Abstract
We showed that renal calpain 10, a mitochondrial and cytosolic Ca2+-regulated cysteine protease, is specifically decreased in kidneys of diabetic rats and mice, and is associated with diabetic nephropathy. The goals of this study were to examine renal calpain 10 and mitochondrial dysfunction in streptozotocin-induced hyperglycemic rats and determine the effects of siRNA-mediated knock down of renal calpain 10 on mitochondrial function. Four weeks after streptozotocin injection, calpain 10 protein and mRNA were decreased and calpain 10 substrates accumulated. We detected increased state 2 respiration in isolated renal mitochondria and increased markers of mitochondrial fission and mitophagy. All changes were prevented by daily insulin injection. Compared to scrambled siRNA, calpain 10 siRNA resulted in a marked decrease in renal calpain 10 at 2, 5 and 7 days. In concert with the loss of renal calpain 10, calpain 10 substrates accumulated, mitochondrial fusion decreased, mitochondrial fission and mitophagy increased. In summary, insulin-sensitive hyperglycemia induced loss of renal calpain 10 is correlated with renal mitochondrial dysfunction, fission and mitophagy, and specific depletion of renal calpain 10 produces similar mitochondrial defects. These results provide evidence that diabetes-induced renal mitochondrial dysfunction and renal injury may directly result from the loss of renal calpain 10.
Keywords: Calpain 10, Kidney, Hyperglycemia, siRNA, Mitochondria, Dysfunction
Introduction
Calpains are a 15 member family of Ca2+-activated cysteine proteases [1–2] that have been shown to be important in many cellular processes and diseases [1]. Calpain 10 is a ubiquitously expressed atypical calpain, lacking the penta-EF hand in domain IV. It has been found in the nucleus, cytosol and mitochondria [3–5] and our laboratory has shown that calpain 10 is found in the mitochondria and cytosol of renal proximal tubular cells (RPTC) and in renal mitochondria of rabbits, mice and rats [5–6]. While much of the physiology of calpain 10 is unknown, our laboratory has shown that both overexpression and knockdown of calpain 10 causes mitochondrial disruption and cell death [5, 7]. A few mitochondrial calpain 10 substrates are known (NDUFB8, NDUFV2, ATP synthase β and ORP150) and Ca2+ overload results in the cleavage of these proteins and leads to reduced state 3 respiration in isolated mitochondria. Finally, cytosolic calpain 10 has been shown to be important in insulin-stimulated glucose uptake and insulin secretion [4, 8–10].
Renal calpain 10 loss has been associated with renal aging [7, 11]. Our laboratory discovered that calpain 10 is down regulated with age in the kidney in rats, mice and humans. Interestingly, two other members of the calpain family, calpain 1 and 2, did not change in the kidney with age providing evidence that the loss of renal calpain 10 with age is specific. While it is known that renal function decreases past 40 years of age in humans [12], it is unknown if loss of renal calpain 10 contributes to the decrease in renal function.
In 2000, Horikawa et al. discovered a single nucleotide polymorphism (SNP) in calpain 10 that made humans with the SNP more susceptible to type II diabetes [11]. Since that report was released there have been many epidemiological studies attempting to determine which ethnic groups are affected by this SNP in calpain 10 and the relative importance to diabetes [13–14]. However, there have been limited studies that address the physiological and pathological roles of calpain 10 in diabetes. We recently reported that primary cultures of RPTC incubated in high (17 mM) glucose media had decreased mitochondrial calpain 10 after three days, while cytosolic calpain 10 did not decrease until five days [16]. The reduction in calpain 10 was the result of decreased calpain 10 transcription. In addition, mitochondrial calpain 10 substrates accumulated, mitochondrial respiration decreased and apoptosis increased. Using an in vivo rat model of streptozotocin (STZ) - induced diabetic nephropathy renal calpain 10, but not calpains 1 and 2, was decreased, mitochondrial calpain 10 substrates accumulated, and apoptosis and renal dysfunction occurred at 10 weeks [15].
To confirm that the loss of renal calpain 10 results in renal dysfunction, we administered a calpain 10 siRNA by tail vein injection and showed knockdown of renal calpain 10 at days 2, 5 and 7 with no effects on calpain 1 and no effects on liver calpain 10 [15]. At day 7, there was a two-fold increase in serum creatinine levels, showing kidney dysfunction occurred after loss of calpain 10. Additionally, we detected increased cleavage of pro-caspase 3 beginning at day 5 and increasing at day 7, and increased TUNEL positive cells on day 7. These data provide evidence that specific loss of calpain 10 leads to increased apoptosis and kidney dysfunction.
Our previous study examined rats 10 weeks after STZ treatment and 7 days after calpain 10 siRNA treatment. In both models we detected decreased calpain 10 and renal dysfunction, suggesting loss of calpain 10 causes kidney dysfunction [15]. The goals of this study were to expand upon our previous in vivo studies and examine renal calpain 10 and mitochondrial dysfunction in streptozotocin-induced hyperglycemic rats after four weeks in the presence and absence of insulin treatment, and determine the effects of siRNA-mediated knock down of renal calpain 10 on mitochondrial function.
Methods
Reagents
The calpain 10, calpain 1, PINK1, ATP synthase β and LC3B antibodies were purchased from Abcam (Cambridge, MA). The calpain 2 and PGC-1α antibodies were obtained from Calbiochem (Gibbstown, NJ). The caspase 3 antibody was purchased from Enzo Life Sciences (Plymouth Meeting, PA). The calpain 10 siRNA, scramble siRNA, TRIZOL, NDUFB8 and COX1 antibodies were obtained from Invitrogen (Carlsbad, CA). The Mfn1, Drp1 and β-actin antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The Mfn2 antibody was obtained from Sigma (St. Louis, MO). The GAPDH antibody was purchased from Fitzgerald (Acton, MA). The Lon antibody was a gracious gift from Dr. Kelvin Davies (University of Southern California). HRP-conjugated goat anti-rabbit/mouse secondary antibodies were obtained from Pierce (Rockford, IL). The reverse transcriptase kit and PCR reagents were obtained from Fermentas (Glen Burnie, MD). All other chemcials were purchased from Sigma (St. Louis, MO).
