Abstract
Staphylococcal entertoxin B (SEB) is a potent exotoxin produced by the Staphylococcus aureus. This toxin is classified as a superantigen because of its ability to directly bind with MHC II class molecules followed by activation of a large proportion of T cells bearing specific Vβ-T cell receptors. Commonly associated with classic food poisoning, SEB has also been shown to induce toxic shock syndrome, and is also considered to be a potential biological warfare agent because it is easily aerosolized. In the present study, we assessed the ability of indole-3-carbinol (I3C) and one of its byproducts, 3,3′-diindolylmethane (DIM), found in cruciferous vegetables, to counteract the effects of SEB-induced activation of T cells in mice. Both I3C and DIM were found to decrease the activation, proliferation, and cytokine production by SEB-activated Vβ8+ T cells in vitro and in vivo. Interestingly, inhibitors of histone deacetylase class I (HDAC-I), but not class II (HDAC-II), showed significant decrease in SEB-induced T cell activation and cytokine production, thereby suggesting that epigenetic modulation plays a critical role in the regulation of SEB-induced inflammation. In addition, I3C and DIM caused a decrease in HDAC-I but not HDAC-II in SEB-activated T cells, thereby suggesting that I3C and DIM may inhibit SEB-mediated T cell activation by acting as HDAC-I inhibitors. These studies not only suggest for the first time that plant-derived indoles are potent suppressors of SEB-induced T cell activation and cytokine storm but also that they may mediate these effects by acting as HDAC inhibitors.
Keywords: staphylococcal enterotoxin B; indole-3-carbinol; 3,3′-diindolylmethane; histone deacetylase; epigenetic regulation; inflammation
Introduction
Staphylococcal entertoxin B (SEB) is a 28-KDa protein belonging to a family of exotoxins secreted by the bacterium Staphylococcus aureus (S. aureus), a ubiquitous Gram-positive coccus that has been found to colonize both human and domestic animals as a common opportunistic pathogen. It is estimated that S. aureus can be found in 20% of the general population, with 60% of those being intermittent carriers, and has become a major cause of nosocomial infections and community-acquired diseases (Pinchuck, 2010). Growing worldwide concern has emerged with the discovery that many incidences of these nosocomial infections involve the methicillin-resistant (MRSA) strain of S. aureus, with a majority of this particularly dangerous antibiotic-resistant strain producing toxins, such as SEB (Boyce and Havill, 2005; Schmitz et al., 1997). Among food-borne diseases, which was estimated by the Centers for Disease Control (CDC) to affect approximately 76 million individuals resulting in 325,000 hospitalizations and 5,000 deaths in the US alone (Mead et al., 1999), staphylococcal entertoxin-contaminated food was reported to be the second most common cause (Pinchuck, 2010). SEB exposure, when ingested or inhaled, can produce mild food poisoning-like symptoms to more severe and potentially fatal conditions, such as toxic shock syndrome (Henghold, 2004).
SEB is an extremely potent antigen, classified as a superantigen, which bypasses normal processing by antigen-presenting cells (APCs), and results in nonspecific binding of the major histocompatibility complex class II (MHC-II) molecule on APCs with the variable region of the β chain of the T cell receptor (TCR) on T cells. This nonspecific binding leads to rapid T cell activation and uncontrolled release of cytokines, also referred to as a cytokine storm, producing an adverse inflammatory response (Baker and Acharya, 2004). It is estimated that while exposure of normal antigens can result in the activation of approximately 0.1% of host T cells, SEB exposure to the host can lead to the activation of 5 to 30% T cells (Reider et al., 2011). In addition to the robust activation of T cells, SEB was found to be remarkably stable in acidic environments, such as in the gastrointestinal tract, and highly resistant to both heat and proteolytic digestion (Ler et al., 2006). These properties of SEB, in addition its ability to become easily aerosolized, led the CDC to classify SEB as a category B priority agent for the potential use as a biological warfare weapon (Henghold, 2004). All of these factors illustrate the importance of discovering new therapies that would counteract the effects of SEB exposure. Inasmuch as, conventional antibiotic therapy may seem futile, given the emergence of the highly antibiotic-resistant strain of S. aureus, it would seem more appropriate to seek out treatments that could reduce the rapid T cell activation and inflammatory response caused by exposure of SEB to the host. In the current study, we investigated the potential role of naturally-occurring indole compounds, indole-3-carbinol (I3C) and one of its byproducts, 3,3′-diindolylmethane (DIM), in suppressing inflammation triggered by SEB.
I3C is an indole compound found in cruciferous vegetables, such as cabbage and broccoli, which is formed by the enzymatic breakdown of glucosinolate glucobrassicin by myrosinase. In acidic environments, I3C undergoes rapid self-condensation reactions that produce a variety of byproducts, with a major component being DIM (Aggarwal and Ichikawa, 2005). In terms of structure, DIM is formed by the combination of two I3C molecules (Fig. 1A) (Sarkar and Li, 2010). I3C and DIM have gained significant attention in the past based on their well-studied anti-cancer effects (Ahmad et al., 2010). However, the role of these compounds in exerting anti-inflammatory effects has emerged more recently (Busbee et al., 2013). DIM was shown to reduce the pro-inflammatory cytokines during dextran sodium sulfate (DSS)-induced experimental colitis in mice (Kim et al., 2009). Recent studies from our laboratory demonstrated that in experimental autoimmune encephalomyelitis (EAE), a mouse model for multiple sclerosis, both I3C and DIM ameliorated the clinical symptoms by reducing the infiltration of T cells into the brain, as well as decreasing the pro-inflammatory cytokines in the serum of diseased mice (Rouse et al., 2013).
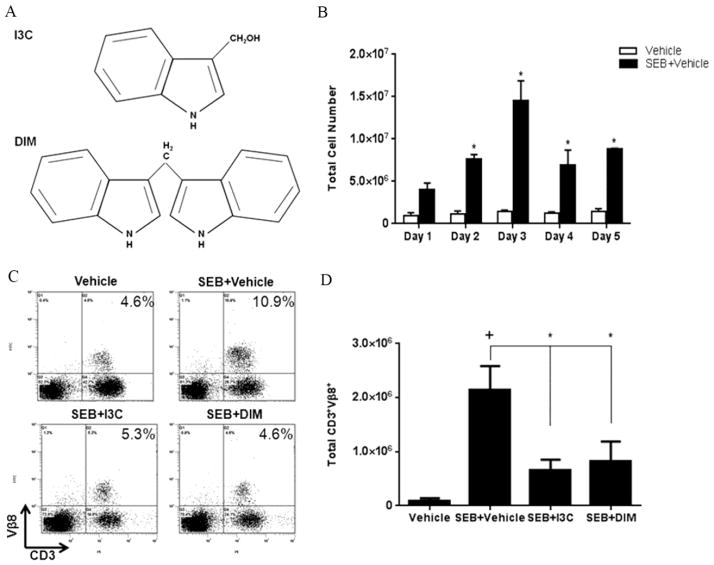
FIGURE 1. Treatment with I3C or DIM in vivo reduces percentage and number of SEB-specific Vβ8 T cells.
(A) Chemical structure of I3C and DIM. (B) C57BL/6 mice were given injections of 10ug of SEB in each hind footpad only once. Total cellularity from popliteal lymph nodes isolated from vehicle-treated versus SEB-treated mice was depicted. Mice were given ip injections of I3C or DIM (40mg/kg) for three consecutive days prior to SEB injection, which was followed by I3C and DIM treatment every other day. During the peak period of cell expansion on day 3, percentages (C) and total cell numbers (D) of CD3+Vβ8+ from popiteal lymph nodes were determined in each experimental group (n=5) using flow cytometry and antibodies for the respective markers. Statistical significance (p-value <0.05) was determined using GraphPad Prism analysis software with one-way ANOVA and Tukey’s multiple comparsion test (+ indicates significance compared to Vehicle group, and * indicates significance compared to SEB+Vehicle).
