Abstract
AIM
To determine whether single nucleotide polymorphism (SNP) rs641153 is associated with the risk of age-related macular degeneration (AMD), we performed a systematic meta-analysis of 15 eligible studies. SNP in the complement factor B (CFB) gene is considered to have significant association with AMD susceptibility, but there is great discrepancy in these results.
METHODS
The eligible studies were identified by searching the databases of PubMed, EMBASE, and Web of Science. Odds ratios (ORs) with 95% confidence intervals (CIs) were used to assess the association. All data were analyzed using Stata software.
RESULTS
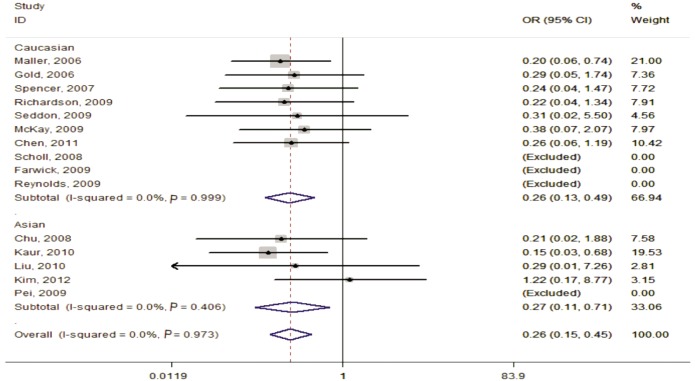
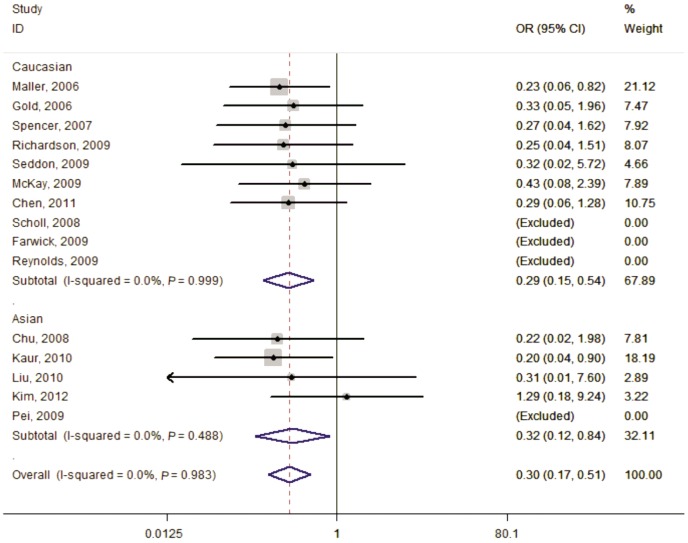
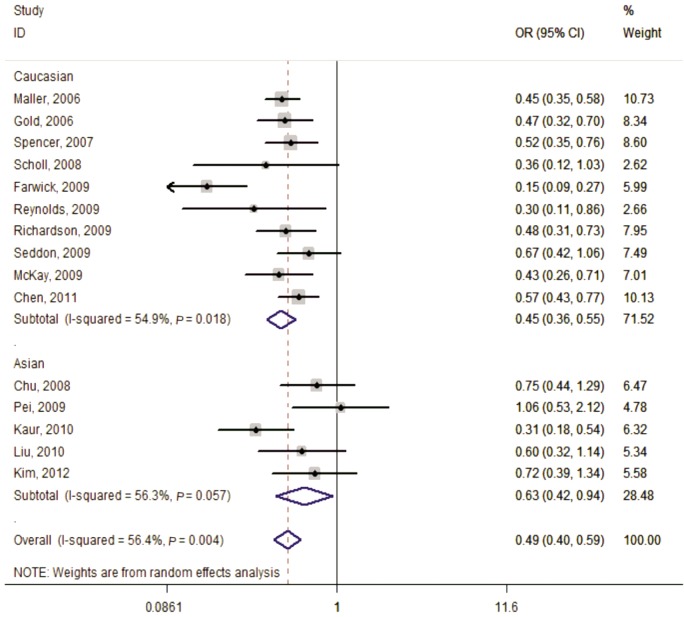
The association between rs641153 and AMD risk was statistically significant under the homozygous model (AA vs GG:OR=0.26, 95%CI=0.15-0.45, Ph=0.973, I2=0.0%, fixed effects), dominant model (AA+GA vs GG:OR=0.49, 95%CI=0.40-0.59, Ph=0.004, I2=56.4%, random effects) and recessive model (AA vs GA+GG:OR=0.30, 95%CI=0.17-0.51, Ph=0.983, I2=0.0%, fixed effects). The same results were also observed in the stratified analyses by ethnicity, source of control and sample size.
CONCLUSION
Our meta-analysis suggests that rs641153 in the CFB gene may play a protective role in AMD susceptibility, the late AMD in particular, both in Caucasians and in Asians.
