Abstract
Background and Objectives
White matter hyperintensities (WMH) on brain MRI are associated with cognitive and mobility impairment in older adults. We examined whether WMH in tracts in older adults with mobility impairment are linked to outcomes of gait rehabilitation interventions.
Design
A 12-week randomized controlled single-blind trial.
Setting
University-based mobility research laboratory.
Participants
Ambulatory adults aged 65 and older with mobility impairment.
Intervention
A conventional gait intervention focusing on walking, endurance, balance, and strength (WEBS, n=21) compared to a task-oriented intervention focused on timing and coordination of gait (TC, n=23).
Measurements
We measured self-paced gait speed over an instrumented walkway, pre and post intervention, and quantified WMH and brain volumes on pre-intervention brain MRI using an automated segmentation process. We overlaid a white matter tract atlas on the segmented images to measure tract WMH volumes and normalized WMH volumes to total brain volume. Aggregate WMH volumes in all white matter tracts and individual WMH volumes in specific longitudinal tracts (the superior longitudinal fasciculus, inferior longitudinal fasciculus and the fronto-occipital fasciculus) and cingulum were obtained.
Results
Gait speed gains in the TC group were of the same magnitude, independent of the WMH volume measures in all except the cingulum. However, in the WEBS group, gain in gait speed was smaller with greater overall tract WMH volumes (P<0.001) and with greater WMH volume in the three longitudinal tracts (P< 0.001 to 0.025).
Conclusion
Gains in gait speed with two types of gait rehabilitation are associated with individual differences in WMH. Task-oriented therapy that targets timing and coordination of gait may particularly benefit older adults with WMH in brain tracts that influence gait and cognition.
INTRODUCTION
White matter hyperintensities (WMH) in the brain are associated with reduced gait speed and increased gait variability in older adults.1-3 WMH burden is also linked to impairments in executive function.4,5 In older adults without cognitive impairment or dementia, gait characteristics are associated with cognitive abilities such as executive function, processing speed and visuospatial attention. 6-8 This association between altered gait and cognition with aging is likely due to disruption of longitudinal tracts within the brain, especially those located in periventricular and subcortical regions that link motor function and executive control of gait. 9-11 WMH are common in older adults. 12 Their presence in older adults with mobility impairment typically involves periventricular and deep white matter regions.9,10,13 WMH in these longitudinal tracts can interfere with bidirectional transfer of information between key motor and cognitive cortical regions.9,11 Since rehabilitation of persons with gait disorders is an important aspect of geriatric care, we are particularly interested in whether WMH in these tracts are linked to benefit from gait rehabilitation, and whether the type of gait intervention matters.
White matter fiber tracts serve to connect different brain regions and likely play an important role in coordinating complex functions. Brain regions that are known to play a major role in motor skill performance include the premotor region, basal ganglia, supplemental motor area, cerebellum, and even the hippocampus,14,15 while those involved in attention and executive functions include the prefrontal cortex, anterior cingulate, inferior parietal lobule, posterior parietal cortex and other subcortical areas. 16 The regions associated with executive function are also associated with gait, possibly due to the need for higher order processing in the face of complex tasks and environments. 7,17,18 With age, information transfer between these regions can be disrupted by age-related damage to any part of the tracts and can lead to decline in their purported functions. The longitudinal tracts that link brain regions involved in executive function and visuoperception with those involved in gait control are the superior longitudinal fasciculus (SLF), inferior longitudinal fasciculus (ILF) and inferior fronto-occipital fasciculus (IFO).19-21 The SLF is made up of three subcomponents that traverse the core of white matter in the parietal and frontal lobe; the ILF lies in the white matter between the striatum and parieto-occipital and temporal lobes; the IFO runs in the white matter above the caudate, between the corpus callosum and corona radiate.22 The superior and inferior portions of the cingulum bundle are major pathways within the limbic system and between the limbic and the prefrontal and premotor cortices, and they play an important role in emotional and cognitive functions. 23 Therefore, we expect that the WMH burden in the SLF, ILF and IFO, but not the cingulum, may be associated with gait rehabilitation outcomes depending on type of rehabilitation.
We previously developed and tested a novel approach to gait rehabilitation that is focused on the timing and coordination of gait (TC) and the motor control aspect of walking, as opposed to traditional gait rehabilitation that focuses on walking practice, endurance, strength and balance (WEBS). We found that the TC approach led to greater gains in gait speed, energy cost of walking and self-reported walking confidence compared to the conventional WEBS approach of rehabilitation.24 The TC interventions incorporate theories of motor-skill learning by focusing on complex pattern walking, adaptive stepping and performing secondary tasks that may facilitate the gait pattern while walking.24 Here, we explored this data to determine whether total WMH burden in all tracts as well as WMH burden specifically in the SLF, ILF, IFO and cingulum (superior and inferior fibers) are associated with gain from gait rehabilitation, and whether the impact is different in the two types of therapy.
METHODS
Study design
We analyzed data from a 12 week randomized controlled trial of two exercise interventions aimed at improving mobility in community-dwelling older adults with mobility impairment. 24 Both interventions were protocol-driven and were conducted by trained physical therapists who conducted sessions in groups of two to three participants, lasting 60 minutes for 12 weeks. The interventions were conducted at separate times to avoid cross contamination. The conventional gait rehabilitation involved walking practice, progressive strengthening exercises, balance tasks, and endurance training (WEBS). This program began with trunk and leg stretches and was followed by strengthening using progressive resistance exercises of lower extremities, balance exercises and endurance training either with a seated, stair climbing-like activity, or a stationary cycle.24-26 The experimental therapy focused on motor control skills of timing and coordination (TC) during stepping and walking, designed to integrate motor planning, muscle activity and limb positioning in phase of gait with the goal of walking. This program of task oriented stepping and walking patterns included secondary tasks while walking to facilitate the task focus. For example, bouncing a ball was added to facilitate forward momentum in gait, and treadmill walking facilitated the timing of stepping and defined the speed for brief walking intervals.24-26 Time spent on walking in the two interventions was monitored to be equal. Study personnel, masked to the treatment arm, collected data before and after the 12-week intervention. The study was approved by the Institutional Review Board and informed consent was obtained from all study participants.
