Summary
Investigators developed chimeric antigen receptors (CARs) for expression on T cells more than 25 years ago. When the CAR is derived from an antibody, the resultant cell should combine the desirable targeting features of an antibody (e.g. lack of requirement for major histocompatibility complex recognition, ability to recognize non-protein antigens) with the persistence, trafficking and effector functions of a T-cell. This article describes how the past two decades have seen a crescendo of research which has now begun to translate these potential benefits into effective treatments for patients with cancer. We describe the basic design of CARs, describe how antigenic targets are selected, and the initial clinical experience with CART cells. Our review then describes our own and other investigators’ work aimed at improving the function of CARs and reviews the clinical studies in hematological and solid malignancies that are beginning to exploit these approaches. Finally, we show the value of adding additional engineering features to CAR-T cells, irrespective of their target, to render them better suited to function in the tumor environment, and discuss how the safety of these heavily modified cells may be maintained.
Keywords: cancer, immunotherapy, T cells, CAR, gene therapy
Introduction
Chimeric antigen receptor-expressing T (CAR-T) cells are examples of adoptive cellular immunotherapies (ACIs) which are themselves a subset of complex biological therapies (CBTs) (1, 2). While such therapies have been available for more than 20 years, it has proved difficult to develop them to a stage at which they can be predictably successful and widely implemented as a standard of care. In this review, we outline some specific approaches to overcome these barriers for CAR-T cells, but it is important also to understand the more general barriers that ACIs face in becoming approved therapies in clinical practice.
Many ACIs have to be individually made for each patient, a challenge to the robust scalability required for late phase clinical studies. Moreover, the standard pharmaceutical business model is to recoup the costs of initial drug development by selling cheap-to-manufacture licensed drugs that ameliorate rather than cure and that are administered over a prolonged period of time with exceedingly high profit margins. Many ACIs will remain expensive to produce even after approval, an effect compounded by the stacked license fees for the many patents covering the multiplicity of intellectual property incorporated in a single product. Unlike many conventional drugs, ACIs, including CAR-T cells, are intended to be curative not ameliorative, so that they will need to be given once, or a few times, only. Finally, the very specificity of these ACIs means that only a small subset of patients with any given cancer may be suited to treatment, making every ACI an orphan drug. In combination, these market issues can lead to an unaffordable pricing structure with little appeal to pharmaceutical companies. In this review, we describe how we are developing a “plug and play” approach to adoptive immunotherapy, using CAR-T cells directed to cancer. With this approach, it will be possible to use a multiplicity of genetic engineering strategies that can enhance access to and killing of many different types of tumor cells. The tumor targeted is then altered simply by changing the specificity of the targeting receptor on the adoptively transferred effector T cells. In other words, broadly applicable strategies will be made specific for individual tumors by coupling the engineered T-cell to specific chimeric antigen receptors.
For this concept to work, we must first define appropriate target antigens within and around tumor cells to provide specificity of action, and devise receptors that can signal to the T cell that it has engaged the appropriate antigen within the tumor or its microenvironment. We have to then develop generic approaches to enhance the anti-tumor effector activity of the adoptively transferred cells, increase their resistance to tumor immune evasion strategies, and allow the immune response to be terminated should it prove toxic or damaging to the host immediately or in the longer term.
Design of the chimeric antigen receptor
CARs combine the antigen-binding property of monoclonal antibodies with the lytic capacity and self-renewal of T cells and have several advantages over conventional T cells (3–5). CAR-T cells recognize and kill tumor cells independently of the major histocompatibility complex (MHC), so that target cell recognition is unaffected by some of the major mechanisms by which tumors avoid MHC-restricted T-cell recognition, such as downregulation of human leukocyte antigen (HLA) class I molecules and defective antigen processing.
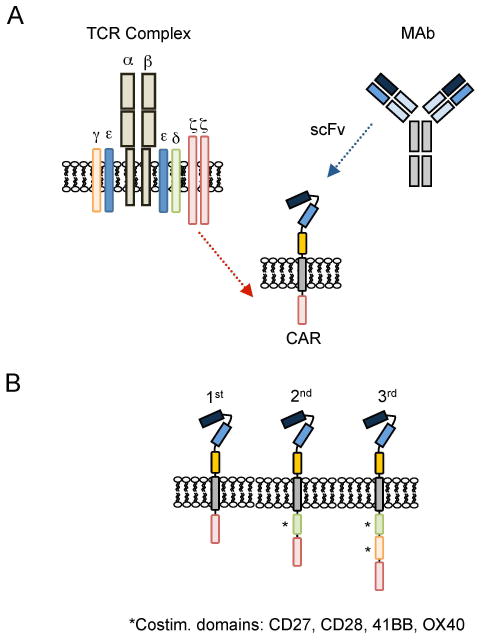
Chimeric immune receptors were first developed in the mid-1980s and initially consisted of the variable (antigen binding) regions of a monoclonal antibody and the constant regions of the T-cell receptor (TCR) α and β chains (6). In 1993, Eshhar et al. modified this design to use an ectodomain, from a single chain variable fragment (scFv), from the antigen binding regions of both heavy and light chains of a monoclonal antibody (Figs 1 and 2), a transmembrane domain, and an endodomain with a signaling domain derived from CD3-ζ. Most CARs subsequently designed and used have followed this same structural pattern, with incorporation of co-stimulatory signaling endodomains, which are described in Fig. 1 and below (Provision of Co-stimulation to enhance T-cell activity after antigen-specific receptor engagement). In this section, we describe how the composition of the ectodomain, hinge and transmembrane domain influences CAR function and the consequent behavior of the T cell that expresses it (Fig. 2).
Fig. 1. CAR Design.
(A) CARs consist of an ectodomain, commonly derived from a single chain variable fragment (scFv), a transmembrane domain, and an endodomain. (B) Depending on the number of signaling domains, CARs are classified into 1st generation (one), 2nd generation (two), or 3rd generation (three) CARs.
