Abstract
BACKGROUND AND PURPOSE
Haemopressin and RVD-haemopressin, derived from the haemoglobin α-chain, are bioactive peptides found in brain and are ligands for cannabinoid CB1 receptors. Activation of brain CB1 receptors inhibited the secretion of adrenal catecholamines (noradrenaline and adrenaline) induced by i.c.v. bombesin in the rat. Here, we investigated the effects of two haemoglobin-derived peptides on this bombesin-induced response
EXPERIMENTAL APPROACH
Anaesthetised male Wistar rats were pretreated with either haemoglobin-derived peptide, given i.c.v., 30 min before i.c.v. bombesin and plasma catecholamines were subsequently measured electrochemically after HPLC. Direct effects of bombesin on secretion of adrenal catecholamines were examined using bovine adrenal chromaffin cells. Furthermore, activation of haemoglobin α-positive spinally projecting neurons in the rat hypothalamic paraventricular nucleus (PVN, a regulatory centre of central adrenomedullary outflow) after i.c.v. bombesin was assessed by immunohistochemical techniques.
KEY RESULTS
Bombesin given i.c.v. dose-dependently elevated plasma catecholamines whereas incubation with bombesin had no effect on spontaneous and nicotine-induced secretion of catecholamines from chromaffin cells. The bombesin-induced increase in catecholamines was inhibited by pretreatment with i.c.v. RVD-haemopressin (CB1 receptor agonist) but not after pretreatment with haemopressin (CB1 receptor inverse agonist). Bombesin activated haemoglobin α-positive spinally projecting neurons in the PVN.
CONCLUSIONS AND IMPLICATIONS
The haemoglobin-derived peptide RVD-haemopressin in the brain plays an inhibitory role in bombesin-induced activation of central adrenomedullary outflow via brain CB1 receptors in the rat. These findings provide basic information for the therapeutic use of haemoglobin-derived peptides in the modulation of central adrenomedullary outflow.
Keywords: haemoglobin-derived peptides, brain, cannabinoid CB1 receptor, bombesin, central adrenomedullary outflow
Introduction
In vertebrates, blood haemoglobin, a heterotetramer of two α and two β haemoglobin chains (α2β2), is responsible for the delivery of oxygen to the respiring tissues of the body, and is a constituent of red blood cells. Surprisingly, haemoglobin has been detected in a range of tissues other than erythroid cells including macrophages, alveolar epithelial cells, lens cells and isolated myelin (Liu et al., 1999; Wride et al., 2003; Newton et al., 2006; Setton-Avruj et al., 2007). Recently, expression of mRNA for haemoglobin α and β and the proteins were detected in substantia nigral, striatal, and cortical pyramidal neurons in the rat (Biagioli et al., 2009; Richter et al., 2009). These haemoglobin chains might function as precursors of numerous bioactive peptides (Gomes et al., 2010). Actually, up-regulation of haemoglobin expression and elevation of haemoglobin processing into peptides were observed in response to global ischaemia in the mouse brain (Gomes et al., 2009; He et al., 2009). These findings suggested that haemoglobin-derived peptides could be endogenous signalling molecules within the brain.
Haemopressin, a nonapeptide (PVNFKFLSH) derived from the haemoglobin α-chain, was originally isolated from rat brain homogenates and so called because it caused hypotension in the rat (Rioli et al., 2003; Blais et al., 2005). In vitro studies showed that haemopressin acts as a selective inverse agonist of cannabinoid CB1 receptors (Heimann et al., 2007), a receptor subtype preferentially expressed in the brain (Howlett et al., 2002; Pertwee et al., 2010; receptor nomenclature follows Alexander et al., 2013). In in vivo studies, centrally administered haemopressin reduced food intake via brain CB1 receptors in the rodent (Dodd et al., 2010). Later, N-terminally extended forms of haemopressin (RVD-haemopressin α, VD-haemopressin α and VD-haemopressin β) were identified in the mouse brain (Gomes et al., 2009). The former two peptides are derived from the haemoglobin α-chain while the last is derived from the β-chain (Gomes et al., 2009). These extended haemopressins (RVD- or VD-haemopressins) are postulated to represent endogenous haemoglobin-derived peptides and haemopressin itself seems likely to be generated from the longer haemopressins (Gomes et al., 2010) because the Asp-Pro peptide bond is labile under acidic conditions (Marcus, 1985), which were used in the extraction of rat brains when haemopressin was originally identified (Rioli et al., 2003). In fact, RVD-haemopressin, but not haemopressin, was found in the mouse brain and was secreted from mouse brain slices (Gelman et al., 2010; 2013). Interestingly, the extended haemopressins exhibited agonist activity at CB1 receptors (Gomes et al., 2009) in contrast to the original haemopressin, while in vitro studies recently showed that RVD-haemopressin functions as a negative allosteric modulator at these receptors (Bauer et al., 2012). Hence, the mechanisms of action of the ‘haemopressin family peptides’ on CB1 receptors are still to be established. Additionally, the distribution of peptides in the brain and physiological roles of these peptides, relative to brain CB1 receptors, are yet to be fully explored.
We previously reported that activation of brain CB1 receptors inhibited the secretion of catecholamines (noradrenaline and adrenaline) from the adrenal medulla, induced by centrally administered bombesin (a stress-related neuropeptide), in the rat (Yokotani et al., 2005; Shimizu et al., 2011). Therefore, in the present study, we hypothesized that brain ‘haemopressin family peptides’ might modulate the bombesin-induced secretion through brain CB1 receptors and we examined the central effects and the possible mechanisms of action of RVD-haemopressin and haemopressin on the elevation of plasma catecholamines induced by centrally administered bombesin in the rat.
Methods
Animals
All animal care and experimental procedures complied with the guiding principles for the care and use of laboratory animals approved by Kochi University (No. D-4, E-4 and F-3), which are in accordance with the ‘Guidelines for proper conduct of animal experiments’ from the Science Council of Japan. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010). All efforts were made to minimize the suffering of animals and the number of animals needed to obtain reliable results. A total of 84 animals were used in the experiments described here. Twelve-week-old male Wistar rats (Japan SLC Inc., Hamamatsu, Japan) weighing 300–350 g, were housed at two per cage and were maintained in an air-conditioned room at 22–24°C under a constant day-night rhythm (14/10 h light-dark cycle, lights on at 05:00) for more than 2 weeks and given food (laboratory chow, CE-2; Clea Japan, Hamamatsu, Japan) and water ad libitum.
Experimental procedures for intracerebroventricular administration
In the morning (09:00–10:00), under urethane anaesthesia (1.2 g·kg−1, i.p.), the femoral vein was cannulated for saline infusion (1.2 mL·h−1) and the femoral artery was cannulated in order to collect blood samples. Subsequently, every rat was placed in a stereotaxic apparatus (SR-6R; Narishige, Tokyo, Japan) until the end of each experiment, as described earlier (Yokotani et al., 1995; Shimizu et al., 2004). The skull was drilled for i.c.v. administration of test reagents using a stainless-steel cannula (outer diameter of 0.3 mm). The stereotaxic coordinates of the tip of the cannula were as follows (in mm): AP −0.8, L 1.5, V 4.0 (AP, anterior from the bregma; L, lateral from the midline; V, below the surface of the brain), according to the rat brain atlas (Paxinos and Watson, 2005). Three hours were allowed to elapse before any further experimental procedures.