Keywords: complement factor B, rs641153, age-related macular degeneration, meta-analysis
INTRODUCTION
Age-related macular degeneration (AMD), also known as age-related maculopathy (ARM), is the leading cause of irreversible blindness and a major public health threat in the elderly of western countries[1]. The prevalence of the disease increases significantly with age, accounting for approximately 4% among the population over 60 years and more than 10% of individuals older than 75[1],[2]. Environmental factors, such as smoking and exposure to chronic infection are involved in the onset of AMD[3]. Genetic susceptibility, however, plays a predominant role in the disease progression and aetiology[4],[5]. The strong genetic association with AMD directs widespread attention to the mechanism underlying the pathogenesis of this aggressive disease.
Common variants in a wide range of genes have been identified to have genetic contributions to AMD susceptibility[6]. The variants in complement factor H (CFH) gene as well as the single nucleotide polymorphisms (SNPs) in HTRA1 and ARMS2 genes are strongly related to AMD[7]-[10]. The discovery of CFH variants and HTRA1 and ARMS2 SNPs in the etiology of AMD has promoted many following investigations on other genes of the complement cascade, including C2, C3 and complement factor B (CFB)[11]-[13].
CFB, located downstream on human chromosome 6p21, contains a common SNP rs641153 that is closely correlated with AMD susceptibility[14]-[20]. Nevertheless, no significant association with the risk of AMD is simultaneously shown in accumulated investigations[21]-[25]. Whether the rs641153 in CFH gene is truly involved in AMD remains to be elucidated. Hence in the present study, we hypothesized that rs641153 might modify the risk of AMD. To test this hypothesis, we performed a meta-analysis of 15 case-control studies.
SUBJECTS AND METHODS
Identification and Eligibility of Relevant Studies
We systematically identified articles pertaining to rs641153 and AMD in PubMed, EMBASE, and Web of Science databases, using the keywords “CFB”, “R32Q” or “rs641153”, “polymorphism” or “polymorphisms”, and “age-related macular degeneration” or “AMD” (the last search was updated on February 19, 2013). The search was restricted to English language. Additional relevant articles were identified through screening the reference lists of the retrieved studies and journals that are known to publish related articles. When several publications included the same population, only the most recent or the largest study was used in this meta-analysis. We selected the studies based on the following criteria: 1) evaluation of rs641153 and AMD risk; 2) a case-control study; 3) the genotype distribution of controls is in accordance with Hardy-Weinberg equilibrium (HWE); and 4) contains sufficient genotype data to calculate the odds ratios (ORs) with 95% confidence intervals (CIs).
Data Extraction
Data extraction was performed by two investigators independently and consensus was reached on all items. The following information was recorded from each article: first author, year of publication, country of study, ethnicity (Caucasian or Asian), source of control (population- or hospital-based controls), allele and genotype frequencies in cases and controls and numbers of cases and controls. For studies including populations of different ethnicities, we extracted the data separately and categorized them into Caucasians or Asians.
Statistical Analysis
The numbers of allele and genotype frequencies in cases and controls were extracted from each study to estimate the risk of AMD development by ORs and 95%CIs. We further performed subgroup analyses by ethnicity, source or controls, stage of AMD (early/late AMD) and sample size (<500 or >500). HWE was detected for control subjects of each study, using the goodness-of-fit χ2-test (P<0.10 was considered representative of departure from HWE). ORs with 95%CIs were used to assess the strength of association between rs641153 and the risk of AMD. The pooled ORs were calculated under homozygous model (AA vs GG), dominant model (AA+GA vs GG) and recessive model (AA vs GA+GG).
The Chi-square-based Q-test was performed to assess heterogeneity across studies and P<0.10 suggested presence of significant heterogeneity[26]. Between-study heterogeneity was also quantified with the I2 statistic, which takes values between 0% and 100% with higher values denoting greater degree of heterogeneity (I2=0-25%: no heterogeneity; I2=25%-50%: moderate heterogeneity; I2=50%-75%: large heterogeneity; I2=75%-100%: extreme heterogeneity)[27]. Both fixed effects model and random effects model were applied for the pooled ORs. The fixed-effects model (Mantel-Haenszel method) was used if P>0.10, which assumes the same homogeneity of effect size across all studies[28]; otherwise, the random-effects model (DerSimonian and Laird method) was more appropriate, which tends to provide wider 95%CIs when differences occur in the results of the constituent studies[29].
To determine the influence of independent studies on the overall AMD risk, sensitivity analysis was performed by meta-analyses of omitting each study, one at a time and recalculating the ORs and 95%CIs. Potential publication bias was assessed by Begg's funnel plot and Egger's test, P<0.10 was considered significant[30].
All statistical data were done using Stata software (version 12.0, Stata Corp LP, College Station, TX, USA). A significant P value was defined at 0.10.
