Abstract
During re-infection high-affinity IgG antibodies form complexes with both soluble antigen and antigen displayed on the surface of infected cells. These interactions regulate cellular activation of both innate cells and B cells, which express specific combinations of activating Fc gamma receptors (FcγRI, FcγRIII, FcγRIV) and/or the inhibitory Fc gamma receptor (FcγRIIB). Direct proof for functional expression of FcγR by antigen-specific CD8 T-cells is lacking. Here, we show that the majority of memory CD8 T-cells generated by bacterial or viral infection express only FcγRIIB and that FcγRIIB could be detected on previously activated human CD8 T-cells. Of note, FcγR stimulation during in vivo antigen challenge not only inhibited the cytotoxicity of memory CD8 T-cells against peptide-loaded or virus-infected targets, but FcγRIIB blockade during homologous virus challenge enhanced the secondary CD8 T-cell response. Thus, memory CD8 T-cells intrinsically express a functional FcγRIIB, permitting antigen-antibody complexes to regulate secondary CD8 T-cell responses.
Introduction
Following acute infection with intracellular pathogens, antigen-specific CD8 T-cells become activated, proliferate, then contract in numbers to generate long-lived memory populations (1-4). By virtue of their enhanced numbers, immediate effector functions and capacity to undergo secondary proliferation, memory CD8 T-cells can play a pivotal role in host protection against re-infection (2, 5, 6).
B cell populations activated by infection also promote protective immunity by maintaining high levels of circulating high-affinity IgG antibody (Ab) (7-9). When Abs complex with soluble antigen (Ag) or with Ag displayed on the surface of infected cells, the Fc fragment regulates the activation status and effector functions of nearby cells that bear Fc receptors (FcR). In mice, there are four FcR for IgG; FcγRI, FcγRIIB, FcγRIII, and FcγRIV (10), which are classified based on their ability to regulate cellular activation. Activating FcγR (FcγRI, FcγRIII, and FcγRIV), which can be expressed by a variety of innate immune cell populations, contain intracellular immunoreceptor tyrosine-based activation motifs (ITAM) and have been shown to increase phagocytosis, release of proinflammatory cytokines, and facilitate antibody-dependent cell-mediated cytotoxicity (ADCC) (11-13). In contrast, FcγRIIB, which is thought to be restricted to innate immune cells and B cells, contains an intracellular immunoreceptor tyrosine-based inhibition motif (ITIM) motif and is important for negatively impacting the signaling capacity of activating FcγR on innate effector cells (11) and B cells and also tempering BCR-mediated signaling (14).
Although FcγRs play a crucial role in regulating the activation of both innate cells and B cells during re-infection, their role in CD8 T-cell biology is unclear and remains controversial. It has been suggested that T-cells do not intrinsically express FcγR (10), but in some instances can acquire FcγR following intercellular transfer from an FcγR-bearing cell (15, 16). We recently showed by microarray analyses that Fcgr2b mRNA, but not mRNA for any other FcγR, is upregulated in memory CD8 T-cells generated after Listeria monocytogenes (LM) infection (17). Here, we address both the protein expression and in vivo function of FcγRIIB in memory CD8 T-cells generated by bacterial and viral infection.
Materials and Methods
Human Blood, Mice, Bone Marrow Chimera, Virus, and Bacteria
Whole blood was acquired from anonymous donors that had consented for blood donation at the DeGowin Blood Center at the University of Iowa. Consent forms were approved by the University of Iowa’s Institutional Review Board (IRB). C57BL/6 (Thy1.2/CD45.2 and CD45.1) were obtained from the National Cancer Institute (Frederick, MD, USA). T-cell receptor transgenic (Tg) OT-I (Thy1.1) and P14 (Thy1.1) mice have been described (18, 19). FcγRIIB KO mice were obtained from Jackson Laboratories (Bar Harbor, ME). WT: FcγRIIB KO bone marrow chimeric mice were generated as previously described (20). LCMV Armstrong (LCMV Arm) and LCMV Clone 13 were propagated according to standard protocols. LCMV Armstrong (LCMV Arm; 2×105 PFU) was injected i.p. while LCMV Clone 13 (2×106 PFU)was injected i.v. Attenuated actA-deficient L. monocytogenes expressing OVA257 (att LM-OVA) or GP33 (att LM-GP33) were propagated and injected i.v. at 1×107 CFU as described (21-23).
