Abstract
AIM
To investigate the effects of bovine pituitary extract on the proliferation of keratocytes and maintaining the keratocyte phenotype in vitro.
METHODS
Single keratocytes were isolated by enzyme digestion for in vitro culture. Three groups were designed according to the different culture media: a bovine pituitary extract (BPE) group, a fetal bovine serum (FBS) group and the control group. The phenotypes and proliferation of cultured cells were evaluated by morphology, immunofluorescent staining and mRNA expression of CD34, Lumican, VSX1, α-SMA and proliferating cell nuclear antigen (PCNA). In the BPE group, cells underwent serial subcultivation, and their phenotypes were identified by immunofluorescent staining. To analyze the proliferation of keratocytes in different concentrations of BPE, six different concentrations were designed to ascertain the most appropriate amount.
RESULTS
In the BPE group, the cells spread out and presented dendritic morphology, and their dendrites connected to one another to form networks. On the third passage, most cells maintained their phenotype. In the FBS group, the cells exhibited a dendritic appearance in early cultured stages, but their morphology subsequently changed into a fibroblast-like shape. The number of dendritic cells in BPE group was more than FBS and control groups. Immunofluorescent staining and real-time polymerase chain reaction (PCR) confirmed that few keratocytes underwent fibroblastic transformation in the BPE and control groups, and that proliferation was higher in the BPE group than in the control group. Although the proliferation was higher in the FBS group, many keratocytes underwent fibroblastic transformation. The analysis of cell morphology and mRNA expressions of CD34, PCNA and VSX1 in six group showed that different concentrations of BPE affected the proliferation obviously but didn't affect the keratocyte phenotype, and the concentration of 40µg/mL was the most appropriate one.
CONCLUSION
BPE can improve the proliferation of keratocytes and maintain their phenotype in vitro. Many keratocytes can be harvested rapidly and provide seeds for the construction of corneal stroma.
Keywords: keratocyte, bovine pituitary extract, proliferation, phenotype, fibroblastic transformation
INTRODUCTION
Keratocytes are mesenchymal-derived cells of the corneal stroma. These cells are normally quiescent and secrete the collagen that composes the corneal matrix and are involved in maintaining the transparent state of the cornea. Following injury to the cornea, keratocytes can readily undergo apoptosis or transition into repair functions that can either promote regeneration or induce fibrotic scar formation, which is significant to corneal wound healing[1],[2]. Keratocytes have two known repair phenotypes: fibroblast and myofibroblast. Fetal bovine serum can promote cell proliferation in vitro, but keratocytes can be easily activated to become fibroblasts and myofibroblasts in serum culture medium, which increases the difficulty of rapid harvesting[1],[3]. Bovine pituitary extract (BPE) is a supplement for serum-free culture with enriching growth factors that highly promote mitotic activity. This study used enzymatic digestion to isolate keratocytes and investigated whether BPE could improve keratocyte proliferation and maintain their phenotype in culture.
SUBJECTS AND METHODS
Subjects
Corneal tissues were obtained from Henan Eye Bank. Bovine pituitary extract, keratinocyte serum-free medium, defined trypsin inhibitor, Trizol reagent, Alexa Fluor 546-phalloidin and Alexa Fluor 568 conjugated goat anti-mouse IgG were obtained from Invitrogen (United States). The fetal bovine serum was from Hyclone (United States). Dispase II, collagenase L, hyaluronidase, Triton X-100, paraformaldehyde and Fluoromount were obtained from Sigma (United States). The primary antibody mouse anti-human CD34 and proliferating cell nuclear antigen (PCNA) were from Thermo Scientific (United States). 4′,6-diamidino-2-phenylindole (DAPI) was from Roche (Germany). TransScript First-Strand cDNA Synthesis SuperMix was from Beijing TransGen Biotech Co. Ltd (China). The SYBR Green Real-Time Polymerase Chain Reaction (PCR) Master Mix was from Toyobo (Japan), and primers were obtained from Shanghai Sangon Biotech (China). The phase contrast microscope IX71 was from Olympus (Japan), and the 7500 Fast Real-Time PCR System was from Applied Biosystems (United States). The laser scanning confocal microscope, 80i, was from Nikon (Japan).
