Abstract
AIM
To examine the association between dietary macronutrient intake and the risk of age-related cataract (ARC) in middle-aged and elderly men.
METHODS
A hospital-based case-control study was conducted from December 2009 to November 2011. Cases (n=360) were patients with cataract aged 45-85 years old, and controls (n=360) were patients who had been admitted to the same hospital for diseases not related with cataract. All subjects were interviewed using a structured interviewer-administrated questionnaire that included information on socio-demographic characteristics, lifestyle habits and detailed medical history, simultaneously, the dietary intakes of nutrients were collected via a valid semi-quantitative food frequency questionnaire (FFQ). The odds ratios (OR) and corresponding 95% confidence intervals (CI) of three types of ARC were estimated using multiple logistic regression models.
RESULTS
After adjusting for multiple potential confounders, total dietary intake of carbohydrate was positively associated with cortical cataract, compared to controls in the lowest quartile, and the OR for cases in the highest quartile of intake was 2.471 (95%CI: 1.348-6.043, P=0.027). Higher dietary intakes of protein were protective for posterior subcapsular cataract (PSC) (OR=0.528, 95%CI: 0.148-0.869, P=0.023). Dietary fat intake was not associated with any type of cataract, however, participants in the highest quartile of polyunsaturated fatty acids intake had 2.7 times the risk of nuclear cataract as did those in the lowest quartile (OR=2.742, 95%CI: 1.790-4.200, P=0.033).
CONCLUSION
A high intake of carbohydrate and polyunsaturated fatty acid may increase the odds of cortical and nuclear cataract, respectively, whereas high intake of protein, especially animal protein, may protect against PSC cataract. It is possible that dietary changes of target population may reduce the risk of ARC.
Keywords: age-related cataract, carbohydrate, protein, polyunsaturated fatty acid
INTRODUCTION
Age-related cataract (ARC) remains the leading cause of loss of useful vision worldwide, accounting for about half the global prevalence of blindness[1],[2]. Because the worldwide burden of blindness is increasing as a result of both growth and ageing of the population, the identification of ARC risk factors has major public health significance. Although little is known about the etiology of ARC except the increasing age, previous studies show that ultraviolet exposure, medical use and genetic factor are associated with ARC[3]-[6]. The role of micronutrient intake and antioxidant supplementation in relation to the pathogenesis of ARC formation has been well studied, but few studies have examined the association of dietary macronutrient intakes in relation to risk for ARC development[7]-[10]. The results of these studies have been mixed, whereas some studies reported a positive association between ARC and dietary macronutrient intake, others found no association for macronutrient intake[7]-[10]. However, these limited data have mainly been derived from white population in the United States and Australia, and few have been conducted in Asia[8]-[10]. Furthermore, there are no studies among Chinese people, the approximately 20% of the world's population. In order to identify the association between dietary macronutrient intake and nuclear, cortical and posterior subcapsular cataract (PSC) in middle-aged and elderly men, a hospital-based case-control study was carried out in Jinzhou area.
