Abstract
Background
Nonfunctioning adrenal incidentalomas are common and many patients undergo adrenalectomy to exclude adrenocortical carcinoma (ACC). Recent studies have shown dysregulated microRNA expression in ACC. The objective of this study was to determine the feasibility and diagnostic accuracy of measuring serum microRNAs in patients with benign and malignant adrenocortical tumors.
Method
Five microRNAs were selected from microRNA profiling studies in ACC (miR-let-7d, -34a, -195, -214, and 483-5p). Total microRNA was extracted from serum samples in patients with malignant and benign adrenal neoplasms. MicroRNAs levels were measured by quantitative RT-PCR and normalized to miR-16. To determine if microRNAs were secreted from ACC cells, we measured microRNA levels in culture.
Results
Serum samples from 22 patients with cortical adenomas and 17 patients with ACC were analyzed and all 5 microRNAs were detected. We found higher levels of miR-34a(p=0.001) and miR-483-5p(p=0.011) in patients with ACC. The AUC was 0.81 for miR-34a and 0.74 for miR-438-5p. MiR-34a and miR-483-5p levels in ACC cells were higher in the supernatant at 48 hours as compared to intracellular levels.
Conclusions
We show dysregulated microRNAs in ACC are detectable in human serum samples. MiR-34a and miR-483-5p are candidate serum biomarkers for distinguishing between benign and malignant adrenocortical tumors.
Keywords: adrenocortical carcinoma, microRNA, serum, diagnosis, biomarker
Introduction
The widespread use of computed tomography (CT) has resulted in an increase in incidentally detected adrenal masses termed adrenal incidentalomas (1). The estimated prevalence of these incidentalomas is 4–7% in the adult population (2). The prevalence of incidentalomas in young patients is 0.2% and increases with age to 6.9% in patients older than 70 years of age (3). These incidentalomas are relatively common, in contrast to adrenocortical carcinoma (ACC). However, many patients with adrenal incidentaloma undergo adrenalectomy to exclude a diagnosis of ACC. The incidence of ACC is estimated to be 1 to 2 cases per million adults (4). ACC accounts for 0.2% of cancer-related deaths per year in the United States and the five year survival varies from 32% to 45% (5). Given the high prevalence of adrenal incidentalomas in the general population, and the poor survival and rarity of ACC, there is a need for noninvasive biomarkers to improve ACC diagnosis and prognosis.
Recently, there has been a growing interest in evaluating microRNAs (miRNAs) as noninvasive biomarkers. MiRNAs are stable in various bodily fluids and can be accurately and precisely tested in a multitude of disease states and cancers (6). MiRNAs are 20–22 nucleotides small non-coding RNAs that regulate gene expression. These small non-coding RNAs have a critical role in many biological pathways and pathological processes such as regulating cell proliferation, apoptosis, and cell differentiation (7). MiRNAs are dysregulated in several types of cancers, including ACC. Microarray profiling studies have shown differing levels of miRNA expression in tissue between benign and malignant adrenocortical tumors (4, 8–10). In addition, recent studies have shown that not only are miRNAs dysregulated in ACC, they may also serve as potential prognostic markers. Soon et al. demonstrated that high expression levels of miR-483-5p and low expression levels of miR-195 in tissue samples was associated with poor prognosis in patients with ACC (10). These dysregulated miRNAs, if detected in the serum, could serve as potential biomarkers for diagnosis, prognosis, and even as therapeutic targets.
The aim of this study was to determine the feasibility and diagnostic accuracy of measuring serum circulating miRNAs dysregulated in ACC in patients’ serum samples with benign and malignant adrenocortical tumors.
Patients and Methods
Serum samples
Fasting serum samples from patients with adrenocortical tumors were obtained the day of surgery and stored at −80°C. Demographic, clinical, and pathologic information and tissue samples were collected under an Institutional Review Board (IRB) approved protocol. Clinical characteristics of the study cohort are summarized in Table 1. Tumors were classified as ACC if the Weiss criteria was ≥3. Tumors were classified as benign if the Weiss criteria was <3.
Table 1.