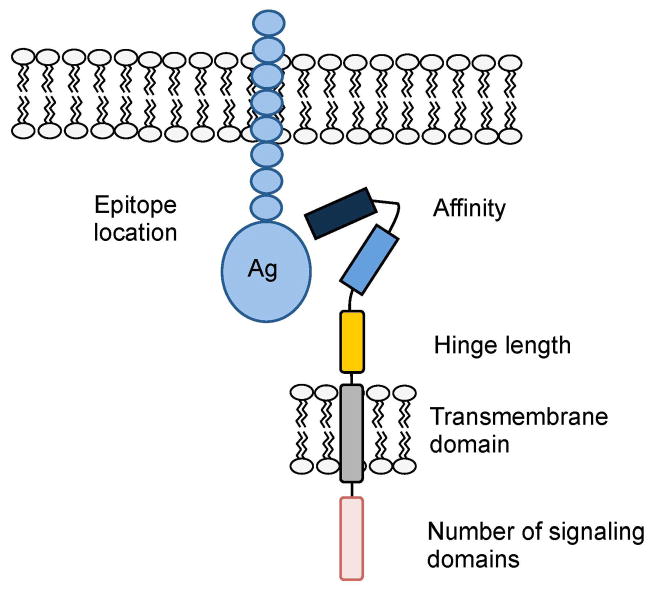
Fig. 2. Critical CAR components.
Optimal CAR activity is determined by epitope location, scFv affinity, hinge and transmembrane domains, and number of signaling domains.
Ectodomain of CARs
ScFvs are the most commonly used ectodomains for CARs, and the affinity of the scFv predicts CAR function (7, 8). For example, T cells expressing CARs containing high affinity ROR1-specific scFv have superior effector function than low affinity scFvs (7). There is, however, a plateau above which further affinity maturation does not increase T-cell activation for any given CAR. The likely explanation for the plateau effect is that the avidity of the CAR needed for maximal T-cell activation is a function of the number and density of the expressed receptors as well as their affinity (8). In addition to CAR affinity, function is also affected by the location of the recognized epitope on the antigen (9, 10). For example, CAR-T cells expressing an scFv that recognized an epitope on CD22 (an antigen expressed by normal and malignant B cells) that was proximal to the B cells’ plasma membrane had superior anti-leukemic activity to CAR-T cells that recognized a membrane-distal epitope (10). Antigen binding and subsequent activation can also be modulated by introducing a flexible linker sequence in the CAR, which will also allow expression of two distinct scFvs that can recognize 2 different antigens (11) (Figs 1 and 2). T cells expressing these so-called tandem CARs (TanCARs) may be better able to kill tumor targets expressing low levels of each antigen individually and may also reduce the risk of tumor immune escape due to the emergence of single antigen loss variants.
Because the scFvs used to date in the clinic have almost all consisted of both heavy and light chain-derived antigen binding domains and are often derived from murine monoclonal antibodies, there is considered to be a significant risk of anti-idiotype or anti-mouse antibodies, either of which can block function. Single domain scFvs have therefore also been used to prepare CARs (12). Their smaller ectodomain may render them less immunogenic, although this may come with the cost of lower affinity/specificity. Another strategy to reduce CAR immunogenicity is to humanize the scFvs, an approach taken for HER2-, EphA2-, and mesothelin-specific CARs (13–15). Unfortunately, this approach does not preclude the development of anti-idiotype antibodies that may be equally inhibitory.
The CAR concept is not confined to using scFvs as the targeting ectodomain, and other ligands and receptors have been substituted. For example, IL13Rα2-specific CARs have been prepared by modifying IL13 molecules to form ectodomains and used clinically (16–18), while NKG2D-ligand and CD70-specific CARs have been constructed by adding a ζ-signaling domain to the cytoplasmic tail of NKG2D or the CD70 receptor (CD27) respectively (19–21). Peptide ligands have also been used as CAR ectodomains. For example, Davies et al. (22) designed a CAR containing the promiscuous T1E peptide ligand that will recognize and bind to target cells expressing the ErbB family of receptors. Finally, multiple antigens can be recognized by so called ‘universal ectodomains’ such as CARs that incorporate an avidin ectodomain to recognize targets that have been incubated with biotinylated monoclonal antibodies (23), or that contain a FITC-specific scFv, which has potent antitumor activity in preclinical animal models when given in combination with FITC-labeled monoclonal antibodies (24). These alternative CAR ectodomains have performed well in preclinical studies, but only the IL13Rα2-specific CAR have been tested in humans (25, 26).
Hinge region of CARs
While the ectodomain is critical for CAR specificity, the connecting sequence between the ectodomain to the transmembrane domain (the hinge region) (Fig. 1), can also profoundly affect CAR-T-cell function by producing differences in the length and flexibility of the resulting CAR. For example Guest et al. (27) compared the influence of adding a CH2CH3 hinge derived from IgG1 to hingeless CARs specific for carcinoembryonic antigen (CEA), neural small adhesion molecule (NCAM), 5T4, or CD19. While 5T4- and CD19-specific CAR-T cells with a CH2CH3 hinge had enhanced effector function, CEA- and NCAM-specific CAR-T cells had optimal activity without a hinge. More recently, Hudecek et al. (7) compared the influence of a CH2-CH3 hinge [229 amino acids (AA)], CH3 hinge (119 AA), and short hinge (12AA) on the effector function of T cells expressing 3rd generation ROR1-specific CARs. They demonstrated that T cells expressing ‘short hinge’ CARs had superior antitumor activity. Conversely, other investigators found that a CH2-CH3 hinge impaired epitope recognition of a 1st generation CD30-specific CAR (28). While these results are somewhat conflicting, they all clearly indicate the potential importance of the hinge region in determining outcome of CAR engagement.
At present we do not know the mechanisms underlying the above observational differences, and our dataset is too small for any general predictive rules or algorithm to have emerged as to which hinge will likely best work with which CAR. For the moment, therefore, empiric testing of scFv/hinge domain combinations is required to determine optimal CAR design, although this will likely change as more experimental data and validation studies are available for analysis.
Transmembrane domain of CARs
Between the hinge and the signaling endodomains lies the transmembrane domain. This is usually derived from CD3-ζ, CD4, CD8, or CD28 molecules. Like hinges, the transmembrane domains were initially viewed as inert structural links between the ectodomain and endodomain of the CAR. It is now evident that the transmembrane domain can indeed influence CAR-T-cell effector function. For example, first generation CD19-specfic CAR which contain a CD3-ζ transmembrane domain are less stable over time on the cell surface of T cells in comparison to 2nd generation CD19-specific CAR-T cells with a CD28 transmembrane domain (29). Simply replacing the CD3-ζ transmembrane domain with a CD28 transmembrane domain renders the expression of 1st generation CARs more stable (Dotti et al., unpublished data). Other investigators have shown that an intact CD3-ζ transmembrane domain is essential for measurable signaling by a 1st generation CEA-specific CAR expressed in a T-cell line (30). CARs incorporating a transmembrane domain from native CD3-ζ chain could dimerize and form complexes with endogenous TCRs resulting in enhanced T-cell activation, while CARs containing mutated CD3-ζ transmembrane lacked these interactions.
