Abstract
Therapeutic options that directly enhance cardiomyocyte contractility in chronic heart failure (HF) therapy are currently limited and do not improve prognosis. In fact, most positive inotropic agents, such as β-adrenoreceptor agonists and PDE inhibitors, which have been assessed in HF patients, cause increased mortality as a result of arrhythmia and sudden cardiac death. Cardiac sarcoplasmic reticulum Ca2+-ATPase2a (SERCA2a) is a key protein involved in sequestration of Ca2+ into the sarcoplasmic reticulum (SR) during diastole. There is a reduction of SERCA2a protein level and function in HF, which has been successfully targeted via viral transfection of the SERCA2a gene into cardiac tissue in vivo. This has enhanced cardiac contractility and reduced mortality in several preclinical models of HF. Theoretical concerns have been raised regarding the possibility of arrhythmogenic adverse effects of SERCA2a gene therapy due to enhanced SR Ca2+ load and induction of SR Ca2+ leak as a result. Contrary to these concerns, SERCA2a gene therapy in a wide variety of preclinical models, including acute ischaemia/reperfusion, chronic pressure overload and chronic myocardial infarction, has resulted in a reduction in ventricular arrhythmias. The potential mechanisms for this unexpected beneficial effect, as well as mechanisms of enhancement of cardiac contractile function, are reviewed in this article.
Keywords: gene therapy, heart failure, arrhythmia, anti-arrhythmic
Introduction
Chronic heart failure (HF) has reached epidemic proportions in Western countries. The Framingham Heart Study measured the lifetime risk for congestive cardiac failure at 20% in both men and women (Lloyd-Jones et al., 2002). Mortality as a result of HF remains high despite the implementation of current evidence-based therapies. Five-year survival is only 32% in patients with systolic dysfunction and little better (35%) in patients with HF and preserved ejection fraction (Owan et al., 2006; Jhund et al., 2009).
Several pharmacological therapies improve both symptoms and prognosis in patients with HF. These include neurohormonal antagonists, such as β-adrenoceptor antagonists (β-blockers), ACE inhibitors and aldosterone antagonists. Recently, it has also been shown that heart rate reduction by specific inhibition of the ‘Funny'-current (If) using ivabradine can also improve prognosis and symptoms (Swedberg et al., 2010). On the contrary, the use of positive inotropes to directly target the ventricular dysfunction that characterizes HF has proven to be counterproductive – many traditional agents increase mortality, principally by increasing sudden cardiac death (Packer et al., 1991; O'Connor et al., 1999; Toma and Starling, 2010) and accelerating progression of left ventricular dysfunction (Packer et al., 1984). Cardiac sarcoplasmic reticulum Ca2+-ATPase2a (SERCA2a) gene therapy is one of a number of promising therapies that target specific protein derangements in HF. Others include myosin activators (Teerlink et al., 2011), ryanodine receptor (RyR) stabilizers (Wehrens et al., 2005) and Ca2+/calmodulin kinase (CaMKII) inhibition (Anderson et al., 1998), but a detailed description of these is beyond the scope of this article.
Our group and others have shown that, despite increasing cardiac contractility, SERCA2a gene therapy reduces the risk of potentially fatal arrhythmias in preclinical HF models (Lyon et al., 2011; Cutler et al., 2012b). Promising data have already been reported from a phase 1/2 trial, which demonstrated no pro-arrhythmic effect in HF patients (Jessup et al., 2011) and an ongoing phase 2b trial will assess this in a larger number of patients. If SERCA2a gene therapy makes the transition to the clinic, it may become the first anti-arrhythmic, positively inotropic, agent available in the treatment of HF.
SERCA2a function
The role of SERCA2a in myocyte contraction
The generation of cardiac contractile force is a complex process that has been reviewed elsewhere (Bers, 2001). A highly simplified scheme of the process is shown in Figure 1. The process of excitation contraction coupling (ECC) in the ventricular myocyte begins with the entry of Ca2+ via the L-type Ca2+ current (ICa) (Bers, 2002). This relatively small Ca2+ influx is amplified by a larger Ca2+ release from the sarcoplasmic reticulum (SR) – so-called Ca2+-induced Ca2+ release (CICR). The resulting increase in intracellular (cytoplasmic) Ca2+ concentration ([Ca2+]i) results in conformational changes in myofilament proteins, which ultimately leads to cross-bridge cycling and myocyte contraction. Other than relatively small areas of the terminal cisternae that are closely apposed to the sarcolemma, known as the junctional SR, most of the SR is specialized for Ca2+ uptake (the longitudinal SR) such that the vast majority of protein particles in the SR membrane are SERCA2a (Stewart and MacLennan, 1974; Franzini-Armstrong, 1975). SERCA2a transports the Ca2+ released during ECC back into the SR lumen against the electrochemical [Ca2+] gradient using chemical energy from ATP. The mechanism of Ca2+ pumping follows the E1-E2 model (Figure 2). An initial state in which 2 Ca2+ ions can be accepted from the cytosol (E1) transitions, via an intermediate occluded state, to a final state (E2) in which the lumen is accessible by the Ca2+ ions concurrent with a sudden reduction in affinity of the protein for Ca2+, thus facilitating its rapid release into the SR (MacLennan and Green, 2000; Wuytack et al., 2002). There are different SERCA isoforms that are functionally adapted to their location: SERCA1a is predominantly expressed in fast-twitch skeletal muscle; SERCA1b is expressed mainly in developing fetal fast-twitch skeletal muscle; SERCA2a is the dominant isoform in cardiomyocytes, but is also expressed in slow-twitch skeletal and smooth muscle; SERCA2b expression occurs in most cell types (the so-called ‘house-keeping’ isoform), and predominates in vascular smooth muscle; and finally, SERCA3a and 3b are expressed in the reticuloendothelial system (Leberer et al., 1989; Schulte et al., 1993).
Figure 1.
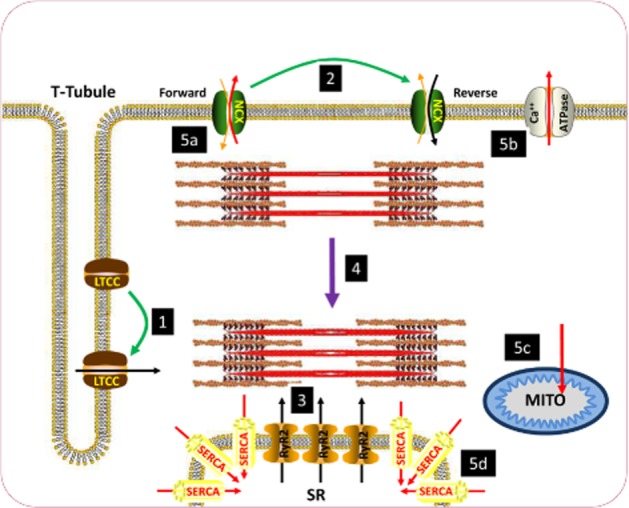
Schematic diagram of excitation-contraction coupling in the ventricular cardiomyocyte. (1) Sarcolemmal depolarization causes L-type Ca2+ channels (LTCC) to open, resulting in influx of Ca2+ during systole. Ca2+ enters initially through the LTCC. (2) During the early stages of the action potential, the Na+–Ca2+ exchanger (NCX) also transitions from functioning in forward mode (Na+ in Ca2+ out) to reverse mode (Ca2+ in Na+ out) to further enhance the rise in [Ca2+]i. (3) The initial rise in [Ca2+]i induces a larger release of Ca2+ from the sarcoplasmic reticulum (SR) via ryanodine receptors (RyR2), resulting in a large rise in [Ca2+]i. (4) The large rise in Ca2+ causes contraction of the myofilaments. (5) [Ca2+] is subsequently reduced to bring about diastole through movement out of the cytosol (a) via NCX in forward mode and (b) the sarcolemmal Ca2+-ATPase. (c) Ca2+ is also buffered by mitochondria. (d) The majority of Ca2+ movement in diastole is into the SR via SERCA2a. Green arrows denote transitions brought about by sarcolemmal depolarization. Black arrows denote routes of influx of Ca2+ to the cytosol during systole. Purple arrow denotes contraction of myofilaments. Red arrows denote routes of efflux of Ca2+ during diastole.
Figure 2.
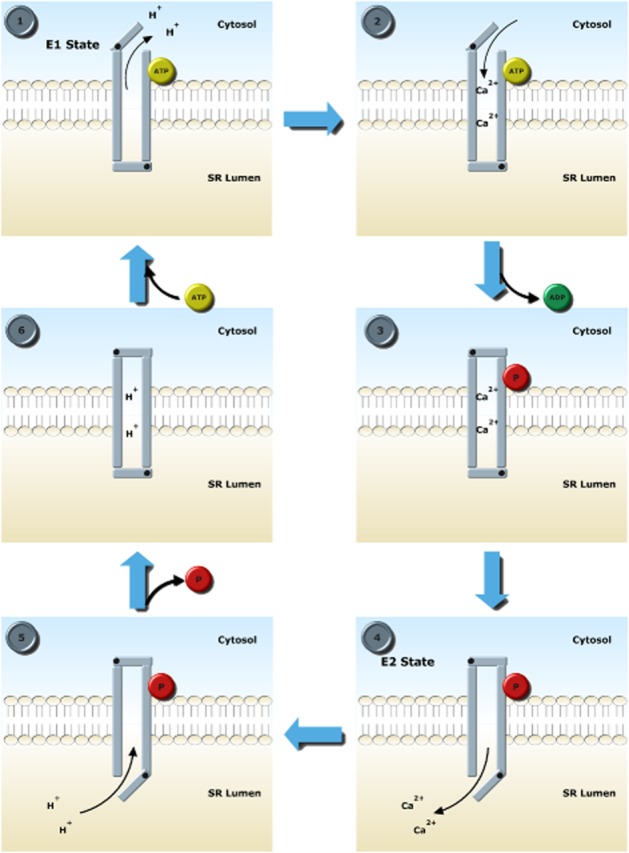
E1-E2 Ca transport scheme via which SERCA2a transports Ca into the sarcoplasmic reticulum (SR) lumen. Stage 1: In the E1 conformation, the high-affinity Ca2+ binding sites are accessible by the cytosol. Two protons leave the pump as ATP binds to allow access to the cytosol. Stage 2: 2 Ca2+ ions bind with high affinity. Stage 3: Bound ATP is used to phosphorylate Asp351, causing a conformational change which occludes Ca2+. At this point, the pump is in the high-energy unstable phosphointermediate condition. Stage 4: A further conformational change brings about the E2 form of SERCA2a where the SR lumen is accessible to Ca2+. Ca2+ ions are released because of a lower affinity in the E2 state. Stage 5: Protons replace Ca2+. Stage 6: Asp351 is dephosphorylated, bringing the pump into a low-energy intermediate form, which is unstable and returns to the E1 state.
SERCA2a is a key regulator of cardiac contractile force. An increase in SERCA2a function augments SR Ca2+ content, which increases Ca2+ transient amplitude (and thus force generated). This is because SR Ca2+ release is steeply dependent on SR Ca2+ content (Gyorke and Gyorke, 1998; Bode et al., 2011). The function of SERCA2a within an organism is dynamic, matching cardiac function to physiological demands by increasing during exercise or other forms of stress. Binding of phospholamban (PLB) to SERCA2a inhibits the pump and reduces Ca2+ influx into the SR lumen by decreasing its Ca2+ affinity two to three times (Tada et al., 1975) and also by reducing energetic efficiency (Frank et al., 2000). At physiologically relevant values of diastolic [Ca2+], the presence of PLB reduces the Ca2+ transport rate from about 90 to 30 μM·s−1 (Bers, 2001). Stimulation of β-adrenoceptors causes a conformational change of PLB secondary to phosphorylation of the Ser16 site by PKA (Talosi et al., 1993). This change reduces the affinity of active monomeric PLB for SERCA2a but enhances affinity for other PLB molecules such that detached PLB forms homopentamers, an inactive reservoir of the protein (Cornea et al., 1997; Kimura et al., 1997; Hou et al., 2008). In this way, the affinity of SERCA2a for Ca2+ is increased and uptake rate is enhanced.
PLB can also be phosphorylated at Thr17 by CaMKII. This site appears to be more important in frequency-dependent responses (Hagemann et al., 2000; Zhao et al., 2004) such as the force–frequency relationship and frequency-dependent acceleration of relaxation, which are often impaired in diseased myocardium (Pieske et al., 1992). Apart from PLB binding, pump function is modulated significantly by the intracellular cytosolic milieu, including ATP, pH, [Mg2+] and [Ca2+] (Dupont, 1977; Bers, 2001), and by post-translational modification, including SUMOylation (see below). Sarcolipin (SLN) is a 31 amino acid structure originally identified in skeletal muscle (MacLennan and Kranias, 2003), but more recent analyses indicate that SLN is also expressed in cardiac tissue, predominantly in the atria (Babu et al., 2007a). Compared with PLB, much less in known about SLN, but they have similar transmembrane sequences, suggesting they share a similar genetic heritage and they may bind to the same regulatory site on SERCA2a. There is evidence that SLN could be an important regulator of atrial Ca2+ transport and may be responsible for mediating at least some of the β-adrenoceptor responses in atria (Babu et al., 2007b).
Transgenic models
Transgenic animal models have further highlighted the importance of normal SERCA2a expression and function in Ca2+ homeostasis. Germline homozygous SERCA2a knockout is embryonically lethal (Periasamy et al., 1999). Heterozygous SERCA2a knockout reduces protein levels to 55% that of normal and results in increased propensity to HF following transverse aortic constriction (TAC) (Schultz et al., 2004). A model of conditional cardiac-specific SERCA2a knockout in adult mice induces an HF phenotype without any superimposed insult (Andersson et al., 2009; Louch et al., 2010). The importance of SERCA2a as the specific cardiac isoform has also been revealed by transgenic mouse studies.
