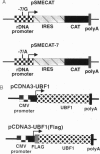
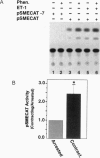
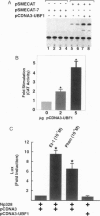
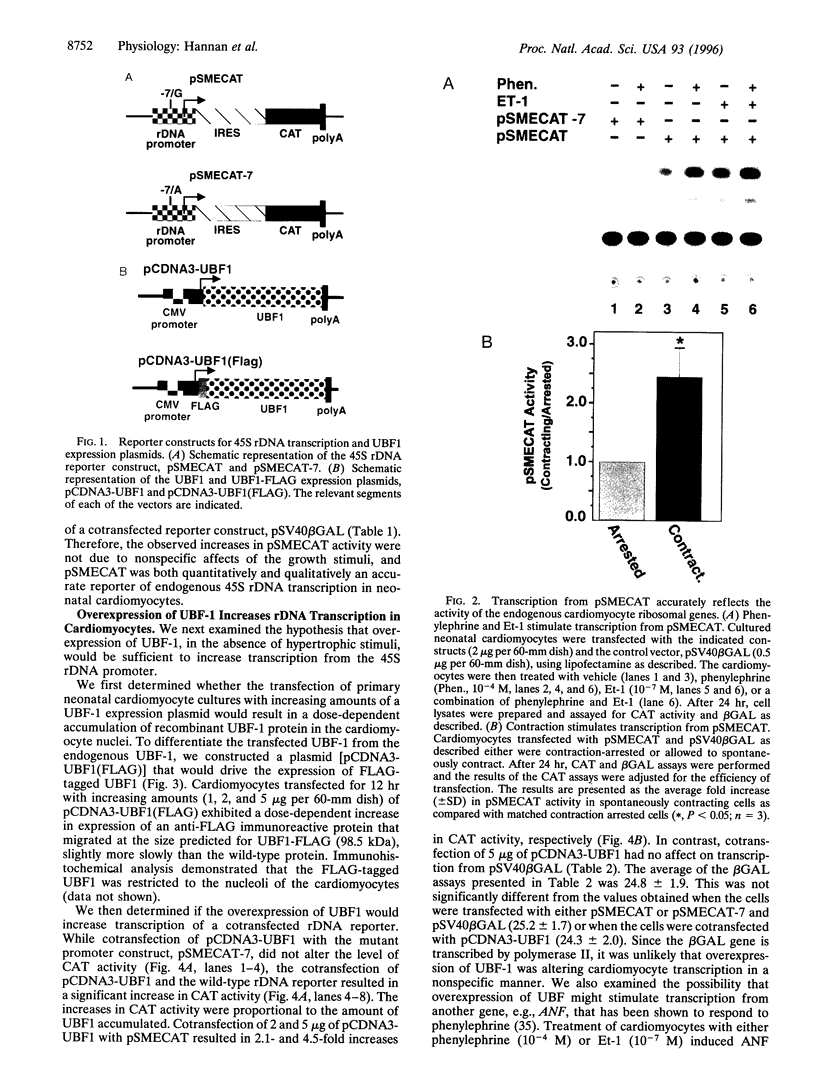
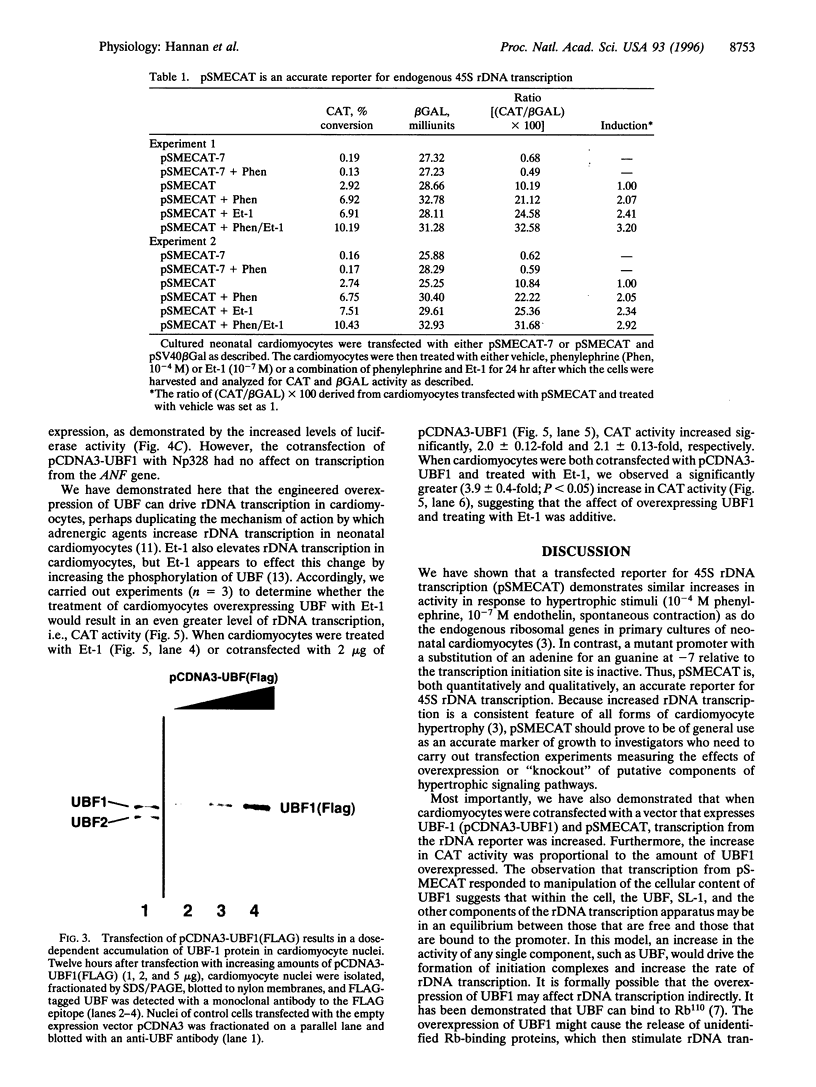
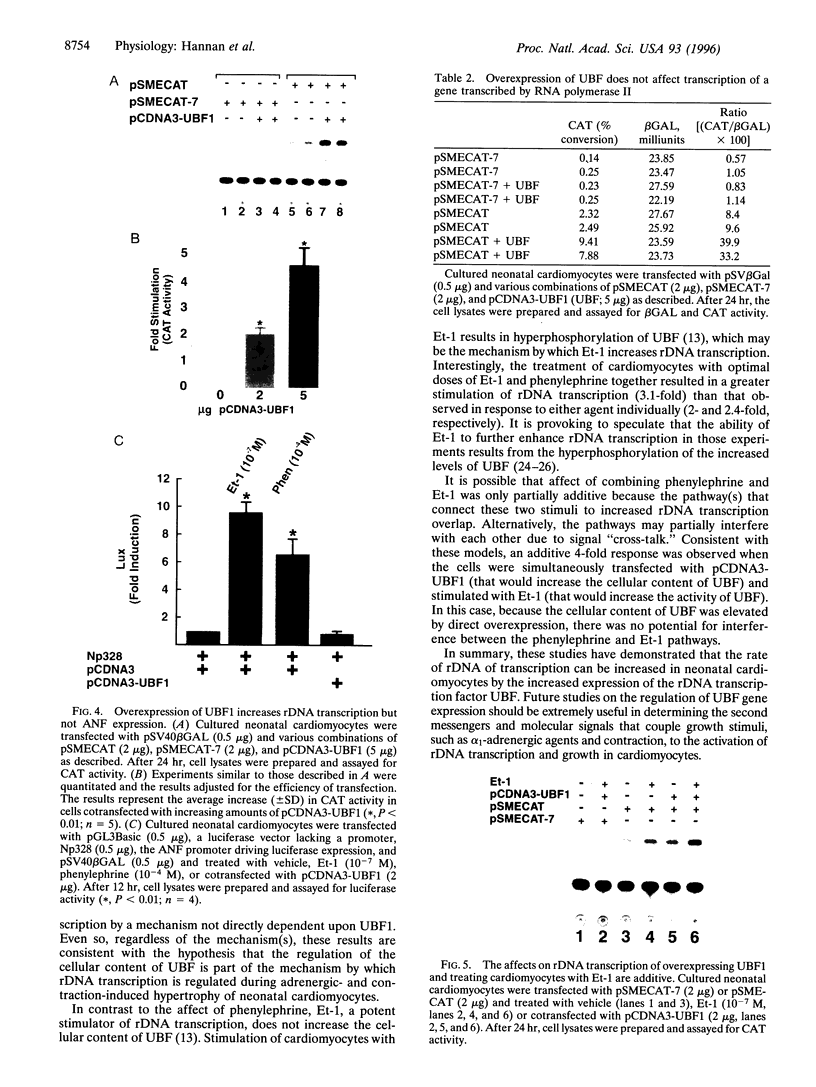
The accelerated protein accumulation characteristic of cardiomyocyte hypertrophy results from increased cellular protein synthetic capacity (elevated ribosome content). The rate limiting step in ribosome accumulation is transcription of the rRNA genes. During neonatal cardiomyocyte hypertrophy induced by norepinephrine or spontaneous contraction, changes in the expression of a ribosomal DNA transcription factor, UBF, correlated with increased rates of ribosome biogenesis. We hypothesized that elevated expression of UBF was part of the mechanism by which these hypertrophic stimuli effected increases in the rate of transcription from the rDNA promoter. In this study, we have examined directly the effect of overexpressing UBF on rDNA transcription in neonatal cardiomyocytes in culture. In control experiments, a novel reporter construct for rDNA transcription (pSMECAT) showed similar increases in activity in response to hypertrophic stimuli (10(-4) M phenylephrine, 10(-7) M endothelin, and spontaneous contraction) as did the endogenous rRNA genes. When contraction-arrested cardiomyocytes were cotransfected with pSMECAT and increasing amounts of a UBF1 expression vector; a dose-dependent (3-5 fold) increase in rDNA transcription was observed. Western blot analysis confirmed that the overexpressed, FLAG-tagged UBF accumulated in the cardiomyocyte nuclei. The observation that overexpression of UBF1 is sufficient to increase rDNA transcription in neonatal cardiomyocytes provides evidence in support of the hypothesis that the regulation of UBF is a key component of the increased ribosome biogenesis and protein accumulation associated with cardiomyocyte hypertrophy.