Methods
Isolation and cultivation of human keratocytes
Human tissue was handled according to the tenets of the Declaration of Helsinki. The remaining donor corneal tissues after penetrating keratoplasty (less than 7mm in diameter) were obtained by dissecting along the limbal boundary and were rinsed with Hank's balanced salt solution containing 1 000U/mL penicillin and 1 000U/mL streptomycin for 30min. The BPE, fetal bovine serum (FBS) and control groups were designed according to different culture media. As the culture medium, the BPE and FBS groups used K-SFM supplemented with 50µg/mL BPE or with 10% FBS, respectively. The control group used K-SFM. Corneal tissues were quickly washed after rinsing and divided equally into three groups. Following a 30min rinse in 5mg/mL Dispase II solution at 37°C, corneal epithelium, endothelium and Descemet's membrane were removed from the corneal tissues. The remaining tissues were washed and cut into small pieces (<2mm) using corneal scissors. To isolate single cells, the fragments were treated with a 78U/mL collagenase and 38U/mL hyaluronidase mixed solution at 37°Cfor 4h[4],[5]. The three different culture media were used to make single cell suspensions, and these suspensions were plated at a density of 1×104/cm2 in 35-mm dishes. Cultures were incubated at 37°C in 5% CO2 and 95% humidity. The medium was changed after 72h and then every 2d thereafter. Changes in morphology and growth were recorded by phase contrast microscopy.
Serial subcultivation of human keratocytes
In the BPE group, keratocytes were passaged when the confluence reached approximately 70% of the total surface area. Cells were washed with Hank's balanced solution to remove dead cells on the surface and treated with a 0.25% trypsin/0.02% ethylene diamine tetraacetic acid (EDTA) solution at 37°C for 5min. When cells shrank and fell off by carefully shaking the dish, equal volumes of defined trypsin inhibitor were used to inhibit the digestion. After collection, single cell suspensions were made in K-SFM supplemented with 50µg/mL BPE and then plated at a proportion of one to three in 35-mm dishes. Cultures were incubated at 37°C in 5% CO2 and 95% humidity. The medium was changed after the first 48h and then every 2d thereafter. When the confluence reached approximately 70%, keratocytes were subcultured again. The CD34 expression and the distribution of F-actin were detected by immunofluorescent staining until the third generation.
Immunofluorescent staining
Using a previously reported method, immunofluorescent staining was performed to evaluate the expression of molecular markers that have been proposed to identify keratocytes and detect proliferation[6],[7]. Briefly, cells were fixed in 2% paraformaldehyde at 4°C for 10min and permeabilized with 0.2% Triton ×-100 in PBS at room temperature for 10min. The non-specific sites were blocked by 5% BSA at room temperature for 15min. Primary antibodies against CD34 (1:100) and PCNA (1:200) were applied and incubated overnight at 4°C. The secondary antibody Alexa Fluor 568 conjugated goat anti-mouse IgG was incubated for 40min at room temperature followed by counterstaining with DAPI (1µg/mL in PBS) for 5min at room temperature. The sections were covered with antifade Fluoromount, then scanned and recorded by laser scanning confocal microscopy. Nikon AR 3.1 software was used to manage the images and perform calculations.
Immunofluorescent staining for F-actin detection
Third generation keratocytes were washed with Hank's balanced solution to remove dead cells on the surface. Cells were fixed in 4% paraformaldehyde at 4°C for 10min and permeabilized with 0.1% Triton ×-100 in PBS at room temperature for 10min. The non-specific sites were blocked by 1% BSA at room temperature for 15min, and Alexa Fluor 543-phalloidin (1:40) was applied and incubated for 2h in a dark box at room temperature[8]. The samples were counterstained with DAPI dye (1µg/mL in PBS) for 5min at room temperature, followed by the application of Fluoromount. The sections were scanned and recorded by laser scanning confocal microscopy.
Real-time quantitative polymerase chain reaction
The total cellular RNA was extracted using the Trizol reagent. Primer sequences and GenBank accession numbers are listed in Table 1. Reverse transcription of mRNA to cDNA was performed in 20µL reaction volumes with random priming using TransScript Reverse Transcriptase and the TransScript First-Strand cDNA Synthesis SuperMix kit. Gene expression was examined in a 7500 Fast Real-Time PCR Detection System using the SYBR Green real-time PCR Master Mix. The cycle number at which the reporter fluorescence reached a threshold (Ct value) was used for quantitative measurement. The relative expression data were determined by normalizing to β-actin expression measured concurrently from the same sample, which enabled the fold-change calculation using the 2−ΔΔCT method[9]-[11].
