Abstract
AIM
To demonstrate the apoptosis-inducing effect of lidocaine on human corneal stromal (HCS) cells in vitro, and provide experimental basis for safety anesthetic usage in clinic of ophthalmology.
METHODS
In vitro cultured HCS cells were treated with lidocaine at different doses and times, and their morphology was monitored successively with inverted phase contrast microscopy. The membrane permeability of them was detected by acridine orange/ethidium bromide (AO/EB) double staining. The DNA fragmentation of them was examined by agarose gel electrophoresis, and their ultrastructure was observed by transmission electron microscopy (TEM), respectively.
RESULTS
Exposure to lidocaine at doses from 0.3125g/L to 20g/L induced morphological changes of HCS cells such as cytoplasmic vacuolation, cellular shrinkage, and turning round, and elevated membrane permeability of these cells in AO/EB staining. The change of morphology and membrane permeability was dose- and time-dependent, while lidocaine at dose below 0.15625g/L could not induce these changes. Furthermore, lidocaine induced DNA fragmentation and ultrastructural changes such as cytoplasmic vacuolation, structural disorganization, chromatin condensation, and apoptotic body appearance of the cells.
CONCLUSION
Lidocaine has significant cytotoxicity on human corneal stromal cells in vitro in a dose- and time-dependent manner by inducing apoptosis of these cells. The established experimental model and findings based on this model here help provide new insight into the apoptosis-inducing effect of local anesthetics in eye clinic.
Keywords: lidocaine, apoptosis-inducing effect, apoptotic body, DNA fragmentation, human corneal stromal cell
INTRODUCTION
Human corneal stroma (HCS), constituting 90% of the corneal volume, is a highly organized and transparent flattened lamellae of collagen fibrils and proteoglycans with HCS cells present between the lamellae[1]. HCS cells, specialized mitotically quiescent fibroblasts, play the major role in keeping HCS transparent, healing its wounds, and synthesizing its components[2]-[5]. The number of HCS cells declines with age, at a rate approximately 0.45% per year[6]. In a healthy cornea, HCS cell apoptosis is a rare occasion, but they undergo apoptosis immediately after an injury to the uppermost layer of HCS[7]. Excessive HCS cell apoptosis is often observed after eye operations and degenerative corneal disorders, and may play a role in the development of post-surgery complications[8],[9].
Lidocaine, a local amide-type anesthetic possessing both lipophilic and hydrophilic properties, is widely used in eye examinations and surgeries[10],[11]. Long term and repeated exposure to lidocaine at the concentrations supplied commercially leads to cytotoxicity on porcine or rabbit corneal endothelial cells, causing significant cell loss, corneal thickening, and opacification[12]-[14]. The toxicity and side effects of lidocaine on HCS cells, and the underlying mechanisms remain unknown. In this study, HCS cells from recently established untransfected HCS cell line were treated by lidocaine, and cell morphology, membrane permeability, DNA fragmentation, and cell ultrastructure were examined. The results demonstrated that we established a useful in vitro experimental model to investigate the cytotoxicity and mechanism by which topical local anesthetics cytotoxic on HCS cells.
MATERIALS and methods
Materials
HCS cells from the untransfected HCS cell line (utHCSC01) established previously in our laboratory, were maintained and cultured in 10% fetal bovine serum (FBS; Hyclone, Logan, Utah, USA)-containing Dulbecco's modified Eagle medium: Ham's nutrient mixture F-12 (1:1) medium (DMEM/F12; Invitrogen, Carlsbad, CA, USA) at 37°C.
Methods
Morphological observation of human corneal stroma cells
HCS cells from the utHCSC01 cell line were inoculated into a 24-well culture plate and cultured in 10% FBS-DMEM/F12 medium (Invitrogen) at 37°C for 24h in a 5% CO2 incubator as described previously. The culture medium of HCS cells at logarithmic phase was replaced with 10% FBS-DMEM/F12 medium containing lidocaine (Hualu Pharmaceutical Co., Ltd, Liaocheng, Shandong, China) at concentrations from 0.078125g/L to 20g/L, using HCS cells without lidocaine treatment as blank controls. The morphology and growth status of the cells were monitored with a TS100 light microscope (Nikon, Tokyo, Japan) at a 3h interval.