Clinical characteristics of study cohort.
| Adrenocortical Carcinoma | Benign Adrenocortical Tumor | |
|---|---|---|
| Number of Patients | 17 | 22 |
| Age (Ave. ± St. Dev.) | 56.0 ± 11.0 | 47.4 ± 18.2 |
| Sex (Female/Male) | 11/6 | 12/10 |
| Syndrome: | ||
| Cushing’s | 5 | 11 |
| Subclinical Cushing’s | 0 | 1 |
| Conn’s | 1 | 9 |
| Nonfunctioning | 11 | 1 |
Serum miRNA extraction
MiRNA was extracted from serum using the Qiagen miRNeasy Kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. In brief, total RNA was isolated from 200μl of serum using QIAzol (Qiagen). After chloroform extraction, 1.5 times the volume of 100% ethanol was added to the aqueous phase. MiRNA was purified from this mixture using the Qiagen miRNeasy Kit (Qiagen), enriched column purification system. Total miRNA was eluted in 28 μl of RNase free water.
Real-Time quantitative RT-PCR analysis
Five miRNAs previously shown to be dysregulated in cancer (8–9, 11–14) were selected from miRNA profiling studies in ACC (miR-let-7d, -34a, -195, -214, and 483-5p) (8–10, 13). Expression of these five miRNAs was measured using TaqMan quantitative real-time RT-PCR (Applied Biosystems, Foster City, CA). Single-stranded cDNA was synthesized from total miRNA using specific miRNA primers (TaqMan MicroRNA Assay, PN 4427975, Applied Biosystems) and the TaqMan MicroRNA Reverse Transcription Kit (PN 4366596, Applied Biosystems). 2.5 μl of cDNA was used as a template in a 10 μl PCR reaction. PCR products were amplified using specific primers (TaqMan MicroRNA Assay) and the TaqMan Universal PCR Master Mix (PN 4324018, Applied Biosystems) and detected using 7900HT Fast Real-Time PCR System (Applied Biosystems). PCR reactions for each sample were run in triplicate. Control reactions included cDNA synthesized without reverse transcriptase enzyme (RNA only) and no cDNA template. The following TaqMan MicroRNA Assays used in this study were obtained from Applied Biosystems: miR-483-5p (002338), miR-214 (002306), miR-195 (000494), miR-34a (000426), and miR-let-7d (002283). MiR-U6 (001973) was used as an endogenous control for data normalization by measuring expression in the cell line samples (15). MiR-16 (001006) was used as an endogenous control for data normalization by measuring expression in all of the serum and cell culture supernatant samples (16).
In vitro Studies
The miRNA expression levels were evaluated in H295R and SW13 ACC cell lines. The H295R and SW13 cells were grown in DMEM media. DMEM media only served as a negative control. The cell lines and negative control were incubated for 48 hours at 37 °C in a 5% CO2 incubator. At 48 hours, the supernatant was collected and centrifuged for 5 minutes at 1500 rpm to remove any cellular debris. The total miRNA was extracted from the supernatant as described above. The cells were trypsinized and resuspended in media. The resuspended cells were centrifuged for 5 minutes at 1500 rpm. The supernatant was aspirated and discarded, and total RNA extracted from the pellet using 1 mL of TRIzol (Ambion, Foster City, CA).
Statistical Analysis
The Mann–Whitney U test was used to compare miRNA expression levels between groups. A P value of <0.05 was considered statistically significant. All calculations were performed using GraphPad Software (La Jolla, CA, USA). Serum and culture miRNA expression levels were calculated according to the 2−ΔCt method. Receiver operating characteristic (ROC) curves and the area under the ROC curve (AUC) were determined using GraphPad Statisical Software.