Drug administration in vivo
Bombesin dissolved in sterile saline was slowly administered into the right lateral ventricle, in a volume of 10 μL·per animal, using a cannula connected to a 50-μL Hamilton syringe, at a rate of 10 μL·min−1, and the cannula was retained until the end of the experiment. RVD-haemopressin or haemopressin dissolved in 10 μL of sterile saline was given i.c.v. using the cannula connected to a 50 μL Hamilton syringe at a rate of 10 μL·min−1, which was retained in the ventricle for 15 min to avoid the leakage of these reagents and then removed from the ventricle. Subsequently, bombesin was slowly administered, as described above, 30 min after the application of the peptides. Rimonabant, dissolved in 3 μL of 100% N,N-dimethylformamide (DMF), was given i.c.v. using the cannula connected to a 10 μL Hamilton syringe at a rate of 10 μL·min−1, which was retained in the ventricle for 15 min to avoid the leakage of the reagent and then removed from the ventricle. Thirty minutes after this administration, RVD-haemopressin and bombesin were administered as described above. The exact location of the cannula was confirmed at the end of each experiment by confirming that Cresyl Violet, injected through the cannula, had spread throughout the entire ventricular system.
Experimental groups for i.c.v. treatments
The 81 rats placed in a stereotaxic apparatus were divided into 16 groups: bombesin treated groups at 0.1 nmol·per animal (n = 5), at 1 nmol·per animal (n = 6), and at 10 nmol·per animal (n = 4); vehicle corresponding to bombesin treated group (n = 4), at 10 μL saline·per animal; RVD-haemopressin (100 nmol·per animal) and vehicle corresponding to bombesin (10 μL saline·per animal) treated group (n = 4); vehicle corresponding to RVD-haemopressin (10 μL saline·per animal) and bombesin (1 nmol·per animal) treated group (n = 5); RVD-haemopressin (30 nmol·per animal) and bombesin (1 nmol·per animal) treated group (n = 6); RVD-haemopressin (100 nmol·per animal) and bombesin (1 nmol·per animal) treated group (n = 7); rimonabant (180 nmol·per animal), RVD-haemopressin (100 nmol·per animal) and vehicle corresponding to bombesin (10 μL saline·per animal) treated group (n = 4); vehicle corresponding to rimonabant (3 μL DMF·per animal), vehicle corresponding to RVD-haemopressin (10 μL saline·per animal) and bombesin (1 nmol·per animal) treated group (n = 6); vehicle corresponding to rimonabant (3 μL DMF·per animal), RVD-haemopressin (100 nmol·per animal) and bombesin (1 nmol·per animal) treated group (n = 7); rimonabant (90 nmol·per animal), RVD-haemopressin (100 nmol·per animal) and bombesin (1 nmol·per animal) treated group (n = 5); rimonabant (180 nmol·per animal), RVD-haemopressin (100 nmol·per animal) and bombesin (1 nmol·per animal) treated group (n = 5); haemopressin (100 nmol·per animal) and vehicle corresponding to bombesin (10 μL saline·per animal) treated group (n = 4); haemopressin (30 nmol·per animal) and bombesin (1 nmol·per animal) treated group (n = 5); haemopressin (100 nmol·per animal) and bombesin (1 nmol·per animal) treated group (n = 4).
Measurement of plasma catecholamines
Blood samples (250 μL) were collected through an arterial catheter at 0, 10, 30, 60, 90 and 120 min after the administration of bombesin or vehicle corresponding to bombesin. The samples were preserved on ice during experiments. Plasma was prepared immediately after the final sampling. Noradrenaline and adrenaline in the plasma were extracted by the method of Anton and Sayre (1962) with a slight modification and were assayed electrochemically after HPLC (Shimizu et al., 2004).
Primary culture of bovine adrenal chromaffin cells and measurement of secretion of catecholamines
Isolated bovine adrenal chromaffin cells were cultured (4 × l06 per dish, Falcon; 35 mm in diameter) under 5% CO2 and 95% air in a CO2 incubator in Eagle's minimum essential medium (Nissui Seiyaku, Tokyo, Japan) containing 10% calf serum and 3 μM cytosine arabinoside (Sigma-Aldrich, St. Louis, MO, USA) to suppress the proliferation of non-chromaffin cells (Yanagita et al., 2007; 2009; 2011). Three days after plating, the cells were incubated with or without bombesin (0.33, 3.3 or 33 μM) for up to 120 min. Then, incubation medium was transferred into a test tube for the catecholamines assay by HPLC (Yanagita et al., 2007; 2009; 2011). In the cells treated with or without bombesin (3.3 μM) for 30 min, the secretion of nicotine- (100 μM) induced catecholamines was also measured by HPLC (Yanagita et al., 2007; 2009; 2011).
Immunohistochemical study on the spinally projecting neurons of the hypothalamic paraventricular nucleus (PVN)
For labelling PVN neurons innervating the spinal cord, a mono-synaptic retrograde tracer Fluoro-Gold (Fluorochrome, Denver, CO, USA) was microinjected into the intermediolateral cell column (IML) of the thoracic spinal cord with a slight modification of previously reported methods (Viñuela and Larsen, 2001; Tanaka et al., 2012; Shimizu et al., 2013). Briefly, under pentobarbital anaesthesia (50 mg·kg−1, i.p.), three rats were placed in a stereotaxic apparatus for the spinal cord (STS-B; Narishige) until the end of surgery. The spinal cord was exposed by dorsal laminectomy through a back midline incision with an aseptic surgical procedure. Fluoro-Gold (4% in sterile saline) was bilaterally microinjected into the IML (0.5 mm lateral from the midline and 1.0 mm below the surface of the spinal cord) at the T8 level in a volume of 200 nL on each side using a 30-G stainless-steel cannula (outer diameter of 0.3 mm) at a rate of 40 nL·min−1. The muscle and skin were sutured, and the rats were returned to their home cages. The exact location of the spinal cord injection was verified by Nissl staining at the end of each experiment described below. Fourteen days after the injection of Fluoro-Gold, the rats were anaesthetized with urethane (1.2 g·kg−1, i.p.) and the femoral vein was cannulated for the infusion of saline (1.2 mL·h−1). Subsequently, the rats were placed in a stereotaxic apparatus, and the skull was drilled and bombesin (1 nmol·per animal) or vehicle (10 μL sterile saline·per animal) was given i.c.v. as described above. One hour after the administration, the rats were perfused through the left cardiac ventricle with 150 mL of 0.1 M PBS (pH 7.4) followed by 500 mL of ice-cold 4% paraformaldehyde in 0.1 M phosphate buffer. Brains and spinal cords were immediately removed, post-fixed in the same fixative overnight, equilibrated in 0.1 M phosphate buffer containing 20% sucrose at 4°C, coronally cut on a freezing cryostat (Cryostat HM505E, Thermo Scientific, Yokohama, Japan) at a thickness of 20 μm, and washed with 0.05 M Tris-buffered saline (pH 7.4). Immunohistochemical analysis was performed with a slight modification of previously reported methods (Tanaka et al., 2010; 2012). Free-floating brain sections containing PVN (−1.80 to −1.88 mm anterior from the bregma) were incubated in a mixed dilution of a goat polyclonal antibody against haemoglobin α (1:500; sc-31333; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and a rabbit polyclonal antibody against Fos (1:500; sc-52; Santa Cruz Biotechnology) for 48 h at 4°C. After washing in 0.05 M Tris-buffered saline, the sections were incubated in a mixed dilution of Cy3-conjugated donkey anti-goat IgG and FITC-conjugated donkey anti-rabbit IgG antibodies (1:1000, each; Jackson ImmunoResearch Laboratories, West Grove, PA, USA) for 2 h at room temperature in the dark, and washed again in 0.05 M Tris-buffered saline. The sections were then mounted on silane-coated slides and coverslipped with VECTASHIELD® mounting medium (Vector Laboratories, Burlingame, CA, USA). Control experiments, which were performed by omitting primary antibodies as a test of cross-reactivity of secondary antibodies, resulted in the absence of staining. Microphotographs from brain sections were captured using a digital camera (DP70; Olympus, Tokyo, Japan) attached to a fluorescent microscope (AX70; Olympus) with appropriate filter sets that allow for the separate visualization of rhodamine (for haemoglobin α-chain), FITC (for Fos) and ultraviolet excitation (for Fluoro-Gold). Fluoro-Gold-labelled neurons were visualized under ultraviolet illumination. Representative microphotographs are shown as qualitative data in the present study.