Histone acetylation is an epigenetic modification that is regulated through histone deacetylases (HDACs). The role of HDACs involves the removal of acetyl groups on lysine residues, and in the case of histone proteins, this action plays a key role in the regulation of gene transcription (Haberland et al., 2009; Strahl and Allis, 2000). The role of HDACs in SEB-induced inflammation as well as the anti-inflammatory properties of dietary indoles has not been previously investigated. In the present study, we investigated the efficacy of I3C and DIM in reducing the activation of T cells stimulated with SEB, with particular emphasis on the role of HDACs. Our data demonstrate for the first time that HDACs play a prominent role in the promotion of activation and pro-inflammatory cytokine release following SEB stimulation. Also, I3C and DIM suppress SEB-induced inflammation by acting as HDAC inhibitors.
Materials and Methods
Animals
Female C57BL/6 mice (aged 8–10 weeks) were purchased from the National Cancer Institute. All mice were housed at the AAALAC-accredited animal facility at the University of South Carolina, School of Medicine (Columbia, SC). All procedures were performed according to NIH guidelines under protocols approved by the Institutional Animal Care and Use Committee.
Effects of I3C and DIM on mice stimulated with SEB in vivo
To test the efficacy of treatment of I3C and DIM in an in vivo SEB mouse model, SEB, in sterile phosphate-buffered saline (PBS), was injected into each hind footpad of mice (10ug/footpad) only once, as previously described (Camacho et al., 2002; Fernández et al., 2006). For treatment groups, I3C and DIM, purchased from Sigma-Aldrich (St. Louis, MO), was administered intraperitonally (ip) at 40mg/kg in a total volume of 100ul in appropriate vehicle (2% DMSO in corn oil). Since SEB is a known superantigen that leads to a robust immune response and resulting cytokine storm, animals were treated with either I3C or DIM 24 hours prior to SEB injection to test whether these compounds could prevent or decrease this response. Subsequent treatments of I3C and DIM were given every other day for up to 5 days. Popiteal lymph nodes were excised from mice and made into single-cell suspensions by a tissue homogenizer. Cells were subjected to red blood cell lysis, counted, and stained with antibodies purchased from Biolegend (San Diego, CA) for CD3 and Vβ8 and analyzed by flow cytometry.
Effects of I3C, DIM, and inhibitors of HDACs on splenocytes in vitro
Spleens were excised from female C57BL/6 mice (aged 8–10 weeks) and placed in complete RPMI 1640 media supplemented with heat inactivated 10% fetal bovine serum, 10mM L-glutamine, 10mM HEPES, 50uM β-mercaptoethanol, and 100ug/ml penicillin/streptomycin. Tissues were homogenized into single-cell suspensions and subjected to red blood cell lysis. Cells were plated in a 96-well plate in 200ul of complete media at 1×106 cells per well in for 3, 6, 12, or 24 hours at 37°C and 5% CO2 with or without SEB-stimulation (1ug/ml) and with vehicle or I3C, DIM (100uM), trichostatin A (TSA) (10nM-1uM), MGCD0103 (1–20uM), or MC1568 (1–20uM). Vehicle for all compounds was dimethyl sulfoxide (DMSO), with a total volume of never exceeding 0.005% DMSO in complete medium per well. TSA, MGCD0103, and MC1568 were purchased from Selleck Chemicals (Houston, TX). Cells were harvested after the indicated time points and stained with CD69 antibody purchased from Biolegend (San Diego, CA) for flow cytometry analysis.
Measurement of cytokines from collected supernatants
Cell culture supernatants were collected after 24 hours from in vitro experiments described above. Cytokines levels were analyzed and quantified using individual enzyme-linked immunosorbent assay (ELISA) kits for interferon-gamma (IFN-γ), tumor necrosis factor-alpha (TNF-α), interleukin-2 (IL-2), and IL-6 purchased from Biolegend (San Diego, CA). All ELISAs were performed as per the manufacturer’s instructions.
RT-PCR for HDAC expression in CD3+cells
Expression of HDAC-I and HDAC-II mRNA from 6-hour in vitro cultures was determined by quantitative real-time PCR. In vitro cultures with or without SEB stimulation in the presence or absence of either I3C or DIM (100uM) were performed as described above. After 6 hour incubation, cells were collected and sorted using EasySep™ Mouse PE Positive Selection Kit from Stem Cell Technologies (Tukwila, WA) for expression of CD3. mRNA was isolated using RNeasy kit from Qiagen (Valencia, CA), and cDNA was synthesized using iScript cDNA synthesis kit from Bio-Rad (Hercules, CA). Quantitative rt-PCR was carried out using SsoAdvanced™ SYBR® Green Supermix from Bio-Rad (Hercules, CA) with mouse primers for HDAC-I and II (HDACs 1–10). Expression levels for all HDACs were normalized to GAPDH mRNA levels.
Western blots for histone H3 and acetylated histone H3 lysine 9 (H3K9Ac)
Whole cell lysates were prepared from sorted CD3+ 6-hour culture conditions mentioned above using RIPA Lysis Buffer System purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Protein concentrations were determined using Pierce BCA Protein Assay kit purchased from Thermo Scientific (Rockford, IL). Proteins were separated by SDS-page and transferred to nitrocellulose membranes using a semi-dry apparatus. Membranes were then placed in 5% dry milk blocking buffer for 1 hour at room temperature on a shaker. Membranes were than washed and incubated overnight at 4°C in primary antibodies for H3 (1:1000 dilution) and H3K9Ac (1:500 dilution), both purchased from Cell Signaling Technology (Beverly, MA). After the overnight incubation, membranes were washed and incubated with secondary antibody (anti-mouse IgG) for 1 hour at room temperature. Lastly, the membranes were washed and incubated in developing solution (Pierce ECL Western Blotting Subrate) purchased from Thermo Scientific (Rockford, IL) for 1 minute. Western blots were quantified using ImageJ software, and relative expression of H3K9Ac was corrected against histone H3 signal as a loading control.
Statistical Analysis
For the in vivo mouse experiments, 5 mice were used per experimental group. For in vitro assays, all experiments were performed in triplicate. For statistical differences, one-way ANOVA was calculated for each experiment. Tukey’s post-hoc test was performed to analyze differences between groups. A p value of ≤ 0.05 was used to determine statistical significance.
Results
I3C and DIM reduce number of T cells specifically stimulated by SEB in vivo
SEB is a superantigen that triggers a strong T cell response. In order to test the efficacy of I3C and DIM against SEB-induced inflammatory response in vivo, we injected C57BL/6 mice with SEB into footpads and studied the cell proliferation in the draining popliteal lymph nodes. The data indicated that the popliteal lymph node cellularity increased dramatically following SEB immunization, when compared to vehicle controls, with the response peaking on day 3 (Fig. 1B). In subsequent experiments, therefore, we used day 3 of SEB immunization to compare the effects of I3C and DIM.
Our lab had previously shown that a dose of 40mg/kg of either I3C or DIM was able to decrease cell-infiltration into the central nervous system in a mouse model of multiple sclerosis (Rouse et al., 2013). Therefore we used this dose to determine how effective these compounds would be against SEB-induced inflammation. We pre-treated mice with ip injections of I3C or DIM 24-hours before mice were given SEB, followed by treatment with I3C or DIM every other day to determine if either compound could decrease SEB-induced T cell proliferation. SEB is known to selectively activate and lead to the expansion of T cells, such as those bearing Vβ8 TCR (Marrack et al., 1990). Therefore, we examined the percentages (Fig. 1C) and total cell numbers (Fig. 1D) of CD3+ Vβ8+ cell populations isolated from popliteal lymph nodes on day 3 following SEB immunization. The CD3+ Vβ8+ T cell percentages expanded in lymph nodes of mice injected with SEB (10.9%) compared to vehicle-treated mice (4.6%), However, in mice treated with SEB+I3C or SEB+DIM, there was marked decrease in the percentages of these T cells (5.3% and 4.6% respectively) (Fig 1C) as well as the total cell numbers (Fig 1D).
