Abstract
BACKGROUND AND PURPOSE
Hypoxia in tumours is known to cause resistance to conventional chemotherapeutic drugs. In contrast, little is known about the effects of hypoxia on targeted anti-cancer drugs. This study evaluated the effect of hypoxia on a series of clinically approved tyrosine kinase inhibitors (TKIs).
EXPERIMENTAL APPROACH
The effect of hypoxia (0.1% oxygen) on the activity of conventional cytotoxic drugs (5-fluorouracil, doxorubicin and vinblastine), the hypoxia-activated prodrug tirapazamine and 9 TKIs was determined in a panel of cell lines. Where hypoxia had a marked effect on chemosensitivity, Western blot analysis was conducted to determine the effect of hypoxia on target expression and the effect of TKIs on cell signalling response under aerobic and hypoxic conditions.
KEY RESULTS
Three patterns of chemosensitivity were observed: resistance under hypoxia, equitoxic activity against hypoxic and aerobic cells, and preferential cytotoxicity to hypoxic cells. Significant hypoxia selectivity (independent of HIF1) was observed in the case of dasatinib and this correlated with the ability of dasatinib to inhibit phosphorylation of Src at tyrosine 530. Sorafenib was significantly less effective under hypoxic conditions but resistance did not correlate with hypoxia-induced changes in Raf/MEK/ERK signalling.
CONCLUSIONS AND IMPLICATIONS
Hypoxia influences the activity of TKIs but in contrast to conventional cytotoxic drugs, preferential activity against hypoxic cells can occur. The search for hypoxia-targeted therapies has been long and fruitless and this study suggests that some clinically approved TKIs could preferentially target the hypoxic fraction of some tumour types.
Keywords: hypoxia, tyrosine kinase inhibitors, Src, dasatinib, sorafenib, HIF1
Introduction
One of the characteristic features of solid tumours is the development of a microenvironment caused by a poor and inefficient vascular supply (Vaupel and Kelleher, 2013). This physiological microenvironment is characterized by gradients of oxygen tension, nutrients, catabolites, cellular metabolites, cell proliferation and extracellular pH, all of which vary as a function of distance from a supporting blood vessel (Brown, 2002; Vaupel, 2010). The presence of hypoxia has significant biological and therapeutic implications and hypoxia is established as a marker of poor prognosis in many cancer types (Vaupel, 2008). Resistance to conventional cytotoxic anti-cancer drugs has been extensively studied and while it is widely acknowledged that resistance is caused by hypoxia-induced reduction in cell proliferation, other mechanisms are emerging (Brown, 2002; Rohwer and Cramer, 2011; Brahimi-Horn et al., 2012; Flamant et al., 2012; Onishi et al., 2012; Wu et al., 2012). In addition, the fact that hypoxic cells reside some distance away from blood vessels introduces drug delivery challenges and this further compounds the resistance of this population of cells (Heldin et al., 2004; Minchinton and Tannock, 2006). The need to develop therapeutic approaches that target the hypoxic fraction was recognized many years ago and seminal studies by Sartorelli's group showing that mitomycin C can target hypoxic cells (Kennedy et al., 1980; Sartorelli et al., 1994) led to the search for more effective hypoxia-selective drugs. Hypoxia-activated bioreductive prodrugs were actively pursued for many years and a number entered clinical trial (Wilson and Hay, 2011). However, while elegant in concept, this approach has proved extremely difficult to translate into clinical efficacy. Novel compounds are still being developed (McKeage et al., 2011; Weiss et al., 2011; Sun et al., 2012) and are in clinical trial, but the fact remains that after 40 years of research and development, no hypoxia-selective bioreductive drugs have been approved for use in humans. The need to develop hypoxia-selective drugs is even more important today as our understanding of the effect that hypoxia has on tumour biology and therapy increases.
In contrast to conventional cytotoxic drugs, little or no information has been published concerning the effects of hypoxia on targeted therapeutics. Hypoxia is known to induce a plethora of biochemical changes, many of which are mediated by hypoxia-inducible factors (Rohwer and Cramer, 2011), and signal transduction pathways are influenced by hypoxia. This is exemplified by studies demonstrating that the expression of Src and phosphorylated Src proteins is elevated in hypoxic regions of pancreatic and cervical carcinoma xenografts (Pham et al., 2009). Furthermore, studies using the Bcl-2 inhibitor ABT-737 clearly demonstrate that targeted therapeutics can selectively kill hypoxic cells (Harrison et al., 2011; Klymenko et al., 2011). The hypoxia-selective activity of ABT-737 was attributed to hypoxia-induced down-regulation of myeloid cell leukaemia sequence-1, a protein that causes resistance to ABT-737 (Harrison et al., 2011). These studies demonstrate that hypoxia can modulate the activity of targeted anti-cancer drugs. The identification of clinically used tyrosine kinase inhibitors (TKIs) that are selectively toxic to hypoxic cells would be beneficial and would stimulate the design of novel combination strategies with drugs that preferentially target the aerobic fraction. In addition, resistance of hypoxic cells to TKIs would significantly influence the outcome of chemotherapy and this knowledge could help tailor chemotherapy to individuals who are most likely to benefit from these drugs. Given the importance of TKIs and other small molecule drugs in treating cancer, it is imperative to characterize the effects of hypoxia on the activity of targeted anti-cancer drugs (Filippi et al., 2011). The aim of this study was to determine whether the response of a panel of cell lines to TKIs was influenced by hypoxia. Where differences between cellular response under aerobic and hypoxic conditions were obtained, additional studies were conducted to identify mechanisms of action.
Methods
All standard cytotoxic drugs [doxorubicin, vinblastine and 5-fluorouracil (5FU)], tirapazamine and TKIs were prepared in dimethylsulphoxide (DMSO), aliquoted and stored at −20°C until required for use. All cell culture reagents were obtained from Sigma Aldrich unless stated otherwise.
Cell lines and culture conditions
As little is known about the effects of hypoxia on TKIs, the experimental design involved the evaluation of a number of TKIs against a panel of human cancer cell lines. As the TKIs evaluated had multiple mechanisms of action, it was not feasible to select cell lines that harboured specific mutations or activated pathways. In the initial phase of the project therefore, the cell lines used were DLD-1 human colorectal carcinoma, H460 and A549 human NSCLC cells, MDA-MB-231 human breast cancer, and TK10 human renal cancer cells. An extended panel of cell lines was used to study the effects of hypoxia on the activity of dasatinib and these consisted of MDA-MB- 468, MDA-MB-453, Hs578T, BT-20, T47D and MCF7, all of which are derived from human breast carcinomas. A549, H460, MDA-MB-231, MDA-MB-453, MCF7 and TK10 cells were obtained from the National Cancer Institute (Bethesda, MD, USA). DLD-1, Hs578T, BT20, T47D and MDA-MB-468 cells were purchased from the American Type Culture Collection (LGC Standards, London, UK).
Chemosensitivity studies
Chemosensitivity under aerobic and hypoxic conditions was determined using the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyltetrazolium bromide) assay as described previously (Phillips et al., 1992). Studies under hypoxia were performed in a Whitley H35 Hypoxystation (Don Whitley Scientific, Shipley, UK). In this study, hypoxia was defined as 0.1% oxygen which is a physiologically relevant level of hypoxia that is associated with resistance to radiotherapy, the induction of HIF1 activation and unfolded protein response (Wilson and Hay, 2011). It is also important to stress that these conditions are distinct from the majority of studies on bioreductive prodrugs where conditions of anoxia are typically used to evaluate compounds (Wilson and Hay, 2011). Initial validation of the MTT assay demonstrated that a linear relationship between cell number and absorbance was obtained under both hypoxic and aerobic conditions. Following a 24 h exposure to 0.1% oxygen, linear regression analysis of the relationship between cell number and absorbance was identical to those obtained under aerobic conditions with the exception of A549 cells where r values of 4.97 × 105 and 2.02 × 105 were obtained under aerobic and hypoxic conditions respectively (data not shown). Even though the efficiency of MTT conversion to formazan was reduced in A549 cells under hypoxia, the relationship between cell number and absorbance is linear. To further confirm the suitability of the MTT assay for use under hypoxic conditions, cellular response was also determined by counting viable cells using a haemocytometer following continuous exposure to dasatinib.
