Abstract
BACKGROUND AND PURPOSE
The cardiovascular effects produced by 3,4-methylenedioxymethamphetamine (MDMA; ‘Ecstasy’) contribute to its acute toxicity, but the potential role of its metabolites in these cardiovascular effects is not known. Here we examined the effects of MDMA metabolites on cardiovascular function in rats.
EXPERIMENTAL APPROACH
Radiotelemetry was employed to evaluate the effects of s.c. administration of racemic MDMA and its phase I metabolites on BP, heart rate (HR) and locomotor activity in conscious male rats.
KEY RESULTS
MDMA (1–20 mg·kg−1) produced dose-related increases in BP, HR and activity. The peak effects on HR occurred at a lower dose than peak effects on BP or activity. The N-demethylated metabolite, 3,4-methylenedioxyamphetamine (MDA), produced effects that mimicked those of MDMA. The metabolite 3,4-dihydroxymethamphetamine (HHMA; 1–10 mg·kg−1) increased HR more potently and to a greater extent than MDMA, whereas 3,4-dihydroxyamphetamine (HHA) increased HR, but to a lesser extent than HHMA. Neither dihydroxy metabolite altered motor activity. The metabolites 4-hydroxy-3-methoxymethamphetamine (HMMA) and 4-hydroxy-3-methoxyamphetamine (HMA) did not affect any of the parameters measured. The tachycardia produced by MDMA and HHMA was blocked by the β-adrenoceptor antagonist propranolol.
CONCLUSIONS AND IMPLICATIONS
Our results demonstrate that HHMA may contribute significantly to the cardiovascular effects of MDMA in vivo. As such, determining the molecular mechanism of action of HHMA and the other hydroxyl metabolites of MDMA warrants further study.
Keywords: MDMA metabolites, heart rate, BP, noradrenergic, telemetry
Introduction
Recreational use of 3,4-methylenedioxymethamphetamine (MDMA; ‘Ecstasy’) remains a significant public health concern. Based on survey data from high school and college students in the USA, the use of MDMA peaked more than a decade ago (Johnston et al., 2010), yet reports from emergency departments show that the incidence of MDMA-induced adverse effects has actually increased over the past few years (Drug Abuse Warning Network, 2010). MDMA can produce robust hyperthermia, and it is well established from animal studies that high doses of the drug can lead to persistent deficits in brain 5-HT systems (Baumann and Rothman, 2009). It is also recognized that cardiovascular effects of MDMA contribute significantly to its adverse health effects in humans (Suarez and Riemersma, 1988; Shenouda et al., 2010). Hypertension is often reported in patients presenting to the emergency room after MDMA use (Halpern et al., 2011), and laboratory studies of MDMA administration in humans have universally shown that doses of 1 mg·kg−1 and above increase both BP and heart rate (HR; Mas et al., 1999; Lester et al., 2000; Kolbrich et al., 2008a). In conscious rats, MDMA increases BP and HR at doses comparable with those used by humans (O'Cain et al., 2000), but at higher doses, the HR actually decreases due to reflex bradycardia (O'Cain et al., 2000; Bexis and Docherty, 2006).
The molecular mechanism of action of MDMA involves the interaction of the drug with monoamine transporter proteins expressed on neurons and other cell types (Green et al., 2003; Baumann and Rothman, 2009). More specifically, MDMA serves as a substrate for monoamine transporters, thereby evoking non-exocytotic release of monoamine transmitters (i.e. noradrenaline, dopamine and 5-HT). MDMA is distinguished from prototypical stimulants like amphetamine by its preferential effects on 5-HT transporters, whereas amphetamine is selective for catecholamine transporters. Nevertheless, an effect of MDMA on noradrenaline transporters in the brain and periphery has been implicated in the mechanism underlying the increases in HR and BP (Fitzgerald and Reid, 1994; McDaid and Docherty, 2000; Shenouda et al., 2010). MDMA and amphetamine display similar in vivo potency in their ability to increase cardiovascular parameters in rats and humans (Mas et al., 1999; O'Cain et al., 2000), even though MDMA is 10-fold less potent than amphetamine as a substrate for noradrenaline transporters (Baumann and Rothman, 2009). Such observations suggest that the cardiovascular effects of MDMA may involve non-catecholamine mechanisms, or perhaps these effects are produced by its bioactive metabolites.
MDMA is rapidly metabolized by hepatic enzymes in vivo to form a variety of metabolites, some of which may possess bioactivity (Maurer et al., 2000; de la Torre et al., 2004). Little information is available regarding the effects of MDMA metabolites on cardiovascular function. As shown in Figure 1, there are two primary pathways for the metabolism of MDMA in humans (de la Torre et al., 2004). For the major O-demethylenation pathway, MDMA is metabolized to 3,4-dihydroxymethamphetamine (HHMA) and then to 4-hydroxy-3-methoxymethamphetamine (HMMA). This pathway accounts for >80% of MDMA metabolism in humans. A minor N-demethylation pathway involves the initial production of 3,4-methylenedioxyamphetamine (MDA), and then follows the O-demethylenation pathway to form 3,4-dihydroxyamphetamine (HHA) and 4-hydroxy-3-methoxyampetamine (HMA). Hydroxyl-containing metabolites of MDMA are conjugated to sulfate or glutathione and excreted. It is noteworthy that the metabolic pathways for MDMA biotransformation in rodents are similar to those described for humans (Baumann et al., 2009; Scheidweiler et al., 2010), although there are species differences in hepatic enzymes involved and time intervals for drug clearance (de la Torre and Farre, 2004).
Figure 1.