Cell lines, Antibodies, Peptides, MHC Class I Tetramers
CH12 B cells were provided by Dr. Gail Bishop (University of Iowa; Iowa City, IA). Antibodies for FACS analysis were used with the indicated specificity and the appropriate combinations of fluorochromes. For FcγRIIB/FcγRIII staining, biotinylated-2.4G2 (BD Bioscience; San Jose, CA) and streptavidin-APC (Invitrogen; Carlsbad, CA) were used. MHC class I tetramers H-2Kb/OVA257-264 and H-2Db/GP33-41 were prepared as described (24-26). Ab treatment during LCMV re-challenge was 400 μg of either Rat IgG (Fischer Scientific; Pittsburgh, PA) or 2.4G2 (prepared in house) for three consecutive days following secondary infection.
Adoptive Transfer and Quantitative/Phenotypic Analysis of Pathogen-Specific CD8 T-cells
103 OT-I (OVA257-264-specific) or 104 P14 (GP33-41-specific) TCR Transgenic (Tg) CD8 T-cells (Thy1.1) (unless otherwise stated) from the spleen or blood of naïve mice were transferred into naïve B6 (Thy1.2) hosts as previously described (27). Recipient mice were then challenged with att LM-OVA or LCMV Arm. The magnitude of pathogen-specific CD8 T-cell response was determined by either tetramer staining of endogenous CD8 T-cells (28) or by staining for Thy1.1 expressing Tg cells (27) or by evaluating changes in CD11a and CD8a expression (29).
RNA Purification and RT-PCR
Cells were sorted based on Thy1.1 (OT-I) and NK1.1 (splenic NK cells) to approximately 99% purity and RNA from three independent pools of purified cells was extracted using the RNEasy kit (QIAGEN). Approximately 50-100 ng of RNA template was converted to cDNA and amplified using Script One-Step RT-PCR Kit with SYBR Green according to manufacturer’s protocol (BIO RAD; Hercules, CA). The following oligonucleotides were used to analyze expression of the following transcripts: 5′-CCCTGGGAACTCTTCTACCC-3′ and 5′- CAGCAGCCAGTCAGAAATCA-3′ for Fcgr2b and 5′-CCTCATGGACTGATTATGGACA-3′ and 5′TATGTCCCCGTTGACTG AT-3′ for Hprt. PCR reaction was carried out using ABI PRISM 7700 Sequence. Expression of transcripts was normalized to controls groups as indicated.
In vivo Cytotoxicity Assay
Splenocytes from CD45.1 mice were harvested and stained with carboxyfluorescein succinimidylester (CFSE) or CellTrace™ Violet Cell Proliferation Kit (CTV; Life Technologies; Carlsbad, CA). CFSEHIGH cells were stained with 1 μM CFSE; CFSELOW cells were stained with 0.04 μM CFSE; CTVHIGH cells were stained with 2.5 μM CTV; CTVLOW cells were stained with 0.25 μM CTV. After 15 minutes at 37°C, staining was quenched 1:1 with FCS then cells were washed three times with RPMI containing 10% (vol/vol) FCS. Stained cells were then coated with the following cocktails at 37°C in an orbital shaker for 1 hour: CTVHIGH cells were incubated with 1 μM GP33-41 and α-H-2Kb, CTVLOW were coated with α-H-2Kb, CFSEHIGH cells were coated with 1 μM GP33-41, CFSELOW cells were left uncoated. After washing, cells were mixed 1:1:1:1 and transferred into CD45.2 hosts that were either naïve or >60 days after LCMV Arm. Recipient mice were pre-treated with 400 μg of rat IgG (IgG) or 2.4G2 i.v. 20 minutes prior to target cell transfer. After 1 hour, % GP33-41/H2-Db-specific lysis was assessed by comparing the presence of CTVLOW vs. CTVHIGH or CFSELOW vs. CFSEHIGH using the following formula: %Specific Lysis = [1-(Naïve transfer Ratio/Infected transfer Ratio)] ×100 (30). For in vivo cytotoxicity assays using LCMV Arm infected target cells, splenocytes were harvested from naïve CD45.1 mice (stained CTVLOW) and from CD45.1 mice that had been infected with LCMV Arm 4 days prior (stained CTVHIGH), mixed 1:1 and injected into LCMV Arm immune or naïve mice. % Specific Lysis was determined 1 hour following transfer. FACS analysis using anti-LCMV NP (113 hybridoma) was as described (31).