Treatment of I3C and DIM leads to decreased activation of SEB-specific Vβ8+ T cells
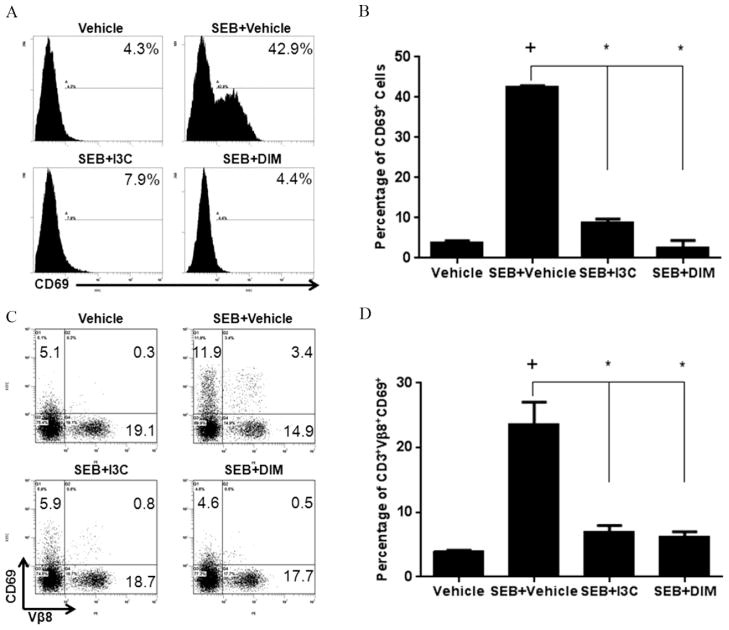
We next tested if I3C and DIM inhibited T cell activation. To that end, we activated spleen cells with SEB and assessed the upregulation of the activation marker CD69 on T cells (Lindsey et al., 2007). After 24-hour stimulation with SEB (1ug/ml), there was a significant increase of CD69 expression, both in density and percentages (Fig 2A and B) compared to vehicle-treated cultures. However, SEB-stimulated cultures treated with I3C or DIM (100uM) showed reduced expression of this surface marker (Fig 2A and B). In addition, we triple-stained cells with antibodies for CD3, Vβ8, and CD69 to access how expression of this surface marker changed in SEB-activated T cells specifically. We gated on CD3+ cell subsets and examined Vβ8 and CD69 expression (Fig. 2C). We noted a marked decline in the induction of CD69 expression on T cells in general, as well as those expressing Vβ8 specifically (Fig. 2D), in SEB-stimulated cultures treated with either I3C or DIM.
FIGURE 2. Treatment with I3C or DIM in vitro reduces the T cell activation with SEB.
Splenocytes from C57BL/6 were cultured in 96-well plates in the presence or absence of SEB (1ug/ml). Treatment groups were cultured with 100uM of I3C or DIM or vehicle. After 24 hours, cells were stained for CD3 and CD69, and cells gated for CD3 were analyzed CD69 by flow cytometry (A, B). Panel A shows a representative experiment and panel B depicts data from 5 mice/group. Cells were also triple-stained with antibodies for CD3, Vβ8, and CD69. Representative dot-plots of CD3-gated cells are shown (C), in addition to percentages of cells gated on CD3+ Vβ8+ that expressed CD69 (D). Statistical significance (p-value <0.05) was determined using GraphPad Prism analysis software with one-way ANOVA and Tukey’s multiple comparsion test (+ indicates significance compared to Vehicle group, and * indicates significance compared to SEB+Vehicle).
I3C and DIM reduce the production of pro-inflammatory cytokines by T cells after SEB stimulation
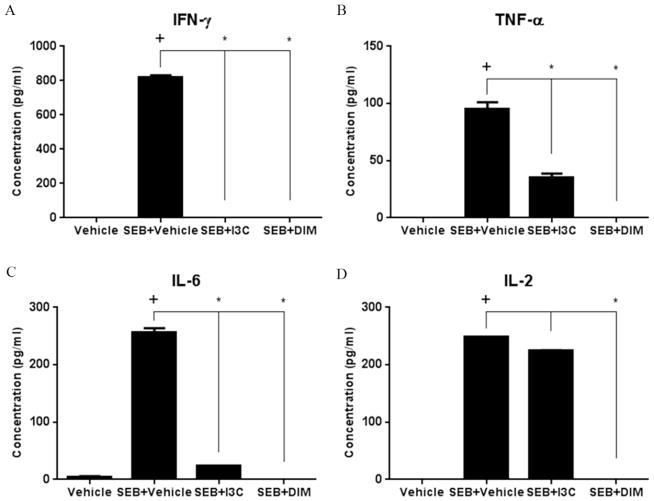
We next investigated the effect of I3C and DIM on pro-inflammatory cytokine production. SEB, being a superantigen, triggers the production of a variety of cytokines by T cells responsible for acute inflammation and shock, which include IFN-γ, TNF-α, IL-2, and IL-6 (Assenmacher et al., 1998; Heidemann et al., 2011; Krakauer et al., 2010). Production of these pro-inflammatory cytokines was measured in supernatants collected from 24-hour in vitro cultures of T cells that were stimulated with or without SEB, along with those that were treated with either I3C or DIM (Fig. 3A–D). As expected, production of all the tested pro-inflammatory cytokines increased in SEB-stimulated cultures compared to vehicle-treated ones. Importantly, cultures treated with I3C or DIM showed marked decrease in all cytokine levels, the exception being I3C which caused a modest decrease in IL-2 levels. Collectively, these studies revealed that both I3C and DIM were very effective at decreasing SEB-induced activation of T cells and production of pro-inflammatory cytokines.
FIGURE 3. Treatment with I3C or DIM decreases pro-inflammatory cytokine release after SEB stimulation.
Splenocytes from C57BL/6 mice were cultured for 24 hours with or without 1ug/ml of SEB, in the absence or presence of I3C or DIM (100uM). Supernatants were collected after 24 hours and ELISA assay was used to detect IL-2, IL-6, TNF-α, and IFN-γ. Statistical significance (p-value <0.05) was determined using GraphPad Prism analysis software with one-way ANOVA and Tukey’s multiple comparsion test (+ indicates significance compared to Vehicle group, and * indicates significance compared to SEB+Vehicle).
Pan-inhibitor of HDACs (TSA) reduces SEB-induced T cell activation and pro-inflammatory cytokine release
Recent studies have indicated that epigenetic regulation, such as histone acetylation and deacetylation, plays a crucial role in gene transcription. HDACs are a family of lysine deacetylases that target histones as well as a significant number of non-histone proteins (Choudhary et al., 2009). The classic HDAC family includes HDAC-I (HDAC1, HDAC2, HDAC3, and HDAC8) and HDAC-II (HDAC4, HDAC5, HDAC6, HDAC7, HDAC9, and HDAC10). Thus, we next considered the possibility that the indoles may suppress cytokine genes or T cell activation markers by modulating HDACs. However, because there are no previous studies on the role of HDACs on SEB-mediated activation of T cells and consequent cytokine storm, we first tested the role of HDACs in SEB-induced inflammation using HDAC inhibitors.
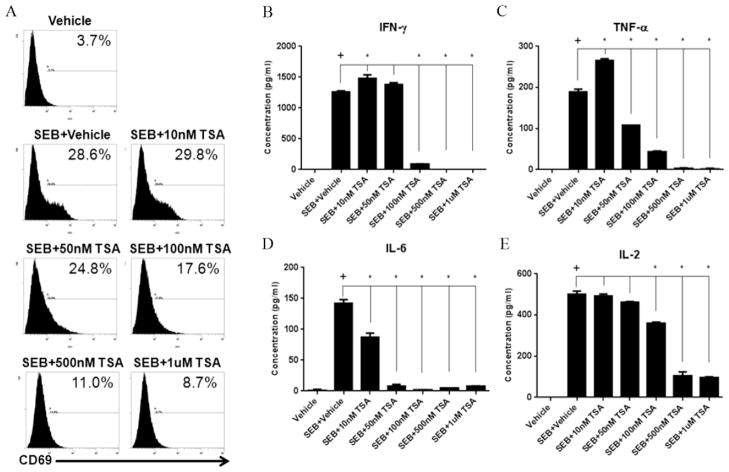
To this end, we investigated the effects of TSA, a pan-inhibitor of mammalian HDAC-I and HDAC-II (Vanhaecke, 2004). For these studies, we activated T cells with SEB in the presence of varying doses of TSA (10nM-1uM), and looked at CD69 expression and pro-inflammatory cytokine release. Interestingly, TSA, in a dose-associated manner, was able to decrease the induction of CD69 in SEB-activated cells (Fig. 4A) as well as the production of inflammatory cytokines such as IFN-γ, TNF-α, IL-2, and IL-6 (Fig. 4B–E). These data together suggested that pan-inhibition of HDACs suppresses SEB-induced T cell activation and cytokine production.