Streptozotocin-Induced Diabetes Model
Male Sprague-Dawley rats, 8 weeks of age (200–250 g), were fasted for 16 hr prior to a single injection with streptozocin (55 mg/kg, tail vein) in sodium citrate buffer [16–18]. To limit mortality from insulin release from damaged pancreatic islets, rats were given sucrose drinking water (15 g/L) for 48 hr. Diabetes was confirmed in STZ-treated rats 24 hr after treatment by measuring tail vein plasma glucose. After two weeks of STZ-treatment, some of the STZ-treated rats received 8 U/day of insulin by IP injection. Four weeks after diabetes initiation, all rats (Control, STZ and STZ + Insulin) were euthanized and blood and kidneys were harvested.
Calpain 10 In Vivo siRNA
Male Fischer 344 rats, 8 weeks of age (200 g), were injected in the tail vein with calpain 10 siRNA or negative (scramble) control (Invitrogen, Carlsbad, CA) as previously described and the same tissues were used in this study [15]. At days 2, 5 and 7 the rats were euthanized and kidneys were harvested for immunoblot analysis.
Immunoblot Analysis
Rat kidney cortex was homogenized, protein concentrations determined, and electrophoresed on SDS-PAGE gels (4–12%) followed by transfer to nitrocellulose membranes. The membranes were blocked in 2.5% non-fat milk/TBST (Tris-buffered saline Tween 20) for 1 hour. All primary antibodies were incubated on a shaker at 4° C overnight. The following antibodies were used at a 1:1000 dilution: calpain 10, calpain 1, calpain 2, caspase 3, ATP synthase β, NDUFB8, GAPDH, Mfn1, Mfn2, Drp1, PGC-1α and COX1. PINK1, LC3B and Lon were used at a 1:500 dilution. Secondary antibody (anti-mouse or anti-rabbit) was used at 1:10,000 dilution for 1 hour at room temperature. An Alpha Innotech imaging system was used to visualize and quantify membranes for immunoreactive proteins using enhanced chemiluminescence detection.
Isolation of Renal Cortical Mitochondria and Oxygen Consumption (QO2)
Renal cortical mitochondria isolation has been described previously [5, 19]. After the final centrifugation, the pellet was resuspended in mitochondrial incubation buffer (130 mM KCl, 9 mM Tris-PO4, 4 mM Tris-HCl and 1 mM EGTA) + 5 mM malate and 6 mM pyruvate for oxygen consumption measurements. QO2 was measured as previously described using a Clark-type electrode [5, 15]. After measurement of state 2 respiration, 1 mM ADP was added to measure state 3 respiration.
Reverse Transcription PCR
Total RNA was isolated from rat kidney cortex samples using TRIZOL (Invitrogen, Carlsbad, CA) and the manufacturer’s protocol. RNA was quantified by measuring absorbance at 260 and 280 nm. The reverse transcriptase kit (Fermentas, Glen Burnie, MD) was used to transform the RNA to cDNA. PCR primers for calpain 10, calpain 1 and β-actin (loading control) have been previously reported [9]. PCR products were electrophoresed on 1.5% agarose gel and stained with ethidium bromide.
Statistical Analysis
A one-way ANOVA with a Student-Newman-Keuls test was used to determine significance between multiple groups. A Student’s t-test was performed to determine significance between two groups. The sample size for each group was ≥ 3 and a p-value ≤ 0.05 was required for statistical significance.
Results
Hyperglycemia downregulates renal calpain 10 protein and mRNA at four weeks
Male Sprague-Dawley rats injected with STZ is a commonly used model to induce hyperglycemia/diabetes [20] and we have previously used this model in our studies [15]. Since we detected a decrease in calpain 10 protein and mRNA 10 weeks after STZ treatment [15], we determined if 4 weeks after STZ treatment produced a similar effect and to determine if insulin would prevent the decrease in calpain 10. Rats were placed in metabolic cages for 24 hr to collect urine and measure water intake. Plasma glucose, water intake and urine output were greater in STZ-treated rats while body weight decreased compared to control rats (Table 1). These changes were prevented with daily insulin injection. It is important to note that at this time point there was no increase in serum creatinine levels (Table 1).
Table 1.
Measurements of weight, plasma glucose, serum creatinine, water intake and urine output and mitochondrial respiration between control, STZ and STZ + insulin groups. Data are represented as means ± SEM of % of control (N ≥ 3 for each group).
| Control | STZ | STZ + Insulin | |
|---|---|---|---|
| Body Weight (g) | 440 ± 19 | 285 ± 22*# | 395 ± 20 |
| Plasma Glucose (mg/dL) | 128 ± 17 | 408 ± 25*# | 189 ± 33 |
| Serum Creatinine (mg/dL) | 0.9 ± 0.3 | 1.0 ± 0.5 | 1.1 ± 0.4 |
| Water Intake (mL) | 40 ± 3 | 125 ± 10*# | 44 ± 5 |
| Urine Output (mL/24 hr) | 10 ± 1 | 42 ± 5*# | 15 ± 3 |
| State 2 Respiration (nmol O2/mg protein/min) | 52 ± 8 | 92 ± 10*# | 49 ± 12 |
| State 3 Respiration (nmol O2/mg protein/min) | 162 ± 12 | 145 ± 15 | 172 ± 11 |
Labels with * are statistically different from control and # are statistically different from STZ + Insulin (p ≤ 0.05).