Cardiac-specific overexpression of the SERCA1a isoform, which has higher kinetic turnover of Ca2+, led to increases in systolic and diastolic cardiac function in the normal mouse heart (Jane Lalli et al., 2001). In contrast, SERCA1a gene transfer into failing rat hearts actually impaired contractile function (O'Donnell et al., 2008). The reason for this unexpected finding may be curtailment of the rise of the Ca2+ transient, which has been observed with high-dose SERCA1a gene transfer [multiplicity of infection (MOI) 50], but not low dose (MOI 10) (Teucher et al., 2004). This highlights the role of SERCA proteins as a buffer of cytosolic Ca2+, which can reduce Ca2+ transient amplitude when faster kinetics of SERCA are included in computational models (Higgins et al., 2006). Such rapid kinetics would be seen with SERCA1a. Overall, this highlights the importance of using the cardiac-specific isoform SERCA2a in gene therapy strategies.
Abnormalities of Ca2+ handling in HF
Abnormal SERCA2a function
One of the hallmarks of the cellular phenotype of HF in animal models and human studies is prolongation of the Ca2+ transient, particularly in the decay phase (Gwathmey and Morgan, 1985; Gwathmey et al., 1987). In human preparations, Beuckelmann et al. (1992) revealed that this occurred even in the absence of changes in ICa. Furthermore, even with intracellular [Na+] controlled and action potential clamped, Ca2+ transient decay was slower and diastolic Ca2+ levels were higher than in control myocytes (Beuckelmann et al., 1992). These changes in Ca2+ handling can, in part, be attributed to reduced SERCA2a gene expression and protein levels, which are reported in many animal models of chronic HF and human failing myocardium (Hasenfuss, 1998). While SERCA2a levels often decrease in HF, most findings indicate that PLB protein levels are unchanged (MacLennan and Kranias, 2003). This implies that the remaining SERCA2a is also more likely to be inhibited by excess monomeric PLB. The phosphorylation state of PLB in HF is complex, with reduced levels of phosphorylation at Ser16 found in human tissue (Schwinger et al., 1999; Ai et al., 2005) but enhanced phosphorylation at Thr17 (Ai et al., 2005; Mills et al., 2006). Although the net effect of the extent of phosphorylation is unclear, the sum of the changes in PLB : SERCA2a expression and phosphorylation is reduced SERCA2a Ca2+ uptake into the SR (del Monte et al., 1995).
Consequences include delayed cytoplasmic Ca2+ clearance, resulting in prolonged relaxation times, increased chamber stiffness and a reduction in the rate of systolic pressure generation. The prolongation of Ca2+ transient decay and reduction in SERCA2a levels appear to occur in human failing hearts regardless of the aetiology of cardiac dysfunction (del Monte et al., 1995; Schmidt et al., 1998). Therefore, an important advantage of SERCA2a gene therapy is that it is expected to be beneficial in a wide range of HF patients. This is in comparison to the relatively limited patient population that might benefit from the targeting of an individual mutation causing dilated cardiomyopathy.
It is important to note that HF is not one single pathological entity and SERCA2a function may not be uniformly impaired in all cases. However, in the later stages, the phenotype may become more uniform. For example, we have found that, regardless of aetiology, the cellular phenotype of end-stage human HF generally includes impaired SERCA2a function (del Monte et al., 1995). This common finding, despite differences in the underlying primary pathology, may relate to neurohormonal changes that occur as a result of reduced cardiac output, including increased adrenergic hormones, B-type natriuretic peptide and hormones of the renin–angiotensin–aldosterone axis, which have all been shown to reduce SERCA2a expression or function with chronic exposure (Flesch et al., 1997; Linck et al., 1998; Sodi et al., 2008). On the other hand, we acknowledge that end-stage HF may not be representative of the whole range of disease. For example, during early stages of pressure overload, SR Ca2+ cycling may be enhanced rather than impaired (Carvalho et al., 2006), with reduced SERCA2a levels ensuing during the transition to cardiac decompensation (Feldman et al., 1993). Even in human end-stage HF, Hasenfuss et al. (1999) identified significant differences between myocardium exhibiting both systolic and diastolic dysfunction [reduced SERCA2a levels, normal Na+–Ca2+ exchanger (NCX) levels] and myocardium with systolic dysfunction but preserved diastolic function (preserved SERCA2a levels, enhanced NCX levels). A potential caveat is that SERCA2a activity was not always reported, and reduced SERCA2a function may reflect not only lower protein levels but also post-translational modification of the protein which is expressed. Given these differences dependent on aetiology and cardiac function, we could speculate that therapies to enhance SERCA2a function would be especially helpful in decompensated HF (rather than compensated hypertrophy) in which both systolic and diastolic function are deranged. Dose titration of SERCA2a gene therapy may therefore be important, with higher doses being required with end-stage disease.
Arrhythmogenic Ca2+ handling abnormalities in HF
The SR is not only an important store of Ca2+ that can be released during contraction, but also the potential origin of spontaneous arrhythmogenic SR Ca2+ release. Such SR Ca2+ leak manifests either as leak, which is visible by high-speed confocal fluorescence imaging as Ca2+ sparks (Cheng et al., 1993) or waves (Bers, 2001), or as more subtle leak, which has been called Ca2+ quarks (Lipp and Niggli, 1996; Lipp and Niggli, 1998) or spark-independent Ca2+ release (Zima et al., 2010). Spontaneous Ca2+ release from the SR can result in triggered cellular activity and subsequent arrhythmia via extrusion of excess cytoplasmic Ca2+ via NCX which exchanges 3 Na+ ions for 1 Ca2+ and is thus electrogenic such that clearance of Ca2+ results in depolarization of myocytes towards the threshold potential for voltage-gated sodium channels. This NCX-mediated current is the basis of delayed afterdepolarizations (DADs). Cellular triggered activity also comes in the form of early afterdepolarizations (EADs). These are a different entity resulting in a second depolarization prior to the end of the action potential, perhaps as a result of reactivation of L-type Ca2+ channels (LTCCs) (Bers, 2001), although more recent evidence also points to an important role for SR Ca2+ leak in EAD genesis (Volders et al., 2000; Zhao et al., 2012).
As discussed previously, SERCA2a function and SR Ca2+ load are reduced in several models of HF and in human HF cardiomyocytes. All else being equal, therefore, it would follow that spontaneous SR Ca2+ leak would also be reduced. On the contrary, many researchers, including our own group (Lyon et al., 2009, 2011), find that leak is enhanced in HF. This forms the basis of a Ca2+ paradox in HF (Kass et al., 2008). The reasons for the paradox remain unclear and may involve increased intracellular [Na+] in HF which could hinder Ca2+ efflux via NCX and thus enhance RyR2 opening via interaction with Ca2+-sensitive sites at the cytosolic domain (Sossalla et al., 2010). Another important mechanism might be a reduced threshold for SR Ca2+ leak in HF similar to that characterized in catecholaminergic polymorphic ventricular tachycardia (VT) (Jiang et al., 2004). This could enhance leak despite lower SR Ca2+ content. The leak threshold may differ in different models and aetiologies of HF (Respress et al., 2012).
There are several modifications to the RyR2 that occur in HF and may reduce the threshold for spontaneous SR Ca2+ release. There is evidence that phosphorylation of RyR2, and reduced binding of the channel's stabilizer FK506-binding protein 12.6 as a result, causes an increase in SR Ca2+ leak in HF (Marx et al., 2000; Kushnir and Marks, 2010). Other post-translational modifications to RyR2 such as nitrosylation and oxidation may also be important and have been reviewed elsewhere (Aracena et al., 2005; Vassort and Lacampagne, 2005; Zissimopoulos et al., 2007). The leaky SR in HF both predisposes to arrhythmias via the depolarizing influence of the NCX current and reduces contractility due to further depletion of SR Ca2+ content. Each occurrence of SR Ca2+ leak could also be more arrhythmogenic in HF than in the healthy heart due to an increase in NCX activity which has been seen in some settings, although this is not always associated with reduced SERCA2a activity (Hasenfuss et al., 1999; Pogwizd et al., 2001). Kho et al. identified a common regulator for SERCA2a and NCX in the small ubiquitin-like modifier type 1 (SUMO1) protein. This protein both up-regulates SERCA2a function and down-regulates NCX function, and is reduced in a mouse model of HF. These mice showed reduced SERCA2a Ca2+ uptake and enhanced NCX function (Kho et al., 2011). In addition, there is down-regulation of inward rectifying potassium channel (IK1) expression (Pogwizd et al., 2001), which normally stabilizes resting membrane potential. The result is that for any given spontaneous Ca2+ leak in HF cells, a greater membrane potential (Vm) depolarization occurs via NCX. In the intact organ, mechanical dysfunction and fibrosis result in a mechanoelectrically heterogeneous ventricular substrate in which triggered arrhythmias can lead to sustained arrhythmias via structural and functional re-entry (Weiss et al., 2005). The transient outward current (Ito) density has also been shown to decrease in HF patients (Beuckelmann et al., 1993). Ito plays a major role in controlling the duration of the action potential and it is thought that such a decline in current is one of the reasons for APD prolongation in HF. APD prolongation will provide a setting for EADs because the opportunity for Ca2+ current reactivation is increased.
Alternative strategies to enhance SERCA2a function in HF
Conventional positive inotropy and arrhythmogenesis in HF
Drugs that have been used conventionally for positive inotropy such as adrenoceptor agonists and PDE inhibitors enhance SERCA2a function via increased PLB phosphorylation. However, the global enhancement of PKA activity phosphorylates numerous other targets and significantly enhances metabolic demand. With respect to Ca2+ cycling, phosphorylation of LTCC, PLB and SR Ca2+ release channels (such as RyR2) increases SR Ca2+ content and causes an increased propensity to diastolic Ca2+ leak from the SR to cause DADs. EADs are also exacerbated by conventional positive inotropes due to enhancement of ICa and reduction in the rate of its inactivation (both Ca2+ and voltage dependent) (Zeng and Rudy, 1995), as well as the increase in SR Ca2+ leak. Hence, as a result of enhancement of cellular triggered activity, positive inotropes can be arrhythmogenic, even in the absence of underlying cardiac disease (Pogwizd et al., 2001; Cox et al., 2005).
In HF, conventional positive inotropy can thus be seen as a perfect storm; the increased tendency for arrhythmias on an already arrhythmic substrate. This may be why, despite improving cardiac contractility and reducing pulmonary venous pressure, agents such as dobutamine and milrinone have been unsuccessful (Packer et al., 1984, 1991; O'Connor et al. 1999; Penson et al., 2007). These agents increase mortality, in large part, due to a rise in ventricular ectopy, arrhythmia and sudden death. SERCA2a gene therapy aims to enhance cardiac contractility but circumvent such problems by targeting derangements of specific molecular pathways in HF.
Specific pharmacological enhancement of SERCA2a function
An alternative to conventional positive inotropy or SERCA2a gene therapy in rescuing impaired SR Ca2+ uptake in HF is direct pharmacological activation of SERCA2a or inhibition of PLB binding, although to date most agents purported to have such activity have had insufficient efficacy or specificity to be considered for clinical use in humans. This is despite the initial promise shown by a group of phytochemicals, including the polyphenol ellagic acid, to increase the maximum reaction velocity of SERCA2a (Antipenko et al., 1999). More recently, istaroxime, a new non-glycoside agent that inhibits the Na+/K+-ATPase, has been shown to elevate SERCA2a activity in addition to its primary mode of action. In vivo studies showed that the incidence of lethal arrhythmias was reduced compared with digoxin at a dose that caused equivalent positive inotropy, probably due to a relative reduction in NCX-mediated transient inward current (ITI) (Rocchetti et al., 2003; 2005). Istaroxime also enhances SERCA2a activity in SR vesicle preparations and increases SR Ca2+ content in isolated myocytes treated with the agent (Rocchetti et al., 2005). Istaroxime infusion in a guinea pig TAC HF model restored echocardiographic measures of systolic and diastolic cardiac function to levels comparable with non-failing controls (Micheletti et al., 2007). A recent phase 2 trial of 120 hospitalized patients with HF revealed that the drug was well tolerated with a modest drop in pulmonary capillary wedge pressure (3–5 mmHg) compared with placebo (Gheorghiade et al., 2008). Istaroxime inhibition of the Na+/K+-ATPase might lead to similar problems found with digoxin therapy where elevated diastolic Ca2+ leads to subsequent impairment of diastolic function and arrhythmia. Its parallel SERCA2a activation may circumvent the potential problem but this will only be resolved with larger trials.
SERCA2a gene therapy aims to rescue the reduction of SERCA2a protein levels in HF and has more extensive safety and efficacy data than any small molecule strategy to enhance SERCA2a function. Even if more specific molecules are discovered, it is currently unclear whether pharmacological stimulation of SERCA2a function will be as effective as gene therapy in HF due to the reduction in levels of the target protein limiting the potential for such an agent to enhance SR Ca2+ uptake.
Gene delivery of SERCA2a
It is important to appreciate the substantial technical difficulties that have been overcome to take SERCA2a gene therapy into man. These issues have been reviewed elsewhere (Schmidt and Hajjar, 2001; Huq et al., 2002; Lyon et al., 2008) and will only be explored briefly here. Viral vectors have shown greater transfection efficacy than non-viral methods (Lyon et al., 2008). The choice of viral vector is important. Retroviruses require active division of the host cell for their genetic material to be incorporated into host DNA and therefore have limited utility in mature myocardium. Adenoviruses have been used successfully in animal models of SERCA2a gene therapy and are appealing because they carry double-stranded (ds)DNA, which does not integrate into the host genome, thus limiting side effects as a result of insertional mutagenesis of host DNA. However, targeted adenoviral-mediated delivery is limited by the virus predilection to infect multiple human organs, in addition to its immunogenicity resulting in both immune myocarditis and a limited duration of therapeutic gene expression after the host's immune response is established (Yang et al., 1994; Calabrese and Thiene, 2003).