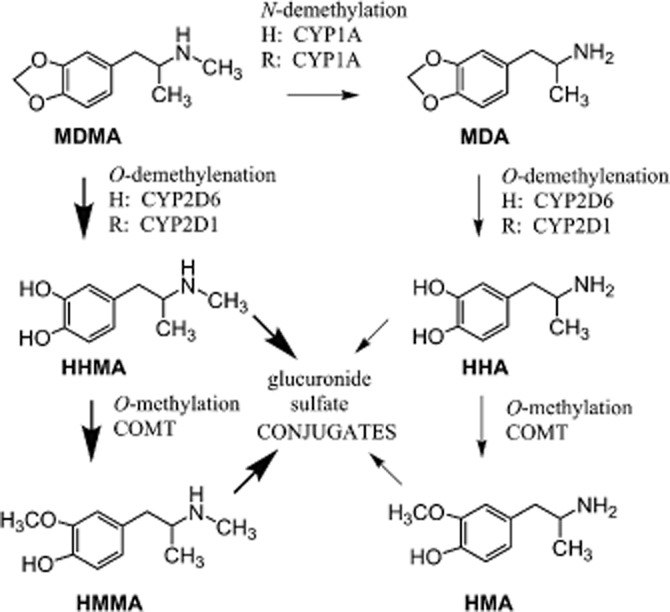
Metabolism of MDMA in humans and rats. Abbreviations for drug names are found in the text. The main cytochrome p450 (CYP) isoforms responsible for specific biotransformation reactions are given for humans (H) and rats (R). Catechol-O-methyltransferase is abbreviated as COMT. Thick arrows represent major pathways of metabolism, whereas thin arrows represent minor pathways.
With the exception of MDA (Bexis and Docherty, 2006), the cardiovascular effects of MDMA metabolites have not been investigated. Bexis and Docherty (2006) found that MDA has similar effects to MDMA, with both drugs increasing BP. However, because these investigators only studied a high dose of MDMA and MDA (20 mg·kg−1), the primary effect on HR was a decrease. The purpose of the present study was to investigate the effects produced by a range of doses of MDMA and its metabolites on BP and HR in conscious animals. Rats received surgically implanted telemetric recording devices that allowed for the long-term measurement of BP and HR, as well as a measurement of gross locomotor activity. Our results show that the hydroxyl metabolites of MDMA, in particular HHMA, may contribute significantly to cardiovascular effects of MDMA in vivo.
Methods
Animals and housing
Nine adult male Sprague–Dawley rats were used in the following experiments. They were individually housed in a temperature- and humidity-controlled room with a 12 h light/dark cycle (lights on at 07:00 h). All animals used in this study were maintained in facilities fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care. All procedures were conducted in accordance with the guidelines of the Institutional Care and Use Committee of the National Institute on Drug Abuse/Intramural Research Program and the Guide for the Care and Use of Laboratory Animals. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2000).
Surgical procedures
Following their arrival in the laboratory, the rats were given 1 week to adapt. Rats were anaesthetized with an isoflurane-oxyen mixture (3% isoflurane for induction, maintenance anaesthesia of 1–2% isoflurane). Depth of anaesthesia was evaluated with tail or paw pinch. After surgery the rats were given 2.5 mg kg-1 Banamine s.c for analgesia. If there were signs of distress the day after surgery, the rat was given a subsequent injection of the same dose of Banamine. Surgery was performed to implant telemetry transmitters (Data Sciences International, St. Paul, MN, USA) for the measurement of BP. Details of the surgery are given elsewhere (Tella et al., 1999). Briefly, under isoflurane anaesthesia, a 4–5 cm long incision was made on the midline of abdomen. The descending aorta was exposed below the level of the renal arteries. A vascular clamp was placed immediately posterior to the renal arteries and a curved 21 G needle was used to puncture the vessel anterior to the bifurcation of the common iliac arteries. The catheter of the transmitter was inserted about 2 cm into the aorta, the area was dried and a drop of adhesive (Vetbond, 3M, St. Paul, MN, USA) was applied to the catheter entry point. The transmitter was then sutured to the abdominal musculature, and the abdominal incision and the skin were closed. An i.p. injection of 50 000 U·kg−1 dual penicillin was given to safeguard against infections.
Telemetric measurements
One to 2 weeks following surgery, experimental procedures began. All testing occurred in a room separate from the housing room. Testing was typically performed 5 days per week (Monday–Friday) at approximately the same time each day for any individual animal. During the experimental session, the animal's entire home cage (with food and water removed) was placed on top of the telemetry receiver. Three telemetry receivers were located in three separate, but identical, sound attenuation (experimental) chambers. Mean arterial BP and HR were sampled for 10 s every minute and were then monitored for up to 2 h. Activity measurements were taken continuously throughout the session with the total activity counts saved every minute. Two antennae within the telemetry receiver (one in the x-axis and one in the y-axis) monitor the signal emitted from the implanted transmitter. As the rat moves, the signal strength changes in relation to the two antennae. This change in signal strength is computed as a count, but has no direct relationship to distance travelled. Therefore, the activity measure presented is a qualitative one with no units. Higher counts simply indicate more activity relative to lower counts. At the onset of the study, rats (in their home cages) were placed into the telemetry chambers without receiving injections, and this acclimatization process continued until cardiovascular parameters were stable from session to session. Animals were then given a s.c. injection of saline just prior to placement of the cage on the telemetry receiver, at least twice a week, until cardiovascular parameters following saline injection remained stable. The cardiovascular response to the injection of saline was indistinguishable from that observed when the animals were placed in the telemetry chamber with no prior injection. Testing then began with MDMA or the metabolites, with test drugs given s.c. no more frequently than twice a week, usually on Tuesdays and Fridays, with control saline injections given typically once a month on Thursdays. All the rats were tested with multiple drugs, but not every rat received every drug (MDMA, rats 31, 32, 35, 38, 43a, 45, 46; HHMA and HMMA, rats 31, 35, 43a, 45, 46; MDA, rats 31, 35, 38, 39, 43a, 45, 46; HHA and HMA, rats 31, 35, 38, 43a; propranolol study, rats 43a, 44a, 45, 46). All rats received multiple injections of saline, but for any one drug not all rats received every dose. When a pretreatment injection of propranolol was given, it was administered s.c. 5 min before either MDMA or HHMA, which were given just prior to placement of the rats in the testing chamber. Appropriate controls for propranolol alone, MDMA or HHMA, and saline were also given in the same manner. Testing continued for up to 5 months, with an average of just over 2 months for any individual rat and up to 30 drug treatments were tested over that period. The rat's food intake was restricted to maintain their wt over that period. The average starting wt was 441.0 ± 29.4 g and the average wt at the end of the experiment was 413.8 ± 11.5 g. Order of drug treatment and dose administered was non-systematic, with the exception of the experiment with propranolol treatments. For example, 10 mg·kg−1 HHMA was given as early as the third drug treatment for one rat or as late as the ninth drug treatment for a different rat, with a variety of other drugs and doses given in between. There was no indication that the order of treatment affected the results. Treatments with propranolol always occurred last for those subjects.