Statistics
Statistical analysis was performed using two-tailed Student T-tests or ANOVA as indicated.
Results
Antigen-Specific CD8 T-cells Express FcγRIIB Following Infection
A transcriptional profiling study performed in our laboratory revealed consistent upregulation of Fcgr2b mRNA, but not other FcγR transcripts, in memory CD8 T-cell populations generated by one or more antigen-stimulation compared to naïve CD8 T-cells (17). To confirm these microarray data, we performed RT-PCR for Fcgr2b mRNA in memory OT-I CD8 T-cells as well as a NK cells (which do not express Fcgr2b) and CH12 B cells (which express Fcgr2b) (10) and normalized expression relative to naïve OT-I CD8 T-cells. Expression of Fcgr2b mRNA in NK cells did not differ from naive OT-I cells whereas both CH12 B cells and memory OT-I cells expressed significantly (p<0.01) more Fcgr2b mRNA compared to naïve OT-I cells (Supplemental Fig. 1A).
To determine if Fcgr2b mRNA expression by memory CD8 T-cells resulted in protein production, we measured FcγRIIB protein expression with the 2.4G2 monoclonal Ab (mAb) on the surface of antigen-specific CD8 T-cells at memory time points following att LM-OVA infection. Indeed, approximately 60% of memory CD8 T-cells stained positive with 2.4G2 (Fig. 1A). 2.4G2 is known to detect (and block) both FcγRIII and FcγRIIB (32). To determine the specificity of 2.4G2 reactivity, we compared 2.4G2 binding on memory CD8 T-cells from WT and FcγRIIB KO mice. As predicted based on lack of mRNA expression for FcγRIII (17), 2.4G2 failed to react with memory CD8 T-cells from FcγRIIB KO mice (Fig. 1A), thus indicating that 2.4G2 exclusively detects surface FcγRIIB and not FcγRIII on memory CD8 T-cells.
Figure 1. Memory CD8 T-cells Express FcγRIIB Following Infection in vivo.
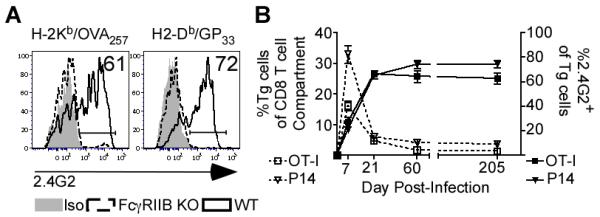
A) Surface protein expression FcγRIIB/FcγRIII (staining with the 2.4G2 mAb) by tetramer+ memory CD8 T-cells from WT (black solid line) or FcγRIIB KO mice (black dotted line) were evaluated in the blood relative to their isotype (filled grey) at >70 days post att LM-OVA or LCMV Arm infection. Histograms are representative of three independent experiments with three mice per group. B) Frequency of Tg (P14 or OT-I) cells of CD8 T-cells (left axis) and FcγRIIB expression (2.4G2 staining, right axis) by Tg cells were measured in blood at the indicated days post infection. Data are depicted of single experiments that are representative of at three independent experiments with at least 3 mice per group.
In order to determine if expression of FcγRIIB is a general feature of memory CD8 T-cells, we generated memory CD8 T-cells specific for LCMV Armstrong (Arm) in WT and FcγRIIB KO mice. Indeed, the majority of GP33-41-specific WT memory CD8 T-cells also reacted with 2.4G2 and thus express FcγRIIB (Fig. 1A). Importantly, surface expression of FcγRIIB by memory CD8 T-cells was not due to trogocytosis, since 2.4G2 failed to react with FcγRIIB KO antigen-specific CD8 T-cells in WT: FcγRIIB KO bone marrow chimeric mice (Supplemental Fig. 1B). Together, these data indicate that in contrast to previous suggestions that T-cells do not express FcγR (10, 11), memory CD8 T-cells generated by both bacterial and viral infection intrinsically express surface FcγRIIB protein.
To address the kinetics of FcγRIIB expression by CD8 T-cells following infection, mice were seeded with naïve P14 or OT-I CD8 T-cells and then given LCMV Arm or att LM-OVA infection, respectively. FcγRIIB protein expression was detected on a minority of effector CD8 T-cells as early as day 7 post-infection, and the fraction of FcγRIIB-expressing CD8 T-cells continued to increase until approximately 21 days post-infection (Fig. 1B). At this point, FcγRIIB expressing CD8 T-cells stabilized at 60-80% and were maintained as late as 200 days post-LCMV Arm or att LM-OVA infection (Fig. 1B). As predicted by mRNA expression analysis by Wirth et al., memory CD8 T-cells do not express either FcγRI or FcγRIV protein relative whereas both receptors were readily detectable on CD3−CD11b+ cells (Supplemental Fig. 1C). These data suggest that some effector CD8 T-cells express FcγRIIB shortly after activation but that the majority of memory CD8 T-cell population maintains high FcγRIIB expression while not expressing other FcγR.
