Abstract
AIM
To analyze cases of obstruction of the nasolacrimal duct which creates a fertile environment for secondary bacterial infection and can result in dacryocystitis, which is a constant threat to cornea and orbital soft tissue and a potential source of endophthalmitis following intraocular surgery. The majority of obstructions of the lacrimal excretory outflow system are acquired ones occurring in adulthood and involving the distal parts of the system. Acquired obstruction may be primary/idiopathic or secondary to a wide variety of infectious, inflammatory, traumatic, mechanical, toxic or neoplastic causes mimicking idiopathic inflammation. These cases are treated by dacryocystorhinostomy (DCR).
METHODS
The present study was conducted to determine the histopathologic, immunohistochemical and current microbiologic characteristics of lacrimal sac specimens in patients undergoing external dacryocystorhinostomy.
RESULTS
Non-specific lacrimal sac pathology was present in all 33 cases and 81.8% of the cases showed moderate chronic inflammation with a chronic inflammatory score (CIS) ranging between 4 and 6, whereas 12.12% showed severe inflammatory changes with a CIS of 7. Mild degree of inflammation was seen in 6.06% with a CIS of 3. The total prevalence of gram-positive, gram-negative, and culture-negative samples were 59.4%, 37.5%, and 3% respectively.
CONCLUSION
Non-specific chronic inflammation with fibrosis is indeed the most commonly reported histopathological finding in lacrimal sac wall biopsy specimens.
Keywords: dacrocystitis, lacrimal sac biopsy, chronic inflammation, lacrimal duct obstruction
INTRODUCTION
Disorders of the lacrimal drainage system which cause epiphora, punctal discharge, or medial canthal swelling, are common ophthalmic complaints that comprise approximately 3% of clinic visits in some series[1],[2]. Obstruction of the nasolacrimal duct from whatever source results in stasis with the accumulation of tears, desquamated cells, and mucoid secretions superior to the obstruction in a pathologically closed lacrimal drainage system. This creates a fertile environment for secondary bacterial infection and can result in dacryocystitis which is a constant threat to cornea and orbital soft tissue[3]. Chronic dacryocystitis is diagnosed in patients with persistent epiphora and regurgitation of mucoid or mucopurulent material on pressure over the sac area, or regurgitation of mucoid or mucopurulent discharge on irrigation of the lacrimal drainage system[4],[5].
Primary acquired nasolacrimal drainage system obstruction is treated by dacryocystorhinostomy (DCR) operation a reliable, effective and well-established standard surgical procedure for the treatment of complete or partial nasolacrimal obstruction for alleviation of epiphora and providing symptomatic relief to patients. It is the approach of choice for suspected lacrimal sac diverticuli or lacrimal sac malignancies where biopsy or removal of the lacrimal sac or duct is planned. Even inflammatory lesions, including nonspecific chronic inflammation or granulomatous diseases, may present as lacrimal sac masses and may be a sign of systemic diseases diagnosed upon lacrimal sac biopsy[6]-[8]
Any reason that causes nasolacrimal duct obstruction (NLDO) generally converts the lacrimal sac into a reservoir of bacterial infection that may lead to chronic dacryocystitis which is a constant threat to cornea and orbital soft tissue[9].
The macroscopic appearance of an inflamed lacrimal sac specimen reveals a thickened and purulent or mucoid material in the lumen. A non-specific chronic inflammation with fibrosis and thickening of the wall due to submucosal lymphocytic infiltration with follicle formation is the most commonly reported histopathological finding in lacrimal sac wall biopsy specimens[9].
Thus, determining the incidence of primary lacrimal sac-specific pathology mimicking primary acquired nasolacrimal duct obstruction is important. It may have implications on whether routine biopsy during DCR is warranted or not, and how great is a risk of missing a clinically non-suspected and intra-operatively non-visible underlying specific non-neoplastic or neoplastic process involving the lacrimal sac in patients not undergoing routine biopsy during DCR[9],[10]. The risk of overlooking a spectrum of lacrimal sac originated specific pathologies, particularly malignancies that cause a nasolacrimal system obstruction, although very low, always exists[9],[10].
Neoplasms that affect the lacrimal drainage system are rare, but potentially life-threatening, so early diagnosis and treatment are particularly important[11],[12]. Epithelial neoplasms are most common (73%), including benign tumors (squamous cell papilloma, transitional cell papilloma, mixed-cell papilloma, oncocytoma) and malignant tumors (squamous cell carcinoma, transitional cell carcinoma, adenocarcinoma, mucoepidermoid carcinoma, oncocytic adenocarcinoma). Mesenchymal tumors such as fibrous histiocytoma, fibroma, hemangioma, hemangiopericytoma, angiosarcoma, or lipoma are less common (14%), and the rarer tumors include malignant melanomas (4%), and neural tumors (1%)[1],[11].
Primary lymphomas of the lacrimal sac are rare, but are a genuine cause of secondary acquired nasolacrimal duct obstruction. Lacrimal sac primary lymphomas are infrequent, not aggressive lesions that evolve rapidly and are not likely to be associated with typical clinical symptoms and signs such as mass extending above medial canthal tendon or bloody tearing suggestive of neoplastic pathology or malignancy. Secondary tumors originating in adjacent structures (paranasal sinuses, orbit and nose) may extend into the lacrimal sac. Metastatic neoplasms confined to the lacrimal sac are extremely rare, and most metastases also affect adjacent structures, such as the eyelid, nose, sinuses, and orbit[11],[12].
These findings have led to the recommendation that lacrimal sac biopsy specimens need not be routinely submitted for pathologic examination during DCR surgery, except for atypical clinical presentations or intraoperative findings, although that recommendation is controversial. Anderson et al[13] thought that all lacrimal sac walls should be biopsied at the time of DCR. Thus there has been debate in the literature regarding the value of lacrimal sac biopsy at the time of DCR surgery as the literature presents this dichotomy of views between “biopsy always” and “biopsy if the sac looks suspicious”[10],[11].
The lacrimal excretory system is prone to infection and inflammation for various reasons. This mucus membrane-lined tract is contagious with two surfaces (conjunctiva and nasal mucosa) that are normally colonized with bacteria. Obstruction of the nasolacrimal duct from whatever source results in stasis with the accumulation of tears, desquamated cells, and mucoid secretions superior to the obstruction in a pathologically closed lacrimal drainage system. This creates a fertile environment for secondary bacterial infection and can result in dacryocystitis.
It is currently believed that the inflammation and fibrosis in patients with nasolacrimal duct obstruction may be secondary to coexisting infectious colonization within the lumen of the lacrimal sac. It is possible that many cases of primary acquired nasolacrimal duct obstruction are in fact secondary to unrecognized low-grade dacryocystitis[14]. Knowledge of the bacteriology of nasolacrimal duct obstruction contributes significantly as well to the choice of prophylactic antimicrobial agents, and it would help in reducing the unnecessary load of anti-microbial agents with subsequent development of resistance patterns in the conjunctival flora with detrementous effects on regimens for prophylaxis on further intraocular surgery[15],[16].
During the past 20 years there have been only a few studies on the bacteriology of dacryocystitis in patients with NLDO. According to them, coagulase-negative staphylococci (CoNS) staphylococcus epidermidis and staphylococcus aureus are the most frequently isolated organisms in lacrimal sac infections[3]. Mixed bacterial isolates are more commonly found in cases of chronic dacryocostitis with the predominance of streptococcus pneumoniae and staphylococcus spp. fungal infections caused by candida albicans and aspergillus spp. occur infrequently[3].
