Abstract
AIM
To study two methods for culturing and purifying Sprague-Dawley (SD) rat retinal Müller cells and determine which one is better.
METHODS
The passage culture method of Müller cells was respectively carried out by complete pancreatic enzyme digestion method and repeated incomplete pancreatic enzyme digestion method. After culturing retinal cells for one month through these two methods, fluorescence-activated cell sorter (FACS), RT-PCR, and immunohistochemistry technology were performed to examine the enrichment and purity of Müller glial cells, and carried out two-sample approximate t test using SSPS 13.0 to further compare the Müller cell positive rate in both methods.
RESULTS
The statistical results showed that the purity of Müller cells was 83.2%±5.16% in group A, and the purity was 98.5%±1.08% in group B. The two-sample approximate t test analysis demonstrated that the difference between group A and group B was statistically significant (t=-9.178, P<0.005). The results clearly exhibited a difference between the purity of Müller cells cultured by the complete pancreatic enzyme digestion method (group A) and the repeated incomplete pancreatic enzyme digestion method (group B).
CONCLUSION
Compared with the complete pancreatic enzyme digestion method, this novel method was more efficient and a higher purity of Müller cells could be obtained using this approach.
Keywords: primary culture, passage, purification, retinal Müller cell, trypsinization
INTRODUCTION
Müller cells are the main neuroglial cells in the retina of humans and other mammals, and are found from the internal limiting membrane to the external limiting membrane. They occupy an important position in retina for its effort to connect other kinds of neurons and nerve fibers[1]. Müller cells are born in the late stages of retinal histogenesis, when the majority of neuronal cell types are already in the process of generation[2]. Traditionally, Müller cells have been considered to play an important role in the maintenance of normal retinal physiological function and the development of various retinal diseases. Recently, many studies have indicated that retinal Müller cells are a resource of retinal progenitor cells (RPCs). In separate research on zebrafish, chickens, and rats, retinal Müller cells have been shown to dedifferentiate into cells with stem cell properties under the injury induced by ouabain or NMDA (N-methyl-D-aspartate)[3],[4]. These dedifferentiated retinal Müller cells exhibit the characteristics of stem cells, and can continue to differentiate into neurons, including retinal ganglion cells, amacrine cells, horizontal cells, bipolar cells, and photoreceptor cells. Therefore, identifying the best way to purify retinal Müller cells quickly in vivo will be significant for the study of neurogenesis in future. At present, the tissue culture and enzyme digestion methods are used to purify Müller cells[5]. The enzyme digestion method can be divided into two approaches: complete pancreatic enzyme digesting and repeated incomplete pancreatic enzyme digesting. It often takes a long time to obtain high-purity Müller cells using the tissue culture method, which is obviously inferior to others. Thus, in the present research, we studied the complete pancreatic enzyme digestion and repeated incomplete pancreatic enzyme digestion methods separately and we found that stable, high-purity Müller cells could be quickly obtained through repeated incomplete pancreatic enzyme digestion.
MATERIALS AND METHODS
Materials
Postnatal day (PN) 20 Sprague-Dawley (SD) rats were obtained from Animal Laboratory Supplies (Xiangya School of Medicine, Changsha, China). The use of animals in this study was in accordance with the Guidelines for Animal Experiments of Central South University, Changsha, China. All animal experiments in this study were conducted with the approval of the Animal Research Committee, Xiangya School of Medicine, Central South University, Changsha, China (permit numbers: SCXK 2006-0002).
Methods
Primary Müller cell culture
The primary culture of Müller cells was carried out according to a previously described method[6]. Twenty SPF SD rats of at PN20 were sacrificed by cutting off the heads in a sterile fashion to obtain the eyes. After washing the eyes in phosphate buffer solution (PBS) for several times, retinas were removed carefully to avoid contamination from the anterior eye segment or retinal pigment epithelium (RPE) in appropriate Dulbecco's modified Eagle's medium (DMEM) containing 20% fetal bovine serum (FBS) and 1:100 penicillin/streptomycin (complete medium). The retina was mechanically dissociated into about 1mm2 aggregates and then trypsinized with 0.25% trypsin-EDTA dissociation solution in a 37°C tank for 15min. Then, an appropriate amount of complete medium was added to stop digestion. After filtering with a 200 well filter, the filtered liquid was centrifuged for 3min at 800r/min. Complete medium was used to suspend cells; the cells were then transferred equally into 10 culture flasks with size of 25mm2. Complete medium was added into each culture flask until there was 2mL of liquid. The 10 culture flasks were randomly divided into 2 groups, group A and group B, with five flasks in each group. The complete pancreatic enzyme digestion method was used in group A, and the repeated incomplete pancreatic digestion method was employed in group B.