Drugs and injection procedures
MDMA, MDA, HHMA, HHA, HMMA and (±)-4-hydroxy-3-methoxyamphetamine HCl (HMA) were synthesized as either racemic HCl or HBr salts and analysed for purity in the laboratory of Bruce E. Blough at RTI. Propranolol was purchased from Sigma Chemical (St. Louis, MO, USA). All drugs were dissolved in sterile saline and administered s.c. in a volume of 1 mL·kg−1 of body wt. We chose to use the s.c. route in these experiments for several reasons: (i) most importantly, the s.c. route minimizes metabolism of the injected drugs or metabolites, which is an essential attribute for the present investigation; (ii) the s.c. route affords the slowest possible pharmacokinetics in rats and is therefore most similar to humans (Baumann et al., 2009); and (iii) because the implanted transmitters were in the peritoneal cavity, we could not use the i.p. route as this would risk damaging the implanted devices. For all drugs tested, baseline values of BP and HR had recovered before the administration of any other treatment. Furthermore, occasionally, treatments or doses were repeated in the same animal and the results of those tests were always in close agreement. As a result, the repeated tests were averaged for final analysis.
Data analysis and statistics
For the time course data presentation, BP and HR were averaged over 5 min periods, and activity was summed over the 5 min period (from the 1 min samples). As time course data showed that effects in general persisted throughout most of the 2 h session, data for statistical comparisons were performed using mean values across the entire session for BP and HR, and summed values for activity (Matthews et al., 1990). Statistical comparisons among different drug treatments were made using anova, followed by Tukey's post hoc test to test for significance compared with saline. For graphic presentation, a change score (Δ) was calculated using the saline average for all the rats tested on a particular drug as the baseline from which the change score was calculated.
Results
Dose-related effects of MDMA and its hydroxyl metabolites
Table 1 presents BP, HR and activity measurements for the averaged saline control sessions for each drug. For HHMA and HMMA, and for HHA and HMA, the same rats were tested with each drug. Due to the substantial overlap in the rats tested across drugs, the values for saline control were remarkably consistent across the drugs. Values for all three measures were also consistent across the multiple injections of saline that each rat received through the course of the study (data not shown).
Table 1.
BP, HR and activity following saline treatment for the rats used in dose–effect studies for specific drugs
| Drug(s) | n | BP (mmHg) | HR (beats per minute) | Activity |
|---|---|---|---|---|
| MDMA | 7 | 96.3 ± 1.9 | 284.8 ± 4.3 | 172.5 ± 13.7 |
| HHMA and HMMA | 5 | 96.8 ± 2.7 | 285.7 ± 6.1 | 186.9 ± 14.6 |
| MDA | 7 | 95.7 ± 2.0 | 282.8 ± 4.7 | 178.7 ± 2.0 |
| HHA and HMA | 4 | 94.3 ± 2.7 | 285.9 ± 7.6 | 170.9 ± 19.0 |
Data are mean ± SEM for n = number of rats tested.
Figure 2 (A, C and E) shows the time courses for BP, HR and activity following saline administration for those rats tested with MDMA and the metabolites generated via the O-demethylenation pathway (HHMA and HMMA). Typically, both BP and HR were slightly elevated after the rats were given an s.c. saline injection and placed in the experimental chamber. Over the first 30 min of the session, both BP and HR decreased steadily and stabilized through the remaining 90 min of the session. Like BP and HR, activity was also elevated at the beginning of the experimental session following saline administration, but then decreased to near zero for the last 90 min of the session.
Figure 2.
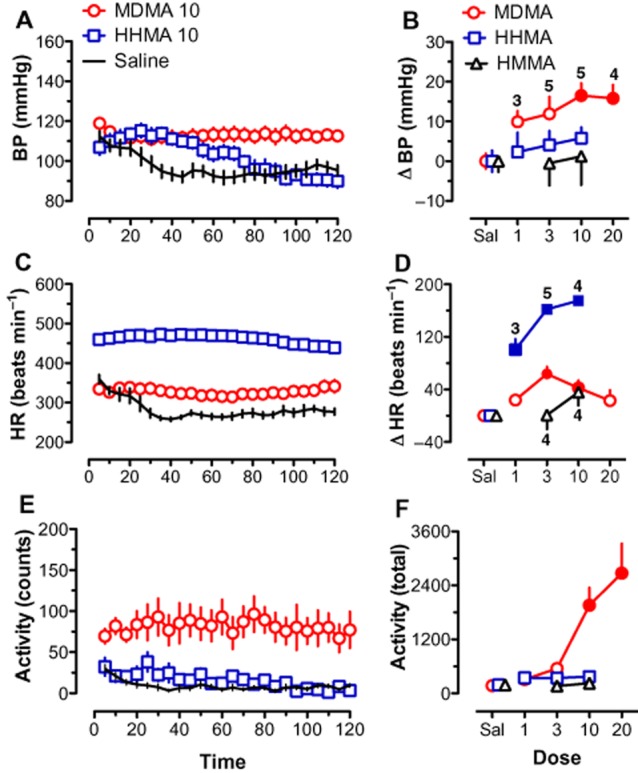
Effects of MDMA, HHMA, HMMA and saline on BP (A, B), HR (C, D) and activity (E, F). Left-hand panels (A, C and E) depict time course data for a selected dose of MDMA (10 mg·kg−1) and HHMA (10 mg·kg−1) compared with saline. Each point represents mean ± SEM during a 5 min period. Right-hand panels (B, D and F) show the mean change scores (Δ) over the entire 120 min session for all doses tested. Change scores are compared with saline control. Filled symbols indicate significant difference (P < 0.05) with respect to saline-treated rats. Number of rats per dose is given for MDMA in (B) and for HHMA and HMMA in (D). Number of rats for saline for each drug is given in Table 1. Both MDMA and HHMA significantly increased HR. Only MDMA significantly increased BP and activity.