Table 1. Real-time PCR primer sequences.
| Primers | Sequence | GenBank accession No. |
| CD34 | ||
| Sense | TTGACAACAACGGTACTGCTAC | NM_001773 |
| Antisense | TGGTGAACACTGTGCTGATTAC | |
| PCNA | ||
| Sense | GGAAATGGAAACATTAAATTGTCAC | NM_002592 |
| Antisense | GAGTGGCTTTTGTAAAGAAGTTCAG | |
| Lumican | ||
| Sense | GGAAAACAATGCCCAGACTC | NM_002345 |
| Antisense | TGTTTACAAAACATTTCCCTCAGA | |
| VSX1 | ||
| Sense | ATTGACCTCTCCAGCTCTGC | NM_014588 |
| Antisense | TGGACAATTTTTGTCTTTTGGA | |
| α-SMA | ||
| Sense | ACTGGGACGACATGGAAAAG | NM_001613 |
| Antisense | TAGATGGGGACATTGTGGGT | |
| β-Actin | ||
| Sense | GCATCCATGAAACTACATTCAATT | NM_001101 |
| Antisense | AATGATCTTGATCTTCATGGTGCTA |
Keratocyte proliferation in different bovine pituitary extract concentrations
To analyze the proliferation of keratocytes in different concentrations of BPE, the following six concentrations were designed: 0µg/mL, 10µg/mL, 20µg/mL, 30µg/mL, 40µg/mL and 50µg/mL. Changes in morphology and growth were recorded by phase contrast microscopy, and the mRNA expression of CD34, PCNA and VSX1was analyzed by real-time quantitative PCR.
Statistical Analysis
The Statistical Package for the Social Sciences, Version 17.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analyses. The data were expressed as the mean±standard deviation. Nikon AR 3.1 software was used for image management and calculations of the average fluorescence intensity. The mRNA expression of CD34, PCNA, Lumican, VSX1 and α-SMA in different groups was analyzed by a one-way ANOVA analysis. A P value less than 0.05 was considered to be significant.
RESULTS
Morphologic Changes and Molecule Detection in Primary Culture
Changes in morphology and growth
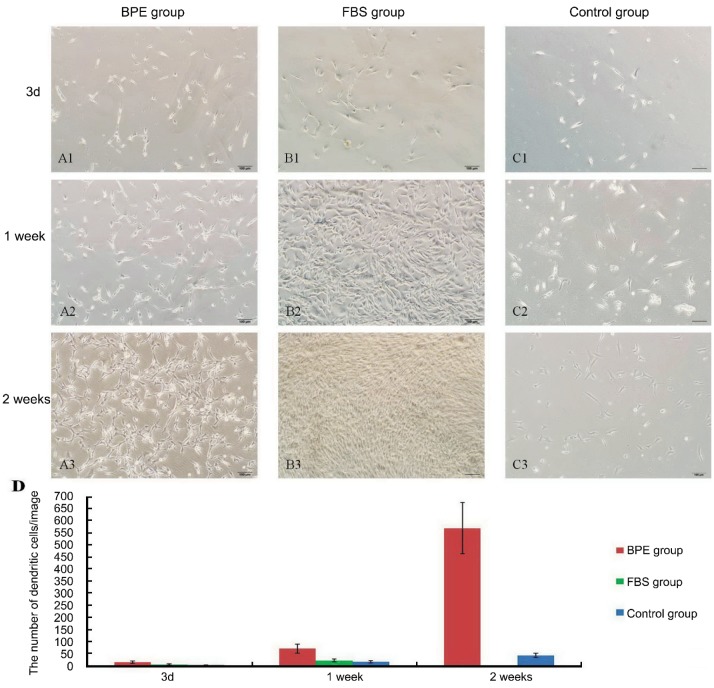
In the BPE group, most cells were attached within 72h. On the third day, cells stretched out into branch-like shapes, and a few cells exhibited a triangular or stellate appearance. After one week, many of them spread out with completely dendritic shapes and proliferated actively. By two weeks, cells proliferated more actively, and their dendrites connected to one another and formed networks (Figure 1 A1-A3). In the FBS group, most cells were attached within 48h. On the third day, many cells stretched out into branch-like, triangular or stellate shapes, and a few displayed a dendritic appearance. By one week, cells proliferated actively, and many of them showed fibroblast-like shapes instead of a dendritic appearance. By two weeks, most cells showed fibroblast-like shapes with crosswise arraying, and dendritic cells weren't found (Figure 1 B1-B3). In the control group, only a few cells attached within 72h. On the third day, many cells displayed elongated shapes. After one week, they gradually stretched out into branched or triangular shapes. By two weeks, many of them showed dendritic shapes, but networks weren't formed (Figure 1 C1-C3). In three groups, the quantity of cells with typical dendritic appearance per image was showed by histogram, and the number of dendritic cells in BPE group was more than other two groups (Figure 1D).
Figure 1. Morphologic changes in primary culture.
A1-A3. BPE group: A1. Many cells stretched out into branch-like shapes. A2. Most cells showed dendritic appearances. A3. By two weeks, networks were formed. B1-B3. FBS group: B1. Multiple morphological cells. B2. Transforming into fibroblast-shapes. B3. Fibroblast-like cells were with crosswise arraying. C1-C3. Control group: C1. On the third day, many cells were elongated. C2. Stretching out into branch-like or triangular shapes. C3. A few dendritic cells. D. The number of dendritic cells in three groups at different time points. The scale bar is 100µm in length.