Complete pancreatic enzyme digestion passage (group A)
This passage method was carried out as previously described[7],[8]. The first complete medium change was arranged on the eighth day, when the retinal tissues were attached to the bottom of the flasks and some irregular cells had emerged from the tissues, to remove the floating aggregates and debris. After this point, the complete medium was changed every other day. When cells attached to the flask bottom became monolayer and confluent (after the medium was changed at least four times), the cells in each flask were trypsinized by 0.5mL 0.25% trypsin-EDTA dissociation solution in a 37°C incubator for about 3min, and 2mL complete medium was added into the flask to stop digestion until the cells became round and some cells were found to be suspended in liquid under microscopy. After repeatedly blowing with Pasteur pipettes to completely detach the cells from the walls of the culture flasks, the liquid was centrifuged for 3min at 800r/min. Supernatant was discarded and 4mL complete medium was used to suspend cells. The cells were transferred equally into two sterile culture flasks and cultured in a 37°C incubator until the medium was changed six days later. Then, the complete medium was changed every other day, and when cells became monolayer and confluent, the complete pancreatic enzyme digestion method was used again for another cell passage. After one month, four flasks of cells were obtained and tested by fluorescence-activated cell sorter (FACS) and immunohistochemistry.
Repeated incomplete pancreatic enzyme digestion passage (group B)
The complete medium was changed on the sixth day. Three days later, cells were trypsinized with 0.3mL 0.25% trypsin-EDTA dissociation solution in a 37°C incubator, and 2mL complete medium was added into the flask to stop digestion until cells became round and a few cells were found to be suspended in liquid under microscopy. The flask was then put on a rotary shaker for 2min. Cells and debris that detached from the walls were thrown away and 2mL complete medium was added. After this point, fluid changing was performed every other day. After the complete medium was changed twice, repeated incomplete pancreatic enzyme digestion was again used when the cells were attached to the wall confluently in a monolayer. At this time, the cells were trypsinized until more cells became round and suspended. After digestion was stopped and cells were swayed for 2min, fluid in the flask was transferred into a centrifuge tube and centrifuged for 3min at 800r/min. The supernatant was discarded and 2mL complete medium was added to the following culture in a new flask. At the same time, 2mL medium was added into the old flask to culture the remaining cells. The method mentioned above was carried out when cells had again grown confluently. After one month, eight flasks of cells were obtained and tested by FACS and immunohistochemistry.
Fluorescence-activated cell sorter analysis
In order to determine the purity of enriched Müller cells, we carried out FACS analysis using glutamine synthetase (GS) antibodies (Sigma, America), which is specific to Müller cells. In brief, cells in the monolayer culture were dissociated into single cells with 0.25% trypsin-EDTA. After cell counting, cells were centrifuged for 3min at 800r/min and fixed with 4% paraformaldehyde for 15min. Then, cells were blocked with PBS containing 2% bovine serum albumin (BSA) and 0.1% Triton×-100 at 4°C for 30min. Cells were divided equally into three tubes which were labeled blank control group, control group, and experimental group, respectively. In the experimental group, the GS antibody was added into the tube. In the meantime, the same volume of PBS was added to the control tube, and nothing was added to the blank tube. Incubation in primary antibodies was carried out at 4°C for 1h. Next, cells were incubated in PBS-BSA solution containing the appropriate secondary antibodies linked to fluorescein isothiocyanate (FITC) (Sigma, America) for 1h at 4°C in the dark. Cells were washed with PBS and resuspended in PBS for FACS analysis.