Participants
This study included community-dwelling ambulatory older adults aged 65 years or older with evidence of mobility impairment. Mobility impairment was defined as both slow gait speed and abnormal gait variability. Eligible gait speed was between 0.6m/s and 1.0m/s, and gait variability was either or both of increased step-length variability (coefficient of variation (CV) of greater than 4.5%) and step-width variability (CV of less than 7% or greater than 30%).24 Additionally, for inclusion in the study participants had to score greater than or equal to 24 on the Mini-mental Status Examination (MMSE)27 and have medical clearance for participation in mild to moderate intensity exercise from their primary physician. Use of an assistive device other than a straight cane for ambulation, persistent lower extremity pain not controlled by medication that limited walking on most days of the week, dyspnea at rest or use of supplemental oxygen, less than functional lower extremity muscle strength,28 progressive neuromuscular disorders, a fused lower extremity joint or amputation, uncontrolled hypertension, depression, recent MI, stroke and a recent hospitalization or major surgery were considered exclusionary. Of the 50 subjects in the trial, 6 either did not have post intervention data or did not undergo MRI due to contraindications for scanning, so were not included in this analysis. All completers in the study participated in a minimum of 22 of the 24 exercise sessions.
Twenty-one participants had the WEBS intervention (mean age, 78 years) and 23 participants had the TC intervention (mean age, 76 years) (Table 1).
Table 1.
Participant characteristics by intervention arm (WEBS: walking, endurance, balance strength training; TC: timing and coordination training).
| WEBS group | TC group |
P value / chi-square |
|
|---|---|---|---|
| N | 21 | 23 | |
| Age, years | 78 ± 5 | 76 ± 6 | 0.2 |
| Female, n (%) | 11 (55%) | 19 (82%) | 0.1 |
| Black, n (%) | 2 (10%) | 4 (17%) | 0.7 |
| Comorbidities, Index (mean ± (SD)) |
2 ± 1 | 3 ± 1 | 0.2 |
| GDS, 0 to 15 (mean ± (SD)) | 1.9 ± 1.4 | 1.4 ± 1.3 | 0.6 |
| MMSE, 0 to 30 (mean ± (SD)) |
29 ± 1 | 29 ± 1 | 0.3 |
| DSST, (number correct, mean ± (SD)) |
45 ± 8 | 45 ± 11 | 0.8 |
| Trails B time, (sec, mean ± (SD)) |
127 ± 56 | 129 ± 73 | 0.9 |
| SPPB, 0 to 12, mean ± (SD)) | 8 ± 2 | 9 ± 2 | 0.3 |
| Pre-intervention gait speed (m/sec, mean ± (SD)) |
0.81 ± 0.12 | 0.87 + 0.12 | 0.1 |
| Post-intervention gait speed (m/sec, mean ± (SD)) |
0.96 ± 0.2 | 1.08 ± 0.13 | 0.02 |
Measures
Brain imaging
We quantified WMH volumes on brain MRI images using a fuzzy-connected algorithm and seed selection that underwent multiple automated processes.29 First, skull and surrounding soft-tissue were stripped from the underlying brain on T1-weighted images using the Brain Extraction Tool,30 which was then used as a mask to remove brain from skull from the correspondingly located T2-FLAIR images at that same voxel-size.31 Subsequently, we used an automated WMH segmentation method that identified and clustered WMH based on voxel intensities on T2-FLAIR images.29 Total brain volume constituted the sum of volumes of individual tissue classes - grey matter, white matter and cerebrospinal fluid obtained using the Automated Labeling Pathway.32
We identified white matter tracts regions using the John Hopkins University (JHU) White Matter Atlas33 and overlaid these on the WMH segmented images to obtain WMH volume for white matter tracts (WMH registered as voxels, were then converted to volume (1 voxel= 4.2 mm3). We aggregated the WMH volumes for all major white matter tracts outlined in the JHU atlas and labeled this as the overall tract WMH volume. For this analysis, we focused on individual WMH volumes in the longitudinal tracts (SLF, ILF, IFO) and the cingulum (superior and inferior portions). WMH volumes for the SLF included all its three subdivisions. We aggregated the WMH volumes within the regions of interest in both cerebral hemispheres. To account for individual differences in brain size and avoid spuriously inflating WMH volume we normalized the WMH volumes to the total brain volume by dividing individual’s WMH volume by his/her total brain volume. Normalized total tract WMH volumes and the individual WMH volumes in bilateral SLF, ILF, IFO and the superior and inferior fibers of the cingulum were used as independent variables in the analysis.
Gait speed
This analysis focuses on gait speed as the primary outcome, because gait speed is associated with white matter disease, executive function and adverse outcome in older adults34-38. We averaged the gait speed over two traverses across a 4m long automated walkway, the GaitMatII (EQ Inc., Chalfonte, PA). Two practice walks preceded data collection. Gait acceleration and deceleration at both ends of the walkway was excluded in the data collection. The change in gait speed following the 12-week rehabilitation intervention was the dependent variable in this analysis.
Mini-Mental Status Examination (MMSE)
The MMSE is a widely used screening instrument for dementia and has a high sensitivity and specificity for distinguishing moderate degree of cognitive impairment from normal cognition.27 It includes brief assessment of orientation, memory, language and visuo-constructive abilities, and scores range from 0 to 30, high scores indicating better cognitive status.
Short Physical Performance Battery (SPPB)
The SPPB provides an objective measure of lower-extremity function in older adults. It is scored on a scale of 0 (unable to perform) to 4 (fastest time) for each of the three timed components namely, gait-speed over 4 meters, standing-balance and chair stand providing a total range of 0 to 12.39
Executive function measures
Executive functions are higher-order cognitive processes that include judgment, decision making, processing information, planning, organization and conceptualization that are required in daily activities. Both the Digit Symbol Substitution Test (DSST)40 and Trail B are timed tasks and measure specific aspects of executive function abilities.41,42
DSST assess speed of processing speed and performance that requires individuals to draw specific symbols for each digit in a series of numbers in a 90 second interval by referring to a list of digits and matching symbols provided on the sheet.40 Participants are instructed to perform the test as fast as possible aiming to fill in the boxes with symbols corresponding to the number. Participants have to discriminate symbols by its spatial organization, process matching symbols to specific digits, memorize the symbols as they perform the task in the time allotted. Higher scores indicate better performance.