Endodomain of CARs
Upon antigen recognition, CAR endodomains transmit activation and costimulatory signals to T cells. T-cell activation relies on the phosphorylation of immunoreceptor tyrosine-based activation motifs (ITAMs) present in the cytoplasmic domain to the cytoplasmic CD3-ζ domain of the TCR complex (31). While the majority of current CAR endomains contain an activation domain derived from CD3-ζ, other ITAM-containing domains have been explored including the Fc receptor for IgE-γ domain, which proved to be less effective (32). The selection of costimulatory signaling domains is described in detail in a later section (Provision of co-stimulation to enhance CAR-T-cell activity after antigen-specific receptor engagement).
In conclusion, there is an intricate interplay between scFVs, hinge, transmembrane domain and endodomain that determines CAR function and there is no single optimal configuration that is ‘one size fits all’. For the moment, therefore, CAR receptor optimization remains largely empirical, with testing required in a range of pre-clinical models.
Antigens suited to CAR-targeted adoptive cellular immunotherapy
Unlike the native TCR, the majority of scFv-based CARs only recognize target antigens expressed on the cell surface, rather than internal antigens that are processed and presented by the cells’ MHC. While this limits the detection of a range of tumor specific antigenic epitopes (for example from mutant oncogenes and translocations), CARs have the advantage over the classical TCR that they can recognize structures other than protein epitopes, including carbohydrates and glycolipids. This increases the pool of potential target antigens. Like other cancer immunotherapy approaches, CARs should ideally target antigens that are only expressed on cancer cells or their surrounding stroma (33), such as the splice variant of EGFR (EGFRvIII), which is specific to glioma cells (34). Few human antigens, however, meet this criterion, and the majority of target antigens (Tables 1 and 2) are expressed either at low levels on normal cells (e.g. GD2, CAIX, HER2) and/or in a lineage restricted fashion (e.g. CD19, CD20).
Table 1.
CAR targets for hematological malignancies
| Antigen | Malignancy | CAR ectodomain, antigen class | Clinical Trial |
|---|---|---|---|
| CD19 (29, 35–44) | B-cell | scFv, protein | published |
| CD20 (36, 45–48) | B-cell | scFv, protein | published |
| CD22 (10) | B-cell | scFv, protein | |
| CD30 (49–51) | B-cell | scFv, protein | ongoing |
| CD33 (52) | Myeloid | scFv, protein | |
| CD70 (21) | B-cell/T-cell | ligand, protein | |
| CD123 (53) | Myeloid | scFv, protein | |
| Kappa (54) | B-cell | scFv, protein | ongoing |
| Lewis Y (55, 56) | Myeloid | scFv, carbohydrate | ongoing |
| NKG2D ligands (20, 57–59) | Myeloid | ligand, protein | |
| ROR1 (7) | B-cell | scFv, protein |
Table 2.
CAR targets for solid tumors
| Antigen | Malignancy* | CAR ectodomain, antigen class | Clinical Trial |
|---|---|---|---|
| B7H3 (60) | sarcoma, glioma | scFv, protein | |
| CAIX (61, 62) | kidney | scFv, protein | published |
| CD44 v6/v7 (63, 64) | cervical | scFv, protein | |
| CD171 (65) | neuroblastoma | scFv, protein | published |
| CEA (66) | colon | scFv, protein | ongoing |
| EGFRvIII (67, 68) | glioma | scFv, protein | ongoing |
| EGP2 (69, 70) | carcinomas | scFv, protein | |
| EGP40 (71) | colon | scFv, protein | |
| EphA2 (14) | glioma, lung | scFv, protein | |
| ErbB2(HER2) (72–79) | breast, lung, prostate, glioma | scFv, protein | published |
| ErbB receptor family (22) | breast, lung, prostate, glioma | ligand, protein | |
| ErbB3/4 (80, 81) | breast, ovarian | scFv, protein | |
| HLA-A1/MAGE1 (82, 83) | melanoma | scFV, peptide/protein complex | |
| HLA-A2/NY-ESO-1 (84) | sarcoma, melanoma | scFV, peptide/protein complex | |
| FR-α (85–88) | ovarian | scFv, protein | published |
| FAP** (89) | cancer associated fibroblasts | scFv, protein | |
| FAR (90) | rhabdomyosarcoma | scFv, protein | |
| GD2 (91–93) | neuroblastoma, sarcoma, melanoma | scFv, ganglioside | published |
| GD3 (94) | melanoma, lung cancer | scFv, ganglioside | |
| HMW-MAA (95) | melanoma | scFv, proteoglycan | |
| IL11Rα (96) | osteosarcoma | ligand, protein | |
| IL13Rα2 (16–18, 25) | glioma | ligand, protein | ongoing |
| Lewis Y (55, 97, 98) | breast/ovarian/pancreatic | scFv, carbohydrate | published |
| Mesothelin (15, 99) | mesothelioma, breast, pancreas | scFv, protein | ongoing |
| Muc1 (100) | ovarian, breast, prostate | scFv, glycosylated protein | |
| NCAM (101) | neuroblastoma, colorectal | scFv, protein | |
| NKG2D ligands (20, 57–59) | ovarian, sacoma | native receptor, protein | |
| PSCA (102, 103) | prostate, pancreatic | scFv, protein | |
| PSMA (104, 105) | prostate | scFv, protein | |
| TAG72 (106, 107) | colon | scFv, carbohydrate | |
| VEGFR-2** (108, 109) | tumor vasculature | ligand/scFv, protein | ongoing |
many antigens are expressed on several malignancies; due to space limitations only examples are listed
expressed on the tumor stroma
Antigens overexpressed by tumor cells
Although the majority of target antigens on tumor cells are shared with normal tissues and are only overexpressed in comparison to normal tissues, many have been targeted by CAR-T cells in preclinical animal models. Unfortunately, these models often cannot accurately predict human toxicities since many of the scFV ectodomains do not recognize non-human counterparts of the targeted antigens, the tissue distribution of which is also frequently species specific. This same limitation, of course, applied to studies with monoclonal antibodies. So to avoid unexpected toxicities, many of the first clinical studies selected target antigens based on the availability of monoclonal antibodies and their safety profile in humans. Since almost no monoclonal antibody has been free of at least some adverse effects, the decision on whether to proceed with a study in which the CAR is derived from a monoclonal antibody has been determined by careful assessment of the likely risk:benefit ratio for any given target antigen. For example, the EGFR monoclonal antibody cetuximab is FDA approved for the treatment of EGFR-positive cancers, but most patients treated with the monoclonal antibody develop a skin rash due to baseline EGFR expression in epithelial cells (110). Since the overall avidity of EGFR-specific CARs arrayed on a T cell would be greater than the avidity of a bivalent soluble antibody, significant safety concerns were raised about the severity and persistence of skin toxicity that have so far precluded clinical trials of EGFR-specific CAR-T cells by systemic administration. Conversely, GD2- and HER2-specific monoclonal antibodies have a favorable safety profile, which led to testing of CAR-T cells in humans (73, 91, 92, 111).