Since FcγRIIB expression can be regulated by cytokine stimulation alone in some immune cell types [e.g. IL-4 and TGF-b (33-36)], it is possible that TCR-mediated activation may not be required for FcγRIIB expression by CD8 T-cells during infection. To determine whether FcγRIIB expression was limited to activated CD8 T-cells, we evaluated the expression of FcγRIIB on “antigen-experienced” and “naïve” polyclonal CD8 T-cells during the course of infection, distinguishing these populations using surrogate activation markers: CD11aHIGHCD8aLOW (antigen-experienced) and CD11aINT/CD8aHIGH (not antigen-experienced)(29). Of note, only CD11aHIGHCD8aLOW T-cells from either LCMV Arm or att LM-OVA infected mice expressed FcγRIIB (Fig. 2A). Interestingly, FcγRIIB expression could also be detected on 5-17% of CD45RO+ (activated/memory) human CD8 T-cells from normal donors while their CD45RO− (naïve) counterpart do not exhibit FcγRIIB expression (Supplemental Fig. 1D,E). Based on these results, FcγRIIB expression is restricted to antigen-experienced CD8 T-cells in both mice and humans.
Figure 2. Characteristics of FcγRIIB-Expressing Memory CD8 T-cells.
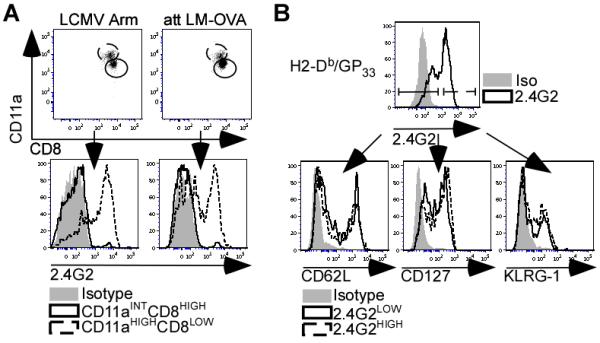
A) At day 65 p.i., FcγRIIB protein expression was evaluated on the surface of blood-derived CD11aHIGHCD8LOWThy1.2+ cells (dotted black line) and CD11aINTCD8HIGHThy1.2+ cells (solid black line) as compared to isotype (filled grey). FACS plots and histograms are representative of two independent experiments with at least 3 mice per group. B) At day 55 post-LCMV Arm infection, 2.4G2HIGH (dotted black line) and 2.4G2LOW (solid black line) H2-Db/GP +33-41-Tetramer CD8 T-cells were assessed for CD62L, CD127, KLRG-1 expression. Filled grey histogram represents isotype controls. Histograms are representative of 2 independent experiments with at least 3 mice in each group per experiment.
Next we determined if FcγRIIB expression was limited to specific memory CD8 T-cell subsets (TEM or TCM). FcγRIIB staining with 2.4G2 reveals bimodal expression on memory CD8 T-cells (Fig. 2B). Importantly, LCMV-specific memory CD8 T-cells with low or high expression of FcγRIIB had similar expression profiles of CD62L, CD127, and KLRG-1 (Fig. 2B). Thus, FcγRIIB expression does not correspond to previously described TEM or TCM memory CD8 T-cell subsets.