Membrane permeability detection of human corneal stroma cells
Plasma membrane permeability was measured by acridine orange (AO)/ethidium bromide (EB) double-fluorescent staining according to the method described previously[15]. The medium of HCS cells at logarithmic phase in a 24-well culture plate were replaced with lidocaine-containing medium and cultured as described above. The cells were harvested every 2h or 4h by 0.25% trypsin digestion (1-2min) and centrifugation (120g, 10min) methods as described previously[16]. After cell pellet was re-suspended with 0.1mL serum-free DMEM/F12 medium, 4µL of AO/EB (Sigma-Aldrich, St. Louis, MO, USA) solution (100µg/mL AO: 100µg/mL EB=1:1) was added, mixed, and stained for 1min at room temperature. About 1 drop of stained cell suspension from each group was dripped onto a glass slide and covered with a coverslip. The AO/EB stained HCS cells were observed under a Ti-S fluorescent microscope (Nikon). The cells with red or orange nuclei were designated as apoptotic cells while the cells with green nuclei designated as non-apoptotic cells, and the apoptotic rate of HCS cells was calculated on condition that at least 400 cells were counted in each group. Data were presented as mean±SEM and analyzed by the Student's t-test using SPSS 17.0 software (SPSS Inc., Chicago, IL, USA). Differences were considered significant at P<0.05.
DNA detection of human corneal stroma cells
DNA fragmentation was examined by agarose gel electrophoresis as described previousl[17]. Briefly, HCS cells in 25cm2 flasks were treated with lidocaine at doses from 0.625g/L to 20g/L for 1h to 12h. Then the cells were harvested with a cell scraper and collected by centrifugation as described above. After washed once with ice-cold phosphate-buffered saline (PBS) by centrifugation, the cells were re-suspended in 200µL PBS and their DNA were isolated with a Quick Tissue/Culture Cells Genomic DNA Extraction Kit (Dongsheng Biotech, Guangzhou, China) following its user's manual. The DNA preparation from each group was electrophoresed on a 1% agarose gel (200mA, 260min). After stained with 0.5mg/L EB, the gel was observed with an EC3 Imaging System (UVP, LLC Upland, CA, USA).
Ultrastructural observation of human corneal stroma cells
After treated with 1.25g/L lidocaine for 10h, HCS cells were collected by trypsin digestion and centrifugation as described above. The cells were fixed with 40g/L glutaraldehyde in 0.1mol/L sucrose with 0.2mol/L sodium cacodylate buffer (pH 7.4) overnight at 4°C. After washing with sodium cacodylate buffer and post-fixing with 10g/L osmium tetroxide for 1.5h, the fixed cells were dehydrated and embedded in epoxy resin. Ultrathin sections were stained with 20g/L uranyl acetate-lead citrate and observed by an H700 transmission electron microscope (TEM; Hitachi, Tokyo, Japan).
RESULTS
Morphological Changes of Human Corneal Stroma Cells
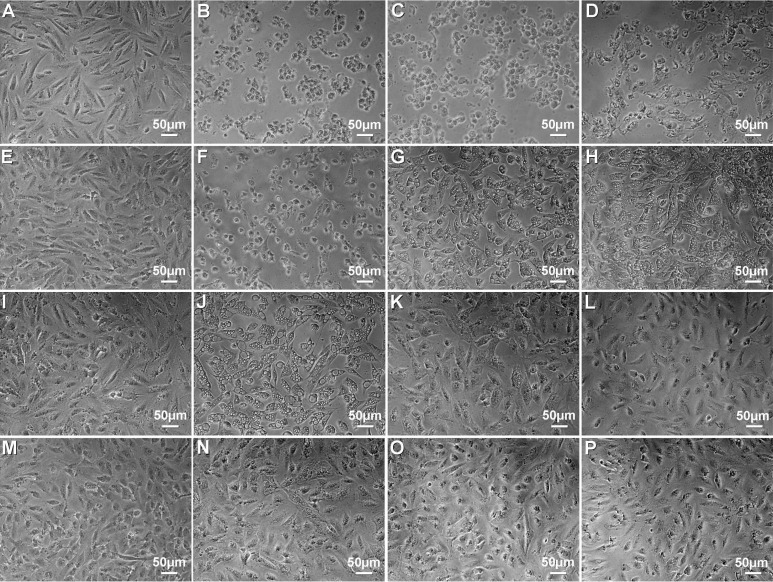
After HCS cells were treated with lidocaine at different doses and times, it was found that the cells treated 12h with lidocaine at doses from 0.3125g/L to 20g/L exhibited morphological changes similar to those of apoptotic cells, including cytoplasmic vacuolation, cellular shrinkage, turning round, and cell death (Figure 1B-D, F-H, J, N). Cells treated 28h at doses of 0.15625g/L and 0.078125g/L showed no morphological features of apoptotic cells (Figure 1K, L, O, P). The extent of morphological changes of HCS cells exposed to lidocaine was dose- and time-dependent. These indicate that lidocaine at dose above 0.3125g/L has obvious toxicity on HCS cells, while that below the dose of 0.15625g/L has not.