Focus has therefore shifted to the recombinant adeno-associated viruses (rAAVs), which are currently the viral vector most suited to myocardial gene delivery (Lyon et al., 2008). Like adenoviruses, they can infect non-dividing cells and have high transduction efficiency without integration into the host genome (Schmidt and Hajjar, 2001). It was hoped that because rAAVs are derived from non-pathogenic parvoviruses, they would be less likely to induce an immune response giving the potential for lifelong gene expression (del Monte and Hajjar, 2003). However, with increasing experience of these vectors, it has been found that the presence of neutralizing anti-AAV antibodies, both pre-existing and generated as a result of therapeutic use, is problematic in up to 50% of patients (Louis et al., 2013). The presence of such antibodies, even at low titres, is associated with reduced or absent therapeutic benefit (Jaski et al., 2009). Strategies to circumvent the immune response via creation of novel, genetically diverse AAV mutants, chemical shielding or pharmacological inhibition of neutralization are reviewed elsewhere (Louis et al., 2013).
An important advantage of the use of rAAVs is their tropism for specific tissues allowing targeting of the diseased organ, in this case the myocardium. Of the 11 serotypes currently identified, AAV1, 6, 8 and 9 have higher myocardial tropism than any of the alternative candidate myocardial vectors (Pacak et al., 2006; Palomeque et al., 2007). However, as there may still be significant infection of other organs (Cheng and Smith, 2013), delivery of the vector has until now usually been achieved in a targeted manner via various methods, including coronary infusions, retrograde venous infusion via the coronary sinus or injection into the pericardial sac (del Monte and Hajjar, 2003). A hope for the future is that more highly cardiotropic rAAVs may be developed that circumvent the need for such invasive techniques by allowing peripheral venous administration (Pacak et al., 2006).
One potential concern in any gene delivery system is that transfection will result in patchy expression of the gene within the myocardium. This could potentially enhance arrhythmias as any electrophysiological heterogeneities caused could induce wavebreak and re-entry. Mechanisms designed to enhance delivery, such as coronary infusions – rather than direct myocardial injection – and high viral tropism may result in global rather than patchy uptake. These approaches appear to have been successful thus far, with the beneficial effects on arrhythmia in preclinical and clinical settings (below) suggesting that enhanced electrophysiological heterogeneity of the myocardium is not a major problem with current gene delivery techniques.
Rescuing EC coupling in HF models with SERCA2a gene therapy
Rescuing contractile impairment in HF
The first cell model used to study SERCA2a gene therapy was neonatal rat cardiomyocytes. This is relevant to HF because the disease process is characterized by a regression to a less mature phenotype. These studies revealed that increased SERCA2a expression increased amplitude of Ca2+ transients, enhanced relaxation kinetics and reduced diastolic Ca2+ (Hajjar et al., 1997). This was only achieved in the absence of elevated PLB – that is, the SERCA2a : PLB ratio was of paramount importance (Giordano et al., 1997; Meyer et al., 1999). Subsequently, it was demonstrated that in vitro transfer and expression of the SERCA2a gene in failing human cardiomyocytes from hearts explanted from transplant recipients could rescue deranged contraction and relaxation parameters, and this was mirrored by improved kinetics of intracellular Ca2+ transients and normalization of diastolic [Ca2+] (Figure 3) (del Monte et al., 1999). The next step in translation to human HF therapy was to assess whether gene transfer could rescue established HF in animal models.
Figure 3.
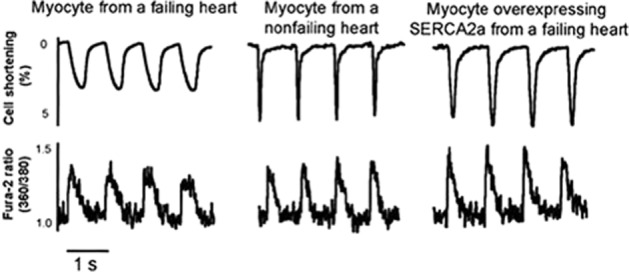
Rescue of human failing ventricular cardiomyocyte contraction and relaxation by in vitro Ad.SERCA2a gene transfer. Analysis of % shortening (above) and Fura-2-reported Ca2+ transients (below) reveals lower amplitude, slower contractions and Ca2+ transients in heart failure cells. This was rescued by SERCA2a gene transfer via an adenoviral vector. Reproduced with permission from Wolters Kluwer Health: Circulation [del Monte et al. (1999)]. © 1999.
Initial in vivo studies of acquired HF focused on the TAC model in the rat (Miyamoto et al., 2000). Adenoviral SERCA2a gene therapy delivered via coronary infusion restored reduced SERCA2a expression to levels comparable with non-failing sham-operated rats. Cardiac contractile parameters, specifically the maximum rate of pressure generation (dP/dt) in the left ventricle (LV) and maximum rate of relaxation (−dP/dt), were also returned to sham-operated levels. In the same model, SERCA2a gene therapy reduced mortality compared with untreated TAC animals. In contrast, TAC animals treated with dobutamine had accelerated mortality, analogous to the clinical experience using this positive inotrope (del Monte et al., 2001). Large animal studies followed, with advancements in vector technology leading to the use of rAAVs in these preclinical experiments. In a porcine model of chronic volume overload-induced HF due to mitral regurgitation, AAV1.SERCA2a restored SERCA2a levels to normal and rescued cardiac contractile function (Kawase et al., 2008). Similar results have been obtained in a variety of large animal models, including pacing-induced HF in sheep (Byrne et al., 2008) and dog (Mi et al., 2009).
These effects may not only be of importance in enhancing impaired contractile function, but also of value in reducing the formation of arrhythmias. Contractile impairment in HF is closely coupled with arrhythmias through mechanoelectrical coupling. Heterogeneity of contractility is common in HF, particularly where there is an ischaemic aetiology. Hence, there are regions in the heart in which acute stretch during systole can lead to activation of depolarizing stretch-activated ion channels to bring about ventricular premature beats (Janse et al., 2003). Rapid relaxation of these segments can also bring about triggered activity via rapid release of Ca2+ from myofilaments, which can induce Ca2+release from the SR and subsequent Ca2+ waves and DADs (ter Keurs, 2011). On a cellular level, it has been found that acute stretch of isolated cardiomyocytes can lead to enhancement of spontaneous SR Ca2+ release via increased production of reactive oxygen species (Byrne et al., 2008; Iribe et al., 2009; Prosser et al., 2011). Chronic stretch of cardiomyocytes, as seen in dilated cardiomyopathies, can also contribute to arrhythmia via altered transcription of ion channels and cellular Ca2+ overload (Janse et al., 2003).
Indirect benefits of SERCA2a gene therapy
In addition to the effects on contractility, reduced SERCA2a levels can have less direct but equally damaging effects that contribute to the pathophysiology of HF. Mitochondrial saturation by the elevated resting Ca2+ levels impairs mitochondrial energetics, increasing cardiomyocyte oxidative stress (Duchen et al., 2008). Myocardial oxidative stress damages numerous effector systems in the failing cardiomyocyte, including central proteins in Ca2+ cycling (Cutler et al., 2012a), leading to a positive feedback cycle between rising calcium levels, oxidative stress, mechanical dysfunction, electrical instability and ultimately cell death. Furthermore, increased diastolic [Ca2+] drives several pathological signalling pathways in ventricular cardiomyocytes, including calcineurin-NFAT, CaMKII-histone deacetylase and stromal interaction molecule 1, which can lead to hypertrophy and adverse cardiac remodelling (Kho et al., 2012).
Abnormalities of both cardiac metabolism (measured by the ratio of myocardial phosphocreatine : ATP using NMR spectroscopy) and the energetic cost of contractility were restored in SERCA2a-treated animals with HF induced by TAC or pacing (del Monte et al., 2001; Sakata et al., 2007a; Byrne et al., 2008; Kawase et al., 2008). Similar findings were reported by Sakata et al. in a rat model of diabetic cardiomyopathy (Sakata et al., 2006) and by our group in a myocardial infarction (MI) rat HF model (Lyon et al., 2011). Enhanced myocardial energetics may be explained, at least partially, by the alteration of balance of cytoplasmic Ca2+ efflux from SERCA2a to NCX in HF and back towards SERCA2a in SERCA2a gene therapy. The stoichiometry of ATP molecules consumed to Ca2+ ions transported is 1:2 (ATP : Ca2+) for SERCA2a and 1:1 for NCX (indirectly via the Na+/K+-ATPase), hence contributing to the reduction in efficiency seen with reduced SERCA2a levels (Sakata et al., 2006). The improvement in oxygen cost of contractility contrasts with the neutral (or negative at higher doses) effects on this parameter by dobutamine and levosimendan (Muller et al., 2010).
In addition, SERCA2a appears to have some effects on myocardial perfusion. Coronary blood flow was shown to increase following its administration in a model of diabetic cardiomyopathy (Sakata et al., 2007b), although it was not clear whether this was related to improved myocardial relaxation facilitating diastolic coronary blood flow or a direct vascular mechanism. More recently, evidence has emerged that SERCA2a is expressed in vascular smooth muscle of treated HF animals and that this is related to restoration of normal levels of endothelial NOS and thus reduced vasoconstriction (Hadri et al., 2010). SERCA2a gene therapy has also been shown to result in reduced smooth muscle proliferation and neointimal thickening in response to injury in rat carotid and human internal mammary artery (Lipskaia et al., 2005; 2013).
Modulation of arrhythmia with SERCA2a gene therapy
Possible detrimental effects of SERCA2a overexpression on failing hearts
SERCA2a, as a key regulator of SR Ca2+ content, is important in dictating Ca2+ available for CICR and thus the cardiac contractile force subsequently generated. This can be understood in the context of the fundamental SR Ca2+ release event: the Ca2+ spark. When summated in their thousands, sparks form the large cytosolic rise in Ca2+ (the Ca2+ transient) that causes contraction (Cannell et al., 1995; Lopez-Lopez et al., 1995). There is a large amount of gain in the system, provided both by the higher Ca2+ flux carried by RyRs compared with LTCCs and by the persistence of Ca2+ release beyond the initial ICa trigger. The quantity of Ca2+ leaving the SR during both individual spark events and their summated transient is steeply dependent on SR Ca2+ content (Gyorke and Gyorke, 1998; Trafford et al., 2000; Bode et al., 2011). Thus, increasing SERCA2a activity increases the amplitude of each spark and the summated systolic Ca2+ transient (Jane Lalli et al., 2001). However, because Ca2+ sparks are also the fundamental building blocks of Ca2+ waves (Cheng et al., 1996), which result in pro-arrhythmic DADs, the beneficial effects on contractility could theoretically result in an increased risk of arrhythmia as seen with conventional positive inotropes. This is particularly concerning in the context of the ‘leaky’ SR in HF. Seemingly supporting the concept of arrhythmogenesis with SERCA2a overexpression, accelerating SR-cytoplasmic Ca2+ cycling above normal levels is deleterious with pro-arrhythmic sequelae in a number of transgenic mouse models (Chopra et al., 2007; Yuan et al., 2007). In addition, in humans with a homozygous PLB null mutation, a fatal dilated cardiomyopathy phenotype results (Haghighi et al., 2003). Also, sudden arrhythmic death was transiently increased in a transgenic SERCA2a overexpressing rat following acute coronary ischaemia and reperfusion (Chen et al., 2004), although surviving animals then had reduced post-infarction LV impairment compared with wild-type controls. This enhanced arrhythmia is in contrast to the majority of studies that have found beneficial effects.
Beneficial effects on SR Ca2+ leak
Contrary to these concerns, we reported that SERCA2a therapy reduced arrhythmia in an established chronic MI model of HF in the rat (Lyon et al., 2011). The HF model was characterized by a high frequency of spontaneous ventricular arrhythmia (VA) with over 4000 spontaneous ventricular ectopic beats per day (corresponding to approximately 1% of all beats). This was significantly reduced in SERCA2a-treated animals. We also assessed the incidence of isoprenaline-induced arrhythmia, a technique used to stress the importance of SR leak : load imbalance in HF. In the untreated HF group, 75% of animals had sustained arrhythmia in the form of sustained VT or ventricular fibrillation (VF), whereas no animals in the HF + SERCA2a gene therapy group had sustained (>4 beats) VT or VF (Figure 4).
Figure 4.