RESULTS
Characteristics of Studies
A total of 97 potentially relevant articles were extracted by initial search in PubMed, EMBASE, and Web of Science. Among them, 23 were subjected to further examination. After reading the full text, eight studies were ultimately excluded. Three of them did not provide available genotype data[11],[31]-[34]; one contained duplicate information that had been included in the present meta-analysis[35]; one was a case-only study[13]; one was a comment letter[36]. The flow chart for study selection is presented in full detail in Figure 1.
Figure 1. The flow diagram of included/excluded studies.
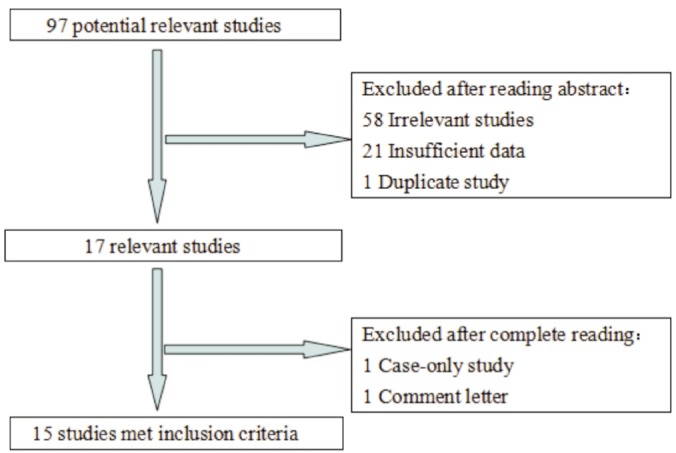
In total, there were 15 eligible studies[14]-[25],[37]-[39] for rs641153 with 6 712 cases and 4 669 controls in this meta-analysis. The main characteristics of all included studies are listed in Table 1, including first author, publication date, ethnicity, source of controls, and allele and HWE. No derivation from HWE was indicated in the controls of each study included in the current meta-analysis.
Table 1. Characteristics of all studies included in meta-analysis.
| First author | Year | Ethnicity | Control source | Case |
Control |
HWE | ||||||||||
| Total | AA | GA | GG | A | G | Total | AA | GA | GG | A | G | |||||
| Maller[37] | 2006 | Caucasian | PCC | 1238 | 3 | 106 | 1129 | 112 | 2364 | 934 | 10 | 171 | 753 | 191 | 1677 | 0.933 |
| Gold[16] | 2006 | Caucasian | PCC | 551 | 2 | 52 | 497 | 56 | 1046 | 269 | 3 | 53 | 213 | 59 | 479 | 0.883 |
| Spencer[17] | 2007 | Caucasian | HCC | 698 | 2 | 66 | 630 | 70 | 1326 | 282 | 3 | 50 | 229 | 56 | 508 | 0.883 |
| Scholl[14] | 2008 | Caucasian | HCC | 112 | 0 | 6 | 106 | 6 | 218 | 67 | 0 | 10 | 57 | 10 | 124 | 0.509 |
| Chu[21] | 2008 | Asian | HCC | 144 | 1 | 30 | 113 | 32 | 256 | 126 | 4 | 32 | 90 | 40 | 212 | 0.582 |
| Farwick[22] | 2009 | Caucasian | PCC | 776 | 0 | 26 | 750 | 26 | 1526 | 119 | 0 | 26 | 93 | 26 | 212 | 0.181 |
| Reynolds[38] | 2009 | Caucasian | ND | 103 | 0 | 6 | 97 | 6 | 200 | 57 | 0 | 11 | 46 | 11 | 103 | 0.420 |
| Richardson[15] | 2009 | Caucasian | PCC | 529 | 2 | 54 | 473 | 58 | 1000 | 199 | 3 | 41 | 155 | 47 | 351 | 0.878 |
| Seddon[23] | 2009 | Caucasian | ND | 279 | 0 | 23 | 256 | 23 | 535 | 1167 | 6 | 138 | 1023 | 150 | 2184 | 0.566 |
| McKay[39] | 2009 | Caucasian | PCC | 271 | 2 | 25 | 244 | 29 | 513 | 235 | 4 | 50 | 181 | 58 | 412 | 0.799 |
| Pei[18] | 2009 | Asian | HCC | 123 | 0 | 18 | 105 | 18 | 228 | 130 | 0 | 18 | 112 | 18 | 242 | 0.396 |
| Kaur[19] | 2010 | Asian | HCC | 162 | 2 | 18 | 142 | 22 | 302 | 158 | 10 | 53 | 95 | 73 | 243 | 0.483 |
| Liu[24] | 2010 | Asian | HCC | 238 | 0 | 17 | 221 | 17 | 459 | 220 | 1 | 25 | 194 | 27 | 413 | 0.841 |
| Chen[20] | 2011 | Caucasian | HCC | 1335 | 3 | 128 | 1204 | 134 | 2536 | 509 | 4 | 83 | 422 | 91 | 927 | 0.971 |
| Kim[25] | 2012 | Asian | PCC | 153 | 2 | 16 | 135 | 20 | 286 | 197 | 2 | 30 | 165 | 34 | 360 | 0.630 |
PCC: Population-based case-control study; HCC: Hospital-based case-control study; ND: Not defined; HWE: Hardy-Weinberg equilibrium.