Figure 1. Effect of lidocaine on the morphology of HCS cells.
A: Control, 4h; B: 20g/L, 4h; C: 10g/L, 4h; D: 5g/L, 4h; E: Control, 16h; F: 2.5g/L, 16h; G: 1.25g/L, 16h; H: 0.625g/L, 16h; I: Control, 24h; J: 0.3125g/L, 24h; K: 0.15625g/L, 24h; L: 0.078125g/L, 24h; M: Control, 28h; N: 0.3125g/L, 28h; O: 0.15625g/L, 28h; P: 0.078125g/L, 28h.
Plasma Membrane Permeability of Human Corneal Stroma Cells
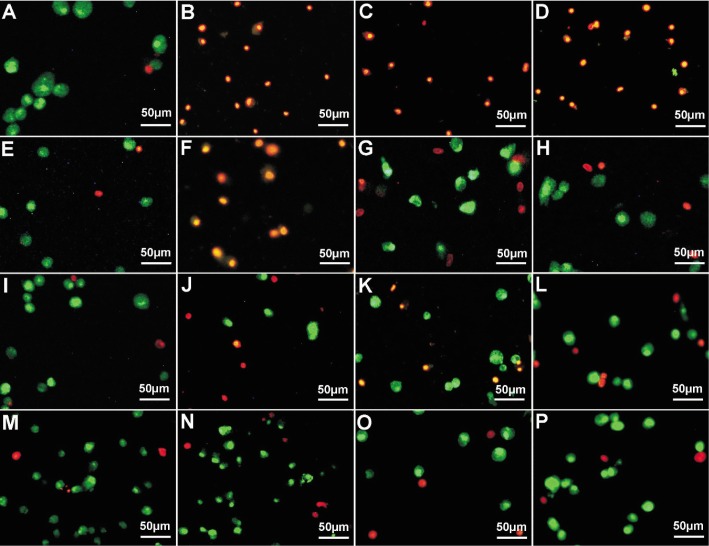
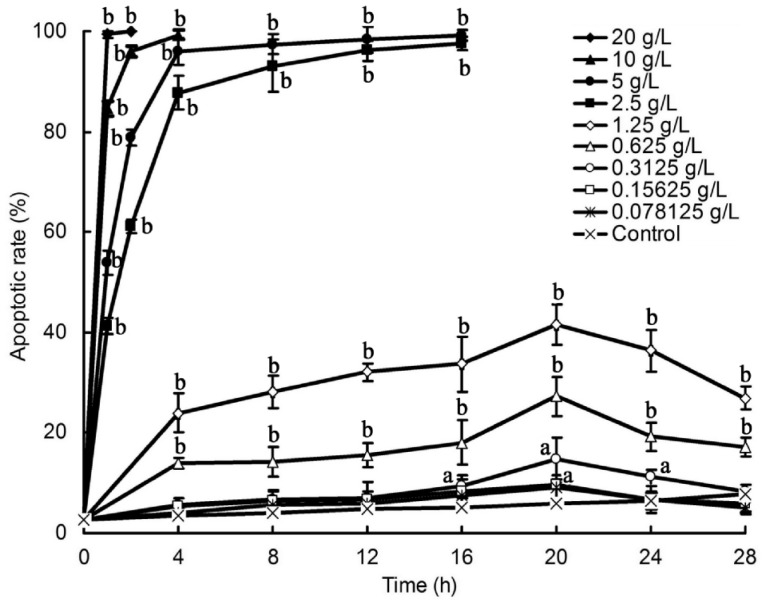
Changes of the plasma membrane permeability of lidocaine-treated HCS cells were examined with AO/EB staining and results were shown in Figure 2. It was found that the membrane integrity of HCS cells treated with 0.3125g/L to 20g/L lidocaine was destroyed, membrane permeability elevated, and nuclear chromatin was stained into orange color by EB incorporation. It was also found that the orange nuclei were all in well-proportioned condensed round or pyknotic morphologies, while the green nuclei were all unevenly stained, indicating that lidocaine might induce apoptosis in HCS cells. The apoptotic rate of these cells, increased with time and dosage, was shown in Figure 3. Among these, the apoptotic rate of HCS cells reached almost to 100% when treated with 20g/L lidocaine for 1h, 10g/L for 4h, 5 and 2.5g/L for 16h (P<0.01). That of HCS cells treated with 1.25g/L and 0.625g/L lidocaine for 20h reached only highest to 41.57% and 27.33%, respectively (P<0.01). Lidocaine at dose of 0.3125g/L exhibited a little apoptosis-inducing effect on HCS cells (P<0.05), and lidocaine at doses of 0.15625g/L and 0.078125g/L