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ardati A., Nemer M. A nuclear pathway for alpha 1-adrenergic receptor signaling in cardiac cells. EMBO J. 1993 Dec 15;12(13):5131–5139. doi: 10.1002/j.1460-2075.1993.tb06208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell S. P., Learned R. M., Jantzen H. M., Tjian R. Functional cooperativity between transcription factors UBF1 and SL1 mediates human ribosomal RNA synthesis. Science. 1988 Sep 2;241(4870):1192–1197. doi: 10.1126/science.3413483. [DOI] [PubMed] [Google Scholar]
- Cavanaugh A. H., Hempel W. M., Taylor L. J., Rogalsky V., Todorov G., Rothblum L. I. Activity of RNA polymerase I transcription factor UBF blocked by Rb gene product. Nature. 1995 Mar 9;374(6518):177–180. doi: 10.1038/374177a0. [DOI] [PubMed] [Google Scholar]
- Garg L. C., Dixit A., Jacob S. T. A 37-base pair element in the far upstream spacer region can enhance transcription of rat rDNA in vitro and can bind to the core promoter-binding factor(s). J Biol Chem. 1989 Jan 5;264(1):220–224. [PubMed] [Google Scholar]
- Gokal P. K., Cavanaugh A. H., Thompson E. A., Jr The effects of cycloheximide upon transcription of rRNA, 5 S RNA, and tRNA genes. J Biol Chem. 1986 Feb 25;261(6):2536–2541. [PubMed] [Google Scholar]
- Hannan R. D., Luyken J., Rothblum L. I. Regulation of rDNA transcription factors during cardiomyocyte hypertrophy induced by adrenergic agents. J Biol Chem. 1995 Apr 7;270(14):8290–8297. doi: 10.1074/jbc.270.14.8290. [DOI] [PubMed] [Google Scholar]
- Hannan R. D., Luyken J., Rothblum L. I. Regulation of ribosomal DNA transcription during contraction-induced hypertrophy of neonatal cardiomyocytes. J Biol Chem. 1996 Feb 9;271(6):3213–3220. doi: 10.1074/jbc.271.6.3213. [DOI] [PubMed] [Google Scholar]
- Hannan R. D., Rothblum L. I. Regulation of ribosomal DNA transcription during neonatal cardiomyocyte hypertrophy. Cardiovasc Res. 1995 Oct;30(4):501–510. [PubMed] [Google Scholar]