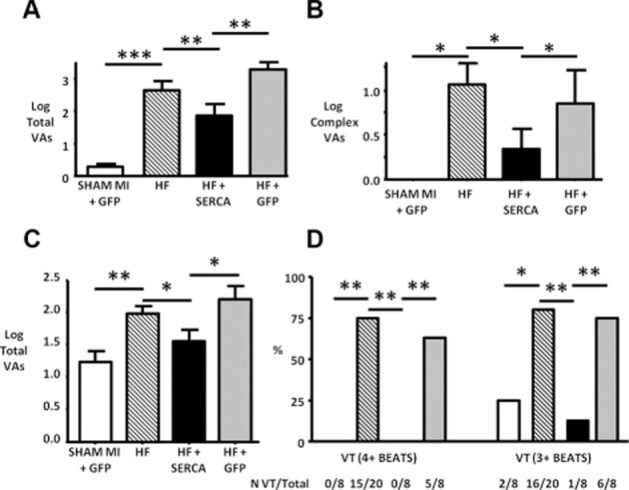
SERCA2a gene therapy rescues pro-arrhythmic phenotype of myocardial infarction (MI)-induced heart failure (HF) in the rat. (A) Simple spontaneous ventricular arrhythmias (VAs) [i.e. ventricular ectopic (VE) beat incidence] during a 24 h study period were significantly increased in HF versus sham animals and rescued in SERCA2a-treated animals. Control virus (HF + GFP) group showed no improvement over HF group. (B) Complex spontaneous VAs [i.e. multiple sequential VEs or ventricular tachycardia (VT)] showed a similar pattern. (C) Total isoprenaline-induced VAs during the 60 min recording period after injection were reduced by SERCA2a gene transfer. (D) The effect on isoprenaline-induced VT was even more marked with 15/20 HF animals suffering isoprenaline-induced VT (4 + beats), but none of the SERCA2a-treated HF animals. *P < 0.05, **P < 0.01, and ***P < 0.001. N VT/Total – number of rats that suffered VT versus total rats assessed with isoprenaline challenge. Reproduced with permission from Wolters Kluwer Health: Circulation Arrhythmia & Electrophysiology [Lyon et al. (2011)]. © 2011.
We proceeded to study the cellular mechanisms underlying this anti-arrhythmic effect. We found that HF cells had a typical ‘leaky’ SR phenotype, in part, due to increased frequency of spontaneous Ca2+ sparks. As a consequence, SR Ca2+ content was reduced in HF cells. Following SERCA2a gene therapy, Ca2+ spark frequency remained high, but spark morphology changed substantially with a reduction of spark mass to normal levels. This resulted in improvements to spark-mediated Ca2+ leak (calculated by spark mass × frequency) to a level not statistically different from that of age-matched control animals. The reduction in mass had compensated for the higher frequency. Another measure of SR Ca2+ leak is total leak, which includes spark and non-spark mediated fluxes and is measured through application of tetracaine (Shannon et al., 2002). This was also reduced to control levels following SERCA2a gene therapy. SR Ca2+ content also returned to normal following SERCA2a treatment, presumably in part due to reduced SR Ca2+ leak and in part due to rescued SERCA2a levels and SR Ca2+ uptake. Isoprenaline-induced triggered cellular activity was also reduced in cardiomyocytes from SERCA2a-treated animals, consistent with the anti-arrhythmic effect observed following in vivo isoprenaline challenge.
Clearly, such changes are not predictable from the increase in SR Ca2+ content induced by SERCA2a gene therapy. There must be additional effects of therapy that compensate for the presumed increase in leak simply induced by elevation of SR Ca2+ load per se. Several possibilities may explain why this might happen. One possibility is direct modification of Ca2+ spark morphology due to the buffering ability of increased SERCA2a. Indeed, pharmacological inhibition of SERCA2a has been shown to prolong sparks (Gomez et al., 1996). If the opposite occurs following therapy, curtailed sparks may result.
Secondly, phosphorylation of RyR2 at Ser2815 was also reduced by SERCA2a gene transfer (Lyon et al., 2011). Phosphorylation at this site is induced by the activity of CaMKII (Yang et al., 2007). As SERCA2a enhances Ca2+ uptake, it could potentially reduce [Ca2+]i in the vicinity of CaMKII, thus reducing its activation via the Ca2+-calmodulin complex. The lower amount of phosphorylation would be expected to increase the threshold for SR Ca2+ release towards normal levels, thus ‘resetting’ the leak threshold and reducing spontaneous Ca2+ release at a given load. We believe a virtuous cycle whereby reduced CaMKII activity is initiated by enhanced SERCA2a activity and reinforced by reduced SR Ca2+ leak may ensue. The effects of SERCA2a therapy on other modifications of RyR2 such as oxidation and nitrosylation have not been specifically assessed but may also be significant, particularly given the importance of diastolic [Ca2+]i in the regulation of mitochondrial function and oxidative stress (Zima and Blatter, 2006; Duchen et al., 2008).
Although not yet studied in detail, it is also possible that SERCA2a therapy is beneficial not only in terms of Ca2+ leak reduction but also in terms of reducing adverse consequences of Ca2+ leak. It is interesting to speculate on what effect SERCA2a therapy would have on Ca2+ waves. On the one hand, the enhanced buffering of cytosolic Ca2+ may mean that it is more difficult for waves to initiate and propagate. On the other hand, there is recent evidence that Ca2+ uptake by SERCA2a at the wavefront might produce luminal sensitization that is necessary for efficient wave propagation (Keller et al., 2007; Maxwell and Blatter, 2012), and could enhance waves in the presence of SERCA2a up-regulation. The consequences of a wave might also be altered since the amplitude of the ensuing DAD could be reduced by the increase in SERCA2a : NCX ratio, or enhanced as a result of increased SR Ca2+ content. These aspects will be the subject of further study.
Beneficial effects on Ca2+ and repolarization alternans
A further mechanism whereby SERCA2a gene therapy can be anti-arrhythmic has been explored extensively by Rosenbaum and co-workers. Beat-to-beat variations in Ca2+ fluxes and action potential duration (APD) within myocytes, known as Ca2+ and electrical alternans, respectively, are known to be associated with arrhythmia susceptibility (Cutler et al., 2009). Cellular alternans occur when the heart rate exceeds the capability of the cardiomyocytes to cycle Ca2+. Hence, particularly in HF, a reduction in SERCA2a can predispose to alternans and arrhythmia. Cutler et al. showed, first in normal myocytes (Cutler et al., 2009) and later in myocytes from a pressure-overload induced HF model in the guinea pig (Cutler et al., 2012b), that enhanced SERCA2a levels reduced the propensity for both Ca2+ and APD alternans. Even under constant action potential clamp conditions, this difference persisted, providing further evidence that the effect was due to SR-dependent effects. As a result, pacing-induced VAs were suppressed in hearts with higher SERCA2a expression, with the lowest SERCA2a levels (as seen in HF) being associated with reduced threshold for alternans. Comparatively higher SERCA2a expression in ascending order was seen in (a) control, (b) SERCA2a-treated HF and (c) SERCA2a-treated controls. VF/VT inducibility was reduced predictably as SERCA2a levels increased (Figure 5). Consistent with our study, these experiments also showed that SERCA2a-treated HF also reduced SR Ca2+ leak in the whole heart, with reduced Ser2814 (the CaMKII site in this species) phosphorylation. Xie et al. have also shown that adenoviral SERCA2a gene transfer in cultured rabbit cardiomyocytes suppresses Ca2+ alternans and that the SERCA2a inhibitor thapsigargin increases susceptibility to alternans (Xie et al., 2008). It is important to note that enhancement of SR Ca2+ leak alone can result in cellular Ca2+ and voltage alternans (Weiss et al., 2011). Thus, the extent to which alternans is a primary event or an epiphenomenon indicating the enhanced SR Ca2+ leak in this setting is unclear. Nevertheless, it is likely to be an important mechanistic connection between spontaneous SR Ca2+ release and possible VT or VF at the level of the organ.
Figure 5.
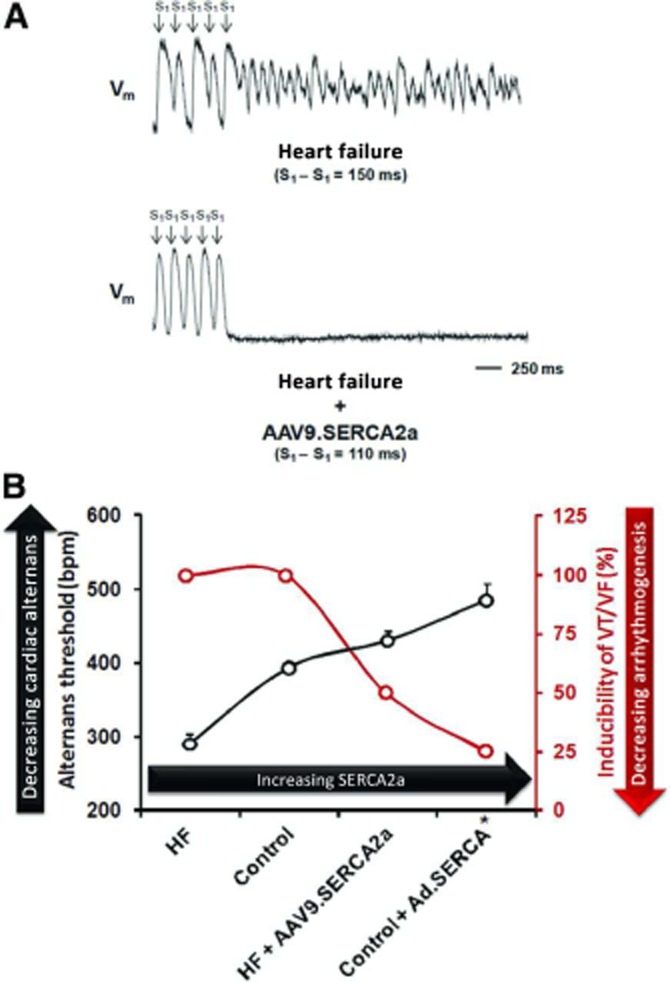
Modulation of alternans-mediated arrhythmia by SERCA2a gene transfer. (A) Optical mapping of a Langendorff-perfused heart failure (HF) heart reveals marked action potential duration (APD) alternans with subsequent induction of ventricular fibrillation (VF). In a HF heart treated with SERCA2a gene therapy (HF + AAV9.SERCA2a), such alternans does not occur despite an even shorter basic cycle length. (B) Graph of increasing threshold for alternans and decreasing inducibility of VT/VF in the presence of higher SERCA2a expression from lowest (HF) to highest (SERCA2a-treated controls – control + Ad.SERCA). Reproduced with permission from Wolters Kluwer Health: Circulation [Cutler et al. (2012b)]. © 2012.
Other anti-arrhythmic mechanisms
APD prolongation in HF is thought to be an important cause of arrhythmia, both through enhancing dispersion of repolarization and favouring the development of EADs (Kaab et al., 1996). Terracciano et al. (2002) showed that SERCA2a gene transfer in cultured rabbit cardiomyocytes reduced APD duration. Consistent with these findings, Davia et al. (2001) showed that SERCA2a gene therapy also reduced the incidence of aftercontractions in the same model. Together, these studies suggest that a reduction in EADs, as well as DADs, may play a role in arrhythmia reduction. APD prolongation can lead to increased QT interval on the ECG of the intact animal. Hence, our finding that QT interval is prolonged in HF and rescued in SERCA2a-treated rats (Lyon et al., 2011) is consistent with these data. The reduction in APD induced by SERCA2a probably has its basis in enhanced Ca2+-dependent inactivation of LTCC that is due to the larger SR Ca2+ release.
Abnormal automaticity is another potentially important mechanism of arrhythmogenicity in HF (Vermeulen, 1998) and may be independent of SR Ca2+ leak (Nuss et al., 1999). This may be enhanced in ventricular cells in HF due to reductions in IK1 and enhanced If. Given recent insights into the role of SR Ca2+ release in normal automaticity, and particularly how it is synergistic with If in bringing the membrane potential to threshold (Maltsev and Lakatta, 2009), it is possible that reduction of abnormal automaticity may also play a role in the anti-arrhythmic effect of SERCA2a in HF.
Modulation of ischaemia–reperfusion (I/R)-induced injury may be another mechanism that confers protection against arrhythmias in vivo. In a rat I/R rat model, SERCA2a gene therapy 2–6 days prior to I/R reduced episodes of VT/VF (del Monte et al., 2004). While the initial ischaemic area of the heart did not differ between treated and control animals in this study, the final MI area was significantly reduced in SERCA2a-treated animals. This suggests enhanced survival of SERCA2a-treated cardiomyocytes in the presence of ischaemia, potentially due to enhanced buffering of the large rise in cytosolic Ca2+, which can be induced by I/R. The reduction in infarct size would be expected to reduce conduction heterogeneity in the ventricle and thus mediate the decline in the number of arrhythmias observed. These findings were later reproduced in a porcine I/R model in which arrhythmias were reduced following I/R, but not in a permanent coronary ligation model in the same species (Prunier et al., 2008).
More general reverse remodelling of the failing heart as a result of improved cardiac mechanics may also play a significant role in the reduction of arrhythmias. We have shown an example of this at the level of the isolated cardiomyocytes whereby the disruptions in surface structure and t-tubule morphology that occur in the rat chronic MI model of HF (Lyon et al., 2009) are rescued in cardiomyocytes isolated from SERCA2a-treated HF rats (Lyon et al., 2012). Such restoration of ultrastructure could improve arrhythmia via a reduction of ‘orphaned’ RyRs (those remote from t-tubules), which have been hypothesized to be particularly important in the pro-arrhythmic consequences of HF (Song et al., 2005; 2006; Keller et al., 2007). Enhancing SERCA2a function via transfection of pseudophosphorylated PLB has also been shown to result in significant reduction of macroscopic tissue heterogeneity via reduction of fibrosis in a cardiomyopathic hamster model (Hoshijima, 2005). These structural improvements could reduce the enhanced electrophysiological heterogeneity and thus decrease the likelihood of re-entry in HF. They also emphasize that SERCA2a gene therapy can result in a broader reversal of the pathophysiology of HF than isolated effects on SR Ca2+ cycling could confer. A summary of the multifaceted mechanism of action of SERCA2a gene therapy in reducing arrhythmia is shown in Figure 6.
Figure 6.
