Abstract
BACKGROUND AND PURPOSE
Studies have demonstrated that a moderate intake of amino acids is associated with development of bone health. Methionine, a sulphur-containing essential amino acid, has been largely implicated for improving cartilage formation, however its physiological significance on bone integrity and functionality have not been elucidated. We investigated whether methionine can prevent osteoporotic bone loss.
EXPERIMENTAL APPROACH
The anti-resorptive effect of methionine, (250 mg kg−1 body wt administered in drinking water for 10 weeks), was evaluated in ovariectomized (OVX) rats by monitoring changes in bone turnover, formation of osteoclasts from blood-derived mononuclear cells and changes in the synthesis of pro-osteoclastogenic cytokines.
KEY RESULTS
Methionine improved bone density and significantly decreased the degree of osteoclast development from blood mononuclear cells in OVX rats, as indicated by decreased production of osteoclast markers tartarate resistant acid phosphatase b (TRAP5b) and MIP-1α. siRNA-mediated knockdown of myeloid differentiation primary response 88 [MyD88], a signalling molecule in the toll-like receptor (TLR) signalling cascade, abolished the synthesis of both TRAP5b and MIP-1α in developing osteoclasts. Methionine supplementation disrupted osteoclast development by inhibiting TLR-4/MyD88/NF-κB pathway.
CONCLUSIONS AND IMPLICATIONS
TLR-4/MyD88/NF-κB signalling pathway is integral for osteoclast development and this is down-regulated in osteoporotic system on methionine treatment. Methionine treatment could be beneficial for the treatment of postmenopausal osteoporosis.
Keywords: methionine, osteoclast, RANKL, MyD88, alendronate, osteoporosis
Introduction
Osteoporosis is a progressive and systemic bone disorder prevalent in the ageing population of both developed and developing countries (Feng and McDonald, 2011). It is characterized by a significant decline in bone mineral density and microarchitectural deterioration of the bone tissue (Nancy, 2006). Clinically this disease is classified as primary or secondary (WHO study group, 1994). Primary osteoporosis consists of two subtypes, type I and type II. Type I progresses due to the decline in oestrogen or testosterone levels in the body; which in women is referred to as postmenopausal osteoporosis (Jakob, 2005; Fink et al., 2006). Type II or senile osteoporosis arises as a result of bone ageing and progresses after the age of 70. A secondary form of the disease, secondary osteoporosis, occurs as a result of long-term intake of glucocorticoids, insulin-dependent diabetes mellitus, hyperthyroidism or hypercortisolism (Wright, 1991; Jakob, 2005).
The prevailing view is that osteoporosis develops because of an imbalance between bone formation and bone resorption (Nancy, 2006). For example, in postmenopausal women, oestrogen deficiency-mediated receptor activator of NF-κB ligand (TNFSF11, also known as RANKL) production by osteoblasts triggers changes in osteoclasts, activating new bone remodelling units within the bone tissue. This accelerated bone remodelling without a commensurate rise in bone formation alters trabecular structure and increases bone fragility (WHO study group, 1994; Seeman, 2003). Osteoporosis can be treated with hormone replacement therapy, bisphosphonates, steroids or monoclonal antibodies (e.g. denosumab). However ulcers, renal toxicity, cancer, eczema, stroke and cardiovascular complications associated with the intake of such medications have restricted their continued usage (Ernesto, 2000; Riggs et al., 2002; Rossouw et al., 2002; Beral and Million Women Study Collaborators, 2003; Handa, 2004; Bridgeman and Pathak, 2011). The development of safer treatment strategies for osteoporosis is therefore warranted.
Therapeutic interventions using amino acids have received much attention in the last few years because of their unique ability to serve as signalling factors in regulatory pathways (Brandy, 1993; Kerstetter et al., 2003; Kimball and Jefferson, 2004, 2006; Ahmed and Hamza, 2009). These studies have shown that arginine, lysine, glutamine, tryptophan and taurine exert diverse pharmacological effects on the bone like increasing NO, type I collagen, GSH and 5-HT synthesis in bone cells and improving calcium and vitamin D absorption (Fini et al., 2001; Ahmed and Hamza, 2009; Pernow et al., 2010; Jiang and Cai, 2011). Wang et al. (2006) demonstrated that the conditionally essential amino acid glutamine improves calcium uptake by accelerating GSH synthesis and activating calcium-sensing receptors in the bone. Given the ample evidence that amino acids restore bone health by well-characterized physiological mechanisms, we explored the possibility that methionine has beneficial effects, as little attention has been paid in evaluating the pharmacological effects of methionine on bone remodelling mediated by osteoblasts and osteoclasts.
Methionine (HO2CCH(NH2)CH2CH2SCH3) is a sulphur-containing essential amino acid critical for the synthesis of vital molecules such as cysteine, carnitine, taurine, lecithin, phosphotidyl choline and other phospholipids. It improves cartilage synthesis and is therefore used for the treatment of arthritis (Ursini and Pipicelli, 2009). Methionine has also been found to be effective against the progression of Parkinson's disease and schizophrenia (Antun et al., 1971; Meininger et al., 1982). A recent report by Cordomi et al. (2013) revealed methionine to be a molecular gear for pharmacology and function. Our preliminary data showed that methionine protects against bone loss in vivo, but has no direct effects on differentiated osteoclasts or osteoblasts in vitro. Since blood-borne osteoclast precursors are involved in replenishing osteoclast population during bone-loss pathology, we speculated whether methionine modulates the development of osteoclasts cells from their blood-borne precursors. Based on this hypothesis, we examined the modulatory effect of methionine in an experimentally induced osteoporotic model. By knocking down myeloid differentiation primary response gene 88 (MyD88) in developing osteoclasts, a potential mechanism accounting for osteoclastogenesis during osteoporosis and how methionine regulates this pathway is described here. Drug/molecular target nomenclature throughout this manuscript conforms to BJP's Concise Guide to PHARMACOLOGY (Alexander et al., 2013).
Methods
Animals
All the animal experiments were approved by the Institute animal ethics committee (IAEC No. 192/08) in accordance with the International Guiding Principles for Biomedical Research Involving Animals issued by the Council for the International Organizations of Medical Sciences. Female Sprague-Dawley rats bred and reared in the institute animal house facility were used in this study. The animals were given standard laboratory chow and water ad libitum, and kept in an ambient room with the following conditions: temperature (22 ± 4°C), humidity 60%∼65% and light (7:00–19:00). A total of 90 rats were used in this study.
Skeletally mature 5-month-old rats weighing 320 ± 10 g were used to generate the ovariectomized (OVX) rat model. Treatments were initiated 1 week from the day of ovariectomy. This period of rest allowed the rats to recover from the stress associated with the surgery.
Rats were divided into the following groups: normal rats; sham rats (in these rats, the ovaries were exposed and not removed); OVX rats; OVX rats fed standard chow and supplemented with methionine in their drinking water (100, 250 and 500 mg·kg−1 body wt); OVX rats administered Fosamax (alendronate sodium; Merck & Co., Whitehouse Station, NJ, USA) at a dose of 100 or 200 μg·kg−1 body wt, p.o. alone or in combination with methionine (250 mg·kg−1 body wt). Both treatments were performed 6 h apart. Alendronate (100 or 200 μg·kg−1 body wt) was administered p.o. to OVX rats at an interval of 5 days for 1 month. Rats that received methionine were caged separately to ensure that each animal received the appropriate dose of methionine daily. The duration of methionine therapy was 10 weeks. Cancellous osteopenia (bone loss) requires at least 4 weeks to develop post-ovariectomy and hence this was a prophylactic regime. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010).
Ovariectomy
This surgical procedure was performed by the same person under aseptic conditions. Before surgery, the rats were anaesthetized with a combination of xylazine (10 mg kg−1) and ketamine (75 mg kg−1) administered i.p. Toe pinch, heart rate and body temperature were consistently checked to measure the depth of anaesthesia. Following anaesthesia, an incision was made in the abdomen to gently exteriorize the ovaries. The fallopian tubes were ligated, ovaries excised and then the peritoneal cavities were closed with absorbable sutures. After surgery, rats were administered Diclofenac sodium injection (20 mg kg−1 body weight) to reduce the pain associated with the surgery. Care was taken to ensure that the animals did not suffer from hypothermia.
Bone imaging and histomorphological analysis
Bone imaging was performed using an In Vivo Imaging System FX PRO (Kodak, Rochester, NY, USA) employing the bone density software module, Carestream Molecular Imaging software version 5.0.7 (Carestream Health Inc., Rochester, NY, USA). Sectioning of bone was done by standard methods as detailed in Supporting Information Appendix S1. Trabecular thickness and trabecular separation were analysed using Bone J and Image J, NIH software (National Institute of Health, Bethesda, MD, USA). For analysis of adipogenesis, four fields were randomly selected from each section at 40× magnification and two sections were collected from each femur. A total of 16 fields were selected from four sections of each rat followed by quantification of adipocytes in the bone marrow. The area of adipocytes in bone marrow were determined and analysed using Image J.
Biochemical analysis
Tartarate-resistant acid phosphatase 5b (TRAP5b) activity and C-terminal telopeptides (CTX) levels were measured using elisa kits from Immuno Diagnostic Systems (Fountain Hills, AZ, USA). Cytokines, osteocalcin and osteopontin levels in serum were measured by multiplex kits from Millipore on Bioplex 200™ (Bio-Rad, Hercules, CA, USA). Osteocalcin in cell culture supernatants was assayed by elisa kit from Biomedical Technologies (Stoughton, MA, USA). Homocysteine levels in serum were measured using a kit from Cusa Biotech, China (Wuhan, China). Soluble (s) TNFSF11, M-CSF and macrophage inhibitory protein-1α (MIP-1α also known as CCL3) levels were assayed by indirect elisa employing 3, 3′, 5, 5′-tetramethyl benzidine as substrate.