Data analysis
All values are expressed as means ± SEM. Statistical differences were determined using repeated-measures (treatment × time) or one-way anova, followed by post hoc analysis with the Bonferroni method. P-values less than 0.05 indicate statistical significance.
Materials
The following materials were used: synthetic bombesin (Peptide Institute, Osaka, Japan); rimonabant [5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-N-1-piperidinyl-1H-pyrazole-3-carboxamide] and synthetic haemopressin (Cayman Chemical, Ann Arbor, MI, USA); synthetic RVD-haemopressin (RVD-Hpα; R&D Systems, Inc., Minneapolis, MN, USA). Nicotine and all other reagents were of the highest grade available and were from Nacalai Tesque (Kyoto, Japan).
Results
Effect of centrally administered bombesin on plasma catecholamines
For plasma levels of catecholamines (noradrenaline and adrenaline), repeated-measures anova showed significant effects of treatment with bombesin [noradrenaline, F(3,15) = 37.20, P < 0.05; adrenaline, F(3,15) = 47.60, P < 0.05], time (a 120 min experimental period) [noradrenaline, F(5,72) = 31.85, P < 0.05; adrenaline, F(5,74) = 29.43, P < 0.05] and the interaction between these two factors [noradrenaline, F(15,72) = 6.52, P < 0.05; adrenaline, F(15,74) = 9.26, P < 0.05] (Figure 1A). One-way anova also revealed significant effects of the treatment on plasma catecholamines [noradrenaline, F(3,15) = 27.29, P < 0.05; adrenaline, F(3,15) = 47.39, P < 0.05] (Figure 1B). These results of anova revealed a main effect of treatment indicating that administration of bombesin significantly increased plasma catecholamines levels, and a main effect of treatment × time indicating that there was significant interaction between the administration and the administration period.
Figure 1.
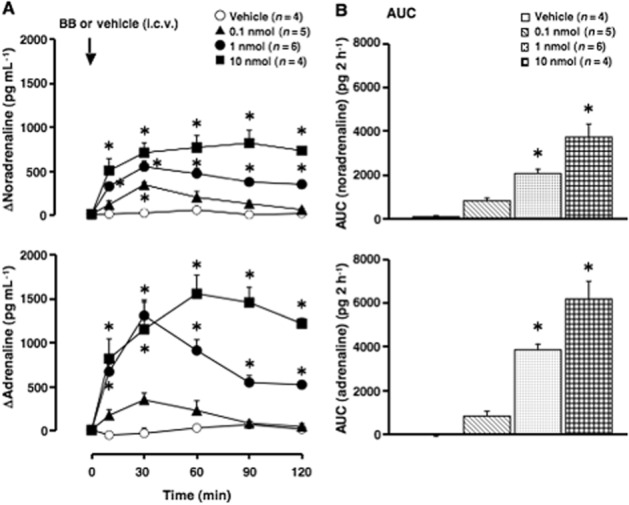
Effect of centrally administered bombesin on plasma catecholamines. Vehicle (10 μL saline·per animal) or bombesin (BB, 0.1, 1 or 10 nmol per animal) was i.c.v. administered. (A) Increments of plasma catecholamines (noradrenaline and adrenaline) above the basal level. ΔNoradrenaline and ΔAdrenaline: increments of noradrenaline and adrenaline above the basal level. Arrow indicates the administration of vehicle or bombesin. The actual values for noradrenaline and adrenaline at 0 min were 277 ± 26 and 193 ± 19 pg·mL−1 (n = 19). (B) The AUC of the elevation of plasma catecholamines above the basal level for each group is expressed as pg·2 h−1. Each point represents the mean ± SEM. *P < 0.05, significantly different from the vehicle-treated group; ANOVA, with Bonferroni post hoc test.
Treatment with vehicle (10 μL saline·per animal, i.c.v.) had no effect on the plasma levels of catecholamines (noradrenaline and adrenaline; Figure 1A and B). Bombesin [0.1, 1 and 10 nmol (0.16, 1.6 and 16 μg)·per animal, i.c.v.] dose-dependently elevated plasma catecholamines (adrenaline>noradrenaline; Figure 1A and B). The levels of catecholamine peaked at 30 min after the administration of bombesin and then declined towards their basal levels, while the highest dose of the peptide (10 nmol·per animal) increased the levels of plasma catecholamines throughout the experimental period (Figure 1A).
Effect of bombesin on the spontaneous and nicotine-induced secretion of catecholamines from cultured bovine adrenal chromaffin cells
Treatment with bombesin (0.33, 3.3 or 33 μM) for up to 120 min had no effect on the spontaneous secretion of catecholamines from the cultures of bovine adrenal chromaffin cells (Figure 2A). Bombesin did not change the content of catecholamines of the cells (data not shown). We also examined the effects of bombesin on the secretion of catecholamines induced by nicotine, an activator of nicotinic cholinoceptor ion channels (Figure 2B). Pretreatment with bombesin (3.3 μM, for 30 min) had no effect on nicotine- (100 μM) induced secretion of catecholamines.
Figure 2.
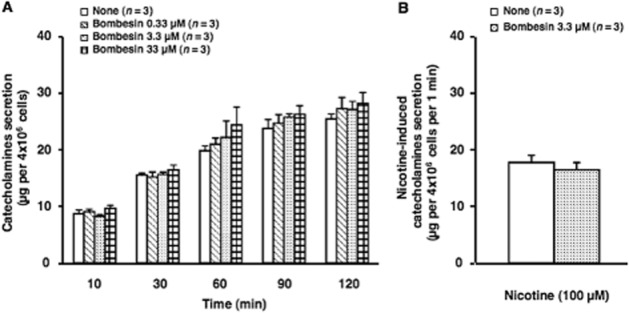
Effect of bombesin on secretion of catecholamines from cultured bovine adrenal chromaffin cells. (A) Cells were incubated with (0.33, 3.3 or 33 μM) or without (none) indicated concentrations of bombesin for up to 120 min at 37°C. Subsequently, both catecholamines secreted spontaneously in the incubation medium were measured by HPLC. Data represent the mean ± SEM. (B) Cells treated with (Bombesin) or without (none) 3.3 μM bombesin for 30 min were washed twice with normal medium, and incubated with medium at 37°C in the absence or presence of 100 μM nicotine for 5 min. Subsequently, catecholamines secreted in the incubation medium were measured. Basal values obtained at 37°C without nicotine were not changed by bombesin treatment, and were thus subtracted. Data represent the mean ± SEM.
Effect of RVD-haemopressin on the elevation of plasma catecholamines induced by centrally administered bombesin
For plasma levels of catecholamines (noradrenaline and adrenaline), repeated-measures anova showed significant effects of treatments with RVD-haemopressin and bombesin [noradrenaline, F(3,18) = 12.46, P < 0.05; adrenaline, F(3,18) = 18.64, P < 0.05], time (a 120 min experimental period) [noradrenaline, F(5,85) = 26.99, P < 0.05; adrenaline, F(5,85) = 45.35, P < 0.05] and the interaction between these two factors [noradrenaline, F(15,85) = 4.24, P < 0.05; adrenaline, F(15,85) = 6.93, P < 0.05] (Figure 3A). One-way anova also revealed significant effects of the treatments on plasma catecholamines [noradrenaline, F(3,16) = 14.47, P < 0.05; adrenaline, F(3,16) = 25.97, P < 0.05] (Figure 3B). These results of anova revealed a main effect of treatment indicating that administration of RVD-haemopressin and bombesin significantly increased plasma catecholamines levels, and a main effect of treatment × time indicating that there was significant interaction between the administration and the administration period.