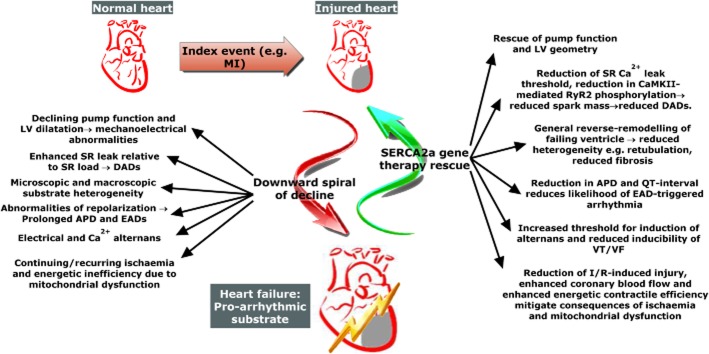
Multiple mechanisms lead to pro-arrhythmic heart failure phenotype and are rescued by SERCA2a gene therapy. An index event causes a degree of cardiac damage and leads to a downward spiral of events which result in impaired pump function and a pro-arrhythmic substrate. Pathological changes leading to arrhythmia, and how they are rescued by SERCA2a gene therapy, are highlighted. APD, action potential duration; CaMKII, Ca2+/calmodulin-dependent protein kinase II; DAD, delayed afterdepolarization; EAD, early afterdepolarization; I/R, ischaemia/reperfusion; LV, left ventricle; MI, myocardial infarction; RyR2, cardiac ryanodine receptor; SR, sarcoplasmic reticulum; VF, ventricular fibrillation; VT, ventricular tachycardia.
Clinical SERCA2a gene therapy trials
Regardless of the beneficial effects of SERCA2a gene therapy in preclinical models, whether the therapy is destined to form part of the management of HF is dependent on the results of ongoing clinical trials. A completed phase 1/2 trial [Calcium Up-regulation by Percutaneous Administration of Gene Therapy in Cardiac Disease (CUPID)] and the current phase 2B trial (CUPID2) among others will help decide whether SERCA2a will be beneficial in the human HF population.
The CUPID trial programme
The phase 1/2 CUPID trial was launched in 2007 (Hajjar et al., 2008). In the initial open-label phase 1 part of this study, safety was established through dose escalation in 12 patients (Jaski et al., 2009). In the second stage, 39 patients with advanced HF were randomized to receive one of three doses of intracoronary AAV1.SERCA2a or placebo saline infusion (Jessup et al., 2011). Dosing protocol for the treatment groups was low dose (6 × 1011 DNase-resistant particles), medium dose (3 × 1012 DNase-resistant particles) or high dose (1 × 1013 DNase-resistant particles). Virus or saline placebo was administered via antegrade coronary infusion over 10 min. All patients were required to have an implantable defibrillator in response to concerns that SERCA2a gene therapy may increase the rate of VAs. Eight patients received low-dose AAV1.SERCA2a, eight were randomized to a mid-level dose and nine to high dose; 14 patients were randomized to placebo with saline infusion.
The primary endpoint in terms of efficacy was evaluated by examining concordant trends in the following endpoints: Patient's symptom questionnaires, functional status [6 min walk test and VO2-max (maximal rate of oxygen consumption)], N-terminal pro–B-type natriuretic peptide (NT-proBNP) levels and echocardiographic measures. The AAV1.SERCA2a high-dose group met the pre-specified criteria for efficacy. Patients in this treatment group demonstrated improvement or stabilization in symptoms, functional class, NT-proBNP levels, and LV end-systolic volumes. There was also a significant increase in time to cardiovascular events and a decreased frequency of cardiovascular events per patient in all patients receiving AAV1.SERCA2a. Importantly, patients in the treatment arm showed no increase in adverse events, disease-related events or arrhythmias, correlating well with the preclinical studies described earlier, and contrary to the perceived concerns. In this trial, a potentially problematic issue of AAV auto-antibodies was identified. Patients with even the lowest titres of AAV auto-antibodies (>1:2) appeared not to receive any therapeutic benefit. This highlights the importance of developing novel vectors that are not neutralized by pre-existing antibodies.
CUPID 2
The CUPID 2 trial is a phase 2b, double-blind, placebo-controlled, multinational, randomized study evaluating the safety and efficacy of intracoronary administration of AAV1.SERCA2a in subjects with HF. Two hundred patients will be recruited with ischaemic or dilated cardiomyopathy with New York Heart Association (NYHA) III/IV class symptoms despite optimal HF treatment. In contrast to CUPID, in this trial patients are not required to have implantable defibrillators, reflecting the balance of evidence thus far in both preclinical and clinical studies regarding arrhythmia risk. Patients will be screened for neutralizing antibodies to AAV1 and must be negative (titre <1:2) to be enrolled. Eligible patients will be randomized in parallel in a 1:1 ratio to receive either AAV1.SERCA2a or placebo. Vector delivery is in the form of a 10 min intracoronary infusion of 1 × 1013 DNase-resistant particles of AAV1.SERCA2a (the high dose in the CUPID trial) with concomitant glyceryl trinitrate to improve vector uptake and transfection efficiency via vasodilatation. To allow sufficient vector delivery, participants must have at least one coronary vessel with angiographically normal flow.
The primary efficacy endpoint is ‘time to recurrent HF hospitalizations in the presence of a terminal event [all-cause death, heart transplantation, left ventricular assist device (LVAD) implantation]’. The secondary endpoint is ‘time to a terminal event (all-cause death, heart transplantation, LVAD implantation)’. Both primary and secondary endpoints will be analysed using the joint frailty model, a method of statistical analysis of endpoints which, in contrast to standard survival analysis, takes into account all events and not just the first (Huang and Liu, 2007). Exploratory assessments include changes from baseline in the following: NYHA class, 6 min walk test and symptom questionnaire.
Safety will be assessed based on the incidence of adverse events, all-cause mortality and laboratory evaluations. Patients have already been treated in the USA, Denmark and at our centre in the UK. An appropriately powered separate trial in a high-risk arrhythmia population (e.g. HF patients with implantable cardioverter-defibrillators) would be necessary to specifically assess anti-arrhythmic efficacy. Further trials may also be necessary to assess whether the dose would need to be adjusted depending on the differences in SERCA2a deficiencies seen in different models of HF.
Conclusion
Myocardial SERCA2a gene therapy is the first of several novel molecular-targeted strategies aimed at improving mechanical function of the failing heart without the detrimental side effects of accelerating energetic inefficiencies, apoptosis and arrhythmogenicity associated with classical positive inotropes which increase PKA activity. Ca2+ has long been identified as a central regulatory and signalling messenger for numerous intracellular pathways in cardiomyocytes, as the fields of excitation-contraction coupling, excitation-energetic coupling and excitation-transcription coupling all testify. As a result, targeting abnormal SR Ca2+ cycling in HF directly confers several simultaneous benefits, including normalized Ca2+ transient kinetics, enhanced energetic efficiency and increased cell survival. Evidence of additional beneficial effects, via reduced SR Ca2+ leak, increased alternans threshold, reduced propensity for injury during I/R and reverse remodelling of the heart suggest that SERCA2a gene therapy has the potential to become the first anti-arrhythmic positively inotropic treatment strategy in HF. This could improve both quality of life and survival in this group of patients.
Acknowledgments
M. B. S. is supported by the Wellcome Trust (WT092852). A. R. L. is supported by a British Heart Foundation Intermediate Research Fellowship (FS/11/67/28954) and the National Institute for Health Research-funded Cardiovascular Biomedical Research Unit at the Royal Brompton Hospital.
Abbreviations
- APD
action potential duration
- [Ca2+]i
intracellular (cytoplasmic) Ca2+ concentration
- CaMKII
Ca2+/calmodulin-dependent protein kinase II
- CUPID
Calcium Up-regulation by Percutaneous Administration of Gene Therapy in Cardiac Disease Trials
- CICR
Ca2+-induced Ca2+ release
- DAD
delayed afterdepolarization
- (−)dP/dt
maximum rate of left ventricular pressure generation in systole (or reduction in diastole)
- EAD
early afterdepolarization
- ECC
excitation contraction coupling
- HF
heart failure
- ICa
L-type Ca2+ current
- If
‘Funny'-current
- IK1
inward rectifying potassium channel
- ITI
transient inward current
- I/R
ischaemia/reperfusion
- LTCC
L-type Ca2+ channel
- LV
left ventricle
- LVAD
left ventricular assist device
- MI
myocardial infarction
- MOI
multiplicity of infection
- NCX
Na+–Ca2+ exchanger
- NT-proBNP
N-terminal pro–B-type natriuretic peptide
- PLB
phospholamban
- rAAV
recombinant adeno-associated virus
- RyR2
cardiac ryanodine receptor
- SERCA
sarcoplasmic reticulum Ca2+-ATPase
- SR
sarcoplasmic reticulum
- SUMO
small ubiquitin-like modifier
- TAC
transverse aortic constriction
- VA
ventricular arrhythmia
- VF
ventricular fibrillation
- VO2-max
maximal rate of oxygen consumption
- VT
ventricular tachycardia
Conflict of interest
Dr. Lyon is the UK national lead investigator for the CUPID2 trial and has a consultancy contract with Celladon Corporation for this role.
References
- Ai X, Curran JW, Shannon TR, Bers DM, Pogwizd SM. Ca2+/calmodulin protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure. Circ Res. 2005;97:1314–1322. doi: 10.1161/01.RES.0000194329.41863.89. [DOI] [PubMed] [Google Scholar]
- Anderson ME, Braun AP, Wu Y, Lu T, Wu Y, Schulman H, et al. KN-93, an inhibitor of multifunctional Ca++/calmodulin-dependent protein kinase, decreases early afterdepolarizations in rabbit heart. J Pharmacol Exp Ther. 1998;287:996–1006. [PubMed] [Google Scholar]
- Andersson KB, Birkeland JAK, Finsen AV, Louch WE, Sjaastad I, Wang Y, et al. Moderate heart dysfunction in mice with inducible cardiomyocyte-specific excision of the Serca2 gene. J Mol Cell Cardiol. 2009;47:180–187. doi: 10.1016/j.yjmcc.2009.03.013. [DOI] [PubMed] [Google Scholar]
- Antipenko AY, Spielman AI, Kirchberger MA. Interactions of 6-gingerol and ellagic acid with the cardiac sarcoplasmic reticulum Ca2+-ATPase. J Pharmacol Exp Ther. 1999;290:227–234. [PubMed] [Google Scholar]
- Aracena P, Tang W, Hamilton SL, Hidalgo C. Effects of S-glutathionylation and S-nitrosylation on calmodulin binding to triads and FKBP12 binding to type 1 calcium release channels. Antioxid Redox Signal. 2005;7:870–881. doi: 10.1089/ars.2005.7.870. [DOI] [PubMed] [Google Scholar]
- Babu GJ, Bhupathy P, Carnes CA, Billman GE, Periasamy M. Differential expression of sarcolipin protein during muscle development and cardiac pathophysiology. J Mol Cell Cardiol. 2007a;43:215–222. doi: 10.1016/j.yjmcc.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu GJ, Bhupathy P, Timofeyev V, Petrashevskaya NN, Reiser PJ, Chiamvimonvat N, et al. Ablation of sarcolipin enhances sarcoplasmic reticulum calcium transport and atrial contractility. Proc Natl Acad Sci USA. 2007b;104:17867–17872. doi: 10.1073/pnas.0707722104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bers DM. Excitation-Contraction Coupling and Cardiac Contractile Force. 2nd edn. Dodrecht, The Netherlands: Kluwer Academic Publishers; 2001. [Google Scholar]
- Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- Beuckelmann DJ, Nabauer M, Erdmann E. Intracellular calcium handling in isolated ventricular myocytes from patients with terminal heart failure. Circulation. 1992;85:1046–1055. doi: 10.1161/01.cir.85.3.1046. [DOI] [PubMed] [Google Scholar]
- Beuckelmann DJ, Nabauer M, Erdmann E. Alterations of K+ currents in isolated human ventricular myocytes from patients with terminal heart failure. Circ Res. 1993;73:379–385. doi: 10.1161/01.res.73.2.379. [DOI] [PubMed] [Google Scholar]
- Bode EF, Briston SJ, Overend CL, O'Neill SC, Trafford AW, Eisner DA. Changes of SERCA activity have only modest effects on sarcoplasmic reticulum Ca2+ content in rat ventricular myocytes. J Physiol. 2011;589(Pt 19):4723–4729. doi: 10.1113/jphysiol.2011.211052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne MJ, Power JM, Preovolos A, Mariani JA, Hajjar RJ, Kaye DM. Recirculating cardiac delivery of AAV2/1SERCA2a improves myocardial function in an experimental model of heart failure in large animals. Gene Ther. 2008;15:1550–1557. doi: 10.1038/gt.2008.120. [DOI] [PubMed] [Google Scholar]