showed no obvious apoptosis-inducing effect on HCS cells when compared with that from blank control. All these indicate that lidocaine at dose above 0.3125g/L might have an apoptosis-inducing effect on HCS cells in a dose- and time-dependent manner.
Figure 2. Fluorescent double staining photographs of lidocaine-treated HCS cells.
A: Control, 4h; B: 20g/L, 1h; C: 10g/L, 4h; D: 5g/L, 4h; E: Control, 16h; F: 2.5 g/L, 16h; G: 1.25g/L, 16h; H: 0.625g/L, 16h; I: Control, 24h; J: 0.3125g/L, 24h; K: 0.15625g/L, 24h; L: 0.078125g/L, 24h; M: Control, 28h; N: 0.3125g/L, 28h; O: 0.15625g/L, 28h; P: 0.07825g/L, 28h.
Figure 3. Effect of lidocaine on the apoptotic rate of HCS cells.
Data are presented as mean±SEM values. aP<0.05; bP<0.01 relative to control cells.
DNA Fragmentation of Human Corneal Stroma Cells
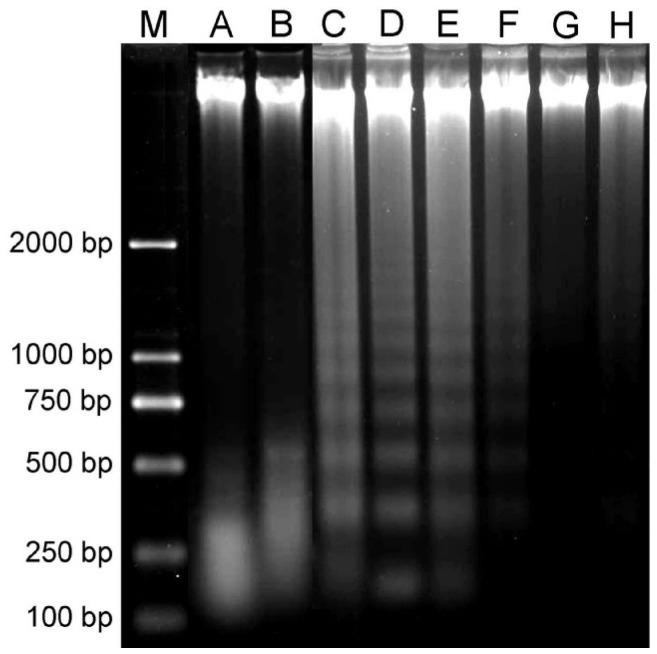
DNA extracted from HCS cells treated with 0.625g/L to 5.0g/L lidocaine for 1h to 12h showed typical DNA ladder in agarose gel electrophoresis in a dose-dependent manner (Figure 4C-F), and DNA extracted from the cells treated with lidocaine at doses of 10g/L and 20g/L for 1h was almost completely fragmented (Figure 4A, B). In other words, DNA fragmentation was found in these cells. No DNA fragmentation was found in HCS cells treated with lidocaine at dose below 0.3125g/L, which was almost the same as the blank control (Figure 4G, H) (Data of 0.15625g/L and 0.078125g/L lidocaine were not shown). All these imply that lidocaine at dose above 0.3125g/L, most probably, has an apoptosis-inducing effect on HCS cells in a dose- and time-dependent manner.
