Abstract
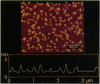
The inwardly rectifying K+ channel ROMK1 has been implicated as being significant in K+ secretion in the distal nephron. ROMK1 has been shown by immunocytochemistry to be expressed in relevant nephron segments. The development of the atomic force microscope has made possible the production of high resolution images of small particles, including a variety of biological macromolecules. Recently, a fusion protein of glutathione S-transferase (GST) and ROMK1 (ROMK1-GST) has been used to produce a polyclonal antibody for immunolocalization of ROMK1. We have used atomic force microscopy to examine ROMK1-GST and the native ROMK1 polypeptide cleaved from GST. Imaging was conducted with the proteins in physiological solutions attached to mica. ROMK1-GST appears in images as a particle composed of two units of similar size. Analyses of images indicate that the two units have volumes of approximately 118 nm3, which is close to the theoretical volume of a globular protein of approximately 65 kDa (the molecular mass of ROMK1-GST). Native GST exists as a dimer, and the images obtained here are consistent with the ROMK1-GST fusion protein's existence as a heterodimer. In experiments on ROMK1 in aqueous solution, single molecules appear to aggregate, but contact to the mica was maintained. Addition of ATP to the solution produced a change in height of the aggregates. This change (which was reversible) suggests that ATP induces a structural change in the ROMK1 protein. The data show that atomic force microscopy is a useful tool for examination of purified protein molecules under near-physiological conditions, and furthermore, that structural alterations in the proteins may be continuously investigated.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Binnig G, Quate CF, Gerber C. Atomic force microscope. Phys Rev Lett. 1986 Mar 3;56(9):930–933. doi: 10.1103/PhysRevLett.56.930. [DOI] [PubMed] [Google Scholar]
- Boim M. A., Ho K., Shuck M. E., Bienkowski M. J., Block J. H., Slightom J. L., Yang Y., Brenner B. M., Hebert S. C. ROMK inwardly rectifying ATP-sensitive K+ channel. II. Cloning and distribution of alternative forms. Am J Physiol. 1995 Jun;268(6 Pt 2):F1132–F1140. doi: 10.1152/ajprenal.1995.268.6.F1132. [DOI] [PubMed] [Google Scholar]
- Fritzsche W., Schaper A., Jovin T. M. Probing chromatin with the scanning force microscope. Chromosoma. 1994 Jul;103(4):231–236. doi: 10.1007/BF00352247. [DOI] [PubMed] [Google Scholar]
- Fritzsche W., Vesenka J., Henderson E. Scanning force microscopy of chromatin. Scanning Microsc. 1995 Sep;9(3):729–739. [PubMed] [Google Scholar]
- Giebisch G. Renal potassium channels: an overview. Kidney Int. 1995 Oct;48(4):1004–1009. doi: 10.1038/ki.1995.382. [DOI] [PubMed] [Google Scholar]
- Guan K. L., Dixon J. E. Eukaryotic proteins expressed in Escherichia coli: an improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Anal Biochem. 1991 Feb 1;192(2):262–267. doi: 10.1016/0003-2697(91)90534-z. [DOI] [PubMed] [Google Scholar]
- Hansma H. G., Hoh J. H. Biomolecular imaging with the atomic force microscope. Annu Rev Biophys Biomol Struct. 1994;23:115–139. doi: 10.1146/annurev.bb.23.060194.000555. [DOI] [PubMed] [Google Scholar]
- Ho K., Nichols C. G., Lederer W. J., Lytton J., Vassilev P. M., Kanazirska M. V., Hebert S. C. Cloning and expression of an inwardly rectifying ATP-regulated potassium channel. Nature. 1993 Mar 4;362(6415):31–38. doi: 10.1038/362031a0. [DOI] [PubMed] [Google Scholar]
- Kukuljan M., Labarca P., Latorre R. Molecular determinants of ion conduction and inactivation in K+ channels. Am J Physiol. 1995 Mar;268(3 Pt 1):C535–C556. doi: 10.1152/ajpcell.1995.268.3.C535. [DOI] [PubMed] [Google Scholar]
- Lal R., John S. A. Biological applications of atomic force microscopy. Am J Physiol. 1994 Jan;266(1 Pt 1):C1–21. doi: 10.1152/ajpcell.1994.266.1.C1. [DOI] [PubMed] [Google Scholar]
- Lang F., Rehwald W. Potassium channels in renal epithelial transport regulation. Physiol Rev. 1992 Jan;72(1):1–32. doi: 10.1152/physrev.1992.72.1.1. [DOI] [PubMed] [Google Scholar]
- McNicholas C. M., Wang W., Ho K., Hebert S. C., Giebisch G. Regulation of ROMK1 K+ channel activity involves phosphorylation processes. Proc Natl Acad Sci U S A. 1994 Aug 16;91(17):8077–8081. doi: 10.1073/pnas.91.17.8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTigue M. A., Williams D. R., Tainer J. A. Crystal structures of a schistosomal drug and vaccine target: glutathione S-transferase from Schistosoma japonica and its complex with the leading antischistosomal drug praziquantel. J Mol Biol. 1995 Feb 10;246(1):21–27. doi: 10.1006/jmbi.1994.0061. [DOI] [PubMed] [Google Scholar]
- Morris V. J. Biological applications of scanning probe microscopies. Prog Biophys Mol Biol. 1994;61(2):131–185. doi: 10.1016/0079-6107(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Schaper A., Starink J. P., Jovin T. M. The scanning force microscopy of DNA in air and in n-propanol using new spreading agents. FEBS Lett. 1994 Nov 21;355(1):91–95. doi: 10.1016/0014-5793(94)01166-4. [DOI] [PubMed] [Google Scholar]
- Schneider S., Folprecht G., Krohne G., Oberleithner H. Immunolocalization of lamins and nuclear pore complex proteins by atomic force microscopy. Pflugers Arch. 1995 Sep;430(5):795–801. doi: 10.1007/BF00386178. [DOI] [PubMed] [Google Scholar]
- Shao Z., Yang J. Progress in high resolution atomic force microscopy in biology. Q Rev Biophys. 1995 May;28(2):195–251. doi: 10.1017/s0033583500003061. [DOI] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Wang W., Giebisch G. Dual effect of adenosine triphosphate on the apical small conductance K+ channel of the rat cortical collecting duct. J Gen Physiol. 1991 Jul;98(1):35–61. doi: 10.1085/jgp.98.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Tamm L. K., Somlyo A. P., Shao Z. Promises and problems of biological atomic force microscopy. J Microsc. 1993 Sep;171(Pt 3):183–198. doi: 10.1111/j.1365-2818.1993.tb03375.x. [DOI] [PubMed] [Google Scholar]
- Zhou H., Tate S. S., Palmer L. G. Primary structure and functional properties of an epithelial K channel. Am J Physiol. 1994 Mar;266(3 Pt 1):C809–C824. doi: 10.1152/ajpcell.1994.266.3.C809. [DOI] [PubMed] [Google Scholar]