Quantitative Synthesis
When pooling all eligible studies into one dataset for the meta-analysis, we found statistical evidence for an association between rs641153 and overall reduced risk of AMD under the homozygous model (AA vs GG: OR=0.26, 95%CI=0.15-0.45, Ph=0.973, I2=0.0%, fixed effects), dominant model (AA+GA vs GG: OR=0.49, 95%CI=0.40-0.59, Ph=0.004, I2=56.4%, random effects) and recessive model (AA vs GA+GG: OR=0.30, 95%CI=0.17-0.51, Ph=0.983, I2=0.0%, fixed effects). Significant decreased risk was also showed under all of the analyzed comparisons in the analysis restrained to late AMD studies (Table 2).
Table 2. Meta-analyses of SNP rs641153 and risk of AMD in each subgroup.
| Variables | n (case/control) | AA vs GG |
AA+GA vs GG |
AA vs GA+GG |
||||||
| OR (95%CI) | Ph | I2 | OR (95%CI) | Ph | I2 | OR (95%CI) | Ph | I2 | ||
| Ethnicity | ||||||||||
| Caucasian | 5892/3838 | 0.26 (0.13, 0.49) | 0.999 | 0.0% | 0.45 (0.36, 0.55) | 0.018 | 54.9% | 0.29 (0.15, 0.54) | 0.999 | 0.0% |
| Asian | 820/831 | 0.27 (0.11, 0.71) | 0.406 | 0.0% | 0.63 (0.42, 0.94) | 0.057 | 56.3% | 0.32 (0.12, 0.84) | 0.488 | 0.0% |
| Source of controls | ||||||||||
| PCC | 3518/1953 | 0.32 (0.15, 0.65) | 0.653 | 0.0% | 0.42 (0.31, 0.57) | 0.008 | 68.2% | 0.35 (0.17, 0.73) | 0.679 | 0.0% |
| HCC | 2812/1492 | 0.21 (0.09, 0.47) | 0.984 | 0.0% | 0.56 (0.44, 0.72) | 0.135 | 38.5% | 0.24 (0.10, 0.54) | 0.997 | 0.0% |
| ND | 382/1224 | 0.31 (0.02, 5.50) | NA | NA | 0.52 (0.25, 1.07) | 0.173 | 46.2% | 0.32 (0.02, 5.72) | NA | NA |
| Sample size | ||||||||||
| <500 | 1585/2357 | 0.23 (0.12, 0.48) | 0.998 | 0.0% | 0.43 (0.33, 0.57) | 0.005 | 69.9% | 0.26 (0.13, 0.53) | 0.998 | 0.0% |
| >500 | 5127/1314 | 0.30 (0.13, 0.66) | 0.705 | 0.0% | 0.56 (0.43, 0.73) | 0.106 | 39.3% | 0.34 (0.15, 0.76) | 0.778 | 0.0% |
| Stage | ||||||||||
| Late | 3646/3355 | 0.31 (0.15, 0.62) | 0.778 | 0.0% | 0.55 (0.48, 0.64) | 0.158 | 33.9% | 0.34 (0.17, 0.68) | 0.789 | 0.0% |
| Total | 6712/4669 | 0.26 (0.15, 0.45) | 0.973 | 0.0% | 0.49 (0.40, 0.59) | 0.004 | 56.4% | 0.30 (0.17, 0.51) | 0.983 | 0.0% |
PCC: Population-based case-control study; HCC: Hospital-based case-control study; ND: Not defined; NA: Not available; Ph: P value of heterogeneity test; CI: Confidence interval; OR: Odds ratio.
In the stratified analysis according to ethnicity, the risks for the AA genotype, compared with the GG genotype, were 0.26 (95%CI=0.13-0.49, Ph=0.999, I2=0.0%, fixed effects) in Caucasians and 0.27 (95%CI=0.11-0.71, Ph=0.406, I2=0.0%, fixed effects) among Asians. A significantly decrease in AMD risk between rs641153 and the analyzed subgroups was also seen in dominant model and recessive model (Table 2).
Stratifying by control source and sample size indicated that the risk of AMD was decreased both in population-based case-control studies and in hospital-based case-control studies, and that for rs641153, the sample sizes was not a modifier of the reduced risk observed in the above analyses (Table 2).
Heterogeneity and Sensitivity Analysis
No substantial heterogeneity was detected in the three genetic models except for the dominant model (Ph=0.004, I2=56.4%) and the random-effects model was selected for the pooled OR. Subsequently, the leave-one-out sensitivity analysis was performed to identify the heterogeneity source. The results indicated two datasets may constitute the main contribution to the obvious heterogeneity across the studies[19],[22]. The exclusion of these two articles increased homogeneity among the remaining studies (Ph=0.438, I2=0.8%). The general result, however, was not significantly changed with or without them (OR=0.53, 95%CI=0.47-0.60) (Figures 2–4).