Figure 4. Effect of lidocaine on the DNA fragmentation of HCS cells.
M: Molecular weight standards marker. A: 20g/L, 1h; B: 10g/L, 1h; C: 5g/L, 1h; D: 2.5g/L, 4h; E: 1.25g/L, 4h; F: 0.625g/L, 12h; G: 0.3125g/L, 12h; H: Control, 12h. One percent of agarose gel was used.
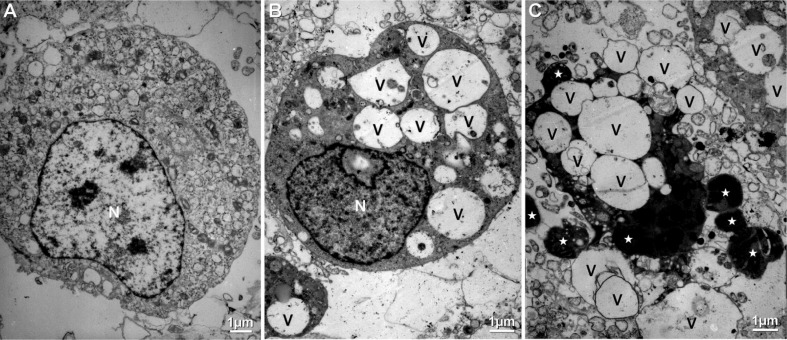
Ultrastructural Changes of Human Corneal Stroma Cells
Since the apoptosis-inducing effect of lidocaine on HCS cells was dose- and time-dependent, the ultrastructures of HCS cells treated 10h with 1.25g/L lidocaine was studied for further verification of the apoptosis-inducing effect of lidocaine. Ultrastructural changes of HCS cells treated 10h with 1.25g/L lidocaine were shown in Figure 5. It was found that the ultrastructure of some HCS cells exhibited early apoptotic characteristics including cytoplasmic vacuolation, structural disorganization, and chromatin condensation (Figure 5B), compared with that of HCS cells in blank control (Figure 5A). The ultrastructure of some cells exhibited late apoptotic characteristics including cytoplasmic vacuolation, disorganization, disaggregation of cell and nucleus, and presence of a lot of apoptotic bodies (Figure 5C). All these indicate that lidocaine does have an apoptosis-inducing effect on HCS cells in vitro.
Figure 5. Effect of lidocaine on the ultrastructure of HCS cells.
A: Control, 10h; B, C: 1.25g/L, 10h; N: Nucleus; V: Vacuole; Asterisk: Apoptotic body.
DISCUSSION
Since HCS cells play key roles in keeping cornea transparent[3],[4], identifying and characterizing the toxicity of topical corneal anesthetics and drugs to these cells will be of great importance in the security of ophthalmic medication[2],[10]. With an untransfected HCS cell line culture system, widely used for cytotoxicity evaluation in most clinically used agents, the cytotoxicity of lidocaine with a clinical dosage of 20g/L was investigated in this study[18].
Light microscopic observation showed that HCS cells, treated with lidocaine at dose above 0.3125g/L, underwent dramatic morphological changes such as cytoplasmic vacuolation, cellular shrinkage, detachment, and death with time and concentration, which was similar to those of apoptotic cells[19]. It implies that lidocaine might have an apoptosis-inducing effect on HCS cells in vitro. To validate the apoptosis-inducing hypothesis, membrane permeability of HCS cells was then examined by AO/EB double staining assay, because increase of membrane permeability is one of the remarkable features of apoptotic cells[20]. Induced elevation of membrane permeability was detected in HCS cells after treated with lidocaine at dose above 0.3125g/L in a dose- and time-dependent manner. Thus it can be postulated that lidocaine probably have an apoptosis-inducing effect on HCS cells. To verify this, DNA fragmentation, one of the most remarkable features of apoptotic cells[21], of lidocaine treated HCS cells was further inspected by agarose electrophoresis. It was found that DNA from lidocaine treated HCS cells appeared as typical DNA ladder in electrophoresed agarose gel. Then it can be concluded that lidocaine, most probably, has an apoptosis-inducing effect on HCS cells. To support this conclusion, ultrastructural changes of HCS cells treated 10h with 1.25g/L lidocaine were further checked by TEM. The results showed that lidocaine treated HCS cells underwent ultrastructural changes such as cytoplasmic vacuolation, structural disorganization, chromatin condensation, disaggregation of cell and nucleus, and presence of a lot of apoptotic bodies, which was similar to those of apoptotic cells[22],[23]. From all of the above, it can be concluded for certain that lidocaine does have an apoptosis-inducing effect on HCS cells in vitro in a dose- and time-dependent manner, especially at its clinical dose of 20g/L, which is coincident to that of oxybuprocaine hydrochloride on human corneal endothelial cells[24]. The time span used in this study was to proclaim the time-dependent cytotoxic effect of lidocaine on HCS cells in vitro, not to induce cytotoxicity and/or create apoptosis. Whether this in vitro data is relevant to actual clinical application of lidocaine or not needs to be further investigated with an in vivo model system.