Isolation and culture of blood mononuclear cells
Blood was collected from rats by retro-orbital puncture into heparin-containing tubes. Briefly, blood was layered on Histopaque 1083 (Sigma-Aldrich, St Louis, MO, USA) and centrifuged at 400x g for 30 min to obtain a buffer coat layer. The mononuclear cells in the buffy coat were enriched by washing with α-minimal essential medium (MEM) (supplemented with 10% fetal calf serum and 1% antibiotic–antifungal solution) and seeding on to type I collagen-coated plates (50 μg·mL−1). Cell viability was ensured to be greater than 90% by trypan blue assay. After 4 h, the culture media was changed and the cells were allowed to proliferate. To initiate differentiation, the mononuclear cells were cultured in the presence of 25 μg·L−1 recombinant mouse M-CSF (days 1–11) and 20 μg·L−1 TNFSF11 (days 6–20). Osteoclastogenic media was replaced every 3 days. The formation of osteoclasts was quantified by measuring the level of TRAP5b (a specific marker of osteoclast activity independent of renal dysfunction that indicates the number of osteoclasts) by immunoassay.
Transient siRNA-mediated knockdown
siRNA transfections were performed with Lipofectamine RNAiMAX (Invitrogen™, Life Technologies, Carlsbad, CA, USA). After stimulating with TNFSF11, cells were transferred to either MyD88 siRNA or scrambled siRNA (40 nmol·mL−1). Knockdown efficiency was measured by Western blot. Before the siRNA transfection, the TRAP5b level in the cell culture supernatant was measured. Only those cultures that showed TRAP5b activity between 0.5 and 2 U·L−1 were used for siRNA transfection.
Western blot
Cells were lysed in radioimmunoprecipitation assay lysis buffer containing complete protease inhibitors and sodium orthovanadate. Equal amounts of samples were electrophoresed on either 12 or 15% SDS-PAGE and then blotted to nitrocellulose transfer membrane. Subsequently, the membrane was incubated at room temperature with Western blocker for 1 h. For probing total protein and the phosphorylated forms, membranes were probed with primary and secondary antibodies. Immunoreactive bands were visualized by enhanced chemiluminescence system in LAS4000 with ImageQuant LAS4000 software (GE Healthcare Life Science, Buckinghamshire, UK) and quantified using Image J.
Osteoblast culture
MC3T3-E1 sub-clone 4 (ATCC CRL2593), a pre-osteoblastic cell line derived from new born mouse calvaria, was purchased from American Type Culture Collection (ATCC, Manassas, VA, USA) and cultured in α-MEM supplemented with 10% fetal calf serum and 1% antibiotics. For cell culture experiments 2 × 106 cells were seeded in 60 mm Petri plates. After attaining 70% confluence, cells were incubated with methionine (1–500 μg) before treatment with LPS (1 μg·mL−1). Alternatively to study the effect of methionine on osteoblast differentiation, cells were treated with methionine with or without 10 mM β-glycerophosphate and 50 μg·mL−1 ascorbic acid for 30 days.
Statistics
The results were analysed using a statistical program Statistical Package for the Social Sciences (SPSS)/PC+, version 11.0 (SPSS Inc., Chicago, IL, USA). All values are expressed as means ± SEM. One-way anova was used to evaluate differences between samples of interest and the corresponding controls. P value less than 0.05 was considered statistically significant.
Results
Methionine inhibits bone loss in OVX rats
As a first step towards asserting the anti-resorptive effect of methionine, three different doses of methionine (100, 250 and 500 mg·kg−1 body wt) were chosen. The results showed that administration of methionine at doses 100, 250 and 500 mg·kg−1 body wt decreased OVX-induced body wt gain (Figure 1). Serum CTX assay indicated that the level of collagen degradation products was high in OVX rats and low in OVX rats administered 250 mg·kg−1 body wt of methionine (Figure 2). CTX level remained high in rats administered 100 and 500 mg·kg−1 body wt of methionine even at the end of the experimental period (Figure 2). TRAP5b assay showed that methionine at doses 100 and 250 mg·kg−1 body wt decreased TRAP5b activity in serum (Figure 3). While 250 mg·kg−1 body wt of methionine inhibited TRAP5b activity as early as four weeks, 100 mg·kg−1 body wt of methionine imparted its effect only after 10 weeks of administration.
Figure 1.
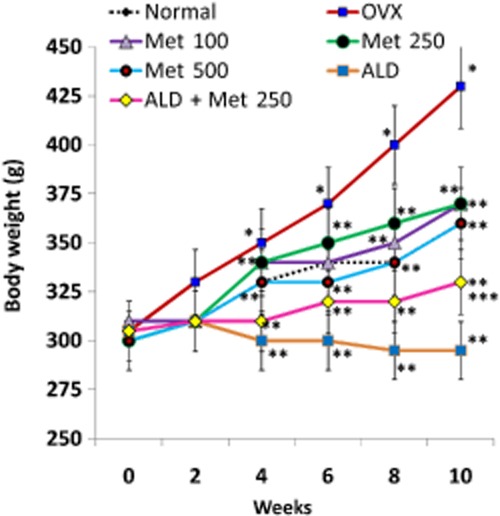
Changes in the body wt of normal, OVX and treatment groups during the experimental period. Methionine at doses 100, 250 and 500 mg·kg−1 body wt was administered in drinking water for 10 weeks. Alendronate (200 μg·kg−1 body wt) was administered p.o. to OVX rats at an interval of 5 days for 1 month. For combination therapy, rats were administered alendronate (100 μg·kg−1 body wt) and methionine (250 mg·kg−1 body wt). Both treatments were performed 6 h apart. Values are mean ± SEM; n = 4. *P < 0.05 compared with normal rat. **P < 0.05 compared with OVX rat. ***P < 0.05 compared with alendronate (200 μg·kg−1 body wt)-treated OVX rats.
Figure 2.
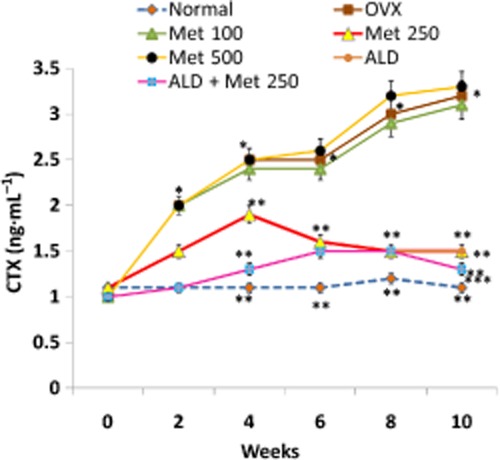
Changes in the level of CTX in serum samples from normal, OVX and treatment groups during the experimental period. Methionine at doses 100, 250 and 500 mg·kg−1 body wt was administered in drinking water for 10 weeks. Alendronate (200 μg·kg−1 body wt) was administered p.o. to OVX rats at an interval of 5 days for 1 month. For combination therapy, rats were administered alendronate (100 μg·kg−1 body wt) and methionine (250 mg·kg−1 body wt). Both treatments were performed 6 h apart. CTX levels in serum were measured by immunoassay. Values are mean ± SEM; n = 4. *P < 0.05 compared with normal rat. **P < 0.05 compared with OVX rat. ***P < 0.05 compared with alendronate (200 μg·kg−1 body wt)-treated OVX rats.
Figure 3.
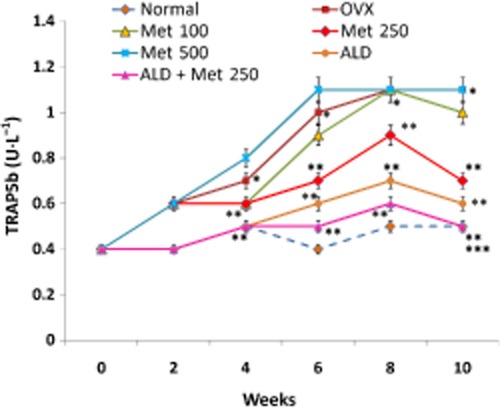
Changes in the activity of TRAP5b in serum of normal, OVX and treatment groups. Methionine at doses 100, 250 and 500 mg·kg−1 body wt was administered in drinking water for 10 weeks. Alendronate (200 μg·kg−1 body wt) was administered p.o. to OVX rats at an interval of 5 days for 1 month. For combination therapy, rats were administered alendronate (100 μg·kg−1 body wt) and methionine (250 mg·kg−1 body wt). Both treatments were performed 6 h apart. TRAP5b activity in serum was measured by immunoassay. Values are mean ± SEM; n = 4. *P < 0.05 compared with normal rat. **P < 0.05 compared with OVX rat. ***P < 0.05 compared with alendronate (200 μg·kg−1 body wt)-treated OVX rats.
To evaluate whether methionine intake at the studied doses raised the level of its toxic metabolite viz. homocysteine, we estimated homocysteine in serum by elisa. It was observed that ovariectomy itself induced a fourfold rise in serum homocysteine (Figure 4). Administration of methionine at doses 100 and 250 mg·kg−1 body wt significantly inhibited OVX-induced homocysteine production. At a higher dose of 500 mg kg−1, a rise in serum homocysteine level to 15 µmol· L−1 was observed (Figure 4) which we presumed may be the cumulative effect of homocysteine augmented upon ovariectomy and that produced by administration of high dose of methionine.