- Hempel W. M., Cavanaugh A. H., Hannan R. D., Taylor L., Rothblum L. I. The species-specific RNA polymerase I transcription factor SL-1 binds to upstream binding factor. Mol Cell Biol. 1996 Feb;16(2):557–563. doi: 10.1128/mcb.16.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob S. T. Regulation of ribosomal gene transcription. Biochem J. 1995 Mar 15;306(Pt 3):617–626. doi: 10.1042/bj3060617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S. K., Davies M. V., Kaufman R. J., Wimmer E. Initiation of protein synthesis by internal entry of ribosomes into the 5' nontranslated region of encephalomyocarditis virus RNA in vivo. J Virol. 1989 Apr;63(4):1651–1660. doi: 10.1128/jvi.63.4.1651-1660.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirshenbaum L. A., MacLellan W. R., Mazur W., French B. A., Schneider M. D. Highly efficient gene transfer into adult ventricular myocytes by recombinant adenovirus. J Clin Invest. 1993 Jul;92(1):381–387. doi: 10.1172/JCI116577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn M., Arnheim N. Nucleotide sequence of the genetically labile repeated elements 5' to the origin of mouse rRNA transcription. Nucleic Acids Res. 1983 Jan 11;11(1):211–224. doi: 10.1093/nar/11.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn A., Voit R., Stefanovsky V., Evers R., Bianchi M., Grummt I. Functional differences between the two splice variants of the nucleolar transcription factor UBF: the second HMG box determines specificity of DNA binding and transcriptional activity. EMBO J. 1994 Jan 15;13(2):416–424. doi: 10.1002/j.1460-2075.1994.tb06276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Learned R. M., Cordes S., Tjian R. Purification and characterization of a transcription factor that confers promoter specificity to human RNA polymerase I. Mol Cell Biol. 1985 Jun;5(6):1358–1369. doi: 10.1128/mcb.5.6.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Learned R. M., Learned T. K., Haltiner M. M., Tjian R. T. Human rRNA transcription is modulated by the coordinate binding of two factors to an upstream control element. Cell. 1986 Jun 20;45(6):847–857. doi: 10.1016/0092-8674(86)90559-3. [DOI] [PubMed] [Google Scholar]