FcγRIIB Inhibits Both Cytotoxicity and Secondary Expansion of Memory CD8 T-cells
Since FcγRIIB is known to have a negative impact on B cell activation (37), we tested whether this Fc receptor was capable of dampening the response of memory CD8 T-cells when co-engaged with T cell receptor (TCR). To address this question, LCMV Arm immune mice (> 60 days p.i.) were used for an in vivo cytotoxicity assay (GP33-41/H2-Db-Specific) that compared the susceptibility of Ag-pulsed targets to lysis when left uncoated or coated with mouse-derived IgG Ab (〈–H2-Kb). To compare these scenarios, the target populations were generated by surface staining and pulsing CD45.1 splenocytes with the following combinations of reagents: 1) CFSELOW = Unpulsed, 2) CFSEHIGH = Pulsed with GP LOW33-41, 3) CTV = Unpulsed with α-H-2Kb, 4) CTVHIGH = α-H2-Kb + Pulsed with GP33-41. Four populations were then mixed into a 1:1:1:1 ratio (Supplemental Fig. 2A) and transferred into either naïve CD45.2 hosts or LCMV Arm immune CD45.2 hosts at day 70 p.i. The percent specific lysis of GP33-41-coated targets in the absence of α-H2-Kb antibody coating was ~55% in LCMV Arm immune mice treated with control rat IgG. Strikingly, specific lysis of cells that were coated with α-H2-Kb+GP33-41 was decreased to ~20% (Fig. 3A), suggesting that the α-H2-Kb antibody on target cells provided an inhibitory signal. In addition, if immune mice were treated with 2.4G2 mAb to block FcγRIIB stimulation (34, 38) prior to transfer of target cells, the specific lysis of cells coated with α-H2-Kb+GP33-41 returned to the level of cells pulsed with GP33-41 alone (Fig. 3A). Additionally, when LCMV Arm immune mice (day>70 p.i., when neutralizing IgG antibodies have developed (39)) were challenged with LCMV Arm infected targets (Supplemental Fig. 2B) in the presence of control IgG or 2.4G2 treatment, specific lysis of infected targets was enhanced by 2.4G2 pre-treatment compared to IgG control treated mice (Fig. 3B). Therefore, these data demonstrate that FcγRIIB negatively regulates the cytotoxicity of memory CD8 T-cells upon encountering mouse IgG-coated targets.
Figure 3. FcγRIIB Inhibits the Cytotoxicity Memory CD8 T-cells.
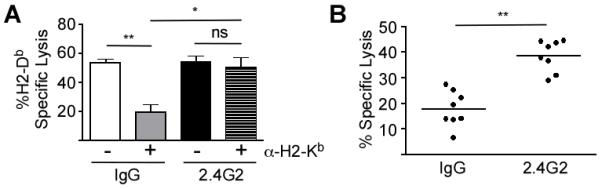
A)CD45.2 mice were injected with P14 CD8 T-cells and subsequently infected with LCMV Arm. At least 70 days after infection, hosts were treated with rat IgG (IgG) or 2.4G2 and then subjected to in vivo GP33-41/H2-Db-specific cytotoxicity assay. Separate CD45.1 target populations were stained and coated in the following combinations: CFSEHIGH (GP33-41), CFSELOW(uncoated), CTVHIGH(GP33-41+α-H2-Kb), CTVLOW (α-H2-Kb). Targets were mixed 1:1:1:1 and then injected into CD45.2 pre-treated mice that were either naïve or LCMV Arm-immune. In vivo cytotoxicity was assessed by calculating % GP33-41/H2-Db-Specific Lysis (CFSELOW vs. CFSEHIGH; CTVLOW vs. CTVHIGH). Displayed data are compiled from two independent experiments, each with three mice per group. Statistical analysis was done using ANOVA. B) Splenocytes from naïve and from mice that had been infected with LCMV Arm 4 days prior were transferred into P14 seeded LCMV Arm immune mice that had received IgG or 2.4G2 pre-treatment. Displayed data are compiled from two independent experiments with a total of eight mice per group. Statistical analysis was done using Student’s T-test. * = p-value <0.05, ** = p-value<0.01, ns = p-value > 0.05.
To further examine a function for FcγRIIB expression on memory CD8 T-cells, we determined if FcγRIIB played a role in regulating the expansion of memory CD8 T-cells during homologous challenge in hosts with circulating levels of pathogen-specific high-affinity IgG Abs. Blocking FcγRIIB significantly (p<0.01) enhanced the accumulation of memory P14 CD8 T-cells following high-dose challenge with LCMV Clone 13 (Fig. 4A,B). However, treatment of LCMV Arm immune mice with 2.4G2 did not significantly enhance secondary accumulation of memory CD8 T-cells when the mice challenged with att LM-GP33, a scenario where the host lacks IgG antibodies to the challenge pathogen (Fig. 4A). Thus, FcγRIIB tempers memory CD8 T-cell responses to re-infection in hosts with pre-existing IgG antibodies to the challenge pathogen.
Figure 4. FcγRIIB Inhibits the Expansion of Memory CD8 T-cells Following Homologous Challenge.