Figure 4.
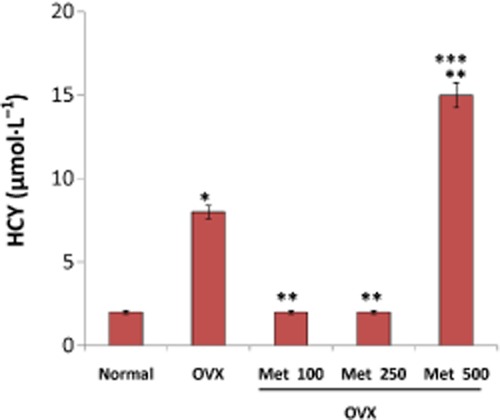
Changes in the level of serum homocysteine (HCY) in OVX rats fed 100, 250 and 500 mg·kg−1 body wt of methionine. Homocysteine in serum was measured by elisa. Values are mean ± SEM; n = 4. *P < 0.05 compared with normal rat. **P < 0.05 compared with OVX rat. ***P < 0.05 compared with methionine (100 and 250 mg·kg−1 body wt)-treated OVX rat.
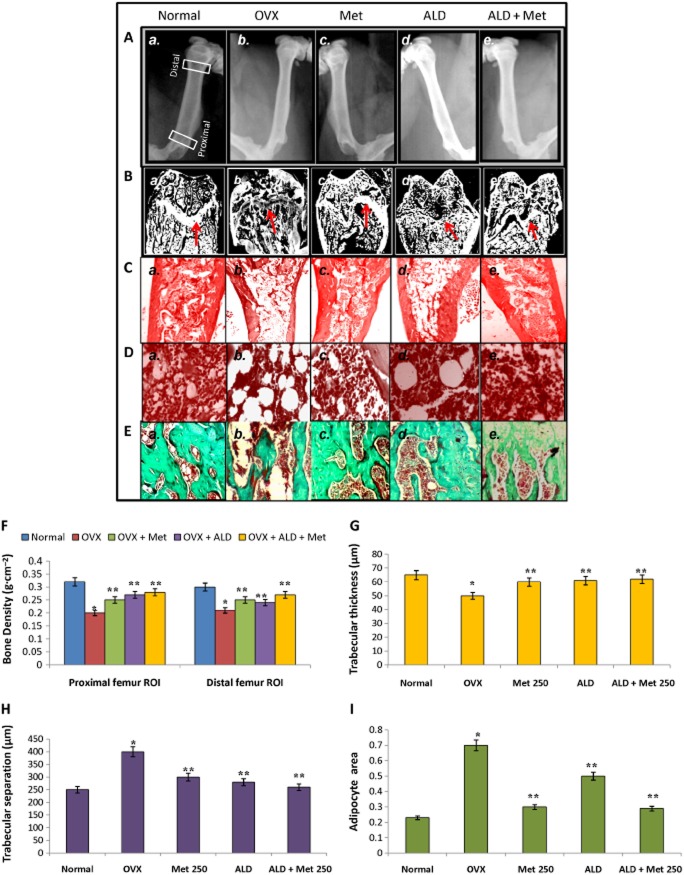
To define the basis of the anti-resorptive effect exerted by methionine at the dose of 250 mg·kg−1 body wt, radiograph analysis and bone density measurements were carried out. Femur bone densitometry measurements showed that methionine (250 mg·kg−1 body wt) significantly improved bone density as evidenced by increased bone density in the proximal and distal regions of femur as compared with OVX rats (Figure 5A, panel c; 5F). Figure 5B shows sections of rat femoral head belonging to different experimental groups. These images acquired and processed by Image J after haematoxylin and eosin staining show changes in bone architecture (growth plate) upon ovariectomy and on methionine (250 mg·kg−1 body wt) and alendronate treatments (200 μg·kg−1 body wt). Figure 5B, panel c shows preservation of trabecular structure in OVX rats on methionine treatment. We observed that OVX rats possessed high bone marrow adiposity with increased trabecular separation (Figure 5B, panel b; 5C, panel b) and low collagen content (Figure 5). After 10 weeks of methionine (250 mg·kg−1 body wt) administration, trabecular separation was reduced (Figure 5B, panel c; 5C, panel c; 5H) while collagen content (Figure 5E, panel c) and trabecular thickness (Figure 5G) were preserved in methionine-administered rats compared with OVX rats. Quantification of trabecular separation, trabecular thickness and adipocyte area showed that bone physiology of methionine-administered rats was comparable with alendronate (200 μg·kg−1 body wt) administered rats (Figure 5B, panel d; 5C, panel d; 5D, panel d; 5E, panel d; 5F–I). Analysis of bone-specific proteins like osteocalcin and osteopontin in serum showed that methionine administration maintained adequate levels of these bone-specific proteins in serum as compared with that of OVX rats (Figure 6). Collectively, the macroscopic and biochemical evaluation revealed that methionine (250 mg·kg−1 body wt) restrains bone loss and confers protection against development of osteoporosis.
Figure 5.
Bone physiology of normal, OVX and treatment groups. Methionine at a dose of 250 mg·kg−1 body wt was administered in drinking water for 10 weeks. Alendronate (200 μg·kg−1 body wt) was administered p.o. to OVX rats at an interval of 5 days for 1 month. For combination therapy, rats were administered alendronate (100 μg·kg−1 body wt) and methionine (250 mg·kg−1 body wt). Both treatments were performed 6 h apart. (A) Radiographs of femur were acquired on Kodak FX Pro in the X-ray mode. Each animal received an approximate radiation dose of 1.4 Rad during imaging. (B) Images of femoral bone head of normal and experimental rats acquired using Image J after haematoxylin and eosin (H & E) staining. Red arrows indicate changes in growth plate. (C) H & E staining of femur showing changes in trabecular structure between normal and experimental rats. (D) H & E staining of femur showing changes in adipogenesis between normal and experimental rats. (E) Masson Trichome staining of femur showing changes in collagen level between normal and experimental rats. (F) Bone density measurements of femoral bone samples from normal and experimental rats. Bone density was measured and analysed by bone density software module employing a Carestream Molecular Imaging software version 5.0.7 (G–I) Changes in trabecular thickness, trabecular separations and adipogenesis in normal and experimental rats analysed using Bone J and Image J, Adipocyte area refers to area of bone marrow adipocytes in units of bone trabecula. Values are mean ± SEM; n = 4. *P < 0.05 compared with normal rat. **P < 0.05 compared with OVX rat.
Figure 6.
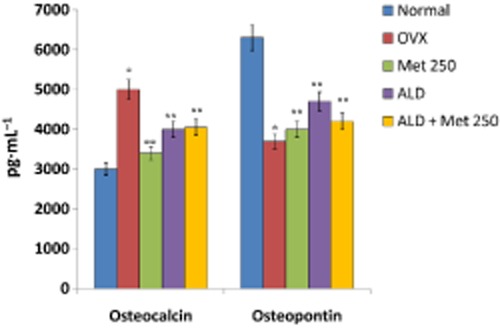
Changes in the levels of osteocalcin and osteopontin in serum of normal, OVX and treatment groups. Methionine at a dose of 250 mg·kg−1 body wt was administered in drinking water for 10 weeks. Alendronate (200 μg·kg−1 body wt) was administered p.o. to OVX rats at an interval of five days for one month. For combination therapy, rats were administered alendronate (100 μg·kg−1 body wt) and methionine (250 mg·kg−1 body wt). Both treatments were performed 6 h apart. Osteocalcin and osteopontin levels in serum were measured by multiplex kits from Millipore on Bioplex 200™ Values are mean ± SEM; n = 4. *P < 0.05 compared with normal rat. **P < 0.05 compared with OVX rat.
Methionine reduces pro-osteoclastogenic and pro-inflammatory cytokines in OVX rats
Pro-inflammatory and pro-osteoclastogenic cytokines possess potential roles in precipitating bone loss (Fuller et al., 1993; Gao et al., 2007; Fine et al., 2009). At the end of the experimental period, OVX rats showed high levels of Th1-derived cytokines (pro-inflammatory) such as IL-1α, IL-1β, G-CSF, GM-CSF, TNF-α, IL-8 and INF-γ in serum and low levels of Th2 cytokines (with anti-inflammatory role) like IL-4 and IL-10 (Figure 7). Administration of methionine effectively decreased the level of Th1 cytokines and increased the level of Th2 cytokines such as IL-4 and IL-10 in serum (Figure 7). We also observed that cytokines integral for osteoclastogenesis such as MIP-1α, M-CSF and RANKL were significantly lower in methionine-administered rats as compared with OVX rats (Figure 8).
Figure 7.