This study was conducted for a further understanding of the pathological changes and the incidence of specific pathology in our cases of presumed primary acquired nasolacrimal duct obstruction, together with the spectrum and antimicrobial susceptibility of microbiologic agents isolated from such cases.
SUBJECTS AND METHODS
Subjects
This prospective analysis included a total of 33 lacrimal sac wall biopsies obtained from 33 patients eligible for the study who underwent external DCR for NLDO during the period from March 2012 to November 2012. Informed consent was obtained from the patients that were included in the study after explanation of the details of the study and of the procedure to be performed.
Methods
All the patients underwent a complete preoperative ophthalmic evaluation and slit-lamp ophthalmologic examination including visual acuity, ocular surface assessment and fundus biomicroscopy to report any posterior segment abnormalities and the demographic data (age and gender) and medical history of the patients and operative information were regularly recorded.
Assessment of lacrimal drainage system abnormalities using probing and syringing of the proximal lacrimal drainage system up to the nasal wall of the lacrimal sac together with lacrimal system irrigation for confirmation of NLDO. All patients underwent external dacryocystorhinostomy under general anesthesia and operative data was recorded. Biopsy specimens obtained from the posterior inferior flap of the lacrimal sac were examined under light microscopy by the same pathologist after being fixated in 4% neutral buffered formalin, paraffin embedded, cut at 5µm and stained with conventional histological stain (haematoxylin and eosin, H&E). All the specimens were examined for the chronic inflammation related histopathological features (inflammatory cell infiltration, fibrosis and capillary proliferation) and graded according to their severity using a “chronic inflammation score” established to determine the intensity of chronic inflammation using the grade of histopathological features[17],[18]. These are: 1) The intensity of inflammatory cell infiltration (number of lymphocytes, histiocytes and plasma cells in a HPF): mild<50 cells, moderate 50-200 cells, severe>200 cells; 2) The density of fibrosis (the amount of fibrotic tissue in a HPF): mild <25%, moderate 25%-50%, severe>50%; 3) The degree of capillary proliferation (number of capillary vessels in a HPF): mild<5, moderate 5-10, severe>10.
In addition, to determine the intensity of chronic inflammation in the lacrimal sac, all these three histopathological features were scored individually according to their severity (mild=1, moderate=2, and severe=3). Thus, a total score (sum) was obtained for each case ranging between 3 and 9 and named “chronic inflammatory score” (CIS).
Finally, every case was grouped according to its CIS as: mild chronic inflammation (CIS<3), moderate chronic inflammation (3<CIS<6) and severe chronic inflammation (CIS>6).
Immunohistochemical staining of the specimens using anti CD3 and CD 20 were done to determine the type of chronic inflammatory infiltrate using Avidin Biotin method according to the following procedure
Positive and negative controls were included in all runs. External positive control cases were used as follow (as recommended by manufacturer's protocols): tonsillar tissues for CD20, CD3. Immunostaining technique was done as recommended by manufacturer's protocols[19],[20]. Clones of antibodies, antigen retrieval and dilutions were as follow: CD20: mouse monoclonal Ab, clone L26, no special pretreatment, dilution 1:100. CD3: mouse monoclonal Ab, clone, antigen retrieval using citrate (10mM, pH6.0), dilution 1:150. Expression of immunohistochemical markers was visualized using the stretavidin-biotin-immunoenzymatic antigen detection system which was performed according to manufacturer's protocol[20]. Positive cell membranous staining in tumor cells for CD3 antibody. Positive cytoplasmic and cell membranous staining in tumor cells for: CD20 antibody.
Microbiology
Specimens for microbiological analysis were obtained by wiping a sterile broth-moistened swab over everted puncta after applying pressure over the lacrimal sac area and allowing the mucopurulent material to reflux through the lacrimal punctum, or by irrigating the lacrimal drainage system with sterile saline and collecting the sample from the refluxing material in cases that did not have regurgitation of mucus or mucopurulent discharge or pus on pressure on the lacrimal sac area e.g. encysted mucoceles and pyoceles. None of the patients had used either antibiotic eye drops or systemic antibiotics for at least a week before sample collection.
Samples were incubated in 2mL of brain-heart infusion broth agar cultured onto blood agar, MacConkey, chocolate agar, Sabouraud dextrose agar and incubated for 4d. The specific identification of bacterial isolates were performed based on microscopic morphology, staining characteristics, and biochemical properties using standard laboratory criteria, such as catalase, oxidase, and coagulase tests. All inoculated media were incubated aerobically. The inoculated Sabouraud's dextrose agar was incubated at 27°C, examined daily, and discarded at 3 weeks if no growth was seen. The inoculated blood agar, chocolate agar, thioglycollate broth, brain-heart infusion broth were incubated at 37°C, examined daily, and discarded at 7d if growth was not seen.
Microbial cultures were considered significant if growth of the same organism was demonstrated on more than one solid-phase medium, and/or if there was confluent growth at the site of inoculation on one solid medium, and/or if growth of one medium was consistent with direct microscopy findings (i.e. appropriate staining and morphology with Gram stain), and/or if the same organism was grown from more than one specimen.
Antibiotic susceptibility testing was done through the conventional Kirby-Bauer disk diffusion method of in vitro antibacterial susceptibility testing for tobramycin, quinolones (ciprofloxacin and ofloxacin), chloramphenicol, doxycycline, vancomycin, amoxicillin clavulinate and cefoxitin.
Statistical Analysis
StatSoft, Inc. (2007). STATISTICA (data analysis software sustem), version 8.0. Chi-square (χ2) distribution was used to test the qualitative distribution. The values of P<0.05 were accepted as statistically significant.
RESULTS
In this prospective interventional case series, 33 lacrimal sac specimens were obtained from a total of 33 consecutive patients who underwent external DCR for clinically presumed acquired NLDO at the time interval from March to December 2012 at Alexandria Main University Hospital.
The cases included 32 females (96.97%) and 1 male (3.03%). The female subjects with chronic dacryocystitis (78.1%) were more in number in than male subjects (0).
The mean age of study group was 50.4 with a range between 6 and 63 years. Patients above the age of 30 years (30 of 33; 91%) were significantly more than patients below 30 years (3 of 33; 9.1%). Four of the 33 patients with lacrimal duct obstruction were bilateral cases (12.1%).
Eight (24.2%) of the patients had previously had at least one attack of acute dacryocystitis. A total of 25 (78.8%) of the cases had chronic dacryocystitis. These were further subdivided into two groups according to the nature of the lacrimal discharge. Fourteen cases (56%) showed copious thick mucopurulent discharge coming from the sac, whereas 7 cases (28%) showed epiphora with clear tear fluid or minor mucopurulent discharge. Four patients (16%) had swellings over the lacrimal sac with characters consistent with mucoceles, of which 2 cases were expressible mucoceles.
Of the 33 cases recruited in this study, 29 (87.9%) had complete NLDO, whereas 4 patients (12.1%) were diagnosed as having functional obstruction of the nasolacrimal passages.