Lineage-specific antigens
Lineage-specific targets have been mainly explored for hematological malignancies (29, 37, 40, 42–44, 112). For example, CAR-T-cell treatment of B-cell malignancies can be used to target a highly and consistently expressed lineage-specific antigen (e.g. CD19, CD20), even though it is also expressed by normal B cells, since replacement therapy using intravenous immune globulin (IVIG) is feasible. In general, however, it might be preferable to target more lineage-associated antigens. For example, in many B-cell malignancies it is possible to target either the κ- or λ-light chain, since normal B cells express one or other of these antigens, while all the cells of the (clonal) malignancy will express a single light chain (54). In addition, as discussed below (Increasing the safety of CAR-T cells), the increasing potency of later generation CARs and their combination with other strategies to improve their function will almost inevitably increase their potential for ‘on-target antigen but off target tissue’, thereby producing unexpected toxicities even when targeting lineage specific antigens, since these might be aberrantly expressed at low levels elsewhere.
Other considerations for antigen selection
Targeting single antigens carries the inherent risk of immune escape (113–115), which can be reduced by targeting multiple antigens. In preclinical studies, targeting HER2 and IL13Rα2 with CAR-T cells resulted in enhanced antitumor effects in comparison to CAR-T cells that targeted a single antigen (116). As discussed below (Increasing the safety of CAR-T cells), expressing multiple CARs in T cells also has the potential to increase safety by generating T cells that recognize a unique antigen pattern that is only present on tumor cells. Targeting antigens exclusively expressed by cancer cells does not, however, address the fundamental problem of selecting variants that lack expression of the target antigen. Such escape variants are common because of the marked genetic instability of most cancer cells (117). One solution is to target antigens expressed on the tumor stroma, a component that is critical for tumor growth and is more genetically stable. As discussed below (Targeting the cellular components of tumor stroma) investigators have therefore targeted fibroblast activation protein (FAP) expressed on cancer associated fibroblasts (CAFs) or vascular endothelial growth factor receptor (VEGFR)-2 expressed on the endothelial cells of the tumor vasculature (89, 108).
Initial clinical experience with CAR-T cells
Initial studies in humans were conducted with T cells expressing the 1st generation CARs illustrated in Fig. 1. Investigators used CAR-T cells to target hematological malignancies as well as solid tumors. Even when these CAR-T cells were combined with lymphodepletion (to reduce Treg mediated inhibition and favor homeostatic expansion of the infused cells), the results were uniformly disappointing, with minimal expansion or persistence in vivo, and with no unequivocal evidence of antitumor activity. For example, 2 patients received CD20-specific CAR-T cells post autologous stem cell transplant, and 4 patients received CD19-specific CAR-T cells in combination with IL-2 outside the transplant setting (36, 45). While high doses of T cells were administered (up to 2×109 cells/m2), T-cell persistence was less than one week. Despite the short persistence, 2 patients developed antibodies directed to the CAR. The clinical efficacy of these CAR-T cells was difficult to assess since patients either received T cells post transplant or received additional therapies post T-cell infusion.
First generation CAR-T cells targeting carbonic anhydrase IX (CAIX), CD171, folate receptor α (FR-α), and GD2 were evaluated in patients with advanced stage solid tumors (61, 87, 91, 92, 118). Lamers et al. (61) infused renal cell carcinoma RCC (RCC) patients with polyclonal T cells expressing a 1st generation CAIX-specific CAR. Two of the first 3 patients developed hepatitis due to CAIX expression on bile ducts. Both patients also developed potent anti-CAR immune responses resulting in limited T-cell persistence (61). Subsequently, pretreatment with CAIX monoclonal antibodies of 4 patients prior to CAR-T-cell transfer prevented hepatitis and abrogated the induction of anti-CAR immune responses (118). While this resulted in prolonged T-cell persistence, no clinical benefit was observed. Six neuroblastoma patients received up to 109/m2 of CD8+ T-cell clones expressing 1st generation CARs specific for CD171 (65). Infusions were well tolerated, but T cells persisted only for 6 weeks, and only 1 out of 6 patients had a partial response 8 weeks post T-cell infusion. Kershaw et al. (87) evaluated the safety and efficacy of 1st generation FR-α CAR-T cells in patients with ovarian cancer. Eight patients received up to 5×1010 CAR-T cells in combination with IL-2, where as 6 patients received CAR-T cells in combination with an allogeneic PBMC vaccine. T cells persisted less than 3 weeks in all but one patient and did not specifically home to tumor sites as judged by 111Indium scintigraphy. No antitumor activity was observed. Because of these disappointing outcomes, extensive efforts have been made to enhance the effector function of CAR-T cells in vivo, which are discussed in detail in the following sections.
Optimal T-cell subset in which to express CARs
The ideal T-cell target in which to graft CAR molecules should be the subset that can traffic to tumor sites, receive appropriate co-stimulation and retain a profile predictive of prolonged in vivo survival. Moreover, the T-cell chosen should preferably not be able to produce toxicity in vivo due to the inappropriate activation of signaling pathways in otherwise quiescent T-cell subtypes, or because of disruption to the otherwise tightly coordinated activation and subsequent contraction process of T cells that are dependent on both antigenic stimulation and physiological costimulation and inhibition (see section “Provision of costimulation to enhance CAR-T-cell activity after antigen-specific receptor engagement”).