- Calabrese F, Thiene G. Myocarditis and inflammatory cardiomyopathy: microbiological and molecular biological aspects. Cardiovasc Res. 2003;60:11–25. doi: 10.1016/s0008-6363(03)00475-9. [DOI] [PubMed] [Google Scholar]
- Cannell MB, Cheng H, Lederer WJ. The control of calcium release in heart muscle. Science. 1995;268:1045–1049. doi: 10.1126/science.7754384. [DOI] [PubMed] [Google Scholar]
- Carvalho BM, Bassani RA, Franchini KG, Bassani JW. Enhanced calcium mobilization in rat ventricular myocytes during the onset of pressure overload-induced hypertrophy. Am J Physiol Heart Circ Physiol. 2006;291:H1803–H1813. doi: 10.1152/ajpheart.01345.2005. [DOI] [PubMed] [Google Scholar]
- Chen Y, Escoubet B, Prunier F, Amour J, Simonides WS, Vivien B, et al. Constitutive cardiac overexpression of sarcoplasmic/endoplasmic reticulum Ca2+-ATPase delays myocardial failure after myocardial infarction in rats at a cost of increased acute arrhythmias. Circulation. 2004;109:1898–1903. doi: 10.1161/01.CIR.0000124230.60028.42. [DOI] [PubMed] [Google Scholar]
- Cheng H, Lederer WJ, Cannell MB. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993;262:740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- Cheng H, Lederer MR, Lederer WJ, Cannell MB. Calcium sparks and [Ca2+]i waves in cardiac myocytes. Am J Physiol. 1996;270(1 Pt 1):C148–C159. doi: 10.1152/ajpcell.1996.270.1.C148. [DOI] [PubMed] [Google Scholar]
- Cheng SH, Smith AE. Gene therapy progress and prospects: gene therapy of lysosomal storage disorders. Gene Ther. 2013;10:1275–1281. doi: 10.1038/sj.gt.3302092. [DOI] [PubMed] [Google Scholar]
- Chopra N, Kannankeril PJ, Yang T, Hlaing T, Holinstat I, Ettensohn K, et al. Modest reductions of cardiac calsequestrin increase sarcoplasmic reticulum Ca2+ leak independent of luminal Ca2+ and trigger ventricular arrhythmias in mice. Circ Res. 2007;101:617–626. doi: 10.1161/CIRCRESAHA.107.157552. [DOI] [PubMed] [Google Scholar]
- Cornea RL, Jones LR, Autry JM, Thomas DD. Mutation and phosphorylation change the oligomeric structure of phospholamban in lipid bilayers. Biochemistry. 1997;36:2960–2967. doi: 10.1021/bi961955q. [DOI] [PubMed] [Google Scholar]
- Cox DE, Farmer LD, Hoyle JR, Wells GL. Prognostic significance of nonsustained ventricular tachycardia during dobutamine stress echocardiography. Am J Cardiol. 2005;96:1293–1298. doi: 10.1016/j.amjcard.2005.06.075. [DOI] [PubMed] [Google Scholar]
- Cutler MJ, Wan X, Laurita KR, Hajjar RJ, Rosenbaum DS. Targeted SERCA2a gene expression identifies molecular mechanism and therapeutic target for arrhythmogenic cardiac alternans. Circ Arrhythmia Electrophysiol. 2009;2:686–694. doi: 10.1161/CIRCEP.109.863118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler MJ, Plummer BN, Wan X, Sun QA, Hess D, Liu H, et al. Aberrant S-nitrosylation mediates calcium-triggered ventricular arrhythmia in the intact heart. Proc Natl Acad Sci USA. 2012a;109:18186–18191. doi: 10.1073/pnas.1210565109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler MJ, Wan X, Plummer BN, Liu H, Deschenes I, Laurita KR, et al. Targeted sarcoplasmic reticulum Ca2+ ATPase 2a gene delivery to restore electrical stability in the failing heart. Circulation. 2012b;126:2095–2104. doi: 10.1161/CIRCULATIONAHA.111.071480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davia K, Bernobich E, Ranu HK, del Monte F, Terracciano CMN, MacLeod KT, et al. SERCA2a overexpression decreases the incidence of aftercontractions in adult rabbit ventricular myocytes. J Mol Cell Cardiol. 2001;33:1005–1015. doi: 10.1006/jmcc.2001.1368. [DOI] [PubMed] [Google Scholar]
- Duchen MR, Verkhratsky A, Muallem S. Mitochondria and calcium in health and disease. Cell Calcium. 2008;44:1–5. doi: 10.1016/j.ceca.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Dupont Y. Kinetics and regulation of sarcoplasmic reticulum ATPase. Eur J Biochem. 1977;72:185–190. doi: 10.1111/j.1432-1033.1977.tb11238.x. [DOI] [PubMed] [Google Scholar]
- Feldman AM, Weinberg EO, Ray PE, Lorell BH. Selective changes in cardiac gene expression during compensated hypertrophy and the transition to cardiac decompensation in rats with chronic aortic banding. Circ Res. 1993;73:184–192. doi: 10.1161/01.res.73.1.184. [DOI] [PubMed] [Google Scholar]
- Flesch M, Schiffer F, Zolk O, Pinto Y, Rosenkranz S, Hirth-Dietrich C, et al. Contractile systolic and diastolic dysfunction in renin-induced hypertensive cardiomyopathy. Hypertension. 1997;30:383–391. doi: 10.1161/01.hyp.30.3.383. [DOI] [PubMed] [Google Scholar]
- Frank K, Tilgmann C, Shannon TR, Bers DM, Kranias EG. Regulatory role of phospholamban in the efficiency of cardiac sarcoplasmic reticulum Ca2+ transport. Biochemistry. 2000;39:14176–14182. doi: 10.1021/bi001049k. [DOI] [PubMed] [Google Scholar]
- Franzini-Armstrong C. Membrane particles and transmission at the triad. Fed Proc. 1975;34:1382–1389. [PubMed] [Google Scholar]
- Gheorghiade M, Blair JE, Filippatos GS, Macarie C, Ruzyllo W, Korewicki J, et al. Hemodynamic, echocardiographic, and neurohormonal effects of istaroxime, a novel intravenous inotropic and lusitropic agent: a randomized controlled trial in patients hospitalized with heart failure. J Am Coll Cardiol. 2008;51:2276–2285. doi: 10.1016/j.jacc.2008.03.015. [DOI] [PubMed] [Google Scholar]
- Giordano FJ, He H, McDonough P, Meyer M, Sayen MR, Dillmann WH. Adenovirus-mediated gene transfer reconstitutes depressed sarcoplasmic reticulum Ca2+-ATPase levels and shortens prolonged cardiac myocyte Ca2+ transients. Circulation. 1997;96:400–403. doi: 10.1161/01.cir.96.2.400. [DOI] [PubMed] [Google Scholar]
- Gomez AM, Cheng H, Lederer WJ, Bers DM. Ca2+ diffusion and sarcoplasmic reticulum transport both contribute to [Ca2+]i decline during Ca2+ sparks in rat ventricular myocytes. J Physiol. 1996;496(Pt 2):575–581. doi: 10.1113/jphysiol.1996.sp021708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwathmey JK, Morgan JP. Altered calcium handling in experimental pressure-overload hypertrophy in the ferret. Circ Res. 1985;57:836–843. doi: 10.1161/01.res.57.6.836. [DOI] [PubMed] [Google Scholar]
- Gwathmey JK, Copelas L, MacKinnon R, Schoen FJ, Feldman MD, Grossman W, et al. Abnormal intracellular calcium handling in myocardium from patients with end-stage heart failure. Circ Res. 1987;61:70–76. doi: 10.1161/01.res.61.1.70. [DOI] [PubMed] [Google Scholar]
- Gyorke I, Gyorke S. Regulation of the cardiac ryanodine receptor channel by luminal Ca2+ involves luminal Ca2+ sensing sites. Biophys J. 1998;75:2801–2810. doi: 10.1016/S0006-3495(98)77723-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadri L, Bobe R, Kawase Y, Ladage D, Ishikawa K, Atassi F, et al. SERCA2a gene transfer enhances eNOS expression and activity in endothelial cells. Mol Ther. 2010;18:1284–1292. doi: 10.1038/mt.2010.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagemann D, Kuschel M, Kuramochi T, Zhu W, Cheng H, Xiao RP. Frequency-encoding Thr17 phospholamban phosphorylation is independent of Ser16 phosphorylation in cardiac myocytes. J Biol Chem. 2000;275:22532–22536. doi: 10.1074/jbc.C000253200. [DOI] [PubMed] [Google Scholar]
- Haghighi K, Kolokathis F, Pater L, Lynch RA, Asahi M, Gramolini AO, et al. Human phospholamban null results in lethal dilated cardiomyopathy revealing a critical difference between mouse and human. J Clin Invest. 2003;111:869–876. doi: 10.1172/JCI17892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajjar RJ, Kang JX, Gwathmey JK, Rosenzweig A. Physiological effects of adenoviral gene transfer of sarcoplasmic reticulum calcium ATPase in isolated rat myocytes. Circulation. 1997;95:423–429. doi: 10.1161/01.cir.95.2.423. [DOI] [PubMed] [Google Scholar]
- Hajjar RJ, Zsebo K, Deckelbaum L, Thompson C, Rudy J, Yaroshinsky A, et al. Design of a phase 1/2 trial of intracoronary administration of AAV1/SERCA2a in patients with heart failure. J Card Fail. 2008;14:355–367. doi: 10.1016/j.cardfail.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Hasenfuss G. Alterations of calcium-regulatory proteins in heart failure. Cardiovasc Res. 1998;37:279–289. doi: 10.1016/s0008-6363(97)00277-0. [DOI] [PubMed] [Google Scholar]
- Hasenfuss G, Schillinger W, Lehnart SE, Preuss M, Pieske B, Maier LS, et al. Relationship between Na+-Ca2+-exchanger protein levels and diastolic function of failing human myocardium. Circulation. 1999;99:641–648. doi: 10.1161/01.cir.99.5.641. [DOI] [PubMed] [Google Scholar]
- Higgins ER, Cannell MB, Sneyd J. A buffering SERCA pump in models of calcium dynamics. Biophys J. 2006;91:151–163. doi: 10.1529/biophysj.105.075747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshijima M. Gene therapy targeted at calcium handling as an approach to the treatment of heart failure. Pharmacol Ther. 2005;105:211–228. doi: 10.1016/j.pharmthera.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Hou Z, Kelly EM, Robia SL. Phosphomimetic mutations increase phospholamban oligomerization and alter the structure of its regulatory complex. J Biol Chem. 2008;283:28996–29003. doi: 10.1074/jbc.M804782200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Liu L. A joint frailty model for survival and gap times between recurrent events. Biometrics. 2007;63:389–397. doi: 10.1111/j.1541-0420.2006.00719.x. [DOI] [PubMed] [Google Scholar]
- Huq F, del Monte F, Hajjar RJ. Modulating signaling pathways in hypertrophy and heart failure by gene transfer. J Card Fail. 2002;8(6, Part 2):S389–S400. doi: 10.1054/jcaf.2002.129249. [DOI] [PubMed] [Google Scholar]
- Iribe G, Ward CW, Camelliti P, Bollensdorff C, Mason F, Burton RA, et al. Axial stretch of rat single ventricular cardiomyocytes causes an acute and transient increase in Ca2+ spark rate. Circ Res. 2009;104:787–795. doi: 10.1161/CIRCRESAHA.108.193334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jane Lalli M, Yong J, Prasad V, Hashimoto K, Plank D, Babu GJ, et al. Sarcoplasmic reticulum Ca2+ ATPase (SERCA) 1a structurally substitutes for SERCA2a in the cardiac sarcoplasmic reticulum and increases cardiac Ca2+ handling capacity. Circ Res. 2001;89:160–167. doi: 10.1161/hh1401.093584. [DOI] [PubMed] [Google Scholar]
- Janse MJ, Coronel R, Wilms-Schopman FJ, de Groot JR. Mechanical effects on arrhythmogenesis: from pipette to patient. Prog Biophys Mol Biol. 2003;82:187–195. doi: 10.1016/s0079-6107(03)00015-4. [DOI] [PubMed] [Google Scholar]
- Jaski BE, Jessup ML, Mancini DM, Cappola TP, Pauly DF, Greenberg B, et al. Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID trial), a first-in-human phase 1/2 clinical trial. J Card Fail. 2009;15:171–181. doi: 10.1016/j.cardfail.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessup M, Greenberg B, Mancini D, Cappola T, Pauly DF, Jaski B, et al. Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease (CUPID): a phase 2 trial of intracoronary gene therapy of sarcoplasmic reticulum Ca2+-ATPase in patients with advanced heart failure. Circulation. 2011;124:304–313. doi: 10.1161/CIRCULATIONAHA.111.022889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhund PS, Macintyre K, Simpson CR, Lewsey JD, Stewart S, Redpath A, et al. Long-term trends in first hospitalization for heart failure and subsequent survival between 1986 and 2003: a population study of 5.1 million people. Circulation. 2009;119:515–523. doi: 10.1161/CIRCULATIONAHA.108.812172. [DOI] [PubMed] [Google Scholar]
- Jiang D, Xiao B, Yang D, Wang R, Choi P, Zhang L, et al. RyR2 mutations linked to ventricular tachycardia and sudden death reduce the threshold for store-overload-induced Ca2+ release (SOICR) Proc Natl Acad Sci USA. 2004;101:13062–13067. doi: 10.1073/pnas.0402388101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaab S, Nuss HB, Chiamvimonvat N, O'Rourke B, Pak PH, Kass DA, et al. Ionic mechanism of action potential prolongation in ventricular myocytes from dogs with pacing-induced heart failure. Circ Res. 1996;78:262–273. doi: 10.1161/01.res.78.2.262. [DOI] [PubMed] [Google Scholar]