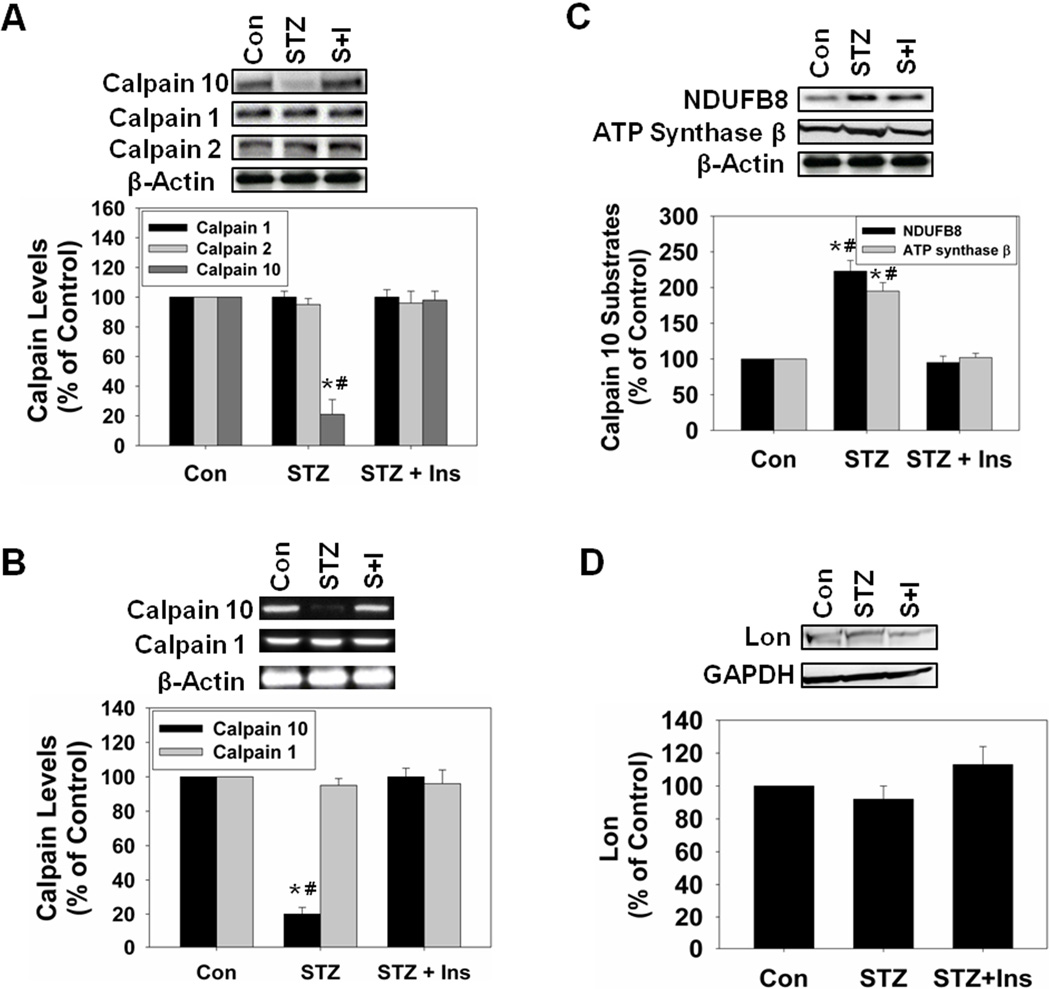
Immunoblot analysis revealed an 80% decrease in calpain 10 protein in the kidney cortex of STZ-treated rats and the loss in calpain 10 protein was attenuated by insulin (Fig. 1A). There were no changes in calpain 1 or calpain 2 protein levels in any of the groups, suggesting that the decrease is specific for calpain 10. Semi-quantitative PCR analysis revealed no alterations in calpain 1 mRNA in any of the groups. Calpain 10 mRNA was decreased 80% in STZ-treated rats and insulin prevented hyperglycemia which maintained calpain 10 mRNA at control levels (Fig. 1B). Thus, four weeks of hyperglycemia decreased renal calpain 10 transcription and protein levels and these decreases were attenuated by insulin.
Figure 1.
Calpain 10 is downregulated and calpain 10 substrates accumulate in STZ rats four weeks after administration. A, Rat kidney cortex was immunoblotted for calpain 10 (75 kDa), calpain 1 (80 kDa), calpain 2 (80 kDa), and β-actin (42 kDa – loading control). B, PCR reactions were performed with cDNA for calpain 10, calpain 1 and for β-actin. C, Rat kidney cortex was immunoblotted for NDUFB8 (18 kDa), ATP synthase β (56 kDa). D, Rat kidney cortex was immunoblotted for Lon (100 kDa) and GAPDH (37 kDa – loading control). All blots were quantified by densitometric analysis. Data are represented as means ± SEM of % of control (N ≥ 3 for each group). Bars with * are statistically different from control and # are statistically different from STZ + Insulin (p ≤ 0.05).
Renal mitochondrial calpain 10 substrates accumulate and mitochondrial function decreases during hyperglycemia
Two known substrates of calpain 10, NDUFB8 and ATP synthase β, are important for proper function of the mitochondrial electron transport chain and production of ATP. Since we previously demonstrated that NDUFB8 and ATP synthase β were increased 10 weeks following STZ treatment [16], we determined if similar changes were observed four weeks following STZ treatment. The STZ-treated group had an increase in NDUFB8 and ATP synthase β and this accumulation was attenuated with insulin (Fig. 1C). Lon protease is a soluble mitochondrial matrix protease that has been shown to be important in degrading oxidized and misfolded proteins, and calpain 10 is a Lon protease substrate [21–22]. We hypothesized that the loss of calpain 10 and the accumulation of calpain 10 substrates in the mitochondria may lead to an upregulation of Lon protease, but there were no changes in this protein in any group (Fig. 1D).
Mitochondria were isolated from rat renal cortex from Control, STZ and STZ + Insulin groups, and state 2 and 3 respiration were measured. State 2 respiration only increased in the STZ group while state 3 respiration was unchanged in all groups (Table 1). These data provide evidence that hyperglycemia-induced loss of renal calpain 10 and the excess accumulation of mitochondrial calpain 10 substrates results in mitochondrial dysfunction.