Identification of optimal T-cell surface phenotype
A number of investigators have tried to identify a phenotypic profile for the optimal subset to allow this component of the T-cell system to be separated and selectively transduced, to produce maximum benefit with minimum adverse effects (119, 120) (Fig. 3). These efforts are based on evidence that signals for memory T-cell development are received during the initial expansion of T cells and that the pattern of development and cell fate are influenced by the stimuli received during initial exposure to antigen (priming). The affinity of the TCR engagement, the balance of costimulatory versus inhibitory cellular and soluble signals, as well as other environmental cues (121, 122) all dictate the diversity of T-cell subsets which generate memory T cells and effector T cells (Teff) either linearly, progressively, or through asymmetric division (123).
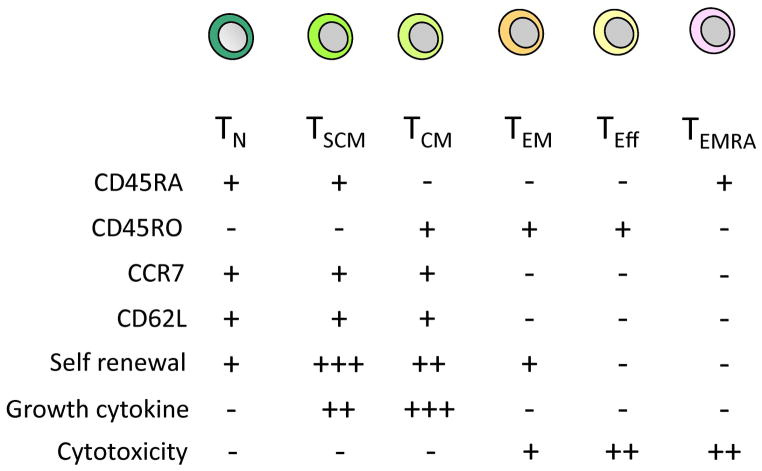
Fig. 3. T-cell subsets.
The phenotypic properties of T-cell subsets. − indicates absence and + presence of the specific cell surface marker. Functional properties for each subset are also shown. − indicates lack of the functional property; +, ++, +++ indicates the degree (low, intermediate, high) of the specific property.
The obvious population in which to express CARs is the Teff, since this subset are potent anti-tumor effector cells (124). Teff cells are, however, less appealing for adoptive immunotherapy because of their limited proliferative capacity and persistence in vivo. Investigators instead are now concentrating on memory T-cell subsets, which are traditionally divided into central and effector memory cells (Tcm and Tem) based upon the expression of CD62L and CCR7 (maintained on Tcm and lost on Tem) (125, 126). These cells are ‘antigen-experienced’, can expand substantially in vivo and are long-lived. Many studies have now shown that a balanced composition of both Tcm and Tem subsets can effectively and rapidly control repeated exposure to pathogens over a prolonged period (127). Because of these characteristics, Tcm and Tem cells have been considered ideal vehicles for grafting CARs. Since CD62L expression can be used to identify T cells with memory characteristics, investigators have used positively selected CD62L cells to express a CAR and shown superior activity in a mouse model (128). A clinical study based on this approach has been opened at the Fred Hutchinson Cancer Research Center.
More recently, a phenotypically defined T-cell subset with true stemness properties (the Tscm) has been identified (129). These cells have enhanced proliferative potential and survival in vivo and can differentiate into memory and effector populations. If they are indeed a truly distinct subset in humans, they may allow investigators to identify a T-cell subset suited to genetic manipulation that will also be best able to mediate complete and durable remission in cancer patients.
Functional T-cell selection
An alternative approach to using surface-phenotype based selection of T-cell subsets prior to CAR expression is to employ T cells already selected in vivo for their established capacity to act as Teff, to enter the memory pool, and to re-expand on re-exposure to antigens. Virus-specific cytotoxic T lymphocytes (CTLs) are an example of such cells, and in addition to their potential for life-long persistence, virus-specific CTLs contain both CD8+ and CD4+ subsets, with the latter compartment critical for long-term persistence of the former (130, 131). Virus-specific CTLs are also well characterized for expression of homing/chemokines receptors commensurate with their capacity for trafficking to and residing in the designated lymphoid or non-lymphoid tissue (132). Their potential value as CAR-expressing effector cells is considered below.
Provision of costimulation to enhance CAR-T-cell activity after antigen-specific receptor engagement
T-cell costimulation is mediated by a multiplicity of receptor-ligand interactions, and plays a fundamental role in preventing the induction of anergy in T lymphocytes upon engagement with the target antigen (121). Tumor cells and the tumor microenvironment are deficient in co-stimulation and favor the induction of T-cell anergy due to their lack of expression of co-stimulatory molecules such as CD80 and CD86 that are ligands for the CD28 receptor expressed by activated effector T cells (133). Adoptively transferred T lymphocytes engineered to express CARs are not immune from tumor-induced anergy. In the absence of CD80/CD86 co-stimulation, engagement of antigen through the CAR produces T-cell hyporesponsiveness (134). In addition, when tumor-specific T cells are expanded ex vivo they may lose expression of CD28, particularly if culture is prolonged, and thus become unresponsive to tumor cells or bystander cells expressing costimulatory molecules.
CAR molecules can be engineered to overcome the lack of co-stimulation by tumor cells. Endodomains derived from well characterized co-stimulatory molecules such as CD28 (54, 104), CD134/OX40 (134), CD137/4-1BB (135, 136), and CD27 (137) can be incorporated within CARs to provide direct T-cell co-stimulation after CAR antigen binding (Fig. 1). Since each of these co-stimulatory molecules activates different signaling pathways (e.g. phosphatidylinositol 3-kinase (PI3K) for CD28 versus tumor necrosis family (TNF)-receptor-associated factor (TRAF) adapter proteins for 4-1BB and OX40), multiple endodomains can be included in a single CAR and thereby recruit multiple signaling pathways, potentially maximizing the co-stimulatory benefits (134, 136). Although the mechanism of the effector cell immunological synapse formation by CAR molecules has not been elucidated, it is likely that CAR cross-linking upon antigen-binding leads to the physical recruitment and dimer formation of costimulatory endodomains incorporated within the CAR and consequent activation of the downstream signaling pathways. An alternative means of providing costimulation, that may occur independently of CAR cross linking relies on the trans- and auto-co-stimulation achieved when CAR-T cells are further engineered to express either CD80 or 4-1BBL molecules that are separated from CAR molecules, but this approach has not yet been clinically tested (138).