SUBJECTS AND METHODS
Subjects
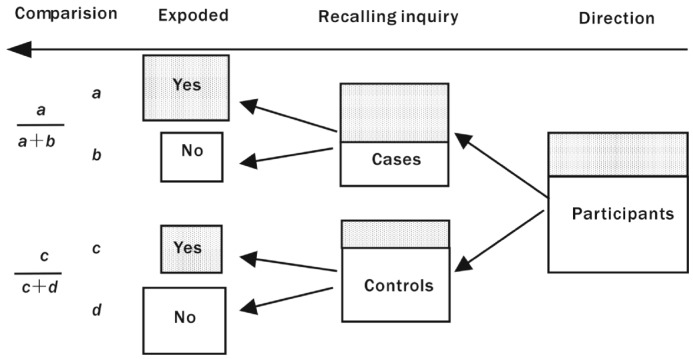
Details of the study design have been published elsewhere, and are briefly described here. From December 2009 to November 2011, 360 patients between the ages of 45 and 85 years and 360 controls frequency-matched on age and gender were interviewed in the Department of Ophthalmology of the First Affiliated Hospital and the Third Affiliated Hospital of Liaoning Medical University and Jinzhou Central Hospital. Cases were patients diagnosed with ARC and admitted to hospital for surgery because of visual loss caused by cataract, meanwhile, for whom ARC was confirmed histologically. Any patient with the following inclusive criteria was included as a case: 1) Patients diagnosed with nuclear, cortical, PSC cataract in at least one eye, based on slit lamp findings; 2) Visual acuity of 0.3 or worse in the affected eye; 3) Intraocular pressure 10-21mmHg; 4) Both eyes had no conditions other than cataract that might account for vision loss; 5) Patients in both eyes had no previous ocular surgery (except cataract surgery in the other eye). Meanwhile, controls were patients who had been admitted to the same hospital for diseases not related with cataract, with no lens opacities in either eye and with good visual acuity. Controls were excluded if they had medical conditions and treatments known to be related to cataract or affect vision (e.g. age-related macular degeneration, diabetic retinopathy, glaucoma, or acute or chronic uveitis). Those controls who received antibiotic treatment or any supplementary vitamins were also excluded. Controls were 1:1 matched on age and gender, and age frequency-matching was carried out in 5-years age groups (45-, 50-, 55-, 60-, 65-, 70-, 75-, and 81-85), i.e., for each case, a control was matched within the same 5-years age group. Participants were selected strictly based on the inclusive and exclusive criteria, and there was no bias in selecting subjects. The subjects-enrolling flowchart (Figure 1) is as follows:
Figure 1. Schematic diagram a case-control study of the association between the number of exposed persons and ARC.
Shaded areas represent subject with a large number of exposed persons, and unshaded areas represent subject with a small number of exposed persons.
Methods
Measurement of nutrient intakes
The participants responded to a structured interviewer-administered questionnaire that included questions on a wide range of possible risk factors for cataract, including demographic characteristics, lifestyles, medical histories and family history of cataract. Simultaneously, data about diet was assessed with a validated 137-item semi-quantitative Food Frequency Questionnaire (FFQ). A common unit or portion size for each food was specified, and participants were asked how often on average, they had consumed each of the food items listed and what was the usual size of each item during the previous year. There were 8 possible responses for each food item, ranging from “<1 time per month” to “≥3 times per day” and 5 possible responses for usual size of each item, for example, the usual size of rice ranging from “<50g” to “≥200g”. The consumption frequency and usual size for each food were assigned corresponding weighting coefficient respectively, with “0, 0.1, 0.3, 0.6, 0.8, 1, 2, 3” for consumption frequency and “0.5, 0.75, 1, 1.5, 2” for usual size. The average daily intake of energy and nutrients was calculated by multiplying the weighting coefficient of frequency of each item by the weighting coefficient of usual size of each food by its nutrient content per standard size on the basis of China Food Composition 2004 and totaling the nutrient intake for all food items.
Assessment of cataract type
Photographs of the lens of each eye were taken after pupil dilation with 1% tropicamide. Lens photography and cataract grading were carried out according to the Lens Opacity Classification System (LOCS III). Color film images were taken with slit-lamp camera to assess the severity of nuclear cataract. Retroillumination photographs of the anterior and posterior lens were taken to assess presence and severity of cortical cataract and PSC cataract. The severity of cataract was assessed by comparing participants' photographs with a set of standard photographs. LOCS III was used to measure the degree of opalescence on a scale of 0.1 to 6.9 for nuclear cataract and on a scale of 0.1 to 5.9 for cortical and PSC cataract. We considered an eye to have nuclear, cortical, or PSC cataract if the corresponding LOCS III grade was ≥4, ≥2, or ≥2, respectively[12]. Patients were classified as having a specific type of cataract when there was the specific type of opacity present alone or at a greater grade than other type of opacity present, as defined by slit lamp examination in the affected eye. Therefore, the nuclear cataract group consisted of patients with nuclear sclerosis type of opacity only or with predominant nuclear opacity type over cortical or PSC opacities in the affected eye. Similar criteria were used to define the cortical group and the PSC group. Individuals, rather than eyes, were the unit of analysis, and analyses involved data from the most severely affected eye only.