Hyperglycemia increases renal mitochondrial fission and mitophagy
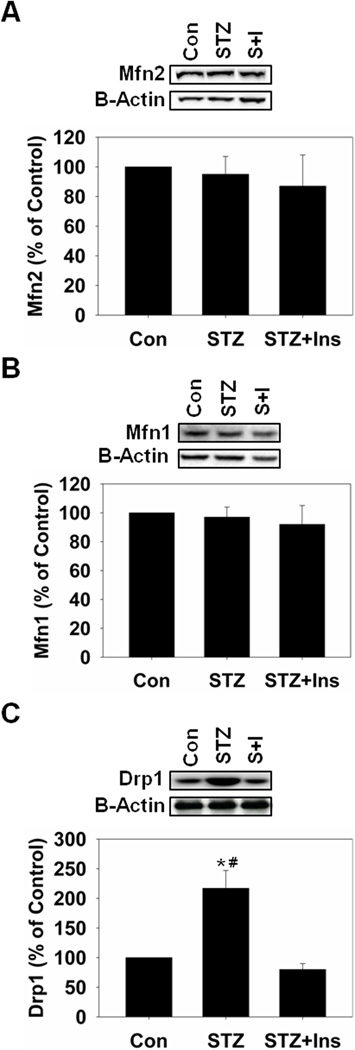
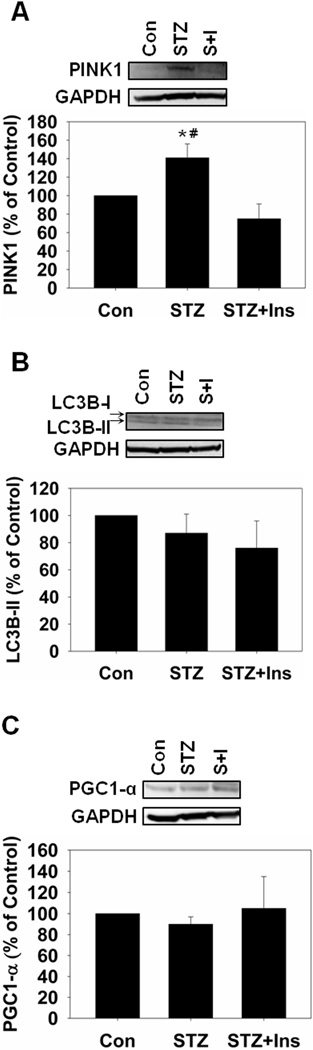
Because we detected dysfunctional mitochondrial respiration we determined if there were alterations in renal mitochondrial dynamics by measuring protein levels of mitofusin 1 (Mfn1), mitofusin 2 (Mfn2) and dynamin-related protein 1 (Drp1), markers of mitochondrial fusion and fission. There were no changes in Mfn1 and Mfn2 in any groups, but there was an increase in Drp1 in STZ rats that was attenuated with insulin (Fig. 2). Increased mitochondrial fission can lead to increased mitophagy and/or apoptosis in the absence of changes in mitochondrial fusion [23–24]. To determine if hyperglycemia increased renal mitophagy we examined PINK1 and LC3B, which are mediators of mitophagy/autophagy, using immunoblot analysis. STZ-treated rats had an approximately 50% increase in PINK1 without a change in LC3B-II (Fig. 3A and B). Insulin attenuated the increase in PINK1 and had no effect on LC3B-II. We did not detect increased cleaved pro-caspase 3 in these samples (data not shown), suggesting that renal mitophagy is occurring in the absence of apoptosis during hyperglycemia.
Figure 2.
Mitochondrial fission is increased in STZ-treated rats four weeks after administration. A, Rat kidney cortex was immunoblotted for Mfn2 (86 kDa) and β-actin (42 kDa – loading control). B, Rat kidney cortex was immunoblotted for Mfn1 (86 kDa). C, Rat kidney cortex was immunoblotted for Drp1 (71 kDa). All blots were quantified by densitometric analysis. Data are represented as means ± SEM of % of control (N ≥ 3 for each group). Bars with * are statistically different from control and # are statistically different from STZ + Insulin (p ≤ 0.05).
Figure 3.
Mitophagy is increased in STZ-treated rats four weeks after administration. A, Rat kidney cortex was immunoblotted for PINK1 (66 kDa) and GAPDH (37 kDa – loading control). B, Rat kidney cortex was immunoblotted for LC3B-I/II (17 kDa/15 kDa). C, Rat kidney cortex was immunoblotted for PGC-1α (92 kDa). All blots were quantified by densitometric analysis. Data are represented as means ± SEM of % of control (N ≥ 3 for each group). Bars with * are statistically different from control and # are statistically different from STZ + Insulin (p ≤ 0.05).
Renal mitochondrial biogenesis does not increase during hyperglycemia
Because we detected dysfunctional mitochondria and mitophagy, we determined if there was an increase in mitochondrial biogenesis. PGC-1α, the master regulator of mitochondrial biogenesis [25], was measured by immunoblot analysis and no changes in PGC-1α were observed in any group (Fig. 3C).
Calpain 10 siRNA induces renal mitochondrial calpain 10 substrate accumulation
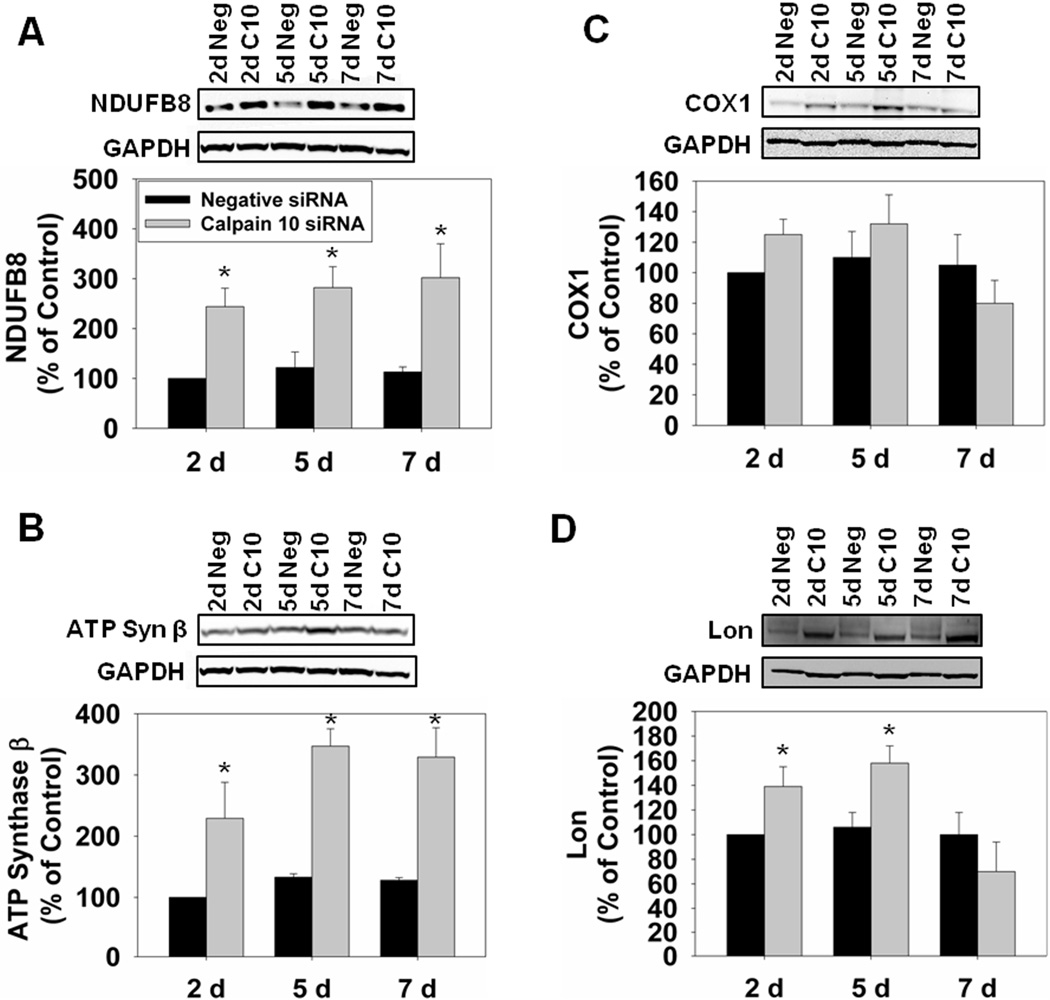