RT-PCR analysis
Total RNA was isolated from cells cultured using TRIZOL reagent according to the manufacturer's protocol[9], and was reversely transcribed to obtain cDNA. Briefly, each 30µL cDNA synthesis mixture contained 9µL total RNA, 12µL 1% DEPC-H2O (diethylpyrocarbonate-treated water), 2µL deoxynucleotide triphosphates (dNTP), 1µL primer oligo (dT), 4µL 5× buffer, 1µL M-MLV, and 1µL RNasin. The sequences of the primers specific for the target genes are given in Table 1. The PCR reaction was carried out in a final volume of 20µL containing the following: 10µL 2× SYBR Green mix, 1µL 10µmol/L forward primer and reverse primer, 2µL diluted cDNA, and 6µL ddH2O (double-distilled H2O). The reaction was carried out for 45 cycles, which consisted of denaturation at 95°C for 5s, annealing, and elongation at 60°C for 30s after an initial denaturation step at 95°C for 15s. The RT-PCR signals were analyzed by ABIViia7 (USA).
Table 1. List of primers for RT-PCR analysis in this study.
| Name | Sequence | Annealing temp (°C) | Size (bp) | Acc. No |
| GS | Forward:5′ TCACAGGGACAAATGCCGAG3′ | 58 | 362 | M96152 |
| Reverse:5′ GTTGATGTTGGAGGTTTCGTGG3′ | ||||
| Vimentin | Forward:5′AAGGCACTAATGAGTCCCTGGAG3′ | 56 | 251 | NM031140 |
| Rev erse:5′ GTTTGGAAGAGGCAGAGAAATCC3′ | ||||
| Clusterin | Forward:5′ CCTCCAGTCCAAGATGCTCAAC3′ | 58 | 292 | NM_053021 |
| Reverse:5′ TTTCCTGCGGTATTCCTGTAGC3′ | ||||
| Carbonic anhydrase | Forward:5′ TTGCCAATGGAGACCGACAG3′ | 58 | 233 | NM_019291 |
| Reverse:5′ TGAGCCCCAGTGAAAGTGAAAC3′ | ||||
| Opsin | Forward:5′ CATGCAGTGTTCATGTGGGA 3′ | 64 | 422 | U22180 |
| Reverse:5′ AGCAGAGGCTGGTGAGCATG3′ | ||||
| mGluR6 | Forward:5′ CACAGCGTGATTGACTACGAG 3′ | 56 | 317 | D13963 |
| Reverse:5′ CTCAGGCTCAGTGACACAGTTAG3′ | ||||
| HPC1 | Forward:5′ AAGAGCATCGAGCAGCAGAGCATC3′ | 60 | 342 | NM016801 |
| Reverse:5′ CATGGCCATGTCCATGAACAT3′ | ||||
| Brn3b | Forward:5′ GGCTGGAGGAAGCAGAGAAATC3′ | 60 | 141 | AF390076 |
| Reverse:5′ TTGGCTGGATGGCGAAGTAG3′ | ||||
| CD31 | Forward:5′ AAGAGCAACTTCCAGACCGTCC3′ | 58 | 222 | NM_031591 |
| Reverse:5′ AAGCACCATTTCATCTCCAGACTG3′ | ||||
| Tyrosinase | Forward:5′ TCAGTCTATGTCATCCCCACAGG3′ | 56 | 252 | NM_011661 |
| Reverse:5′ GTTCTCATCCCCAGTTAGTTCTCG3′ | ||||
| β-actin | Forward:5′TCACAGGGACAAATGCCGAG3′ | 50 | 548 | XM_037235 |
| Reverse:5′GTTGATGTTGGAGGTTTCGTGG3′ |
Immunohistochemistry
Immunohistochemistry analysis was carried for the detection of Müller cell-specific marker glutamine synthetase (GS)[10]. Briefly, 0.4% paraformaldehyde-fixed cells were incubated in PBS containing 3% BSA, 5% goat serum, and 0.3% Triton×-100 at 37°C for 1h, followed by overnight incubation at 4°C in the primary antibodies. The secondary antibodies used were as follows: anti-rabbit IgG conjugated with FITC (1:100; Sigma). Incubation in the secondary antibodies was carried out in the dark at room temperature for 1h. After incubation in DAPI for 5min, images were captured using fluorescence microscopy (Leica DMI4000B).