- Luyken J., Hannan R. D., Cheung J. Y., Rothblum L. I. Regulation of rDNA transcription during endothelin-1-induced hypertrophy of neonatal cardiomyocytes. Hyperphosphorylation of upstream binding factor, an rDNA transcription factor. Circ Res. 1996 Mar;78(3):354–361. doi: 10.1161/01.res.78.3.354. [DOI] [PubMed] [Google Scholar]
- Mishima Y., Financsek I., Kominami R., Muramatsu M. Fractionation and reconstitution of factors required for accurate transcription of mammalian ribosomal RNA genes: identification of a species-dependent initiation factor. Nucleic Acids Res. 1982 Nov 11;10(21):6659–6670. doi: 10.1093/nar/10.21.6659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss T., Stefanovsky V. Y. Promotion and regulation of ribosomal transcription in eukaryotes by RNA polymerase I. Prog Nucleic Acid Res Mol Biol. 1995;50:25–66. doi: 10.1016/s0079-6603(08)60810-7. [DOI] [PubMed] [Google Scholar]
- O'Mahony D. J., Rothblum L. I. Identification of two forms of the RNA polymerase I transcription factor UBF. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3180–3184. doi: 10.1073/pnas.88.8.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Mahony D. J., Smith S. D., Xie W., Rothblum L. I. Analysis of the phosphorylation, DNA-binding and dimerization properties of the RNA polymerase I transcription factors UBF1 and UBF2. Nucleic Acids Res. 1992 Mar 25;20(6):1301–1308. doi: 10.1093/nar/20.6.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Mahony D. J., Xie W. Q., Smith S. D., Singer H. A., Rothblum L. I. Differential phosphorylation and localization of the transcription factor UBF in vivo in response to serum deprivation. In vitro dephosphorylation of UBF reduces its transactivation properties. J Biol Chem. 1992 Jan 5;267(1):35–38. [PubMed] [Google Scholar]
- Pikaard C. S., Smith S. D., Reeder R. H., Rothblum L. rUBF, an RNA polymerase I transcription factor from rats, produces DNase I footprints identical to those produced by xUBF, its homolog from frogs. Mol Cell Biol. 1990 Jul;10(7):3810–3812. doi: 10.1128/mcb.10.7.