Because male Fischer 344 rats are a commonly used model to study age-related renal dysfunction [26] and we have previously shown that aged Fischer 344 rats have decreased renal calpain 10 [7], we continued to use this rat strain for our siRNA studies. Because we detected apoptosis and kidney dysfunction in calpain 10 siRNA treated rats previously [16], we sought to determine the direct effects of calpain 10 loss on renal mitochondrial homeostasis and determine whether these mitochondrial changes are similar to those observed in hyperglycemic rats described above. Male Fischer 344 rats were treated with either 20 nmol of calpain 10 or negative (scramble) control siRNA and euthanized at days 2, 5 and 7 [16]. We previously showed that calpain 10 siRNA causes ~50% knockdown of calpain 10 protein by day 2, that further decreases to ~80% knockdown by day 7, and only renal calpain 10 is affected [15]. The reduction in calpain 10 protein resulted in the accumulation of mitochondrial calpain 10 substrates, NDUFB8 and ATP synthase β two days after siRNA administration and remained elevated through seven days (Fig. 4A and B). We also measured cytochrome c oxidase subunit 1 (COX1), a component of complex IV that is not a mitochondrial calpain 10 substrate, and detected no changes at any time point (Fig. 4C). These data reveal that specific loss of renal calpain 10 leads to accumulation of mitochondrial calpain 10 substrates. Since we detected an accumulation of mitochondrial calpain 10 substrates, we determined if Lon protease was upregulated. We detected increases at days 2 and 5 in the calpain 10 siRNA-treated rats, suggesting that Lon may be upregulated to degrade the accumulation of mitochondrial calpain 10 substrates (Fig. 4D).
Figure 4.
Calpain 10 substrates accumulate in calpain 10 siRNA-treated rats. A, Rat kidney cortex was immunoblotted for NDUFB8 (18 kDa) and GAPDH (37 kDa – loading control). B, Rat kidney cortex was immunoblotted for ATP synthase β (18 kDa). C, Rat kidney cortex was immunoblotted for COX1 (35 kDa). D, Rat kidney cortex was immunoblotted for Lon (100 kDa). All blots were quantified by densitometric analysis. Data are represented as means ± SEM of % of control (N ≥ 3 for each group). Bars with * are statistically different from controls (p ≤ 0.05).
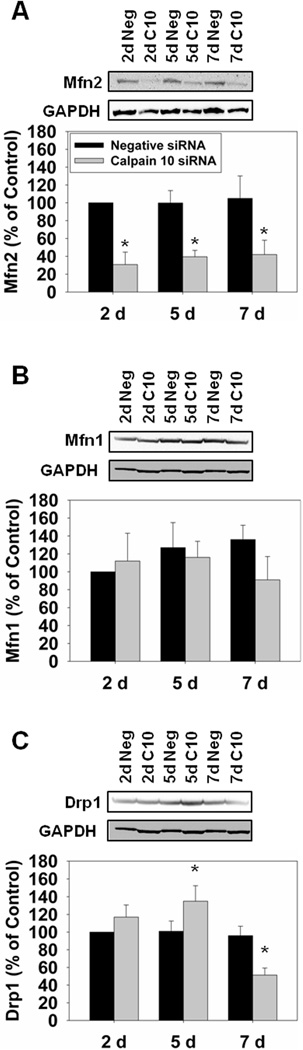
Renal calpain 10 reduction increases mitochondrial fission and mitophagy and has mixed effects on mitochondrial fusion
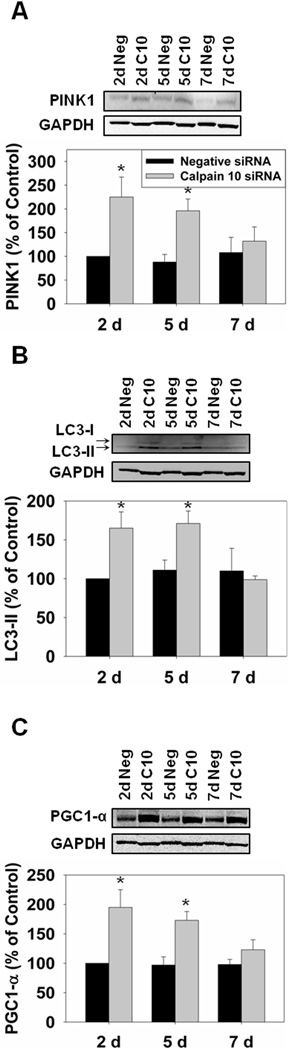
Since STZ-treated rats had increased mitochondrial fission and mitophagy we determined if specific calpain 10 loss resulted in similar changes. There was a reduction in Mfn2 at all time points, Mfn1 did not change, and Drp1increased at day 5 prior to decreasing at day 7 in the calpain 10 siRNA-treated rats (Fig. 5). The reduction in fusion and increase in fission provides evidence that mitophagy may be occurring. In the calpain 10 siRNA-treated rats we detected increases in PINK1 and LC3B-II at days 2 and 5 (Fig. 6A and B). These data provide evidence that loss of calpain 10 leads to increased mitochondrial fission and mitophagy.
Figure 5.