FIGURE 4. pan-HDAC inhibitor (TSA) reduces SEB-induced T cell activation and proinflammatory cytokine release.
Splenocytes from C57BL/6 female mice were cultured in 96-well plates in the presence or absence of SEB (1ug/ml). Treatment groups were given a range of TSA doses (10nM-1uM). After 24 hours, cells were stained with antibodies for CD69, followed by analysis using flow cytometry (A). Supernatants were collected after 24 hours and ELISA assay was performed to detect IL-2, IL-6, and TNF-α, and IFN-γ(B). Statistical significance (p-value <0.05) was determined using GraphPad Prism analysis software with one-way ANOVA and Tukey’s multiple comparsion test (+ indicates significance compared to Vehicle group, and * indicates significance compared to SEB+Vehicle).
Role of HDAC-I and II in SEB-induced T cell activation and pro-inflammatory cytokine release
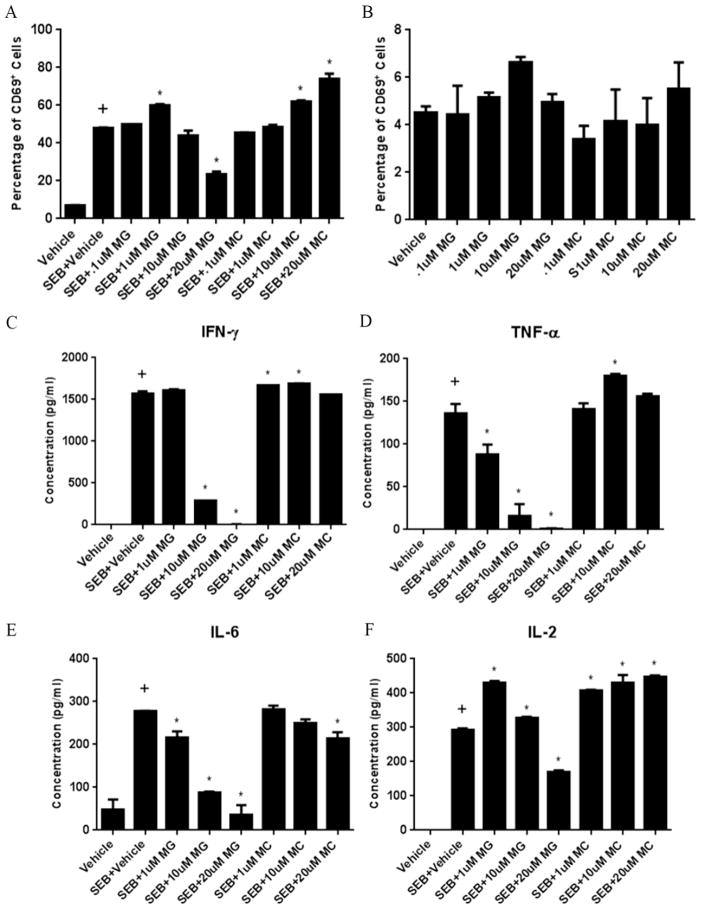
To further understand the contributions of different classes of HDACs in SEB-mediated activation of T cells, we incorporated the use of class-specific inhibitors in our in vitro studies. We used both MGCD0103 (MG), an inhibitor of isoforms of HDAC-I (Fournel et al., 2008), and MC1568 (MC), a potent and selective HDAC-II inhibitor (Duong et al., 2008), to ascertain any distinct effects that these two different classes of HDACs may have on cells stimulated with SEB. For these studies, we used the approximate half-maximal inhibitory concentrations (IC50) reported for these inhibitors, which consisted of a range of doses from 0.1–20uM. The data indicated that with increasing doses of the HDAC-I inhibitor, there was a marked decrease in CD69 expression. The only exception was the lower dose (1uM) of MGCD0103. Interesting the HDAC-II inhibitor showed opposite effects on CD69 expression inasmuch as with increasing doses of MC1568, there was a significant upregulation of CD69, particularly at the higher doses (10 and 20uM) (Fig 5A). In order to make sure that the observed effects were not due to the direct action of inhibitors themselves, and were specific to SEB-stimulated cultures, we also cultured MGCD0103 and MC1568 with naïve splenocytes using the same dose range (Fig. 5B). There were no significant changes in CD69 expression under these conditions, which showed that the downregulation of CD69 by MGCD0103 and the upregulation of this activation marker by MC1568 were dependent on the cells being activated by SEB.
FIGURE 5. Effect of specific inhibitors of Class I and Class II HDACs on SEB-induced T cell activation and proinflammatory cytokine release.
Splenocytes were cultured in 96-well plates in the presence or absence of SEB (1ug/ml). In addition, cultures received Class I HDAC inhibitor, MGCD0103 (MG), or Class II inhibitor, MC1568 (MC), at varying doses (0.1uM-20uM). After 24 hours, cells were stained using antibodies for CD69 and analyzed by flow cytometry (A). Additionally, naïve whole splenocytes cultures were analyzed for CD69 expression after 24 hours in the presence of the MGCD0103 and MC1568 at the various doses (B). Supernatants were collected after 24 hours and analyzed for IL-2, IL-6, TNF-a, IFN-γ (C). Statistical significance (p-value <0.05) was determined using GraphPad Prism analysis software with one-way ANOVA and Tukey’s multiple comparison test (+ indicates significance compared to Vehicle group, and * indicates significance compared to SEB+Vehicle).
Next, we looked at pro-inflammatory cytokine release in these culture conditions (Fig. 5C–F). The same distinct and opposite effects of HDAC-I and HDAC-II inhibitors was noted in cytokine responses, where increasing doses of the HDAC-I inhibitor decreased SEB-induced pro-inflammatory cytokine production, and higher doses of the HDAC-II inhibitor often caused increased induction of pro-inflammatory cytokines. The exception of this general trend was with IL-6, in which both inhibitors decreased production of this cytokine. The impact of this on inflammation was not clear inasmuch as IL-6 has been shown to exert both pro-inflammatory and anti-inflammatory effects (Wood et al., 2011).
Taken together, these studies demonstrated that SEB-induced activation of T cells and cytokine production can be regulated by specific HDAC-I and HDAC-II inhibitors. Furthermore, class I HDACs may play a role in promoting T cell activation and pro-inflammatory cytokine release by SEB, whereas class II HDACs may serve to downregulate this response.
I3C and DIM reduce the expression of class I HDACs in T cells stimulated with SEB
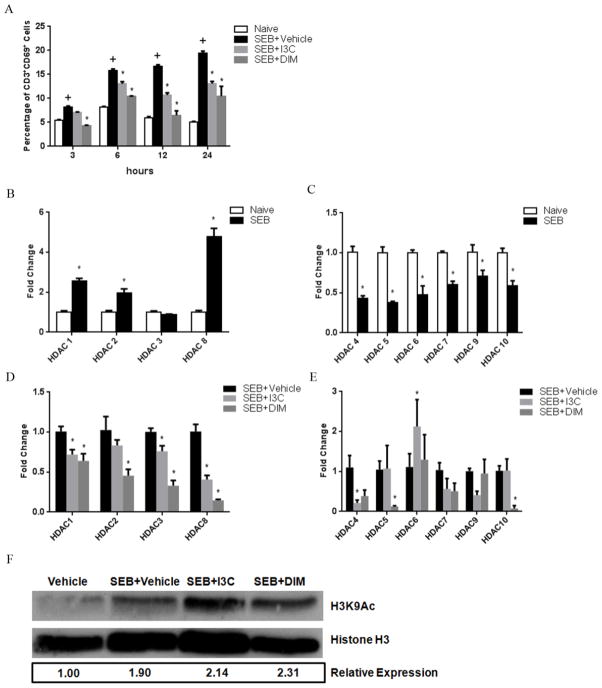
Next, we determined if I3C and DIM were modulating the expression of HDACs in SEB-activated T cells. For this purpose, we examined CD69 expression on T cells by culturing spleen cells with SEB in the presence of vehicle, I3C, or DIM at various time points (3, 6, 12, and 24 hrs) to determine what time would be best to examine HDAC mRNA expression. The basis for selecting this time point was to determine the earliest point at which not only SEB-stimulated cultures showed marked increase in CD69 compared to unstimulated cultures, but also when these SEB cultures treated with I3C or DIM showed significant reduction in the expression of this activation maker. As shown in Fig. 6A, there was a significant increase in CD69 expression when comparing naïve to SEB-treated cultures as early as 3 hours. However, it was not until 6 hours that both I3C and DIM were able to reduce the increase of CD69 expression in SEB-activated conditions (Fig. 6A). Therefore, we chose the 6-hour time point to investigate HDAC expression levels.