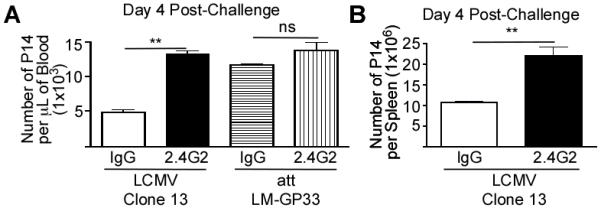
(A, B)Mice were injected with P14 CD8 T-cells and subsequently infected with LCMV Arm. At >60 days p.i., LCMV Arm immune mice were challenged with LCMV Clone 13 or att LM-GP33. Groups received IgG or 2.4G2 treatment 20 minutes prior to and two consecutive days following challenge. The number of memory P14 CD8 T-cells were measured per μL sample of blood (A) or as number in the spleen at day 4 post-challenge (B). All displayed data are compiled from a two independent experiments, each with three mice per group. Statistical analysis was done using Student’s T-test. **= p-value<0.01, ns = p-value<0.05.
Discussion
Expression of FcγR by T-cells has been questioned in the literature (10). Here, we confirm that both viral and bacterial infections specifically promote the expression of FcγRIIB, and not other FcγR, by previously activated human and mouse CD8 T-cells. Previous studies have indicated that FcγR expression can be endowed upon CD8 T-cells by intercellular transfer from FcγR-bearing cells in vitro, often termed trogocytosis (15, 16). Since Fcgr2b mRNA expression is elevated in memory CD8 T-cells, and FcγRIIB KO antigen-specific CD8 T-cells do not acquire FcγRIIB expression after infection of mixed-bone marrow chimeras we conclude that the presence of FcγRIIB on the surface of memory CD8 T-cells is cell intrinsic.
Acquisition of FcγR expression on CD8 T-cells by trogocytosis has yet to associated with a functional consequence in vivo (15). In contrast, our results indicate that FcγRIIB plays a discernable role in dampening not only the cytotoxicity of memory CD8 T-cells upon encountering Ab/Ag-coated cells and virus-infected cells in vivo, but also by limiting the expansion of memory CD8 T-cells during re-infection in hosts with an established IgG Ab response. These findings confirm that CD8 T-cells do, in fact, express FcγRIIB and this receptor can play an important role in tempering secondary responses during re-infection. This concept may useful for future and current therapies that aim to optimally regulate memory CD8 T-cell expansion following homologous booster vaccinations.
Supplementary Material
Acknowledgements
The authors thank members of the Harty lab for helpful discussions. GRSM designed and performed experiments, analyzed data, and wrote the paper. VPB designed experiments and wrote the paper. DLB designed experiments. JTH designed and analyzed experiments, and wrote the paper.
This work was supported by the National Institute of Health Grants T32 AI 007511 (to GRSM), AI083286 and AI096850 to (VPB) and AI42676, AI85515, AI90850, AI95178, AI96850 (to JTH).
Footnotes
Disclosures
The authors have no financial conflicts of interests.
References
- 1.Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002;2:251–262. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- 2.Badovinac VP, Harty JT. Programming, demarcating, and manipulating CD8+ T-cell memory. Immunol Rev. 2006;211:67–80. doi: 10.1111/j.0105-2896.2006.00384.x. [DOI] [PubMed] [Google Scholar]
- 3.Marshall DR, Turner SJ, Belz GT, Wingo S, Andreansky S, Sangster MY, Riberdy JM, Liu T, Tan M, Doherty PC. Measuring the diaspora for virus-specific CD8+ T cells. Proc Natl Acad Sci U S A. 2001;98:6313–6318. doi: 10.1073/pnas.101132698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harty JT, Badovinac VP. Shaping and reshaping CD8+ T-cell memory. Nat Rev Immunol. 2008;8:107–119. doi: 10.1038/nri2251. [DOI] [PubMed] [Google Scholar]
- 5.Hikono H, Kohlmeier JE, Ely KH, Scott I, Roberts AD, Blackman MA, Woodland DL. T-cell memory and recall responses to respiratory virus infections. Immunol Rev. 2006;211:119–132. doi: 10.1111/j.0105-2896.2006.00385.x. [DOI] [PubMed] [Google Scholar]