Results
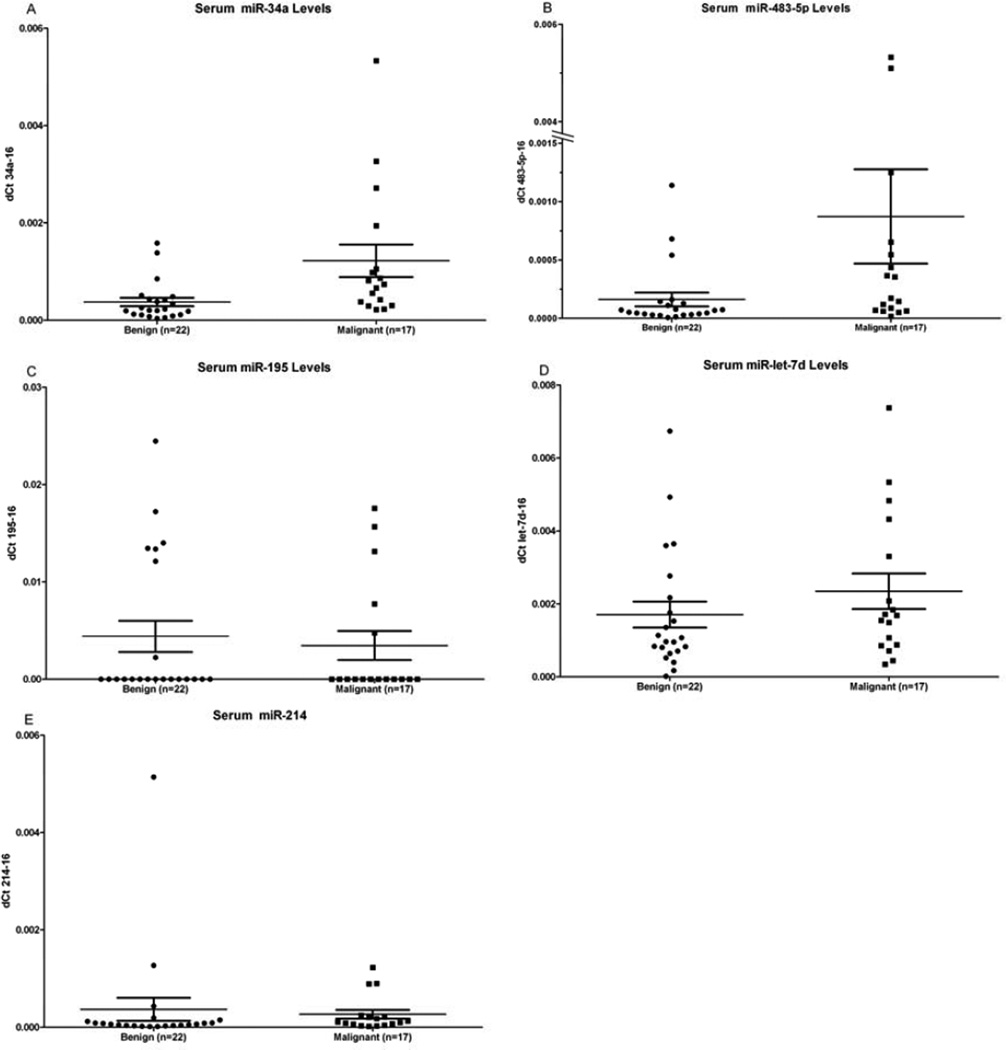
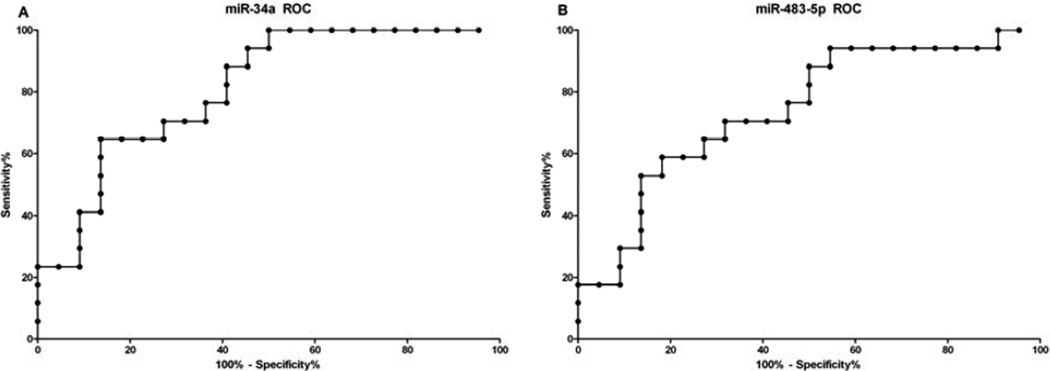
Five miRNAs were measured in serum samples from patients with adrenocortical tumors. All 5 miRNAs were detected in serum and were normalized to miR-16, a miRNA that is ubiquitously present in serum (16). There was significantly higher levels of miR-34a (p=0.001) and miR-483-5p (p=0.011) in patients with ACC (Figure 1). Mir-let-7d (p=0.1975), miR-214 (p=0.1370), and miR-195 (p=0.9210) levels in serum were not significantly different between patients with malignant and benign adrenocortical tumors (Figure 1). To determine the diagnostic accuracy of the miRNAs which were significantly different, the area under the ROC curve (AUC) was determined for miR-34a (0.83, p=0.001) and for miR-483-5p (0.74, p=0.011) (Figure 2). There was no significant association in miRNA serum expression levels by extent of disease, disease-free survival and PET scan avidity (tumor SUV) in patients with ACC. There was no significant difference in miRNA serum levels by functional status in patients with benign or malignant adrenocortical tumors.