The time course data in Figure 2 show that MDMA (10 mg·kg−1) and HHMA (10 mg·kg−1) produced significant effects. MDMA increased BP, HR and activity. HHMA increased only HR, but to a much greater extent than that seen for MDMA. The significant effects on BP and HR persisted through the session. Figure 2 (B, D and F) present change scores from saline baseline for all doses of MDMA, HHMA and HMMA tested. For BP, significant increases from saline were seen only for MDMA [F(4,19) = 5.59, P < 0.01] at the two highest doses (10 and 20 mg·kg−1). Neither HHMA nor HMMA significantly changed BP. MDMA increased HR compared with saline [F(4,19) = 6.99, P < 0.01] at the intermediate doses (3 and 10 mg·kg−1), but not at the higher dose, suggesting that MDMA had a biphasic effect on HR. In contrast, large increases in HR were observed for every dose of HHMA [F(3,13) = 107.69, P < 0.0001]. The increase in HR induced by HHMA was larger than that induced by MDMA. HMMA did not increase HR. Neither HHMA nor HMMA increased activity, but MDMA [F(4,19) = 12.77, P < 0.0001] produced a clear dose-dependent increase in activity, with significant effects seen at the higher doses.
Dose-related effects of MDA and its hydroxyl metabolites
Figure 3 presents the results for those drugs in the N-demethylation metabolic pathway (MDA, HHA and HMA). The time course data shown in Figure 3, A, C and E, reveal that the effects of saline administration were nearly identical to those seen in Figure 2. These results illustrate the stability of the responses to saline injections between testing sessions over time, as many of the same rats were used for the MDMA and MDA treatment studies. The time course data are also shown for doses of MDA (10 mg·kg−1) and HHA (10 mg·kg−1) that had significant effects. Like MDMA, MDA produced increases in BP, HR and activity. Unlike HHMA, HHA increased BP as well as HR. Figure 3, B, D and F, show the change scores for the three measures for this set of drugs. Both MDA [F(3,15) = 9.43, P < 0.01] and HHA [F(2,9) = 18.10, P < 0.001] increased BP compared to saline at the 10 mg·kg−1 dose. Like MDMA, the effect of MDA on HR peaked at an intermediate dose and then decreased at the highest dose tested. This effect of MDA on HR failed to reach significance [F(3,15) = 2.73, P = 0.08]. Like HHMA, HHA also increased HR [F(2,9) = 6.86, P < 0.05], with significant differences from saline seen at both doses tested. Only MDA produced a significant increase in activity [F(3,15) = 13.93, P < 0.001], with the effect at the highest dose being significantly different from saline. Similar to HMMA, HMA had no significant effects on any of the variables.
Figure 3.
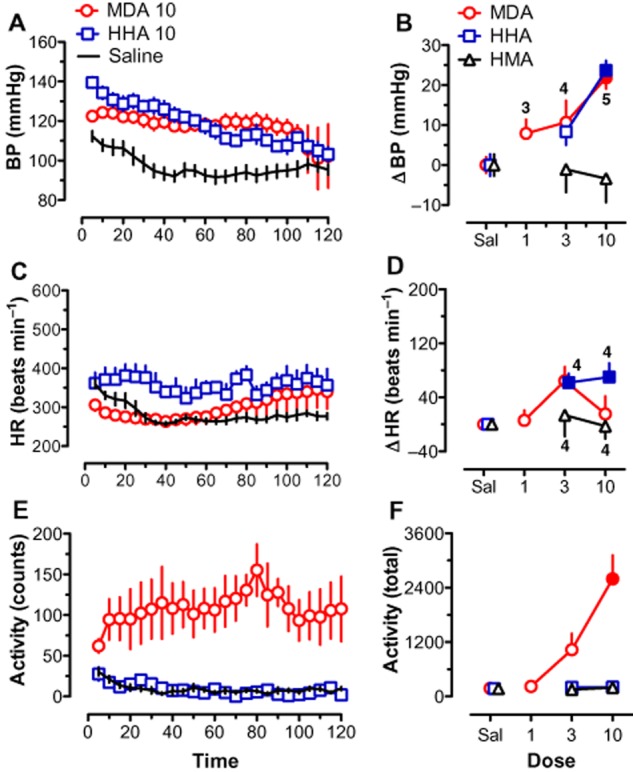
Effects of MDA, HHA, HMA and saline on BP (A, B), HR (C, D) and activity (E, F). Left-hand panels (A, C and E) depict time course data for a selected dose of MDA (10 mg·kg−1) and HHA (10 mg·kg−1) compared with saline. Each point represents mean ± SEM during a 5 min period. Right-hand panels (B, D and F) show the mean change scores (Δ) over the entire 120 min session for all drugs tested. Change scores are compared with saline control. Filled symbols indicate significant difference (P < 0.05) with respect to saline-treated rats. Number of rats per dose is given for MDA in (B) and for HHA and HMA in (D). Number of rats for saline for each drug is given in Table 1. Both MDA and HHA significantly increased BP. HHA also increased HR and MDA significantly increased activity.
