Abstract
AIM
To determine the clinical features, diagnosis and treatment of the primary Sjögren syndrome (SS) related optic neuritis.
METHODS
The clinical data of 8 patients (12 eyes) with primary SS related optic neuritis were analyzed retrospectively.
RESULTS
Eight of 128 consecutive patients with optic neuritis resulted from varied causes fulfilled the diagnostic criteria for the primary SS. They presented initially with the signs and symptoms of non-specific optic neuritis, and 5 patients presenting without dryness showed a chronic inflammation of submandibular gland or parotid gland, and lymphocyte infiltration was demonstrated by labial gland biopsy in 2 patients. There were serum positive titers for anti-Sjögren syndrome A (SSA) in 7 patients and anti-Sjögren syndrome B (SSB) in 8 patients. Anti-aquaporin-4 (AQP4) antibody was negative in all the 8 patients. Both glucocorticoids and immunosuppressive agent were administered, and visual acuity elevated in 8 eyes (66.7%), 3 patients (37.5%) recurred in the follow-up.
CONCLUSION
Primary SS related optic neuritis is less common and easily misdiagnosed. The conventional therapies for optic neuritis could not control the recurrence.
Keywords: optic neuritis, primary Sjögren's syndrome, anti-aquaporin-4
INTRODUCTION
Sjögren's syndrome (SS) is a chronic, progressive autoimmune disease characterized by periductal lymphocytic infiltration of the secretory exocrine glands, in particular the salivary and lacrimal glands[1],[2]. The disease can be alone as primary SS or in a background of other autoimmune diseases as secondary SS[3]. Despite the dryness of the mucous membranes throughout the body are usually prominent features of the disease, the systemic manifestations resulted from inflammation or vasculitis, including neurological disorders, may also be found in patients with SS[4],[5]. In this paper, we retrospectively studied the clinical features, diagnosis, treatment and follow-up in the primary SS related optic neuritis.
SUBJECTS AND METHODS
Subjects
Totally 128 patients with optic neuritis resulted from varied causes were treated in the Neuro ophthalmology of the General Hospital of Chinese People's Liberation Army (PLA) from February 2011 to November 2012. Among 128 patients, 8 patients (6.3%, 12 eyes) diagnosed as the primary SS related optic neuritis were recruited in the present study. They were all females and ranged in age from 15 to 56 years (average 34.7±18.4 years). Patients were diagnosed with primary SS according to the new international consensus for diagnosis[6]. This study was approved by the PLA General Hospital Institutional Review Board and informed consent was obtained from all patients.
Methods
Besides ophthalmology examinations, laboratory blood tests including rheumatoid factor (RF), anti-nuclear antibodies (ANA), anti-Sjögren syndrome A (SSA), anti-Sjögren syndrome B (SSB) antigen, and the water channel aquaporin-4 (AQP4) antibody, were performed on admission. The other auxiliary examination mainly included the schirmer's test, submandibular gland ultrasonic inspection, labial gland biopsy, and magnetic resonance imaging of head and spinal cord.
All 128 patients received a standard treatment based on the Optic Neuritis Treatment Trial (ONTT), i.e. a 250-1000mg daily intravenous methylprednisolone over 3 consecutive days, followed by oral prednisone initial with 1mg/kg per day for 11d and gradually reduced their dosage[7],[8]. Once diagnosed of primary SS, they also received an oral administration of azathioprine. After being discharged, all patients were followed up 3 months to 1 year (7.8±2.1months).
Statistical Analysis
All quantitative data were expressed as the mean±SD and analyzed using a Student's t-test, P<0.05 was considered statistically significant.
RESULTS
In this study, all the 8 patients presented with the initial acute symptom of blurred vision, and the painful visual loss occurred in 3 eyes (25%, 3 of 12). The central or pericentral scotoma of the visual deficit occurred in 7 eyes (58.3%). No other systematic performance was seen besides dry eye and dry mouth in 3 patients. The relative afferent pupillary defect (RAPD) presented in 8 eyes (66.7%), and the visual evoked potential (VEP) testing showed that mean VEP P100 latency values were 127.8±11.7ms for these 12 eyes, which was significantly prolonged when compared to our laboratory references of 103.4±8.9ms (P<0.01).