Pathological Findings
Of all the study cases, none showed normal histology. Non-specific lacrimal sac pathology was present in all 33 cases including varying degrees of non-specific non-granulomatous chronic inflammation, whereas specific lacrimal sac pathology was not found in any of the biopsied cases.
All the obtained specimens were further examined for certain chronic inflammation related histopathological features (inflammatory cell infiltration, fibrosis and capillary proliferation) and graded according to their severity. Moreover, a chronic inflammation score CIS was used to determine the intensity of chronic inflammation using the grade of these histopathological features (Table 1).
Table 1. Showing the histopathologic grading system for chronic inflammation scoring CIS.
| Mild | Moderate | Severe | |
| Intensity of inflammatory cell infiltration (number of lymphocytes, histiocytes and plasma cells in a HPF) | <50 cells | 50-200 cells | severe>200 cells |
| Density of fibrosis (amount of fibrotic tissue in a HPF) | <25% | 25-50% | >50% |
| Degree of capillary proliferation (number of capillary vessels in a HPF) | <5 | 5-10 | >10 |
In addition, to determine the intensity of chronic inflammation in the lacrimal sac, all these three histopathological features were scored individually according to their severity (mild=1, moderate=2, and severe=3). Thus, a total score (sum) was obtained for each case ranging between 3 and 9, and named “chronic inflammatory score” (CIS). Finally, every case was grouped according to its CIS as: mild chronic inflammation (CIS<3), moderate chronic inflammation (3<CIS<6) and severe chronic inflammation (CIS>6).
The results were evaluated using StatSoft, Inc. (2007). STATISTICA (data analysis software sustem), version 8.0. Chi-square (χ2) distribution was used to test the qualitative distribution. The values of P<0.05 were accepted as statistically significant.
All lacrimal sac biopsies demonstrated variable degrees of inflammatory cell infiltration. When the groups were stratified according to their intensity, 22 (66.67%) had mild (Figure 1), 8 (24.2%) had moderate (Figure 2) and 3 (9.1%) had severe inflammatory cell infiltration (Figure 3).
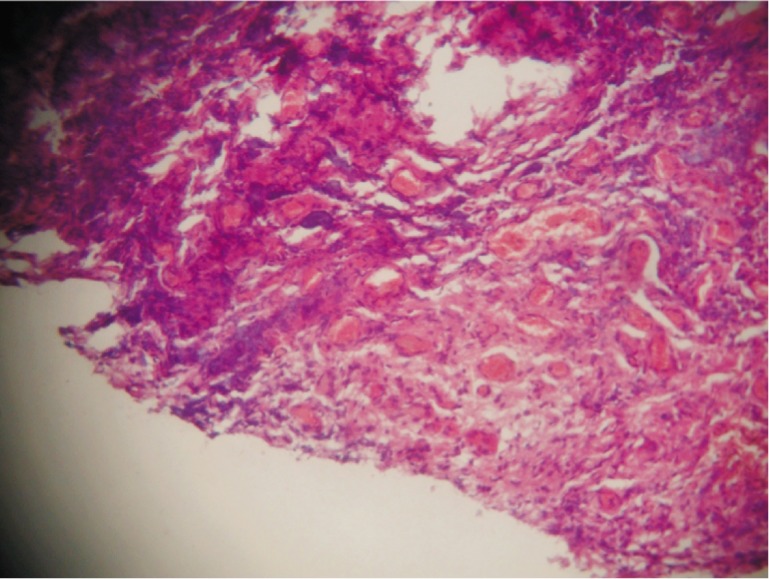
Figure 1. Mild lymphocytic infiltration and mild angiogenesis. H&E ×100.
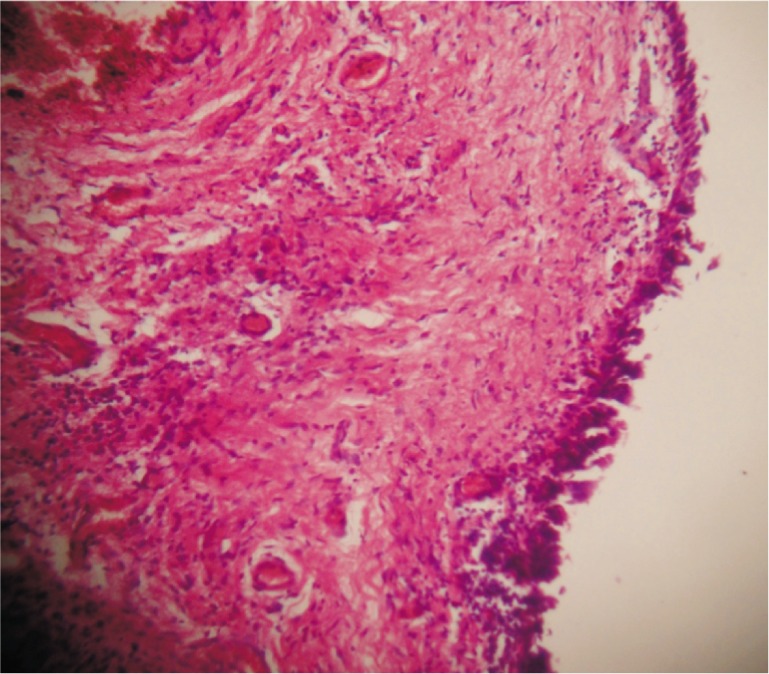
Figure 2. Intact linning with moderate inflammation. H&E ×100.
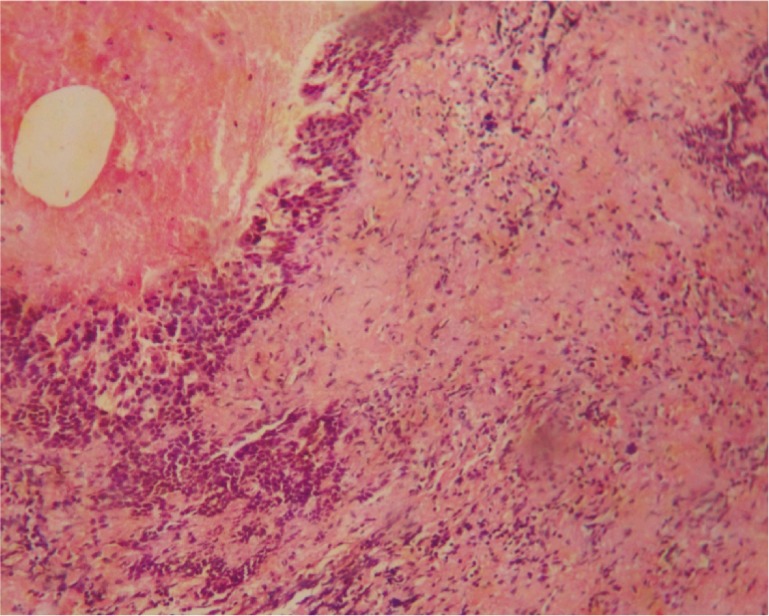
Figure 3. Severe lymphocytic infiltrate and fibrosis. H&E ×100.
In this study, the density of fibrosis was mild in 5 cases (15.2%), moderate in 6 cases (18.2%) and severe in 22 cases (66.67%).
The degree of capillary proliferation was mild in 21 cases (66.67%), moderate in 12 cases (36.4%) (Figure 4) and was not found to be severe in any of the cases.