Figure 1.
A comparison of the normalized miRNA expression of (A) miR-34a (p=0.0011), (B) miR-483-5p (p=0.0113), (C) miR-195 (p=0.9210), (D) miR-let-7d (p=0.1975), (E) miR-214 (p=0.1370) in patients with benign adrenocortical tumors and malignant adrenocortical carcinoma.
Figure 2.
Receiver operator curve for (A) miR-34a (AUC=0.8102) and (B) miR-483-5p (AUC=0.7406).
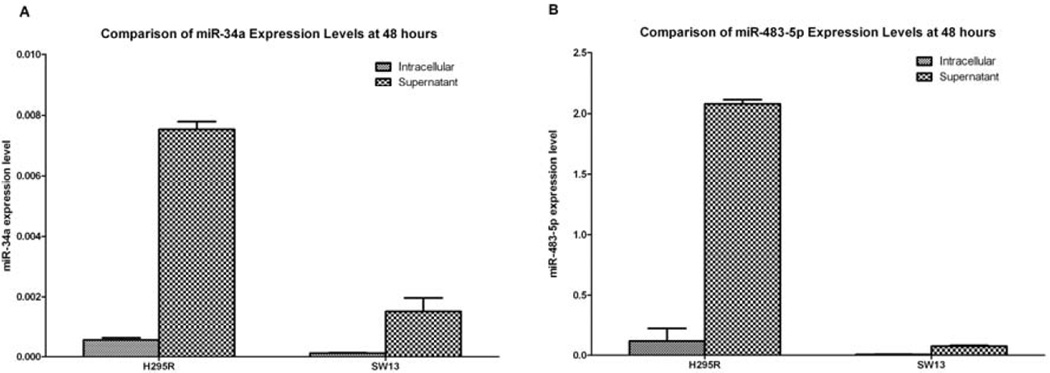
In order to determine if miR-34a and miR-483-5p were secreted by ACC cells, we measured intracellular and culture media expression levels by RT-PCR in the ACC cell lines, H295R and SW13. The expression level of miR-34a and miR-483-5p in both cell lines was higher in the supernatant at 48 hours compared to the intracellular expression levels (Figure 3). The media only negative control did not show expression of either miR-34a or miR-483-5p.
Figure 3.
A comparison of intracellular (normalized to miR-U6) and supernatant (normalized to miR-16) miRNA expression levels of (A) miR-34a and (B) miR-483-5p in H295R and SW13 cell lines, respectively.
Discussion
To the authors knowledge there have been no studies evaluating miRNAs levels in serum samples of patients with ACC. We show that miRNAs, which are dysregulated in adrenocortical tumors are detectable in human serum samples. Moreover, miR-34a and miR-483-5p are candidate serum biomarkers for distinguishing between benign and malignant adrenocortical tumors. Furthermore, we found that miR-34a and miR-483-5p were secreted by ACC cells. These miRNAs, dysregulated in ACC, could potentially be useful for ACC diagnosis, prognosis and as therapeutic targets.
Patterson et. al. (9) showed that the miR-34a was found to have a significantly lower expression in ACC tissue by microRNA profiling studies. However, validation of miR-34a by quantitative PCR did not show a difference between malignant and benign samples (9). But we were interested in evaluating miR-34a levels in serum because it targets p53 and ectopic expression of miR-34a has been shown to induce apoptosis (14). Inactivating mutations of the tumor suppressor gene TP53 have been shown to be present in 25% to 70% of sporadic ACCs (4). There is a positive feedback loop via the myc and SIRT1 pathways, whereby p53 promotes expression of miR-34a, which leads to apoptosis and cell cycle arrest (14). The serum data in our study showed higher levels of miR-34a compared to benign, which may indicate that miR-34a is actively secreted by malignant cells to evade apoptosis and cell cycle arrest.
MiR-483-5p has been shown to be dysregulated in ACC and is a diagnostic and prognostic marker of ACC in tumor samples (10, 13, 17). It is located within the second intron of IGF2 at 11p15.5 and is coexpressed with IGF2 9). The IGF2 protein has been shown to be overexpresseded in ACCs and has been shown in immunohistochemical studies to differentiate between benign and malignant adrenal tumors (13). Since miR-483-5p and IGF2 are co-expressed, the detection in serum and differential expression serves as a first step in a possible noninvasive biomarker that could not only diagnosis ACC, but also serve as a biomarker of recurrence.