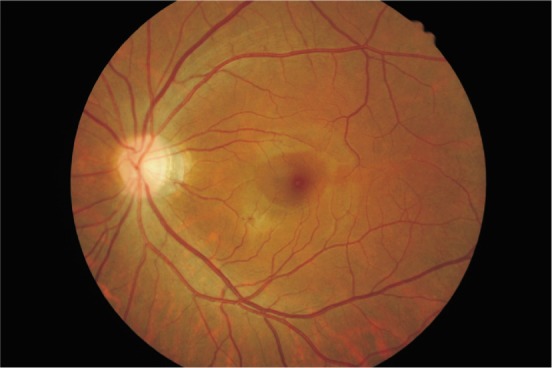
The course of disease varied from 2 weeks to 11 years. Three patients suffered the first monocular onset, 1 patient developed 2 times of the monocular relapse, and another 4 patients showed 2-4 times of binocular relapse. Three eyes recurring repeatedly developed optic atrophy, and optic disc vasculitis was seen in another 9 eyes (Figures 1, 2).
Figure 1. The optic disk was pale in the right eye of a patient with 3 times of relapse.
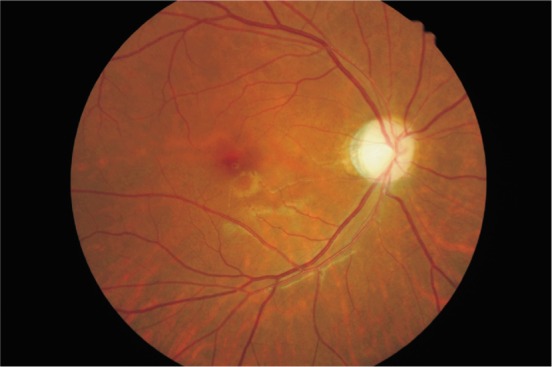
Figure 2. The optic disk was congested in a patient.
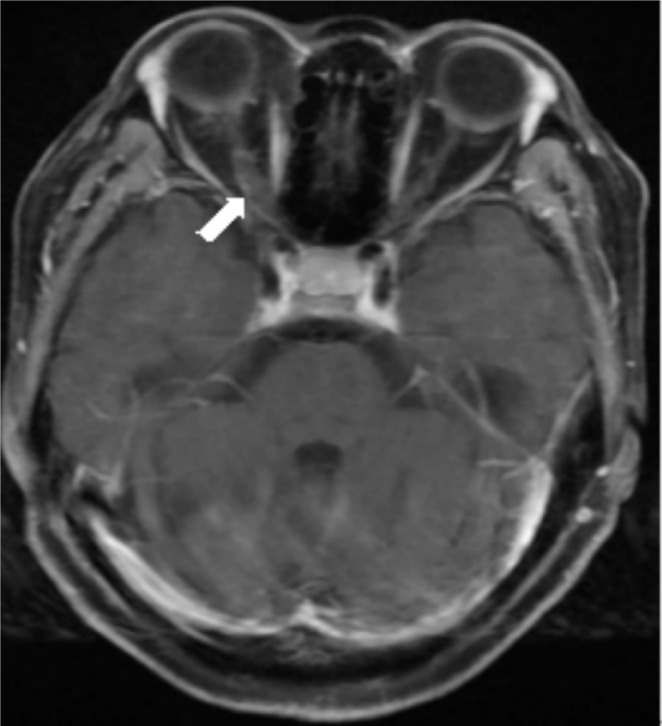
Ophthalmology examination revealed a positive schirmer's test in 3 patients (4 eyes) with dry eye. Chronic inflammation of submandibular gland or parotid gland was detected by ultrasonic testing in another 5 patients (8 eyes) displaying no dryness, and the multifocal lymphocytic infiltration of labial salivary gland was found in 2 patients in lip biopsy. Main laboratory findings on admission were as follows (Table 1): Positive serum ANA were in 6 patients, anti-SSA antibody in 7 patients, anti-SSB antibody in 8 patients, and RF in 6 patients. AQP4 antibodies were negative in all 8 patients. MRI showed an asymmetric coarsening of binocular optic nerve in one patient (Figure 3).