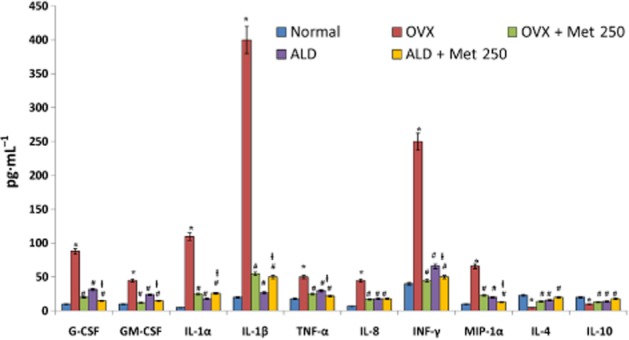
Changes in the levels of cytokine/chemokines in serum of normal, OVX and treatment groups. Methionine at a dose of 250 mg·kg−1 body wt was administered in drinking water for 10 weeks. Alendronate (200 μg·kg−1 body wt) was administered p.o. to OVX rats at an interval of 5 days for 1 month. For combination therapy, rats were administered alendronate (100 μg·kg−1 body wt) and methionine (250 mg·kg−1 body wt). Both the treatments were performed 6 h apart. Cytokines and chemokines in serum were measured using a multiplex kit from Millipore on Bioplex 200™. Values are mean ± SEM; n = 4. *P < 0.05 compared with normal rat. #P < 0.05 compared with OVX rat. ƚP < 0.05 compared with alendronate (200 μg·kg−1 body wt) administered rat.
Figure 8.
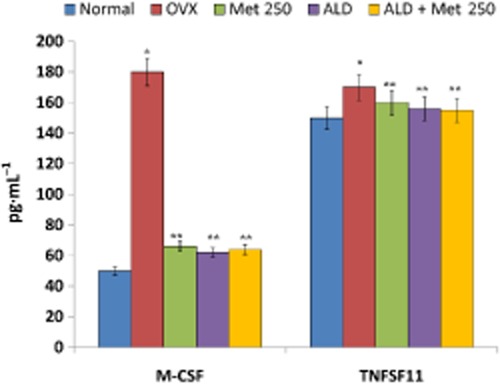
Changes in the level of M-CSF and TNFSF11 in the serum of normal, OVX and treatment groups. Methionine at a dose of 250 mg·kg−1 body wt was administered in drinking water for 10 weeks. Alendronate (200 μg·kg−1 body wt) was administered p.o. to OVX rats at an interval of 5 days for 1 month. For combination therapy, rats were administered alendronate (100 μg·kg−1 body wt) and methionine (250 mg·kg−1 body wt). Both the treatments were performed 6 h apart. M-CSF and soluble TNFSF11 levels in serum were measured by elisa. Values are mean ± SEM; n = 4. *P < 0.05 compared with normal rat. **P < 0.05 compared with OVX rat.
Effect of methionine on osteoblasts
To determine whether methionine modulates TNFSF11 production (a prime factor that regulates osteoclast activity) by osteoblasts, LPS-stimulated osteoblasts were used as model system. Pre-osteoblastic MC3T3E1 cells differentiated using ascorbic acid (50 μg·mL−1) and β-glycerophosphate (10 mM) for 5 days were incubated with methionine (1–500 μg·mL−1; 24 h) prior to LPS (1 μg·mL−1; 3 h) treatment and soluble TNFSF11 (sTNFSF11) release in cell culture supernatants was analysed by elisa. It was found that methionine inhibited LPS-induced sTNFSF11 release in osteoblast cultures at a dose of 50 μg·mL−1 (Figure 9A).
Figure 9.
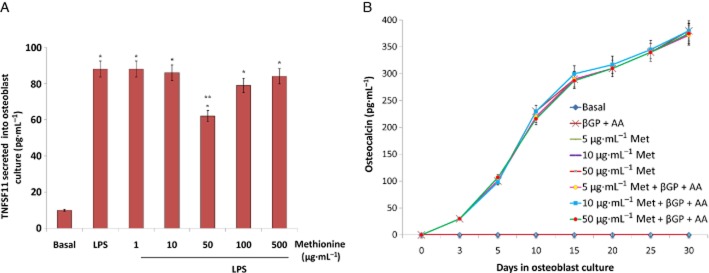
Effect of methionine on osteoblastogenesis in vitro. (A) An in vitro study showing changes in the level of soluble TNFSF11 in methionine pretreated (1–500 μg·mL−1) osteoblast cultures stimulated with LPS endotoxin (1 μg·mL−1). Osteoblast differentiation was induced by adding osteogenic media containing β-glycerophosphate (10 mM) and ascorbic acid (50 μg·mL−1) to pre-osteoblast cultures (cell line passage no. 9). Soluble TNFSF11 in cell culture supernatants was assayed by elisa. (B) Effect of methionine on osteoblast differentiation with or without β-glycerophosphate (10 mM) and ascorbic acid (50 μg·mL−1). Osteoblast differentiation was evaluated by measuring the level of osteocalcin, a bone matrix protein by elisa. Values are mean ± SEM of four independent experiments. *P < 0.05 compared with basal. **P < 0.05 compared with LPS control.
To evaluate whether methionine affected osteoblast differentiation, pre-osteoblasts were treated with methionine (5, 10 and 50 μg·mL−1) for 30 days. Ascorbic acid and β-glycerophosphate induced cell differentiation was evident on day 5 by the presence of osteocalcin, a non-collagenous bone matrix protein in culture supernatants. Methionine treatment neither induced cell differentiation (not even after 30 days) nor enhanced cell differentiation potential of ascorbic acid and β-glycerophosphate (Figure 9B).
Methionine suppresses the formation of osteoclasts from blood-derived mononuclear cells
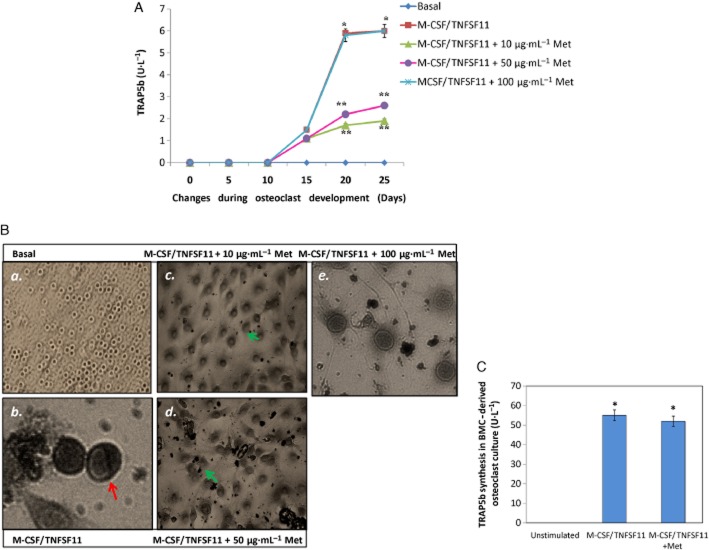
To assess the anti-osteoclastogenic potential of methionine, blood mononuclear cells (derived from normal rats) were cultured with TNFSF11 (20 μg·L−1) and M-CSF (25 μg·L−1) for 25 days. Methionine (10, 50 and 100 μg·mL−1) was added to cell culture from day 0 and replaced with fresh cell culture media every other 3 days. M-CSF/ TNFSF11-induced TRAP5b release was evident from day 15 onwards. Methionine at doses 10 and 50 μg·mL−1 inhibited osteoclast activity as evidenced by reduced TRAP5b release in cell culture supernatants (Figure 10A, B). This inhibition of TRAP5b synthesis was not observed at a concentration of 100 μg·mL−1. Interestingly methionine had no inhibitory effect on differentiated osteoclasts in vitro (Figure 10C).
Figure 10.
Effect of methionine on osteoclastogenesis in vitro. (A) Changes in the level of TRAP5b in blood borne-derived osteoclast cultures grown (developing osteoclasts) in osteoclastogenic media (M-CSF: 25 μg·L−1, days 1–11 and TNFSF11: 20 μg·L−1, days 6–20) in the presence and absence of methionine (10, 50 and 100 μg·mL−1). (B, panels a–e) Photographs showing changes in developing osteoclasts grown in osteoclastogenic media (M-CSF 25 μg·L−1, days 1–11 and TNFSF11 20 μg·L−1, days 6–20), in the presence and absence of methionine. Red arrow indicates developing osteoclast. Green arrows show absence of osteoclast morphology in methionine (10 and 50 μg·mL−1) treated cell culture. (C) TRAP5b level in matured osteoclast cultures (developed osteoclasts) in the presence and absence of methionine. Osteoclasts were grown in (M-CSF, 25 μg L−1 for days 1–11 and TNFSF11, 20 μg L−1 for days 6–20) for 25 days and then incubated with methionine (50 μg·mL−1) for 5 days. Values are mean ± SEM of four independent experiments. *P < 0.05 compared with basal. **P < 0.05 compared with M-CSF/TNFSF11 control. BMC, blood mononuclear cell.
To evaluate whether administration of methionine modulated the intrinsic ability of blood mononuclear cells to form TRAP5b-secreting osteoclasts, mononuclear cells were subjected to an osteoclastogenic environment ex vivo. Blood mononuclear cells from normal, OVX and methionine-administered rats were enriched and cultured ex vivo in the presence of 25 μg·L−1 M-CSF and 20 μg·L−1 sTNFSF11 for 25 days. Our results show that mononuclear cells from OVX rats had greater propensity to secrete TRAP5b (Figure 11) while cell cultures derived from blood mononuclear cells of methionine- and alendronate-treated rats showed much lower predisposition to form TRAP5b-secreting osteoclasts. We also observed that the cell cultures derived from methionine-treated OVX rats showed reduced levels of IL-1α, IL-1β, TNF-α and MIP-1α whereas these were significantly high in osteoclast cultures derived from OVX rats (Figure 12). The results therefore indicate that the anti-resorptive nature of methionine was more due to its ability to restrain formation of osteoclasts from blood-borne precursors than its direct effects on functional osteoclasts.
Figure 11.