Trails B is a timed-test of speed for visual search, attention, mental flexibility and motor function. Trails B tests the ability to shift attention during an on-going activity and also reallocate attention to more than one stimulus at a time.41,42 Higher scores indicate poorer performance.
Comorbidities Index
Information on comorbidities was based on self-report of the 18 most common medical conditions. Eight domains (cardiovascular, respiratory, musculoskeletal, neurological, cancer, diabetes, sensory) were derived to obtain the comorbidity index.26
Geriatric Depression Scale (GDS)
The GDS is a screening tool ranging from 0 to 30 and was used to detect depressive symptoms in this sample at baseline. It is simple to administer and does not require any specific skills in its administration.43
Statistical analysis
We used independent samples t- and chi-square tests, as appropriate, to compare baseline differences between the two intervention groups. We fit a series of linear models with pre- to post-intervention change in gait speed as the dependent variable; intervention (WEBS/TC), each measure of WMH volume (overall and individual tracts) and their interaction term as independent variables of primary interest; and pre-intervention gait speed as a covariate. Statistical significance of the interaction term was interpreted as evidence of differential associations between WMH volume and gait speed change in the two intervention groups. Subsequently, from the same models, we extracted regression coefficients corresponding to intervention group specific slopes (β) and their statistical significance to quantify the association between WMH volume and gait speed change. We performed the statistical analysis using SAS® version 9.2 (SAS Institute, Inc., Cary, North Carolina).
RESULTS
There were no significant differences between the two study arms in the demographic measures, physical function, general cognitive and executive function measures, or tract WMH burden (Table 1). Overall tract WMH volume ranged from almost none to 5.6% of total brain volume, while the individual tract WMH volumes ranged from almost none to 2.5% of total brain volume (Table 2).
Table 2.
White matter hyperintensities (WMH) volume normalized to total brain volume in the two groups (WEBS: walking, endurance, balance strength training; TC: timing and coordination training).
| Tracts | WEBS group | TC group | P value |
|---|---|---|---|
| Overall tracts | 5.4 × 10−3 ± 6.4 × 10−3 |
7.1 × 10−3 ±11.2 × 10−3 |
0.6 |
| ILF | 1.3 × 10−3 ± 1.2 × 10−3 |
1.3 × 10−3 ±2.1 × 10−3 |
0.9 |
| IFO | 2.4 × 10−3 ± 2.2 × 10−3 |
2.8 × 10−3 ± 4.2 × 10−3 |
0.7 |
| SLF | 0.7 × 10−3 ± 0.9 × 10−3 |
1.6 × 10−3 ± 5.1 × 10−3 |
0.4 |
| Cingulum (superior fibers) |
0.3 × 10−3 ± 0.7 × 10−3 |
0.4 × 10−3 ± 1.0 × 10−3 |
0.5 |
| Cingulum (inferior fibers) |
0.2 × 10−3 ± 0.4 × 10−3 |
0.2 × 10−3 ± 0.6 × 10−3 |
0.4 |
WMH: white matter hyperintensities; SLF: superior longitudinal fasciculus; ILF: inferior longitudinal fasciculus; IFO: inferior frontooccipital fasciculus.
The pre-intervention gait speed in both groups was not significantly different (Table 1). Within each group, gait speed following the intervention was significantly greater than the pre-intervention gait speed (0.21 m/sec in TC and 0.15 m/sec in WEBS group, P<0.001). However, the TC group had a greater gain in gait speed following the intervention than the WEBS group)24(P=0.02, effect size (Cohen’s d= 0.2)).
Across groups at baseline (n=44) we found a significant correlation between DSST score and total tract WMH volume (r=−0.332, P=0.028) and WMH volume in ILF (r=−0.497, P=0.001), IFO (r=−0.391, P= 0.009), inferior cingulum (r=−0.322, P= 0.033) but not in the SLF (r=−0.224, P=0.143) and superior cingulum (r=−205, P=0.182). Baseline Trails B score correlated with total tract WMH volume (r=0.370, P=0.014) and WMH volume in SLF (r=0.368, P=0.014), ILF (r=0.491, P=0.001), IFO (r=0.421, P= 0.004), superior cingulum (r=0.276, P=0.070) but not with the inferior superior cingulum (P=0.5). Baseline gait speed did not correlate with any of the baseline executive function measures or WMH tract volume measures.
Association of total WMH burden with response to interventions
The overall tract WMH volume was differentially associated in the two interventions with outcome of intervention as measured by increase in gait speed (group × WMH interaction, P =0.009). There was no significant association between improvement in gait speed and overall tract WMH volume in those receiving the TC intervention (slope β=−2.633, P=0.3). However, in those receiving the WEBS intervention, each additional 1% increase in overall tract WMH volume was associated with a reduction in gait speed improvement by 0.16 m/s (β=−16.441, P<0.001) (Figure 1).
Figure 1.
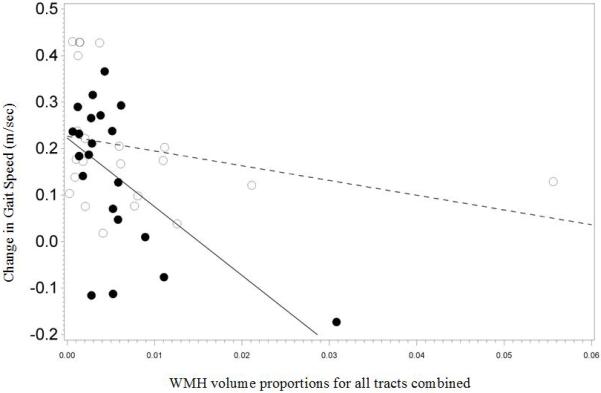
Association between improvement in gait speed and overall tract white matter hyperintensities (WMH) volume proportional to total brain volume in the two intervention arms (Timing and coordination training (TC): open circles, walking, endurance, balance strength training (WEBS): closed circles).