Cell Counting and Statistical Analysis
To determine the percentage of Müller cells on the 30th day, the cells were fixed using cold 4% paraformaldehyde for immunocytochemical analysis to calculate the ratio of Müller cells. Ten non-overlapping visual fields in each group were randomly selected from a culture flask under a fluorescence microscope (×100) to count the number of Müller cells with positive green fluorescence staining and the total number of the cells with positive DAPI staining (blue fluorescence). The cell positive rate equals the number of cells expressing GS/number of cells dyed by DAPI ×100%. All data were expressed as the mean±SD. Statistical analysis was performed with a two-sample approximate t test in SPSS 13.0. The difference between the two samples was statistically significant if P<0.05.
RESULTS
Characteristics of Müller Cells
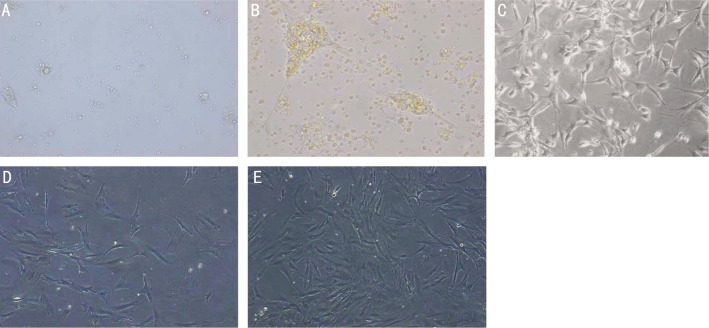
After culturing for 48h in complete medium, some retinal tissues were attached to the bottom of the flasks and parts of the aggregates were floating in the medium (Figure 1A). Four to five days later, most of the tissues were attached to the bottom, while some irregular cells emerged from the tissues and began to proliferate (Figure 1B). At the same time, they displayed an elongated and flattened morphology. After 6-8d's culture, most of cells were adherent and spindly (Figure 1C). Cell debris and a few non-adherent cells were found suspended in the medium, which turned lighter, although the medium of both groups was first changed to clear away debris. Cells were passaged using the complete pancreatic enzyme digestion and repeated incomplete pancreatic digestion methods mentioned above. One month later, the cells of both groups were observed to be proliferating confluently and displaying a flattened morphology. However, compared with group A (Figure 1D), the cells of group B (Figure 1E) exhibited a more regular shaped morphology and had a higher density.
Figure 1. Phase-contrast microscopy of cells isolated from the neural retina.
A: Retinal tissues cultured in complete medium for 48h. Some were attached to the bottom of the flasks when others were still floating; B: Retinal tissues cultured for 4d were adherent when some irregular cells emerged from them and began to proliferate; C: Six to eight days later, cells were adherent and most of them were spindly; D: When cultured by the complete pancreatic enzyme digestion method for one month, cells attached, acquired a flattened morphology, and proliferated; E: When cultured by the repeated incomplete pancreatic enzyme digestion method for one month, cells were adherent, proliferated confluently, and displayed a flattened morphology. Comparing (E) with (D), the cells in group B are denser than those in group A.
Comparison of Müller Cells Cultured by the Complete Pancreatic Enzyme Digestion Method and the Repeated Incomplete Pancreatic Enzyme Digestion Method
To compare the purity of Müller cells cultured using the complete pancreatic enzyme digestion method (group A) with that of Müller cells cultured using the repeated incomplete pancreatic enzyme digestion method (group B), we carried out FACS, immunocytochemical analysis, and RT-PCR. We chose the GS antibody to react with the cells, as GS is a specific marker of Müller cells[10]. It was observed that 75.41% of cells in group A were Müller cells, as revealed by the proportion of cells expressing GS immunoreactivity, and 98.01% of the cells in group B were Müller cells (Figure 2A, B). It was demonstrated that cells in both group A and group B expressed GS abundantly after culture for one month (Figure 2C-H). However, the cells in group A were distributed with a low density in the culture flask (Figure 2C-E), while the cells in group B exhibited a high density and uniform distribution (Figure 2F-H). This suggests that we can obtain higher purity and density Müller cells through the repeated incomplete pancreatic enzyme digestion method. In order to further detect the proliferation and purity of Müller cells, we examined the expression of certain cell-specific markers. RT-PCR analysis revealed that cultured cells expressed a series of transcripts characteristic of Müller cells, including GS, vimentin, clusterin, and carbonic anhydrase (Figure 2I). In contrast, markers corresponding to other kinds of cells such as rod photoreceptors (opsin), bipolar cells (mGluR6), amacrine cells (HPC1), RCGs or retinal ganglion cells (Brn3b), RPE/pigmented ciliary epithelium (tyrosinase), and endothelial cells (CD31) were expressed very little, meaning that the monolayer-cultured cells were Müller cells of high purity, and were not contaminated by the cells mentioned above (Figure 2J).