Figure 2. Forest plot of AMD risk associated with SNP rs641153 (AA vs GG) in the stratified analyses by ethnicity.
The squares and horizontal lines correspond to the study-specific OR and 95%CI. The area of the squares reflects the weight (inverse of the variance). The diamond represents the summary OR (0.26) and 95%CI (0.15, 0.45).
Figure 4. Forest plot of AMD risk associated with SNP rs641153 (AA vs GA+GG) in the stratified analyses by ethnicity.
The squares and horizontal lines correspond to the study-specific OR and 95%CI. The area of the squares reflects the weight (inverse of the variance). The diamond represents the summary OR (0.30) and 95%CI (0.17, 0.51).
Figure 3. Forest plot of AMD risk associated with SNP rs641153 (AA+GA vs GG) in the stratified analyses by ethnicity.
The squares and horizontal lines correspond to the study-specific OR and 95%CI. The area of the squares reflects the weight (inverse of the variance). The diamond represents the summary OR (0.49) and 95%CI (0.40, 0.59).
Publication Bias
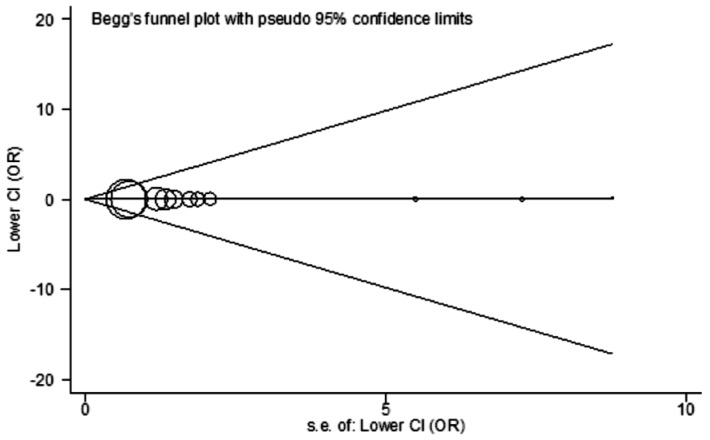
Begg's funnel plot and Egger's test were performed to determine the publication bias of included studies. Statistical evidence of publication bias was showed neither in the funnel plot nor in the Egger's test (t=0.45, P=0.666 for AA vs GG) (Figure 5).
Figure 5. Begg's funnel plot for publication bias test (AA vs GG).
Each point represents a separate study for the indicated association. Log[OR]: Natural logarithm of the odds ratio; Horizontal line: Mean effect size.
DISCUSSION
We performed a systematic meta-analysis of 15 eligible studies involving 6 712 cases and 4 669 controls. We found a significant decrease of AMD risk in the general analysis (26% under the homozygous model, 49% under the dominant model and 32% under the recessive model). Due to the incomplete data from three studies for analyses of the AA vs GG and the AA vs GA+GG genetic models (for example, in the AA vs GG genetic model, AA genotype frequency for all of the studies are 0 in both cases and controls), they were automatically excluded when performing meta-analysis and the results were summarized in pooling data consisting of 12 publications[14],[22],[38]. The protective effects remained stable when analysis was restrained to the studies focusing on late AMD. Several reports have indicated that inflammatory processes may play a central role in the development of AMD by inducing the formation of drusen, which is an important characteristic of early AMD[40],[41]. Therefore, we can conclude that rs641153 in the CFB gene may act as an inhibitor against AMD progression, the late AMD in particular by suppressing inflammation.
Meta-analysis is deemed as an important tool with strong statistical power in defining the association of selected genetic polymorphisms with the risk of disease and identifying potential between-study heterogeneity. A previously published meta-analysis based on 8 case-control studies, showed a tendency for strong protective effects on AMD risk, which was especially exhibited in Caucasians[42]. Following this study, a larger assessment including 14 case-control publications provided a robust estimate of the protective association of rs641153 with AMD, with an absolute lowering risk among Caucasian populations[43]. However, in our meta-analysis, we found strongly protective impact on the risk of AMD in Caucasians, as well as in Asians. Since rs641153 is common among Caucasians and Asians, and there is no obvious ethnic difference in the associations with AMD risk, the biological significance of this SNP may be comparable in different ethnic groups.
While most studies in current meta-analysis have already been included in the meta-analysis of the two published papers and no obvious new information is provided, there are some differences. First, compared to the reference 43 (the larger study of the two published papers), new data have been included in our meta-analysis, which helps to enlarge the sample size of the general and subgroup analysis, thus deriving a more estimate of the true association[25]. Second, for a meta-analysis involving a large number of subjects, accuracy of data is critically important. By carefully checking the published papers, especially the reference 43, some data included in the meta-analysis are not matching those provided in the original article (for example, reference 39), which may increase the chance of false positives.