Identification of optimal endodomains
That incorporation of co-stimulatory endodomains in CARs enhances the proliferation and activation of CAR-T cells in humans in vivo has been clearly demonstrated by direct comparison of the fate of CAR-T cells with and without an included co-stimulatory endodomain (29) (see below ‘Direct comparison of co-stimulatory endodomain in humans’). Efforts to predict in advance which endodomains or combination of endodomains will prove to be optimal in humans has been much more problematic. In vitro experiments and xenogeneic mouse models have been used to dissect and compare the effects of incorporating each co-stimulatory endodomain into CARs, and it has become evident that the results are often contradictory and may not predict events in clinical studies. For example, when CD28, 4-1BB or combinations of multiple co-stimulatory endodomains were compared, the relative potency of each was dependent on the target antigen, the biology of the tumor cells and the mouse strains used (139, 140). Ultimately, identification of the optimum choice of costimulatory endodomains(s) for a given CAR and tumor cell target may have to be resolved in clinical trials.
Direct comparison of costimulatory endodomain activity in humans
One means of directly determining the value of each specific CAR costimulatory moiety in patients is to simultaneously infuse each patient with two or more T-cell products, each expressing CARs with identical specificity and sequence, but with different costimulatory components. Using this approach, we infused six lymphoma patients simultaneously with two T-cell products expressing the same CD19-specific CAR but encoding either the CD3-ζ chain of the TCR alone or both the CD3-ζ chain and the CD28 endodomain. We demonstrated that CD28 costimulation within the CAR indeed promotes superior in vivo expansion of CD19-specific CAR-T cells (29). In a similar current study at Memorial Sloan-Kettering and University of Pennsylvania, investigators are comparing the simultaneous infusion of T cells expressing CD19-specific CARs incorporating either the CD28 or 4-1BB endodomains. We will initiate a new study to compare CD19-specific CARs incorporating either CD28 alone or the combination of CD28/4-1BB endodomains. Although impressive results have already been reported in B-cell malignancies incorporating the 4-1BB endodomain within a CD19-specific CAR (41, 42, 44), these direct comparator studies retain fundamental importance if we are to know definitively how CAR-mediated T-cell costimulation affects T-cell fate and anti-tumor activity, since this study design avoids the inevitable and significant confounding variables associated with small phase I studies enrolling heterogeneous patients with heterogeneous disease.
Role of lymphodepletion
The expansion, persistence and anti-tumor activity of CAR-T cells may be enhanced if the cells are given after administration of lymphodepleting drugs (141). These benefits may result from reduction of Treg in the lymphoid tissues and in tumor and from the production of cytokines such as IL-7 and IL-15 that may favor expansion of infused cells. At present, however, there is no unequivocal evidence that lymphodepletion benefits the outcome, and the potential toxicities may ultimately negate the putative benefits (38, 73).
Clinical experience with 2nd and 3rd generation CAR-T cells: Hematological malignancies
The most impressive clinical results with CAR-T cells so far has been achieved with polyclonal T cells expressing CD19-specific CARs either with CD28.ζ or 41BB.ζ signaling domains (37, 40–44). These studies have been recently reviewed in detail elsewhere (142). Complete responses were observed post infusion of 2nd generation CAR-T cells in patients with CD19+ hematological malignancies including NHL, chronic lymphocytic leukemia (CLL) and acute lymphoblastic leukemia (ALL). Antitumor activity was dependent on significant T-cell expansion in vivo, which was associated in several patients with a life-threatening cytokine storm (42, 44). The clinical picture was reminiscent of hemophagocytic syndrome and patients responded either to a combination of steroids, TNF-α antibody (infliximab), and IL-6 receptor antibody (tocilizumab) or to monotherapy with tocilizumab (42, 44). While addition of co-stimulatory domains dramatically increased the expansion and persistence of polyclonal T cells, this benefit was not observed with T-cell clones. Three patients received T-cell clones expressing 3rd generation CD20-specific CARs after lymphodepletion with cytoxan (143). T-cell persistence was limited; 2 patients with no evaluable disease remained disease free for 12 and 24 months, and a 3rd patient had a partial response that lasted for 12 months. This study indicates that T-cell clones, regardless of the endodomain used, can have limited T-cell function in vivo.
Besides targeting hematological malignancies with CD19 and CD20 with CAR T cells, we are currently conducting clinical studies with polyclonal T cells expressing 2nd generation CARs specific for the κ-light chain of human immunoglobulin (see ‘Control of toxicities’) or for CD30. We have generated CARs specific to selectively target κ+ lymphoma/leukemia cells, while sparing the normal B cells expressing the non-targeted λ-light chain, thus minimizing the impairment of humoral immunity associated with the depletion of all normal B lymphocytes (54). This approach is now in clinical trial using a 2nd generation CAR encoding the CD28 endodomain and clinical responses including CRs have been observed (144). We have also implemented clinical trials using a 2nd generation CAR specific for the CD30 antigen (50), which is present on most Hodgkin’s lymphoma (HL) and some non Hodgkin’s lymphoma (NHL) cells. The generation of functional CAR-T cells has been successful in the patients with refractory/relapsed diseases, of whom 4 have been treated so far without toxicities.
In contrast to B-cell malignancies, limited clinical experience is available for CARs redirected to T-cell or myeloid-derived malignancies. While preclinical studies have demonstrated the potent anti-acute myeloid leukemia (AML) effects of CD123-specific or Lewis-Y antigen-specific CAR T cells (53, 55), only one clinical study with Lewis-Y antigen-specific CAR T cells is in progress for AML patients (56).
Solid tumors
Limited clinical experience is currently available with targeting solid tumor antigens using 2nd or 3rd generation CARs. One patient who received 1010 T cells expressing a 3rd generation HER2-specific CAR and a lymphodepleting chemotherapy regimen consisting of cytoxan and fludarabine, in combination with IL-2 rapidly developed acute respiratory distress syndrome and died (73). We have infused up to 108/m2 T cells expressing a 2nd generation HER2-specific CAR to patients with osteosarcoma. While no overt toxicities were observed, the antitumor activity has so far been limited (111). Clinical studies with T cells expressing a 3rd generation EGFRvIII-specific CAR for glioma patients are in progress, and another study has treated patients with pancreatic cancer with a 2nd generation mesothelin-specific CAR, one of whom developed an anaphylactic reaction (145).