Medical ethics
The hospital-based case control study was conducted by the Department of Epidemiology in Liaoning Medical University and had approval from the Liaoning Medical University, the First Affiliated Hospital and the Third Affiliated Hospital of Liaoning Medical College. The study protocol adhered to the tenets of the Declaration of Helsinki and consent for participation was obtained from the subjects and relatives to commencement of the survey, and informed, oral consent was obtained from all willing participants.
Statistical Analysis
Initially descriptive analyses were performed for general characteristics of cases and controls, and differences among these factors were assessed using t-test or χ2 test. Energy-adjusted macronutrient intakes were divided into quartiles, and associations between the relative level of nutrient intake and the incidence of three types of cataract were assessed comparing higher intake quartiles with the lowest quartile in logistic regression models. Tests of linear trend across increasing quartiles of macronutrient intakes were conducted by assigning the medians of intakes in quartiles treated as a continuous variable in the logistic regression models. Odds ratios (OR) and 95% confidence intervals (CI) were presented. All analyses were performed using SPSS version 13.0. All reported P-values were based on two-sided tests and compared with a significance lever of 0.05.
RESULTS
A total of 360 cases and 360 controls, frequency-matched on sex and age, were recruited. Both cases and controls were the local population aged 45-85 years who have been living in Jinzhou for more than 10 years. The most frequent type of cataract was cortical (n=204) followed by nuclear (n=110) and PSC (n=46). The socio-demographic, lifestyle characteristics and medical history conditions of the cases and controls included in the study are shown in Table 1. Cases and controls were comparable on the two matching variables with similar age distributions and mean ages (69.20 years in cases and 69.62 years in controls) and gender proportions. The two groups were also similar on current occupational status, on long-term residence and alcohol intake. Educational level was significantly lower in cases than controls (χ2=8.663, P=0.034), with fewer cases having higher education, compared to controls. Cases and controls also differed significantly in family monthly economic income, smoking, physical activity habit, BMI, hypertension and diabetes history (P<0.05). The family economic income of cases was lower and more cases didn't take exercises than controls. The prevalence of current smokers was higher in case patients (33.33%) relative to control subjects (24.72%). Cases were more frequently overweight (BMI: 24.0-27.99) and obese (BMI≥28) than were control subjects. For cases there were a greater percentage of hypertension and diabetes. Furthermore, the total energy intakes of cases (2 612.96±622.91)kcal/d were significantly higher than controls (2 483.97±655.96)kcal/d (t=2.299, P=0.022).
Table 1. The distribution of general characteristics for cases and controls.
| Characteristics | Cases | Controls | χ2 | P |
| Age (a) | 1.599 | 0.660 | ||
| 45- | 49 (13.61) | 51 (14.17) | ||
| 55- | 71 (19.72) | 63 (17.50) | ||
| 65- | 112 (31.11) | 126 (35.00) | ||
| 75-85 | 128 (35.56) | 120 (33.33) | ||