FIGURE 6. Effects of I3C and DIM on Class I and II HDACs in SEB-stimulated T cells.
Splenocytes were cultured in 96-well plates in the presence or absence of SEB (1ug/ml). Cultures also received I3C or DIM (100uM). The expression of CD69 on CD3+ was analyzed at various time points using antibody staining and flow cytometry among the experimental groups (A). After determining 6 hours was an appropriate time point to examine HDAC expression, RT-PCR was performed on samples. Samples consisted of RNA collected from PE-conjugated magnetic bead separated CD3+ T cell cultures for HDAC-I or HDAC-II. HDAC expression was compared between unstimulated and SEB-stimulated cultures for HDAC-I (B) and HDAC-II (C), followed by comparison between SEB-stimulated cultures to those treated with SEB+I3C or DIM for HDAC-I (D) and HDAC-II (E). Statistical significance (p-value <0.05) was determined using GraphPad Prism analysis software with one-way ANOVA and Tukey’s multiple comparison test (+ indicates significance compared to naive group, and * indicates significance compared to SEB+Vehicle). Western blot was performed for histone H3 and H3K9Ac in proteins isolated using RIPA buffer from CD3+ cells in all experimental groups (F). Numbers below the blots are relative expressons of H3K9 levels of experimental groups compared to vehicle group after normalization to histone H3 levels. Levels were determined using ImageJ software.
To determine any differences in HDAC expression, RNA was isolated from cells under the previously mentioned conditions after 6 hours. T cells (CD3+) were separated, and RT-PCR was performed looking at the expression of HDAC-I and HDAC-II (Fig. 6B–E). Interestingly, the data showed that after 6 hours of culture, SEB-activated T cells showed a significant upregulation in HDAC-I expression compared to unstimulated T cells (Fig. 6B). However, expression of all HDAC-II was significantly downregulated (Fig. 6C) in T cells activated with SEB. Even more so, SEB-activated T cells that were treated with I3C or DIM had a significant downregulation of HDAC-I expression when compared to SEB-activated T cells treated with only vehicle (Fig. 6D). However, their reduction of HDAC-II expression was mostly modest, with little to no significance observed (Fig. 6E). Together, these data indicated that not only did HDAC-I expression increase and HDAC-II decrease in T cells stimulated with SEB, but also I3C and DIM may act as HDAC-I inhibitors in SEB-activated T cells. Lastly, we wanted to determine if the decreased HDAC expression we observed in CD3+ T cells correlated with increased acetylation of H3K9, an important epigenetic modification often associated with actively transcribed promoter regions (Allan et al., 2012) and modified in T cells that become activated (Fields et al., 2002). To this end, we isolated proteins from sorted T cells for western blots to look at H3K9Ac expression in previously described 6-hour cultures. As shown in Fig. 6F, there was increased expression of H3K9Ac in T cells stimulated with SEB compared to unstimulated cells, which is to be expected since the expression of a majority of the HDACs, particularly HDAC-II, were shown to be decreased in SEB-stimulated T cells in Fig. 6C. More importantly, levels of H3K9Ac increased further in T cells stimulated with SEB when treated with either I3C or DIM. This increase in H3K9Ac with I3C and DIM treatment correlates well with these compounds decreasing HDAC expression (mainly HDAC-I) in T cells activated with SEB.
Discussion
In this study, we were able to demonstrate for the first time how effective I3C and DIM can be against T cell activation by SEB exposure. SEB is a super antigen that triggers a large proportion of T cells leading to cytokine storm and pathogenesis. Thus, it was remarkable to note that both in vivo and in vitro, I3C and DIM could decrease T cell activation and production of pro-inflammatory cytokines. In the current study, we also demonstrated for the first time that SEB-induced inflammation may be regulated by HDACs inasmuch as HDAC inhibitors were very effective in decreasing SEB-induced T cell activation and pro-inflammatory cytokine production. Additionally, we also noted that I3C and DIM were able to inhibit HDAC activity in SEB-triggered T cells, thereby suggesting that these indoles may act as HDAC inhibitors, which may account for their anti-inflammatory properties.
Much of the research involving I3C and DIM has concentrated on their anti-cancer properties, but more recently there is growing evidence that these compounds can exert anti-inflammatory effects, as shown in the current study. Chronic inflammation is thought to be a key factor in tumor progression, and often, inflammatory cells are active and abundant in tumor microenvironments (Mantovani et al., 2008; Coussens and Werb, 2002). This helps explain how DIM can be so effective at reducing both colonic inflammation and the development of tumorgenesis within the colon (Kim et al., 2009). Our laboratory recently reported that I3C and DIM were able to suppress the inflammatory response in the CNS of mice developing experimental autoimmune encephalitis (EAE) through the induction of regulatory T cells (Tregs) and suppression of Th17 cells (Rouse et al., 2013). Another report indicated that DIM was able exert anti-arthritic effects in a rat model of adjuvant-induced arthritis (AIA) by attenuating clinical indices indicative of suppression of inflammation in addition to reducing inflammatory cytokine production (Dong et al., 2010). DIM was also found to be effective in mice given topical applications of 12-O-tetradecanoylphorbol-13-acetate (TPA) on the ear or skin to induce inflammation (Kim et al., 2010). In these studies, DIM was able to reduce nuclear factor-kappa B (NF-κB) activation leading to the reduction of inflammatory mediators such as cyclooxygenase-2 (COX-2), IL-6, and inducible nitric oxide synthase (iNOS). The increased accumulation of macrophages in the epididymal adipose tissue of mice fed high-fat diets was reduced with i.p. injections of I3C, and in vitro studies also showed that I3C mixed in co-cultures of macrophages and adipocytes, led to decreases in inflammatory factors, IL-6 and nitrite production (Chang et al., 2011).
Several studies have linked the effects of I3C and DIM through their interaction with the aryl hydrocarbon receptor (AhR). Interaction with the AhR has been shown to play an important role in immune responses, particularly in T cell differentiation (Marshall and Kerkvliet, 2010), and ligands for this particular receptor show potential in acting as anti-inflammatory agents (Busbee et al., 2013). Our laboratory showed that the ability of I3C and DIM to suppress Th17 cells and induce Tregs was dependent on their interaction with the AhR in the EAE model (Rouse et al., 2013). More recently, in a study with the oxazolone-induced colitis model, a mouse model of human ulcerative colitis, researchers found DIM alleviated the disease by reducing Th2/Th17 responses and upregulating Tregs via the AhR signaling pathway (Huang et al., 2013). In dendritic cells (DCs) stimulated with LPS, I3C exhibited immunosuppressive and anti-inflammatory effects by reducing pro-inflammatory cytokine (IL-1β, IL-6, IL-12) release (Benson and Shepherd, 2011). In the same study, co-cultures with naïve T cells using DCs derived from bone marrow resulted in increased Treg frequency and IL-10 production when treated with I3C. In addition to the AhR, I3C and DIM have been known to interact with the estrogen receptor (ER), though many of these studies are restricted to the effects these dietary indoles have on various cancer cells, such as cell cycle arrest and apoptosis (Auborn et al., 2003; Wang et al., 2006; Mulvey et al., 2007).