Propranolol pretreatment experiments
Because of the large and sustained increases in HR observed with HHMA, the pharmacological mechanism for this effect was investigated using the β-antagonist propranolol (1 mg·kg−1) as a pretreatment. For comparison, propranolol was also used as a pretreatment for MDMA. For the rats tested with propranolol, BP following saline (Sal + Sal) ranged from 94.8 to 106.3 mmHg and HR ranged from 258.5 to 287.8. Figure 4 shows the results for HR for both HHMA (3 mg·kg−1) and MDMA (3 mg·kg−1). For statistical analysis, the results for HHMA and MDMA were analysed separately. HHMA significantly increased HR compared with the saline (Sal + Sal) condition [F(3,12) = 117.71, P < 0.0001], an effect that was antagonized by pretreatment with propranolol. Like HHMA, 3 mg·kg−1 MDMA increased HR [F(3,12) = 57.62, P < 0.0001], an effect that was also blocked by propranolol. Although propranolol (Prop + Sal) itself slightly decreased HR, this effect was not significantly different from saline. Propranolol did not alter the effect of MDMA on BP or activity (data not shown).
Figure 4.
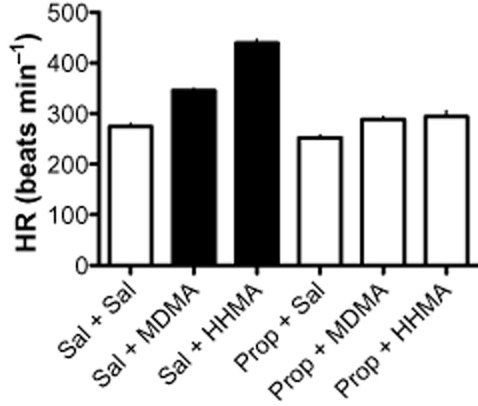
Effects of MDMA (3 mg·kg−1) and HHMA (3 mg·kg−1) alone and in combination with propranolol (1 mg·kg−1) on HR. The filled bars indicate that both MDMA and HHMA significantly (P < 0.05) increased HR compared with saline-treated controls. Significant increases in HR were not observed for MDMA and HHMA following pretreatment with propranolol. Propranolol alone did not significantly affect HR. n = 4 for every condition.
Discussion
A major aim of the present experiments was to characterize the cardiovascular effects of MDMA and its phase I metabolites in conscious rats. For MDMA, the peak increase in HR was observed at 3 mg·kg−1, with higher doses producing smaller effects. The biphasic dose–response for HR could be the result of robust elevations in BP after high doses producing reflex bradycardia that overrides the tachycardia seen at low doses. However, this explanation may not totally explain the biphasic effect, as the 20 mg·kg−1 dose of MDMA showed a continued decrease in HR despite no further increase in BP. Similar cardiovascular effects of MDMA have been reported in both conscious (Badon et al., 2002; Bexis and Docherty, 2006) and anaesthetized (O'Cain et al., 2000; McDaid and Docherty, 2000) rats. For BP and HR measures, our time course data show that effects often lasted for the entire length of the 2 h session, indicating a long duration of action. The cardiovascular effects of MDMA seen here in rats closely resemble those observed in humans given recreational doses of the drug under controlled laboratory conditions (Vollenweider et al., 1998; Mas et al., 1999; Liechti et al., 2001a,b; de la Torre et al., 2004).
Like MDMA, MDA also increased BP. The effect of MDA on HR was less clear, but an intermediate dose of MDA (3 mg·kg−1) increased HR analogous to MDMA, although the rise did not reach statistical significance. The effect of MDA on BP had returned to saline control levels by the end of the 2 h session, suggesting that MDA might have a shorter duration of action than MDMA. However, MDA and MDMA have similar t1/2 when administered systemically to rats (Fonsart et al., 2009), therefore, the differences in the cardiovascular effects between these drugs must be due to factors other than pharmacokinetics.
The effects of MDMA and MDA on BP and HR are similar to other sympathomimetic agents. In particular, elevations in BP and biphasic HR responses have been seen with both amphetamine (O'Cain et al., 2000) and cocaine (Kiritsy-Roy et al., 1990; Branch and Knuepfer, 1992). The mechanism of cardiovascular stimulation produced by amphetamine and cocaine involves central and peripheral sympathetic components (Schindler et al., 1992a,b; Schindler, 1996). Similarly, pharmacological studies in animals and humans suggest that sympathetic activation is responsible for the cardiovascular effects of MDMA. Hysek et al. (2010) found that the β-adrenoceptor antagonist pindolol blocks the HR increase observed following MDMA administration in humans. The present data showing that propranolol reduces MDMA-induced tachycardia in rats are consistent with these human data. Hysek et al. (2011) also showed that the noradrenaline uptake blocker reboxetine reduces elevations in BP and HR seen following MDMA administration in human volunteers, indicating that noradrenaline transporters are involved in these effects. Vandeputte and Docherty (2002) concluded that α-adrenergic mechanisms contribute to BP increases following MDMA in humans. Previously, O'Cain et al. (2000) reported that the α-antagonist phentolamine reduces the duration of action of MDMA on BP in rats, but it does not reduce the peak effects. McDaid and Docherty (2000) showed that the pressor response to MDMA in rats could be changed to a depressor response by treatment with prazosin. Taken together, these data indicate that the adrenergic system is a primary mediator of MDMA-induced cardiovascular effects, but the underlying receptor mechanisms are complex. Moreover, the 5-hydroxytryptaminergic system may also influence the cardiovascular actions of the drug in humans (Liechti and Vollenweider, 2000b; Liechti and Vollenweider, 2001; Tancer and Johanson, 2007).
In addition to their effects on BP and HR, MDMA and MDA dose-dependently increased locomotor activity. The method used here to measure locomotor activity with telemetry does not allow for an accurate determination of distanced travelled or the determination of finer detailed measures of activity like stereotypy. Nevertheless, the increase in activity observed with MDMA agrees with results from other studies where infrared photo-beams were used to measure locomotion (Bankson and Cunningham, 2001; 2002; Baumann et al., 2008). In general, forward locomotion produced by MDMA appears to involve a primary dopaminergic component (Baumann et al., 2008), but actions at 5-HT transporters and receptors also contribute to the motor effects of the drug (Bankson and Cunningham, 2001; 2002). In humans, dopamine receptor blockade can reduce the subjective effects of MDMA, but dopaminergic mechanisms do not appear to contribute to cardiovascular effects (Liechti and Vollenweider, 2000a; Liechti et al., 2001b).