Association of WMH burden by individual tracts with response to interventions
Pre-intervention WMH volume measures in the SLF, ILF and IFO was associated with gain in gait speed following intervention (group × WMH interaction P =0.023 for SLF, 0.007 for ILF and 0.008 for IFO; Figure 2). There were no significant associations in the TC-group (β=−3.577, P=0.5 for SLF, β=−14.620, P=0.3 for ILF, β=−8.647, P=0.2 for IFO). However, in those receiving the WEBS intervention, for each additional 0.1% increase in WMH volume in the SLF, ILF and IFO was associated with a reduction in gait speed improvement by 0.087 m/s (β=−87.592, P=0.017), 0.088 m/s (β=−88.223, P<0.001) and 0.047 m/s (β=−47.051, P<0.001) respectively. WMH volume in the cingula was not associated with gain in gait speed following intervention (group × WMH interaction P= 0.5 (superior fibers) and P=0.4 (inferior fibers)). There was no significant association between gain in gait speed and WMH in superior or inferior portions of the cingulum in the TC (P=0.3 and 0.5 (superior and inferior respectively)) or the WEBS group (P=0.3 for both; figures not shown).
Figure 2.
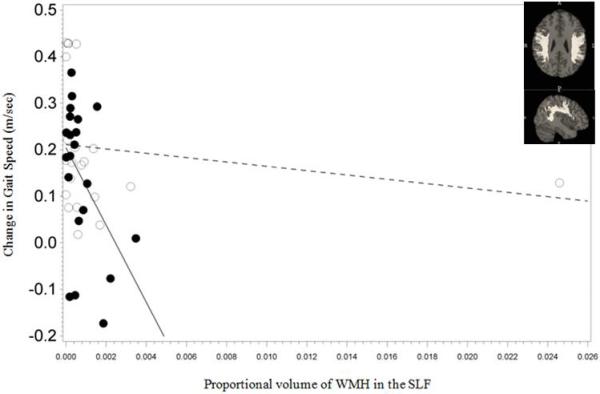
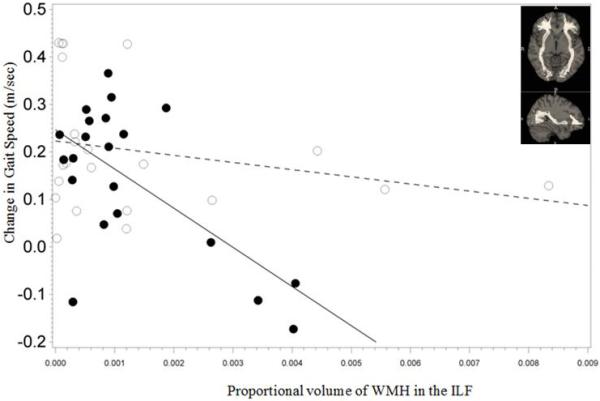
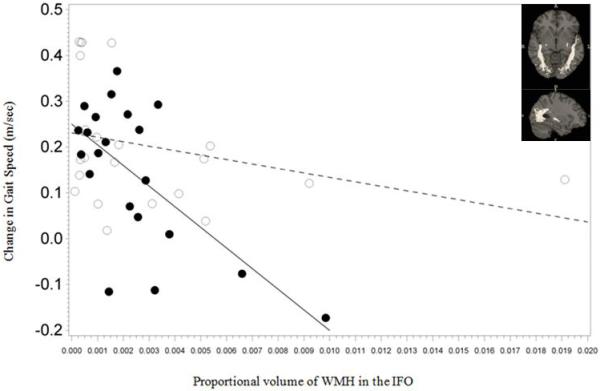
Association between improvement in gait speed and white matter hyperintensities (WMH) volume proportional to total brain volume in the three longitudinal tracts (depicted as figure inserts) in the two intervention arms (Timing and coordination training (TC): open circles, walking, endurance, balance strength training (WEBS): closed circles).
2a: Superior Longitudinal Fasciculus (SLF)
2b: Inferior longitudinal fasciculus (ILF)
2c.Inferior fronto-occipital fasciculus (IFO):
Given the distribution of WMH tract burden among participants, with two subjects (one in each treatment arm) having especially high volumes, we considered that the results might have been distorted by these two outliers. Hence, as a sensitivity analysis, we re-examined our findings without the data from these two subjects. For those receiving the TC intervention, there was no significant association between magnitude of gain in gait speed and increase in overall tract WMH volume (β=−9.877, P=0.1) or individual WMH volumes in the 3 longitudinal tracts (β=−21.913 to −63.051, P= 0.1 to 0.2). For those receiving the WEBS intervention, each 1% increase in overall tract WMH volume was associated with a reduced improvement in gain in gait speed by 0.24 m/sec (β=−24.367, P=0.03) and each 0.1% increase in WMH volume in the ILF and IFO was associated with a reduced improvement in gain in gait speed by 0.07 m/sec (β=−70.938, P=0.009) and 0.043 m/sec (β=−43.453, P=0.026) respectively. Each 0.1% increase in WMH volume in the SLF was associated with a reduced gain in gait speed of 0.064 m/sec (β=−64.833, P=0.06).
CONCLUSION
In older adults with mobility impairment who undergo gait rehabilitation, the severity of subcortical vascular disease in white matter tracts is associated with gait outcome, depending on the type of intervention. Specifically, the volume of WMH in longitudinal fiber tracts in the brains of older adults is differentially associated with gain in gait speed from two types of interventions. Gain in gait speed following the TC intervention appears to be consistent across the range of WMH, whereas with the WEBS intervention, gait speed improvement is reduced for those with greater WMH load. These results suggest that a task-directed motor skill learning approach to gait rehabilitation may be better suited to older adults across the range of WMH burden while an impairment-based approach (e.g., WEBS) appears to have beneficial effects on gait speed only in the older adults with a lesser severity of WMH burden.