Mitochondrial fission is increased in calpain 10 siRNA-treated rats. A, Rat kidney cortex was immunoblotted for Mfn2 (86 kDa) and GAPDH (37 kDa – loading control). B, Rat kidney cortex was immunoblotted for Mfn1 (86 kDa). C, Rat kidney cortex was immunoblotted for Drp1 (71 kDa). All blots were quantified by densitometric analysis. Data are represented as means ± SEM of % of control (N ≥ 3 for each group). Bars with * are statistically different from controls (p ≤ 0.05).
Figure 6.
Mitophagy and PGC-1α are increased in calpain 10 siRNA-treated rats. A, Rat kidney cortex was immunoblotted for PINK1 (66 kDa) and GAPDH (37 kDa – loading control). B, Rat kidney cortex was immunoblotted for LC3B-I/II (17 kDa/15 kDa). C, Rat kidney cortex was immunoblotted for PGC-1α (92 kDa). All blots were quantified by densitometric analysis. Data are represented as means ± SEM of % of control (N ≥ 3 for each group). Bars with * are statistically different from controls (p ≤ 0.05).
Mitochondrial Biogenesis increases in calpain 10 siRNA-treated rats
Since we detected increased mitophagy we determined if mitochondrial biogenesis was upregulated. Immunoblot analysis for PGC-1α revealed increases at days 2 and 5 before returning to normal at day 7 (Fig. 6C).
Discussion
This study reveals that four weeks after STZ-induced hyperglycemia there is down regulation of renal calpain 10 mRNA and protein without changes in calpain 1 or 2. We think hyperglycemia down regulates calpain 10 because high glucose down regulates calpain 10 in primary cultures of RPTC [15]. These results are similar to those reported recently in the same model but for 10 weeks [15]. However, at four weeks, there is no increase in serum creatinine and the amount of overt renal damage and dysfunction is limited, thereby allowing examination of earlier renal events.
Our results demonstrate there are extensive changes in renal mitochondrial homeostasis and dysfunction after four weeks of hyperglycemia that were attenuated by insulin treatment. The insulin sensitivity supports the idea that the observed renal events are primarily due to hyperglycemia. In the STZ-treated rats, we observed accumulation of renal mitochondrial calpain 10 substrates, increased state 2 respiration, mitochondrial fission and mitophagy with the loss of renal calpain 10. The increase in mitochondrial calpain 10 substrates is similar to our observation in renal proximal tubular cells (RPTC) exposed to 17 mM glucose for 4 days and STZ rats after 10 weeks [15]. To determine whether the loss of calpain 10 alone could produce these renal mitochondrial changes, calpain 10 siRNA was used to specifically knockdown renal calpain 10 as shown recently by Covington and Schnellmann [16]. We detected increased mitochondrial calpain 10 substrates, decreased mitochondrial fusion, increased mitochondrial fission, mitophagy and mitochondrial biogenesis. Therefore, we conclude that mitochondrial dysfunction detected during chronic hyperglycemia can be produced by the loss of renal calpain 10.
Hyperglycemia has previously been reported to cause mitochondrial dysfunction [27–28]. We detected no changes between control and STZ + insulin rats in state 2 or state 3 respiration, but the STZ group had increased state 2 respiration. Our measured state 2 and state 3 rates for kidney mitochondria from Sprague-Dawley rats are similar to those reported by another group [29]. Chronic hyperglycemia causes increased ROS in the kidney, with much of the ROS being created by the electron transport chain [30–34]. Uncoupling protein-2 (UCP-2) allows mitochondrial respiration to occur without the creation of ATP to reduce excess ROS. Recently, it was shown that UCP-2 mRNA and protein were 3- and 2-fold higher, respectively, after STZ-treatment in the renal cortex [35]. Thus, renal cortical cells upregulate UCP-2 to remove ROS and increase state 4 respiration [34–35]. This phenomenon was also reported in heart mitochondria in four week STZ-treated rats [36].
We examined multiple markers of mitochondrial homeostasis in these animal models because it provides greater information of the events that are occurring in vivo. Under control conditions, there is a balance between mitochondrial fusion and fission. For example, an increase in mitochondrial fission has been associated with increased mitophagy and apoptosis [23–24]. In the STZ-treated rats, we detected no changes in mitochondrial fusion, but an increase in mitochondrial fission. In concert, there was an increase in mitophagy as indicated by an increase in PINK1 in the STZ-treated rats. PINK1 is a serine/threonine protein kinase that binds to the inner mitochondrial membrane and leads to the recruitment of Parkin, initiating mitophagy [37–38]. Recently another PINK1 function was discovered; PINK1 quarantines damaged mitochondria prior to mitophagy [39]. LC3B-II is a protein that is involved in autophagy (including mitophagy) and is found on the autophagosome just before the organelle is degraded [40]. We determined LC3B-II protein levels and there were no differences between any groups. Despite not detecting an increase in LC3B-II in the STZ group, we think that there is increased mitophagy and/or quarantining of damaged mitochondria since PINK1 is increased.
We analyzed mitochondrial dynamics in the siRNA model and determined that calpain 10 siRNA-treated rats had decreased Mfn2 at days 2, 5 and 7, but not Mfn1, and an increase in Drp1 at day 5. These results signify a pro-fission environment where mitophagy and apoptosis occur. We have previously shown that there is increased apoptosis at days 5 and 7 [15], but had not explored mitophagy. PINK1 and LC3B-II increased at days 2 and 5 before returning to normal at day 7. The results from the STZ rats and the calpain 10 siRNA rats are similar in that they both have increased fission and mitophagy, but the STZ-treated rats did not display changes in mitochondrial fusion like the calpain 10 siRNA rats. There are several possible reasons for this difference. The calpain 10 siRNA-treated rats have a rapid reduction in calpain 10 mRNA and protein over seven days, unlike the STZ-treated rats where hyperglycemic down regulation of calpain 10 may take several weeks. The slow versus rapid reduction may allow the kidney to adapt to loss of calpain 10 and mitochondrial fusion is unaffected. Also, chronic hyperglycemia may have other effects on the kidney that could play a role in mitochondrial dynamics.