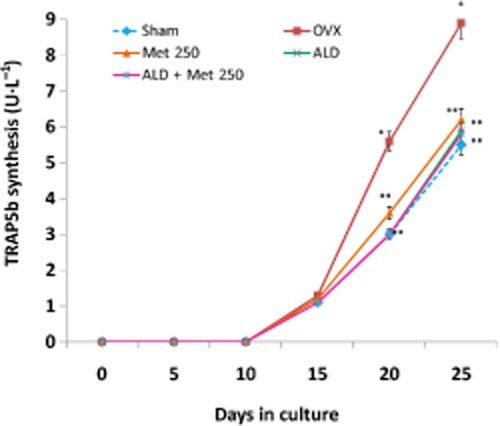
Effect of methionine on osteoclastogenesis in vivo. Methionine at a dose of 250 mg·kg−1 body wt was administered in drinking water for 10 weeks. Alendronate (200 μg·kg−1 body wt) was administered p.o. to OVX rats at an interval of 5 days for 1 month. For combination therapy, rats were administered alendronate (100 μg·kg−1 body wt) and methionine (250 mg·kg−1 body wt). Both treatments were performed 6 h apart. Blood mononuclear cells isolated from these rats were cultured in the presence of M-CSF (25 μg·L−1, days 1–11) and TNFSF11 (20 μg·L−1, days 6–20) ex vivo. Values are mean ± SEM; n = 4. *P < 0.05 compared with normal rat. **P < 0.05 compared with OVX rat.
Figure 12.
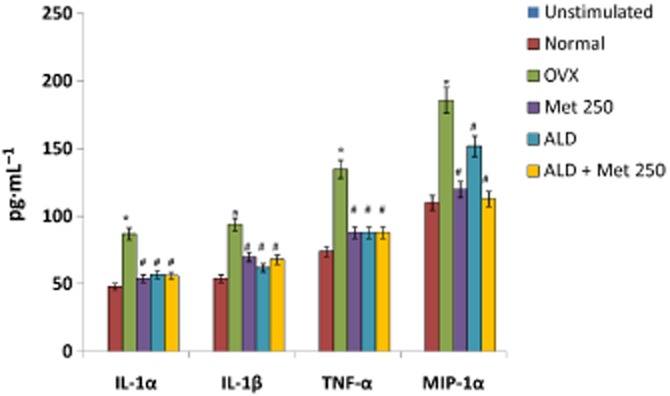
Effect of methionine on pro-inflammatory cytokine production in blood-derived osteoclast cultures ex vivo. Methionine at a dose of 250 mg·kg−1 body wt was administered in drinking water for 10 weeks. Alendronate (200 g·kg−1 body wt) was administered p.o. to OVX rats at an interval of 5 days for 1 month. For combination therapy, rats were administered alendronate (100 μg·kg−1 body wt) and methionine (250 mg·kg−1 body wt). Both treatments were performed 6 h apart. Blood mononuclear cells isolated from rats were cultured in the presence of M-CSF (25 μg L−1, days 1–11) and TNFSF11 (20 μg L−1, days 6–20) ex vivo. Pro-inflammatory cytokines in developing blood-derived osteoclast cultures were assayed by multiplex kits from Millipore on Bioplex 200™. Values are mean ± SEM; n = 4. *P < 0 05 compared with normal rat. #P < 0.05 compared with OVX rat.
Methionine inhibits TLR-4/MyD88/NF-κB signalling to reduce osteoclast formation from blood mononuclear cells
The responsiveness of mononuclear cells to differentiate to osteoclast lineage in response to a pathophysiological stimulus varies in terms of pro-inflammatory cytokine production (Itoh et al., 2003). Hence we closely evaluated changes in the developing osteoclasts at days 15–17, a time-point when mononuclear cells shifted to the osteoclast phenotype as evident from the expression of TRAP5b. During this time frame, we observed that the level of MIP-1α, a chemokine essential for osteoclast development from mononuclear cells, was significantly reduced in cell culture isolated from methionine-treated rats as compared with OVX-derived cell cultures. To elucidate the underlying molecular mechanism for this observed effect, we checked whether methionine administration modulated NF of activated T-cells (NFATc1) expression in developing osteoclasts. It was observed that methionine treatment did not induce any change in the expression status of this master regulator of osteoclastogenesis (Figure 13). Since osteoclast survival during pathological conditions also involves toll-like receptor (TLR) signalling, we monitored the changes in TLR-4 expression in developing osteoclast cultures and found that ovariectomy increased the expression of TLR4 in developing osteoclasts, a change that was not observed in sham-operated rats (Figure 14). Quantification of Western blots showed that TLR4 expression was low in developing osteoclast cultures isolated from methionine-treated rats (Figure 14). Because TLR signalling involves the recruitment of MyD88 adapter protein and finally activation of NF-κB, we checked the expression of these associated downstream signalling molecules in cell cultures by Western blot. It was observed that ovariectomy not only increased the expression of TLR4 and MyD88, but also elevated the level of nuclear p65 in the developing osteoclasts (Figure 14). Ovariectomy reduced the expression of IκB in the cytosol (Figure 14). Methionine treatment effectively down-regulated OVX-induced up-regulation of TLR4, MyD88 and nuclear localization of p65 subunit of NF-κB and restored the level of IκB in cytosol (Figure 14). Taken together, the results indicate that methionine modulated TLR4/MyD88/NF-κB signalling during osteoclastogenesis.
Figure 13.
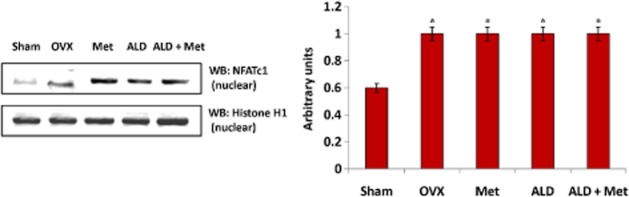
Changes in the expression of NFATc1 transcription factor in developing osteoclasts following ovariectomy and methionine treatment. Methionine at a dose of 250 mg·kg−1 body wt was administered in drinking water for 10 weeks. Alendronate (200 μg·kg−1 body wt) was administered p.o. to OVX rats at an interval of 5 days for 1 month. For combination therapy alendronate was administered at a dose of 100 μg·kg−1 body wt. Both treatments for combination therapy were performed 6 h apart. NFATc1 expression in nuclear extract of blood-derived osteoclast cultures from different experimental rats was analysed by Western blot. One of the four representative Western blots is given. Values are mean ± SEM of four independent experiments. *P < 0.05 compared with basal.
Figure 14.
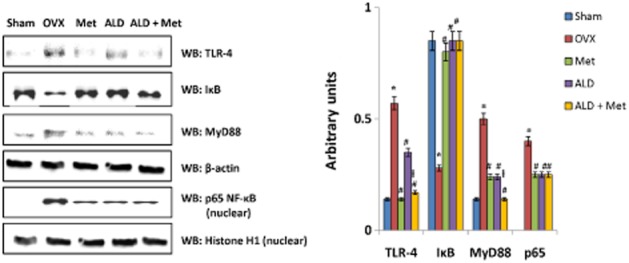
Changes in the expression of TLR4, MyD88 and NF-κB signalling factors in developing osteoclasts following ovariectomy and methionine treatment. Methionine at a dose of 250 mg·kg−1 body wt was administered in drinking water for 10 weeks. Alendronate (200 μg·kg−1 body wt) was administered p.o. to OVX rats at an interval of 5 days for 1 month. For combination therapy alendronate was administered at a dose of 100 μg·kg−1 body wt. Both treatments for combination therapy were performed 6 h apart. The expressions of TLR4, MyD88, IκB and NF-κB signalling proteins were analysed by Western blot. One of the four representative Western blots is given. *P < 0.05 compared with basal. Values are mean ± SEM; n = 4. #P < 0.05 compared with OVX rat. ƚP < 0.05 compared with alendronate (200 μg·mL−1)-treated rat.
Since MyD88 signalling was down-regulated following methionine administration, we questioned whether MyD88 has any role in the synthesis of TRAP5b or development of osteoclasts. We therefore knocked down MyD88 in developing osteoclasts (isolated from experimental rats and stimulated with TNFSF11/M-CSF) by siRNA-mediated transfection and checked osteoclast functionality by monitoring the level of TRAP5b and MIP-1α in the cell culture supernatants. As seen in Figure 15A, MyD88 siRNA transfection significantly down-regulated MyD88 expression, which was not observed in scrambled siRNA knockout cells (Figure 15A). Figure 15B shows that siRNA-mediated knockdown of MyD88 abolished TRAP5b and MIP-1α levels synthesis in culture supernatants, a phenomenon not observed in scrambled siRNA transfected cells. These results show that MyD88 is essential for the synthesis of TRAP5b and MIP-1α in developing osteoclastogenesis. To summarize: (i) OVX (oestrogen deficit condition) induces TLR4 signalling involving MyD88 in developing osteoclasts; (ii) TLR4/MyD88 signalling in developing osteoclasts is critical for secretion of factors like TRAP5b and MIP-1α; and (iii) methionine down-regulates osteoclast function by inhibiting TLR4/MyD88/NF-κB/MIP-1α signalling in developing osteoclasts.
Figure 15.