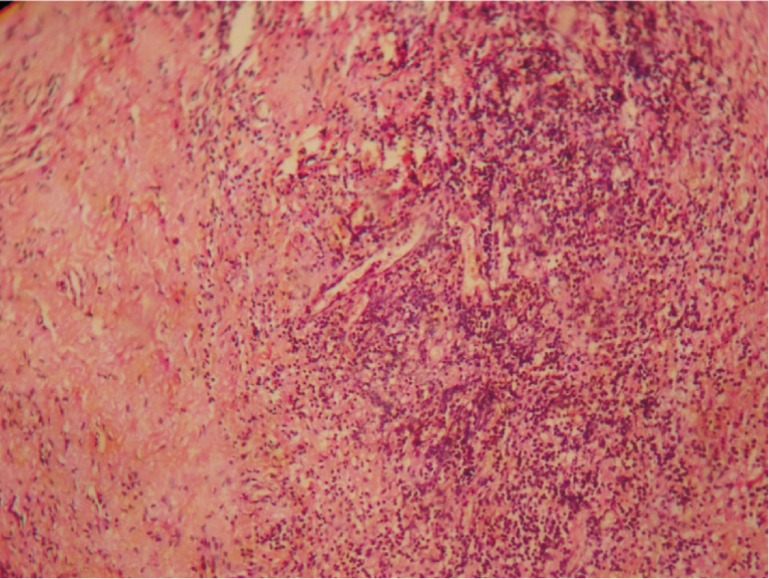
Figure 4. Moderate angiogenesis. H&E ×100.
On applying the CIS system to the aforementioned results, 27 (81.8%) of the cases showed moderate chronic inflammation with a CIS of a range between 4 and 6, whereas 4 cases (12.12%) showed severe inflammatory changes with a CIS of 7. Mild degree of inflammation was seen in 2 cases (6.06%) with a CIS of 3. The distribution of histopathological features and chronic inflammation is illustrated in Table 2.
Table 2. Histopathology findings in 33 lacrimal sac biopsy specimens from cases undergoing dacryocystorhinostomy.
| Inflammatory cell infiltrate | Fibrosis | Capillary proliferation | Chronic inflammation score | |
| Mild | 22 | 5 | 21 | 2 |
| Moderate | 8 | 6 | 12 | 27 |
| Severe | 3 | 22 | 0 | 4 |
Results were satisfactory in 2 (100%) patients with mild score, 24 (92.3%) patients with moderate score and 3 (60%) patients with severe score, while unsatisfactory results were obtained in 0 (0), 2 (7.7%) and 2 (40%) patients with mild, moderate and severe score respectively. However, these differences were statistically insignificant (MCp=0.110) (Table 3).
Table 3. Relation between CIS score and surgical outcome.
| Score | n | Outcome |
MCp | |
| Satisfactory n (%) | Unsatisfactory n (%) | |||
| Mild | 2 | 2 (100) | 0 (0) | 0.110 |
| Moderate | 26 | 24 (92.3) | 2 (7.7) | |
| Sever | 5 | 3 (60) | 2 (40) | |
Correlations between inflammatory infiltrate, fibrosis and capillary proliferation and surgical outcome of the cases were found to be statistically insignificant (Tables 4–6).
Table 4. Relation between inflammatory cell infiltrate and surgical outcome.
| Inflammatory cell infiltrate | n | Outcome |
MCp | |
| Satisfactoryn (%) | Unsatisfactoryn (%) | |||
| Mild | 22 | 21 (95.5) | 1 (4.5) | 0.070 |
| Moderate | 8 | 5 (62.5) | 3 (37.5) | |
| Sever | 3 | 3 (100) | 0 (0) | |
Table 6. Relation between degree of capillary proliferation and surgical outcome.
| Capillary proliferation | n | Score |
P | |
| Satisfactoryn (%) | Unsatisfactoryn (%) | |||
| Mild | 21 | 19 (90.5) | 2 (9.5) | 0.610 |
| Moderate | 12 | 10 (83.3) | 2 (16.7) | |
| Sever | 0 | 0 (0) | 0 (0) | |
Table 5. Relation between degree of fibrosis and surgical outcome.
| Fibrosis | n | Outcome |
MCp | |
| Satisfactoryn (%) | Unsatisfactoryn (%) | |||
| Mild | 5 | 5 (100) | 0 (0) | 0.830 |
| Moderate | 6 | 5 (83.3) | 1 (16.7) | |
| Sever | 22 | 19 (86.9) | 3 (13.6) | |
Immunohistochemical Results
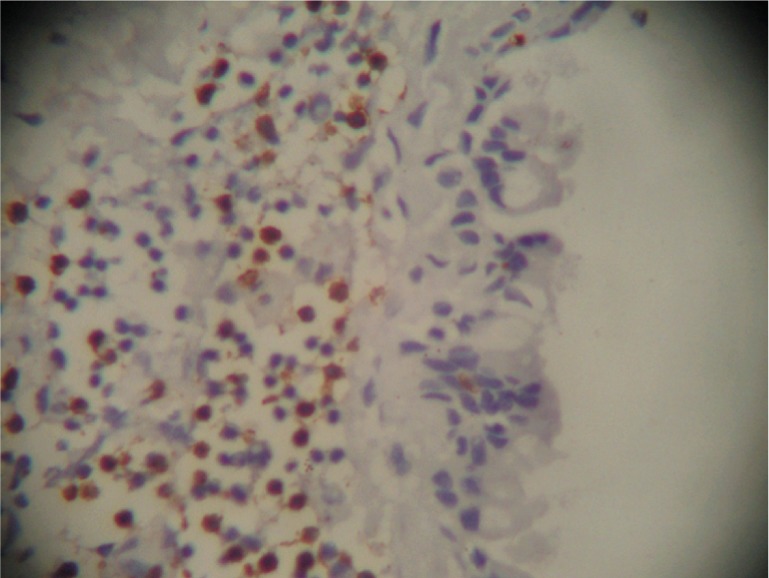
All the lymphocytic infiltrate proved to be T lymphocytes (CD 3+ve) (Figure 5).
Figure 5. CD3 positive membranous immunostaining in the lymphocytic infiltrate.
Bacteriological Findings
Another purpose of this study was to identify the spectrum of bacterial pathogens in dacryocystitis and to determine their in vitro antibiotic susceptibility to commonly used antibacterial agents.