Consistent with the protective effects indicated in the general analysis, stratification analyses according to source of control and sample size suggested that there was significant association between rs641153 and susceptibility to AMD. It is especially important to use typically representative populations and large sample sizes for genetic association studies, which could contribute to a more precise estimation. There was no apparent difference in the results in the subgroups of control source and sample size, implying that rs641153 itself may have a strong protective role in the development and progression of AMD.
Although our meta-analysis is based on all eligible case-control studies to date and indicated no significant publication bias facilitating the accuracy and credibility of the results, some limitations should be highlighted. First, lack of the original data from the included studies limited further assessment of potential interactions between gene-to-gene and gene-to-environment, because such interactions may modify AMD risk. Second, significant heterogeneity detected in the dominant model may have influenced the results, although no statistical evidence was indicated in the meta-analysis. Third, the included case-control studies were carried out among Caucasians or Asians, thus the results from this meta-analysis may not be applicable to other ethnic groups.
Despite these limitations, our meta-analysis provided evidence of significant association between rs641153 and AMD risk, supporting the hypothesis that this SNP confers protection to the susceptibility to AMD, especially the late AMD. To confirm our findings, further well-designed studies including gene-to-gene and gene-to-environment interactions in diverse ethnic populations are necessary.
REFERENCES
- 1.Friedman DS, O'Colmain BJ, Muñoz B, Tomany SC, McCarty C, de Jong PT, Nemesure B, Mitchell P, Kempen J. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122(4):564–572. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 2.Vingerling JR, Dielemans I, Hofman A, Grobbee DE, Hijmering M, Kramer CF, de Jong PT. The prevalence of age-related maculopathy in the Rotterdam Study. Ophthalmology. 1995;102(2):205–210. doi: 10.1016/s0161-6420(95)31034-2. [DOI] [PubMed] [Google Scholar]
- 3.Baird PN, Robman LD, Richardson AJ, Dimitrov PN, Tikellis G, McCarty CA, Guymer RH. Gene-environment interaction in progression of AMD: the CFH gene, smoking and exposure to chronic infection. Hum Mol Genet. 2008;17(9):1299–1305. doi: 10.1093/hmg/ddn018. [DOI] [PubMed] [Google Scholar]
- 4.Klaver CC, Wolfs RC, Assink JJ, van Duijn CM, Hofman A, de Jong PT. Genetic risk of age-related maculopathy. Population-based familial aggregation study. Arch Ophthalmol. 1998;116(12):1646–1651. doi: 10.1001/archopht.116.12.1646. [DOI] [PubMed] [Google Scholar]
- 5.Seddon JM, Cote J, Page WF, Aggen SH, Neale MC. The US twin study of age-related macular degeneration: relative roles of genetic and environmental influences. Arch Ophthalmol. 2005;123(3):321–327. doi: 10.1001/archopht.123.3.321. [DOI] [PubMed] [Google Scholar]
- 6.Swaroop A, Chew EY, Rickman CB, Abecasis GR. Unraveling a multifactorial late-onset disease: from genetic susceptibility to disease mechanisms for age-related macular degeneration. Annu Rev Genomics Hum Genet. 2009;10:19–43. doi: 10.1146/annurev.genom.9.081307.164350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haines JL, Hauser MA, Schmidt S, Scott WK, Olson LM, Gallins P, Spencer KL, Kwan SY, Noureddine M, Gilbert JR, Schnetz-Boutaud N, Agarwal A, Postel EA, Pericak-Vance MA. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308(5720):419–421. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- 8.Klein RJ, Zeiss C, Chew EY, Tsai JY, Sackler RS, Haynes C, Henning AK, SanGiovanni JP, Mane SM, Mayne ST, Bracken MB, Ferris FL, Ott J, Barnstable C, Hoh J. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308(5720):385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dewan A, Liu M, Hartman S, Zhang SS, Liu DT, Zhao C, Tam PO, Chan WM, Lam DS, Snyder M, Barnstable C, Pang CP, Hoh J. HTRA1 promoter polymorphism in wet age-related macular degeneration. Science. 2006;314(5801):989–992. doi: 10.1126/science.1133807. [DOI] [PubMed] [Google Scholar]
- 10.Rivera A, Fisher SA, Fritsche LG, Keilhauer CN, Lichtner P, Meitinger T, Weber BH. Hypothetical LOC387715 is a second major susceptibility gene for age-related macular degeneration, contributing independently of complement factor H to disease risk. Hum Mol Genet. 2005;14(21):3227–3236. doi: 10.1093/hmg/ddi353. [DOI] [PubMed] [Google Scholar]