For chemosensitivity studies, cells were plated into 96-well plates at a density of 2 × 103 cells per well and incubated under aerobic or hypoxic conditions (0.1% oxygen) at 37°C in a CO2-enriched atmosphere overnight to allow cells to attach. On the following day, media was removed from each well and replaced with media containing test compounds at a range of concentrations (8 wells per drug concentration). For hypoxia studies, drugs were diluted in media that had been conditioned under hypoxia for at least 24 h prior to the start of the experiment. Following a 5 days incubation at 37°C in a CO2-enriched atmosphere, MTT (0.5 mg·mL−1) was immediately added to each well and incubated for 4 h. Formazan crystals were dissolved in DMSO and the absorbance of the resulting solution was measured at 570 nm. Cell survival was determined as the true absorbance of the treated wells divided by the controls and expressed as a percentage. The effect of hypoxia on the activity of drugs was expressed as the hypoxic cytotoxicity ratio (HCR), which is defined as the ratio of IC50 values under aerobic to hypoxic conditions.
Antibodies and Western blot analysis
Antibodies to Src and p-Src (Src antibody sampler kit), ErbB (EGF) receptor and p-ErbB receptor signalling pathways (Phosph-EGFR antibody sampler kit), Raf signalling (Raf family antibody sampler kit) and phospo-ERK1/2 signalling (phosphor-ERK1/2 pathway sampler kit) were obtained from Cell Signalling Technology® (Beverly, MA, USA). Antibodies to β-actin were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Secondary antibodies were horseradish peroxidase-linked anti-mouse or anti-rabbit. To determine the effect of selected TKIs on cell signalling, cells were conditioned to hypoxia (0.1% oxygen) and normoxia for 24 h before drug exposure. Cells were exposed to a range of drug concentrations for a further 24 h before cell lysis and analysis by Western blotting. Images of immunoblots were digitally captured on Molecular Imager FX and individual bands were quantified using Quantity One software (Biorad, Hemel Hempstead, UK).
Role of HIF1 in the hypoxia-selective activity of dasatinib
HIF1α protein expression was determined by Western blot analysis following the incubation of MDA-MB-231, MDA-MB-468 and MCF7 cells in 0.1% oxygen for 24 h. Western blot analysis was performed using a purified mouse anti-HIF1α antibody obtained from BD Biosciences (Oxford, UK) and band intensities from three independent experiments were quantified using Quantity One software (Biorad). Under aerobic conditions, MDA-MB-231, MDA-MB-468 and MCF7 cells were exposed to CoCl2 for 6 h (150 μM) or 24 h (100 μM) and HIFα expression determined as above. Once HIF1α expression had been confirmed, cells were exposed to dasatinib for 1 h in the presence or absence of CoCl2 and cell survival determined using the MTT assay following a 5 days recovery period at 37°C.
Quantification of apoptosis following exposure of cells to dasatinib under aerobic and hypoxic conditions
MCF7, MDA-MB-468 and MDA-MB-231 cells were exposed to a range of dasatinib concentrations under aerobic and hypoxic conditions for 24 h. Cells were harvested by trypsinization and the percentage of cells undergoing apoptosis was determined by flow cytometry following Anexin-V-FLUOS staining (Roche, Penzberg, Germany) as described previously (Allison et al., 2009).
Analysis of data
Statistical analysis of the results was conducted using Student's t test.
Results
Chemosensitivity studies
The response of cell lines to standard classic anti-cancer drugs (doxorubicin, 5-FU and vinblastine) and the hypoxia-selective agent tirapazamine are presented in Table 1 and HCR values are presented graphically in Figure 1. The classic anti-cancer drugs tested were generally more active against cells under aerobic conditions compared with hypoxic (0.1% oxygen) conditions with some exceptions. In the case of doxorubicin, activity against MDA-MB-231 cells was similar under aerobic and hypoxic conditions (IC50 = 0.42 ± 0.01 and 0.46 ± 0.05 μM, respectively) and TK10 cells were marginally more responsive under hypoxic conditions (IC50 = 0.34 ± 0.03 and 0.26 ± 0.05 μM respectively). H460 cells were also equally sensitive to vinblastine under aerobic and hypoxic conditions (IC50 = 1.23 ± 0.03 and 1.36 ± 0.10 μM respectively). The magnitude of the effect of hypoxia on the cellular response to each drug was cell line dependent (Figure 1). The hypoxia-selective agent tirapazamine was preferentially active against all cell lines under hypoxic conditions although HCR values varied across the panel of cell lines (2.71–9.63 in MDA-MB-231 and A549 cells, respectively, Figure 1). Exposure to 0.1% oxygen induced a partial G1 arrest in the majority of cell lines within 24–48 h (data not shown). These results are consistent with reduced cell growth observed under hypoxia (data not shown) and resistance to the conventional cytotoxic drugs evaluated in this study.
Table 1.
Response of a panel of cell lines to conventional cytotoxic drugs, tirapazamine and targeted therapeutics under normoxic (N) and hypoxic (H) conditions
| Drug | A549 | H460 | MDA MB231 | DLD1 | TK10 |
|---|---|---|---|---|---|
| 5FU (N) | 2.28 ± 0.56 | 1.97 ± 1.27 | 14.61 ± 9.23 | 3.03 ± 0.59 | 41.35 ± 7.92 |