In conclusion, lidocaine has strong cytotoxicity on HCS cells in vitro, especially at the concentration supplied commercially. Its cytotoxicity on HCS cells, in a dose- and time-dependent manner, is acomplished by inducing apoptosis of these cells. The experimental model we established and our observations based on this model help provide new insight into the apoptosis-inducing effect on eye examination and surgery. It should be noted that the usage of this agent in eye clinic may be a potential risk factor for corneal stromal injury. Further studies elucidating the apoptosis inducing mechanism of lidocaine in corneal cells would be intriguing and could be exploited for overcoming its cytotoxicity in eye clinic.
Footnotes
Foundation item: National High Technology Research and Development Program (“863” Program) of China (No.2006AA02A132)
REFERENCES
- 1.DelMonte DW, Kim T. Anatomy and physiology of the cornea. J Cataract Refract Surg. 2011;37(3):588–598. doi: 10.1016/j.jcrs.2010.12.037. [DOI] [PubMed] [Google Scholar]
- 2.Jester JV, Barry PA, Lind GL, Petroll WM, Garana R, Cavanagh HD. Corneal keratocytes: in situ and in vitro organization of cytoskeletal contractile proteins. Invest Ophthalmol Vis Sci. 1994;35(2):730–735. [PubMed] [Google Scholar]
- 3.Fini ME. Keratocyte and fibroblast phenotypes in the repairing cornea. Prog Retin Eye Res. 1999;18(4):529–551. doi: 10.1016/s1350-9462(98)00033-0. [DOI] [PubMed] [Google Scholar]
- 4.Hillenaar T, van Cleynenbreugel H, Verjans GM, Wubbels RJ, Remeijer L. Monitoring the inflammatory process in herpetic stromal keratitis: the role of in vivo confocal microscopy. Ophthalmology. 2012;119(6):1102–1110. doi: 10.1016/j.ophtha.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 5.West-Mays JA, Dwivedi DJ. The keratocyte: corneal stromal cell with variable repair phenotypes. Int J Biochem Cell Biol. 2006;38(10):1625–1631. doi: 10.1016/j.biocel.2006.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel S, McLaren J, Hodge D, Bourne W. Normal human keratocyte density and corneal thickness measurement by using confocal microscopy in vivo. Invest Ophthalmol Vis Sci. 2001;42(3):333–339. [PubMed] [Google Scholar]
- 7.Wilson SE, He YG, Weng J, Li Q, McDowall AW, Vital M, Chwang EL. Epithelial injury induces keratocyte apoptosis: hypothesized role for the interleukin-1 system in the modulation of corneal tissue organization and wound healing. Exp Eye Res. 1996;62(4):325–327. doi: 10.1006/exer.1996.0038. [DOI] [PubMed] [Google Scholar]
- 8.Erie JC, McLaren JW, Hodge DO, Bourne WM. Long-term corneal keratoctye deficits after photorefractive keratectomy and laser in situ keratomileusis. Trans Am Ophthalmol Soc. 2005;103:56–66, discussion 67–68. [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson SE, Chaurasia SS, Medeiros FW. Apoptosis in the initiation, modulation and termination of the corneal wound healing response. Exp Eye Res. 2007;85(3):305–311. doi: 10.1016/j.exer.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manu RK, Mathew FRCS, Lennox A. Surgeon experience and patient comfort during clear corneal phacoemulsification under topical local anesthesia. J Cataract Refract Surg. 2002;28(11):1977–1981. doi: 10.1016/s0886-3350(02)01369-x. [DOI] [PubMed] [Google Scholar]