Table 1. Laboratory findings on admission and changes in visual acuity at final follow-up.
| Patient (eye) | Times of relapse | Blood tests for antibodies |
Best corrected visual acuity |
|||||
| ANA | RF | SSA | SSB | AQP4 | On admission | Final follow-up | ||
| 1 (R) | 0 | + | + | + | + | – | Counting fingers | 0.6 |
| 2 (R) | 0 | – | + | + | + | – | 0.2 | 1.0 |
| 3 (R) | 2 | + | – | + | + | – | 0.15 | 0.6 |
| 4 (L) | 0 | – | + | + | + | – | 0.4 | 0.8 |
| 5 (R) | 2 | + | + | – | + | – | 0.12 | 0.1 |
| 5 (L) | 2 | 0.1 | 0.25 | |||||
| 6 (R) | 2 | + | + | + | + | – | 0.2 | 0.4 |
| 6 (L) | 4 | Counting fingers | Counting fingers | |||||
| 7 (R) | 3 | + | + | + | + | – | 0.02 | 0.02 |
| 7 (L) | 2 | Counting fingers | 0.04 | |||||
| 8 (R) | 3 | + | – | + | + | – | 0.1 | 0.12 |
| 8 (L) | 3 | Counting fingers | Counting fingers | |||||
Figure 3. MRI findings at binocular optic neuritis. T1-weighted imaging showed the right optic nerve was much thickening.
The best-corrected visual acuity of 3 patients (3 eyes) suffering the first monocular attack significantly elevated (3 lines or more of E acuity). Among the 9 eyes with relapsing optic neuritis, 5 eyes had an improvement of one or more lines of E acuity, 3 eyes had no changes in vision, one eye lost one line (Table 1). The final follow-up showed 3 recurrences among 8 patients (37.5%).
DISCUSSION
Although optic neuritis is mostly idiopathic, it can be associated with variable causes of the demyelinating lesions, autoimmune disorders, infectious and inflammatory conditions[9]. In this study, 8 patients presented initially with the signs and symptoms of non-specific optic neuritis, and although 5 of 8 patients showed no dryness of the main mucosal surfaces, these patients were diagnosed as primary SS related optic neuritis by the further laboratory tests and other auxiliary examination. First, laboratory examinations, mainly of the serum ANA, anti-SSA antibody, anti-SSB antibody and RF, suggested the presence of an autoimmune-associated cause. Studies have demonstrated that the anti-SSA and anti-SSB antibody is the hallmark antibodies in the diagnosis of SS, and they associate with an earlier disease onset and the presence of extraglandular manifestations[10],[11]. Second, ultrasonic testing and lip biopsy further confirmed the cause of primary SS. The signs of dryness lack specificity in the diagnosis of primary SS for the salivary and lachrymal secretory function may be impaired from other medical conditions, however, the salivary gland biopsy is highly specific for the diagnosis[2].
The neurological findings associated with suspicious nervous system involvement in SS patients have been gained wide attention, and may be classified as either the central nervous system (CNS) involvements or peripheral nervous system (PNS) involvements, the CNS involvement is less common than PNS disease[12]-[14]. Massara et al[15] retrospectively analyzed the CNS involvement in a total of 424 primary SS patients whom were diagnosed according to the 2002 criteria proposed by the American-European Consensus Group, and found that CNS involvement was detected in 25 patients (5.8%) including one case of isolated optic neuritis. Along with this series, we showed a low frequency of 6.3% (8 of 128) optic neuritis from the primary SS. For the neurological involvement occurs usually preceding the diagnosis of primary SS, the periodical screening of the blood anti-SSA and anti-SSB antibody should be performed routinely in patients of optic neuritis[16].