| 5FU (H) | 15.13 ± 3.72++ | 6.54 ± 1.13++ | 39.17 ± 5.21++ | 12.62 ± 3.02++ | 72.99 ± 25.38+ |
| Dox (N) | 0.25 ± 0.04 | 0.023 ± 0.006 | 0.42 ± 0.01 | 0.074 ± 0.004 | 0.34 ± 0.03 |
| Dox (H) | 1.00 ± 0.14++ | 0.066 ± 0.011+ | 0.46 ± 0.05 | 0.43 ± 0.05++ | 0.26 ± 0.05 |
| Vinblastine (N) | 0.72 ± 0.05 | 1.23 ± 0.03 | 0.55 ± 0.06 | 2.47 ± 0.79 | 2.40 ± 0.17 |
| Vinblastine (H) | 2.13 ± 0.69++ | 1.36 ± 0.10 | 0.85 ± 0.10 | 5.28 ± 0.44+ | 7.43 ± 1.43++ |
| Tirapazamine(N) | 23.13 ± 3.41 | 22.85 ± 1.12 | 7.84 ± 0.86 | 15.91 ± 1.23 | 19.69 ± 2.54 |
| Tirapazamine(H) | 2.40 ± 1.34++ | 2.53 ± 0.92++ | 2.89 ± 0.65++ | 2.95 ± 1.68++ | 3.38 ± 1.15++ |
| Sunitinib (N) | 4.82 ± 0.31 | 3.73 ± 2.00 | 8.17 ± 1.13 | 2.85 ± 0.73 | 8.02 ± 0.63 |
| Sunitinib (H) | 4.97 ± 0.42 | 2.54 ± 0.47 | 5.08 ± 0.49 | 0.50 ± 0.09++ | 5.92 ± 0.62 |
| Sorafenib (N) | 2.74 ± 1.50 | 1.42 ± 0.13 | 0.51 ± 0.34 | 1.16 ± 0.53 | 0.25 ± 0.05 |
| Sorafenib (H) | 21.63 ± 6.12++ | 5.49 ± 0.95++ | 2.10 ± 1.38++ | 2.90 ± 0.77+ | 9.02 ± 3.05++ |
| Gefitinib (N) | 23.32 ± 3.62 | 14.90 ± 1.11 | 10.62 ± 0.82 | 10.46 ± 1.94 | 0.97 ± 0.27 |
| Gefitinib (H) | 16.69 ± 2.41 | 22.34 ± 2.12++ | 11.85 ± 0.78 | 7.22 ± 1.81+ | 9.10 ± 3.50++ |
| Masatinib (N) | 18.17 ± 0.51 | 12.47 ± 1.52 | 8.39 ± 2.02 | 16.33 ± 3.10 | 14.72 ± 3.65 |
| Masatinib (H) | 18.75 ± 2.81 | 18.47 ± 1.04++ | 12.93 ± 2.23+ | 5.34 ± 0.61++ | 10.40 ± 0.78 |
| Vandetinib (N) | 7.60 ± 3.77 | 8.75 ± 0.78 | 8.12 ± 0.99 | 6.68 ± 1.90 | 2.35 ± 1.92 |
| Vandetinib (H) | 8.07 ± 0.84 | 10.54 ± 0.37 | 5.56 ± 2.56+ | 4.25 ± 0.53+ | 1.82 ± 0.39 |
| Dasatinib (N) | 0.17 ± 0.01 | 14.52 ± 1.48 | 33.0 ± 9.53* | 2.25 ± 0.04 | 3.62 ± 1.08* |
| Dasatinib (H) | 23.92 ± 8.58++ | 11.18 ± 1.27 | 6.0 ± 4.35*++ | 25.09 ± 4.43++ | 12.17 ± 2.55*++ |
| Nilotinib (N) | 7.61 ± 0.86 | 5.86 ± 1.89 | 2.81 ± 1.00 | 8.08 ± 2.64 | 1.12 ± 0.68 |
| Nilotinib (H) | 9.98 ± 1.66 | 9.04 ± 0.94+ | 1.73 ± 1.02 | 5.39 ± 0.42 | 24.53 ± 0.99++ |
| Imatinib (N) | 32.77 ± 0.85 | 23.76 ± 2.15 | 18.28 ± 0.68 | 21.58 ± 1.68 | 15.90 ± 3.17 |
| Imatinib (H) | 33.28 ± 3.85 | 22.89 ± 3.10 | 19.69 ± 1.30 | 17.54 ± 1.20 | 18.96 ± 1.71 |
| Erlotinib (N) | 3.74 ± 0.67 | 11.58 ± 0.85 | 15.70 ± 0.36 | 10.33 ± 0.65 | 4.71 ± 2.12 |
| Erlotinib (H) | 33.83 ± 3.23++ | 29.58 ± 10.99++ | 20.11 ± 0.54+ | 7.56 ± 0.81+ | 47.25 ± 8.41++ |
Cells were pre-incubated under normoxic and hypoxic (0.1% oxygen) conditions for 24 h before a 96 h exposure to each drug. Cell survival was determined using the MTT assay and results are expressed as IC50 values. The units are μM in all cases with the exception of those marked by an asterisk where the units are in nM. Each value represents the mean ± SD of three independent experiments. Statistical analysis comparing response under hypoxia to aerobic conditions was performed using Student's t-test and P-values of <0.05 and 0.01 are represented as + and ++ respectively.
Figure 1.
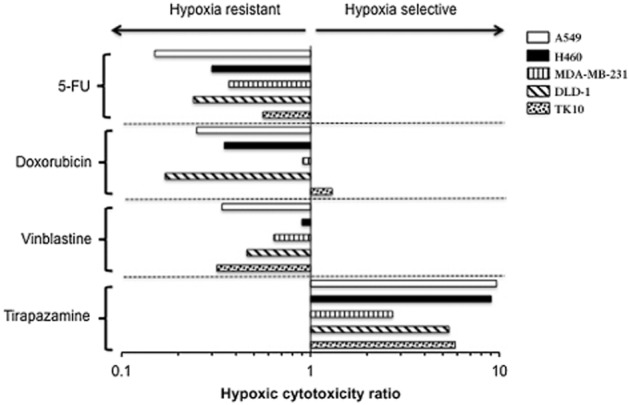
HCRs for 5FU, doxorubicin, vinblastine and tirapazamine. The centre line represents a HCR of 1, which indicates that the response of cells under aerobic and hypoxia is equal. A HCR value greater than 1 indicates sensitivity under hypoxia. HCR of less than 1 indicates resistance under hypoxia. Each HCR is calculated from mean IC50 values obtained from three independent experiments (Table 1). Experimental error in the form of standard deviations for IC50 values is presented in Table 1.
The response of cell lines to TKIs under hypoxic and aerobic conditions is reported in Table 1 and HCR values are presented in Figure 2. Three patterns of response were observed: resistance under hypoxia (HCR < 1), equipotent activity under aerobic and hypoxic conditions (HCR ≈ 1), and sensitivity under hypoxia (HCR > 1). With the exception of sorafenib, which was more resistant under hypoxia, and imatinib, which was equally active under aerobic and hypoxic conditions against all five cell lines, considerable heterogeneity in HCR values to individual drugs exists across the panel of cell lines tested (Table 1, Figure 2). This is illustrated most prominently in the case of dasatinib. Comparing the response of cells to dasatinib under aerobic and hypoxic conditions, A549 cells are resistant to dasatinib under hypoxic conditions (HCR = 0.007) whereas MDA-MB-231 cells were significantly (P < 0.01) more sensitive under hypoxia compared with oxygenated cells (HCR = 5.50). Haemocytometer counts of control and dasatinib-treated MDA-MB-231 cells gave a HCR of 6.49 providing further verification that the MTT assay is appropriate for use under these experimental conditions. Other examples where all three patterns of response exist include erlotinib, sunitinib, nilotinib and masatinib (Figure 2). In contrast to the conventional drugs tested, gefitinib, vandetinib and imatinib generally have equipotent activity under hypoxic and aerobic conditions (Table 1, Figure 2).
Figure 2.
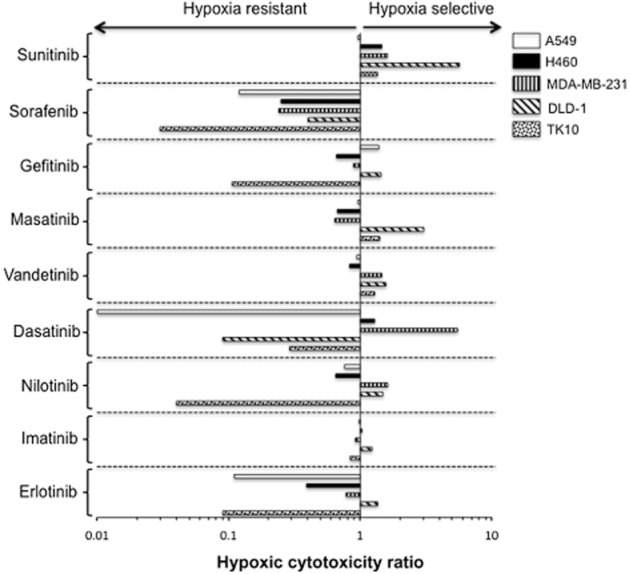
Influence of hypoxia (0.1% oxygen) on the response of a panel of human cancer cells lines to tyrosine kinase inhibitors. Each HCR is calculated from mean IC50 values obtained from three independent experiments (Table 1). Experimental error for IC50 values is presented in Table 1.