Immunofluorescent staining for CD34 and proliferating cell nuclear antigen
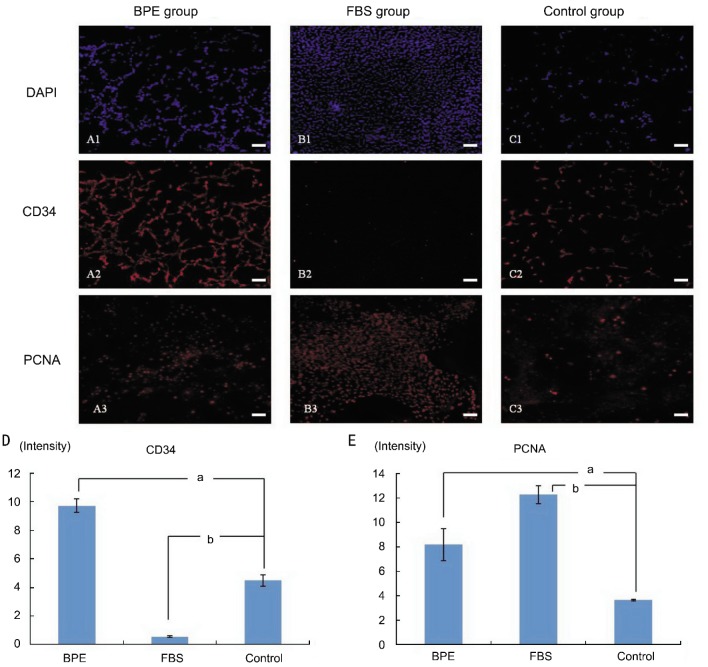
On the fourteenth day, immunofluorescent staining was used to detect the expression of CD34 and PCNA. The BPE group exhibited high expression of CD34 and PCNA, and their average fluorescence intensities were 6.07±2.04 and 8.19±1.31, respectively (Figure 2 A1-A3, Figure D, E). In the FBS group, fewer cells expressed CD34, but they exhibited a higher expression of PCNA than in the other two groups, and their average fluorescence intensities were 0.56±0.05 and 12.27±0.73, respectively (Figure 2 B1-B3, D, E). In the control group, the expression of CD34 was intermediate among the groups, but the expression of PCNA was lower; their average fluorescence intensities were 4.50±0.39 and 3.65±0.07, respectively (Figure 2 C1-C3, D, E).
Figure 2. Immunofluorescent staining of CD34 and PCNA in primary culture.
A1-A3. BPE group: A1. Nuclear counterstaining (blue) with DAPI. A2. Most cells (red) expressed CD34. A3. Staining of PCNA in the nucleus (red). B1-B3. FBS group: B1. Nuclear counterstaining (blue). B2. Fewer cells expressed CD34 (red). B3. PCNA was expressed in most nuclei (red). C1-C3: Control group: C1. Nuclear counterstaining (blue). C2. A few cells expressed CD34 (red). C3. PCNA was expressed in a few nuclei (red). D. The average fluorescence intensity of the CD34: BPE group was higher than in the other two groups. E. The average fluorescence intensity of PCNA was higher in the BPE and FBS groups than in the control group. aP<0.05 and bP<0.01 versus the control group. The scale bar is 100µm in length.
Real-time quantitative polymerase chain reaction
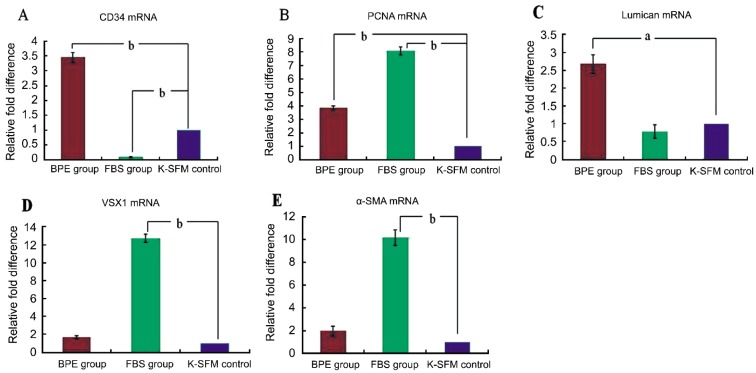
On the fourteenth day, the relative differences in mRNA expression of CD34, PCNA, Lumican and VSX1 were analyzed by real-time quantitative PCR. In the BPE group, the mRNA expression of CD34 was higher than those in the control group (P<0.01), but the mRNA expression of CD34 was lower in the FBS group than those in the control group (P<0.01) (Figure 3A). The mRNA expression of PCNA in the BPE and FBS groups was higher than those in the control group (P<0.05) (Figure 3B). The mRNA expression of Lumican in the BPE group was higher than in the control group (P<0.05), but no obvious difference was observed between the FBS and control groups (Figure 3C). The mRNA expression of VSX1 and α-SMA in the FBS group was higher than in the control group (P<0.01), and no difference was detected between the BPE group and the control group (Figure 3D, 3E).