One of our 33 patients was culture negative, whereas cultures were positive from 32 samples (97%). Of the 32 samples with positive culture results, 2 (6.25%) had mixed culture results with more than one gram negative organism isolated
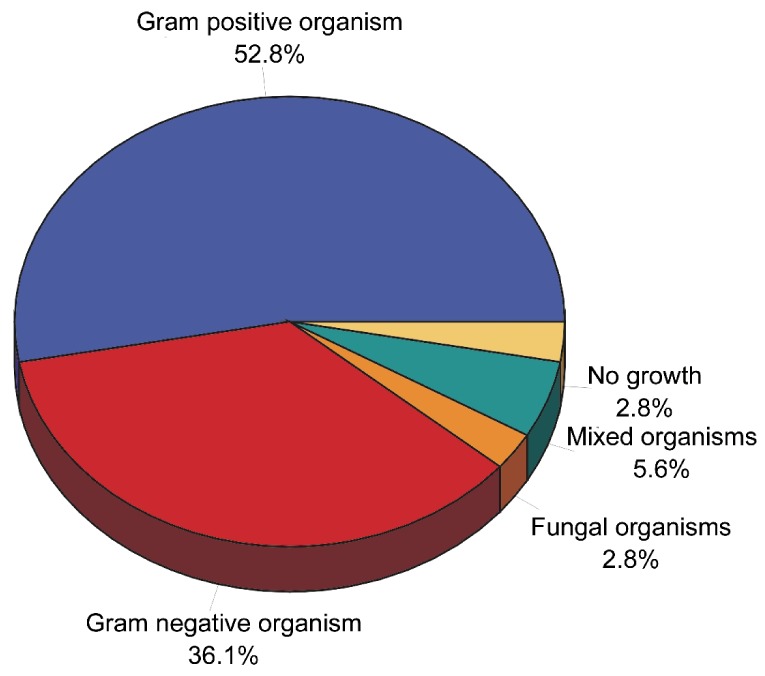
Altogether, ten types of bacterial isolates were recovered from the 32 positive culture samples. The most frequently cultured bacterial species of all was staphylococcus epidermidis, which was isolated in 13 samples, accounting for 39.4% of all the isolates (Table 7, Figure 6).
Table 7. Bacteriological findings from the culture of lacrimal sac biopsy specimens obtained from 33 patients undergoing dacryocystorhinostomy for nasolacrimal duct obstruction.
| Micro-organisms isolated | n of isolates |
| Gram positive organisms | 19 |
| Staphylococcus epidermidis | 13 |
| Staphylococcus aureus | 4 |
| Other staphylococcus sp. | 1 |
| Corynebacterium sp. | 1 |
| Gram negative organisms | 14 |
| Pseudomonas aeruginosa | 5 |
| Klebsiella pneumoniae | 4 |
| Escherichia coli | 1 |
| Enterobacter sp. | 3 |
| Acinetobacter lwoffii | 1 |
| Fungal organisms | 1 |
| Candida sp. | 1 |
| Mixed flora | 2 |
| No growth | 1 |
Figure 6. Bacteriological findings from the culture of lacrimal sac biopsy specimens obtained from 33 patients undergoing dacryocystorhinostomy for nasolacrimal duct obstruction.
The prevalence of bacterial pathogens responsible for acute dacryocystitis and chronic dacryocystitis differed from each other in this study. The predominant bacterial pathogens isolated from acute dacryocystitis were staphylococcus spp. (50%) followed by pseudomonas aeruginosa (37.5%), and from chronic dacryocystitis were coagulase-negative staphylococci (CoNS) (52.2%) and klebsiella pneumoniae (17.4%) respectively. One case with chronic dacryocystitis showed fungal growth, and from one patient in each group two gram negative microorganisms were isolated yielding a mixed culture result.
Gram negative organisms were also isolated more often (62.5%) in the cases with copious mucopurulent discharge from chronic dacryocystitis than in the cases with simple epiphora (0). There were more cases with epiphora or minor discharge in which no micro-organism was found (14.3%) than cases with chronic dacryocystitis with mucopurulent discharge (0). More than half (62.5%) of the samples of the cases with copious purulent or mucopurulent discharge showed Gram negative organisms, whereas these bacteria were isolated in none of the samples of the cases with simple epiphora or minor mucopurulent discharge. This difference was statistically highly significant (P=0.000), suggesting that the bacterial flora of this group may be comparable with those of normal conjunctiva (Table 8).
Table 8. Distribution of microorganisms in cases of acute and chronic dacryocystitis.
| Micro-organisms isolated | Acute dacryocystitis | Chronic dacryocystitis |
| Gram positive organisms | 6 | 14 |
| Staphylococcus epidermidis | 1 | 12 |
| Staphylococcus aureus | 3 | 1 |
| Other staphylococcus sp. | 1 | 0 |
| Corynebacterium sp. | 1 | 1 |
| Gram negative organisms | 4 | 10 |
| Pseudomonas aeruginosa | 3 | 2 |
| Klebsiella pneumoniae | 0 | 4 |
| Escherichia coli | 0 | 1 |
| Enterobacter sp. | 1 | 2 |
| Acinetobacter lwoffii | 0 | 1 |
| Fungal organisms | 0 | 1 |
| Candida sp. | 0 | 1 |
| Mixed flora | 1 | 1 |
| No micro-organism | 0 | 1 |
DISCUSSION
Determining the incidence of primary lacrimal sac-specific pathology mimicking primary acquired lacrimal duct obstruction (PALDO) is important, because it may have implications whether routine biopsy during DCR is warranted or not, and how great is a risk of missing a clinically non-suspected and intra-operatively non-visible underlying specific non-neoplastic or neoplastic process involving the lacrimal sac in patients not undergoing routine biopsy during DCR[9]. The risk of overlooking a spectrum of lacrimal sac originated specific pathologies, particularly neoplastic malignant lesions that cause nasolacrimal system obstruction, although very low, always exists[21].
These primary lacrimal sac neoplasms are in majority of reported cases malignant in biological behavior, emphasizing the importance of early diagnosis. However, there has been debate in the literature regarding the value of lacrimal sac biopsy at the time of DCR surgery[9]. Anderson et al[13] thought that all lacrimal sac walls should be biopsied at the time of DCR. Many others argue that biopsy of the lacrimal sac wall at DCR is not indicated routinely and is only indicated if there is a reason to suspect pathology other than chronic inflammation preoperatively or intraoperatively, although that recommendation is still controversial[13].
Nevertheless, some lacrimal sac tumors such as primary lymphomas, although infrequent, are not likely to be associated with typical clinical symptoms and signs such as mass extending above medial canthal tendon or bloody tearing suggestive of neoplastic pathology or malignancy. Some other lesions are also so incipient to produce a grossly visible abnormality. The value of lacrimal sac biopsy and histological examination of the lacrimal sac wall at DCR, in those cases, is undoubtful[22]. This prospective study tests the possible value of routine lacrimal sac biopsy during surgery for clinically presumed PALDO and also adds to our knowledge more about the nature and prevalence of lacrimal sac non-specific and specific pathologic features in our patients.
Our study is based on a consecutive series of lacrimal sac biopsy specimens obtained from patients with clinically presumed nasolacrimal duct obstruction and submitted to a single pathology laboratory. Non granulomatous inflammation was the most common histopathologic diagnosis, as previously reported in many other similar series[21]. Of all the study cases, none showed normal histology. Non-specific lacrimal sac pathology was present in all 33 cases including varying degrees of non-specific non-granulomatous chronic inflammation, whereas specific lacrimal sac pathology was not found in any of the biopsied cases.
Specific pathology was found in 31 out of 377 specimens in Anderson's series[13] including eight sarcoidosis, seven lymphoma, four papilloma, four lymphoplasmacytic infiltrate, two transitional cell carcinoma, one oncocytoma, one granular cell tumour, one adenocarcinoma, one poorly differentiated carcinoma, one plasmacytoma, and one leukaemia; and in 10 out of 302 specimens in Bernardini's series (four sarcoidosis, three squamous papilloma, two lymphoma, one leukaemia)[23].