| Gender | <0.001 | 1.000 | ||
| M | 185 (51.39) | 185 (51.39) | ||
| F | 175 (48.61) | 175 (48.61) | ||
| Occupation | 4.133 | 0.247 | ||
| Worker | 144 (40.00) | 149 (41.39) | ||
| Farmer | 57 (15.83) | 70 (19.44) | ||
| Intellectual | 105 (29.17) | 83 (23.06) | ||
| Other | 54 (15.00) | 58 (16.11) | ||
| Education | 8.663 | 0.034 | ||
| Illiteracy | 55 (15.28) | 38 (10.56) | ||
| Primary school | 108 (30.00) | 101 (28.05) | ||
| Junior high school | 131 (36.39) | 126 (35.00) | ||
| ≥High school | 66 (18.33) | 95 (26.39) | ||
| Long-term residence | 2.920 | 0.232 | ||
| Urban | 275 (76.39) | 282 (78.33) | ||
| Rural | 41 (11.39) | 28 (7.78) | ||
| Other | 44 (12.22) | 50 (13.89) | ||
| Family economic income(¥/month) | 15.452 | 0.001 | ||
| ≤1 000 | 45 (12.50) | 21 (5.83) | ||
| 1 001-2 000 | 128 (35.55) | 107 (29.72) | ||
| 2 001-3 000 | 119 (33.06) | 149 (41.39) | ||
| ≥3 001 | 68 (18.89) | 83 (23.06) | ||
| Smoking | 6.298 | 0.043 | ||
| Never | 181 (50.28) | 217 (60.28) | ||
| Past | 59 (16.39) | 54 (15.00) | ||
| Current | 120 (33.33) | 89 (24.72) | ||
| Alcohol consumption | 5.217 | 0.074 | ||
| Never | 239 (66.39) | 264 (73.33) | ||
| Past | 17 (4.72) | 9 (2.50) | ||
| Current | 104 (28.89) | 87 (24.17) | ||
| Physical activity | 10.407 | 0.005 | ||
| Never | 207 (57.50) | 164 (45.55) | ||
| Occasionally | 88 (24.44) | 109 (30.28) | ||
| Often | 65 (18.06) | 87 (24.17) | ||
| BMI | 11.430 | 0.01 | ||
| <18.5 | 32 (8.89) | 22 (6.11) | ||
| 18.5- | 169 (46.94) | 208 (57.78) | ||
| 24.0- | 114 (31.67) | 104 (28.89) | ||
| ≥28 | 45 (12.50) | 26 (7.22) | ||
| Hypertension | 7.881 | 0.005 | ||
| Yes | 131 (26.67) | 96 (36.39) | ||
| No | 229 (73.33) | 264 (63.61) | ||
n (%)
Table 2 presents the multiple logistic regression-derived ORs and 95%CI for macronutrient intake and each type of cataract. A significant positive association was observed between carbohydrate intake and cortical cataract. Cases in the highest quartile had 2.471 times the risk of controls in the lowest quartile of carbohydrate intake after adjustment for other potential confounders, and the test of linear trend across quartiles was statistically significant (P=0.027). No association was found between total fat intake and any type of ARC (P>0.05). Participants with higher intake of total protein had approximately a 50% reduction in the risk of cortical cataract (quartile 4 vs quartile 1, OR=0.528, 95%CI: 0.148-0.869), and there was statistically significant trend of decreasing risk across increasing quartiles of intake (P=0.023). However, total protein intake was not significantly associated with nuclear and PSC cataract (P>0.05).
Table 2. The OR and 95%CI between energy-adjusted intakes of macronutrient and three types of cataract.
| Nutrients | Casesn (%) | Controlsn (%) | OR (95%CI) of three types of cataract1 |
||
| Nuclear | Cortical | Posterior subcapsular | |||
| Carbohydrate (g/d) | |||||
| Q1 | 65 (18.06) | 113 (31.39) | 1.0 | 1.0 | 1.0 |
| Q2 | 88 (24.44) | 91 (25.28) | 0.821 (0.346-1.941) | 1.439 (0.417-4.724) | 2.435 (1.313-4.127) |
| Q3 | 86 (23.89) | 94 (26.11) | 0.895 (0.327-2.416) | 2.225 (1.035-5.769) | 1.620 (0.859-2.878) |
| Q4 | 121 (33.61) | 62 (17.22) | 0.447 (0.136-1.572) | 2.471 (1.348-6.043) | 1.239 (0.751-2.632) |
| P | 0.127 | 0.027 | 0.889 | ||
| Total fat (g/d) | |||||
| Q1 | 73 (20.28) | 104 (28.89) | 1.0 | 1.0 | 1.0 |