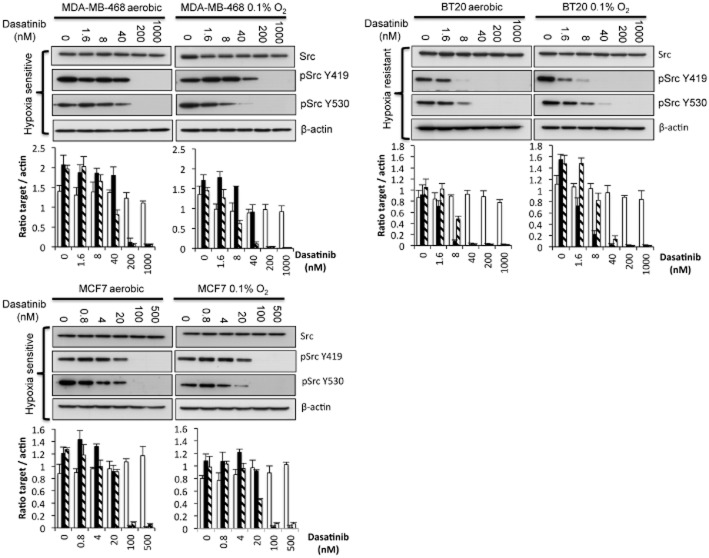
As dasatinib induced a significant increase in response under hypoxia in the MDA-MB-231 breast cancer cell line and c-Src is a potential target in breast cancer (Tryfonopoulos et al., 2011), the effect of dasatinib against an extended panel of breast cancer cell lines was determined. The results are presented in Table 2. As in the MDA-MB-231 cell line, dasatinib was significantly (P < 0.01) more potent under hypoxia against MDA-MB-468 and MCF7 cell lines with HCR values of 5.23 and 15.00 respectively. The response of MDA-MB-453 and Hs578T cells was similar under aerobic and hypoxic conditions with HCR values of 1.38 and 0.91 respectively. In contrast, T47D and BT20 cells were significantly (P < 0.01) more resistant under hypoxic conditions with HCR values of 0.62 and 0.12 respectively (Table 2). Whereas basal levels of apoptosis in MDA-MB-468 cells were similar under aerobic and hypoxic conditions, dasatinib induced significantly (P < 0.01) more apoptosis across a range of doses in hypoxic cells (Figure 3). This would account for the increased potency of dasatinib observed under hypoxia. Similar results were obtained for MDA-MB-231 and MCF7 cells (data not shown).
Table 2.
Response of a panel of breast carcinoma cell lines exposed to dasatinib under aerobic and hypoxic conditions for 96 h
| Cell line | Aerobic IC50 (μM) | Hypoxic IC50 (μM) | HCR |
|---|---|---|---|
| MCF7 | 0.18 ± 0.11 | 0.012 ± 0.007++ | 15.00 |
| MDA-MB-231 | 0.033 ± 0.009 | 0.006 ± 0.004++ | 5.50 |
| MDA-MB-468 | 16.58 ± 2.47 | 3.17 ± 1.70++ | 5.23 |
| MDA-MB-453 | 8.27 ± 3.36 | 5.97 ± 1.45 | 1.38 |
| Hs578T | 0.044 ± 0.016 | 0.048 ± 0.013 | 0.91 |
| T47D | 10.45 ± 2.98 | 16.85 ± 2.53+ | 0.62 |
| BT20 | 1.14 ± 0.59 | 9.63 ± 4.06++ | 0.12 |
Each value represents the mean ± SD of at least three independent experiments. Statistical analysis comparing response under hypoxia to aerobic conditions was performed using Student's t-test and P-values of <0.05 and 0.01 are represented as + and ++ respectively.
Figure 3.
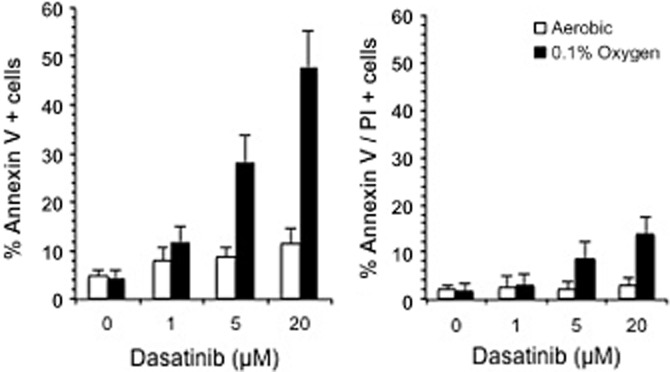
Induction of cell death following the treatment of MDA-MB-468 cells with dasatinib. Cells were exposed to dasatinib under aerobic and hypoxic (0.1% oxygen) conditions for 24 h before analysis of early apoptotic cells (annexin V positive cells) and cells undergoing late apoptosis/necrosis [annexin V and propidium iodide (PI) positive cells]. Each value represents the mean ± SD for three independent experiments.
Based on the results, three compounds were selected for further studies designed to explore the mechanistic basis for hypoxia sensitivity or resistance. These were dasatinib, erlotinib and sorafenib.
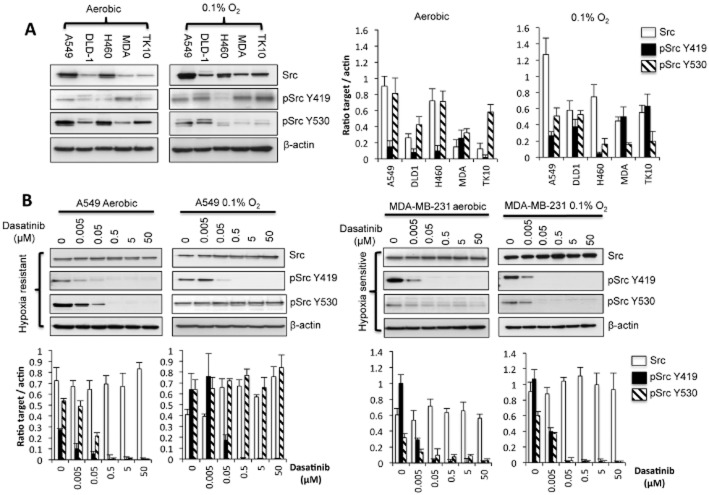
Influence of hypoxia on the expression of Src
The effect of 24 h exposure to 0.1% oxygen on Src and p-Src levels in the panel of cell lines tested is presented in Figure 4A. Under normoxic conditions, Src protein expression ranged from high (A549 and H460) to comparatively low (DLD-1, MDA-MB-231 and TK10). Exposure to hypoxia increased levels of Src expression in DLD-1, MDA MB 231, A549 and TK10 but had little effect of Src levels in H460 cells. Levels of pSrcY419 were influenced by exposure to hypoxia with increased expression observed in all cell lines except H460 cells (Figure 4A). Expression of pSrcY530 decreased under hypoxia in all cells with the exception of DLD-1 cells where hypoxia had no obvious effect on pSrcY530 expression (Figure 4A).
Figure 4.
Influence of hypoxia and dasatinib on Src and p-Src levels. The influence of a 24 h exposure to 0.1% oxygen on the levels of Src, pSrcY419 and pSrcY530 in five cell lines is presented in panel (A). In panel (A), MDA refers to the MDA-MB-231 cell line. The effect of dasatinib (in aerobic and hypoxic conditions) on Src, pSrcY419 and pSrcY530 in the hypoxia-sensitive MDA-MB-231 and hypoxia-resistant A549 cells is presented in panel (B). In both cases, cells were exposed to dasatinib for 24 h before harvesting for immunoblotting. Graphs show the quantification (±SD) of Src, pSrc Y419 and pSrc Y530 after normalization to β-actin for a minimum of n = 3 experiments.
Influence of dasatinib on Src signalling under aerobic and hypoxic conditions
The effect of dasatinib on Src and pSrc expression has been determined in hypoxia-sensitive and resistant cells taken from both the initial panel of cell lines evaluated (Figure 4B) and the extended panel of breast cancer lines (Figure 5). In the initial panel of cell lines, the effect of hypoxia on Src and p-Src expression 24 h after treatment with a range of dasatinib concentrations in A549 (hypoxia-resistant) and MDA-MB-231 (hypoxia-sensitive) cells is presented in Figure 4B. Under aerobic conditions, expression of pSrcY419 is reduced by dasatinib at doses as low as 5 nM (Figure 4B). There was no significant difference in the inhibition of pSrcY419 under hypoxia and aerobic conditions in either cell line (Figure 4B). A similar pattern of response was observed in the breast cancer panel of cells with no major differences in the ability of dasatinib to inhibit pSrcY419 expression under aerobic and hypoxic conditions (Figure 5).
Figure 5.
Influence of hypoxia and dasatinib on Src and pSrc expression in a hypoxia-sensitive and resistant breast cancer cell lines. Western blot analysis of Src, pSrcY419 and pSrcY530 following the treatment of hypoxia-sensitive (MDA-MB-468 and MCF7) and resistant (BT20) cells with dasatinib for 24 h under aerobic and hypoxic conditions. Graphs show the quantification (±SD) of Src, pSrcY419 and pSrcY530 (key to shading of columns same as Figure 4) after normalization to β-actin for a minimum of n = 3 experiments.