Figure 2. Comparison between cells passaged by the complete pancreatic enzyme digestion method and the repeated incomplete pancreatic enzyme digestion method.
FACS showed that (A) 75.41% of cells in group A were Müller cells and (B) 98.01% of cells in group B were Müller cells when cultured for one month; purified Müller cells expressed immunoreactivity corresponding to the Müller cell marker GS; C-H: Immunocytochemical analysis showed that more 90% of the cells expressed GS; C-E: Cells passaged by the complete pancreatic enzyme digestion method exhibited low density and did not proliferate very well when cultured for one month; F-H: Cells passaged by repeated incomplete pancreatic enzyme method had high density and proliferated confluently; I-J: RT-PCR analysis was carried out to detect the expression of Müller and other cells. Lane M: DNA marker; Lane 1: Purified Müller cells in group A; Lane 2: purified Müller cells of group B.
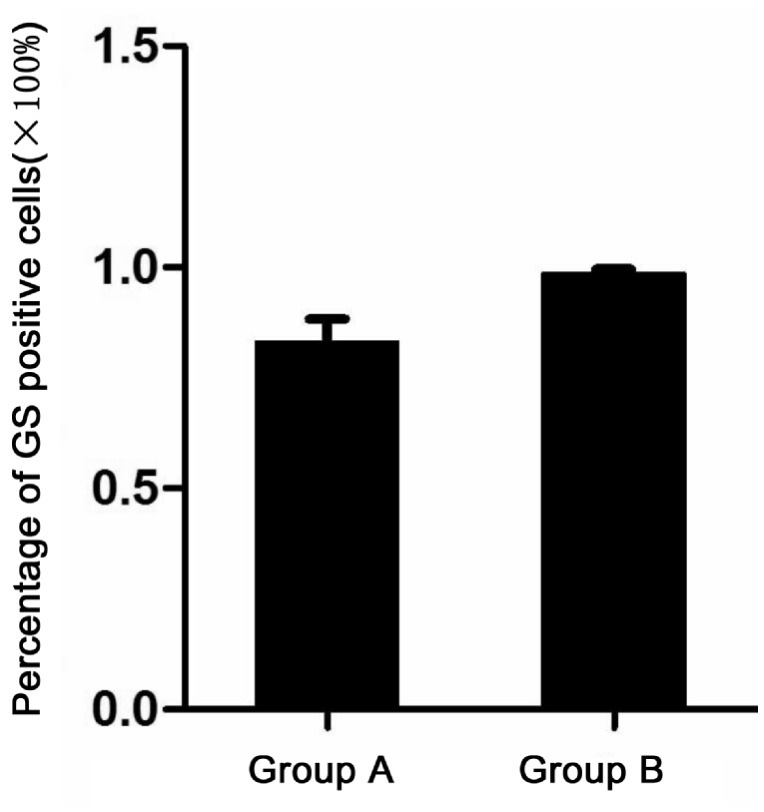
We chose 10 non-overlapping visual fields under 10 multiple fluorescence microscopes and observed that the positive rate of cells expressing GS obtained using the complete pancreatic enzyme digestion method was 83.2%±5.16%, while with the repeated incomplete pancreatic enzyme digestion method, it was 98.5%±1.08%. Using the two-sample approximate t test, the statistical results were t=-9.178, P<0.005, indicating that the difference between the two methods mentioned above was statistically significant (Figure 3).