Why would the TC intervention benefit gait speed independent of WMH burden? The TC intervention focused on gait control by providing training to adapt to diverse conditions while walking, such as performing secondary tasks while walking, walking in complex patterns and pacing on a treadmill.24 The TC intervention was designed to re-program the control of gait through task-oriented motor sequence learning, which involves the use of limb positioning and a defined goal for the movement task to elicit the appropriate pattern and timing of muscle activity for the task. The stepping and walking program embedded in the TC intervention were used to help coordinate limb positions and muscle activity in synchrony with postures and phases of the gait cycle and restore automaticity of gait.24 TC intervention also included reinforcing the timing of stepping through treadmill-assisted walking. The treadmill-assisted walking practice was initially at the older adult’s preferred speed, followed by brief intervals of increased speed, alternating with return to the preferred speed. Since TC generated the same magnitude of gain in gait speed independent of WMH burden, we interpret these findings to mean that the task-oriented motor learning intervention provided the brain the information needed for the reacquisition of the motor skill of walking, and improved efficiency of gait. The motor skill of walking was not addressed by the impairment-based intervention (WEBS) designed to increase capacity in body systems contributing to gait performance. Improvements in gait speed in the WEBS intervention most likely resulted from the increased capacities of muscle strength, limb motion and endurance which enabled the older adults to use more capacity to walk faster. The TC intervention, by focusing on task-monitoring, motor-planning and motor-sequencing along with mobility training, trained the individuals on executive control of gait and improved gait automaticity and therefore greater gait speed gain.
Cognitive training may be an important component of gait training. Executive function, when combined with mobility training has a greater impact on gait compared to a mobility training alone.44 Dual-task gait exercise that incorporates specific executive functions while walking, improves gait more than single-task low-intensity physical exercise.45 Even executive function training for 8 weeks without any physical exercise leads to benefits in gait speed.46 Cognitive rehabilitation also improves executive function in normal aging.47 Our study adds to the evidence that gait training should include elements of cognitively oriented motor planning, especially in persons with high WMH burden.
WMH burden in specific longitudinal tracts can have a bearing on gait intervention in a number of ways. The SLF, ILF, and IFO are important to motor planning as they relay motor, cognitive, perceptual and sensory signals between brain regions. White matter disease in frontal-subcortical regions affects executive function, manifesting as slow speed of processing and impaired motor learning.48,49 The SLF and IFO connect the frontal-subcortical regions with the parietal and occipital regions. These tracts are critical to executive functioning.19,22 Posterior parietal cortex is involved in integrating information from the external environment into signals that trigger motor intensions such as placing of limb to reach a particular point.50,51 These areas are connected to the motor cortex via SLF.21,22 The ILF is also associated with processing speed and visuomotor dexterity.22,52 The IFO connects the parieto-occipital regions with the dorsal premotor and prefrontal regions and mediates interactions between visual, spatial and executive functions.22,52 White matter disease can particularly affect these tracts and disrupt signal transmission leading to mobility impairment in older adults.53,54 Our finding that TC but not WEBS can promote gain in gait speed in the presence of high WMH suggests that impaired motor control due to disrupted signal transmission may be modifiable with interventions that focus on motor control and planning. The TC intervention emphasized modifications to motor control and motor learning via self-monitoring of progressively complex gait activities. This type of intervention may have facilitated the executive, visuoperceptual and spatial abilities involved in higher order control of gait, signaling of which is mediated through the longitudinal tracts (SLF, ILF and IFO). The cingulum bundle connects the limbic and entorrhinal cortex that is involved in emotional and cognitive regulation with indirect links to gait control through the premotor cortex. In contrast to the SLF, ILF and IFO, the WMH burden in the cingulum showed no significant association with gain in gait speed in the TC intervention. The WEBS conventional intervention focused on building endurance and strength, but not on the cognitive elements of gait control. WEBS training may have acted at peripheral cardiovascular, muscular and proprioceptive level, and failed to address the impairments in the central nervous system that affect gait in older adults with greater white matter disease.
Therefore, at least two possible explanations for TC mediated effects are possible. First, TC training may have facilitated gain in gait speed by enhancing cognitive abilities mediated by the longitudinal tracts such as the SLF, ILF and IFO. Second, the TC intervention could have enhanced resilience in some way and enabled the individual to adapt to the deficits caused by disruptions in signal processing. Alternatively, there could be another mechanism that may have played a role. The baseline executive function and WMH volume measures were significantly correlated across groups but the baseline gait speed did not correlate with any of the executive function measures or WMH measures possibly due to the eligibility restrictions limiting the range of gait speed. This study collected data on baseline structural MRI and cognitive measures and the study was not designed to study brain changes associated with the two interventions. Therefore, future studies should examine brain changes with the two interventions.
The findings of this study are clinically relevant to older adults with mobility impairment. WMH are common in older adults, with some degree of WMH in up to 95% of older adult population.55,56 Even with more conservative criteria for WMH volume, the prevalence reaches up to 32%.57 Since subcortical vascular disease is so prevalent in older adults and is related to gait and executive dysfunction, it is important to understand the influence of WMH load on mobility interventions. Subcortical vascular disease portends a poor rehabilitation outcome in older adults - severity of subcortical vascular disease predicts falls in mobility impaired older adults within one year of completing conventional gait rehabilitation, even after accounting for demographic, cognitive and functional status.58 Since high WMH burden is common with aging, and motor learning interventions appear to equally benefit all older adults, motor learning strategies might be appropriately incorporated into routine gait rehabilitation practice for all older adults. Alternatively, either traditional or motor learning interventions for gait could benefit gait speed in older persons with low WMH burden, while motor learning interventions could be especially targeted at persons with high WMH burden. However, this would require that therapists have information on WMH burden to plan treatment. In the future, it is possible that gait rehabilitations might be more individualized that include brain characteristics such as location and burden of WMH in older adults.