- 11.Nakata I, Yamashiro K, Yamada R, Gotoh N, Nakanishi H, Hayashi H, Akagi-Kurashige Y, Tsujikawa A, Otani A, Saito M, Iida T, Oishi A, Matsuo K, Tajima K, Matsuda F, Yoshimura N. Significance of C2/CFB variants in age-related macular degeneration and polypoidal choroidal vasculopathy in a Japanese population. Invest Ophthalmol Vis Sci. 2012;53(2):794–798. doi: 10.1167/iovs.11-8468. [DOI] [PubMed] [Google Scholar]
- 12.Yates JR, Sepp T, Matharu BK, Khan JC, Thurlby DA, Shahid H, Clayton DG, Hayward C, Morgan J, Wright AF, Armbrecht AM, Dhillon B, Deary IJ, Redmond E, Bird AC, Moore AT. Complement C3 variant and the risk of age-related macular degeneration. N Engl J Med. 2007;357(6):553–561. doi: 10.1056/NEJMoa072618. [DOI] [PubMed] [Google Scholar]
- 13.Mantel I, Ambresin A, Moetteli L, Droz I, Roduit R, Munier FL, Schorderet DF. Complement factor B polymorphism and the phenotype of early age-related macular degeneration. Ophthalmic Genet. 2013 doi: 10.3109/13816810.2013.766217. [DOI] [PubMed] [Google Scholar]
- 14.Scholl HP, Charbel Issa P, Walier M, Janzer S, Pollok-Kopp B, Börncke F, Fritsche LG, Chong NV, Fimmers R, Wienker T, Holz FG, Weber BH, Oppermann M. Systemic complement activation in age-related macular degeneration. PLoS One. 2008;3(7):e2593. doi: 10.1371/journal.pone.0002593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richardson AJ, Islam FM, Guymer RH, Baird PN. Analysis of rare variants in the complement component 2 (C2) and factor B (BF) genes refine association for age-related macular degeneration (AMD) Invest Ophthalmol Vis Sci. 2009;50(2):540–543. doi: 10.1167/iovs.08-2423. [DOI] [PubMed] [Google Scholar]
- 16.Gold B, Merriam JE, Zernant J, Hancox LS, Taiber AJ, Gehrs K, Cramer K, Neel J, Bergeron J, Barile GR, Smith RT, AMD Genetics Clinical Study Group. Hageman GS, Dean M, Allikmets R. Variation in factor B (BF) and complement component 2 (C2) genes is associated with age-related macular degeneration. Nat Genet. 2006;38(4):458–462. doi: 10.1038/ng1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spencer KL, Hauser MA, Olson LM, Schmidt S, Scott WK, Gallins P, Agarwal A, Postel EA, Pericak-Vance MA, Haines JL. Protective effect of complement factor B and complement component 2 variants in age-related macular degeneration. Hum Mol Genet. 2007;16(16):1986–1992. doi: 10.1093/hmg/ddm146. [DOI] [PubMed] [Google Scholar]
- 18.Pei XT, Li XX, Bao YZ, Yu WZ, Yan Z, Qi HJ, Qian T, Xiao HX. Association of c3 gene polymorphisms with neovascular age-related macular degeneration in a chinese population. Curr Eye Res. 2009;34(8):615–622. doi: 10.1080/02713680903003484. [DOI] [PubMed] [Google Scholar]
- 19.Kaur I, Katta S, Reddy RK, Narayanan R, Mathai A, Majji AB, Chakrabarti S. The involvement of complement factor B and complement component C2 in an Indian cohort with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2010;51(1):59–63. doi: 10.1167/iovs.09-4135. [DOI] [PubMed] [Google Scholar]
- 20.Chen Y, Zeng J, Zhao C, Wang K, Trood E, Buehler J, Weed M, Kasuga D, Bernstein PS, Hughes G, Fu V, Chin J, Lee C, Crocker M, Bedell M, Salasar F, Yang Z, Goldbaum M, Ferreyra H, Freeman WR, Kozak I, Zhang K. Assessing susceptibility to age-related macular degeneration with genetic markers and environmental factors. Arch Ophthalmol. 2011;129(3):344–351. doi: 10.1001/archophthalmol.2011.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chu J, Zhou CC, Lu N, Zhang X, Dong FT. Genetic variants in three genes and smoking show strong associations with susceptibility to exudative age-related macular degeneration in a Chinese population. Chin Med J (Engl) 2008;121(24):2525–2533. [PubMed] [Google Scholar]
- 22.Farwick A, Dasch B, Weber BH, Pauleikhoff D, Stoll M, Hense HW. Variations in five genes and the severity of age-related macular degeneration: results from the Muenster aging and retina study. Eye (Lond) 2009;23(12):2238–2244. doi: 10.1038/eye.2008.426. [DOI] [PubMed] [Google Scholar]
- 23.Seddon JM, Reynolds R, Maller J, Fagerness JA, Daly MJ, Rosner B. Prediction model for prevalence and incidence of advanced age-related macular degeneration based on genetic, demographic, and environmental variables. Invest Ophthalmol Vis Sci. 2009;50(5):2044–2053. doi: 10.1167/iovs.08-3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu X, Zhao P, Tang S, Lu F, Hu J, Lei C, Yang X, Lin Y, Ma S, Yang J, Zhang D, Shi Y, Li T, Chen Y, Fan Y, Yang Z. Association study of complement factor H, C2, CFB, and C3 and age-related macular degeneration in a Han Chinese population. Retina. 2010;30(8):1177–1184. doi: 10.1097/IAE.0b013e3181cea676. [DOI] [PubMed] [Google Scholar]