- Kass RS, Lindegger N, Hagen B, Lederer WJ. Another calcium paradox in heart failure. J Mol Cell Cardiol. 2008;45:28–31. doi: 10.1016/j.yjmcc.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Kawase Y, Ly HQ, Prunier F, Lebeche D, Shi Y, Jin H, et al. Reversal of cardiac dysfunction after long-term expression of SERCA2a by gene transfer in a pre-clinical model of heart failure. J Am Coll Cardiol. 2008;51:1112–1119. doi: 10.1016/j.jacc.2007.12.014. [DOI] [PubMed] [Google Scholar]
- Keller M, Kao JP, Egger M, Niggli E. Calcium waves driven by ‘sensitization’ wave-fronts. Cardiovasc Res. 2007;74:39–45. doi: 10.1016/j.cardiores.2007.02.006. [DOI] [PubMed] [Google Scholar]
- ter Keurs HE. Electromechanical coupling in the cardiac myocyte; stretch-arrhythmia feedback. Pflugers Arch. 2011;462:165–175. doi: 10.1007/s00424-011-0944-3. [DOI] [PubMed] [Google Scholar]
- Kho C, Lee A, Jeong D, Oh JG, Chaanine AH, Kizana E, et al. SUMO1-dependent modulation of SERCA2a in heart failure. Nature. 2011;477:601–605. doi: 10.1038/nature10407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kho C, Lee A, Hajjar RJ. Altered sarcoplasmic reticulum calcium cycling – targets for heart failure therapy. Nat Rev Cardiol. 2012;9:717–733. doi: 10.1038/nrcardio.2012.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura Y, Kurzydlowski K, Tada M, MacLennan DH. Phospholamban inhibitory function is activated by depolymerization. J Biol Chem. 1997;272:15061–15064. doi: 10.1074/jbc.272.24.15061. [DOI] [PubMed] [Google Scholar]
- Kushnir A, Marks AR. The ryanodine receptor in cardiac physiology and disease. Adv Pharmacol. 2010;59:1–30. doi: 10.1016/S1054-3589(10)59001-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leberer E, Hartner KT, Brandl CJ, Fujii J, Tada M, MacLennan DH, et al. Slow/cardiac sarcoplasmic reticulum Ca2+-ATPase and phospholamban mRNAs are expressed in chronically stimulated rabbit fast-twitch muscle. Eur J Biochem. 1989;185:51–54. doi: 10.1111/j.1432-1033.1989.tb15080.x. [DOI] [PubMed] [Google Scholar]
- Linck B, Boknik P, Baba HA, Eschenhagen T, Haverkamp U, Jackel E, et al. Long-term beta adrenoceptor-mediated alteration in contractility and expression of phospholamban and sarcoplasmic reticulum Ca(++)-ATPase in mammalian ventricle. J Pharmacol Exp Ther. 1998;286:531–538. [PubMed] [Google Scholar]
- Lipp P, Niggli E. Submicroscopic calcium signals as fundamental events of excitation–contraction coupling in guinea-pig cardiac myocytes. J Physiol. 1996;492(Pt 1):31–38. doi: 10.1113/jphysiol.1996.sp021286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipp P, Niggli E. Fundamental calcium release events revealed by two-photon excitation photolysis of caged calcium in guinea-pig cardiac myocytes. J Physiol. 1998;508:801–809. doi: 10.1111/j.1469-7793.1998.801bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipskaia L, del Monte F, Capiod T, Yacoubi S, Hadri L, Hours M, et al. Sarco/endoplasmic reticulum Ca2+-ATPase gene transfer reduces vascular smooth muscle cell proliferation and neointima formation in the rat. Circ Res. 2005;97:488–495. doi: 10.1161/01.RES.0000180663.42594.aa. [DOI] [PubMed] [Google Scholar]
- Lipskaia L, Hadri L, Le PP, Esposito B, Atassi F, Liang L, et al. SERCA2a gene transfer prevents intimal proliferation in an organ culture of human internal mammary artery. Gene Ther. 2013;20:396–406. doi: 10.1038/gt.2012.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Jones DM, Larson MG, Leip EP, Beiser A, D'Agostino RB, Kannel WB, et al. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation. 2002;106:3068–3072. doi: 10.1161/01.cir.0000039105.49749.6f. [DOI] [PubMed] [Google Scholar]
- Lopez-Lopez JR, Shacklock PS, Balke CW, Wier WG. Local calcium transients triggered by single L-type calcium channel currents in cardiac cells. Science. 1995;268:1042–1045. doi: 10.1126/science.7754383. [DOI] [PubMed] [Google Scholar]
- Louch WE, Hougen K, Mork HK, Swift F, Aronsen JM, Sjaastad I, et al. Sodium accumulation promotes diastolic dysfunction in end-stage heart failure following Serca2 knockout. J Physiol. 2010;588:465–478. doi: 10.1113/jphysiol.2009.183517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis JV, Joergensen JA, Hajjar RJ, Weber T. Pre-existing anti-adeno-associated virus antibodies as a challenge in AAV gene therapy. Hum Gene Ther Methods. 2013;24:59–67. doi: 10.1089/hgtb.2012.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon AR, Sato M, Hajjar RJ, Samulski RJ, Harding SE. Gene therapy: targeting the myocardium. Heart. 2008;94:89–99. doi: 10.1136/hrt.2007.116483. [DOI] [PubMed] [Google Scholar]
- Lyon AR, MacLeod KT, Zhang Y, Garcia E, Kanda GK, Lab MJ, et al. Loss of T-tubules and other changes to surface topography in ventricular myocytes from failing human and rat heart. Proc Natl Acad Sci USA. 2009;106:6854–6859. doi: 10.1073/pnas.0809777106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon AR, Bannister ML, Collins T, Pearce E, Sepehripour AH, Dubb SS, et al. SERCA2a gene transfer decreases sarcoplasmic reticulum calcium leak and reduces ventricular arrhythmias in a model of chronic heart failure. Circ Arrhythm Electrophysiol. 2011;4:362–372. doi: 10.1161/CIRCEP.110.961615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon AR, Nikolaev VO, Miragoli M, Sikkel MB, Paur H, Benard L, et al. Plasticity of surface structures and β(2)-adrenergic receptor localization in failing ventricular cardiomyocytes during recovery from heart failure. Circ Heart Fail. 2012;5:357–365. doi: 10.1161/CIRCHEARTFAILURE.111.964692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLennan DH, Green NM. Structural biology: pumping ions. Nature. 2000;405:633–634. doi: 10.1038/35015206. [DOI] [PubMed] [Google Scholar]
- MacLennan DH, Kranias EG. Phospholamban: a crucial regulator of cardiac contractility. Nat Rev Mol Cell Biol. 2003;4:566–577. doi: 10.1038/nrm1151. [DOI] [PubMed] [Google Scholar]
- Maltsev VA, Lakatta EG. Synergism of coupled subsarcolemmal Ca2+ clocks and sarcolemmal voltage clocks confers robust and flexible pacemaker function in a novel pacemaker cell model. Am J Physiol Heart Circ Physiol. 2009;296:H594–H615. doi: 10.1152/ajpheart.01118.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, et al. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- Maxwell JT, Blatter LA. Facilitation of cytosolic calcium wave propagation by local calcium uptake into the sarcoplasmic reticulum in cardiac myocytes. J Physiol. 2012;590(Pt 23):6037–6045. doi: 10.1113/jphysiol.2012.239434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M, Bluhm WF, He H, Post SR, Giordano FJ, Lew WYW, et al. Phospholamban-to-SERCA2 ratio controls the force-frequency relationship. Am J Physiol Heart Circ Physiol. 1999;276:H779–H785. doi: 10.1152/ajpheart.1999.276.3.H779. [DOI] [PubMed] [Google Scholar]
- Mi YF, Li XY, Tang LJ, Lu XC, Fu ZQ, Ye WH. Improvement in cardiac function after sarcoplasmic reticulum Ca2+-ATPase gene transfer in a beagle heart failure model. Chin Med J (Engl) 2009;122:1423–1428. [PubMed] [Google Scholar]
- Micheletti R, Palazzo F, Barassi P, Giacalone G, Ferrandi M, Schiavone A, et al. Istaroxime, a stimulator of sarcoplasmic reticulum calcium adenosine triphosphatase isoform 2a activity, as a novel therapeutic approach to heart failure. Am J Cardiol. 2007;99(2, Suppl):S24–S32. doi: 10.1016/j.amjcard.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Mills GD, Kubo H, Harris DM, Berretta RM, Piacentino V, III, Houser SR. Phosphorylation of phospholamban at threonine-17 reduces cardiac adrenergic contractile responsiveness in chronic pressure overload-induced hypertrophy. Am J Physiol Heart Circ Physiol. 2006;291:H61–H70. doi: 10.1152/ajpheart.01353.2005. [DOI] [PubMed] [Google Scholar]
- Miyamoto MI, del Monte F, Schmidt U, DiSalvo TS, Kang ZB, Matsui T, et al. Adenoviral gene transfer of SERCA2a improves left-ventricular function in aortic-banded rats in transition to heart failure. Proc Natl Acad Sci USA. 2000;97:793–798. doi: 10.1073/pnas.97.2.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Monte F, Hajjar RJ. Targeting calcium cycling proteins in heart failure through gene transfer. J Physiol. 2003;546:49–61. doi: 10.1113/jphysiol.2002.026732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Monte F, O'Gara P, Poole-Wilson PA, Yacoub M, Harding SE. Cell geometry and contractile abnormalities of myocytes from failing human left ventricle. Cardiovasc Res. 1995;30:281–290. [PubMed] [Google Scholar]
- del Monte F, Harding SE, Schmidt U, Matsui T, Kang ZB, Dec GW, et al. Restoration of contractile function in isolated cardiomyocytes from failing human hearts by gene transfer of SERCA2a. Circulation. 1999;100:2308–2311. doi: 10.1161/01.cir.100.23.2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Monte F, Williams E, Lebeche D, Schmidt U, Rosenzweig A, Gwathmey JK, et al. Improvement in survival and cardiac metabolism after gene transfer of sarcoplasmic reticulum Ca(2+)-ATPase in a rat model of heart failure. Circulation. 2001;104:1424–1429. doi: 10.1161/hc3601.095574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Monte F, Lebeche D, Guerrero JL, Tsuji T, Doye AA, Gwathmey JK, et al. Abrogation of ventricular arrhythmias in a model of ischemia and reperfusion by targeting myocardial calcium cycling. Proc Natl Acad Sci USA. 2004;101:5622–5627. doi: 10.1073/pnas.0305778101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller S, How OJ, Jakobsen O, Hermansen SE, Rosner A, Stenberg TA, et al. Oxygen-wasting effect of inotropy: is there a need for a new evaluation? An experimental large-animal study using dobutamine and levosimendan. Circ Heart Fail. 2010;3:277–285. doi: 10.1161/CIRCHEARTFAILURE.109.865519. [DOI] [PubMed] [Google Scholar]
- Nuss HB, Kaab S, Kass DA, Tomaselli GF, Marban E. Cellular basis of ventricular arrhythmias and abnormal automaticity in heart failure. Am J Physiol. 1999;277(1 Pt 2):H80–H91. doi: 10.1152/ajpheart.1999.277.1.H80. [DOI] [PubMed] [Google Scholar]
- O'Connor CM, Gattis WA, Uretsky BF, Adams KF, Jr, McNulty SE, Grossman SH, et al. Continuous intravenous dobutamine is associated with an increased risk of death in patients with advanced heart failure: insights from the Flolan International Randomized Survival Trial (FIRST) Am Heart J. 1999;138(1 Pt 1):78–86. doi: 10.1016/s0002-8703(99)70250-4. [DOI] [PubMed] [Google Scholar]
- O'Donnell JM, Fields A, Xu X, Chowdhury SA, Geenen DL, Bi J. Limited functional and metabolic improvements in hypertrophic and healthy rat heart overexpressing the skeletal muscle isoform of SERCA1 by adenoviral gene transfer in vivo. Am J Physiol Heart Circ Physiol. 2008;295:H2483–H2494. doi: 10.1152/ajpheart.01023.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- Pacak CA, Mah CS, Thattaliyath BD, Conlon TJ, Lewis MA, Cloutier DE, et al. Recombinant adeno-associated virus serotype 9 leads to preferential cardiac transduction in vivo. Circ Res. 2006;99:e3–e9. doi: 10.1161/01.RES.0000237661.18885.f6. [DOI] [PubMed] [Google Scholar]
- Packer M, Medina N, Yushak M. Hemodynamic and clinical limitations of long-term inotropic therapy with amrinone in patients with severe chronic heart failure. Circulation. 1984;70:1038–1047. doi: 10.1161/01.cir.70.6.1038. [DOI] [PubMed] [Google Scholar]
- Packer M, Carver JR, Rodeheffer RJ, Ivanhoe RJ, DiBianco R, Zeldis SM, et al. Effect of oral milrinone on mortality in severe chronic heart failure. The PROMISE Study Research Group. N Engl J Med. 1991;325:1468–1475. doi: 10.1056/NEJM199111213252103. [DOI] [PubMed] [Google Scholar]
- Palomeque J, Chemaly ER, Colosi P, Wellman JA, Zhou S, del Monte F, et al. Efficiency of eight different AAV serotypes in transducing rat myocardium in vivo. Gene Ther. 2007;14:989–997. doi: 10.1038/sj.gt.3302895. [DOI] [PubMed] [Google Scholar]
- Penson PE, Ford WR, Broadley KJ. Vasopressors for cardiopulmonary resuscitation. Does pharmacological evidence support clinical practice? Pharmacol Ther. 2007;115:37–55. doi: 10.1016/j.pharmthera.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Periasamy M, Reed TD, Liu LH, Ji Y, Loukianov E, Paul RJ, et al. Impaired cardiac performance in heterozygous mice with a null mutation in the sarco(endo)plasmic reticulum Ca2+-ATPase isoform 2 (SERCA2) gene. J Biol Chem. 1999;274:2556–2562. doi: 10.1074/jbc.274.4.2556. [DOI] [PubMed] [Google Scholar]