Figure 3. The relative differences in cytokine mRNA expression in primary culture.
Values are given relative to an increase or decrease from the control value in three groups (mean±SD) on the fourteenth day. aP<0.05 and bP<0.01versus the control group. A: CD34; B: PCNA; C: Lumican; D: VSX1; E: α-SMA.
Changes in Serial Subcultivation and Molecule Detection
Keratocytes in the BPE group were subcultured further, and the cells attached within 24h. In every passage, most cells spread out with dendritic shapes and proliferated actively, and their dendrites contacted each other and formed networks (Figure 4 A1, B1). On the seventh day of the third generation, immunofluorescent staining demonstrated that many cells expressed CD34 (Figure 4 B2). Detection of F-actin with Alexa Fluor 543-phalloidin showed it was mainly distributed in the periphery of cell bodies and didn't form tension fibers (Figure 4 B3).
Figure 4. Phenotype change in serial subcultivation.
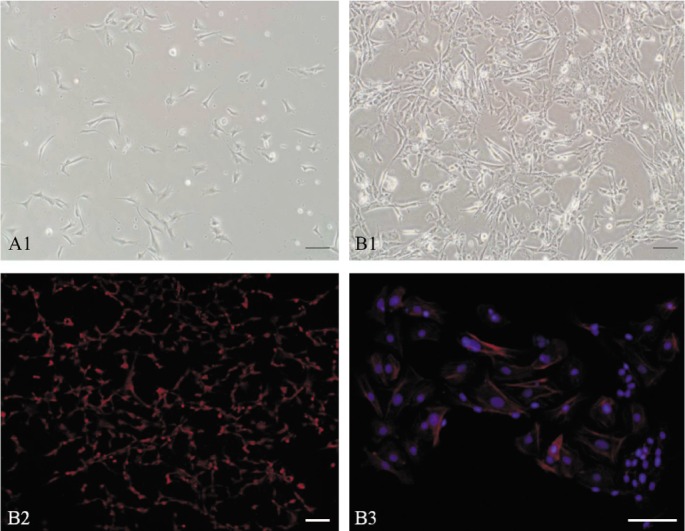
A and B1: Morphologic change. A: On the first day, cells spread out with a dendritic shape; B1: On the seventh day, networks were formed by the connection of their branches; B2: Immunofluorescent staining of CD34 (red); B3: The distribution of F-actins (red). The scale bar is 100 µm in length.
Effect of Different Bovine Pituitary Extract Concentrations on Keratocyte Proliferation
On the fourteenth day, many cells exhibited dendritic shapes, and fewer cells appeared fibroblast-like. The proliferation of cells in the 40µg/mL and 50µg/mL groups was more active than in the other four groups. Real-time quantitative PCR was employed to detect mRNA expressions of CD34, PCNA and VSX1. CD34 mRNA expression was higher in the 40µg/mL and 50µg/mL groups than in the control group (0µg/mL BPE) (P<0.05) (Figure 5A). When compared to the control group, the mRNA expression of PCNA in the 30µg/mL, 40µg/mL and 50µg/mL groups was higher (P<0.05) (Figure 5B). No differences in VSX1 mRNA expression were detected between each group and the control group (P>0.05) (Figure 5C), so the phenotype of keratocytes didn't undergo fibroblastic phenotype transition with the increasing of BPE concentration. In six groups, the concentration of 40µg/mL had already increased proliferation of keratocytes obviously and was the most appropriate amount.
Figure 5. The relative changes in cytokine mRNA expression in different concentration of BPE group. Values are given relative to an increase from the control value in six groups (mean ± SD).