It has been demonstrated that neuromyelitis optica (NMO) is associated with SS, and the serum autoantibodies against the AQP4 is the biomarker of NMO[17],[18]. Yang et al[19] evaluated the presence of AQP4 antibodies in 210 chinese patients with NMO, high-risk NMO, classic multiple sclerosis and other neurologic diseases, and they concluded that the specificity of AQP4 antibodies in NMO was 91%. For the serum anti-AQP4 antibody titers were found to be negative in all 8 patients in our study, NMO is not taken into consideration for our patients.
In our study, the corticosteroids and immunosuppressive drug are effective. Visual acuity increased to varying degree in 8 eyes (66.7%), however, 3 patients (37.5%) experienced recurrence during the follow-up. Studies had suggested that a number of mechanisms attribute to the neurological involvements in primary SS rather than vasculitis and ganglioneuronitis, and specific autoantibodies may directly induce injury of the nervous system[20]. Although conventional therapies such as glucocorticoids and immunosuppressive drugs be helpful, the role of biologic therapies specifically targeted against those autoantibodies involved in disease pathogenesis may represent a more targeted approach[21],[22].
In summary, the primary SS related optic neuritis is less common, which may be characterized by a frequent relapses of binocular optic neuritis. A periodical screening of the blood anti-SSA and anti-SSB antibody, and salivary gland biopsy play a crucial role in avoiding misdiagnoses. Although conventional therapies, such as glucocorticoids and immunosuppressive drugs, have been used with benefits, the biologic therapies are expected for improving the prognosis. Only 8 patients (12 eyes) with primary SS related optic neuritis were summarized in this study because of its low incidence, however, this preliminary conclusion could be as the basis for further population-based study and provide options to ophthalmologists to improve the prognosis of patients.
Acknowledgments
The authors wish to thank Dr. Hongyang Li and Dr. Yan Gong for their help in sorting medical records. The authors of this study indicated no financial conflicts of interest, and all authors read and approved the final manuscript.
Footnotes
Foundation item: The 12th Five-Year Plan National Science and Technology Support Program, China (No.2012BAI08B06)
REFERENCES
- 1.Kim CS, Choi YD, Choi JS, Bae EH, Ma SK, Kim SW. EBV-positive diffuse large B-cell lymphoma in a patient with primary Sjögren's syndrome and membranous glomerulonephritis. BMC Nephrol. 2012;13:149. doi: 10.1186/1471-2369-13-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hu S, Vissink A, Arellano M, Roozendaal C, Zhou H, Kallenberg CG, Wong DT. Identification of autoantibody biomarkers for primary Sjögren's syndrome using protein microarrays. Proteomics. 2011;11(8):1499–1507. doi: 10.1002/pmic.201000206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamza N, Bos NA, Kallenberg CG. B-cell populations and sub-populations in Sjögren's syndrome. Presse Med. 2012;41(9 Pt 2):e475–483. doi: 10.1016/j.lpm.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 4.Seror R, Bootsma H, Bowman SJ, Dörner T, Gottenberg JE, Mariette X, Ramos-Casals M, Ravaud P, Theander E, Tzioufas A, Vitali C. Outcome measures for primary Sjögren's syndrome. J Autoimmun. 2012;39(1–2):97–102. doi: 10.1016/j.jaut.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 5.Theander E, Vasaitis L, Baecklund E, Nordmark G, Warfvinge G, Liedholm R, Brokstad K, Jonsson R, Jonsson MV. Lymphoid organisation in labial salivary gland biopsies is a possible predictor for the development of malignant lymphoma in primary Sjögren's syndrome. Ann Rheum Dis. 2011;70(8):1363–1368. doi: 10.1136/ard.2010.144782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fox RI. Sjögren's syndrome. Lancet. 2005;366(9482):321–331. doi: 10.1016/S0140-6736(05)66990-5. [DOI] [PubMed] [Google Scholar]