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnapp A., Clos J., Hädelt W., Schreck R., Cvekl A., Grummt I. Isolation and functional characterization of TIF-IB, a factor that confers promoter specificity to mouse RNA polymerase I. Nucleic Acids Res. 1990 Mar 25;18(6):1385–1393. doi: 10.1093/nar/18.6.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnapp A., Schnapp G., Erny B., Grummt I. Function of the growth-regulated transcription initiation factor TIF-IA in initiation complex formation at the murine ribosomal gene promoter. Mol Cell Biol. 1993 Nov;13(11):6723–6732. doi: 10.1128/mcb.13.11.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson P., McGrath A., Savion S. Myocyte hypertrophy in neonatal rat heart cultures and its regulation by serum and by catecholamines. Circ Res. 1982 Dec;51(6):787–801. doi: 10.1161/01.res.51.6.787. [DOI] [PubMed] [Google Scholar]
- Smith S. D., O'Mahony D. J., Kinsella B. T., Rothblum L. I. Transcription from the rat 45S ribosomal DNA promoter does not require the factor UBF. Gene Expr. 1993;3(3):229–236. [PMC free article] [PubMed] [Google Scholar]
- Smith S. D., Oriahi E., Lowe D., Yang-Yen H. F., O'Mahony D., Rose K., Chen K., Rothblum L. I. Characterization of factors that direct transcription of rat ribosomal DNA. Mol Cell Biol. 1990 Jun;10(6):3105–3116. doi: 10.1128/mcb.10.6.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voit R., Schnapp A., Kuhn A., Rosenbauer H., Hirschmann P., Stunnenberg H. G., Grummt I. The nucleolar transcription factor mUBF is phosphorylated by casein kinase II in the C-terminal hyperacidic tail which is essential for transactivation. EMBO J. 1992 Jun;11(6):2211–2218. doi: 10.1002/j.1460-2075.1992.tb05280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W. Q., Rothblum L. I. Rapid, small-scale RNA isolation from tissue culture cells. Biotechniques. 1991 Sep;11(3):324, 326-7. [PubMed] [Google Scholar]
- Xie W., O'Mahony D. J., Smith S. D., Lowe D., Rothblum L. I. Analysis of the rat ribosomal DNA promoter: characterization of linker-scanning mutants and of the binding of UBF. Nucleic Acids Res. 1992 Apr 11;20(7):1587–1592. doi: 10.1093/nar/20.7.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]