After finding a significantly different level of miR-34a and miR-483-5p in serum samples from patients with malignant and benign adrenocortical tumors, we set out to better understand whether these miRNAs could potentially be secreted from tumor cells in vitro. Our in vitro data in the H295R cell line shows that miR-483-5p is highly expressed and is secreted into the supernatant. This would be consistent with overexpression of miR-483-5p in ACC (4, 9), extracellular secretion of miR-483-5p and thus higher serum levels in patients with ACC. This is consistent with studies which demonstrated active cellular secretion of miRNAs via exosomes (18). An alternative explanation consistent with a high expression of miR-483-5p in the serum would be the presence of apoptotic cell bodies due to tumor necrosis (11).
In contrast, the miR-34a data was not what we expected. The expression levels of miR-34a in malignant serum samples were high, even though the ACC tissue expression levels were low in previous studies. A potential explanation for these findings could be that miR-34a is actively secreted from the cell thereby depleting intracellular levels. Indeed, Ohshima et. al. (11) demonstrated that miR-let-7, a tumor suppressive miRNA, is selectively secreted into the extracellular environment via exosomes in a metastatic gastric cancer cell line. The investigators proposed that tumor suppressive miRNAs may be actively secreted by cancer cells (11). Our in vitro data in the H295R and SW13 cell line shows that the expression level of miR-34a in the supernatant is higher compared to intracellular levels.
Although our results indicate that miR-483-5p and miR-34a could serve as potential diagnostic biomarkers, the study has several limitations. The majority of our patients with benign tumors had functioning tumors and therefore would undergo surgery regardless of their serum miRNA level. This limits the diagnostic applicability of these miRNAs in patients with nonfunctional tumors. A larger samples size will be needed to confirm our findings and to validate the diagnostic accuracy of these miRNAs. Furthermore, serum samples before and after resection of ACC and with adequate follow up would be helpful in unequivocally demonstrating that miR-483-5p and miR-34a levels are directly related to the burden of diseases.
In conclusion, we have shown that miRNA can be detected in serum samples of patients with adrenocortical tumors, and that miR-483-5p and miR-34a are significantly higher in the serum of patients with ACC. These findings suggest that miR-483-5p and miR-34a may be helpful biomarkers for ACC diagnosis and prognosis.
Acknowledgments
Financial Support: This research was supported by the intramural research program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Toniato A, Merante-Boschin I, Opocher G, Pelizzo MR, Schiavi F, Ballotta E. Surgical versus conservative management for subclinical Cushing syndrome in adrenal incidentalomas: a prospective randomized study. Ann Surg. 2009 Mar;249(3):388–391. doi: 10.1097/SLA.0b013e31819a47d2. [DOI] [PubMed] [Google Scholar]
- 2.Chiodini I. Clinical review: Diagnosis and treatment of subclinical hypercortisolism. J Clin Endocrinol Metab. 2011 May;96(5):1223–1236. doi: 10.1210/jc.2010-2722. [DOI] [PubMed] [Google Scholar]
- 3.NIH state-of-the-science statement on management of the clinically inapparent adrenal mass ("incidentaloma") NIH Consens State Sci Statements. 2002 Feb 4–6;19(2):1–25. [PubMed] [Google Scholar]
- 4.Lehmann T, Wrzesinski T. The molecular basis of adrenocortical cancer. Cancer Genet. 2012 Apr;205(4):131–137. doi: 10.1016/j.cancergen.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 5.Icard P, Goudet P, Charpenay C, Andreassian B, Carnaille B, Chapuis Y, Cougard P, Henry JF, Proye C. Adrenocortical carcinomas: surgical trends and results of a 253-patient series from the French Association of Endocrine Surgeons study group. World J Surg. 2001 Jul;25(7):891–897. doi: 10.1007/s00268-001-0047-y. [DOI] [PubMed] [Google Scholar]