In contrast, differences in the ability of dasatinib to inhibit the expression of pSrcY530 between aerobic and hypoxic cells were observed. In the hypoxia-resistant A549 cells, pSrcY530 was effectively inhibited by dasatinib under aerobic conditions but not under hypoxic conditions (Figure 4B). In the hypoxia-sensitive MDA-MB-231 cells, pSrcY530 expression was preferentially inhibited by dasatinib under hypoxic conditions (Figure 4B). A similar pattern of response was observed in the extended panel breast cancer cell lines but the differential effects of dasatinib on pSrc530 expression were less extreme (Figure 5). These results suggest that the effects of dasatinib on pSrcY530 expression have a greater impact on cell response under hypoxic conditions than dephosphorylation of pSrcY419.
Role of HIF1 in the hypoxia-selective activity of dasatinib
The role of HIF1 in the hypoxic potentiation of dasatinib activity was determined in MCF7, MDA-MB-231 and MDA-MB-468 cells (Figure 6). HIF1α expression was undetectable in all three cell lines under aerobic conditions. Following a 24 h exposure to 0.1% oxygen, a prominent increase in HIF1α levels was observed in the MCF7 and MDA-MB-468 cell lines whereas only a very minor increase in HIF1α was observed in MDA-MB-231 cells (Figure 6A). Under aerobic conditions, treatment of cells with either 150 or 100 μM CoCl2 for 6 or 24 h, respectively, induced HIF1α in all three cell lines (Figure 6A). As treatment of cells with 150 μM CoCl2 for 6 h induced HIF1α expression significantly in all cell lines, these conditions were used to determine the effect of HIF1α on cellular response to dasatinib. As illustrated in Figure 6B, the response of cell lines to dasatinib in the presence of CoCl2 was reduced (MCF7) or equivalent (MDA-MB-231 and MDA-MB-468) to the response of cells treated with dasatinib alone. These results, together with the very modest induction of HIF1α under hypoxic conditions in the MDA-MB-231 cells, suggest that the hypoxia-selective activity of dasatinib is independent of HIF1.
Figure 6.
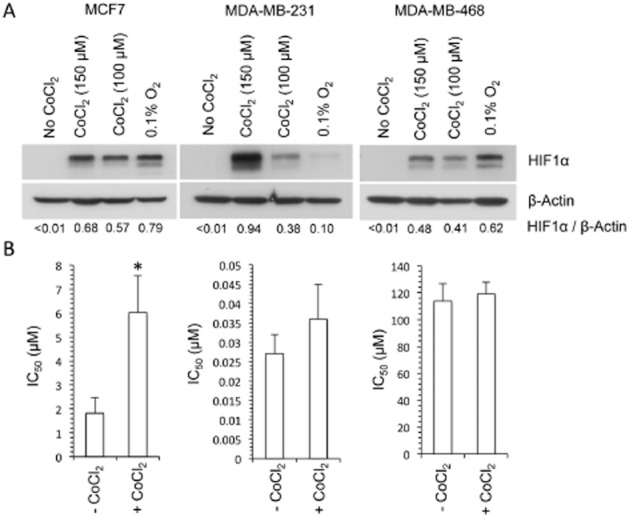
The role of HIF1 in the activity of dasatinib. Western blot analysis of the effect of hypoxia (24 h exposure to 0.1% oxygen) and CoCl2 (6 h exposure to 150 μM and 24 h exposure to 100 μM CoCl2 under aerobic conditions) on the expression of HIF1α is presented in panel (A). The values presented below each lane represent the ratio of HIF1α to β-actin expression as determined by densitometry. The response of cells to dasatinib in the absence and presence of CoCl2 is presented in panel (B). Cells were pretreated with 150 μM CoCl2 for 6 h before a 1 h exposure to dasatinib and 150 μM CoCl2. Each value presented represents the mean ± SD for three independent experiments. Statistical significance (P < 0.01) between the control (no CoCl2) and the CoCl2-treated groups is indicated by *.
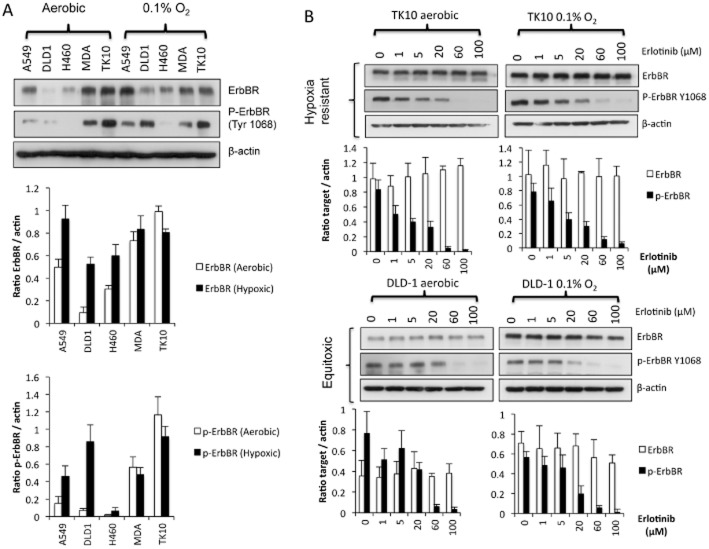
Influence of hypoxia and exposure to erlotinib on the expression of ErbB receptors
Under aerobic conditions, the expression of ErbB receptor protein expression was highest in TK10, MDA-MB-231 and A549 cells (Figure 7A). Under hypoxic conditions, there was a significant increase in ErbB receptor levels in A549, DLD-1 and H460, whereas hypoxia had little effect in MDA-MB-231 and TK10 cells (Figure 7A). The expression of phosphorylated ErbB receptor (p-ErbB receptor Y1068) shows marked differences under hypoxia. In TK10 cells, p-ErbB receptor was strongly expressed under both aerobic and hypoxic conditions, whereas in DLD-1 and A549 cells, there was a significant increase in p-ErbB receptor under hypoxic conditions (Figure 7A). No differences in the expression of p-ErbB receptor occurred between aerobic and hypoxic conditions in MDA-MB-231 cells and p-ErbB receptor was barely detectable in H460 cells under both aerobic and hypoxic conditions (Figure 7A).
Figure 7.
Influence of hypoxia and erlotinib treatment on the expression of ErbB receptors and p-ErbB receptors. The expression of ErbB receptors and p- ErbB receptors (Y1068) under aerobic conditions and following 24 h exposure to 0.1% oxygen is presented in panel (A). The expression of ErbB receptors and p- ErbB receptors in TK10 (hypoxia resistant, HCR = 0.09) and DLD-1 cells (hypoxia sensitive, HCR = 1.36) following 24 h exposure to erlotinib (1–100 μM) under aerobic and hypoxic conditions is presented in panel (B). Graphs show the quantification (±SD) of ErbB receptors and p- ErbB receptors after normalization to β-actin for a minimum of n = 3 experiments.
The effect of erlotinib on ErbB receptor and p-ErbB receptor levels in TK10 (hypoxia resistant) and DLD-1 (marginally hypoxia sensitive) is presented in Figure 7B. In the hypoxia-resistant TK10 cell line, treatment with erlotinib for 24 h did not affect the levels of ErbB receptors but caused a dose-dependent reduction in levels of p-ErbB receptors under both hypoxic and aerobic conditions (Figure 7B). Under aerobic conditions, levels of p-ErbB receptors were undetectable in cells treated with 60 μM erlotinib, whereas under hypoxic conditions, p-ErbB receptors were still detectable at 100 μM (Figure 7B). In DLD1 cells where the effects of hypoxia on the activity of erlotinib were marginally in favour of hypoxia selectivity (HCR = 1.36), no significant differences in the ability of erlotinib to inhibit p-ErbB receptors were observed, despite the increased expression of ErbB receptors under hypoxia (Figure 7B).