Since we detected mitochondrial dysfunction and increased mitophagy, we determined if mitochondrial biogenesis was upregulated. PGC-1α increased in calpain 10 siRNA-treated rats at days 2 and 5 but did not change in the STZ-treated rats. Again, this could be related to a temporal effect where an earlier time point may reveal an increase in PGC-1α or it could relate to the rate of calpain 10 loss. It would seem that rapid reduction of calpain 10 would cause greater mitochondrial stress than a tapered decrease which may signal upregulation of mitochondrial biogenesis. It is also likely that hyperglycemia may block renal mitochondrial biogenesis directly [41–42].
Since we detected an accumulation of mitochondrial calpain 10 substrates we determined if Lon protease was upregulated to degrade the accumulated proteins. Lon protease is a mitochondrial matrix protease that has been shown to be important in mitochondrial protein quality control [21] and calpain 10 is a substrate of Lon protease [22]. Lon protease degrades oxidized and misfolded proteins and prevents accumulation of damaged proteins. Lon protease increased at days 2 and 5 prior to returning to control levels by day 7 following exposure to calpain 10 siRNA. These increases were not detected in the STZ-treated rats. Similar to the other model differences discussed above, Lon protease may increase in response and degrade accumulated mitochondrial proteins when calpain 10 is not present. However, Lon protease may not respond to an extended loss of calpain 10. The decrease in Lon to control levels on day 7 following calpain siRNA exposure supports this idea.
This research examines loss of calpain 10 in two in vivo models that lead to similar mitochondrial defects in each. In summary, we detected accumulation of mitochondrial calpain 10 substrates, increased state 2 respiration, mitochondrial fission and mitophagy in the four week STZ-treated rats. We detected similar results in the calpain 10 siRNA-treated rats of accumulation of mitochondrial calpain 10 substrates, decreased mitochondrial fusion, increased mitochondrial fission, mitophagy and mitochondrial biogenesis. Therefore, the mitochondrial deficits that occur in chronic hyperglycemia can be at least partially attributed to calpain 10 loss. This is the first study that relates calpain 10 loss to mitochondrial dysfunction in vivo. Additionally, it provides greater insight to how calpain 10 is important for proper renal function and provides evidence to how calpain 10 may be important for diabetes and diabetic nephropathy.
Highlights.
Renal calpain 10 decreased 4 weeks after streptozotocin-induced diabetes in rats
Renal mitochondrial dysfunction was detected in diabetic rats
siRNA knockdown of renal calpain 10 in rats revealed similar mitochondrial defects
Renal calpain 10 loss may be responsible for mitochondrial dysfunction in diabetes
Acknowledgements
This study was supported by NIH Grant [GM 084147], the NIEHS Grant [ES-012239], the NIH/NIEHS Training Program in Environmental Stress Signaling [T32ES012878HS Training Program in Environmental Stress Sig and Development Program of the Department of Veterans Affairs. Animal facilities were funded by NIH grant [C06 RR-015455].
Abbreviations
- STZ
streptozotocin
- QO2
oxygen consumption
- Mfn1
mitofusin 1
- Mfn2
mitofusin 2
- Drp1
dynamin-related protein 1
- COX1
cytochrome c oxidase subunit 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer
The article content does represent the views of the Department of Veterans Affairs of the US Government.
References
- 1.Goll DE, Thompson VF, Li H, Wei W, Cong J. Physiol. Rev. 2003;83:731–801. doi: 10.1152/physrev.00029.2002. [DOI] [PubMed] [Google Scholar]
- 2.Suzuki K, Hata S, Kawabata Y, Sorimachi H. Diabetes. 2004;53:S12–S18. doi: 10.2337/diabetes.53.2007.s12. [DOI] [PubMed] [Google Scholar]
- 3.Ma H, Fukiage C, Kim YH, Duncan MK, Reed NA, Shih M, Azuma M, Shearer TR. J. Biol. Chem. 2001;276:28525–28531. doi: 10.1074/jbc.M100603200. [DOI] [PubMed] [Google Scholar]
- 4.Marshall C, Hitman GA, Partridge CJ, Clark A, Ma H, Shearer TR, Turner MD. Mol. Endocrinol. 2005;19:213–224. doi: 10.1210/me.2004-0064. [DOI] [PubMed] [Google Scholar]
- 5.Arrington DD, Van Vleet TR, Schnellmann RG. Am. J. Physiol. Cell Physiol. 2006;291:C1159–C1171. doi: 10.1152/ajpcell.00207.2006. [DOI] [PubMed] [Google Scholar]
- 6.Giguere CJ, Covington MD, Schnellmann RG. Biochem. Biophys. Res. Commun. 2008;366:258–262. doi: 10.1016/j.bbrc.2007.11.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Covington MD, Arrington DD, Schnellmann RG. Am. J. Physiol. Renal Physiol. 2009;296:F478–F486. doi: 10.1152/ajprenal.90477.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cushman SW, Wardzala LJ. J. Biol. Chem. 1980;255:4758–4762. [PubMed] [Google Scholar]
- 9.Otani K, Han D-H, Ford EL, Garcia-Roves PM, Ye H, Horikawa Y, Bell GI, Holloszy JO, Polonsky KS. J. Biol. Chem. 2004;279:20915–20920. doi: 10.1074/jbc.M400213200. [DOI] [PubMed] [Google Scholar]
- 10.Sreenan SK, Zhou Y-P, Otani K, Hansen PA, Currie KPM, Pan C-Y, Lee J-P, Ostrega DM, Pugh W, Horikawa Y, Cox NJ, Hanis CL, Burant CF, Fox AP, Bell GI, Polonsky KS. Diabetes. 2001;50:2013–2020. doi: 10.2337/diabetes.50.9.2013. [DOI] [PubMed] [Google Scholar]