- Pieske B, Hasenfuss G, Holubarsch C, Schwinger R, Bohm M, Just H. Alterations of the force-frequency relationship in the failing human heart depend on the underlying cardiac disease. Basic Res Cardiol. 1992;87(Suppl. 1):213–221. doi: 10.1007/978-3-642-72474-9_17. [DOI] [PubMed] [Google Scholar]
- Pogwizd SM, Schlotthauer K, Li L, Yuan W, Bers DM. Arrhythmogenesis and contractile dysfunction in heart failure: roles of sodium-calcium exchange, inward rectifier potassium current, and residual beta-adrenergic responsiveness. Circ Res. 2001;88:1159–1167. doi: 10.1161/hh1101.091193. [DOI] [PubMed] [Google Scholar]
- Prosser BL, Ward CW, Lederer WJ. X-ROS signaling: rapid mechano-chemo transduction in heart. Science. 2011;333:1440–1445. doi: 10.1126/science.1202768. [DOI] [PubMed] [Google Scholar]
- Prunier F, Kawase Y, Gianni D, Scapin C, Danik SB, Ellinor PT, et al. Prevention of ventricular arrhythmias with sarcoplasmic reticulum Ca2+ ATPase pump overexpression in a porcine model of ischemia reperfusion. Circulation. 2008;118:614–624. doi: 10.1161/CIRCULATIONAHA.108.770883. [DOI] [PubMed] [Google Scholar]
- Respress JL, van Oort RJ, Li N, Rolim N, Dixit SS, deAlmeida A, et al. Role of RyR2 phosphorylation at S2814 during heart failure progression. Circ Res. 2012;110:1474–1483. doi: 10.1161/CIRCRESAHA.112.268094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocchetti M, Besana A, Mostacciuolo G, Ferrari P, Micheletti R, Zaza A. Diverse toxicity associated with cardiac Na+/K+ pump inhibition: evaluation of electrophysiological mechanisms. J Pharmacol Exp Ther. 2003;305:765–771. doi: 10.1124/jpet.102.047696. [DOI] [PubMed] [Google Scholar]
- Rocchetti M, Besana A, Mostacciuolo G, Micheletti R, Ferrari P, Sarkozi S, et al. Modulation of sarcoplasmic reticulum function by Na+/K+ pump inhibitors with different toxicity: digoxin and PST2744 [(E,Z)-3-((2-aminoethoxy)imino)androstane-6,17-dione hydrochloride] J Pharmacol Exp Ther. 2005;313:207–215. doi: 10.1124/jpet.104.077933. [DOI] [PubMed] [Google Scholar]
- Sakata S, Lebeche D, Sakata Y, Sakata N, Chemaly ER, Liang LF, et al. Mechanical and metabolic rescue in a type II diabetes model of cardiomyopathy by targeted gene transfer. Mol Ther. 2006;13:987–996. doi: 10.1016/j.ymthe.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Sakata S, Lebeche D, Sakata N, Sakata Y, Chemaly ER, Liang LF, et al. Restoration of mechanical and energetic function in failing aortic-banded rat hearts by gene transfer of calcium cycling proteins. J Mol Cell Cardiol. 2007a;42:852–861. doi: 10.1016/j.yjmcc.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata S, Lebeche D, Sakata Y, Sakata N, Chemaly ER, Liang L, et al. Transcoronary gene transfer of SERCA2a increases coronary blood flow and decreases cardiomyocyte size in a type 2 diabetic rat model. Am J Physiol Heart Circ Physiol. 2007b;292:H1204–H1207. doi: 10.1152/ajpheart.00892.2006. [DOI] [PubMed] [Google Scholar]
- Schmidt U, Hajjar RJ. Gene therapy in heart failure. Best Pract Res Clin Anaesthesiol. 2001;15:301–312. [Google Scholar]
- Schmidt U, Hajjar RJ, Helm PA, Kim CS, Doye AA, Gwathmey JK. Contribution of abnormal sarcoplasmic reticulum ATPase activity to systolic and diastolic dysfunction in human heart failure. J Mol Cell Cardiol. 1998;30:1929–1937. doi: 10.1006/jmcc.1998.0748. [DOI] [PubMed] [Google Scholar]
- Schulte LM, Navarro J, Kandarian SC. Regulation of sarcoplasmic reticulum calcium pump gene expression by hindlimb unweighting. Am J Physiol. 1993;264(5 Pt 1):C1308–C1315. doi: 10.1152/ajpcell.1993.264.5.C1308. [DOI] [PubMed] [Google Scholar]
- Schultz JJ, Glascock BJ, Witt SA, Nieman ML, Nattamai KJ, Liu LH, et al. Accelerated onset of heart failure in mice during pressure overload with chronically decreased SERCA2 calcium pump activity. Am J Physiol Heart Circ Physiol. 2004;286:H1146–H1153. doi: 10.1152/ajpheart.00720.2003. [DOI] [PubMed] [Google Scholar]
- Schwinger RHG, M3nch G, B÷lck B, Karczewski P, Krause EG, Erdmann E. Reduced Ca2+-sensitivity of SERCA 2a in failing human myocardium due to reduced serin-16 phospholamban phoshorylation. J Mol Cell Cardiol. 1999;31:479–491. doi: 10.1006/jmcc.1998.0897. 3nch, B÷lck, Karczewski, Krause, Erdmann; 1999) [DOI] [PubMed] [Google Scholar]
- Shannon TR, Ginsburg KS, Bers DM. Quantitative assessment of the SR Ca2+ leak-load relationship. Circ Res. 2002;91:594–600. doi: 10.1161/01.res.0000036914.12686.28. [DOI] [PubMed] [Google Scholar]
- Sodi R, Dubuis E, Shenkin A, Hart G. B-type natriuretic peptide (BNP) attenuates the L-type calcium current and regulates ventricular myocyte function. Regul Pept. 2008;151:95–105. doi: 10.1016/j.regpep.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Song LS, Pi Y, Kim SJ, Yatani A, Guatimosim S, Kudej RK, et al. Paradoxical cellular Ca2+ signaling in severe but compensated canine left ventricular hypertrophy. Circ Res. 2005;97:457–464. doi: 10.1161/01.RES.0000179722.79295.d4. [DOI] [PubMed] [Google Scholar]
- Song LS, Sobie EA, McCulle S, Lederer WJ, Balke CW, Cheng H. Orphaned ryanodine receptors in the failing heart. Proc Natl Acad Sci USA. 2006;103:4305–4310. doi: 10.1073/pnas.0509324103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sossalla S, Fluschnik N, Schotola H, Ort KR, Neef S, Schulte T, et al. Inhibition of elevated Ca2+/calmodulin-dependent protein kinase II improves contractility in human failing myocardium. Circ Res. 2010;107:1150–1161. doi: 10.1161/CIRCRESAHA.110.220418. [DOI] [PubMed] [Google Scholar]
- Stewart PS, MacLennan DH. Surface particles of sarcoplasmic reticulum membranes. Structural features of the adenosine triphosphatase. J Biol Chem. 1974;249:985–993. [PubMed] [Google Scholar]
- Swedberg K, Komajda M, Bohm M, Borer JS, Ford I, Dubost-Brama A, et al. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet. 2010;376:875–885. doi: 10.1016/S0140-6736(10)61198-1. [DOI] [PubMed] [Google Scholar]
- Tada M, Kirchberger MA, Katz AM. Phosphorylation of a 22,000-dalton component of the cardiac sarcoplasmic reticulum by adenosine 3′:5′-monophosphate-dependent protein kinase. J Biol Chem. 1975;250:2640–2647. [PubMed] [Google Scholar]
- Talosi L, Edes I, Kranias EG. Intracellular mechanisms mediating reversal of beta-adrenergic stimulation in intact beating hearts. Am J Physiol. 1993;264(3 Pt 2):H791–H797. doi: 10.1152/ajpheart.1993.264.3.H791. [DOI] [PubMed] [Google Scholar]
- Teerlink JR, Clarke CP, Saikali KG, Lee JH, Chen MM, Escandon RD, et al. Dose-dependent augmentation of cardiac systolic function with the selective cardiac myosin activator, omecamtiv mecarbil: a first-in-man study. Lancet. 2011;378:667–675. doi: 10.1016/S0140-6736(11)61219-1. [DOI] [PubMed] [Google Scholar]
- Terracciano CM, Hajjar RJ, Harding SE. Overexpression of SERCA2a accelerates repolarisation in rabbit ventricular myocytes. Cell Calcium. 2002;31:299–305. doi: 10.1016/s0143-4160(02)00058-1. [DOI] [PubMed] [Google Scholar]
- Teucher N, Prestle J, Seidler T, Currie S, Elliott EB, Reynolds DF, et al. Excessive sarcoplasmic/endoplasmic reticulum Ca2+-ATPase expression causes increased sarcoplasmic reticulum Ca2+ uptake but decreases myocyte shortening. Circulation. 2004;110:3553–3559. doi: 10.1161/01.CIR.0000145161.48545.B3. [DOI] [PubMed] [Google Scholar]
- Toma M, Starling RC. Inotropic therapy for end-stage heart failure patients. Curr Treat Options Cardiovasc Med. 2010;12:409–419. doi: 10.1007/s11936-010-0090-9. [DOI] [PubMed] [Google Scholar]
- Trafford AW, Diaz ME, Sibbring GC, Eisner DA. Modulation of CICR has no maintained effect on systolic Ca2+: simultaneous measurements of sarcoplasmic reticulum and sarcolemmal Ca2+ fluxes in rat ventricular myocytes. J Physiol. 2000;522(Pt 2):259–270. doi: 10.1111/j.1469-7793.2000.t01-2-00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassort G, Lacampagne A. The role of ryanodine receptors and consequences of their alterations during cardiac insufficiency. Exp. Clin Cardiol. 2005;10:196–199. [PMC free article] [PubMed] [Google Scholar]
- Vermeulen JT. Mechanisms of arrhythmias in heart failure. J Cardiovasc Electrophysiol. 1998;9:208–221. doi: 10.1111/j.1540-8167.1998.tb00902.x. [DOI] [PubMed] [Google Scholar]
- Volders PGA, Vos MA, Szabo B, Sipido KR, de Groot SHM, Gorgels APM, et al. Progress in the understanding of cardiac early afterdepolarizations and torsades de pointes: time to revise current concepts. Cardiovasc Res. 2000;46:376–392. doi: 10.1016/s0008-6363(00)00022-5. [DOI] [PubMed] [Google Scholar]
- Wehrens XH, Lehnart SE, Reiken S, van der Nagel R, Morales R, Sun J, et al. Enhancing calstabin binding to ryanodine receptors improves cardiac and skeletal muscle function in heart failure. Proc Natl Acad Sci USA. 2005;102:9607–9612. doi: 10.1073/pnas.0500353102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss JN, Qu Z, Chen PS, Lin SF, Karagueuzian HS, Hayashi H, et al. The dynamics of cardiac fibrillation. Circulation. 2005;112:1232–1240. doi: 10.1161/CIRCULATIONAHA.104.529545. [DOI] [PubMed] [Google Scholar]
- Weiss JN, Nivala M, Garfinkel A, Qu Z. Alternans and arrhythmias: from cell to heart. Circ Res. 2011;108:98–112. doi: 10.1161/CIRCRESAHA.110.223586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuytack F, Raeymaekers L, Missiaen L. Molecular physiology of the SERCA and SPCA pumps. Cell Calcium. 2002;32:279–305. doi: 10.1016/s0143416002001847. [DOI] [PubMed] [Google Scholar]
- Xie LH, Sato D, Garfinkel A, Qu Z, Weiss JN. Intracellular Ca alternans: coordinated regulation by sarcoplasmic reticulum release, uptake, and leak. Biophys J. 2008;95:3100–3110. doi: 10.1529/biophysj.108.130955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Zhu WZ, Xiao B, Brochet DX, Chen SR, Lakatta EG, et al. Ca2+/calmodulin kinase II-dependent phosphorylation of ryanodine receptors suppresses Ca2+ sparks and Ca2+ waves in cardiac myocytes. Circ Res. 2007;100:399–407. doi: 10.1161/01.RES.0000258022.13090.55. [DOI] [PubMed] [Google Scholar]
- Yang Y, Nunes FA, Berencsi K, Furth EE, Gonczol E, Wilson JM. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proc Natl Acad Sci USA. 1994;91:4407–4411. doi: 10.1073/pnas.91.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Q, Fan GC, Dong M, Altschafl B, Diwan A, Ren X, et al. Sarcoplasmic reticulum calcium overloading in junctin deficiency enhances cardiac contractility but increases ventricular automaticity. Circulation. 2007;115:300–309. doi: 10.1161/CIRCULATIONAHA.106.654699. [DOI] [PubMed] [Google Scholar]
- Zeng J, Rudy Y. Early afterdepolarizations in cardiac myocytes: mechanism and rate dependence. Biophys J. 1995;68:949–964. doi: 10.1016/S0006-3495(95)80271-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Uehara Y, Chu G, Song Q, Qian J, Young K, et al. Threonine-17 phosphorylation of phospholamban: a key determinant of frequency-dependent increase of cardiac contractility. J Mol Cell Cardiol. 2004;37:607–612. doi: 10.1016/j.yjmcc.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Wen H, Fefelova N, Allen C, Baba A, Matsuda T, et al. Revisiting the ionic mechanisms of early afterdepolarizations in cardiomyocytes: predominant by Ca waves or Ca currents? Am J Physiol Heart Circ Physiol. 2012;302:H1636–H1644. doi: 10.1152/ajpheart.00742.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zima AV, Blatter LA. Redox regulation of cardiac calcium channels and transporters. Cardiovasc Res. 2006;71:310–321. doi: 10.1016/j.cardiores.2006.02.019. [DOI] [PubMed] [Google Scholar]
- Zima AV, Bovo E, Bers DM, Blatter LA. Ca2+ spark-dependent and -independent sarcoplasmic reticulum Ca2+ leak in normal and failing rabbit ventricular myocytes. J Physiol. 2010;588:4743–4757. doi: 10.1113/jphysiol.2010.197913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zissimopoulos S, Docrat N, Lai FA. Redox sensitivity of the ryanodine receptor interaction with FK506-binding protein. J Biol Chem. 2007;282:6976–6983. doi: 10.1074/jbc.M607590200. [DOI] [PubMed] [Google Scholar]