On combining the results from the previous studies, 50 out of 1 294 specimens (3.9%) in these seven series showed specific pathology. Only seven out of 1 294 specimens (0.5%) in these seven series showed specific pathology which was definitely unsuspected preoperatively, and in only one of these was this malignant. In Lindberg's series, of the two specimens with specific pathology this was unsuspected in one specimen with sarcoidosis. In Tucker's series, of the four specimens with specific pathology this was unsuspected in one specimen with oncocytoma. In Bernardini's series, of the 10 specimens with specific pathology this was suspected in all specimens either before or during the surgery. Two of the 3 cases of specific pathology in Merkonidis' series was unsuspected[24]. In Anderson's series, of the 31 specimens with specific pathology it was stated this was unsuspected preoperatively in at least three[13].
Findings from our study together with previous series from the literature have led to the recommendation that lacrimal sac biopsy specimens need not be routinely submitted for pathologic examination during DCR surgery, except for atypical clinical presentations or intraoperative findings.
This recommendation may be argued by the small sample size in some of the mentioned studies and the present study. Again, ethnic heterogeneity may contribute to the results in some studies[24],[25]. Another limitation is the fact that it should always be kept in mind that obtaining a representative biopsy of the lesion is not always easy and sometimes is challenging. If a peripheral portion of specific lesion or inadequate specimen is taken, or if pathologic tissue is not recognized as abnormal, tumor or any other pathologic process may not be presented in the biopsy specimen, so biopsy may yield a false-negative result. In a case of lacrimal drainage system obstruction, a misdiagnosis of chronic inflammation may occur. In addition, intra-operative normal appearance of the lacrimal sac is not an absolute guarantee that the sac is devoid of pathological process other than chronic inflammation and/ or fibrosis in the early phase of clinical evolution of lacrimal sac tumors (stage 1, according to Cook and Olver[26]) even in the eyes of experienced ophthalmic lacrimal surgeons. Because of the absence of definite tumor on palpation, it is difficult to clinically differentiate a lacrimal sac tumor from chronic dacryocystitis, so routine blind lacrimal sac wall biopsy during DCR may not be the best choice.
To minimize the risk of overlooking specific pathology it is important to inquire about symptoms or history of systemic disease preoperatively, to assess the appearance of the lacrimal sac intraoperatively, and to biopsy the lacrimal sac in those cases where specific pathology is suspected.
In our study, the role of chronic inflammation on DCR outcome was also evaluated, using histopathological features of chronic inflammation such as inflammatory cell infiltration, fibrosis and capillary proliferation.
All lacrimal sac biopsies demonstrated variable degrees of inflammatory cell infiltration. When the groups were stratified according to their intensity, 22 (66.67%) had mild, 8 (24.2%) had moderate and 3 (9.1%) had severe inflammatory cell infiltration.
In this study, the density of fibrosis was mild in 5 cases (15.2%), moderate in 6 cases (18.2%) and severe in 22 cases (66.67%). It was noted that in our two cases of revision DCR, there was a marked increase in the density of fibrosis, which is consistent with previous findings in a study that examined the silicone tube intubation related histopathological changes in revision DCR cases, where a marked increase in the density of fibrosis was observed and the role of fibrosis on recurrence was emphasized[27]. Our cases had had silicone tubes inserted during their primary surgeries. Nevertheless, we believe that further studies are required to determine the effect of fibrosis on DCR outcome.
The degree of capillary proliferation was mild in 21 cases (66.67%), moderate in 12 cases (36.4%) and was not found to be severe in any of the cases.
On applying the CIS system to the aforementioned results, 27 (81.8%) of the cases showed moderate chronic inflammation with a CIS of a range between 4 and 6, whereas 4 (12.1%) cases showed severe inflammatory changes with a CIS of 7. Mild degree of inflammation was seen in 2 cases (6.06) with a CIS of 3. A quantitative and statistical analysis of histopathological features and chronic inflammation was performed between patients with satisfactory and unsatisfactory outcome, and of patients with unsatisfactory outcome.
Another purpose of this study was to identify the bacterial aetiology behind dacryocystitis and to determine the in vitro antibacterial susceptibility and resistance of bacterial pathogens to commonly used antibacterial agents.
Using direct biopsy methods, we found culture-positive lacrimal sac specimens in a large proportion of patients undergoing DCR surgery. These organisms were found to be present in patients with and without a history of infection.
In this study Gram positive bacteria were found in 59.4% of the isolates. This is in close agreement with the observation of 65% of Gram positive organisms by Coden et al[28]The most common organisms cultured in our study were staphylococcus species, accounting for 54.5% of the isolates. This percentage compares fairly well with the results of Thicker and Buffam[29], Huber-Spitzy et al[30] and Coden et al[28] (their percentages being 73%, 51%, and 49% respectively). Staphylococcus epidermidis and staphylococcus aureus represented 40% and 12.1% of all the isolates in our study, which is higher than what was reported in previous studies for staphylococcus epidermidis, and the same for staphylococcus aureus (their percentages being 26.9% and 12.3% respectively)[5].
Gram negative organisms represented 37.5% of the isolates of the total material in this study, the most frequently isolated species being pseudomonus aeroginosa (38.5%) followed by klebsiella pneumonia spp. (30.8%). Previously, Huber-Spitzy et al[30] reported Gram negative organisms accounting for 26% of isolates, the most frequent species being escherichia coli (12%). Coden et al[28] observed Gram negative organisms in 27% of all isolates, including pseudomonas aeruginosa in 9% and haemophilus species in 6% of isolates.
These findings demonstrate some discrepancy in the spectrum of gram negative isolates cultured from our study as compared to other similar series. This may be attributed to the injudicious use of antibiotics in our community, particularly broad spectrum antibiotics, in the treatment of non-infectious conditions. More virulent organisms have replaced the flora and anticipated organisms in culture results from such cases.
One of the cases showed fungal growth in the form of candida albicans. Isolation of fungi from the normal conjunctival sac occurs in about 6 to 25% of normal patients[31]. Perhaps the high incidence of fungal isolation from the conjunctiva of humans is related to the frequency of cosmetics use and the chronic use of topical ophthalmic antibiotics, which predisposes humans to fungal carriage in the conjunctiva. This change in flora may be important if followed by trauma or contact lens wear, thus allowing saprophytic fungi direct ingress to the cornea. Infection and obstruction of the lacrimal duct system or dacryocystitis may be due to fungal infection as well. Yeasts such as candida spp. have been implicated[32]. Fungi can be isolated from approximately 30% of eyes with congenital dacryocystitis, and candida albicans is most often cultured[33].
Three cases showed spores from their cultured swab samples, the significance of which still remains unclear. It is postulated that contamination may have occurred during the swab collection process, spores being saprophytes normally occurring on the skin and adnexa. These results, after conducting tests of agreement, suggest that sample collection by either way be recommended with care for cleaning of the skin and adnexa before swab collection.
Another interesting finding in our study was that Gram negative organisms occurred with high statistical significance more frequently in cases with copious purulent or mucous discharge than in cases with minor discharge. All these findings suggest that the antibiotic treatment protocol before and after lacrimal surgery should be reconsidered according to the subgroup of patients.