- 11.Horikawa Y, Oda N, Cox NJ, Li X, Orho-Melander M, Hara M, Hinokio Y, Lindner TH, Mashima H, Schwarz PEH, del Bosque-Plata L, Horikawa Y, Oda Y, Yoshiuchi I, Colilla S, Polonsky KS, Wei S, Concannon P, Iwasaki N, Schulze J, Baier LJ, Bogardus C, Groop L, Boerwinkle E, Hanis CL, Bell GI. Nat. Genet. 2000;26:163–175. doi: 10.1038/79876. [DOI] [PubMed] [Google Scholar]
- 12.Coca SG. Am. J. Kidney Dis. 2010;56:122–131. doi: 10.1053/j.ajkd.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Demirci H, Yurtcu E, Ergun MA, Yazici AC, Karasu C, Yetkin I. Genet. Test. 2008;12:305–309. doi: 10.1089/gte.2007.0118. [DOI] [PubMed] [Google Scholar]
- 14.Bodhini D, Radha V, Ghosh S, Sanapala KR, Majumder PP, Rao MRS, Mohan V. Metabolism. 2011;60:681–688. doi: 10.1016/j.metabol.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Covington MD, Schnellmann RG. Kidney Int. 2011 doi: 10.1038/ki.2011.356. [DOI] [PubMed] [Google Scholar]
- 16.Frier B, Noble E, Locke M. Cell Stress Chaperones. 2008;13:287–296. doi: 10.1007/s12192-008-0062-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gülen Ş, Dinçer S. Mol. Cell. Biochem. 2007;302:59–65. doi: 10.1007/s11010-007-9426-5. [DOI] [PubMed] [Google Scholar]
- 18.Tobin BW, Lewis JT, Chen DZ, Finegood DT. Diabetes. 1993;42:98–105. doi: 10.2337/diab.42.1.98. [DOI] [PubMed] [Google Scholar]
- 19.Schnellmann RG, Cross TJ, Lock EA. Toxicol. Appl. Pharmacol. 1989;100:498–505. doi: 10.1016/0041-008x(89)90297-4. [DOI] [PubMed] [Google Scholar]
- 20.Chen Y, Li X-X, Lin H-C, Qiu X-F, Gao J, Dai Y-T, Wang R. Asian J. Androl. 2012 [Google Scholar]
- 21.Bota DA, Davies KJA. Nat. Cell Biol. 2002;4:674–680. doi: 10.1038/ncb836. [DOI] [PubMed] [Google Scholar]
- 22.Smith MA, Schnellmann RG. Arch. Biochem. Biophys. 2012;517:144–152. doi: 10.1016/j.abb.2011.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brooks C, Wei Q, Cho S-G, Dong Z. J. Clin. Invest. 2009;119:1275–1285. doi: 10.1172/JCI37829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee Y, Lee H-Y, Hanna RA, Gustafsson ÅB. Am. J. Physiol. Heart Circ. Physiol. 2011;301:H1924–H1931. doi: 10.1152/ajpheart.00368.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fernandez-Marcos P, Auwerx J. Am. J. Clin. Nutr. 2011;93:884S–890S. doi: 10.3945/ajcn.110.001917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Corman B, Owen R. In: Pathobiology of Aging Rats. Mohr U, Dungworth D, Capen C, editors. Washington DC: ILSI Press; 1992. pp. 195–209. [Google Scholar]
- 27.Lowell B, Shulman G. Science. 2005;307:384–387. doi: 10.1126/science.1104343. [DOI] [PubMed] [Google Scholar]
- 28.Rolo AP, Palmeira CM. Toxicol. Appl. Pharmacol. 2006;212:167–178. doi: 10.1016/j.taap.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 29.Moralejo DH, Ogino T, Zhu M, Toide K, Wei S, Wei K, Yamada T, Mizuno A, Matsumoto K, Shima K. J. Vet. Med. Sci. 1998;60:1157–1160. doi: 10.1292/jvms.60.1157. [DOI] [PubMed] [Google Scholar]
- 30.Nishikawa T, Edelstein D, Brownlee M. Kidney Int. 2000;58:S26–S30. doi: 10.1046/j.1523-1755.2000.07705.x. [DOI] [PubMed] [Google Scholar]
- 31.Robertson RP. J. Biol. Chem. 2004;279:42351–42354. doi: 10.1074/jbc.R400019200. [DOI] [PubMed] [Google Scholar]
- 32.Brownlee M. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 33.Palm F, Cederberg J, Hansell P, Liss P, Carlsson PO. Diabetologia. 2003;46:1153–1160. doi: 10.1007/s00125-003-1155-z. [DOI] [PubMed] [Google Scholar]
- 34.Friederich M, Olerud J, Fasching A, Liss P, Hansell P, Palm F. In: Oxygen Transport to Tissue XXIX. Kang KA, Harrison DK, Bruley DF, editors. US: Springer; 2008. pp. 37–43. [Google Scholar]
- 35.Friederich M, Nordquist L, Olerud J, Johansson M, Hansell P, Palm F. In: Oxygen Transport to Tissue XXX. Liss P, Hansell P, Bruley DF, Harrison DK, editors. US: Springer; 2009. pp. 205–212. [Google Scholar]
- 36.Lashin O, Romani A. Mol. Cell. Biochem. 2004;267:31–37. doi: 10.1023/b:mcbi.0000049360.75392.89. [DOI] [PubMed] [Google Scholar]
- 37.Matsuda N, Sato S, Shiba K, Okatsu K, Saisho K, Gautier CA, Sou Y-s, Saiki S, Kawajiri S, Sato F, Kimura M, Komatsu M, Hattori N, Tanaka K. J. Cell Biol. 2010;189:211–221. doi: 10.1083/jcb.200910140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Narendra DP, Jin S, Tanaka A, Suen D-F, Gautier CA, Shen J, Cookson M, Youle RJ. PloS Biol. 2010;26 doi: 10.1371/journal.pbio.1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang X, Winter D, Ashrafi G, Schlehe J, Wong Yao L, Selkoe D, Rice S, Steen J, LaVoie Matthew J, Schwarz Thomas L. Cell. 2011;147:893–906. doi: 10.1016/j.cell.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Geisler S, Holmstrom KM, Skujat D, Fiesel FC, Rothfuss OC, Kahle PJ, Springer W. Nat. Cell Biol. 2010;12:119–131. doi: 10.1038/ncb2012. [DOI] [PubMed] [Google Scholar]
- 41.Palmeira CM, Rolo AP, Berthiaume J, Bjork JA, Wallace KB. Toxicol. Appl. Pharmacol. 2007;225:214–220. doi: 10.1016/j.taap.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 42.Yadav H, Quijano C, Kamaraju Anil K, Gavrilova O, Malek R, Chen W, Zerfas P, Zhigang D, Wright Elizabeth C, Stuelten C, Sun P, Lonning S, Skarulis M, Sumner Anne E, Finkel T, Rane Sushil G. Cell Metab. 2011;14:67–79. doi: 10.1016/j.cmet.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]