This study has limitations. We did not measure WMH in lobar regions such as in frontal or parietal cortices nor did we study WMH burden in all tracts that traverse the white matter such as the projection and commissural fibers. We also did not study the integrity of tracts in normal appearing white matter of the brain. Additionally, we used an automated segmentation method to measure WMH and in doing so did not address some of the heterogeneity underlying WMH. It is also likely that WMH located within the tract ROI were not necessarily specific to that tract but could involve other fibers that traverse the same brain regions but were spuriously attributed to the tract only because of its proximity within the chosen ROI. Finally, this was a secondary analysis of a pilot study with small sample sizes and brief duration of intervention (12 weeks). Despite these limitations, the study highlights the potential influence on outcomes of gait interventions from WMH burden overall, and in three important longitudinal tracts. To our knowledge, this is one of the first studies to address the association of WMH with outcome of gait rehabilitation strategies.
In summary, WMH tract burden was associated with gait speed gain with traditional, impairment-based, but not in task-oriented motor learning intervention. Task-oriented motor learning and skill acquisition may be critical components of gait rehabilitation in persons with high WMH burden.
ACKNOWLEDGMENTS
This study was supported by the Pittsburgh Claude D. Pepper Older American’s Independence Center grant (P30 AG024827). We appreciate the assistance of Rebecca L. MacCloud for providing the figure-inserts for the brain tracts.
Sponsors role:
The sponsor had no involvement in any aspect of the research or in preparation of this manuscript.
Funding source: Pittsburgh Claude D. Pepper Older American’s Independence Center grant (P30 AG024827)
Appendix
Conflict of Interest Disclosures:
| Elements of Financial/Personal Conflicts |
Neelesh K. Nadkarni |
Subhashan Perera | Caterina Rosano |
Howard J. Aizenstein |
||||
|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | |
| Employment or Affiliation | X | X | X | X | ||||
| Grants/Funds | X | X | X | X | ||||
| Honoraria | X | X | X | X | ||||
| Speaker Forum | X | X | X | X | ||||
| Consultant | X | X | X | X | ||||
| Stocks | X | X | X | X | ||||
| Royalties | X | X | X | X | ||||
| Expert Testimony | X | X | X | X | ||||
| Board Member | X | X | X | X | ||||
| Patents | X | X | X | X | ||||
| Personal Relationship | X | X | X | X | ||||
| Elements of Financial/Personal Conflicts |
Jennifer S. Brach | Jessie, M. VanSwearingen |
Stephanie A. Studenski |
|||||
|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | |
| Employment or Affiliation | X | X | X | |||||
| Grants/Funds | X | X | X | |||||
| Honoraria | X | X | X | |||||
| Speaker Forum | X | X | X | |||||
| Consultant | X | X | X | |||||
| Stocks | X | X | X | |||||
| Royalties | X | X | X | |||||
| Expert Testimony | X | X | X | |||||
| Board Member | X | X | X | |||||
| Patents | X | X | X | |||||
| Personal Relationship | X | X | X | |||||
Authors can be listed by abbreviations of their names.
For “yes” x mark(s): give brief explanation below: Subhashan Perera and Stephanie A. Studenski have received past research grants/funding from Ortho Biotech, Lilly and Merck.
Footnotes
Conflict of interest:
None of the authors have any financial, personal or potential conflict of interest with the material presented in this article.
An abstract of the preliminary report was presented at the American Geriatrics Society annual meeting, May 2012, Seattle, WA.
Author’s contribution:
NKN: study concept and design, acquisition of data, analysis and interpretation of data, and preparation of manuscript.
SAS: study concept and design, acquisition of subjects and/or data, analysis and interpretation of data, and preparation of manuscript
SKP: study concept and design, acquisition of subjects and/or data, analysis and interpretation of data, and preparation of manuscript
JSB: study concept and design, acquisition of data
HJA: study concept and design, acquisition of data and preparation of manuscript
CR: study concept and design, acquisition of data, interpretation of data
JMV: study concept and design, acquisition of subjects and/or data, analysis and interpretation of data, and preparation of manuscript
REFERENCES
- 1.Whitman GT, Tang Y, Lin A, et al. A prospective study of cerebral white matter abnormalities in older people with gait dysfunction. Neurology. 2001 Sep 25;57(6):990–994. doi: 10.1212/wnl.57.6.990. [DOI] [PubMed] [Google Scholar]
- 2.Wolfson L, Wei X, Hall CB, et al. Accrual of MRI white matter abnormalities in elderly with normal and impaired mobility. J Neurol Sci. 2005 May 15;232(1-2):23–27. doi: 10.1016/j.jns.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 3.Starr JM, Leaper SA, Murray AD, et al. Brain white matter lesions detected by magnetic resonance [correction of resosnance] imaging are associated with balance and gait speed. J Neurol Neurosurg Psychiatry. 2003 Jan;74(1):94–98. doi: 10.1136/jnnp.74.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tullberg M, Fletcher E, DeCarli C, et al. White matter lesions impair frontal lobe function regardless of their location. Neurology. 2004 Jul 27;63(2):246–253. doi: 10.1212/01.wnl.0000130530.55104.b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marshall GA, Hendrickson R, Kaufer DI, et al. Cognitive correlates of brain MRI subcortical signal hyperintensities in non-demented elderly. Int J Geriatr Psychiatry. 2006 Jan;21(1):32–35. doi: 10.1002/gps.1419. [DOI] [PubMed] [Google Scholar]