The lack of Gram negative bacteria in the cases with epiphora or minor discharge, suggested that the bacterial flora of this group are comparable with those of normal conjunctiva. Normal flora of human conjunctiva mostly consist of Gram positive bacteria, which represent up to 97% of cultured aerobic isolates[33]. The most common bacterium is staphylococcus epidermidis, accounting for 57%-87% of isolates, while streptococcus species account only for 6% of all aerobic isolates of normal conjunctiva. Gram negative bacteria represent 0-5% of aerobic isolates[34]. Thus the increased proportion of Gram negative bacterial isolates from cases of chronic dacryocystitis in adults is clearly not related to conjunctival flora.
These Gram negative bacteria are potential pathogens in postoperative infections, both in intraocular and lacrimal drainage surgery. This conforms to the practice that chronic dacryocystitis with mucous or purulent discharge is a contraindication for elective intraocular surgery, whereas patients with simple epiphora did not appear to have an increased risk for endophthalmitis after intraocular surgery in previous studies[34]. For this reason, in lacrimal drainage surgery of such cases, the antimicrobial prophylaxis should also cover Gram negative organisms.
The analysis of the in vitro susceptibility showed that the highest percentages of bacterial isolates were most susceptible to vancomycin (95.1%), gatifloxacin (91.8%), cefotaxime (91.8%), and amikacin (91.1%), tobramycin (88.5%) and ofloxacin (88.5%), while the highest percentage of bacterial isolates were resistant to macrolides (42.3%) and amoxicillin (37.7%). Of all antibacterial agents tested, gatifloxacin and ofloxacin showed lowest percentage of resistance to all categories of bacterial species recovered from both acute and chronic infections of the lacrimal apparatus (8.2% and 11.5% respectively)
The analysis of the in vitro resistance pattern showed variation in the resistance of isolates recovered from acute and chronic dacryocystitis. The percentage of resistance of bacterial isolates recovered from chronic infections to tobramycin (5 of 35; 14.3%), gatifloxacin (5 of 35; 14.3%), ciprofloxacin (8 of 35; 22.9%), ofloxacin (7 of 35; 20%) and amoxicillin clavulinate (22.9% 8 of 35) was found to be higher than the percentage of resistance of bacterial isolates recovered from acute infection to tobramycin (12.5% 2of 16), gatifloxacin (0), ciprofloxacin (0), ofloxacin (0) and amoxicillin clavulinate (3 of 16 18.75%).
Thus our data revealed that the emergence of drug-resistance takes place among bacterial isolates recovered from chronic cases. The reason for the emergence of resistance may be attributed to the prophylactic use of antibiotics for longer periods of time, or using different antibiotics for different ocular infections, chronic antibiotic therapy for non-infectious ocular diseases, and prolonged unnecessary therapy before (several days) and after (weeks) surgery[10]. Bacterial flora is abundant at the eyelid margin, and the setting is conducive to a possible spontaneous mutation that can cause antibiotics resistance[34].
It must be noted that in this present study, all inoculated culture media were incubated at aerobic conditions. Thus, the spectrum of bacterial pathogens recovered from eyes with acute and chronic dacryocystitis in this study showed the complete profile of aerobic and facultative organisms. Although the bacterial aetiology of dacryocystitis includes a spectrum of bacterial species belonging to both aerobic and anaerobic group, the present study highlights the potential importance of aerobic bacterial pathogens and their susceptibility to commonly used antibacterial agents[10].
It must also be noted that the conventional Kirby-Bauer disc diffusion method of in vitro antibacterial susceptibility testing may not directly apply to ocular pathogens, since the ocular antibacterial level achievable by topical administration may be considerably higher than the level attained at the ocular tissue by systemic administration. Indeed, there have been many studies that have reported susceptible and resistant pattern of ocular pathogens with conventional in vitro antibacterial susceptibility testing, and these in vitro susceptible and resistant patterns have been successfully treated in vivo by those antibacterials[35]. These results still do provide information that allows a clinician to make rationale-based decisions in choosing a primary treatment regimen which provide coverage for common ocular pathogens in each subgroup of patients[10].
In conclusion, the proportion of staphylococcus aureus and pseudomonas spp. is higher in causing acute dacryocystitis, while CoNS are frequently associated with chronic dacryocystitis. Of all antibacterials tested, gatifloxacin, ofloxacin, and amikacin show greater efficacy against bacterial isolates from dacryocystitis. Bacterial species isolated from chronic dacryocystitis show higher resistance to broad-spectrum antibiotics than those from acute cases.
REFERENCES
- 1.Heindl Ludwig M., Dr. med . Surgical Anatomy and Pathology in Surgery of the Eyelids, Lacrimal System, Orbit and Conjunctiva. In: Naumann GOH, Holbach L, Kruse FE, editors. Applied pathology for ophthalmic microsurgeons. Berlin: Springer; 2008. pp. 29–75. [Google Scholar]
- 2.Mandal R, Banerjee AR, Biswas MC, Mondal A, Kundu PK, Sasmal NK. Clinicobacteriological study of chronic dacryocystitis in adults. J Indian Med Assoc. 2009;106(5):296–298. [PubMed] [Google Scholar]
- 3.Iliff NT. Infections of the lacrimal drainage system. In: Peopse JS, Holland GN, Wilhelmus KR, editors. Ocular Infection and Immunity. Mosby: St Louis, MO; 2010. pp. 1346–1355. [Google Scholar]
- 4.Bartley GB. Acquired lacrimal drainage obstruction: an etiologic classification system, case reports, and a review of the literature. Part 1. Ophthal Plast Reconstr Surg. 2003;8(4):237–242. [PubMed] [Google Scholar]
- 5.Hartikainen J, Lehtonen OP, Saari KM. Bacteriology of lacrimal duct obstruction in adults. Br J Ophthalmol. 2009;81(1):37–40. doi: 10.1136/bjo.81.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stefanyszyn MA, Hidayat AA, Pe'er JJ, Flanagan JC. Lacrimal sac tumors. Ophthal Plast Reconstr Surg. 2010;10(3):169–184. doi: 10.1097/00002341-199409000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Tucker N, Chow D, Stockl F, Codère F, Burnier M. Clinically suspected primary acquired nasolacrimal duct obstruction. Clinicopathologic review of 150 patients. Ophthalmology. 2009;104(11):1882–1886. doi: 10.1016/s0161-6420(97)30012-8. [DOI] [PubMed] [Google Scholar]