Figure 3. Two-sample approximate t test between group A and group B.
The rate of GS positive cells: Group A was 83.2%±5.16% and Group B was 98.5%±1.08%. The statistical results were t=-9.178, P<0.005.
DISCUSSION
Glaucoma is a complex, multivariate, irreversible blinding eye disease that is induced by several risk factors. These include elevated IOP, increased oxidative stress and free radicals, the release of neurotransmitters (NO and glutamate, amongst others)[11]-[13], increased calcium concentration, the depletion of neurotrophins and growth factors, and the initiation of apoptotic signals[14]-[16]. These factors create a hostile microenvironment that contributes to secondary damage and results in the massive death of retinal ganglion cells. The blockade of the signaling pathway of retinal ganglion cell apoptosis, reduction of IOP, and nourishment of the optic nerve are known to be somewhat effective in prolonging the life of ganglion cells and retarding disease progression for patients with early glaucoma or progressing glaucoma. However, such treatment strategies are ineffective for patients with advanced and absolute glaucoma. This discrepancy lies in the fact that most or all retinal ganglion cells have already undergone apoptosis in patients with advanced and absolute glaucoma, and that there are too few surviving ganglion cells to reverse pathological changes resulting from glaucoma. Therefore, there is a definite need to look for new ways to regenerate retinal ganglion cells to arrest disease progression or even restore vision.
The emergence of cell engineering, particularly stem cell engineering, makes it possible to fulfill this need[17]. By incorporating stem cells into the retina and inducing their proliferation and differentiation into target cells, it is possible to replenish retinal neurons and restore retinal function, thereby bringing hope to patients with irreversibly blinding eye diseases. Retinal Müller cells, the glial cells in the retina, retain proliferation potential and offer an abundant source for cell engineering[18]-[20]. In addition, Müller cells span the entire width of the retina and are widely distributed among ganglion cells. This increases the chance to better integrate the cells converted from Müller cells into the ganglion cell layer. All these features suggest that retinal Müller cells may prove to be the most promising source of stem cells in the treatment of glaucoma[21].
Müller cells represent a special type of neuroglial cells that form over 90% of the retinal cells in vertebrates. Müller cells morphologically resemble radial glia[22]. They carry out processes radiating toward the outer and inner surfaces of retina, and their nucleus is found in the center of the inner nuclear layer. In the past, retinal neurons were considered irreproducible. However, much recent work has indicated that injury could induce retinal Müller cells to dedifferentiate and perform characteristics of stem cells to differentiate into types of neurons. Thus, the role of Müller cells in the regeneration process of retinal neurons has attracted broad attention. To study the characteristics of Müller cells deeply, it is important to find a simple, rapid way to culture and purify Müller cells.
Retinal cells include Müller cells, ganglion cells, bipolar cells, photoreceptor cells, amacrine cells, and horizontal cells. Identifying a simple and rapid method of culturing and purifying Müller cells will form the foundation of future work. At present, the tissue culture and enzyme digestion methods are the main ways of purifying Müller cells. With the tissue culture method, retinal tissue is dissociated into small aggregates and the cells attached to the walls, which have been taken from tissues, are passaged. This method generally does not yield high-purity Müller cells, or if it does, the culturing time required is too long. In the enzyme digestion method, cells are passaged with pancreatic enzyme, which is able to detach cells completely from walls. In the process of purifying retinal Müller cells from SD rats, after complete enzyme digestion, it takes a long time for cells to reattach to the walls, and many Müller cells are been dissociated; such results may induce experimental failure. In this study, we developed an approach called repeated incomplete pancreatic enzyme digestion to purify Müller cells. Through repeated light pancreatic enzyme digestion, we efficiently passaged Müller cells and removed the other retinal cells based on the characteristics of Müller cells such that they can attach to walls quickly and are firm, whereas other cells are fragile. Using this method, we were able to obtain plenty of high-purity Müller cells, as mentioned in the results.