- 6.Etheridge A, Lee I, Hood L, Galas D, Wang K. Extracellular microRNA: a new source of biomarkers. Mutat Res. 2011 Dec 1;717(1–2):85–90. doi: 10.1016/j.mrfmmm.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gilad S, Meiri E, Yogev Y, Benjamin S, Lebanony D, Yerushalmi N, Benjamin H, Kushnir M, Cholakh H, Melamed N, Bentwich Z, Hod M, Goren Y, Chajut A. Serum microRNAs are promising novel biomarkers. PLoS One. 2008;3(9):e3148. doi: 10.1371/journal.pone.0003148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tombol Z, Szabo PM, Molnar V, Wiener Z, Tolgyesi G, Horanyi J, Riesz P, Reismann P, Patocs A, Liko I, Gaillard RC, Falus A, Racz K, Igaz P. Integrative molecular bioinformatics study of human adrenocortical tumors: microRNA, tissue-specific target prediction, and pathway analysis. Endocr Relat Cancer. 2009 Sep;16(3):895–906. doi: 10.1677/ERC-09-0096. [DOI] [PubMed] [Google Scholar]
- 9.Patterson EE, Holloway AK, Weng J, Fojo T, Kebebew E. MicroRNA profiling of adrenocortical tumors reveals miR-483 as a marker of malignancy. Cancer. 2011 Apr 15;117(8):1630–1639. doi: 10.1002/cncr.25724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soon PS, Tacon LJ, Gill AJ, Bambach CP, Sywak MS, Campbell PR, Yeh MW, Wong SG, Clifton-Bligh RJ, Robinson BG, Sidhu SB. miR-195 and miR-483-5p Identified as Predictors of Poor Prognosis in Adrenocortical Cancer. Clin Cancer Res. 2009 Dec 15;15(24):7684–7692. doi: 10.1158/1078-0432.CCR-09-1587. [DOI] [PubMed] [Google Scholar]
- 11.Ohshima K, Inoue K, Fujiwara A, Hatakeyama K, Kanto K, Watanabe Y, Muramatsu K, Fukuda Y, Ogura S, Yamaguchi K, Mochizuki T. Let-7 microRNA family is selectively secreted into the extracellular environment via exosomes in a metastatic gastric cancer cell line. PLoS One. 2010;5(10):e13247. doi: 10.1371/journal.pone.0013247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang CJ, Hsu CC, Chang CH, Tsai LL, Chang YC, Lu SW, Yu CH, Huang HS, Wang JJ, Tsai CH, Chou MY, Yu CC, Hu FW. Let-7d functions as novel regulator of epithelial-mesenchymal transition and chemoresistant property in oral cancer. Oncol Rep. 2011 Oct;26(4):1003–1010. doi: 10.3892/or.2011.1360. [DOI] [PubMed] [Google Scholar]
- 13.Singh P, Soon PS, Feige JJ, Chabre O, Zhao JT, Cherradi N, Lalli E, Sidhu SB. Dysregulation of microRNAs in adrenocortical tumors. Mol Cell Endocrinol. 2012 Mar 31;351(1):118–128. doi: 10.1016/j.mce.2011.09.041. [DOI] [PubMed] [Google Scholar]
- 14.Chen F, Hu SJ. Effect of microRNA-34a in cell cycle, differentiation, and apoptosis: a review. J Biochem Mol Toxicol. 2012 Feb;26(2):79–86. doi: 10.1002/jbt.20412. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Jia Y, Zheng R, Guo Y, Wang Y, Guo H, Fei M, Sun S. Plasma microRNA-122 as a biomarker for viral-, alcohol-, and chemical-related hepatic diseases. Clin Chem. 2010 Dec;56(12):1830–1838. doi: 10.1373/clinchem.2010.147850. [DOI] [PubMed] [Google Scholar]
- 16.Song J, Bai Z, Han W, Zhang J, Meng H, Bi J, Ma X, Han S, Zhang Z. Identification of suitable reference genes for qPCR analysis of serum microRNA in gastric cancer patients. Dig Dis Sci. 2012 Apr;57(4):897–904. doi: 10.1007/s10620-011-1981-7. [DOI] [PubMed] [Google Scholar]
- 17.Ozata DM, Caramuta S, Velazquez-Fernandez D, Akcakaya P, Xie H, Hoog A, Zedenius J, Backdahl M, Larsson C, Lui WO. The role of microRNA deregulation in the pathogenesis of adrenocortical carcinoma. Endocr Relat Cancer. 2011 Oct;18(6):643–655. doi: 10.1530/ERC-11-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kharaziha P, Ceder S, Li Q, Panaretakis T. Tumor cell-derived exosomes: a message in a bottle. Biochim Biophys Acta. 2012 Aug;1826(1):103–111. doi: 10.1016/j.bbcan.2012.03.006. [DOI] [PubMed] [Google Scholar]