- 6.Lefrancois L. Development, trafficking, and function of memory T-cell subsets. Immunol Rev. 2006;211:93–103. doi: 10.1111/j.0105-2896.2006.00393.x. [DOI] [PubMed] [Google Scholar]
- 7.Kaur K, Sullivan M, Wilson PC. Targeting B cell responses in universal influenza vaccine design. Trends Immunol. 2011;32:524–531. doi: 10.1016/j.it.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plotkin SA. Vaccines: correlates of vaccine-induced immunity. Clin Infect Dis. 2008;47:401–409. doi: 10.1086/589862. [DOI] [PubMed] [Google Scholar]
- 9.Ballow M. Primary immunodeficiency disorders: antibody deficiency. J Allergy Clin Immunol. 2002;109:581–591. doi: 10.1067/mai.2002.122466. [DOI] [PubMed] [Google Scholar]
- 10.Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol. 2008;8:34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- 11.Nimmerjahn F, Ravetch JV. FcgammaRs in health and disease. Curr Top Microbiol Immunol. 2011;350:105–125. doi: 10.1007/82_2010_86. [DOI] [PubMed] [Google Scholar]
- 12.Hogarth PM. Fc receptors are major mediators of antibody based inflammation in autoimmunity. Curr Opin Immunol. 2002;14:798–802. doi: 10.1016/s0952-7915(02)00409-0. [DOI] [PubMed] [Google Scholar]
- 13.Takai T. Roles of Fc receptors in autoimmunity. Nat Rev Immunol. 2002;2:580–592. doi: 10.1038/nri856. [DOI] [PubMed] [Google Scholar]
- 14.Bolland S, Ravetch JV. Inhibitory pathways triggered by ITIM-containing receptors. Adv Immunol. 1999;72:149–177. doi: 10.1016/s0065-2776(08)60019-x. [DOI] [PubMed] [Google Scholar]
- 15.Hudrisier D, Clemenceau B, Balor S, Daubeuf S, Magdeleine E, Daeron M, Bruhns P, Vie H. Ligand binding but undetected functional response of FcR after their capture by T cells via trogocytosis. J Immunol. 2009;183:6102–6113. doi: 10.4049/jimmunol.0900821. [DOI] [PubMed] [Google Scholar]
- 16.Lee ST, Paraskevas F. Macrophage--T cell interactions. I. The uptake by T cells of Fc receptors released from macrophages. Cell Immunol. 1978;40:141–153. doi: 10.1016/0008-8749(78)90322-2. [DOI] [PubMed] [Google Scholar]
- 17.Wirth TC, Xue HH, Rai D, Sabel JT, Bair T, Harty JT, Badovinac VP. Repetitive antigen stimulation induces stepwise transcriptome diversification but preserves a core signature of memory CD8(+) T cell differentiation. Immunity. 2010;33:128–140. doi: 10.1016/j.immuni.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 19.Pircher H, Baenziger J, Schilham M, Sado T, Kamisaku H, Hengartner H, Zinkernagel RM. Characterization of virus-specific cytotoxic T cell clones from allogeneic bone marrow chimeras. Eur J Immunol. 1987;17:159–166. doi: 10.1002/eji.1830170202. [DOI] [PubMed] [Google Scholar]
- 20.Pham NL, Badovinac VP, Harty JT. Differential role of “Signal 3” inflammatory cytokines in regulating CD8 T cell expansion and differentiation in vivo. Front Immunol. 2011;2:4–4. doi: 10.3389/fimmu.2011.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pope C, Kim SK, Marzo A, Masopust D, Williams K, Jiang J, Shen H, Lefrancois L. Organ-specific regulation of the CD8 T cell response to Listeria monocytogenes infection. J Immunol. 2001;166:3402–3409. doi: 10.4049/jimmunol.166.5.3402. [DOI] [PubMed] [Google Scholar]
- 22.Wirth TC, Harty JT, Badovinac VP. Modulating numbers and phenotype of CD8+ T cells in secondary immune responses. Eur J Immunol. 2010;40:1916–1926. doi: 10.1002/eji.201040310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ashton-Rickardt PG, Bandeira A, Delaney JR, Van Kaer L, Pircher HP, Zinkernagel RM, Tonegawa S. Evidence for a differential avidity model of T cell selection in the thymus. Cell. 1994;76:651–663. doi: 10.1016/0092-8674(94)90505-3. [DOI] [PubMed] [Google Scholar]