One property that could explain why I3C and DIM are so effective in counteracting the activation and pro-inflammatory release by T cells stimulated with SEB is their ability to modulate HDAC expression, which was the focus of the current study. There are currently eighteen identified mammalian HDACs that are classified into 4 different groups based on their structure and homology to certain yeast proteins: class I HDACs (HDAC1, HDAC2, HDAC3, and HDAC8), class II HDACs (HDAC4, HDAC5, HDAC6, HDAC7, HDAC9, and HDAC10), class III HDACs (SIRT1-SIRT7), and class IV HDAC (HDAC11) (Haberland et al., 2009). HDACs have been shown to play an important roles in regulating inflammatory responses. Most of the studies which highlight the significance of HDACs in the inflammatory response used inhibitors of these particular proteins. Small molecule inhibitors of HDACs, such as TSA and suberoylanilide hydroxamic acid (SAHA), have been shown to exert anti-inflammatory effects in a variety of inflammatory models by several mechanisms that include the reduction of proinflammatory cytokine production (Dinarello et al., 2011). For example, in murine bone marrow-derived macrophages stimulated with lipopolysacchride (LPS), TSA was found to reduce the mRNA and protein levels of pro-inflammatory cytokines TNF-α, IL-6, and IL-1beta (IL-1β) (Han and Lee, 2009). TSA was found to suppress IL-6 production in rheumatoid arthritis synovial cells by mRNA decay as well (Grabiec et al., 2012). SAHA was able to reduce a plethora of pro-inflammatory cytokines, such as TNF-α, IL-1β, IL-6, and IFN-γ, in mice challenged with LPS (Leoni et al., 2002). Inhibitors of HDACs were also shown to be quite effective at ameliorating inflammatory models such as murine systemic lupus erythematosus (Mishra et al., 2003) and ulcerative colitis (Lührs et al., 2002). However, many of the HDAC inhibitors studied so far are broad spectrum inhibitors of HDACs, with few possessing any class-specific inhibition (Balasubramanian et al., 2009).
There is growing evidence that HDAC-I and HDAC-II may have, in addition to distinct structures and tissue distributions, unique functional roles (Morris and Monteggia, 2013). For example, in cancer cells, HDAC-I seem to be more important in promoting survival and proliferation (Dokmanovic and Marks, 2005). In both renal and prostate cancer patients, HDAC-I (HDAC1, HDAC2, and HDAC3) were found to be more highly expressed in these patients, and this was found to correlate with a poor prognosis (Weichert et al., 2008; Fritzsche et al., 2008). On the other hand, reduced expression of HDAC-II were indicative of poor prognosis in lung cancer patients (Osada et al., 2004). In our present study, we observed this same dual role of HDAC-I and HDAC-II in T cells stimulated with SEB, in which class HDAC-I appeared to be more important in promoting activation and pro-inflammatory cytokine release by SEB. It is interesting to note that DIM selectively induces proteasome-mediated degradation of HDAC-I, thereby downregulating their expression in human colon cancer cells, while having little to no effect on HDAC-II (Yongming et al., 2010). We observed the same trend in our SEB-activated T cells that were treated with either I3C or DIM. However, our study also showed that I3C and DIM were able to significantly modify some HDAC-II expression as well. I3C was shown to decrease HDAC4 expression, which could further explain the effectiveness of this compound as an anti-inflammatory agent since HDAC4 has been shown to mediate hypertension through vascular inflammation (Usui et al., 2012). Conversely, DIM was shown to decrease HDAC5 and I3C induced HDAC6 in SEB-activated T cells, which could indicate a more pro-inflammatory response. HDAC5 has been shown to act as a repressor of angiogenesis in endothelial cells (Urbich et al. 2009), and HDAC6 was linked to the promotion of acute inflammation and production of pro-inflammatory cytokines in colonocytes and mouse intestine (Nam et al., 2010). It is possible that these observations are very cell-type specific and not necessarily connected to how modulation of these HDACs by I3C and DIM in SEB-activated T cells would act. In support of this is our data showing that all of HDAC-II were downregulated in T cells activated by SEB, and thus, any changes in their expression by I3C and DIM may have minimal to no effects in promoting inflammation.
SEB and other staphylococcal entertoxins (SEs), are potent stimulators of the immune system and can cause a range of diseases in humans which include food poisoning, sepsis, and toxic shock syndrome (Le Loir et al., 2003). It is estimated that exposure to SEs can cause disease in concentrations as low as only 1ug (Pinchuk et al., 2010). Humans are particularly sensitive to SEB intoxication and when exposed to this toxin via the respiratory route, even low doses can cause lethal shock (Madsen, 2001). There are very few effective treatments currently available to treat something like SEB-induced toxic shock, and often treatments such as intravenous injections of immunoglobulins require treatment to occur close to the time of toxin exposure (Darenberg et al., 2004). With the growing occurrence of heavily antibiotic-resistant strains of bacteria producing toxins like SEB, it becomes important to find treatments and agents that would counteract the rapid T cell activation and cytokine storm that occurs when humans are exposed to such superantigens. Our current study presents two naturally-occurring products, I3C and DIM, as being very effective at reducing the effects of SEB exposure, particularly to T cells, the main components involved in promoting toxicity. With the growing occurrence of such toxin exposure in the human population and implications of SEB as a potential bioweapon, the discovery of just how effective these compounds are presents a significant finding in how to possibly counteract this potential problem.
Highlights.
I3C and DIM reduce SEB-induced T cell activation and inflammatory cytokines
Inhibiting class I HDACs reduce T cell activation and inflammatory cytokines
Inhibiting class II HDACs increase T cell activation and inflammatory cytokines
I3C and DIM selectively reduce mRNA expression of class I HDACs
Novel use and mechanism to counteract SEB with I3C and DIM
Acknowledgments
This work was supported by NIH grants [P01AT003961, R01AT006888, R01ES019313, R01MH094755] and VA Merit Award [1I01BX001357]. The funding agency had no role in the experimental design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations
- SEB
staphylococcal entertoxin B
- I3C
indole-3-carbinol
- DIM
3,3′-diindolylmethane
- HDAC
histone deacetylase
- HDAC-I
class I histone deacetylase
- HDAC-II
class II histone deacetylase
- TSA
trichostatin A
- MG
MGCD0103
- MC
MC1568
Footnotes
Conflict of Interest Statement
The authors declare no conflict of interests exist.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggarwal BB, Ichikawa H. Molecular targets and anticancer potential of indole-3-carbinol and its derivaties. Cell Cycle. 2005;4(9):1201–1215. doi: 10.4161/cc.4.9.1993. [DOI] [PubMed] [Google Scholar]
- Ahmad A, Sakr WA, Rahman KM. Anticancer properties of indole compounds: mechanism of apoptosis induction and role in chemotherapy. Curr Drug Targets. 2010;11(6):652–666. doi: 10.2174/138945010791170923. [DOI] [PubMed] [Google Scholar]