Relationship between cellular response to sorafenib and inhibition of Raf/MEK/ERK signalling pathways
Chemosensitivity studies demonstrated that sorafenib was the only drug that was less effective under hypoxic conditions across the panel of cell lines evaluated. HCR values ranged from 0.40 to 0.027 in DLD-1 and TK10 cells respectively (Figure 2). The influence of hypoxia on the expression of Raf/MEK/ERK signalling pathways is presented in Figure 8. Exposure to 0.1% oxygen for 24 h affects the expression of p-cRaf, p-MEK1,2, p44/42 MAPK and p90RSK in a cell line-dependent manner (Figure 8) but these changes do not correlate with the emergence of resistance to sorafenib under hypoxia.
Figure 8.
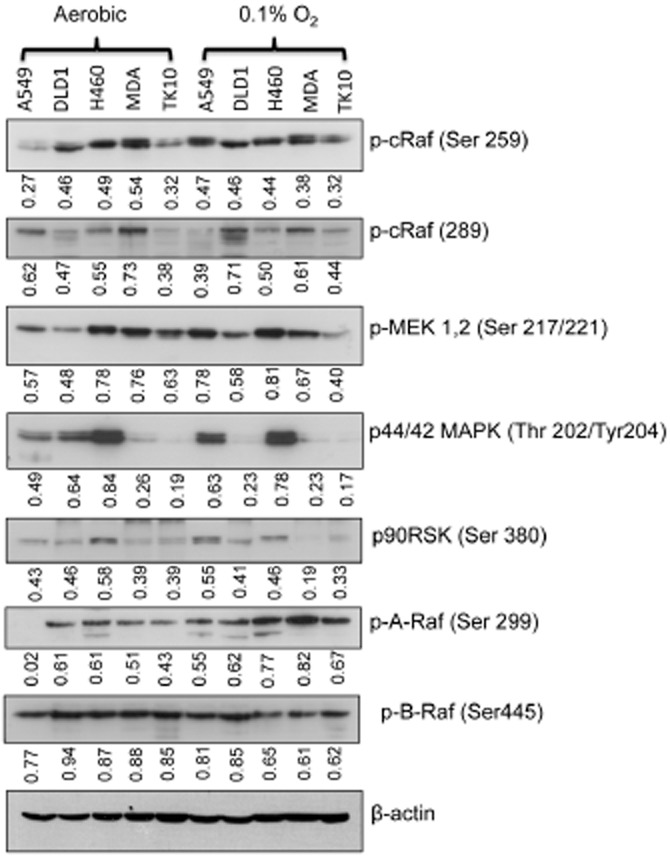
Influence of hypoxia on Raf/MEK/ERK signalling. The expression of proteins involved in Raf/MEK/ERK signalling in five cell lines was determined following a 24 h exposure to either aerobic or hypoxic (0.1% oxygen). The lane labelled MDA refers to the cell line MDA-MB-231. The values quoted directly under each blot represent quantification of band intensities after normalization to β-actin. The values presented represent the mean of three independent experiments but error bars have been omitted in the interests of clarity of presentation.
Discussion
The resistance of hypoxic cells to the classic cytotoxic anti-cancer drugs has been broadly attributed to hypoxia-induced changes in the cell cycle but more recent studies have demonstrated that other mechanisms exist (Brahimi-Horn et al., 2012; Flamant et al., 2012; Onishi et al., 2012; Wu et al., 2012). With the exception of doxorubicin that can be equally or more effective against certain cell types under hypoxic conditions (Teicher et al., 1981; Luk et al., 1990), the majority of cell lines used were resistant to classic cytotoxic drugs under hypoxic conditions (Figure 1, Table 1). In contrast, the response of cells to TKIs under aerobic and hypoxic conditions varied in a cell line-dependent manner with three main patterns of response observed (Table 1, Figure 2). These were resistance under hypoxia, equitoxic effects under aerobic and hypoxic conditions, and sensitivity under hypoxia. With the exception of sorafenib (which was resistant under hypoxia in all cell lines tested) and imatinib (which was equally active against hypoxic and aerobic cells), cellular response under hypoxia was strongly cell line dependent with all three patterns of response observed in many cases (Figure 2). Responses to dasatinib, for example, ranged from hypoxia resistance in the case of A549 cells (HCR = 0.007) to hypoxia sensitivity in MDA-MB-231, MDA-MB-468 and MCF7 cells (HCR = 5.50, 5.23 and 15.00, respectively, Figure 2, Table 2). Increased sensitivity to dasatinib under hypoxia was with increased cell kill as opposed to reduced cell growth, with a significant increase in apoptosis observed in MDA-MB-468, MDA-MB-231 and MCF7 (Figure 3 and data not shown) cell lines.
Dasatinib is an orally active inhibitor of Abl and Src kinases (Lombardo et al., 2004) and in broad agreement with previous studies (Pham et al., 2009), an increase in total Src expression was observed under hypoxic conditions in all cell lines with the exception of H460 cells (Figure 4A). Human c-Src signalling output is controlled post-translationally by phosphorylation at Y530 and Y419 (Yeatman, 2004). Phosphorylation at Y530 inactivates c-Src whereas full activation requires phosphorylation at Y419. Under hypoxic conditions, cell line-dependent changes in the expression of both pSrcY419 and pSrcY530 occurred (Figure 4A) but no clear relationship between c-Src and p-Src and hypoxia resistance or sensitivity exists (Figures 4 and 5). However, the ability of dasatinib treatment to dephosphorylate pSrcY530 did correlate with cellular response and in the hypoxia-sensitive cell lines, greater dephosphorylation of the inhibitory pSrcY530 occurred (Figures 4B and 5) making cells more sensitive to dasatinib. In the hypoxia-resistant cell lines, the dephosphorylation of pSrcY530 was reduced under hypoxia conditions (Figures 4B and 5), thereby keeping c-Src in an inactive state. The ability of dasatinib to dephosphorylate pSrcY530 (rather than pSrcY419) contributes significantly to the differential response of cells to dasatinib. Further studies to determine the effect of dasatinib on Csk and Chk proteins that inactivate Src by phosphorylation at Y530 and the activating Src activator protein tyrosine phosphatase 1B (PTP1B) (Yeatman, 2004; Roskoski, 2005) are required. Hypoxic sensitization of cells to dasatinib appears to be independent of HIF1 (Figure 6). This is consistent with other studies showing that forced expression of HIF1 repressed Src signalling output by repressing transcription of PTP1B, thereby conferring resistance to dasatinib (Suwaki et al., 2011). As cobalt chloride is known to stabilize HIF2α in aerobic conditions (Chan et al., 2002), our results (Figure 6) also suggest that HIF2 is not involved in the hypoxic sensitization of cells to dasatinib. Another potential mechanism that may influence response in normoxia and hypoxia is higher drug efflux capacity in hypoxic stem cell populations (Challen and Little, 2006). Further studies are required to verify these and other potential mechanisms of resistance.
The effect of hypoxia on ErbB receptor (EGFR) signalling pathways is of current interest (Wouters et al., 2013) and resistance to gefitinib under hypoxia has been reported in NSCLC cell lines that harbour both mutant and wild-type ErbB receptors (Minakata et al., 2012). The results of our study are broadly in agreement with that resistance to erlotinib, and gefitinib was observed in some cell lines although HCR values close to 1 were seen in others (Table 1, Figure 2). The effect of hypoxia on the expression of ErbB receptors and p- ErbB receptors varied depending on the cell line (Figure 7A) with increased expression seen in some cells (e.g. DLD1) contrasting with no significant change in other cells (e.g. TK10). Increased expression of ErbB receptors under hypoxia has been reported previously (Wang and Schneider, 2010; Misra et al., 2012), but it is not clear why induction is cell line dependent. A trend between the ability of erlotinib to suppress p- ErbB receptors and response under aerobic and hypoxic conditions exists. In hypoxia-resistant TK10 cells, reduced dephosphorylation of ErbB receptors was observed under hypoxic conditions, whereas little difference in the suppression of p- ErbB receptor expression was observed in DLD-1 cells where erlotinib was equally active against aerobic and hypoxic cells (Figure 7B). Our results further support the view that tumour hypoxia is an important factor that should be considered when treating patients (Minakata et al., 2012). Further work is required to identify the mechanisms responsible for the cell-dependent nature of the hypoxia response to ErbB receptor inhibitors.