Using physiological costimulation through the native T-cell receptor
The costimulation provided by second or later generation CARs is non-physiological, since it does not occur in the same regulated tempero-spatial sequence that follows a T-cell encounter with antigen on professional antigen-presenting cells. Indeed, the overabundance of stimulation from a 2nd or 3rd generation CAR may be toxic to the T-cell itself (for example by activation induced cell death) or to the recipient of the T cells (for example by the rapid production of pro-inflammatory cytokines). An alternative approach outlined in the section ‘Optimal T-cell subset in which to express CARs’ relies instead on restricting expression of CARs to a specific T-cell subset such as virus-specific CTLs for which physiologic CD80/CD86 costimulation is provided by professional antigen-presenting cells when viral latent antigens processed by these APCs are encountered by CTLs through their native TCRs (35, 146, 147). While attractive in principle, the choice of suitable viral antigen specificity of a CTL is limited, as the virus associated antigens need to be encountered relatively frequently and in the presence of a broad array of costimulatory molecules. Epstein Barr virus (EBV)-infected cells may be a valuable resource for this purpose. B lymphocytes expressing EBV-antigens persist lifelong in seropositive individuals and boost both MHC class-I and class-II EBV-specific CTLs targeting latent antigens (132). Importantly, ex vivo expanded EBV-specific CTLs retain the same property of longevity as circulating cells and persist long term after adoptive transfer (130, 131). Based on this evidence, we demonstrated preclinically (146, 147) and then clinically that EBV-specific CTLs engrafted with CARs produce antitumor effects against tumors targeted by the CAR, while retaining their physiological costimulation through their native antigen-specific αβTCRs (91, 92).
Clinical experience with CAR-engrafted virus-specific CTLs
Our group expressed a 1st generation CAR directed to GD2 on EBV-specific CTLs and gave them to 11 children with advanced neuroblastoma (91, 92). By comparing EBV-specific CTLs and activated T cells expressing the same but distinguishable 1st generation CAR, we found that CAR-expressing EBV-specific CTLs initially persisted in the circulation at a higher level and longer than activated T cells and that 5 of the 11 patients with active disease showed tumor responses or necrosis. Three of them had complete responses (sustained in 2), while an additional 2 with bulky tumors showed substantial tumor necrosis. Nevertheless, neither of the CAR-T cell populations expanded in vivo, and patients with massive tumor burdens were helped little. Subsequent GD2-CAR studies are using lymphodepletion in combination with second and third generation vectors and are described in the next sections.
We next designed a study in which adult patients with B-cell malignancies were infused simultaneously with EBV-specific CTLs engrafted with a 1st generation CD19-specific CAR and activated T cells expressing a 2nd generation CD19-specific CAR encoding the CD28 endodomains. Major limits, however, quickly emerged for this study. EBV-specific CTLs could be manufactured only in a minority of potential referrals, since the majority of the lymphoma patients had received anti-CD20 antibody (rituximab) as part of their standard of care, so eliminating B cells and precluding generation of the EBV+ B-lymphoblastoid cell lines required the ex vivo expansion of CTLs. Both products were available for 5 patients, but CD19-specific CAR-expressing EBV-specific CTLs did not persist longer than activated T cells expressing the 2nd generation CAR, likely because EBV-infected B cells in these patients were lacking (authors, manuscript in preparation). Recognizing that EBV-expressing target cells will not be available to use as stimulators in lymphoma patients, we broadened the virus specificity of our cells to include cytomegalovirus (CMV) that also has chronic persistence. This clinical trial has been opened for allogeneic stem cell transplant recipients (148). Similarly, a trial using CTLs specific for three viruses, adenovirus, EBV and CMV, engrafted with a GD2-specific CAR has been recently initiated in the post allogeneic transplant setting in patients with refractory/relapsed neuroblastoma (149). We are also exploring the use of CMV-specific T cells that are genetically modified with a 2nd generation HER2-specific CAR for the adoptive immunotherapy of glioblastoma (GBM). Since CMV antigens are present in latently infected leukocytes and are also detected in the tumor itself (150–153), this approach may enhance in vivo persistence and expansion of adoptively transferred T cells and also directly increase the anti-tumor activity of transferred T cells.
Beyond costimulation and other approaches to improving expansion, persistence and function of CAR-T cells
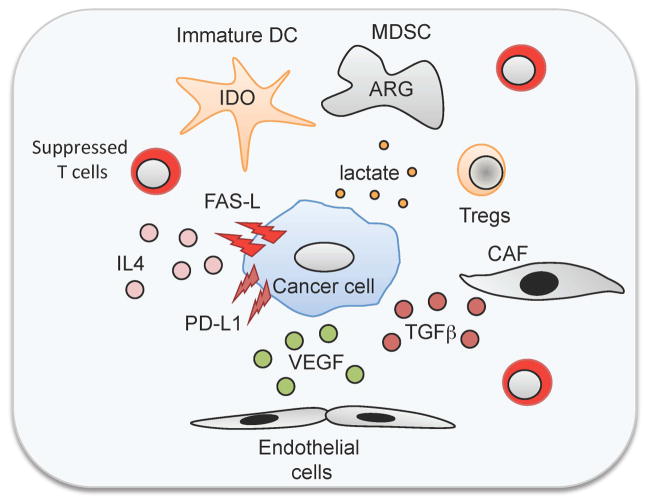
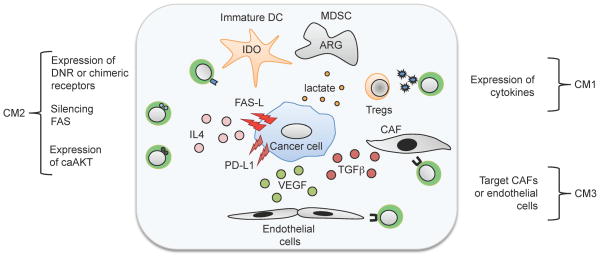
The clinical studies described above confirm the pre-clinical data showing that introducing costimulation for CARs is necessary but insufficient per se to reverse all the immune inhibitory mechanisms of cancer. This is particularly apparent when solid tumors rather than hematologic malignancies are being targeted. Solid tumors and their microenvironment lack the conventional costimulatory molecules that may be present on (for example) malignant and normal B lymphocyte targets and have developed intricate systems to suppress the immune system (133, 154, 155) (Fig. 4). Thus, malignant cells and their supporting stroma secrete immunosuppressive cytokines such as transforming growth factor-β (TGFβ) or IL-10, attract immunosuppressive cells such Tregs or MSDCs, inhibit dendritic cell maturation, express molecules on the cell surface that suppress immune cells including FAS-L and PD-L1, and create a metabolic environment (e.g. high lactate, low tryptophan) that is immunosuppressive.