- Allan RS, Zueva E, Cammas F, Schreiber HA, Masson V, Belz GT, Roche D, Maison C, Quivy JP, Almouzni G, Amigorena S. An epigenetic silencing pathway controlling T helper 2 cell lineage commitment. Nature. 2012;487(7406):249–253. doi: 10.1038/nature11173. [DOI] [PubMed] [Google Scholar]
- Assenmacher M, Löhning M, Scheffold A, Manz RA, Schmitz J, Radbruch A. Sequential production of IL-2, IFN-gamma and IL-10 by individual staphylococcal enterotoxin B-activated T helper lymphocytes. Eur J Immunol. 1998;28(5):1534–1543. doi: 10.1002/(SICI)1521-4141(199805)28:05<1534::AID-IMMU1534>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Auborn KJ, Fan S, Rosen EM, Goodwin L, Chandraskaren A, Williams DE, Chen D, Carter TH. Indole-3-carbinol is a negative regulator of estrogen. J Nutr. 2003;133(7 Suppl):2470S–2475S. doi: 10.1093/jn/133.7.2470s. [DOI] [PubMed] [Google Scholar]
- Baker MD, Acharya KR. Superantigens: structure-function relationships. Int J Med Microbiol. 2004;293:529–537. doi: 10.1078/1438-4221-00298. [DOI] [PubMed] [Google Scholar]
- Balasubramanian S, Verner E, Buggy JJ. Isoform-specific histone deacetylase inhibitors: the next step? Cancer Lett. 2009;280:211–221. doi: 10.1016/j.canlet.2009.02.013. [DOI] [PubMed] [Google Scholar]
- Benson JM, Shepherd DM. Dietary ligands of the aryl hydrocarbon receptor induce anti-inflammatory and immunoregulatory effects on murine dendritic cells. Toxicol Sci. 2011;124(2):327–338. doi: 10.1093/toxsci/kfr249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce JM, Havill NL. Nosocomial antibiotic-associated diarrhea associated with enterotoxin-producing strains of methicillin-resistant staphylococcus aureus. Am J Gastroenterol. 2005;100:1828–1834. doi: 10.1111/j.1572-0241.2005.41510.x. [DOI] [PubMed] [Google Scholar]
- Busbee PB, Rouse M, Nagarkatti M, Nagarkatti PS. Use of natural AhR ligands as potential therapeutic modalities against inflammatory disorders. Nutr Rev. 2013;71(6):353–369. doi: 10.1111/nure.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho IA, Nagarkatti M, Nagarkatii PS. 2,3,7,8-Tetrachlorodibenzo-p -dioxin (TCDD) induces Fas-dependent activation-induced cell death in superantigen-primed T cells. Arch Toxicol. 2002;76:570–580. doi: 10.1007/s00204-002-0390-2. [DOI] [PubMed] [Google Scholar]
- Chang HP, Wang ML, Hsu CY, Liu ME, Chan MH, Chen YH. Suppression of inflammation-associated factors by indole-3-carbinol in mice fed high-fat diets and in isolated, co-cultured macrophages and adipocytes. Int J Obes (Lond) 2011;35(12):1530–1538. doi: 10.1038/ijo.2011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary C, Kumar C, Gnad F, Nielsen ML, Rehman M, Walther TC, Olsen JV, Mann M. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science. 2009;325(5942):834–840. doi: 10.1126/science.1175371. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darenberg J, Söderquist B, Normark BH, Norrby-Teglund A. Differences in potency of intravenous polyspecific immunoglobulin G against streptococcal and staphylococcal superantigens: implications for therapy of toxic shock syndrome. Clin Infec Dis. 2004;38(6):836–842. doi: 10.1086/381979. [DOI] [PubMed] [Google Scholar]
- Dinarello CA, Fossati G, Mascagni P. Histone deacetylase inhibitors for treating a spectrum of diseases not related to cancer. Mol Med. 2011;17(5–6):333–352. doi: 10.2119/molmed.2011.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dokmanovic M, Marks PA. Prospects: histone deacetylase inhibitors. J Cell Biochem. 2005;96:293–304. doi: 10.1002/jcb.20532. [DOI] [PubMed] [Google Scholar]
- Dong L, Xia S, Gao F, Zhang D, Chen J, Zhang J. 3,3′-Diindolylmethane attenuates experimental arthritis and osteoclastogenesis. Biochem Pharmacol. 2010;79(5):715–721. doi: 10.1016/j.bcp.2009.10.010. [DOI] [PubMed] [Google Scholar]
- Duong V, Bret C, Altucci L, Mai A, Duraffourd C, Loubersac J, Harmand PO, Bonnet S, Valente S, Maudelonde T, Cavailles V, Boulle N. Specific activity of class II histone deacetylases in human breast cancer cells. Mol Cancer Res. 2008;6(12):1908–1919. doi: 10.1158/1541-7786.MCR-08-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández MM, De Marzi MC, Berguer P, Burzyn D, Langley RJ, Piazzon I, Mariuzza RA, Malchiodi EL. Binding of natural variants of staphylococcal superantigens SEG and SEI to TCR and MHC class II molecule. Mol Immunol. 2006;43(7):927–938. doi: 10.1016/j.molimm.2005.06.029. [DOI] [PubMed] [Google Scholar]
- Fields PE, Kim ST, Flavell RA. Cutting edge: changes in histone acetylation at the IL-4 and IFN-gamma loci accompany Th1/Th2 differentiation. J Immunol. 2002;169(2):647–650. doi: 10.4049/jimmunol.169.2.647. [DOI] [PubMed] [Google Scholar]
- Fournel M, Bonfils C, Hou Y, Yan PT, Trachy-Bourget MC, Kalita A, Liu J, Lu AH, Zhou NZ, Robert MF, Gillespie J, Wang JJ, Ste-Croix H, Rahil J, Lefebvre S, Moradei O, Delorme D, Macleod AR, Besterman JM, Li Z. MGCD0103, a novel isotype-selective histone deacetylase inhibitor, has broad spectrum antitumor activity in vitro and in vivo. Mol Cancer Ther. 2008;7(4):759–768. doi: 10.1158/1535-7163.MCT-07-2026. [DOI] [PubMed] [Google Scholar]
- Fritzsche FR, Weichert W, Röske A, Gekeler V, Beckers T, Stephan C, Jung K, Scholman K, Denkert C, Dietel M, Kristiansen G. Class I histone deacetylases 1, 2 and 3 are highly expressed in renal cell cancer. BMC Cancer. 2008;8:381. doi: 10.1186/1471-2407-8-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabiec AM, Korchynskyi O, Tak PP, Reedquist KA. Histone deacetylase inhibitors suppress rheumatoid arthritis fibroblast-like synoviocyte and macrophage IL-6 production by accelerating mRNA decay. Ann Rheum Dis. 2012;71(3):424–431. doi: 10.1136/ard.2011.154211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberland M, Montgomery RL, Olson EN. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat Rev Genet. 2009;10:32–42. doi: 10.1038/nrg2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SB, Lee JK. Anti-inflammatory effect of trichostatin-A on murine bone marrow-derived macrophages. Arch Pharm Res. 2009;32(4):613–624. doi: 10.1007/s12272-009-1418-4. [DOI] [PubMed] [Google Scholar]
- Heidemann SM, Sandhu H, Kovacevic N, Phumeetham S, Solomon R. Detection of tumor necrosis factor-α and interleukin-6 in exhaled breath condensate of rats with pneumonia due to staphylococcal enterotoxin B. Exp Lung Res. 2011;37(9):563–567. doi: 10.3109/01902148.2011.611963. [DOI] [PubMed] [Google Scholar]
- Henghold WB., 2nd Other biologic toxin bioweapons: ricin, staphylococcal enterotoxin B, and trichothecene mycotoxins. Dermatol Clin. 2004;22:257–262. doi: 10.1016/j.det.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Huang Z, Jiang Y, Yang Y, Shao J, Sun X, Chen J, Dong L, Zhang J. 3,3′-Diindolylmethane alleviates oxazolone-induced colitis through Th2/Th17 suppression and Treg induction. Mol Immunol. 2013;53(4):335–344. doi: 10.1016/j.molimm.2012.09.007. [DOI] [PubMed] [Google Scholar]
- Kim EJ, Park H, Kim J, Park JH. 3,3′-diindolylmethane suppresses 12-O-tetradecanoylphorbol-13-acetate-induced inflammation and tumor promotion in mouse skin via the downregulation of inflammatory mediators. Mol Carcinog. 2010;49(7):672–683. doi: 10.1002/mc.20640. [DOI] [PubMed] [Google Scholar]
- Kim YH, Kwon HS, Kim DH, Shin EK, Kang YH, Park JH, Shin HK, Kim JK. 3,3′-diindolylmethane attenuates colonic inflammation and tumorigenesis in mice. Inflamm Bowel Dis. 2009;15(8):1164–1173. doi: 10.1002/ibd.20917. [DOI] [PubMed] [Google Scholar]