In contrast to MDMA and MDA, none of the hydroxylated metabolites altered locomotor activity. Furthermore, the O-methylated metabolites HMMA and HMA did not affect any cardiovascular parameters measured. However, the dihydroxy metabolites HHMA and HHA displayed powerful cardiovascular actions. Both compounds significantly increased HR, with the rise in HR produced by HHMA clearly exceeding that of the parent compound MDMA. This effect of HHMA on HR was mediated by a mechanism involving β-adrenoceptors, as propranolol was able to completely block the effect. Although HHMA had no significant effect on BP, HHA also significantly increased BP at the highest dose tested comparable with the effects of MDA. The fact that both HHMA and HHA had sympathomimetic effects on cardiovascular function, but failed to affect locomotor activity, indicates that these metabolites do not readily cross the blood–brain barrier, as suggested by others (Escobedo et al., 2005; Mueller et al., 2009). Little information is available regarding the molecular mechanism of action of HHMA and HHA, but the potent tachycardia produced by these compounds suggests they might target noradrenaline transporters or receptors. One recent study reported that HHA interacts with dopamine transporters more potently than MDMA, and this effect appears to be long lasting (Escubedo et al., 2011).
An important limitation of our study is the lack of pharmacokinetic data for circulating concentrations of MDMA or its metabolites after systemic injection, and future studies of this type should be carried out. Nevertheless, the results with HHMA and HHA raise the question of whether cardiovascular effects of these metabolites might contribute significantly to the overall profile of actions induced by systemically administered MDMA. As the N-demethylation pathway leading to the formation of MDA accounts for less than 20% of MDMA metabolism in humans (de la Torre et al., 2004; Kolbrich et al., 2008b), the role of HHA is likely to be minimal. However, the effect of HHMA could be significant. Pharmacokinetic studies in human subjects receiving recreational doses of MDMA have shown that circulating levels of HHMA are nearly equivalent to those of MDMA (Segura et al., 2001; de la Torre et al., 2004) and reach plasma concentrations of 150 ng·mL−1 (∼1 μM). Furthermore, concentrations of MDMA and HHMA follow a parallel time course of elimination in men. We found that 1 mg·kg−1 HHMA produced more robust effects on HR than 3 mg·kg−1 MDMA in rats, therefore, it seems feasible that HHMA formed in vivo contributes to the effects of systemically administered MDMA in humans. Additionally, the effect of HHMA on HR was longer lasting than that of MDMA, with HR still elevated 2 h following the administration of HHMA.
To summarize, in agreement with previous human and animal studies, MDMA increases BP, HR and locomotor activity. The possible role of MDMA metabolites in mediating the cardiovascular effects of this drug has not been well studied. Here, we show that the N-demethylated metabolite MDA has similar effects to MDMA. Perhaps, more importantly, the dihydroxy metabolite HHMA induced powerful and sustained tachycardia, and HHA increased both HR and BP. Plasma concentrations of HHMA are similar to those of the parent compound in humans receiving psychoactive doses of MDMA, which suggests that the effects of HHMA could contribute significantly to the tachycardia produced by systemically administered MDMA. Elevations in HR produced by MDMA and HHMA were reversed by propranolol pretreatment indicating a role for β-adrenoceptors in this effect. Based on the in vivo data presented here, determining the precise molecular mechanism of action of HHMA and the other hydroxylated metabolites of MDMA warrants further investigation.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institutes of Health, National Institute on Drug Abuse. The views expressed are those of the authors and do not necessarily represent the views of the Drug Enforcement Administration, the US Department of Justice or an officer or entity of the USA.
Abbreviations
- HHA
3,4-dihydroxyamphetamine
- HHMA
3,4-dihydroxymethamphetamine
- HMA
4-hydroxy-3-methoxyamphetamine
- HMMA
4-hydroxy-3-methoxymethamphetamine
- HR
heart rate
- MDA
3,4-methylenedioxyamphetamine
- MDMA
3,4-methylenedioxymethamphetamine
Conflict of interest
The authors have no conflicts to report.
References
- Badon LA, Hicks A, Lord K, Ogden BA, Meleg-Smith S, Varner KJ. Changes in cardiovascular responsiveness and cardiotoxicity elicited during binge administration of Ecstasy. J Pharmacol Exp Ther. 2002;302:898–907. doi: 10.1124/jpet.302.3.898. [DOI] [PubMed] [Google Scholar]
- Bankson MG, Cunningham KA. 3,4-Methylenedioxymethamphetamine (MDMA) as a unique model of serotonin receptor function and serotonin–dopamine interactions. J Pharmacol Exp Ther. 2001;297:846–852. [PubMed] [Google Scholar]