- 6.Rosano C, Studenski SA, Aizenstein HJ, et al. Slower gait, slower information processing and smaller prefrontal area in older adults. Age Ageing. 2012 Jan;41(1):58–64. doi: 10.1093/ageing/afr113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yogev-Seligmann G, Hausdorff JM, Giladi N. The role of executive function and attention in gait. Mov Disord. 2008 Feb 15;23(3):329–342. doi: 10.1002/mds.21720. quiz 472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hausdorff JM, Yogev G, Springer S, et al. Walking is more like catching than tapping: gait in the elderly as a complex cognitive task. Exp Brain Res. 2005 Aug;164(4):541–548. doi: 10.1007/s00221-005-2280-3. [DOI] [PubMed] [Google Scholar]
- 9.Koo BB, Bergethon P, Qiu WQ, et al. Clinical prediction of fall risk and white matter abnormalities: a diffusion tensor imaging study. Arch Neurol. 2012 Jun;69(6):733–738. doi: 10.1001/archneurol.2011.2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moscufo N, Guttmann CR, Meier D, et al. Brain regional lesion burden and impaired mobility in the elderly. Neurobiol Aging. 2011 Apr;32(4):646–654. doi: 10.1016/j.neurobiolaging.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Srikanth V, Phan TG, Chen J, et al. The location of white matter lesions and gait--a voxel-based study. Ann Neurol. 2010 Feb;67(2):265–269. doi: 10.1002/ana.21826. [DOI] [PubMed] [Google Scholar]
- 12.Longstreth WT, Jr, Cardiovascular Health Study Collaborative Research Group Brain abnormalities in the elderly: frequency and predictors in the United States (the Cardiovascular Health Study) J Neural Transm Suppl. 1998;53:9–16. doi: 10.1007/978-3-7091-6467-9_2. [DOI] [PubMed] [Google Scholar]
- 13.de Laat KF, Tuladhar AM, van Norden AG, et al. Loss of white matter integrity is associated with gait disorders in cerebral small vessel disease. Brain. 2011 Jan;134(Pt 1):73–83. doi: 10.1093/brain/awq343. [DOI] [PubMed] [Google Scholar]
- 14.Doyon J. Motor sequence learning and movement disorders. Curr Opin Neurol. 2008 Aug;21(4):478–483. doi: 10.1097/WCO.0b013e328304b6a3. [DOI] [PubMed] [Google Scholar]
- 15.Guillot A, Collet C, Nguyen VA, et al. Functional neuroanatomical networks associated with expertise in motor imagery. Neuroimage. 2008 Jul 15;41(4):1471–1483. doi: 10.1016/j.neuroimage.2008.03.042. [DOI] [PubMed] [Google Scholar]
- 16.Mesulam MM. Spatial attention and neglect: parietal, frontal and cingulate contributions to the mental representation and attentional targeting of salient extrapersonal events. Philos Trans R Soc Lond B Biol Sci. 1999 Jul 29;354(1387):1325–1346. doi: 10.1098/rstb.1999.0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosano C, Brach J, Studenski S, et al. Gait variability is associated with subclinical brain vascular abnormalities in high-functioning older adults. Neuroepidemiology. 2007;29(3-4):193–200. doi: 10.1159/000111582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iseki K, Hanakawa T, Shinozaki J, et al. Neural mechanisms involved in mental imagery and observation of gait. Neuroimage. 2008 Jul 1;41(3):1021–1031. doi: 10.1016/j.neuroimage.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 19.Turken A, Whitfield-Gabrieli S, Bammer R, et al. Cognitive processing speed and the structure of white matter pathways: convergent evidence from normal variation and lesion studies. Neuroimage. 2008 Aug 15;42(2):1032–1044. doi: 10.1016/j.neuroimage.2008.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Charlton RA, Barrick TR, McIntyre DJ, et al. White matter damage on diffusion tensor imaging correlates with age-related cognitive decline. Neurology. 2006 Jan 24;66(2):217–222. doi: 10.1212/01.wnl.0000194256.15247.83. [DOI] [PubMed] [Google Scholar]
- 21.Makris N, Kennedy DN, McInerney S, et al. Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cereb Cortex. 2005 Jun;15(6):854–869. doi: 10.1093/cercor/bhh186. [DOI] [PubMed] [Google Scholar]
- 22.Schmahmann JD, Smith EE, Eichler FS, et al. Cerebral white matter: neuroanatomy, clinical neurology, and neurobehavioral correlates. Ann N Y Acad Sci. 2008 Oct;1142:266–309. doi: 10.1196/annals.1444.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kantarci K, Senjem ML, Avula R, et al. Diffusion tensor imaging and cognitive function in older adults with no dementia. Neurology. 2011 Jul 5;77(1):26–34. doi: 10.1212/WNL.0b013e31822313dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.VanSwearingen JM, Perera S, Brach JS, et al. A randomized trial of two forms of therapeutic activity to improve walking: effect on the energy cost of walking. J Gerontol A Biol Sci Med Sci. 2009 Nov;64(11):1190–1198. doi: 10.1093/gerona/glp098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.VanSwearingen JM, Perera S, Brach JS, et al. Impact of exercise to improve gait efficiency on activity and participation in older adults with mobility limitations: a randomized controlled trial. Phys Ther. 2011 Dec;91(12):1740–1751. doi: 10.2522/ptj.20100391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rigler SK, Studenski SA, Wallace D, et al. Comorbidity adjustments for functional outcomes in community-dwelling older adults. Clinical Rehabilitation. 2002;16:420–428. doi: 10.1191/0269215502cr515oa. [DOI] [PubMed] [Google Scholar]
- 27.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975 Nov;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 28.Smith LK, Iddings DM, Spencer WA, et al. Muscle testing. Part 1. Description of a numerical index for clinical research. Physical Therapy. 1961;41:99–105. [PubMed] [Google Scholar]
- 29.Wu M, Rosano C, Butters M, et al. A fully automated method for quantifying and localizing white matter hyperintensities on MR images. Psychiatry Res. 2006 Dec 1;148(2-3):133–142. doi: 10.1016/j.pscychresns.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002 Nov;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jenkinson M, Pechaud M, Smith S. BET2:MR-based estimation of brain, skull and scalp surfaces; Eleventh Annual Meeting of the Organization for Human Brain Mapping; Toronto, Ontario, Canada. 2005. [Google Scholar]