- Krakauer T, Buckley M, Fisher D. Murine models of staphylococcal enterotoxin B-induced toxic shock. Mil Med. 2010;175(11):917–922. doi: 10.7205/milmed-d-10-00148. [DOI] [PubMed] [Google Scholar]
- Le Loir Y, Baron F, Gautier M. Staphylococcus aureus and food poisoning. Genet Mol Res. 2003;2(1):63–76. [PubMed] [Google Scholar]
- Leoni F, Zaliani A, Bertolini G, Porro G, Pagani P, Pozzi P, Donà G, Fossati G, Sozzani S, Azam T, Bufler P, Fantuzzi G, Goncharov I, Kim SH, Pomerantz BJ, Reznikov LL, Siegmund B, Dinarello CA, Mascagni P. The antitumor histone deacetylase inhibitor suberoylanilide hydroxamic acid exhibits antiinflammatory properties via suppression of cytokines. Proc Natl Acad Sci USA. 2002;99(5):2995–3000. doi: 10.1073/pnas.052702999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ler SG, Lee FK, Gopalakrishnakone P. Trends in detection of warfare agents. Detection methods for ricin, staphylococcal enterotoxin B, and T-2 toxin. J Chromatogr A. 2006;1133:1–12. doi: 10.1016/j.chroma.2006.08.078. [DOI] [PubMed] [Google Scholar]
- Lindsey WB, Lowdell MW, Marti GE, Abbasi F, Zenger V, King KM, Lamb LS., Jr CD69 expression as an index of T-cell function: assay standardization, validation and use in monitoring immune recovery. Cytotherapy. 2007;9(2):123–132. doi: 10.1080/14653240601182838. [DOI] [PubMed] [Google Scholar]
- Lührs H, Gerke T, Müller JG, Melcher R, Schauber J, Boxberge F, Scheppach W, Menzel T. Butyrate inhibits NF-kappaB activation in lamina propria macrophages of patients with ulcerative colitis. Scand J Gastroenterol. 2002;37(4):458–466. doi: 10.1080/003655202317316105. [DOI] [PubMed] [Google Scholar]
- Madsen JM. Toxins as weapons of mass destruction. A comparison and contrast with biological-warfare and chemical-warfare agents. Clin Lab Med. 2001;21(3):593–605. [PubMed] [Google Scholar]
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- Marrack P, Blackman M, Kusnhir E, Kappler J. The toxicity of staphylococcal enterotoxin b in mice is mediated by T cells. J Exp Med. 1990;171:455–464. doi: 10.1084/jem.171.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall NB, Kerkvliet NI. Dioxin and immune regulation: emerging role of aryl hydrocarbon receptor in the generation of regulatory T cells. Ann NY Acad Sci. 2010;1183:25–37. doi: 10.1111/j.1749-6632.2009.05125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead PS, Slutsker L, Dietz V, McCaig LF, Bresee JS, Shapiro C, Griffin PM, Tauxe RV. Food-related illness and death in the united states. Emerg Infect Dis. 1999;5:607–625. doi: 10.3201/eid0505.990502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra N, Reilly CM, Brown DR, Ruiz P, Gilkeson GS. Histone deacetylase inhibitors modulate renal disease in the MRL-lpr/lpr mouse. J Clin Invest. 2003;111(4):539–552. doi: 10.1172/JCI16153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris MJ, Monteggia LM. Unique functional roles of class I and class II histone deacetylases in central nervous system development and function. Int J Dev Neurosci. 2013;31(6):370–381. doi: 10.1016/j.ijdevneu.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvey L, Chandrasekaran A, Liu K, Lombardi S, Wang XP, Auborn KJ, Goodwin L. Interplay of genes regulated by estrogen and diindolylmethane in breast cancer cell lines. Mol Med. 2007;13(1–2):69–78. doi: 10.2119/2006-00038.Mulvey. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam HJ, Kang JK, Kim SK, Ahn KJ, Seok H, Park SJ, Chang JS, Pothoulakis C, Lamont JT, Kim H. Clostridium difficile toxin A decreases acetylation of tubulin, leading to microtubule depolymerization through activation of histone deacetylase 6, and this mediates acute inflammation. J Biol Chem. 2010;285(43):32888–32866. doi: 10.1074/jbc.M110.162743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osada H, Tatematsu Y, Saito H, Yatabe Y, Mitsudomi T, Takahashi T. Reduced expression of class II histone deacetylase genes is associated with poor prognosis in lung cancer patients. Int J Cancer. 2004;112(1):26–32. doi: 10.1002/ijc.20395. [DOI] [PubMed] [Google Scholar]
- Pinchuk IV, Beswick EJ, Reyes VE. Staphylococcal enterotoxins. Toxins. 2010;2:2177–2197. doi: 10.3390/toxins2082177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reider SA, Nagarkatti P, Nagarkatti M. CD1d-independent activation of invariant natural killer T cells by staphylococcal enterotoxin B through major histocompatibility complex class II/T cell receptor interaction results in acute lung injury. Infect Immunity. 2011;79(8):3141–3148. doi: 10.1128/IAI.00177-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse M, Singh NP, Nagarkatti PS, Nagarkatti M. Indoles mitigate the development of experimental autoimmune encephalomyelitis by induction of reciprocal differentiation of regulatory T cells and Th17 cells. Br J Pharmacol. 2013;169(6):1305–1321. doi: 10.1111/bph.12205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar FH, Li Y. Indole-3-carbinol and prostate cancer. J Nutr. 2004;134:3493S–3498S. doi: 10.1093/jn/134.12.3493S. [DOI] [PubMed] [Google Scholar]
- Schmitz FJ, MacKenzie CR, Geisel R, Wagner S, Idel H, Verhoef J, Hadding U, Heinz HP. Enterotoxin and toxic shock syndrome toxin-1 production of methicillin resistant and methicillin sensitive staphylococcus aureus Strains. Eur J Epidemiol. 1997;13:699–708. doi: 10.1023/a:1007357206672. [DOI] [PubMed] [Google Scholar]
- Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403:41–45. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- Urbich C, Rössig L, Kaluza D, Potente M, Boeckel JN, Knau A, Diehl F, Geng JG, Hofmann WK, Zeiher AM, Dimmeler S. HDAC5 is a repressor of angiogenesis and determines the angiogenic gene expression pattern of endothelial cells. Blood. 2009;113(22):5669–5679. doi: 10.1182/blood-2009-01-196485. [DOI] [PubMed] [Google Scholar]
- Usui T, Okada M, Mizuno W, Oda M, Ide N, Morita T, Hara Y, Yamawaki H. HDAC4 mediates development of hypertension via vascular inflammation in spontaneous hypertensive rats. Am J Physiol Heart Circ Physiol. 2012;302(9):H1894–1904. doi: 10.1152/ajpheart.01039.2011. [DOI] [PubMed] [Google Scholar]
- Vanhaecke T, Papeleu P, Elaut G, Rogiers V. Trichostatin A-like hydroxamate histone deacetylase inhibitors as therapeutic agents: toxicological point of view. Curr Med Chem. 2004;11(12):1629–1643. doi: 10.2174/0929867043365099. [DOI] [PubMed] [Google Scholar]
- Wang TT, Milner MJ, Milner JA, Kim YS. Estrogen receptor alpha as a target for indole-3-carbinol. J Nutr. 2006;17(10):659–664. doi: 10.1016/j.jnutbio.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Weichert W, Röske A, Gekeler V, Beckers T, Stephan C, Jung K, Fritzsche FR, Niesporek S, Denkert C, Dietel M, Kristiansen G. Histone deacetylases 1, 2 and 3 are highly expressed in prostate cancer and HDAC2 expression is associated with shorter PSA relapse time after radical prostatectomy. Br J Cancer. 2008;98(3):604–610. doi: 10.1038/sj.bjc.6604199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood MW, Breitschwerdt EB, Gookin JL. Autocrine effects of interleukin-6 mediate acute-phase proinflammatory and tissue-reparative transcriptional responses of canine bladder mucosa. Infect Immun. 2011;79(2):708–715. doi: 10.1128/IAI.01102-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yongming L, Xia L, Bin G. Chemopreventative agent 3,3′-diindolylmethane selectively induces proteasomal degradation of class I histone deacetylases. Cancer Res. 2010;70(2):646–654. doi: 10.1158/0008-5472.CAN-09-1924. [DOI] [PMC free article] [PubMed] [Google Scholar]