FACS showed that after one month's culture, through repeated incomplete pancreatic enzyme digestion, we were able to obtain higher-purity Müller cells: 75.41% cells of group A (complete pancreatic enzyme digestion) expressed GS, while 98.01% cells of group B (repeated incomplete pancreatic enzyme digestion) expressed GS. As we know, Müller cells express GS, vimentin, clusterin, carbonic anhydrase, and so on. We carried out RT-PCR to compare the expression of these specific markers in two methods mentioned above; the results indicated that the purity of Müller cells in group B was higher than in group A. GS is a key enzyme that can transfer glutamic acid into glutamine, and only Müller cells express it. For this reason, GS is chosen as a specific marker to identify Müller cells. Immunocytochemical analysis showed that more 99% cells of both groups expressed GS, but the density of cells obtained by repeated incomplete pancreatic enzyme digestion was obviously higher than that by complete pancreatic enzyme digestion.
The statistical results showed that the percentage of Müller cells of group A was 83.2%±5.16%, and the percentage in group B was 98.5%±1.08%. The two-sample approximate t test analysis demonstrated that the difference between group A and group B was statistically significant (t=-9.178, P<0.005). Thus, we concluded that the repeated incomplete pancreatic enzyme digestion method is a simple and efficient way to purify retinal Müller cells, and should be used for future research on the characteristics of Müller cells.
Acknowledgments
We conducted all our experiments in the State Key Lab of Medical Genetics of China (Grant number 1989DA105084) and the Medical Science Experimental Center of the Xiangya Hospital, Central South University. We are grateful to Dr. Qi Zeng, for her valuable advice and guidance; Dr. Xia Zhou, for her helpful suggestions; Professor Hong Shen, Dr. Jingyu He and Dr. Sai Zhang for their technical assistance.
Footnotes
Foundation item: National Natural Scientific Foundation of China (No.81170844)
REFERENCES
- 1.Bringmann A, Pannicke T, Grosche J, Francke M, Wiedemann P, Skatchkov SN, Osborne NN, Reichenbach A. Müller cells in the healthy and diseased retina. Prog Retin Eye Res. 2006;25(4):397–424. doi: 10.1016/j.preteyeres.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 2.Joly S, Pernet V, Samardzija M, Grimm C. Pax6-positive müller glia cells express cell cycle markers but do not proliferate after photoreceptor injury in the mouse retina. Glia. 2011;59(7):1033–1046. doi: 10.1002/glia.21174. [DOI] [PubMed] [Google Scholar]
- 3.Fimbel SM, Montgomery JE, Burket CT, Hyde DR. Regeneration of inner retinal neurons after intravitreal injection of ouabain in zebrafish. J Neurosci. 2007;27(7):1712–1724. doi: 10.1523/JNEUROSCI.5317-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yurco P, Cameron DA. Responses of Müller glia to retinal injury in adult zebrafish. Vision Res. 2005;45(8):991–1002. doi: 10.1016/j.visres.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 5.Lawrence JM, Singhal S, Bhatia B, Keegan DJ, Reh TA, Luthert PJ, Khaw PT, Limb GA. MIO-M1 cells and similar muller glial cell lines derived from adult human retina exhibit neural stem cell characteristics. Stem Cells. 2007;25(8):2033–2043. doi: 10.1634/stemcells.2006-0724. [DOI] [PubMed] [Google Scholar]
- 6.Fischer AJ, Reh TA. Potential of Müller glia to become neurogenic retinal progenitor cells. Glia. 2003;43(1):70–76. doi: 10.1002/glia.10218. [DOI] [PubMed] [Google Scholar]
- 7.Hicks D, Courtois Y. The growth and behaviour of rat retinal Müller cells in vitro: 1. An improved method for isolation and culture. Exp Eye Res. 1990;51(2):119–129. doi: 10.1016/0014-4835(90)90063-z. [DOI] [PubMed] [Google Scholar]