- 7.Foroozan R, Buono LM, Savino PJ, Sergott RC. Acute demyelinating optic neuritis. Curr Opin Ophthalmol. 2002;13(6):375–380. doi: 10.1097/00055735-200212000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Menon V, Saxena R, Misra R, Phuljhele S. Management of optic neuritis. Indian J Ophthalmol. 2011;59(2):117–122. doi: 10.4103/0301-4738.77020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoorbakht H, Bagherkashi F. Optic neuritis, its differential diagnosis and management. Open Ophthalmol J. 2012;6:65–72. doi: 10.2174/1874364101206010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.ter Borg EJ, Risselada AP, Kelder JC. Relation of systemic autoantibodies to the number of extraglandular manifestations in primary Sjögren's syndrome: a retrospective analysis of 65 patients in the Netherlands. Semin Arthritis Rheum. 2011;40(6):547–551. doi: 10.1016/j.semarthrit.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 11.Hernández-Molina G, Leal-Alegre G, Michel-Peregrina M. The meaning of anti-Ro and anti-La antibodies in primary Sjögren's syndrome. Autoimmun Rev. 2011;10(3):123–125. doi: 10.1016/j.autrev.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Ozgocmen S, Gur A. Treatment of central nervous system involvement associated with primary Sjögren's syndrome. Curr Pharm Des. 2008;14(13):1270–1273. doi: 10.2174/138161208799316366. [DOI] [PubMed] [Google Scholar]
- 13.Lafitte C, Amoura Z, Cacoub P, Pradat-Diehl P, Picq C, Salachas F, Léger JM, Piette JC, Delattre JY. Neurological complications of primary Sjögren's syndrome. J Neurol. 2001;248(7):577–584. doi: 10.1007/s004150170135. [DOI] [PubMed] [Google Scholar]
- 14.Tobón GJ, Pers JO, Devauchelle-Pensec V, Youinou P. Neurological disorders in primary Sjögren's syndrome. Autoimmune Dis. 2012;2012:645967. doi: 10.1155/2012/645967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Massara A, Bonazza S, Castellino G, Caniatti L, Trotta F, Borrelli M, Feggi L, Govoni M. Central nervous system involvement in Sjögren's syndrome: unusual, but not unremarkable-clinical, serological characteristics and outcomes in a large cohort of Italian patients. Rheumatology (Oxford) 2010;49(8):1540–1549. doi: 10.1093/rheumatology/keq111. [DOI] [PubMed] [Google Scholar]
- 16.Niţescu D, Nicolau A, Caraiola S, Predeţeanu D, Ionescu R, Tănăsescu C. Neuromyelitis optica-complication or comorbidity in primary Sjögren's syndrome? Rom J Intern Med. 2011;49(4):295–300. [PubMed] [Google Scholar]
- 17.Tan P, Yu WY, Umapathi T, Lim SA. Severe optic neuritis in a patient with combined neuromyelitis optica spectrum disease and primary Sjögren's syndrome: a case report. J Med Case Rep. 2012;6(1):401. doi: 10.1186/1752-1947-6-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kahlenberg JM. Neuromyelitis optica spectrum disorder as an initial presentation of primary Sjögren's syndrome. Semin Arthritis Rheum. 2011;40(4):343–348. doi: 10.1016/j.semarthrit.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 19.Yang Y, Huang DH, Wu WP, Wu L, Chen LF, Wu Q. The role of aquaporin-4 antibodies in Chinese patients with neuromyelitis optica. J Clin Neurosci. 2013;20(1):94–98. doi: 10.1016/j.jocn.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Gono T, Kawaguchi Y, Katsumata Y, Takagi K, Tochimoto A, Baba S, Okamoto Y, Ota Y, Yamanaka H. Clinical manifestations of neurological involvement in primary Sjögren's syndrome. Clin Rheumatol. 2011;30(4):485–490. doi: 10.1007/s10067-010-1458-7. [DOI] [PubMed] [Google Scholar]
- 21.Bowman S, Barone F. Biologic treatments in Sjögren's syndrome. Presse Med. 2012;41(9 Pt 2):e495–509. doi: 10.1016/j.lpm.2012.05.024. [DOI] [PubMed] [Google Scholar]
- 22.Ramos-Casals M, Brito-Zerón P, Sisó-Almirall A, Bosch X, Tzioufas AG. Topical and systemic medications for the treatment of primary Sjögren's syndrome. Nat Rev Rheumatol. 2012;8(7):399–411. doi: 10.1038/nrrheum.2012.53. [DOI] [PubMed] [Google Scholar]