Figure 3.
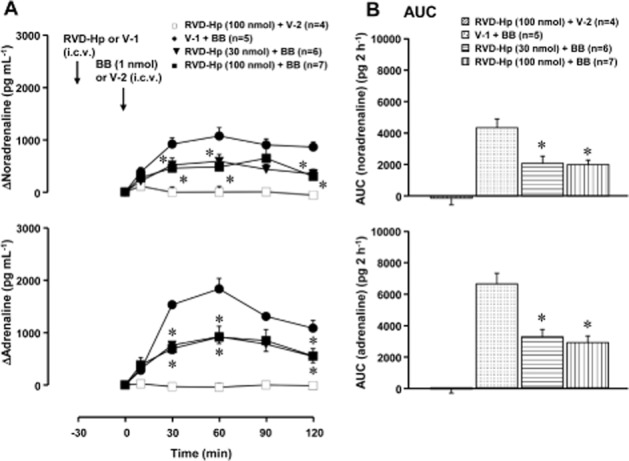
Effect of RVD-haemopressin (RVD-Hp) on the bombesin-induced elevation of plasma catecholamines. RVD-Hp (a peptide agonist of CB1 receptors; 30 or 100 nmol·per animal) or vehicle-1 (V-1; 10 μL saline·per animal) was given i.c.v. 30 min before the administration of bombesin (BB, 1 nmol·per animal, i.c.v.) or vehicle-2 (V-2; 10 μL saline·per animal, i.c.v.). (A) Increments of plasma catecholamines above the basal level. Arrows indicate the administration of RVD-Hp/V-1 and bombesin/V-2. The actual values for noradrenaline and adrenaline at 0 min were 289 ± 53 pg·mL−1 and 72 ± 10 pg·mL−1 in the V-1-pretreated group (n = 5), 494 ± 46 pg·mL−1 and 158 ± 31 pg·mL−1 in the RVD-Hp- (30 nmol·per animal) pretreated group (n = 6) and 600 ± 63 pg·mL−1 and 230 ± 53 pg·mL−1 in the RVD-Hp- (100 nmol·per animal) pretreated group (n = 11) respectively. (B) The AUC of the elevation of plasma catecholamines above the basal level for each group. *P < 0.05, significantly different from the V-1- and bombesin-treated group; ANOVA, with Bonferroni post hoc test. Other conditions are the same as those of Figure 1.
In preliminary experiaments, we found that i.c.v. treatment with vehicle-1 (10 μL saline·per animal) and vehicle-2 (10 μL saline·per animal) had no effect on plasma levels of catecholamines (data not shown). We also found that i.c.v. treatment with RVD-haemopressin [100 nmol (142 μg)·per animal] alone did not affect plasma levels of catecholamines (data not shown). Treatment with i.c.v. RVD-haemopressin (100 nmol·per animal) and vehicle-2 also had no effect on plasma catecholamines (Figure 3A and B), although the actual value for noradrenaline at 0 min was significantly elevated when pretreated with RVD-haemopressin at 100 nmol (legend of Figure 3A). Pretreatment with RVD-haemopressin [30 and 100 nmol (43 and 142 μg)·per animal, i.c.v.] dose-dependently inhibited the elevation of plasma catecholamines induced by bombesin (1 nmol·per animal, i.c.v.) (Figure 3A and B).
Effect of rimonabant on the RVD-haemopressin-induced inhibition of plasma catecholamines elevation induced by centrally administered bombesin
For plasma levels of catecholamines (noradrenaline and adrenaline), repeated measures anova showed significant effects of treatment with rimonabant, RVD-haemopressin and bombesin [noradrenaline, F(4,22) = 8.40, P < 0.05; adrenaline, F(4,22) = 15.91, P < 0.05], time (a 120 min experimental period) [noradrenaline, F(5,109) = 55.18, P < 0.05; adrenaline, F(5,106) = 44.99, P < 0.05] and the interaction between these two factors [noradrenaline, F(20,109) = 4.50, P < 0.05; adrenaline, F(20,106) = 5.12, P < 0.05] (Figure 4A). One-way anova also revealed significant effects of the treatments on plasma catecholamines [noradrenaline, F(4,22) = 8.33, P < 0.05; adrenaline, F(4,22) = 17.14, P < 0.05] (Figure 4B). These results of anova revealed a main effect of treatment indicating that administration of rimonabant, RVD-haemopressin and bombesin significantly increased plasma catecholamines levels, and a main effect of treatment × time indicating that there was significant interaction between the administration and the administration period.
Figure 4.
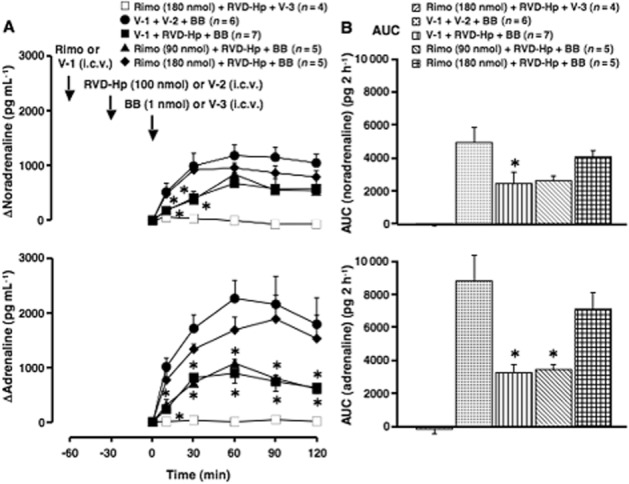
Effect of rimonabant on the inhibition by RVD-haemopressin (RVD-Hp) of the elevation of plasma catecholamines induced by centrally administered bombesin. Rimonabant (Rimo; an inverse agonist of CB1 receptors; 90 or 180 nmol·per animal) or vehicle-1 (V-1; 3 μL DMF·per animal) was given i.c.v. 30 min before the administration of RVD-Hp (a peptidic agonist of CB1 receptors; 100 nmol·per animal) or vehicle-2 (V-2; 10 μL saline·per animal). Subsequently, bombesin (BB, 1 nmol·per animal) or vehicle-3 (V-3; 10 μL saline·per animal) was given i.c.v. 30 min after the administration of the peptidic agonist. (A) Increments of plasma catecholamines above the basal level (at 0 min after the administration of bombesin or V-3). Arrows indicate the administration of Rimo/V-1, RVD-Hp/V-2 and bombesin/V-3. The actual values for noradrenaline and adrenaline at 0 min were 679 ± 93 pg·mL−1 and 415 ± 78 pg·mL−1 in the V-1- and V-2-pretreated group (n = 6), 657 ± 59 pg·mL−1 and 290 ± 33 pg·mL−1 in the V-1- and RVD-Hp-pretreated group (n = 7), 600 ± 96 pg·mL−1 and 179 ± 46 pg·mL−1 in the Rimo- (90 nmol) and RVD-Hp-pretreated group (n = 5), 624 ± 73 pg·mL−1 and 277 ± 47 pg·mL−1 in the Rimo- (180 nmol) and RVD-Hp-pretreated group (n = 9) respectively. (B) The AUC of the elevation of plasma catecholamines above the basal level for each group. *P < 0.05, significantly different from the V-1-, V-2- and bombesin-treated groups; ANOVA, with Bonferroni post hoc test. Other conditions are the same as those of Figures 1 and 3.