| Q2 | 79 (21.94) | 105 (29.17) | 1.328 (0.679-2.366) | 0.572 (0.364-1.821) | 1.323 (0.562-2.935) |
| Q3 | 102 (28.33) | 78 (21.66) | 1.032 (0.511-1.761) | 0.843 (0.458-1.736) | 0.716 (0.321-1.879) |
| Q4 | 106 (29.45) | 73 (20.28) | 0.921 (0.451-1.723) | 1.027 (0.548-1.882) | 0.531 (0.083-1.527) |
| P | 0.576 | 0.527 | 0.245 | ||
| Protein (g/d) | |||||
| Q1 | 81 (22.50) | 102 (28.33) | 1.0 | 1.0 | 1.0 |
| Q2 | 81 (22.50) | 100 (27.78) | 0.729 (0.515-1.643) | 1.014 (0.517-1.746) | 0.563 (0.118-1.649) |
| Q3 | 89 (24.72) | 83 (23.06) | 0.620 (0.316-0.993) | 1.349 (0.791-2.649) | 0.238 (0.079-0.886) |
| Q4 | 109 (30.28) | 75 (20.83) | 0.648 (0.382-1.611) | 0.723 (0.442-1.279) | 0.528 (0.148-0.869) |
| P | 0.233 | 0.445 | 0.023 | ||
1Adjusted for age, gender, smoking, alcohol intake, hypertension, diabetes, BMI and the level of family economic income.
Further analysis with respect to various fatty acids and protein intake suggested that monounsaturated fat and animal protein intake were significantly higher in cases than controls (P<0.05) (Table 3). Neither monounsaturated fat nor saturated fat intake was associated with the risk of ARC after controlling for multiple potential confounders in logistic regression models (P>0.05). Increasing dietary intakes of polyunsaturated fat intake showed a positive association with nuclear cataract risk, with 2.7 times the risk of nuclear cataract as did those in the lowest quartile (OR=2.742, 95%CI: 1.790-4.200). The data suggested that higher polyunsaturated fat intake was associated with a higher prevalence of nuclear cataract (P=0.033), but found no significant positive or negative association with cortical or PSC cataract. Analyses that separately examined the intake of animal and vegetable protein indicated an inverse association between animal protein and cataract. For the highest compared with the lowest quartile of animal protein intake, the OR of nuclear and PSC cataract was 0.841 (95%CI: 0.416-0.963, P=0.038) and 0.761 (95%CI: 0.457-0.973, P=0.043), respectively. There was no significant association of vegetable protein intake with any of the three cataract types.
Table 3. The OR and 95%CI between energy-adjusted intakes of different fatty acids, protein and three types of cataract.
| Nutrients | Median intake |
OR (95%CI) of three types of cataract1 |
|||
| Cases | Controls | Nuclear | Cortical | Posterior Subcapsular | |
| Saturated fat (g/d) | 14.01 | 13.85 | |||
| Q1 | 10.09 | 9.97 | 1.0 | 1.0 | 1.0 |
| Q2 | 12.23 | 12.38 | 0.931(0.535-1.621) | 1.248 (0.596-2.706) | 1.632 (0.541-3.676) |
| Q3 | 14.88 | 14.53 | 1.137(0.580-2.229) | 1.134 (0.440-3.285) | 1.307 (0.346-3.568) |
| Q4 | 18.84 | 18.52 | 0.853(0.742-3.131) | 2.128 (1.067-3.548) | 2.208 (0.694-4.494) |
| P | 0.778 | 0.227 | 0.450 | ||
| Monounsaturated fat (g/d) | 22.61 | 21.65 | |||
| Q1 | 15.78 | 15.46 | 1.0 | 1.0 | 1.0 |
| Q2 | 19.42 | 18.92 | 0.676(0.446-1.025) | 0.925(0.610-1.404) | 0.640(0.466-1.795) |
| Q3 | 23.75 | 22.54 | 1.308(0.863-1.980) | 1.918(1.261-2.916) | 1.241(0.547-2.604) |
| Q4 | 31.47 | 29.69 | 1.131(0.747-1.711) | 1.512(0.998-2.293) | 1.344(0.815-2.379) |
| P | 0.614 | 0.120 | 0.736 | ||
| Polyunsaturated fat (g/d) | 22.15 | 23.24 | |||
| Q1 | 14.10 | 15.59 | 1.0 | 1.0 | 1.0 |
| Q2 | 21.05 | 21.08 | 0.977(0.641-1.490) | 1.013(0.831-1.735) | 0.950(0.625-1.445) |