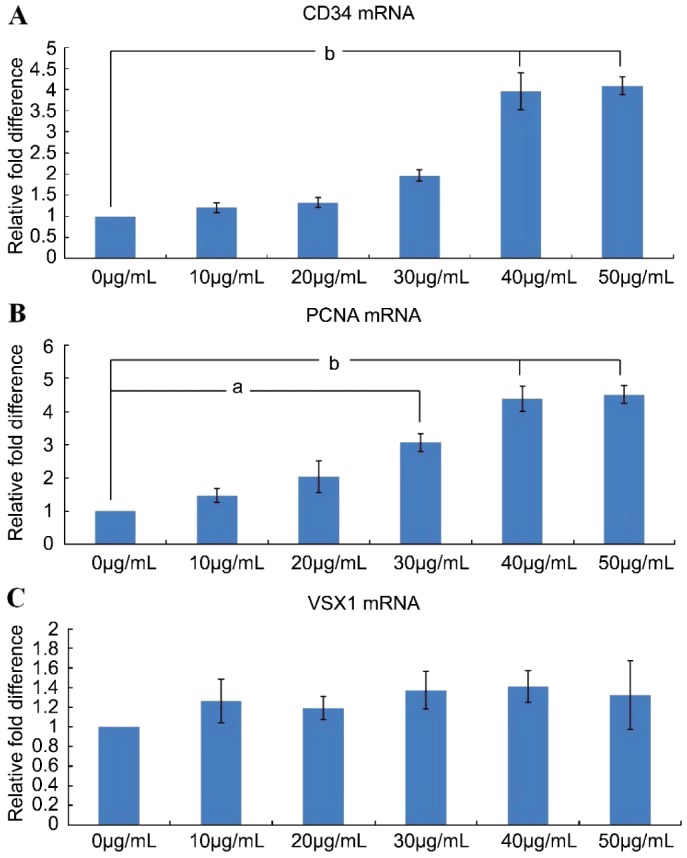
aP<0.05 and bP<0.01 versus the control group. A. CD34; B: PCNA; C: VSX1.
DISCUSSION
Corneal stroma cells have three different phenotypes: keratocyte, fibroblast and myofibroblast. These cells are quiescent and generally display the keratocyte phenotype. When the cornea is injured, these cells are stimulated to undergo apoptosis and transition into fibroblasts and myofibroblasts. Ambrósio et al[12] confirmed that keratocyte apoptosis was an early response to corneal epithelial injury not only in animals but also in humans. In culture, serum and some cytokines such as TGF-β can easily activate keratocytes to transform into fibroblasts and myofibroblasts. To inhibit this transformation, some researchers lowered the concentration of serum and refined serum to remove stimulating factor. Long et al[13] and Funderburgh et al[14] used platelet-poor horse serum and insulin as main culture supplements to decrease transition and increase proliferation.
Compared to serum, serum-free cultures have been reported to be more effective in the maintenance of the keratocyte phenotype by multiple research groups. Though some researchers only used culture medium without supplements to maintain the dendritic morphology of keratocytes successfully, there were a few of inadequacies: poor efficiency of cell attachment and low proliferation of culture cells[15],[16]. Many investigators added some supplements to overcome the inadequacy of serum-free medium. Yoshida et al[17] used epidermal growth factor, fibroblast growth factor 2 and B27 supplement to proliferate keratocyte and maintain their phenotype successfully. Indeed, these methods could improve the culture, but many cytokines need be added into culture medium, and a lot of tests need be administrated for a proper concentration for each cytokine before culture. Bovine pituitary extract contains a unique mixture of native growth factors with enriching growth factors that highly promote mitotic activity. Therefore, bovine pituitary extract could be a good supplement for increasing cell proliferation and avoiding serum side effects in vitro.
In our study, although many cells exhibited dendritic shapes in the control group, cells required a long time to attach and their density was lower than those in the other two groups. In the FBS group, cells attached within a short time and proliferated actively. However, the cell phenotype changed given that most cells displayed a fibroblast-like appearance and fewer cells exhibited a dendritic appearance. In the BPE group, most cells still appeared to have a typical dendritic shape, and the detection of PCNA clearly showed that the proliferation of cells was higher than in the control group. BPE was used as a serum-free supplement to increase keratocyte proliferation and successfully maintain their phenotype in vitro.
CD34 is known as a hematopoietic stem cell marker[18]-[20]. However, studies have verified that quiescent keratocytes also express CD34 normally, and the expression of CD34 had a drastic reduction in corneal stromal cells following injury to the cornea[4]. Therefore, CD34 was also selected as a keratocyte marker[21],[22]. VSX1 is a member of the Vsx1 group of vertebrate paired-like homeodomain transcription factors[23],[24]. Barbaro and Pellegrini showed that keratocytes expressed VSX1 at very low levels, and the expression of VSX1 increased sharply when they used serum or injury to stimulate keratocytes to transit into fibroblasts and myofibroblasts[4]. In this study, immunofluorescent staining and real-time quantitative PCR showed that cells in the BPE group expressed CD34 at a high level and VSX1 at a very low level, which confirmed that fewer keratocytes underwent a phenotype transition. However, in the FBS group, the expression of CD34 and VSX1 underwent the opposite effect; the expression of VSX1 reached a high level, and many cells underwent fibroblastic transformation.