In control experiments, we found that i.c.v. treatment with vehicle-1 (3 μL DMF·per animal), vehicle-2 (10 μL saline·per animal) and vehicle-3 (10 μL saline·per animal) had no effect on plasma levels of catecholamines (data not shown). We also found that i.c.v. treatment with rimonabant [180 nmol (83 μg)·per animal] and RVD-haemopressin [100 nmol (142 μg)·per animal] did not affect plasma levels of catecholamines (data not shown). Treatment with rimonabant (180 nmol·per animal, i.c.v.), RVD-haemopressin (100 nmol·per animal, i.c.v.) and vehicle-3 also had no effect on the plasma levels of catecholamines (Figure 4A and B). RVD-haemopressin (100 nmol·per animal, i.c.v.) significantly inhibited bombesin- (1 nmol·per animal, i.c.v.) induced elevation of plasma catecholamines (Figure 4A and B). This inhibition by RVD-haemopressin was significantly reversed in animals pretreated with rimonabant, in a dose-dependent manner [90 and 180 nmol (42 and 83 μg)·per animal, i.c.v.] (Figure 4A and B).
Effect of haemopressin on the elevation of plasma catecholamines induced by centrally administered bombesin
For plasma levels of catecholamines (noradrenaline and adrenaline), repeated-measures anova showed significant effects of treatments with haemopressin and bombesin [noradrenaline, F(3,14) = 15.94, P < 0.05; adrenaline, F(3,14) = 29.79, P < 0.05], time (a 120 min experimental period) [noradrenaline, F(5,69) = 43.70, P < 0.05; adrenaline, F(5,65) = 30.89, P < 0.05] and the interaction between these two factors [noradrenaline, F(15,69) = 6.18, P < 0.05; adrenaline, F(15,65) = 4.23, P < 0.05] (Figure 5A). One-way anova also revealed significant effects of the treatments on plasma catecholamines [noradrenaline, F(3,12) = 12.28, P < 0.05; adrenaline, F(3,13) = 15.06, P < 0.05] (Figure 5B). These results of anova revealed a main effect of treatment indicating that administration of haemopressin and bombesin significantly increased plasma catecholamines levels, and a main effect of treatment × time indicating that there was significant interaction between the administration and the administration period.
Figure 5.
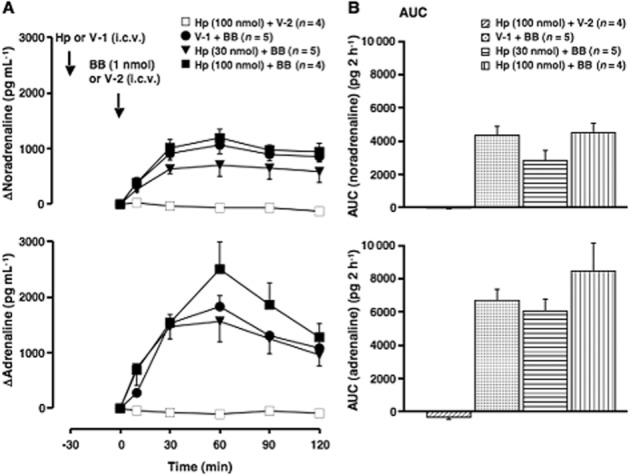
Effect of haemopressin on the elevation of plasma catecholamines induced by i.c.v. bombesin. Haemopressin (Hp; a peptide inverse agonist of CB1 receptors; 30 or 100 nmol·per animal) or vehicle-1 (V-1; 10 μL saline·per animal) was given i.c.v. 30 min before the administration of bombesin (BB, 1 nmol·per animal, i.c.v.) or vehicle-2 (V-2; 10 μL saline·per animal, i.c.v.). (A) Increments of plasma catecholamines above the basal level. Arrows indicate the administration of Hp/V-1 and bombesin/V-2. The V-1-treated group is the same as that in Figure 3A. The actual values for noradrenaline and adrenaline at 0 min were 201 ± 18 pg·mL−1 and 120 ± 35 pg·mL−1 in the Hp- (30 nmol·per animal) pretreated group (n = 5), and 532 ± 81 pg·mL−1 and 194 ± 45 pg·mL−1 in the Hp- (100 nmol·per animal) pretreated group (n = 8) respectively. (B) The AUC of the elevation of plasma catecholamines above the basal level for each group. Other conditions are the same as those of Figures 1, 3 and 4.
Treatment i.c.v. with haemopressin [100 nmol (109 μg)·per animal] alone, did not affect plasma levels of catecholamines (data not shown). Treatment with haemopressin (100 nmol·per animal) and vehicle-2 (10 μL saline·per animal) also had no effect on the plasma levels of catecholamines (Figure 5A and B). The bombesin- (1 nmol·per animal, i.c.v.) induced elevation of plasma catecholamines was not significantly influenced by haemopressin [30 and 100 nmol (33 and 109 μg)·per animal, i.c.v.] (Figure 5A and B).
Immunohistochemical identification of haemoglobin α-chain in the spinally projecting PVN neurons activated by bombesin
In the rats microinjected with a retrograde tracer (Fluoro-Gold) into the spinal cord, Fluoro-Gold-labelled neurons exhibited gold fluorescent granules in the cytoplasm with a heterogeneous distribution in the PVN. These labelled neurons were abundant in the dorsal cap and ventral part of the PVN (Figure 6A). However, Fluoro-Gold-labelled neurons were not detected in other subnuclei such as the medial and lateral parts of the PVN. Immunoreactive haemoglobin α-chain was detected in the cytoplasm of cells in the dorsal cap (Figure 6B) and ventral part (Figure 6C) of the PVN in both vehicle- (10 μL saline·per animal, i.c.v.) and bombesin- (1 nmol·per animal, i.c.v.) treated animals. In the dorsal cap and ventral part of the PVN, immunoreactive Fos was present in parts of the haemoglobin α-immunopositive cells after treatment with bombesin, but was almost absent after treatment with vehicle (Figure 6B and C). Treatment with bombesin clearly increased the number of triple-labelled (with Fluoro-Gold, haemoglobin α and Fos) neurons in the dorsal cap (Figure 6B) and ventral part (Figure 6C) of the PVN.
Figure 6.
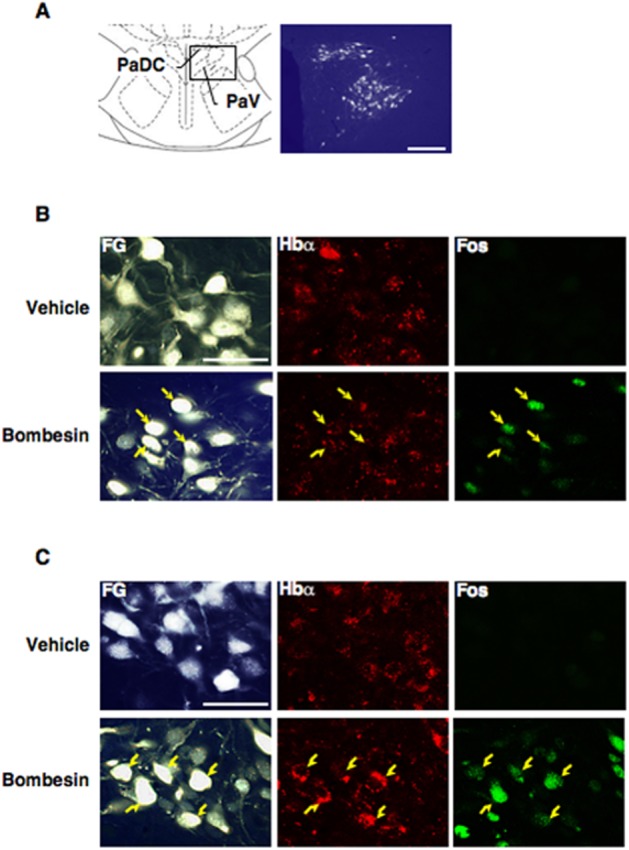
Immunohistochemical identification of haemoglobin α-chain (Hbα) on the spinally projecting hypothalamic PVN neurons activated by bombesin. (A) Left panel shows a diagram of the PVN (–1.80 mm anterior from the bregma) based on the rat brain atlas of Paxinos and Watson (2005). Enlargement of the box in the diagram is shown in the right panel as a representative microphotograph of Fluoro-Gold-labelled (FG-labelled) neurons in the PVN. The FG-labelled neurons were abundantly distributed in two PVN subdivisions, dorsal cap (PaDC) and ventral part (PaV). Scale bar = 200 μm. (B) Microphotographs present the FG-labelling (left) and the immunoreactivity of Hbα (middle) and Fos (right) in the PaDC of the vehicle- (10 μL saline per animal, i.c.v.; upper) and bombesin- (1 nmol·per animal, i.c.v.; lower) treated animals. Scale bar = 50 μm. (C) Microphotographs present the FG-labelling (left) and the immunoreactivity of Hbα (middle) and Fos (right) in the PaV of the vehicle- (upper) and bombesin- (lower) treated animals. Arrows indicate triple-labelled neurons. Scale bar = 50 μm.