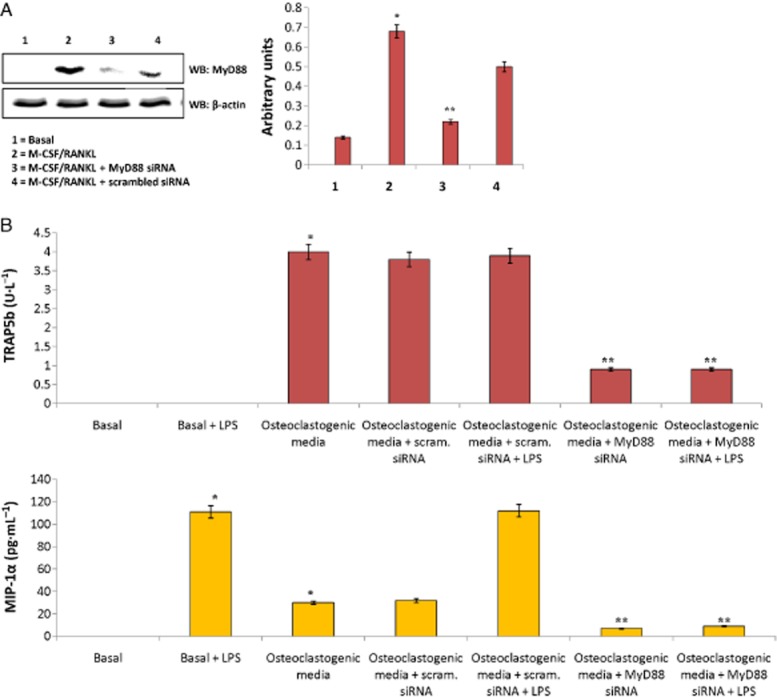
Effect of siRNA mediated knockdown of MyD88 on TRAP5b and MIP-1α synthesis in developing osteoclast culture. (A) Western blot showing changes in the expression of MyD88 before and after siRNA transfection. siRNA transfections were performed with Lipofectamine RNAiMAX. Cells after stimulation with M-CSF (25 μg·L−1, days 1–11) and TNFSF11 (20 μg·L−1, days 6–20) were transfected either with MyD88 siRNA or scrambled siRNA at concentration of 40 nmol·mL−1. Values are mean ± SEM of four independent experiments. One of the four representative Western blots is given. (B) Changes in the levels of TRAP5b and MIP-1α in response to MyD88 siRNA interference in developing osteoclasts stimulated with M-CSF (25 μg·L−1, days 1–11) and TNFSF11 (20 μg·L−1, days 6–20) ex vivo. Values are mean ± SEM of four independent experiments. *P < 0.05 compared with basal. **P < 0.05 compared with scrambled siRNA.
Co-administration of methionine with alendronate is a superior therapeutic regime for treatment of ovariectomy-induced bone loss
Although amino acid interventions alone have the potential to inhibit the progression of bone loss, it is imperative to demonstrate the potency of a combination therapy consisting of methionine together with a known anti-osteoporotic drug. We therefore investigated the potency of a combination therapy of methionine with alendronate, a nitrogen-containing bisphosphonate. Alendronate (100 μg·kg−1 body wt) administration alongside methionine (250 mg·kg−1 body wt) for 1 month significantly improved bone density and bone architecture as compared with alendronate (200 μg·kg−1 body wt) alone (Figure 5F–I). The body wt (Figure 1), serum bone protein levels (Figure 6), serum pro-osteoclastogenic cytokine levels (Figure 7) and the intrinsic ability of blood mononuclear cells to form osteoclasts (Figures 1 and 2) were observed to be near normal values in alendronate + methionine-treated rats.
Discussion
The results of our study demonstrated that administration of methionine at a dose of 250 mg kg−1 body wt effectively inhibits bone loss induced by ovariectomy. To define the cellular basis of this protection rendered by methionine, we first evaluated the effect of this amino acid on differentiated osteoclasts and osteoblasts in vitro and found that methionine posed no direct effects on mature bone cells. This apparent discrepancy prompted us to assess whether the amino acid modulated precursor forms of these cell types. The influence of methionine on osteoclast maturation was analysed by isolating blood mononuclear cells from OVX- and methionine-administered OVX rats and subjecting them to an osteoclastogenic environment, TNFSF11 and M-CSF ex vivo. Although such an ex vivo condition is quite the reverse of an in vivo scenario (e.g. presence of other cell types in the vicinity and presence of cytokines apart from TNFSF11 and M-CSF), this process enabled us to evaluate whether methionine administration during the progression of bone loss influenced the intrinsic ability of blood mononuclear cells to form osteoclasts. Blood mononuclear cells normally differentiate to macrophages or dendritic cells and form osteoclasts only in response to reparative (bone remodelling or fracture) and pathological processes (Roato et al., 2005; D'Amelio et al., 2013; Ritchlin, 2009). By employing an ELISA that specifically detected only TRAP5b (not TRAP5a by macrophages), we were able to determine the functionality of osteoclasts during the 25 days of culture (Alatalo et al., 2003). It was found that methionine treatment down-regulated the ability of blood-derived osteoclast precursors to form functional osteoclasts, as indicated by the reduced activity of TRAP5b, a protein synthesized and secreted only by matured and functional osteoclasts (Alatalo et al., 2003).
We presumed that methionine hindered a specific signalling cascade in the osteoclastogenesis process associated with TRAP5b synthesis. Osteoclastogenesis is a multistep process dependent on the cellular interaction of myeloid pre-osteoclast precursors with either osteoblasts or stromal cells under the influence of a wide range of local autocrine and/or paracrine factors such as M-CSF and TNFSF11 and several intracellular signalling molecules and transcription factors like NFATc1 and NF-κB (Yoshida et al., 1990; Lacey et al., 1998; Novack, 2011). Previous studies showed that TNFSF11 elicited by a deficiency in oestrogen epigenetically regulates osteoclast differentiation by modulating H3K27 demethylase and demethylating histone3-K27me3 in NFATc1 gene (Yasui et al., 2011). Although methionine regulated excessive TNFSF11 production, it was unable to modulate the expression of NFATc1, the master regulator of osteoclastogenesis. It, therefore, became apparent that methionine does not affect the overall function of osteoclasts (as NFATc1 is unchanged), but modulates its activity through a unique signalling mechanism. Interestingly, we found that methionine administration down-regulated ovariectomy-induced TLR4 expression in osteoclast precursors. TLR4 is a pathogen recognition receptor with roles not only in inflammation, insulin resistance and wt gain, but also in osteoclastogenesis (Shi et al., 2006). Previously it was reported that surgically-induced menopause augments TLR4 expression in the frontal cortex of the brain (Sarvari et al., 2012). In this study, an up-regulation in TLR4 expression was observed in developing osteoclasts from OVX rats. The up-regulation of this receptor may be the end-result of oestrogen deficiency alone (induced upon ovariectomy) as it was absent in sham-operated rats. Interestingly methionine treatment suppressed this augmentation in TLR4 expression. To confirm that methionine inhibited osteoclast development through TLR4 signalling, a downstream adapter protein that interacts with the conserved cytoplasmic regions of TLR4 was silenced. Knockdown of MyD88 abolished synthesis of both TRAP5b and MIP-1α, a cytokine imperative for osteoclastogenesis and confirmed the crucial role of MyD88 signalling in osteoclast development. Indeed, an earlier work of Sato et al. showed that both bone formation and resorption are reduced in MyD88–/– mice (Sato et al., 2004). Further, downstream, methionine inhibited TNFSF11-induced nuclear localization of NF-κB. NF-κB is a crucial transcription factor essential for osteoclastogenesis after the interaction of TNFSF11 with its receptor TNFRSF11A (RANK; Novack, 2011). Studies have shown that mice defective in p50 and p52 subunits of NF-κB suffer extreme osteopetrosis due to impairments in osteoclast differentiation (Iotsova et al., 1997). Our results demonstrate that methionine inhibits TRAP5b synthesis in the osteoclast by affecting TLR4/MyD88/NF-κB signalling without modulating the expression of NFATc1. Since methionine is also a proven modulator of DNA methylation (Rowan, 2006), we cannot exclude the possibility that methionine may also epigenetically regulate osteoclast development. Nevertheless further experiments are required to confirm this.
Several lines of research have shown that the maturation of pre-osteoclasts from its haematopoietic stem cells to its functional resorptive stage is regulated by an array of pro-resorptive cytokines in circulation (Fuller et al., 1993; Gao et al., 2007; Fine et al., 2009). We found that methionine induced inhibition of transdifferentiation to osteoclasts (during oestrogen deficiency) may also involve down-regulation of synthesis of pro-inflammatory cytokines such as IL-1α, IL-1β, TNF-α and MIP-1α. Among these pro-osteoclastogenic cytokines up-regulated by ovariectomy, MIP-1α is a proven stimulator of bone resorption (Choi et al., 2000; 2001; Okamatsu et al., 2004). MIP-1α, a chemotactic pro-inflammatory cytokine regulated by NF-κB possesses the ability to stimulate the formation of osteoclast-like multi-nucleated cells (Choi et al., 2001; Brueckmann et al., 2004). Oh et al. (2010) demonstrated that MIP-1 expression stimulated by TNFSF11 facilitates osteoclast survival by activating NF-κB. Our results showed that methionine administration inhibited TNFSF11-induced MIP-1α secretion by modulating MyD88 signalling, as knockdown of this adapter protein eliminated synthesis of MIP-1α in developing osteoclast cultures. These ex vivo studies indicate that pro-inflammatory cytokines also have a major role in determining the fate of the osteoclast. Accordingly, it is possible that the reduced synthesis of pro-resorptive Th1 cytokines in methionine-administered OVX rats and increased synthesis of Th2 anti-inflammatory cytokines are reasons for suppressed osteoclastogenesis.