Sorafenib inhibits Raf/MEK/ERK signalling (Liu et al., 2006) but no obvious correlation was observed between resistance under hypoxia and the expression of proteins involved in Raf/MEK/ERK signalling (Figure 8). However, sorafenib is a multi-kinase inhibitor (Matsuda and Fukumoto, 2011) and further studies are required to determine the role of Raf/MEK/ERK independent pathways in conferring hypoxia resistance. The resistance of cells under hypoxia is however potentially significant as sorafenib, by virtue of its anti-angiogenic properties, has been shown to induce hypoxia in renal cell carcinoma xenografts (Murakami et al., 2012). Recent studies have demonstrated that the development of a resistance to sorafenib is not due to permanent genetic changes or selection of resistant clones but is due to reversible changes that occur within the tumour or its microenvironment (Tang et al., 2010; Zhang et al., 2011). It is conceivable that the induction of hypoxia by sorafenib could generate a resistance phenotype, an effect that would be transient in nature depending upon oxygenation levels. Alternative dosing strategies including low-dose metronomic (Tang et al., 2010) and high-dose intermittent dosing (Wang et al., 2011) have shown improved efficacy in preclinical models, but it is not clear whether this improved efficacy is due to reducing the induction of tumour hypoxia.
In conclusion, the results of this study demonstrate that cell lines exposed to normoxic and hypoxic conditions can induce differential responses to clinically used TKIs. This includes preferential toxicity towards hypoxic cells as exemplified by dasatinib. In the context of the failure to develop therapies that selectively target hypoxic cells (Semenza, 2012), this study suggests that TKIs that show preferential toxicity towards hypoxic cells should be evaluated as potential hypoxia-selective therapies. In the case of dasatinib, the activity of single-agent dasatinib against solid tumours in the clinic has so far proved disappointing (Zhang and Yu, 2012), but further evaluation of dasatinib as a hypoxia-selective drug could be valuable. In this context, combination strategies using agents that target the aerobic fraction of tumours would be required and various preclinical studies have provided evidence that this is an attractive strategy (Montero et al., 2011; Raju et al., 2012). While translating the results of this in vitro study into in vivo efficacy against hypoxic cells is challenging, this study suggests that certain TKIs should be re-evaluated as potential hypoxia-selective therapies designed to overcome hypoxia-induced drug resistance.
Acknowledgments
The authors would like to acknowledge partial financial support from Yorkshire Cancer Research (pump priming grant BPP028).
Abbreviations
- 5FU
5-fluorouracil
- DMSO
dimethylsulphoxide
- HCR
hypoxic cytotoxicity ratio
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyltetrazolium bromide
- TKI
tyrosine kinase inhibitor
Conflict of interest
None.
References
- Allison SJ, Jiang M, Milner J. Oncogenic viral protein HPV E7 up-regulates the SIRT1 longevity protein in human cervical cancer cells. Aging. 2009;1:316–327. doi: 10.18632/aging.100028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahimi-Horn MC, Ben-Hail D, Ilie M, Gounon P, Rouleau M, Hofman V, et al. Expression of a truncated active form of VDAC1 in lung cancer associates with hypoxic cell survival and correlates with progression to chemotherapy resistance. Cancer Res. 2012;72:2140–2150. doi: 10.1158/0008-5472.CAN-11-3940. [DOI] [PubMed] [Google Scholar]
- Brown JM. Tumor microenvironment and the response to anticancer therapy. Cancer Biol Ther. 2002;1:453–458. doi: 10.4161/cbt.1.5.157. [DOI] [PubMed] [Google Scholar]
- Challen GA, Little MH. A side order of stem cells: the SP phenotype. Stem Cells. 2006;24:3–12. doi: 10.1634/stemcells.2005-0116. [DOI] [PubMed] [Google Scholar]
- Chan DA, Sutphin PD, Denko NC, Giaccia AJ. Role of prolyl hydroxylation in oncogenically stabilized hypoxia-inducible factor-1alpha. J Biol Chem. 2002;277:40112–40117. doi: 10.1074/jbc.M206922200. [DOI] [PubMed] [Google Scholar]
- Filippi I, Naldini A, Carraro F. Role of the hypoxic microenvironment in the antitumor activity of tyrosine kinase inhibitors. Curr Med Chem. 2011;18:2885–2892. doi: 10.2174/092986711796150540. [DOI] [PubMed] [Google Scholar]
- Flamant L, Roegiers E, Pierre M, Hayez A, Sterpin C, De Backer O, et al. TMEM45A is essential for hypoxia-induced chemoresistance in breast and liver cancer cells. BMC Cancer. 2012;12:391. doi: 10.1186/1471-2407-12-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison LR, Micha D, Brandenburg M, Simpson KL, Morrow CJ, Denneny O, et al. Hypoxic human cancer cells are sensitized to BH-3 mimetic-induced apoptosis via downregulation of the Bcl-2 protein Mcl-1. J Clin Invest. 2011;121:1075–1087. doi: 10.1172/JCI43505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin CH, Rubin K, Pietras K, Ostman A. High interstitial fluid pressure – an obstacle in cancer therapy. Nat Rev Cancer. 2004;4:806–813. doi: 10.1038/nrc1456. [DOI] [PubMed] [Google Scholar]
- Kennedy KA, Rockwell S, Sartorelli AC. Preferential activation of mitomycin C to cytotoxic metabolites by hypoxic tumor cells. Cancer Res. 1980;40:2356–2360. [PubMed] [Google Scholar]
- Klymenko T, Brandenburg M, Morrow C, Dive C, Makin G. The novel Bcl-2 inhibitor ABT-737 is more effective in hypoxia and is able to reverse hypoxia-induced drug resistance in neuroblastoma cells. Mol Cancer Ther. 2011;10:2373–2383. doi: 10.1158/1535-7163.MCT-11-0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Cao Y, Chen C, Zhang X, McNabola A, Wilkie D, et al. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. 2006;66:11851–11858. doi: 10.1158/0008-5472.CAN-06-1377. [DOI] [PubMed] [Google Scholar]
- Lombardo LJ, Lee FY, Chen P, Norris D, Barrish JC, Behnia K, et al. Discovery of N-(2-chloro-6-methyl- phenyl)-2-(6-(4-(2-hydroxyethyl)- piperazin-1-yl)-2-methylpyrimidin-4- ylamino)thiazole-5-carboxamide (BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays. J Med Chem. 2004;47:6658–6661. doi: 10.1021/jm049486a. [DOI] [PubMed] [Google Scholar]
- Luk CK, Veinot-Drebot L, Tjan E, Tannock IF. Effect of transient hypoxia on sensitivity to doxorubicin in human and murine cell lines. J Natl Cancer Inst. 1990;82:684–692. doi: 10.1093/jnci/82.8.684. [DOI] [PubMed] [Google Scholar]
- McKeage MJ, Gu Y, Wilson WR, Hill A, Amies K, Melink TJ, et al. A phase I trial of PR-104, a pre-prodrug of the bioreductive prodrug PR-104A, given weekly to solid tumour patients. BMC Cancer. 2011;11:432. doi: 10.1186/1471-2407-11-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda Y, Fukumoto M. Sorafenib: complexities of Raf-dependent and Raf-independent signaling are now unveiled. Med Mol Morphol. 2011;44:183–189. doi: 10.1007/s00795-011-0558-z. [DOI] [PubMed] [Google Scholar]
- Minakata K, Takahashi F, Nara T, Hashimoto M, Tajima K, Murakami A, et al. Hypoxia induces gefitinib resistance in non-small-cell lung cancer with both mutant and wild-type epidermal growth factor receptors. Cancer Sci. 2012;103:1946–1954. doi: 10.1111/j.1349-7006.2012.02408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minchinton AI, Tannock IF. Drug penetration in solid tumours. Nat Rev Cancer. 2006;6:583–592. doi: 10.1038/nrc1893. [DOI] [PubMed] [Google Scholar]