| Q3 | 24.89 | 24.49 | 1.899(1.248-2.888) | 1.434(0.547-1. 740) | 0.870(0.575-1.316) |
| Q4 | 28.55 | 31.82 | 2.742(1.790-4.200) | 1.670(0.525-2.862) | 0.696(0.457-1.059) |
| P | 0.033 | 0.210 | 0.332 | ||
| Animal Protein (g/d) | 48.57 | 43.52 | |||
| Q1 | 25.35 | 25.92 | 1.0 | 1.0 | 1.0 |
| Q2 | 44.04 | 42.46 | 0.528(0.261-1.123) | 0.781 (0.357-1.842) | 0.724 (0.397-1.532) |
| Q3 | 51.70 | 52.38 | 1.347(1.142-2.853) | 1.073 (0.625-1.963) | 0.793 (0.382-0.954) |
| Q4 | 68.59 | 69.39 | 0.841(0.416-0.963) | 0.723 (0.349-1. 538) | 0.761 (0.457-0.973) |
| P | 0.038 | 0.320 | 0.043 | ||
| Vegetable Protein (g/d) | 30.84 | 31.86 | |||
| Q1 | 20.20 | 20.68 | 1.0 | 1.0 | 1.0 |
| Q2 | 27.69 | 27.43 | 0.924(0.717-1.185) | 1.000(0.781-1.283) | 0.973(0.725-1.254) |
| Q3 | 35.80 | 35.45 | 0.842(0.655-1.096) | 0.942(0.723-1.216) | 1.005(0.817-1.356) |
| Q4 | 52.27 | 46.52 | 0.851(0.662-1.119) | 0.876(0.674-1.129) | 0.952(0.745-1.238) |
| P | 0.183 | 0.450 | 0.712 | ||
DISCUSSION
A hospital-based case-control study was conducted to identify the association between total carbohydrate, fat, protein intake and ARC in Jinzhou area. In this present population, total carbohydrate intake showed a significant positive association with cortical cataract, and total fat intake was not significantly related to the presence of any of the three cataract types, nevertheless, higher dietary intake of protein was protective for PSC cataract. To our knowledge, Epidemiologic studies show mixed results with respect to the association between carbohydrate and ARC[7],[8],[10],[12]-[14]. Although some studies show a positive association between carbohydrate and cortical cataract or no association between carbohydrate and nuclear and PSC cataract, others show no association for any type of cataract[7],[8],[10],[12]-[14]. Data from the present study suggested that total carbohydrate intake was associated with the prevalence of cortical cataract, with a significant positive trend in risk. It is possible that higher carbohydrate intake may play a role in accelerating the pathophysiologic process of cataract formation, through inducing hyperglycemia, hyperinsulinemia and increasing concentrations of the inflammatory biomarker C-reactive protein[15]. Not only the quantity of carbohydrate, but quality of this nutrient was related to ARC[4],[16]. Therefore, further study is needed to clarify the relation between dietary glycemic load, which represents the quantity and quality of carbohydrates in the overall diet and their interaction and cataract risk.
There was no evidence of a significant association of total fat intake with ARC, which was compatible with that reported from a previous investigation[10]. Analyses that separately investigated the intakes of various fatty acids indicated that higher polyunsaturated fat increased the risk of nuclear cataract, consistent with findings from Lu et al [9]. Conversely, a prospective cohort study in Australia found a decreased risk of nuclear cataract[10]. It was failed to find significant associations between fat intakes of polyunsaturated fat and cortical or PSC cataract in this present study, which may be due to the different amounts of antioxidants available to prevent lipid peroxidation in these locations[9]. It was speculated that oxidative damage of ocular lens components may be correlated with cataract formation[17],[18].