To further confirm that serum-free medium containing BPE could maintain the keratocyte phenotype in vitro, keratocyte serial subcultivation was performed until the third generation. Most cells still held their dendritic shape and expressed the CD34 marker, which indicated that fewer cells underwent phenotype transition. F-actin is a cytoskeletal protein that is mainly distributed in the periphery of the keratocyte cell body and doesn't form tension fibers. However, F-actins form tension fibers across the cell bodies of fibroblast and myofibroblast cells[25],[26]. Given that phalloidin has the capacity to specifically bind F-actin, we used Alexa Fluor 546-phalloidin to detect its distribution. In this study, F-actin was mainly distributed in the periphery of cell bodies and didn't form tension fibers, which also confirmed that the keratocyte phenotype had been maintained.
In this study, we used enzymatic digestion to successfully isolate keratocytes. Using K-SFM containing BPE can improve the proliferation capability and maintain the keratocyte phenotype throughout primary culture and subcultivation. This culture system can also be used to research the mechanism of keratocyte phenotype transition. Most importantly, plentiful keratocytes can be harvested rapidly and can provide seeds for the construction of corneal stroma in vitro.
Acknowledgments
We thank Yue-Qin Zhang, Ping-Ling Shi and Xiao-Feng Du, Henan Eye Institute, who were ophthalmologist and technicians for this study and we acknowledge our colleagues who contributed to our study.
Footnotes
Foundation item: National Natural Science Foundation of China (No.81170831)
REFERENCES
- 1.West-Mays JA, Dwivedi DJ. The keratocyte: corneal stromal cell with variable repair phenotypes. Int J Biochem Cell Biol. 2006;38(10):1625–1631. doi: 10.1016/j.biocel.2006.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilson SE, Chaurasia SS, Medeiros FW. Apoptosis in the initiation, modulation and termination of the corneal wound healing response. Exp Eye Res. 2007;85(3):305–311. doi: 10.1016/j.exer.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petrescu MS, Larry CL, Bowden RA, Williams GW, Gagen D, Li ZJ, Smith CW, Burns AR. Neutrophil interactions with keratocytes during corneal epithelial wound healing: a role for CD18 integrins. Invest Ophthalmol Vis Sci. 2007;48(11):5023–5029. doi: 10.1167/iovs.07-0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barbaro V, Di Iorio E, Ferrari S, Bisceglia L, Ruzza A, De Luca M, Pellegrini G. Expression of VSX1 in human corneal keratocytes during dfifferentiation into myofibroblasts in response to wound healing. Invest Ophthalmol Vis Sci. 2006;47(12):5243–5250. doi: 10.1167/iovs.06-0185. [DOI] [PubMed] [Google Scholar]
- 5.Branch MJ, Hashmani K, Dhillon P, Jones DR, Dua HS, Hopkinson A. Mesenchymal stem cells in the human corneal limbal stroma. Invest Ophthalmol Vis Sci. 2012;53(9):5109–5116. doi: 10.1167/iovs.11-8673. [DOI] [PubMed] [Google Scholar]
- 6.Park SH, Kim KW, Chun YS, Kim JC. Human mesenchymal stem cells differentiate into keratocyte-like cells in keratocyte-conditioned medium. Exp Eye Res. 2012;101:16–26. doi: 10.1016/j.exer.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 7.Liu H, Zhang J, Liu CY, Wang IJ, Sieber M, Chang J, Jester JV, Kao WW. Cell therapy of congenital corneal diseases with umbilical mesenchymal stem cells: lumican null mice. PLoS One. 2010;5(5):e10707. doi: 10.1371/journal.pone.0010707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lakshman N, Kim A, Petroll WM. Characterization of corneal keratocyte morphology and mechanical activity within 3-D collagen matrices. Exp Eye Res. 2010;90(2):350–359. doi: 10.1016/j.exer.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guerriero E, Chen J, Sado Y, Mohan RR, Wilson SE, Funderburgh JL, Sundarraj N. Loss of Alpha3(IV) Collagen Expression Associated with Corneal Keratocyte Activation. Invest Ophthalmol Vis Sci. 2007;48(2):627–635. doi: 10.1167/iovs.06-0635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Y, Jiang J, Xiao H, Wang X, Li Y, Gong Y, Wang D, Huang Y. Topical application of FTY720 and cyclosporin A prolong corneal graft survival in mice. Mol Vis. 2012;18:624–633. [PMC free article] [PubMed] [Google Scholar]