- 11.Kiliç A, Gürler B. Subtenon lidocaine vs topical proparacaine in adult strabismus surgery. Ann Ophthalmol (Skokie) 2006;38(3):201–206. doi: 10.1007/s12009-006-0005-2. [DOI] [PubMed] [Google Scholar]
- 12.Eggeling P, Pleyer U, Hartmann C, Rieck PW. Corneal endothelial toxicity of different lidocaine concentrations. J Cataract Refract Surg. 2000;26(9):1403–1408. doi: 10.1016/s0886-3350(00)00379-5. [DOI] [PubMed] [Google Scholar]
- 13.Guzey M, Satici A, Dogan Z, Karadede S. The effects of bupivicaine and lidocaine on the corneal endothelium when applied into the anterior chamber at the concentrations supplied commercially. Ophthalmologica. 2002;216(2):113–117. doi: 10.1159/000048309. [DOI] [PubMed] [Google Scholar]
- 14.Chang YS, Tseng SY, Tseng SH, Wu CL. Cytotoxicity of lidocaine or bupivacaine on corneal endothelial cells in a rabbit model. Cornea. 2006;25(5):590–596. doi: 10.1097/01.ico.0000220775.93852.02. [DOI] [PubMed] [Google Scholar]
- 15.Leite M, Quinta-Costa M, Leite PS, Guimarães JE. Critical evaluation of techniques to detect and measure cell death-study in a model of UV radiation of the leukaemic cell line HL60. Anal Cell Pathol. 1999;19(3–4):139–151. doi: 10.1155/1999/176515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fan TJ, Ren BX, Geng XF, Yu QT, Wang LY. Establishment of a turbot fin cell line and its susceptibility to turbot reddish body iridovirus. Cytotechnology. 2010;62(3):217–223. doi: 10.1007/s10616-010-9281-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee SV, Bahaman AR. Modified gel preparation for distinct DNA fragment analysis in agarose gel electrophoresis. Trop Biomed. 2010;27(2):351–354. [PubMed] [Google Scholar]
- 18.Rajasekar S, Park da J, Park C, Park S, Park YH, Kim ST, Choi YH, Choi YW. In vitro and in vivo anticancer effects of Lithospermum erythrorhizon extract on B16F10 murine melanoma. J Ethnopharmacol. 2012;144(2):335–345. doi: 10.1016/j.jep.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 19.Vaux DL. Apoptosis timeline. Cell Death Differ. 2002;9(4):349–354. doi: 10.1038/sj.cdd.4400990. [DOI] [PubMed] [Google Scholar]
- 20.Leite M, Quinta-Costa M, Leite PS, Guimarães JE. Critical evaluation of techniques to detect and measure cell death-study in a model of UV radiation of the leukaemic cell line HL60. Anal Cell Pathol. 1999;19(3–4):139–151. doi: 10.1155/1999/176515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D'Emilio A, Biagiotti L, Burattini S, Battistelli M, Canonico B, Evangelisti C, Ferri P, Papa S, Martelli AM, Falcieri E. Morphological and biochemical patterns in skeletal muscle apoptosis. Histol Histopathol. 2010;25(1):21–32. doi: 10.14670/HH-25.21. [DOI] [PubMed] [Google Scholar]
- 22.Menna-Barreto RF, Salomão K, Dantas AP, Santa-Rita RM, Soares MJ, Barbosa HS, de Castro SL. Different cell death pathways induced by drugs in Trypanosoma cruzi: an ultrastructural study. Micron. 2009;40(2):157–168. doi: 10.1016/j.micron.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 23.Wong RS, Radhakrishnan AK, Ibrahim TA, Cheong SK. δ- and γ-tocotrienols induce classical ultrastructural apoptotic changes in human T lymphoblastic leukemic cells. Microsc Microanal. 2012;18(3):462–469. doi: 10.1017/S1431927612000177. [DOI] [PubMed] [Google Scholar]
- 24.Fan TJ, Wen Q, Yu MM, Ge Y, Miao Y, Wang DP. Experimental studies on the effect of oxybuprocaine hydrochloride on human corneal endothelial cells. Int Eye Sci. 2013;13(6):1069–1072. [Google Scholar]