- 32.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002 Jan;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 33.Wakana S, Jiang H, Nagae-Poetscher LM, et al. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004 Jan;230(1):77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- 34.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011 Jan 5;305(1):50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cesari M, Kritchevsky SB, Penninx BW, et al. Prognostic value of usual gait speed in well-functioning older people--results from the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2005 Oct;53(10):1675–1680. doi: 10.1111/j.1532-5415.2005.53501.x. [DOI] [PubMed] [Google Scholar]
- 36.Fitzpatrick AL, Buchanan CK, Nahin RL, et al. Associations of gait speed and other measures of physical function with cognition in a healthy cohort of elderly persons. J Gerontol A Biol Sci Med Sci. 2007 Nov;62(11):1244–1251. doi: 10.1093/gerona/62.11.1244. [DOI] [PubMed] [Google Scholar]
- 37.Kuo HK, Leveille SG, Yu YH, et al. Cognitive function, habitual gait speed, and late-life disability in the National Health and Nutrition Examination Survey (NHANES) 1999-2002. Gerontology. 2007;53(2):102–110. doi: 10.1159/000096792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Newman AB, Simonsick EM, Naydeck BL, et al. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 2006 May 3;295(17):2018–2026. doi: 10.1001/jama.295.17.2018. [DOI] [PubMed] [Google Scholar]
- 39.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994 Mar;49(2):M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 40.Wechsler D. Wechsler Adult Intelligence Scale-Revised. Psychological Corp.; New York, NY: 1981. [Google Scholar]
- 41.Baillon S, Muhommad S, Marudkar M, et al. Neuropsychological performance in Alzheimer’s disease and vascular dementia: comparisons in a memory clinic population. Int J Geriatr Psychiatry. 2003 Jul;18(7):602–608. doi: 10.1002/gps.887. [DOI] [PubMed] [Google Scholar]
- 42.Amieva H, Lafont S, Auriacombe S, et al. Analysis of error types in the trial making test evidences an inhibitory deficit in dementia of the Alzheimer type. J Clin Exp Neuropsychol. 1998 Apr;20(2):280–285. doi: 10.1076/jcen.20.2.280.1161. [DOI] [PubMed] [Google Scholar]
- 43.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric deprexsion screening scale: a preliminary report. Journal of Psychiatry Research. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 44.Liu-Ambrose T, Davis JC, Nagamatsu LS, et al. Changes in executive functions and self-efficacy are independently associated with improved usual gait speed in older women. BMC Geriatr. 2010;10:25. doi: 10.1186/1471-2318-10-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schwenk M, Zieschang T, Oster P, et al. Dual-task performances can be improved in patients with dementia: a randomized controlled trial. Neurology. 2010 Jun 15;74(24):1961–1968. doi: 10.1212/WNL.0b013e3181e39696. [DOI] [PubMed] [Google Scholar]
- 46.Verghese J, Mahoney J, Ambrose AF, et al. Effect of cognitive remediation on gait in sedentary seniors. J Gerontol A Biol Sci Med Sci. 2010 Dec;65(12):1338–1343. doi: 10.1093/gerona/glq127. [DOI] [PubMed] [Google Scholar]
- 47.Levine B, Stuss DT, Winocur G, et al. Cognitive rehabilitation in the elderly: effects on strategic behavior in relation to goal management. J Int Neuropsychol Soc. 2007 Jan;13(1):143–152. doi: 10.1017/S1355617707070178. [DOI] [PubMed] [Google Scholar]
- 48.Wolfe N, Linn R, Babikian VL, et al. Frontal systems impairment following multiple lacunar infarcts. Arch Neurol. 1990 Feb;47(2):129–132. doi: 10.1001/archneur.1990.00530020025010. [DOI] [PubMed] [Google Scholar]
- 49.Venkatraman VK, Aizenstein H, Guralnik J, et al. Executive control function, brain activation and white matter hyperintensities in older adults. Neuroimage. 2010 Feb 15;49(4):3436–3442. doi: 10.1016/j.neuroimage.2009.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seemungal BM, Rizzo V, Gresty MA, et al. Perceptual encoding of self-motion duration in human posterior parietal cortex. Ann N Y Acad Sci. 2009 May;1164:236–238. doi: 10.1111/j.1749-6632.2009.03772.x. [DOI] [PubMed] [Google Scholar]
- 51.Battaglia-Mayer A, Ferraina S, Mitsuda T, et al. Early coding of reaching in the parietooccipital cortex. J Neurophysiol. 2000 Apr;83(4):2374–2391. doi: 10.1152/jn.2000.83.4.2374. [DOI] [PubMed] [Google Scholar]
- 52.Voineskos AN, Rajji TK, Lobaugh NJ, et al. Age-related decline in white matter tract integrity and cognitive performance: a DTI tractography and structural equation modeling study. Neurobiol Aging. 2012 Jan;33(1):21–34. doi: 10.1016/j.neurobiolaging.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nutt JG, Marsden CD, Thompson PD. Human walking and higher-level gait disorders, particularly in the elderly. Neurology. 1993 Feb;43(2):268–279. doi: 10.1212/wnl.43.2.268. [DOI] [PubMed] [Google Scholar]
- 54.Masdeu JC, Wolfson L, Lantos G, et al. Brain white-matter changes in the elderly prone to falling. Arch Neurol. 1989 Dec;46(12):1292–1296. doi: 10.1001/archneur.1989.00520480034016. [DOI] [PubMed] [Google Scholar]
- 55.Longstreth WT, Jr., Manolio TA, Arnold A, et al. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. The Cardiovascular Health Study. Stroke. 1996 Aug;27(8):1274–1282. doi: 10.1161/01.str.27.8.1274. [DOI] [PubMed] [Google Scholar]
- 56.de Leeuw FE, de Groot JC, Achten E, et al. Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study. The Rotterdam Scan Study. J Neurol Neurosurg Psychiatry. 2001 Jan;70(1):9–14. doi: 10.1136/jnnp.70.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Launer LJ, Berger K, Breteler MM, et al. Regional variability in the prevalence of cerebral white matter lesions: an MRI study in 9 European countries (CASCADE) Neuroepidemiology. 2006;26(1):23–29. doi: 10.1159/000089233. [DOI] [PubMed] [Google Scholar]
- 58.Guerini F, Frisoni GB, Marre A, et al. Subcortical vascular lesions predict falls at 12 months in elderly patients discharged from a rehabilitation ward. Arch. Phys. Med. Rehabil. 2008 Aug;89(8):1522–1527. doi: 10.1016/j.apmr.2008.01.018. [DOI] [PubMed] [Google Scholar]