Discussion and conclusions
In this study, we demonstrated that bombesin, given i.c.v., elevated plasma catecholamines via central – but not peripheral – mechanisms, and that pretreatment with i.c.v. RVD-haemopressin, but not haemopressin, inhibited the bombesin-induced response involving brain cannabinoid CB1 receptor-mediated mechanisms in the rat. Furthermore, our immunohistological results suggest that centrally administered bombesin activated the spinally projecting PVN neurons expressing the haemoglobin α-chain, a precursor of the haemopressins.
Bombesin, a tetradecapeptide originally isolated from the skin of the European frog Bombina bombina (Anastasi et al., 1971), is a ligand for bombesin receptors. This peptide is not expressed in mammals, while the mammalian counterparts and the receptors are widely distributed in the mammalian brain (Jensen et al., 2008). Using bombesin, we have examined the central regulatory mechanisms of sympatho-adrenomedullary outflow. Previously, we reported that i.c.v. bombesin induced a rise in plasma catecholamines (noradrenaline and adrenaline), which was abolished by acute bilateral adrenalectomy in the rat (Yokotani et al., 2005), suggesting that bombesin activated the central adrenomedullary outflow. In the present experiment, bombesin given i.c.v. (0.1, 1 and 10 nmol·per animal) dose-dependently elevated plasma catecholamines. Considering the volume of the cerebrospinal fluid in the rat (about 300 μL), the amounts of bombesin given i.c.v. in our study would result in a cerebrospinal fluid concentration of 0.33, 3.3 and 33 μM respectively. In an in vitro assay, treatment with 0.33 to 33 μM of bombesin had no direct effect on the spontaneous secretion of catecholamines and on the nicotine-induced secretion of catecholamines from the bovine adrenal chromaffin cells. Considering that secretion of adrenal catecholamines was evoked by activation of adrenal nicotinic cholinoceptors in vivo, these results suggested that centrally administered bombesin acted in the brain and not directly in the adrenal medulla, thereby activating preganglionic sympathetic neurons and thus stimulating release of catecholamines from the adrenal medulla. In addition, we previously reported that bombesin, given i.c.v., but not that given i.v., increased nerve activity in the adrenal branch of the splanchnic nerves in the rat (Okuma et al., 1995), and that i.v. bombesin (1 nmol·per animal) had no effect on plasma levels of catecholamines in the rat (Shimizu et al., 2013). These earlier results support the present suggestion that centrally administered bombesin acts directly in the brain.
In the brain, the CB1 receptor is one of the most abundant receptors while CB2 receptors are primarily expressed in peripheral tissues (Howlett et al., 2002; Pertwee et al., 2010). The CB receptors are activated by lipophilic compounds including the well-characterized endocannabinoids, anandamide and 2-arachidonoylglycerol (Luchicchi and Pistis, 2012). Recently, we reported that central pretreatment with AM 404, an endocannabinoid-uptake inhibitor inducing the accumulation of endocannabinoids by inhibiting cellular uptake and inactivation (Bisogno et al., 2001; Giuffrida et al., 2001), or AM 251, a synthetic inverse agonist of CB1 receptors (Lan et al., 1999), reduced or potentiated respectively, the elevation of plasma catecholamines induced by centrally administered bombesin (Shimizu et al., 2011). These results suggested that brain CB1 receptors stimulated by endocannabinoids played an endogenous inhibitory role in the bombesin-induced response. In the present experiments, centrally administered RVD-haemopressin effectively inhibited this bombesin-induced response, and this inhibition was attenuated by central treatment with rimonabant, a synthetic inverse agonist of CB1 receptors (Rinaldi-Carmona et al., 1994). These results are in agreement with our previous results showing the inhibitory effect of centrally administered 2-arachidonoylglycerol ether, an analogue of endocannabinoid, 2-arachidonoylglycerol (Hanus et al., 2001), on the bombesin-induced response with rimonabant-sensitive brain mechanisms in the rat (Shimizu et al., 2013). Taken together, our present results suggest that central administration of exogenous RVD-haemopressin inhibited the bombesin-induced activation of central adrenomedullary outflow via brain CB1 receptors, in addition to the endogenous inhibition mediated by endocannabinoid-induced stimulation of CB1 receptors in the brain.
We also examined the effect of exogenous haemopressin on this bombesin-induced elevation of plasma catecholamines. Unlike AM 251, central pretreatment with haemopressin had no potentiating effect on the bombesin-induced response. Although, in the present study, we used a sub-maximal bombesin dose (1 nmol·per animal), it is possible that haemopressin might show more obvious potentiation if we had used a much lower dose of bombesin (0.1 nmol·per animal). Recently, Bomar et al. (2012) reported that haemopressin, but not RVD-haemopressin, self-assembles to form nanostructure fibrils under physiologically relevant conditions in vitro. If this were to happen in cerebrospinal fluid, centrally administered haemopressin might lose activity against brain CB1 receptors by the formation of such fibrils. However, physiological implications of the self-assembly remain unclear. Further studies are needed to examine the physiological role of haemopressin in the bombesin-induced activation of central adrenomedullary outflow in vivo.
Finally, we examined the distribution of haemoglobin α-chain, a precursor of the haemopressin family peptides, in the hypothalamic PVN, which is considered to be a regulatory centre of the central sympatho-adrenomedullary outflow (Swanson and Sawchenko, 1980; Jansen et al., 1995). Pre-sympathetic neurons in the PVN send mono- and poly-synaptic projections to the sympathetic preganglionic neurons in the IML of the spinal cord (Pyner, 2009). The preganglionic neurons innervating the adrenal medulla are located in T7-9 levels (Pyner and Coote, 1994; 2000). Therefore, in the present study, we microinjected Fluoro-Gold, a mono-synaptic retrograde tracer, into the rat spinal cord at the T8 level for labelling the pre-sympathetic PVN neurons innervating the adrenal medulla, and subsequently examined the distribution of haemoglobin α-chain in the Fluoro-Gold-labelled neurons using immunohistochemical procedures. The Fluoro-Gold-labelled neurons were localized in the dorsal cap and ventral part of the PVN, in agreement with previous studies showing that these regions contain neurons projecting to the spinal cord (Swanson and Sawchenko, 1980; Sawchenko and Swanson, 1982). In these Fluoro-Gold-labelled spinally projecting PVN neurons, haemoglobin α was detected, and bombesin given i.c.v. actually activated the spinally projecting PVN neurons expressing haemoglobin α. These results suggest that centrally administered bombesin activated, at least in part, the spinally projecting PVN neurons expressing haemoglobin α, thereby endogenously generating haemopressin family peptides to modulate the bombesin-induced activation of central adrenomedullary outflow in the rat. Further studies are required to identify which haemopressins might be endogenously generated in the spinally projecting PVN neurons, in response to centrally administered bombesin.