- Misra A, Pandey C, Sze SK, Thanabalu T. Hypoxia activated EGFR signaling induces epithelial to mesenchymal transition (EMT) Plos ONE. 2012;7:e49766. doi: 10.1371/journal.pone.0049766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero JC, Seoane S, Ocana A, Pandiella A. Inhibition of SRC family kinases and receptor tyrosine kinases by dasatinib: possible combinations in solid tumors. Clin Cancer Res. 2011;17:5546–5552. doi: 10.1158/1078-0432.CCR-10-2616. [DOI] [PubMed] [Google Scholar]
- Murakami M, Zhao S, Zhao Y, Chowdhury NF, Yu W, Nishijima K, et al. Evaluation of changes in the tumor microenvironment after sorafenib therapy by sequential histology and 18F-fluoromisonidazole hypoxia imaging in renal cell carcinoma. Int J Oncol. 2012;41:1593–1600. doi: 10.3892/ijo.2012.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onishi H, Morifuji Y, Kai M, Suyama K, Iwasaki H, Katano M. Hedgehog inhibitor decreases chemosensitivity to 5-fluorouracil and gemcitabine under hypoxic conditions in pancreatic cancer. Cancer Sci. 2012;103:1272–1279. doi: 10.1111/j.1349-7006.2012.02297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham NA, Magalhaes JM, Do T, Schwock J, Dhani N, Cao PJ, et al. Activation of Src and Src-associated signaling pathways in relation to hypoxia in human cancer xenograft models. Int J Cancer. 2009;124:280–286. doi: 10.1002/ijc.23912. [DOI] [PubMed] [Google Scholar]
- Phillips RM, Hulbert PB, Bibby MC, Sleigh NR, Double JA. In vitro activity of the novel indoloquinone EO-9 and the influence of pH on cytotoxicity. Br J Cancer. 1992;65:359–364. doi: 10.1038/bjc.1992.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju U, Riesterer O, Wang ZQ, Molkentine DP, Molkentine JM, Johnson FM, et al. Dasatinib, a multi-kinase inhibitor increased radiation sensitivity by interfering with nuclear localization of epidermal growth factor receptor and by blocking DNA repair pathways. Radiother Oncol. 2012;105:241–249. doi: 10.1016/j.radonc.2012.08.010. [DOI] [PubMed] [Google Scholar]
- Rohwer N, Cramer T. Hypoxia-mediated drug resistance: novel insights on the functional interaction of HIFs and cell death pathways. Drug Resist Updat. 2011;14:191–201. doi: 10.1016/j.drup.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Roskoski R., Jr Src kinase regulation by phosphorylation and dephosphorylation. Biochem Biophys Res Commun. 2005;331:1–14. doi: 10.1016/j.bbrc.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Sartorelli AC, Hodnick WF, Belcourt MF, Tomasz M, Haffty B, Fischer JJ, et al. Mitomycin C: a prototype bioreductive agent. Oncol Res. 1994;6:501–508. [PubMed] [Google Scholar]
- Semenza GL. Molecular mechanisms mediating metastasis of hypoxic breast cancer cells. Trends Mol Med. 2012;18:534–543. doi: 10.1016/j.molmed.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JD, Liu Q, Wang J, Ahluwalia D, Ferraro D, Wang Y, et al. Selective tumor hypoxia targeting by hypoxia-activated prodrug TH-302 inhibits tumor growth in preclinical models of cancer. Clin Cancer Res. 2012;18:758–770. doi: 10.1158/1078-0432.CCR-11-1980. [DOI] [PubMed] [Google Scholar]
- Suwaki N, Vanhecke E, Atkins KM, Graf M, Swabey K, Huang P, et al. A HIF-regulated VHL-PTP1B-Src signaling axis identifies a therapeutic target in renal cell carcinoma. Sci Transl Med. 2011;3:85ra47. doi: 10.1126/scitranslmed.3002004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang TC, Man S, Xu P, Francia G, Hashimoto K, Emmenegger U, et al. Development of a resistance-like phenotype to sorafenib by human hepatocellular carcinoma cells is reversible and can be delayed by metronomic UFT chemotherapy. Neoplasia. 2010;12:928–940. doi: 10.1593/neo.10804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher BA, Lazo JS, Sartorelli AC. Classification of antineoplastic agents by their selective toxicities toward oxygenated and hypoxic tumor cells. Cancer Res. 1981;41:73–81. [PubMed] [Google Scholar]
- Tryfonopoulos D, Walsh S, Collins DM, Flanagan L, Quinn C, Corkery B, et al. Src: a potential target for the treatment of triple-negative breast cancer. Ann Oncol. 2011;22:2234–2240. doi: 10.1093/annonc/mdq757. [DOI] [PubMed] [Google Scholar]
- Vaupel P. Hypoxia and aggressive tumor phenotype: implications for therapy and prognosis. Oncologist. 2008;13(Suppl 3):21–26. doi: 10.1634/theoncologist.13-S3-21. [DOI] [PubMed] [Google Scholar]
- Vaupel P. Metabolic microenvironment of tumor cells: a key factor in malignant progression. Exp Oncol. 2010;32:125–127. [PubMed] [Google Scholar]
- Vaupel P, Kelleher DK. Blood flow and oxygenation status of prostate cancers. Adv Exp Med Biol. 2013;765:299–305. doi: 10.1007/978-1-4614-4989-8_42. [DOI] [PubMed] [Google Scholar]
- Wang X, Schneider A. HIF-2alpha-mediated activation of the epidermal growth factor receptor potentiates head and neck cancer cell migration in response to hypoxia. Carcinogenesis. 2010;31:1202–1210. doi: 10.1093/carcin/bgq078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhang L, Goldberg SN, Bhasin M, Brown V, Alsop DC, et al. High dose intermittent sorafenib shows improved efficacy over conventional continuous dose in renal cell carcinoma. J Transl Med. 2011;9:220–228. doi: 10.1186/1479-5876-9-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss GJ, Infante JR, Chiorean EG, Borad MJ, Bendell JC, Molina JR, et al. Phase 1 study of the safety, tolerability, and pharmacokinetics of TH-302, a hypoxia-activated prodrug, in patients with advanced solid malignancies. Clin Cancer Res. 2011;17:2997–3004. doi: 10.1158/1078-0432.CCR-10-3425. [DOI] [PubMed] [Google Scholar]
- Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat Rev Cancer. 2011;11:393–410. doi: 10.1038/nrc3064. [DOI] [PubMed] [Google Scholar]
- Wouters A, Boeckx C, Vermorken JB, Van den Weyngaert D, Peeters M, Lardon F. The intriguing interplay between therapies targeting the epidermal growth factor receptor, the hypoxic microenvironment and hypoxia-inducible factors. Curr Pharm Des. 2013;19:907–917. [PubMed] [Google Scholar]
- Wu YC, Ling TY, Lu SH, Kuo HC, Ho HN, Yeh SD, et al. Chemotherapeutic sensitivity of testicular germ cell tumors under hypoxic conditions is negatively regulated by SENP1-controlled sumoylation of OCT4. Cancer Res. 2012;72:4963–4973. doi: 10.1158/0008-5472.CAN-12-0673. [DOI] [PubMed] [Google Scholar]
- Yeatman TJ. A renaissance for SRC. Nat Rev Cancer. 2004;4:470–480. doi: 10.1038/nrc1366. [DOI] [PubMed] [Google Scholar]
- Zhang L, Bhasin M, Schor-Bardach R, Wang X, Collins MP, Panka D, et al. Resistance of renal cell carcinoma to sorafenib is mediated by potentially reversible gene expression. PLoS ONE. 2011;6:e19144. doi: 10.1371/journal.pone.0019144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Yu D. Targeting Src family kinases in anti-cancer therapies: turning promise into triumph. Trends Pharmacol Sci. 2012;33:122–128. doi: 10.1016/j.tips.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]