- 8.Wang RB. Repeated pancreatic enzyme-degesting method for culturing Müller cells of SD rats. J Shenyang Yixueyuan Xuebao. 2009;11(3):171–173. [Google Scholar]
- 9.Fang JH, Wang XH, Xu ZR, Jiang FG. Neuroprotective effects of bis(7)-tacrine against glutamate-induced retinal ganglion cells damage. BMC Neurosci. 2010;11:31. doi: 10.1186/1471-2202-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vardimon L, Fox LE, Moscona AA. Development regulation of glutamine synthetase and carbonic anhydrase II in neural retina. Proc Natl Acad Sci USA. 1986;83(23):9060–9064. doi: 10.1073/pnas.83.23.9060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tezel G. Oxidative stress in glaucomatous neurodegeneration: mechanisms and consequences. Prog Retin Eye Res. 2006;25(5):490–513. doi: 10.1016/j.preteyeres.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coassin M, Lambiase A, Sposato V, Micera A, Bonini S, Aloe L. Retinal p75 and bax overexpression is associated with retinal ganglion cells apoptosis in a rat model of glaucoma. Graefes Arch Clin Exp Ophthalmol. 2008;246(12):1743–1749. doi: 10.1007/s00417-008-0913-5. [DOI] [PubMed] [Google Scholar]
- 13.Danesh-Meyer HV. Neuroprotection in glaucoma: recent and future directions. Curr Opin Ophthalmol. 2011;22(2):78–86. doi: 10.1097/ICU.0b013e32834372ec. [DOI] [PubMed] [Google Scholar]
- 14.Kanamori A, Naka M, Fukuda M, Nakamura M, Negi A. Tafluprost protects rat retinal ganglion cells from apoptosis in vitro and in vivo. Graefes Arch Clin Exp Ophthalmol. 2009;247(10):1353–1360. doi: 10.1007/s00417-009-1122-6. [DOI] [PubMed] [Google Scholar]
- 15.Giannelli SG, Demontis GC, Pertile G, Rama P, Broccoli V. Adult human Müller glia cells are a highly efficient source of rod photoreceptors. Stem Cells. 2011;29(2):344–356. doi: 10.1002/stem.579. [DOI] [PubMed] [Google Scholar]
- 16.Das AV, Mallya KB, Zhao X, Ahmad F, Bhattacharya S, Thoreson WB, Hegde GV, Ahmad I. Neural stem cell properties of Müller glia in the mammalian retina: regulation by Notch and Wnt signaling. Dev Biol. 2006;299(1):283–302. doi: 10.1016/j.ydbio.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 17.Bull ND, Limb GA, Martin KR. Human Müller stem cell (MIO-M1) transplantation in a rat model of glaucoma: survival, differentiation, and integration. Invest Ophthalmol Vis Sci. 2008;49(8):3449–3456. doi: 10.1167/iovs.08-1770. [DOI] [PubMed] [Google Scholar]
- 18.Fischer AJ, Reh TA. Potential of Müller glia to become neurogenic retinal progenitor cells. Glia. 2003;43(1):70–76. doi: 10.1002/glia.10218. [DOI] [PubMed] [Google Scholar]
- 19.Yan RT, Li XM, Huang J, Guidry C, Wang SZ. Photoreceptor-like cells from reprogramming cultured mammalian RPE cells. Mol Vis. 2013;19:1178–1187. [PMC free article] [PubMed] [Google Scholar]
- 20.Yanai A, Laver CRJ, Joe AW, Viringipurampeer IA, Wang X, Gregory-Evans CY, Gregory-Evans K. Differentiation of human embryonic stem cells using size-controlled embryoid bodies and negative cell selection in the production of photoreceptor precursor cells. Tissue Eng Part C Methods. 2013;19(10):755–764. doi: 10.1089/ten.tec.2012.0524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hlawatsch J, Karlstetter M, Aslanidis A, Lückoff A, Walczak Y, Plank M, Böck J, Langmann T. Sterile alpha motif containing 7 (samd7) is a novel crx-regulated transcriptional repressor in the retina. PloS ONE. 2013;8(4):e60633. doi: 10.1371/journal.pone.0060633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pearson RA, Barber AC, West EL, MacLaren RE, Duran Y, Bainbridge JW, Sowden JC, Ali RR. Targeted disruption of outer limiting membrane junctional proteins (Crb1 and ZO-1) increases integration of transplanted photoreceptor precursors into the adult wild-type and degenerating retina. Cell Transplant. 2010;19(4):487–503. doi: 10.3727/096368909X486057. [DOI] [PMC free article] [PubMed] [Google Scholar]