In conclusion, this study demonstrates, for the first time, that a haemoglobin α-derived peptide, RVD-haemopressin, given i.c.v., inhibited the bombesin-induced activation of central adrenomedullary outflow, via brain CB1 receptor-mediated mechanisms in the rat. Recently, Dodd et al. (2013) reported that, using MRI, haemopressin given systemically did not activate the rat brain reward centres (e.g. the ventral tegmental area), which were activated by systemically administered AM 251 (a synthetic inverse agonist of CB1 receptors). Taken together, our results indicate that, unlike conventional synthetic ligands for CB1 receptors, these peptides derived from the haemoglobin α-chain may open new avenues of therapeutic intervention to modulate central adrenomedullary outflow via brain CB1 receptors, without addictive side effects.
Acknowledgments
This work was supported in part by a Grant-in-Aid for Young Scientists (B) (No. 23790745 to K. T. and No. 23790744 to T. S.) and a Grant-in-Aid for Challenging Exploratory Research (No. 25670354 to K. T.) from the Japan Society for the Promotion of Science, a grant from The Smoking Research Foundation in Japan and a Discretionary Grant of the President of the Kochi University.
Abbreviations
- DMF
N,N-dimethylformamide
- IML
intermediolateral cell column
- PVN
paraventricular nucleus
Conflict of interest
None declared.
References
- Alexander SPH, et al. The Concise Guide to PHARMACOLOGY 2013/14: Overview. Br J Pharmacol. 2013;170:1449–1867. doi: 10.1111/bph.12444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasi A, Erspamer V, Bucci M. Isolation and structure of bombesin and alytesin, 2 analogous active peptides from the skin of the European amphibians Bombina and Alytes. Experientia. 1971;27:166–167. doi: 10.1007/BF02145873. [DOI] [PubMed] [Google Scholar]
- Anton AH, Sayre DF. A study of the factors affecting the aluminum oxide-trihydroxyindole procedure for the analysis of catecholamines. J Pharmacol Exp Ther. 1962;138:360–375. [PubMed] [Google Scholar]
- Bauer M, Chicca A, Tamborrini M, Eisen D, Lerner R, Lutz B, et al. Identification and quantification of a new family of peptide endocannabinoids (Pepcans) showing negative allosteric modulation at CB1 receptors. J Biol Chem. 2012;287:36944–36967. doi: 10.1074/jbc.M112.382481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biagioli M, Pinto M, Cesselli D, Zaninello M, Lazarevic D, Roncaglia P, et al. Unexpected expression of α- and β-globin in mesencephalic dopaminergic neurons and glial cells. Proc Natl Acad Sci U S A. 2009;106:15454–15459. doi: 10.1073/pnas.0813216106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisogno T, MacCarrone M, De Petrocellis L, Jarrahian A, Finazzi-Agrò A, Hillard C, et al. The uptake by cells of 2-arachidonoylglycerol, an endogenous agonist of cannabinoid receptors. Eur J Biochem. 2001;268:1982–1989. doi: 10.1046/j.1432-1327.2001.02072.x. [DOI] [PubMed] [Google Scholar]
- Blais PA, Côté J, Morin J, Larouche A, Gendron G, Fortier A, et al. Hypotensive effects of hemopressin and bradykinin in rabbits, rats and mice. A comparative study. Peptides. 2005;26:1317–1322. doi: 10.1016/j.peptides.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Bomar MG, Samuelsson SJ, Kibler P, Kodukula K, Galande AK. Hemopressin forms self-assembled fibrillar nanostructures under physiologically relevant conditions. Biomacromolecules. 2012;13:579–583. doi: 10.1021/bm201836f. [DOI] [PubMed] [Google Scholar]
- Dodd GT, Mancini G, Lutz B, Luckman SM. The peptide hemopressin acts through CB1 cannabinoid receptors to reduce food intake in rats and mice. J Neurosci. 2010;30:7369–7376. doi: 10.1523/JNEUROSCI.5455-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd GT, Worth AA, Hodkinson DJ, Srivastava RK, Lutz B, Williams SR, et al. Central functional response to the novel peptide cannabinoid, hemopressin. Neuropharmacology. 2013;71:27–36. doi: 10.1016/j.neuropharm.2013.03.007. [DOI] [PubMed] [Google Scholar]
- Gelman JS, Sironi J, Castro LM, Ferro ES, Fricker LD. Hemopressins and other hemoglobin-derived peptides in mouse brain: comparison between brain, blood, and heart peptidome and regulation in Cpefat/fat mice. J Neurochem. 2010;113:871–880. doi: 10.1111/j.1471-4159.2010.06653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman JS, Dasgupta S, Berezniuk I, Fricker LD. Analysis of peptides secreted from cultured mouse brain tissue. Biochim Biophys Acta. 2013;1834:2408–2417. doi: 10.1016/j.bbapap.2013.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuffrida A, Beltramo M, Piomelli D. Mechanisms of endocannabinoid inactivation: biochemistry and pharmacology. J Pharmacol Exp Ther. 2001;298:7–14. [PubMed] [Google Scholar]
- Gomes I, Grushko JS, Golebiewska U, Hoogendoorn S, Gupta A, Heimann AS, et al. Novel endogenous peptide agonists of cannabinoid receptors. FASEB J. 2009;23:3020–3029. doi: 10.1096/fj.09-132142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes I, Dale CS, Casten K, Geigner MA, Gozzo FC, Ferro ES, et al. Hemoglobin-derived peptides as novel type of bioactive signaling molecules. AAPS J. 2010;12:658–669. doi: 10.1208/s12248-010-9217-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanus L, Abu-Lafi S, Fride E, Breuer A, Vogel Z, Shalev DE, et al. 2-arachidonyl glyceryl ether, an endogenous agonist of the cannabinoid CB1 receptor. Proc Natl Acad Sci USA. 2001;98:3662–3665. doi: 10.1073/pnas.061029898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Hua Y, Liu W, Hu H, Keep RF, Xi G. Effects of cerebral ischemia on neuronal hemoglobin. J Cereb Blood Flow Metab. 2009;29:596–605. doi: 10.1038/jcbfm.2008.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimann AS, Gomes I, Dale CS, Pagano RL, Gupta A, de Souza LL, et al. Hemopressin is an inverse agonist of CB1 cannabinoid receptors. Proc Natl Acad Sci U S A. 2007;104:20588–20593. doi: 10.1073/pnas.0706980105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- Jansen AS, Nguyen XV, Karpitskiy V, Mettenleiter TC, Loewy AD. Central command neurons of the sympathetic nervous system: basis of the fight-or-flight response. Science. 1995;270:644–646. doi: 10.1126/science.270.5236.644. [DOI] [PubMed] [Google Scholar]
- Jensen RT, Battey JF, Spindel ER, Benya RV. International Union of Pharmacology. LXVIII. Mammalian bombesin receptors: nomenclature, distribution, pharmacology, signaling, and functions in normal and disease states. Pharmacol Rev. 2008;60:1–42. doi: 10.1124/pr.107.07108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: Reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan R, Liu Q, Fan P, Lin S, Fernando SR, McCallion D, et al. Structure-activity relationships of pyrazole derivatives as cannabinoid receptor antagonists. J Med Chem. 1999;42:769–776. doi: 10.1021/jm980363y. [DOI] [PubMed] [Google Scholar]
- Liu L, Zeng M, Stamler JS. Hemoglobin induction in mouse macrophages. Proc Natl Acad Sci U S A. 1999;96:6643–6647. doi: 10.1073/pnas.96.12.6643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchicchi A, Pistis M. Anandamide and 2-arachidonoylglycerol: pharmacological properties, functional features, and emerging specificities of the two major endocannabinoids. Mol Neurobiol. 2012;46:374–392. doi: 10.1007/s12035-012-8299-0. [DOI] [PubMed] [Google Scholar]