- 25.Kim SJ, Lee SJ, Kim NR, Chin HS. Association of polymorphisms in C2, CFB and C3 with exudative age-related macular degeneration in a Korean population. Exp Eye Res. 2012;96(1):42–47. doi: 10.1016/j.exer.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 26.Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127(9):820–826. doi: 10.7326/0003-4819-127-9-199711010-00008. [DOI] [PubMed] [Google Scholar]
- 27.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22(4):719–748. [PubMed] [Google Scholar]
- 29.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 30.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cipriani V, Matharu BK, Khan JC, Shahid H, Stanton CM, Hayward C, Wright AF, Bunce C, Clayton DG, Moore AT, Yates JR. Genetic variation in complement regulators and susceptibility to age-related macular degeneration. Immunobiology. 2012;217(2):158–161. doi: 10.1016/j.imbio.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hughes AE, Mullan GM, Bradley DT. Complement factor B polymorphism 32W protects against age-related macular degeneration. Mol Vis. 2011;17:983–988. [PMC free article] [PubMed] [Google Scholar]
- 33.Yu Y, Reynolds R, Rosner B, Daly MJ, Seddon JM. Prospective assessment of genetic effects on progression to different stages of age-related macular degeneration using multistate Markov models. Invest Ophthalmol Vis Sci. 2012;53(3):1548–1556. doi: 10.1167/iovs.11-8657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van de Ven JP, Smailhodzic D, Boon CJ, Fauser S, Groenewoud JM, Chong NV, Hoyng CB, Klevering BJ, den Hollander AI. Association analysis of genetic and environmental risk factors in the cuticular drusen subtype of age-related macular degeneration. Mol Vis. 2012;18:2271–2278. [PMC free article] [PubMed] [Google Scholar]
- 35.Liu K, Chen LJ, Tam PO, Shi Y, Lai TY, Liu DT, Chiang SW, Yang M, Yang Z, Pang CP. Associations of the C2-CFB-RDBP-SKIV2L locus with age-related macular degeneration and polypoidal choroidal vasculopathy. Ophthalmology. 2013;120(4):837–843. doi: 10.1016/j.ophtha.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 36.Hughes AE, Bradley DT. RE: “The association between complement component 2/complement factor B polymorphisms and age-related macular degeneration: a huge review and meta-analysis’’. Am J Epidemiol. 2013 doi: 10.1093/aje/kwt067. [DOI] [PubMed] [Google Scholar]
- 37.Maller J, George S, Purcell S, Fagerness J, Altshuler D, Daly MJ, Seddon JM. Common variation in three genes, including a noncoding variant in CFH, strongly influences risk of age-related macular degeneration. Nat Genet. 2006;38(9):1055–1059. doi: 10.1038/ng1873. [DOI] [PubMed] [Google Scholar]
- 38.Reynolds R, Hartnett ME, Atkinson JP, Giclas PC, Rosner B, Seddon JM. Plasma complement components and activation fragments: associations with age-related macular degeneration genotypes and phenotypes. Invest Ophthalmol Vis Sci. 2009;50(12):5818–5827. doi: 10.1167/iovs.09-3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McKay GJ, Silvestri G, Patterson CC, Hogg RE, Chakravarthy U, Hughes AE. Further assessment of the complement component 2 and factor B region associated with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2009;50(2):533–539. doi: 10.1167/iovs.08-2275. [DOI] [PubMed] [Google Scholar]
- 40.Anderson DH, Mullins RF, Hageman GS, Johnson LV. A role for local inflammation in the formation of drusen in the aging eye. Am J Ophthalmol. 2002;134(3):411–431. doi: 10.1016/s0002-9394(02)01624-0. [DOI] [PubMed] [Google Scholar]
- 41.Bok D. Evidence for an inflammatory process in age-related macular degeneration gains new support. Proc Natl Acad Sci U S A. 2005;102(20):7053–7054. doi: 10.1073/pnas.0502819102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun C., Zhao M., Li X. CFB/C2 gene polymorphisms and risk of age-related macular degeneration: a systematic review and meta-analysis. Curr Eye Res. 2012;37(4):259–271. doi: 10.3109/02713683.2011.635401. [DOI] [PubMed] [Google Scholar]
- 43.Thakkinstian A, McEvoy M, Chakravarthy U, Chakrabarti S, McKay GJ, Ryu E, Silvestri G, Kaur I, Francis P, Iwata T, Akahori M, Arning A, Edwards AO, Seddon JM, Attia J. The association between complement component 2/complement factor B polymorphisms and age-related macular degeneration: a HuGE review and meta-analysis. Am J Epidemiol. 2012;176(5):361–372. doi: 10.1093/aje/kws031. [DOI] [PMC free article] [PubMed] [Google Scholar]