The levels of anti-inflammatory cytokines also play vital roles in preventing disease development. It was shown earlier that IL-4 promotes the production of osteoprotegerin, a decoy receptor for TNFSF11 in osteoblasts, in addition to directly inhibiting the differentiation of osteoclast precursors towards diminished osteoclastogenesis (Yamada et al., 2007). Therefore the ability of methionine to improve anti-inflammatory cytokines such as IL-4 in circulation is another mechanism for its anti-resorptive effect.
In addition, our results showed that although methionine could unlikely have any positive effect on osteoblast differentiation, it can prevent untoward TNFSF11 induced by inflammatory agents and therefore render protection during pathological conditions. Accordingly the decreased level of TNFSF11 in the serum of methionine-treated OVX rats may be attributed to this beneficial property of methionine.
In this study, the preservation of trabecular architecture achieved with methionine treatment was comparable with that obtained with alendronate. Alendronate, a nitrogen-containing bisphosphonate with high affinity for bone, acts directly on the osteoclast and inhibits geranylgeranyl diphosphate, the substrate for prenylation of GTP-binding proteins that control cytoskeletal reorganization and vesicular fusion, processes involved in osteoclast activation (Fisher et al., 1999). The main advantage of the combination treatment (methionine + alendronate) over that of alendronate alone was that the former provided the same degree of protection as the latter, but at a lower dose. We deduce that the superior effect induced by combination therapy must be due to the direct osteoclast inhibitory effect of alendronate and an additive effect exerted by alendronate and methionine on the regulation of osteoclast formation from blood-borne precursors.
To summarize, the findings reported in this study are in concurrence with a clinical study by Pernow et al. (2010) which demonstrated that the maintenance of the correct essential/non-essential amino acid ratio is critical for preservation of bone density. Although the dose of 250 mg·kg−1 body wt used in rodents is higher than the recommended dietary supplement for humans, this dose of methionine does not induce hyperhomocysteinaemia but interestingly inhibits hyperhomocysteinaemia induced upon ovariectomy. This may be due to: (i) the antioxidant nature of methionine that nullifies the oxidative effect of homocysteine or (ii) the adequate folate (cofactor of methyl-folate-homocysteine-methyl-transferase in methionine metabolism that converts homocysteine back to methionine) available in the rodent diet (4 ppm). Beyond this dose (lower or higher), no protective effect is seen. Methionine therefore appeared to exert a biphasic effect as after a deflection point, it turned from an anti-resorptive pharmacological agent to a hyperhomocysteinemia inducing substance. Although this is an anticipated risk associated with methionine therapy, the potent anti-resorptive pharmacological effect it possesses cannot be ignored. Methionine is an essential amino acid required for the biosynthesis of many vital molecules in the body and can be acquired only through diet. Over the years, the consumption of rich sources of methionine like beans, lentils, yoghurt, onions, eggs and fish have only enriched human health. Therefore appropriate consumption of methionine-containing foods (in adequate amounts) can never pose any untoward effects but may promote bone health and serve as a prophylaxis against the development of osteoporosis. However, for therapeutic purpose in humans, the dose of methionine has to be standardized by independent human clinical trials for optimization of a dose that has anti-resorptive effects without inducing hyperhomocysteinaemia. Although a clinical study by Delrieu et al. (1988) showed that methionine does not produce any adverse effect in arthritic patients at doses 5–10 g·day−1, an effective prophylactic anti-resorptive dose of methionine in humans, remains to be established.
Conclusion
Methionine has an anti-resorptive effect by inhibiting the propensity of mononuclear cells to develop into functional osteoclasts via down-regulation of TLR4/MyD88/NF-κB signalling (Figure 16), a novel therapeutic target that can be useful for the development of treatment regimens against bone disorders.
Figure 16.
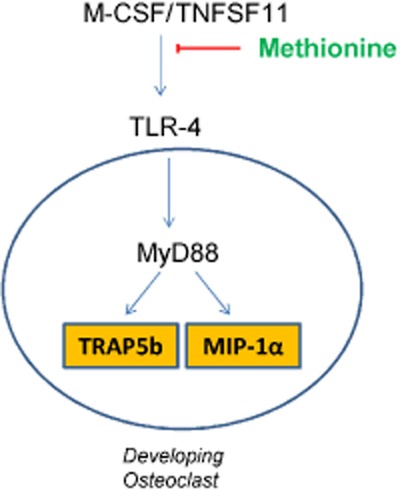
Graphical representation showing the effect of methionine on osteoclast development. Methionine inhibits the propensity of blood mononuclear cells to develop into functional osteoclasts in osteoporotic animals by down-regulation of the TLR4/MyD88/NF-κB signalling pathway.
Author contributions
VV, AS and SG developed the concept and designed the experiment.
VV performed animal experiments, X-ray imaging, elisa, cell culture experiments and interpreted the data.
MK performed animal experiments and immunoblot analysis.
KM performed animal experiments and multiplex analysis.
The article was drafted by VV, SG and AS.
Acknowledgments
The authors thank Ramprakash Singh and Jayaram V for technical assistance, Rajiv Ranjan Singh for acquiring photographs of osteoclasts and Samit Chatteerjee for help with multiplex analysis. AS is a JC Bose Fellow of the Department of Science and Technology, Government of India. VV and KM were supported with DBT-Postdoctoral Fellowship, Department of Biotechnology and Council of Scientific and Industrial Research, University Grants Commission, India, respectively. The work was financially supported by funding from National Institute of Immunology Core.
Abbreviations
- CTX
C-terminal telopeptides
- MIP-1α
macrophage inhibitory protein-1α
- MyD88
myeloid differentiation primary response gene 88
- NFATc1
nuclear factor of activated T-cells
- OVX
ovariectomized;
- TNFSF11
receptor activator of NF-κB ligand (also known as RANKL)
- TLR
toll-like receptor
- TRAP5b
tartrate-resistant acid phosphatase 5b
Conflict of interest
The authors declare that there are no conflicts of interest.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
http://dx.doi.org/10.1111/bph.12434
Appendix S1 Supplementary material.
References
- Ahmed HH, Hamza AH. Potential role of arginine, glutamine and taurine in ameliorating osteoporotic markers in ovariectomized rats. Report and Opinion. 2009;1:24–35. [Google Scholar]
- Alatalo SL, Penq Z, Janckila AJ, Kaija H, Vihko P, Vaananen HK, et al. A novel immunoassay for the determination of tartrate-resistant acid phosphatase 5b from rat serum. J Bone Miner Res. 2003;18:134–139. doi: 10.1359/jbmr.2003.18.1.134. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, et al. The Concise Guide to PHARMACOLOGY 2013/14: Overview. Br J Pharmacol. 2013;170:1449–1867. doi: 10.1111/bph.12444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antun FT, Burnett GB, Cooper AJ, Daly RJ, Smythies JR, Zealley AK. The effects of methionine on schizophrenia. J Psychatr Res. 1971;8:63–71. doi: 10.1016/0022-3956(71)90009-4. [DOI] [PubMed] [Google Scholar]
- Beral V Million Women Study Collaborators. Breast cancer and hormone replacement therapy in the Million Women Study. Lancet. 2003;362:419–427. doi: 10.1016/s0140-6736(03)14065-2. [DOI] [PubMed] [Google Scholar]
- Brandy ML. New treatment strategies: ipriflavone, strontium, vitamin D metabolites and analogs. Am J Med. 1993;95:69S–74S. doi: 10.1016/0002-9343(93)90386-4. [DOI] [PubMed] [Google Scholar]
- Bridgeman MB, Pathak R. Denosumab for the reduction of bone loss in post menopausal osteoporosis: a review. Clin Ther. 2011;33:1547–1549. doi: 10.1016/j.clinthera.2011.10.008. [DOI] [PubMed] [Google Scholar]
- Brueckmann M, Hoffmann U, Dvortsak E, Lang S, Kaden JJ, Borggrefe M, et al. Drotrecogin alfa (activated) NF-KB activation and MIP-α release from isolated mononuclear cells of patients with severe sepsis. Inflam Res. 2004;53:528–533. doi: 10.1007/s00011-004-1291-z. [DOI] [PubMed] [Google Scholar]
- Choi SJ, Cruz JC, Craig FC, Chung H, Devlin RD, Roodman D, et al. Macrophage inflammatory protein-1α is a potential osteoclast stimulatory factor in multiple myeloma. Blood. 2000;96:671–675. [PubMed] [Google Scholar]
- Choi SJ, Oba Y, Gazitt Y, Alsina M, Cruz J, Anderson J, et al. Antisense inhibition of macrophage inflammatory protein-1α blocks bone destruction in a model of myeloma bone disease. J Clin Invest. 2001;108:1833–1841. doi: 10.1172/JCI13116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordomi A, Tamayo J, Gigoux V, Fourmy D. Sulphur containing amino acids in 7-TMRs: molecular gears for pharmacology and function. Trends in Pharmacol. 2013;34:320–331. doi: 10.1016/j.tips.2013.03.008. [DOI] [PubMed] [Google Scholar]