- Marcus F. Preferential cleavage at aspartyl-prolyl peptide bonds in dilute acid. Int J Pept Protein Res. 1985;25:542–546. doi: 10.1111/j.1399-3011.1985.tb02208.x. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton DA, Rao KM, Dluhy RA, Baatz JE. Hemoglobin is expressed by alveolar epithelial cells. J Biol Chem. 2006;281:5668–5676. doi: 10.1074/jbc.M509314200. [DOI] [PubMed] [Google Scholar]
- Okuma Y, Yokotani K, Osumi Y. Centrally applied bombesin increases nerve activity of both sympathetic and adrenal branch of the splanchnic nerves. Jpn J Pharmacol. 1995;68:227–230. doi: 10.1254/jjp.68.227. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. In: The Rat Brain in Stereotaxic Coordinates. Paxinos G, Watson C, editors. Burlington: Elsevier Academic Press; 2005. [Google Scholar]
- Pertwee RG, Howlett AC, Abood ME, Alexander SP, Di Marzo V, Elphick MR, et al. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB1 and CB2. Pharmacol Rev. 2010;62:588–631. doi: 10.1124/pr.110.003004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyner S. Neurochemistry of the paraventricular nucleus of the hypothalamus: implications for cardiovascular regulation. J Chem Neuroanat. 2009;38:197–208. doi: 10.1016/j.jchemneu.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Pyner S, Coote JH. Evidence that sympathetic preganglionic neurones are arranged in target-specific columns in the thoracic spinal cord of the rat. J Comp Neurol. 1994;342:15–22. doi: 10.1002/cne.903420103. [DOI] [PubMed] [Google Scholar]
- Pyner S, Coote JH. Identification of branching paraventricular neurons of the hypothalamus that project to the rostroventrolateral medulla and spinal cord. Neuroscience. 2000;100:549–556. doi: 10.1016/s0306-4522(00)00283-9. [DOI] [PubMed] [Google Scholar]
- Richter F, Meurers BH, Zhu C, Medvedeva VP, Chesselet MF. Neurons express hemoglobin α- and β-chains in rat and human brains. J Comp Neurol. 2009;515:538–547. doi: 10.1002/cne.22062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldi-Carmona M, Barth F, Héaulme M, Shire D, Calandra B, Congy C, et al. SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett. 1994;350:240–244. doi: 10.1016/0014-5793(94)00773-x. [DOI] [PubMed] [Google Scholar]
- Rioli V, Gozzo FC, Heimann AS, Linardi A, Krieger JE, Shida CS, et al. Novel natural peptide substrates for endopeptidase 24.15, neurolysin, and angiotensin-converting enzyme. J Biol Chem. 2003;278:8547–8555. doi: 10.1074/jbc.M212030200. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW. Immunohistochemical identification of neurons in the paraventricular nucleus of the hypothalamus that project to the medulla or to the spinal cord in the rat. J Comp Neurol. 1982;205:260–272. doi: 10.1002/cne.902050306. [DOI] [PubMed] [Google Scholar]
- Setton-Avruj CP, Musolino PL, Salis C, Alló M, Bizzozero O, Villar MJ, et al. Presence of α-globin mRNA and migration of bone marrow cells after sciatic nerve injury suggests their participation in the degeneration/regeneration process. Exp Neurol. 2007;203:568–578. doi: 10.1016/j.expneurol.2006.09.024. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Okada S, Yamaguchi-Shima N, Yokotani K. Brain phospholipase C-diacylglycerol lipase pathway is involved in vasopressin-induced release of noradrenaline and adrenaline from adrenal medulla in rats. Eur J Pharmacol. 2004;499:99–105. doi: 10.1016/j.ejphar.2004.07.087. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Lu L, Yokotani K. Endogenously generated 2-arachidonoylglycerol plays an inhibitory role in bombesin-induced activation of central adrenomedullary outflow in rats. Eur J Pharmacol. 2011;658:123–131. doi: 10.1016/j.ejphar.2011.02.023. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Tanaka K, Yokotani K. Stimulatory and inhibitory roles of brain 2-arachidonoylglycerol in bombesin-induced central activation of adrenomedullary outflow in rats. J Pharmacol Sci. 2013;121:157–171. doi: 10.1254/jphs.12208fp. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE. Paraventricular nucleus: a site for the integration of neuroendocrine and autonomic mechanisms. Neuroendocrinology. 1980;31:410–417. doi: 10.1159/000123111. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Osako Y, Yuri K. Juvenile social experience regulates central neuropeptides relevant to emotional and social behaviors. Neuroscience. 2010;166:1036–1042. doi: 10.1016/j.neuroscience.2010.01.029. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Shimizu T, Lu L, Nakamura K, Yokotani K. Centrally administered bombesin activates COX-containing spinally projecting neurons of the PVN in anesthetized rats. Auton Neurosci. 2012;169:63–69. doi: 10.1016/j.autneu.2012.03.006. [DOI] [PubMed] [Google Scholar]
- Viñuela MC, Larsen PJ. Identification of NPY-induced c-Fos expression in hypothalamic neurones projecting to the dorsal vagal complex and the lower thoracic spinal cord. J Comp Neurol. 2001;438:286–299. doi: 10.1002/cne.1316. [DOI] [PubMed] [Google Scholar]
- Wride MA, Mansergh FC, Adams S, Everitt R, Minnema SE, Rancourt DE, et al. Expression profiling and gene discovery in the mouse lens. Mol Vis. 2003;9:360–396. [PubMed] [Google Scholar]
- Yanagita T, Maruta T, Uezono Y, Satoh S, Yoshikawa N, Nemoto T, et al. Lithium inhibits function of voltage-dependent sodium channels and catecholamine secretion independent of glycogen synthase kinase-3 in adrenal chromaffin cells. Neuropharmacology. 2007;53:881–889. doi: 10.1016/j.neuropharm.2007.08.018. [DOI] [PubMed] [Google Scholar]
- Yanagita T, Maruta T, Nemoto T, Uezono Y, Matsuo K, Satoh S, et al. Chronic lithium treatment up-regulates cell surface NaV1.7 sodium channels via inhibition of glycogen synthase kinase-3 in adrenal chromaffin cells: enhancement of Na+ influx, Ca2+ influx and catecholamine secretion after lithium withdrawal. Neuropharmacology. 2009;57:311–321. doi: 10.1016/j.neuropharm.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Yanagita T, Satoh S, Uezono Y, Matsuo K, Nemoto T, Maruta T, et al. Transcriptional up-regulation of cell surface NaV1.7 sodium channels by insulin-like growth factor-1 via inhibition of glycogen synthase kinase-3β in adrenal chromaffin cells: enhancement of 22Na+ influx, 45Ca2+ influx and catecholamine secretion. Neuropharmacology. 2011;61:1265–1274. doi: 10.1016/j.neuropharm.2011.07.029. [DOI] [PubMed] [Google Scholar]
- Yokotani K, Nishihara M, Murakami Y, Hasegawa T, Okuma Y, Osumi Y. Elevation of plasma noradrenaline levels in urethane-anaesthetized rats by activation of central prostanoid EP3 receptors. Br J Pharmacol. 1995;115:672–676. doi: 10.1111/j.1476-5381.1995.tb14985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokotani K, Okada S, Nakamura K, Yamaguchi-Shima N, Shimizu T, Arai J, et al. Brain prostanoid TP receptor-mediated adrenal noradrenaline secretion and EP3 receptor-mediated sympathetic noradrenaline release in rats. Eur J Pharmacol. 2005;512:29–35. doi: 10.1016/j.ejphar.2005.02.027. [DOI] [PubMed] [Google Scholar]