- 11.Myrna KE, Mendonsa R, Russell P, Pot SA, Liliensiek SJ, Jester JV, Nealey PF, Brown D, Murphy CJ. Substratum topography modulates corneal fibroblast to myofibroblast transformation. Invest Ophthalmol Vis Sci. 2012;53(2):811–816. doi: 10.1167/iovs.11-7982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ambrósio R, Jr, Kara-José N, Wilson SE. Early keratocyte apoptosis after epithelial scrape injury in the human cornea. Exp Eye Res. 2009;89(4):597–599. doi: 10.1016/j.exer.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Long CJ, Roth MR, Tasheva ES, Funderburgh M, Smit R, Conrad GW, Funderburgh JL. Fibroblast growth factor-2 promotes keratan sulfate proteoglycan expression by keratocytes in vitro. J Biol Chem. 2000;275(18):13918–13923. doi: 10.1074/jbc.275.18.13918. [DOI] [PubMed] [Google Scholar]
- 14.Funderburgh JL, Funderburgh ML, Mann MM, Prakash S, Conrad GW. Synthesis of corneal keratan sulfate proteoglycans by bovine keratocytes in vitro. J Biol Chem. 1996;271(49):31431–31436. doi: 10.1074/jbc.271.49.31431. [DOI] [PubMed] [Google Scholar]
- 15.Funderburgh JL, Mann MM, Funderburgh ML. Keratocyte phenotype mediates proteoglycan structure: a role for fibroblasts in corneal fibrosis. J Biol Chem. 2003;278(46):45629–45637. doi: 10.1074/jbc.M303292200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berryhill BL, Kader R, Kane B, Birk DE, Feng J, Hassell JR. Partial restoration of the keratocyte phenotype to bovine keratocytes made fibroblastic by serum. Invest Ophthalmol Vis Sci. 2002;43(11):3416–3421. [PubMed] [Google Scholar]
- 17.Yoshida S, Shimmura S, Shimazaki J, Shinozaki N, Tsubota K. Serum-free spheroid culture of mouse corneal keratocytes. Invest Ophthalmol Vis Sci. 2005;46(5):1653–1658. doi: 10.1167/iovs.04-1405. [DOI] [PubMed] [Google Scholar]
- 18.Gangenahalli GU, Singh VK, Verma YK, Gupta P, Sharma RK, Chandra R, Luthra PM. Hematopoietic stem cell antigen CD34: role in adhesion or homing. Stem Cells Dev. 2006;15(3):305–313. doi: 10.1089/scd.2006.15.305. [DOI] [PubMed] [Google Scholar]
- 19.McKinney-Freeman SL, Naveiras O, Yates F, Loewer S, Philitas M, Curran M, Park PJ, Daley GQ. Surface antigen phenotypes of hematopoietic stem cells from embryos and murine embryonic stem cells. Blood. 2009;114(2):268–278. doi: 10.1182/blood-2008-12-193888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gori JL, Chandrasekaran D, Kowalski JP, Adair JE, Beard BC, D'Souza SL, Kiem HP. Efficient generation, purification, and expansion of CD34(+) hematopoietic progenitor cells from nonhuman primate-induced pluripotent stem cells. Blood. 2012;120(13):e35–44. doi: 10.1182/blood-2012-05-433797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perrella G, Brusini P, Spelat R, Hossain P, Hopkinson A, Dua HS. Expression of haematopoietic stem cell markers, CD133 and CD34 on human corneal keratocytes. Br J Ophthalmol. 2007;91(1):94–99. doi: 10.1136/bjo.2006.097352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Builles N, Bechetoille N, Justin V, André V, Burillon C, Damour O. Variations in the characteristics of keratocytes in culture in relation to their location in human cornea. Biomed Mater Eng. 2008;18(1 Suppl):S87–98. [PubMed] [Google Scholar]
- 23.Ohtoshi A, Wang SW, Maeda H, Saszik SM, Frishman LJ, Klein WH, Behringer RR. Regulation of retinal cone bipolar cell differentiation and photopic vision by the CVC homeobox gene Vsx1. Curr Biol. 2004;14(6):530–536. doi: 10.1016/j.cub.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 24.Shi Z, Jervis D, Nickerson PE, Chow RL. Requirement for the paired-like homeodomain transcription factor VSX1 in type 3a mouse retinal bipolar cell terminal differentiation. J Comp Neurol. 2012;520(1):117–129. doi: 10.1002/cne.22697. [DOI] [PubMed] [Google Scholar]
- 25.Pot SA, Liliensiek SJ, Myrna KE, Bentley E, Jester JV, Nealey PF, Murphy CJ. Nanoscale topography-induced modulation of fundamental cell behaviors of rabbit corneal keratocytes, fibroblasts, and myofibroblasts. Invest Ophthalmol Vis Sci. 2010;51(3):1373–1381. doi: 10.1167/iovs.09-4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pan HW, Xu JT, Chen JS. Pioglitazone inhibits TGFβ induced keratocyte transformation to myofibroblast and extracellular matrix production. Mol Biol Rep. 2011;38(7):4501–4508. doi: 10.1007/s11033-010-0581-5. [DOI] [PubMed] [Google Scholar]