A significant inverse association between PSC cataract and intakes of total protein was reported in our study, which have been consistently addressed in previous studies[10],[12]. Increasing dietary intakes of animal protein showed an inverse association with nuclear and PSC cataract after adjustment for multiple cofounders, but there was no significant relation of dietary vegetable protein intake to risk of any type of cataract. The mechanism for the effects of protein consumption on cataract formation had been speculated. Protein is required for the production of albumin, and the serum albumin-globulin ratio can be used to indicate protein nutritional status. Albumin plays an essential role in the osmotic fluid balance of the body, and osmotic stress of the lens has been associated with cataract formation[10].
Strengths of this study include its interviewer-administrated questionnaire, which permitted careful documentation of potential confounders that were controlled for in the multivariate models. On the other hand, this interviewer-administrated questionnaire was submitted to cases and controls by the same interviewer under the same conditions. Moreover, we chose to use cataract extraction as the endpoint for this analysis as a way to minimize misclassification of disease status, and cataract extraction should be a relatively good proxy for the more visually significant cataracts of greatest public health importance.
Several possible limitations of the study need to be considered. Whereas we controlled for the most likely known or suspected determinants of cataract risk, it is also possible that we have not adequately controlled for some unmeasured confounders. Another potential limitation is measurement error, because participants may have had difficulty in accurate classification of intakes across a wide range of food products. The resulting error in measuring macronutrient intakes from the FFQ would be expected to lead to a dilution of the magnitude of association, a well-known phenomenon in epidemiological studies. Of more concern is recall bias, where errors in reporting food intakes occur because of biased reporting due to knowledge of being a case. However, recall bias of diet between cases and controls seems unlikely, since doctors in Jinzhou do not give dietary advice to patients with cataracts, and the possible relationship between diet and ARC was generally unknown to subjects. Cases and controls were specifically asked whether they had changed their diets recently and if so, when and why. No person reported changes in diet due to eye problems.
In summary, the present study showed that a higher dietary carbohydrate and polyunsaturated fat intake may be a risk factor for cortical and nuclear cataract. Protein, especially the animal protein would be inversely associated with PSC cataract. Changes in dietary practices could contribute to the protection of ARC in middle-aged and elderly population. Given the limited data currently available, further intervention studies on dietary macronutrient intakes are needed.
Acknowledgments
Grateful acknowledgments for the study participants and staff of Department of Ophthalmology in the First and Third Affiliated Hospital of Liaoning Medical University and Jinzhou Central Hospital.
Footnotes
Foundation item: Science and Technology Planning Project, Liaoning Province Education Administration, China (No.2008-424)
REFERENCES
- 1.Congdon NG, Friedman DS, Lietman T. Important causes of visual impairment in the world today. JAMA. 2003;290(15):2057–2060. doi: 10.1001/jama.290.15.2057. [DOI] [PubMed] [Google Scholar]
- 2.Resnikoff S, Pascolini D, Etya'ale D, Kocur I, Pararajasegaram R, Pokharel GP, Mariotti SP. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82(11):844–851. [PMC free article] [PubMed] [Google Scholar]