- Bankson MG, Cunningham KA. Pharmacological studies of the acute effects of (+)-3,4-methylenedioxymethamphetamine on locomotor activity: role of 5-HT(1B/1D) and 5-HT(2) receptors. Neuropsychopharmacology. 2002;26:40–52. doi: 10.1016/S0893-133X(01)00345-1. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Rothman RB. Neural and cardiac toxicities associated with 3,4-methylenedioxymethamphetamine (MDMA) Int Rev Neurobiol. 2009;88:257–296. doi: 10.1016/S0074-7742(09)88010-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Clark RD, Rothman RB. Locomotor stimulation produced by 3,4-methylenedioxymethamphetamine (MDMA) is correlated with dialysate levels of serotonin and dopamine in rat brain. Pharmacol Biochem Behav. 2008;90:208–217. doi: 10.1016/j.pbb.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Zolkowska D, Kim I, Scheidweiler KB, Rothman RB, Huestis MA. Effects of dose and route of administration on pharmacokinetics of (+ or -)-3,4-methylenedioxymethamphetamine in the rat. Drug Metab Dispos. 2009;37:2163–2170. doi: 10.1124/dmd.109.028506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bexis S, Docherty JR. Effects of MDMA, MDA and MDEA on blood pressure, heart rate, locomotor activity and body temperature in the rat involve alpha-adrenoceptors. Br J Pharmacol. 2006;147:926–934. doi: 10.1038/sj.bjp.0706688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branch CA, Knuepfer MM. Adrenergic mechanisms underlying cardiac and vascular responses to cocaine in conscious rats. J Pharmacol Exp Ther. 1992;263:742–751. [PubMed] [Google Scholar]
- Drug Abuse Warning Network. 2009: Selected Tables of National Estimates of Drug-Related Emergency Department Visits. Rockville, MD: Center for Behavioral Health Statistics and Quality, SAMHSA; 2010. [Google Scholar]
- Escobedo I, O'Shea E, Orio L, Sanchez V, Segura M, de la Torre R, et al. A comparative study on the acute and long-term effects of MDMA and 3,4-dihydroxymethamphetamine (HHMA) on brain monoamine levels after i.p. or striatal administration in mice. Br J Pharmacol. 2005;144:231–241. doi: 10.1038/sj.bjp.0706071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escubedo E, Abad S, Torres I, Camarasa J, Pubill D. Comparative neurochemical profile of 3,4-methylenedioxymethamphetamine and its metabolite alpha-methyldopamine on key targets of MDMA neurotoxicity. Neurochem Int. 2011;58:92–101. doi: 10.1016/j.neuint.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Fitzgerald JL, Reid JJ. Sympathomimetic actions of methylenedioxymethamphetamine in rat and rabbit isolated cardiovascular tissues. J Pharm Pharmacol. 1994;46:826–832. doi: 10.1111/j.2042-7158.1994.tb03738.x. [DOI] [PubMed] [Google Scholar]
- Fonsart J, Menet MC, Debray M, Hirt D, Noble F, Scherrmann JM, et al. Sprague–Dawley rats display sex-linked differences in the pharmacokinetics of 3,4-methylenedioxymethamphetamine (MDMA) and its metabolite 3,4-methylenedioxyamphetamine (MDA) Toxicol Appl Pharmacol. 2009;241:339–347. doi: 10.1016/j.taap.2009.09.008. [DOI] [PubMed] [Google Scholar]
- Green AR, Mechan AO, Elliott JM, O'Shea E, Colado MI. The pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, ‘ecstasy’) Pharmacol Rev. 2003;55:463–508. doi: 10.1124/pr.55.3.3. [DOI] [PubMed] [Google Scholar]
- Halpern P, Moskovich J, Avrahami B, Bentur Y, Soffer D, Peleg K. Morbidity associated with MDMA (ecstasy) abuse: a survey of emergency department admissions. Hum Exp Toxicol. 2011;30:259–266. doi: 10.1177/0960327110370984. [DOI] [PubMed] [Google Scholar]
- Hysek CM, Vollenweider FX, Liechti ME. Effects of a beta-blocker on the cardiovascular response to MDMA (Ecstasy) Emerg Med J. 2010;27:586–589. doi: 10.1136/emj.2009.079905. [DOI] [PubMed] [Google Scholar]
- Hysek CM, Simmler LD, Ineichen M, Grouzmann E, Hoener MC, Brenneisen R, et al. The norepinephrine transporter inhibitor reboxetine reduces stimulant effects of MDMA (‘ecstasy’) in humans. Clin Pharmacol Ther. 2011;90:246–255. doi: 10.1038/clpt.2011.78. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future National Results on Adolescent Drug Use: Overview of Key Findings. Ann Arbor, MI: Institute for Social Research, The University of Michigan; 2011. [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: Reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiritsy-Roy JA, Halter JB, Gordon SM, Smith MJ, Terry LC. Role of the central nervous system in hemodynamic and sympathoadrenal responses to cocaine in rats. J Pharmacol Exp Ther. 1990;255:154–160. [PubMed] [Google Scholar]
- Kolbrich EA, Goodwin RS, Gorelick DA, Hayes RJ, Stein EA, Huestis MA. Physiological and subjective responses to controlled oral 3,4-methylenedioxymethamphetamine administration. J Clin Psychopharmacol. 2008a;28:432–440. doi: 10.1097/JCP.0b013e31817ef470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolbrich EA, Goodwin RS, Gorelick DA, Hayes RJ, Stein EA, Huestis MA. Plasma pharmacokinetics of 3,4-methylenedioxymethamphetamine after controlled oral administration to young adults. Ther Drug Monit. 2008b;30:320–332. doi: 10.1097/FTD.0b013e3181684fa0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester SJ, Baggott M, Welm S, Schiller NB, Jones RT, Foster E, et al. Cardiovascular effects of 3,4-methylenedioxymethamphetamine. A double-blind, placebo-controlled trial. Ann Intern Med. 2000;133:969–973. doi: 10.7326/0003-4819-133-12-200012190-00012. [DOI] [PubMed] [Google Scholar]
- Liechti ME, Vollenweider FX. Acute psychological and physiological effects of MDMA (‘Ecstasy’) after haloperidol pretreatment in healthy humans. Eur Neuropsychopharmacol. 2000a;10:289–295. doi: 10.1016/s0924-977x(00)00086-9. [DOI] [PubMed] [Google Scholar]