- 8.Valenzuela AA, McNab AA, Selva D, O'Donell BA, Whitehead KJ, Sullivan TJ. Clinical features and management of tumors affecting the lacrimal drainage apparatus. Ophthal Plast Reconstr Surg. 2009;22(2):96–101. doi: 10.1097/01.iop.0000198457.71173.7b. [DOI] [PubMed] [Google Scholar]
- 9.Salour H, Hatami MM, Parvin M, Ferdowsi AA, Abrishami M, Bagheri A, Aletaha M, Yazdani S. Clinicopathological study of lacrimal sac specimens obtained during DCR. Orbit. 2010;29(5):250–253. doi: 10.3109/01676830.2010.485720. [DOI] [PubMed] [Google Scholar]
- 10.Altan-Yaycioglu R, Canan H, Sizmaz S, Bal N, Pelit A, Akova YA. Nasolacrimal duct obstruction: clinicopathologic analysis of 205 cases. Orbit. 2010;29(5):254–259. doi: 10.3109/01676831003739699. [DOI] [PubMed] [Google Scholar]
- 11.Parmar DN, Rose GE. Management of lacrimal sac tumors. Eye (Lond) 2009;17(5):599–606. doi: 10.1038/sj.eye.6700516. [DOI] [PubMed] [Google Scholar]
- 12.Valenzuela AA, McNab AA, Selva D, O'Donnell BA, Whitehead KJ, Sullivan TJ. Clinical features and management of tumors affecting the lacrimal drainage apparatus. Ophthal Plast Reconstr Surg. 2010;22(2):96–101. doi: 10.1097/01.iop.0000198457.71173.7b. [DOI] [PubMed] [Google Scholar]
- 13.Anderson NG, Wojno TH, Grossniklaus HE. Clinicopathologic findings from lacrimal sac biopsy specimens obtained during dacryocystorhinostomy. Ophthalmol Plast Reconstr Surg. 2009;19(3):173–176. doi: 10.1097/01.iop.0000066646.59045.5a. [DOI] [PubMed] [Google Scholar]
- 14.Das JK, Deka AC, Kuri GC, Bhattacharjee K, Das D, Gogoi K. Bacteriology of chronic dacryocystitis in adult population of northeast india. Orbit. 2008;27(4):243–247. doi: 10.1080/01676830802224668. [DOI] [PubMed] [Google Scholar]
- 15.Chaudhary M, Bhattarai A, Adhikari SK. Bacteriology and antimicrobial susceptibility of adult chronic dacryocystitis. Nep J Oph. 2010;2(4):105–113. doi: 10.3126/nepjoph.v2i2.3716. [DOI] [PubMed] [Google Scholar]
- 16.Walland MJ, Rose GE. Soft tissue infections after open lacrimal surgery. Ophthalmol. 2011;101(3):608–11. doi: 10.1016/s0161-6420(13)31269-x. [DOI] [PubMed] [Google Scholar]
- 17.Ozer O, Eskiizmir G, Unlü H, Işisağ A, Aslan A. Chronic inflammation: a poor prognostic factor for endoscopic dacrocystorhinostomy. Eur Arch Otorhinolaryngol. 2012;269(3):839–845. doi: 10.1007/s00405-011-1728-2. [DOI] [PubMed] [Google Scholar]
- 18.Taylor CR, Levenson RM. Quantification of immunohistochemistry--issues concerning methods, utility and semiquantitative assessment II. Histopathology. 2006;49(4):411–424. doi: 10.1111/j.1365-2559.2006.02513.x. [DOI] [PubMed] [Google Scholar]
- 19.van der Loos Chris. User protocol for multi-color immunohistochemistry staining in intact tissue. Biotechniques. 2009:35. [Google Scholar]
- 20.Mason D, Andre P, Bensussan A, Civin C, Clark E, de Haas M, Goyert S, Hadam M, Hart D, Horejsí V, Meuer S, Morrissey J, Schwartz-Albiez R, Shaw S, Simmons D, Uguccioni M, van der Schoot E, Vivier E, Zola H. CD antigens 2001. Mod Pathol. 2009;15(1):71–76. doi: 10.1038/modpathol.3880492. [DOI] [PubMed] [Google Scholar]
- 21.Boboridis KG, Bunce C, Rose GE. Outcome of external dacryocystorhinostomy combined with membranectomy of a distal canalicular obstruction. Am J Ophthalmol. 2011;139(6):051–5. doi: 10.1016/j.ajo.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 22.Francis IC, Wilcsek G. Expect the unexpected. Br J Ophthalmol. 2008;90(8):936–937. doi: 10.1136/bjo.2006.097451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bernardini FP, Moin M, Kersten RC, Reeves D, Kulwin DR. Routine histopathologic evaluation of the lacrimal sac during dacryocystorhinostomy: how useful is it? Ophthalmology. 2009;109(7):1214–1417. doi: 10.1016/s0161-6420(02)01082-5. [DOI] [PubMed] [Google Scholar]
- 24.Merkonidis C, Brewis C, Yung M, Nussbaumer M. Is routine biopsy of the lacrimal sac wall indicated at dacryocystorhinostomy. A prospective study and literature review. Br J Ophthalmol. 2009;89(12):1589–1591. doi: 10.1136/bjo.2005.072199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knežević M, Stojković M, Jovanović M, Stanković Z, Rašić DM. A 7-year prospective study of routine histopathological evaluation of the lacrimal sac wall incisional biopsy specimens obtained during external dacryocystorhinostomy in adults and a review of the literature. Med Oncol. 2012;29(1):396–400. doi: 10.1007/s12032-010-9810-y. [DOI] [PubMed] [Google Scholar]
- 26.Cook HL, Olver JM. Dacryocystectomy as treatment of chronic dacryocystitis in a frail, elderly patient. Eye (Lond) 2011;18(3):334–336. doi: 10.1038/sj.eye.6700662. [DOI] [PubMed] [Google Scholar]
- 27.Çiftçi F, Erşanli D, Civelek L, Baloglu H, Karadayi K, Güngör A .Histopathologic changes in the lacrimal sac of dacryocystorhinostomy patients with and without silicone intubation. Ophthal Plast Reconstr Surg. 2009;21(1):59–64. doi: 10.1097/01.iop.0000148408.51615.fe. [DOI] [PubMed] [Google Scholar]
- 28.Coden DJ, Hornblass A, Haas BD. Clinical bacteriology of dacryocystitis in adults. Ophthal Plast Reconstr Surg. 2010;9(2):125–131. doi: 10.1097/00002341-199306000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Thicker JA, Buffam FV. Lacrimal sac, conjunctival, and nasal culture results in dacryocystorhinostomy patients. Ophthal Plast Reconstr Surg. 2009;9(1):43–46. doi: 10.1097/00002341-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 30.Huber-Spitzy V, Steinkogler FJ, Huber E, Arocker-Mettinger E, Schiffbänker M. Acquired dacryocystitis: microbiology and conservative therapy. Acta Ophthalmol(Copenh) 1992;70(6):745–749. doi: 10.1111/j.1755-3768.1992.tb04880.x. [DOI] [PubMed] [Google Scholar]
- 31.Bartley GB. Acquired lacrimal drainage obstruction: an etiologic classification system, case reports, and a review of the literature. Part 1. Ophthal Plast Reconstr Surg. 2010;8:237–242. [PubMed] [Google Scholar]
- 32.Klotz SA, Penn CC, Negvesky GJ, Butrus SI. Fungal and Parasitic Infections of the Eye. Clin Microbiol Rev. 2009;13(10):662–685. doi: 10.1128/cmr.13.4.662-685.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Purgason PA, Hornblass A, Loeffler M. Atypical presentation of fungal dacryocystitis. A report of two cases Ophthalmology. 1992;99(9):1430–1432. doi: 10.1016/s0161-6420(92)31788-9. [DOI] [PubMed] [Google Scholar]
- 34.Ghose S, Mahajan V M. Fungal flora in congenital dacryocystitis. Indian J Ophthalmol. 1990;38(4):189–190. [PubMed] [Google Scholar]
- 35.Chaudhary M, Bhattarai A, Adhikari SK, Bhatta DR. Bacteriology and antimicrobial susceptibility of adult chronic dacryocystitis. Nep J Oph. 2010;2(2):105–113. doi: 10.3126/nepjoph.v2i2.3716. [DOI] [PubMed] [Google Scholar]