- 3.Roberts JE. Ultraviolet radiation as a risk factor for cataract and macular degeneration. Eye Contact Lens. 2011;37(4):246–249. doi: 10.1097/ICL.0b013e31821cbcc9. [DOI] [PubMed] [Google Scholar]
- 4.Klein BE, Klein R. Lifestyle exposures and eye diseases in adults. Am J Ophthalmol. 2007;144(6):961–969. doi: 10.1016/j.ajo.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abraham AG, Condon NG, West Gower E. The new epidemiology of cataract. Ophthalmol Clin North Am. 2006;19(4):415–425. doi: 10.1016/j.ohc.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 6.West S. Epidemiology of cataract: accomplishments over 25 years and future directions. Ophthalmic Epidemiol. 2007;14(4):173–178. doi: 10.1080/09286580701423151. [DOI] [PubMed] [Google Scholar]
- 7.Chiu CJ, Robman L, McCarty CA, Mukesh BN, Hodge A, Taylor HR, Taylor A. Dietary carbohydrate in relation to cortical and nuclear lens opacities in the melbourne visual impairment project. Invest Ophthalmol Vis Sci. 2010;51(6):2897–2905. doi: 10.1167/iovs.08-2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan J, Wang JJ, Flood V, Kaushik S, Barclay A, Brand-Miller J, Mitchell P. Carbohydrate nutrition, glycemic index, and the 10-y incidence of cataract. Am J Clin Nutr. 2007;86(5):1502–1508. doi: 10.1093/ajcn/86.5.1502. [DOI] [PubMed] [Google Scholar]
- 9.Lu M, Taylor A, Chylack LT, Jr, Rogers G, Hankinson SE, Willett WC, Jacques PF. Dietary fat intake and early age-related lens opacities. Am J Clin Nutr. 2005;81(4):773–739. doi: 10.1093/ajcn/81.4.773. [DOI] [PubMed] [Google Scholar]
- 10.Townend BS, Townend ME, Flood V, Burlutsky G, Rochtchina E, Wang JJ, Mitchell P. Dietary macronutrient intake and five-year incident cataract: the Blue Mountains Eye Study. Am J Ophthalmol. 2007;143(6):932–939. doi: 10.1016/j.ajo.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 11.Seah SK, Wong TY, Foster PJ, Ng TP, Johnson GJ. Prevalence of lens opacity in Chinese residents of Singapore: the tanjong pagar survey. Ophthalmology. 2002;109(11):2058–2064. doi: 10.1016/s0161-6420(02)01221-6. [DOI] [PubMed] [Google Scholar]
- 12.Cumming RG, Mitchell P, Smith W. Diet and cataract: the Blue Mountains Eye Study. Ophthalmology. 2000;107(3):450–456. doi: 10.1016/s0161-6420(99)00024-x. [DOI] [PubMed] [Google Scholar]
- 13.Chiu CJ, Milton RC, Gensler G, Taylor A. Dietary carbohydrate and glycemic index in relation to cortical and nuclear lens opacities in the Age-Related Eye Disease Study. Am J Clin Nutr. 2006;83(5):1177–1184. doi: 10.1093/ajcn/83.5.1177. [DOI] [PubMed] [Google Scholar]
- 14.Chiu CJ, Morris MS, Rogers G, Jacques PF, Chylack LT, Jr, Tung W, Hankinson SE, Willett WC, Taylor A. Carbohydrate intake and glycemic index in relation to the odds of early cortical and nuclear lens opacities. Am JClin Nutr. 2005;81(6):1411–1416. doi: 10.1093/ajcn/81.6.1411. [DOI] [PubMed] [Google Scholar]
- 15.Liu S, Manson JE, Buring JE, Stampfer MJ, Willett WC, Ridker PM. Relation between a diet with a high glycemic load and plasma concentrations of high-sensitivity C-reactive protein in middle-aged women. Am J Clin Nutr. 2002;75(3):492–498. doi: 10.1093/ajcn/75.3.492. [DOI] [PubMed] [Google Scholar]
- 16.Schaumberg DA, Liu S, Seddon JM, Willett WC, Hankinson SE. Dietary glycemic load and risk of age-related cataract. Am J Clin Nutr. 2004;80(2):489–495. doi: 10.1093/ajcn/80.2.489. [DOI] [PubMed] [Google Scholar]
- 17.Spiteller G. Lipid peroxidation in aging and age-dependent diseases. Exp Gerontol. 2001;36(9):1425–1457. doi: 10.1016/s0531-5565(01)00131-0. [DOI] [PubMed] [Google Scholar]
- 18.Cekić S, Zlatanović G, Cvetković T, Petrović B. Oxidative stress in cataractogenesis. Bosn J Basic Med Sci. 2010;10(3):265–269. doi: 10.17305/bjbms.2010.2698. [DOI] [PMC free article] [PubMed] [Google Scholar]