- 24.Altman JD, Moss PA, Goulder PJ, Barouch DH, McHeyzer-Williams MG, Bell JI, McMichael AJ, Davis MM. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- 25.Busch DH, Pilip IM, Vijh S, Pamer EG. Coordinate regulation of complex T cell populations responding to bacterial infection. Immunity. 1998;8:353–362. doi: 10.1016/s1074-7613(00)80540-3. [DOI] [PubMed] [Google Scholar]
- 26.Murali-Krishna K, Altman JD, Suresh M, Sourdive DJ, Zajac AJ, Miller JD, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 27.Badovinac VP, Haring JS, Harty JT. Initial T cell receptor transgenic cell precursor frequency dictates critical aspects of the CD8(+) T cell response to infection. Immunity. 2007;26:827–841. doi: 10.1016/j.immuni.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Badovinac VP, Porter BB, Harty JT. Programmed contraction of CD8(+) T cells after infection. Nat Immunol. 2002;3:619–626. doi: 10.1038/ni804. [DOI] [PubMed] [Google Scholar]
- 29.Rai D, Pham NL, Harty JT, Badovinac VP. Tracking the total CD8 T cell response to infection reveals substantial discordance in magnitude and kinetics between inbred and outbred hosts. J Immunol. 2009;183:7672–7681. doi: 10.4049/jimmunol.0902874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Durward M, Harms J, Splitter G. Antigen specific killing assay using CFSE labeled target cells. J Vis Exp. 2010;45:2250–2250. doi: 10.3791/2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Korns Johnson D, Homann D. Accelerated and improved quantification of lymphocytic choriomeningitis virus (LCMV) titers by flow cytometry. PLoS One. 2012;7:5–5. doi: 10.1371/journal.pone.0037337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Unkeless JC. Characterization of a monoclonal antibody directed against mouse macrophage and lymphocyte Fc receptors. J Exp Med. 1979;150:580–596. doi: 10.1084/jem.150.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okayama Y, Kirshenbaum AS, Metcalfe DD. Expression of a functional high-affinity IgG receptor, Fc gamma RI, on human mast cells: Up-regulation by IFN-gamma. J Immunol. 2000;164:4332–4339. doi: 10.4049/jimmunol.164.8.4332. [DOI] [PubMed] [Google Scholar]
- 34.Pricop L, Redecha P, Teillaud JL, Frey J, Fridman WH, Sautes-Fridman C, Salmon JE. Differential modulation of stimulatory and inhibitory Fc gamma receptors on human monocytes by Th1 and Th2 cytokines. J Immunol. 2001;166:531–537. doi: 10.4049/jimmunol.166.1.531. [DOI] [PubMed] [Google Scholar]
- 35.Radeke HH, Janssen-Graalfs I, Sowa EN, Chouchakova N, Skokowa J, Loscher F, Schmidt RE, Heeringa P, Gessner JE. Opposite regulation of type II and III receptors for immunoglobulin G in mouse glomerular mesangial cells and in the induction of anti-glomerular basement membrane (GBM) nephritis. J Biol Chem. 2002;277:27535–27544. doi: 10.1074/jbc.M200419200. [DOI] [PubMed] [Google Scholar]
- 36.Tridandapani S, Wardrop R, Baran CP, Wang Y, Opalek JM, Caligiuri MA, Marsh CB. TGF-beta 1 suppresses [correction of supresses] myeloid Fc gamma receptor function by regulating the expression and function of the common gamma-subunit. J Immunol. 2003;170:4572–4577. doi: 10.4049/jimmunol.170.9.4572. [DOI] [PubMed] [Google Scholar]
- 37.Pearse RN, Kawabe T, Bolland S, Guinamard R, Kurosaki T, Ravetch JV. SHIP recruitment attenuates Fc gamma RIIB-induced B cell apoptosis. Immunity. 1999;10:753–760. doi: 10.1016/s1074-7613(00)80074-6. [DOI] [PubMed] [Google Scholar]
- 38.Kurlander RJ, Ellison DM, Hall J. The blockade of Fc receptor-mediated clearance of immune complexes in vivo by a monoclonal antibody (2.4G2) directed against Fc receptors on murine leukocytes. J Immunol. 1984;133:855–862. [PubMed] [Google Scholar]
- 39.Rajini B, Zeng J, Suvas PK, Dech HM, Onami TM. Both systemic and mucosal LCMV immunization generate robust viral-specific IgG in mucosal secretions, but elicit poor LCMV-specific IgA. Viral Immunol. 2010;23:377–384. doi: 10.1089/vim.2010.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