- D'Amelio P, Grimaldi A, Di Bella S, Tamone C, Brianza SZM, Ravazzoli MGA, et al. Risedronate reduces osteoclast precursors and cytokine production in postmenopausal osteoporotic women. J Bone Mineral Res. 2008;23:373–379. doi: 10.1359/jbmr.071031. [DOI] [PubMed] [Google Scholar]
- Delrieu F, Ferrand B, Amor B. Preliminary study of L-methionine in the treatment of rheumatoid polyarthritis. Rev Rhum Mal Osteoartic. 1988;55:995–997. [PubMed] [Google Scholar]
- Ernesto C. Novel treatment for osteoporosis. J Clin Invest. 2000;106:177–179. doi: 10.1172/JCI10584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X, McDonald JM. Disorders of bone remodeling. Annu Rev Pathol. 2011;6:121–145. doi: 10.1146/annurev-pathol-011110-130203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine DH, Markowitz K, Furgang D, Fairlie K, Ferrandiz J, Nasri C. Macrophage inflammatory protein-1α: a salivary biomarker of bone loss in a longitudinal cohort study of children at risk for aggressive periodontal disease? J Periodontol. 2009;80:106–113. doi: 10.1902/jop.2009.080296. [DOI] [PubMed] [Google Scholar]
- Fini M, Torricelli P, Giavaresi G, Carpi A, Nicolini A, Giardino R. Effect of L-lysine and L-arginine on primary osteoblast cultures from normal and osteopenic rats. Biomed Pharmacother. 2001;55:213–220. doi: 10.1016/s0753-3322(01)00054-3. [DOI] [PubMed] [Google Scholar]
- Fink HA, Ewing SK, Ensrud KE, Barrett-Connor E, Taylor BC, Cauley JA, et al. Association of testosterone and estradiol deficiency with osteoporosis and rapid bone loss in older men. J Clin Endocrinol Metab. 2006;91:3908–3915. doi: 10.1210/jc.2006-0173. [DOI] [PubMed] [Google Scholar]
- Fisher JE, Rogers MJ, Halasy JM, Luckman SP, Hughes DE, Masarachia PJ, et al. Alendronate mechanism of action: geranylgeraniol, an intermediate in the mevalonate pathway, prevents inhibition of osteoclast formation, bone resorption, and kinase activation in vitro. Proc Natl Acad Sci U S A. 1999;96:133–138. doi: 10.1073/pnas.96.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller K, Owens JM, Jagger CJ, Wilson A, Moss R, Chambers TJ. Macrophage colony-stimulating factor stimulates survival and chemotactic behavior in isolated osteoclasts. J Exp Med. 1993;178:1733–1740. doi: 10.1084/jem.178.5.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Grassi F, Ryan MR, Terauchi M, Page K, Yang X, et al. IFN-γ stimulates osteoclast formation and bone loss in vivo via antigen-driven T cell activation. J Clin Invest. 2007;117:122–132. doi: 10.1172/JCI30074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa R. Management of osteoporosis, the Indian perspective. Clin Calcium. 2004;14:100–105. [PubMed] [Google Scholar]
- Iotsova V, Caamaño J, Loy J, Yang Y, Lewin A, Bravo R. Osteopetrosis in mice lacking NF-κB1 and NF-κB2. Nat Med. 1997;11:1285–1289. doi: 10.1038/nm1197-1285. [DOI] [PubMed] [Google Scholar]
- Itoh K, Udagawa N, Kobayashi K, Suda K, Li X, Takami M, et al. Lipopolysaccharide promotes the survival of osteoclasts via toll like receptor-4, but cytokine production in response to lipopolysaccharide is different from that of macrophages. J Immunol. 2003;170:3688–3695. doi: 10.4049/jimmunol.170.7.3688. [DOI] [PubMed] [Google Scholar]
- Jakob F. Primary and secondary osteoporosis. The important role of internal medicine in its differential diagnosis. Internist (Berlin) 2005;46:S24–S30. doi: 10.1007/s00108-005-1417-6. [DOI] [PubMed] [Google Scholar]
- Jiang MY, Cai DP. Oral arginine improves linear growth of long bones and neuroendocrine mechanism. Neurosci Bull. 2011;27:156–162. doi: 10.1007/s12264-011-1051-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerstetter JE, O'Brien K, Insogna KL. Dietary protein, calcium metabolism and skeletal homeostasis revisited. Am J Clin Nutr. 2003;78:584S–5592. doi: 10.1093/ajcn/78.3.584S. [DOI] [PubMed] [Google Scholar]
- Kimball SR, Jefferson LS. Molecular mechanisms through which amino acids mediate signaling through the mammalian target of rapamycin. Curr Opin Clin Nutr Metab Care. 2004;7:39–44. doi: 10.1097/00075197-200401000-00008. [DOI] [PubMed] [Google Scholar]
- Kimball SR, Jefferson LS. New functions for amino acids: effects on gene transcription and translation. Am J Clin Nutr. 2006;83:500S–507S. doi: 10.1093/ajcn/83.2.500S. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meininger V, Flamier A, Phan T, Ferris O, Uzan A, Lefur G. L-methionine treatment of Parkinson's disease: preliminary results. Rev Neurol (Paris) 1982;138:297–303. [PubMed] [Google Scholar]
- Nancy EL. Epidemiology, etiology and diagnosis of osteoporosis. Am J Obstet Gynecol. 2006;194:S3–11. doi: 10.1016/j.ajog.2005.08.047. [DOI] [PubMed] [Google Scholar]
- Novack DV. Role of NF-KB in the skeleton. Cell Res. 2011;21:169–182. doi: 10.1038/cr.2010.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh S, Sul O, Kim Y, Kim H, Yu R, Suh J, et al. Saturated fatty acids enhance osteoclast survival. J Lipid Res. 2010;51:892–899. doi: 10.1194/jlr.M800626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamatsu Y, Kim D, Battaglino R, Sasaki H, Spate U, Stashenko P. MIP-1γ, promotes receptor activator of NF-κB ligand-induced osteoclast formation and survival. J Immunol. 2004;173:2084–2090. doi: 10.4049/jimmunol.173.3.2084. [DOI] [PubMed] [Google Scholar]
- Pernow Y, Thorén M, Sääf M, Fernholm R, Anderstam B, Hauge EM, et al. Associations between amino acids and bone mineral density in men with idiopathic osteoporosis. Bone. 2010;47:959–965. doi: 10.1016/j.bone.2010.08.017. [DOI] [PubMed] [Google Scholar]
- Riggs BL, Khosla S, Melton LJ. 3rd Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev. 2002;23:279–302. doi: 10.1210/edrv.23.3.0465. [DOI] [PubMed] [Google Scholar]
- Ritchlin C. The quest for a biomarker of circulating osteoclast precursors. Arthritic Res Ther. 2009;11:113–114. doi: 10.1186/ar2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roato I, Grano M, Brunetti G, Colucci S, Mussa A, Bertetto O, et al. Mechanisms of spontaneous osteoclastogenesis in cancer with bone involvement. FASEB J. 2005;19:228–230. doi: 10.1096/fj.04-1823fje. [DOI] [PubMed] [Google Scholar]
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanic ML. Writing group for the Women's Health Initiative Investigators. Risks and benefits of estrogen plus progesterone in healthy post menopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- Rowan A. Neurogenetics: undoing epigenetics. Nat Rev Neurosci. 2006;7:3–7. [Google Scholar]
- Sarvari M, Hrabovszky E, Kallo I, Solymosi N, Liko I, Berchtold N, et al. Menopause leads to elevated expression of macrophage associated genes in the aging frontal cortex: rat and human studies identify strikingly similar changes. J Neuroinflammation. 2012;9:264–277. doi: 10.1186/1742-2094-9-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato N, Takahashi N, Suda K, Nakamura M, Yamaki M, Ninomiya T, et al. MyD88 but not TRIF is essential for osteoclastogenesis induced by lipopolysaccharide, diacyl lipopeptide and IL-1α. J Exp Med. 2004;200:601–611. doi: 10.1084/jem.20040689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman E. The structural and biomechanical basis of the gain and loss of bone strength in women and men. Endocrinol Metab Clin North Am. 2003;32:25–38. doi: 10.1016/s0889-8529(02)00078-6. [DOI] [PubMed] [Google Scholar]
- Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursini F, Pipicelli G. Nutritional supplementation for osteoarthritis: alternative and complementary therapies. Altern Complement Ther. 2009;15:173–177. [Google Scholar]
- Wang M, Yao Y, Kuang D, Hampson DR. Activation of family of G-protein coupled receptors by the tripeptide glutathione. J Biol Chem. 2006;281:8864–8870. doi: 10.1074/jbc.M512865200. [DOI] [PubMed] [Google Scholar]
- WHO Study group. Assessment of fracture risk and its applications to screening for post menopausal osteoporosis. World Health Organ Tech Rep Ser. 1994;843:1–129. [PubMed] [Google Scholar]
- Wright K. Slowing down the sands of time gerontological research, noting growth hormone, exercise, food restriction, osteoporosis and osteoarthritis. Discover. 1991;12:84–85. [Google Scholar]
- Yamada A, Takami M, Kawawa T, Yasuhara R, Zhao B, Michizuki A, et al. Interleukin-4 inhibition of osteoclast differentiation is stronger than that of interleukin-13 and they are equivalent for induction of osteoprotegerin production from osteoblasts. Immunology. 2007;120:573–579. doi: 10.1111/j.1365-2567.2006.02538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui T, Hirose J, Abutatani H, Tanaka S. Epigenetic regulation of osteoclast differentiation. Ann NY Acad Sci. 2011;1240:7–13. doi: 10.1111/j.1749-6632.2011.06245.x. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Hayashi S, Kunisada T, Ogawa M, Nishikawa S, Okamura H, et al. The murine mutation of osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature. 1990;345:442–444. doi: 10.1038/345442a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.