- Liechti ME, Vollenweider FX. The serotonin uptake inhibitor citalopram reduces acute cardiovascular and vegetative effects of 3,4-methylenedioxymethamphetamine (‘Ecstasy’) in healthy volunteers. J Psychopharmacol. 2000b;14:269–274. doi: 10.1177/026988110001400313. [DOI] [PubMed] [Google Scholar]
- Liechti ME, Vollenweider FX. Which neuroreceptors mediate the subjective effects of MDMA in humans? A summary of mechanistic studies. Hum Psychopharmacol. 2001;16:589–598. doi: 10.1002/hup.348. [DOI] [PubMed] [Google Scholar]
- Liechti ME, Gamma A, Vollenweider FX. Gender differences in the subjective effects of MDMA. Psychopharmacology (Berl) 2001a;154:161–168. doi: 10.1007/s002130000648. [DOI] [PubMed] [Google Scholar]
- Liechti ME, Geyer MA, Hell D, Vollenweider FX. Effects of MDMA (ecstasy) on prepulse inhibition and habituation of startle in humans after pretreatment with citalopram, haloperidol, or ketanserin. Neuropsychopharmacology. 2001b;24:240–252. doi: 10.1016/S0893-133X(00)00199-8. [DOI] [PubMed] [Google Scholar]
- Mas M, Farre M, de la Torre R, Roset PN, Ortuno J, Segura J, et al. Cardiovascular and neuroendocrine effects and pharmacokinetics of 3, 4-methylenedioxymethamphetamine in humans. J Pharmacol Exp Ther. 1999;290:136–145. [PubMed] [Google Scholar]
- Matthews JN, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. BMJ. 1990;300:230–235. doi: 10.1136/bmj.300.6719.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer HH, Bickeboeller-Friedrich J, Kraemer T, Peters FT. Toxicokinetics and analytical toxicology of amphetamine-derived designer drugs (‘Ecstasy’) Toxicol Lett. 2000;112-113:133–142. doi: 10.1016/s0378-4274(99)00207-6. [DOI] [PubMed] [Google Scholar]
- McDaid J, Docherty JR. Vascular actions of MDMA involve alpha1 and alpha2-adrenoceptors in the anaesthetized rat. Br J Pharmacol. 2001;133:429–437. doi: 10.1038/sj.bjp.0704094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller M, Yuan J, Felim A, Neudorffer A, Peters FT, Maurer HH, et al. Further studies on the role of metabolites in (+/–)-3,4-methylenedioxymethamphetamine-induced serotonergic neurotoxicity. Drug Metab Dispos. 2009;37:2079–2086. doi: 10.1124/dmd.109.028340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Cain PA, Hletko SB, Ogden BA, Varner KJ. Cardiovascular and sympathetic responses and reflex changes elicited by MDMA. Physiol Behav. 2000;70:141–148. doi: 10.1016/s0031-9384(00)00235-3. [DOI] [PubMed] [Google Scholar]
- Scheidweiler KB, Ladenheim B, Cadet JL, Huestis MA. Mice lacking multidrug resistance protein 1a show altered dopaminergic responses to methylenedioxymethamphetamine (MDMA) in striatum. Neurotox Res. 2010;18:200–209. doi: 10.1007/s12640-009-9124-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler CW. Cocaine and cardiovascular toxicity. Addict Biol. 1996;1:31–47. doi: 10.1080/1355621961000124676. [DOI] [PubMed] [Google Scholar]
- Schindler CW, Tella SR, Goldberg SR. Adrenoceptor mechanisms in the cardiovascular effects of cocaine in conscious squirrel monkeys. Life Sci. 1992a;51:653–660. doi: 10.1016/0024-3205(92)90238-k. [DOI] [PubMed] [Google Scholar]
- Schindler CW, Zheng JW, Tella SR, Goldberg SR. Pharmacological mechanisms in the cardiovascular effects of methamphetamine in conscious squirrel monkeys. Pharmacol Biochem Behav. 1992b;42:791–796. doi: 10.1016/0091-3057(92)90031-a. [DOI] [PubMed] [Google Scholar]
- Segura M, Ortuno J, Farre M, McLure JA, Pujadas M, Pizarro N, et al. 3,4-Dihydroxymethamphetamine (HHMA). A major in vivo 3,4-methylenedioxymethamphetamine (MDMA) metabolite in humans. Chem Res Toxicol. 2001;14:1203–1208. doi: 10.1021/tx010051p. [DOI] [PubMed] [Google Scholar]
- Shenouda SK, Carvalho F, Varner KJ. The cardiovascular and cardiac actions of ecstasy and its metabolites. Curr Pharm Biotechnol. 2010;11:470–475. doi: 10.2174/138920110791591526. [DOI] [PubMed] [Google Scholar]
- Suarez RV, Riemersma R. ‘Ecstasy’ and sudden cardiac death. Am J Forensic Med Pathol. 1988;9:339–341. doi: 10.1097/00000433-198812000-00015. [DOI] [PubMed] [Google Scholar]
- Tancer M, Johanson CE. The effects of fluoxetine on the subjective and physiological effects of 3,4-methylenedioxymethamphetamine (MDMA) in humans. Psychopharmacology (Berl) 2007;189:565–573. doi: 10.1007/s00213-006-0576-z. [DOI] [PubMed] [Google Scholar]
- Tella SR, Schindler CW, Goldberg SR. Cardiovascular responses to cocaine self-administration: acute and chronic tolerance. Eur J Pharmacol. 1999;383:57–68. doi: 10.1016/s0014-2999(99)00582-8. [DOI] [PubMed] [Google Scholar]
- de la Torre R, Farre M. Neurotoxicity of MDMA (ecstasy): the limitations of scaling from animals to humans. Trends Pharmacol Sci. 2004;25:505–508. doi: 10.1016/j.tips.2004.08.001. [DOI] [PubMed] [Google Scholar]
- de la Torre R, Farre M, Roset PN, Pizarro N, Abanades S, Segura M, et al. Human pharmacology of MDMA: pharmacokinetics, metabolism, and disposition. Ther Drug Monit. 2004;26:137–144. doi: 10.1097/00007691-200404000-00009. [DOI] [PubMed] [Google Scholar]
- Vandeputte C, Docherty JR. Vascular actions of 3,4-methylenedioxymethamphetamine in alpha(2A/D)-adrenoceptor knockout mice. Eur J Pharmacol. 2002;457:45–49. doi: 10.1016/s0014-2999(02)02661-4. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Gamma A, Liechti M, Huber T. Psychological and cardiovascular effects and short-term sequelae of MDMA (‘ecstasy’) in MDMA-naive healthy volunteers. Neuropsychopharmacology. 1998;19:241–251. doi: 10.1016/S0893-133X(98)00013-X. [DOI] [PubMed] [Google Scholar]