Fig. 4. Immune evasion strategies of tumors.
Malignant cells and their supporting stroma 1) secrete immunosuppressive cytokines such as transforming growth factor-β (TGFβ) or interleukin-10 (IL-10); 2) attract immunosuppressive cells such Tregs or MSDCs; 3) inhibit dendritic cell maturation; 4) express molecules on the cell surface that suppress immune cells including FAS-L and PD-L1, and; 5) create a metabolic environment (e.g. high lactate, low tryptophan) that is immunosuppressive.
While T-cell costimulation mediated by CD28 and 4-1BB endodomains in CAR molecules may overcome some of the above inhibitory effects, other causes of T-cell anergy are more resistant. Additional engineering of CAR molecules has therefore been exploited as countermeasures to the inhibitory mechanisms developed by tumor cells and their microenvironment and to further enhance CAR-T-cell activity.
Three broad approaches have been adopted as countermeasures to overcome tumor immunosuppression: (i) increasing the level of CAR-T cell activation or decreasing physiological downregulation, (ii) engineering the CAR-T cells to be resistant to the immune evasion strategies used by the tumor, or (iii) targeting the cellular components of tumor stroma (Fig. 5). Any one countermeasure may affect more than one mechanism of tumor immunosuppression.
Fig. 5. Overcoming tumor-mediated immunosuppression.
Countermeasures can be divided into the following strategies: 1) increasing the level of CAR-T-cell activation or decreasing physiological downregulation; 2) engineering the CAR-T cells to be resistant to the immune evasion strategies used by the tumor; and 3) targeting the cellular components of tumor stroma.
Addition of immunostimulatory cytokines/cytokine receptor genes
IL-2 is released by 2nd generation CAR-T cells following receptor engagement, but is not by itself sufficient to reverse T-cell anergy (156, 157). Moreover, this cytokine may increase the number and activity of local Tregs (158). Cytokines that are not directly produced by T cells upon antigen stimulation but are biologically active in T cells may be better placed to accomplish the goal of selective CAR-T-cell activation and reversal of anergy without Treg recruitment.
Of these cytokines, IL-15 is perhaps the most promising. IL-15, is mainly produced by monocytes/macrophages and dendritic cells. It shares a common γ-chain with IL-2 and IL-7 but also requires a private chain in the receptor, IL-15Rα, that is expressed on antigen-presenting cells, monocytes, and macrophages (159). IL-15 selectively stimulates these target cells through a cross presentation mechanism (160). IL-15 promotes the proliferation of T lymphocytes and also prevents apoptosis and exhaustion (156, 161), reverses anergy (156), stimulates long-lasting antigen-experienced memory cells (162), and overcomes Treg-mediated inhibition (157, 163–165). IL-15 can be used either as a growth factor for the ex vivo expansion of CAR-T cells, where it may ‘imprint’ long-lasting resistance to Tregs (39, 166), or as a recombinant protein in vivo to support T-cell expansion after adoptive transfer (166), thereby enhancing the antitumor activity of adoptively transferred T cells in animal models. Preliminary clinical studies showed that systemic administration of recombinant IL-15 may be highly toxic, and the cytokine may be better tolerated if production is confined to the tumor location. We have described how CAR-T cells can be genetically modified to produce their own IL-15 and achieve the hoped-for benefits at the tumor site while avoiding systemic toxicity (157, 164). Locally produced IL-15 improves CAR-T-cell expansion and persistence in vivo and renders CAR-T cells resistant to the inhibitory effects of Tregs by activation of the phosphoinositide 3-kinase (PI3K) pathway resulting in increased expression of anti-apoptotic molecules such BCL-2 (CM2). We plan to test this approach in a clinical trial.
Local production of other cytokines such as IL-7 and IL-12 may also be beneficial. IL-7 shares the γ-chain of its receptor with IL-2 and IL-15 and plays a crucial role in maintaining the homeostasis of mature T cells and the maintenance of memory T cells (167). Clinical studies have shown a good safety profile compared to the other γ-chain cytokines. Since systemic administration of recombinant IL-7 is well tolerated (168), we and other investigators are manipulating the IL-7/IL-7Rα signaling axis in antigen-specific T cells to selectively promote a robust and selective expansion of these cells following IL-7 exposure (169). Although this approach has not yet reached clinical trial, studies of a third cytokine, IL-12, are more advanced. IL-12 is a pro-inflammatory cytokine that promotes Th1 differentiation and links innate and adaptive immunity (170). Like IL-15, the transgenic expression of IL-12 in tumor-specific T cells significantly increases their anti-tumor activity (171, 172) by directly enhancing T-cell activity and by increasing their production of Th1 cytokines (173). In addition, IL-12 may help to reverse the immunosuppressive tumor environment by triggering the apoptosis of inhibitory tumor-infiltrating macrophages, dendritic cells, and myeloid derived dendritic cells (MSDCs) through a FAS-dependent pathway (174). While there are safety concerns in regards to constitutive IL-12 expression, there are several mechanisms available to restrict IL-12 production to activated T cells at tumor site by using inducible expression systems (172). Transgenic IL-12 expression by tumor-infiltrating T lymphocytes is currently being tested in a clinical trial at the National Cancer Institute.
Ultimately, it may be possible to bypass the use of cytokines/cytokine receptors and to directly manipulate T-cell pathways that influence cell growth and survival. For example, expression of a constitutively active form of serine/threonine AKT (caAKT), which is a major component of the PI3K pathway, improves T-cell function and survival, since caAKT-expressing T cells have sustained higher levels of NF-κB and of anti-apoptotic genes such as Bcl-2, resulting in resistance to tumor inhibitory mechanisms (175). This approach is at an early stage and may lack the selectivity necessary for safe use.
Blocking the immune check points
Instead of adding stimulatory signals (costimulation, cytokines/cytokine receptors), the function of CAR-T cells may be enhanced by blocking downregulatory signals. Antibodies that block the cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) or the programmed death-1 (PD-1) receptor or the PD-L1 ligand have produced encouraging clinical results as single agents (176, 177). CTLA-4 is expressed by activated T cells and acts as a co-inhibitory molecule of T cells after engaging CD80 and CD86 expressed by antigen-presenting cells (178). PD-1 also acts as an inhibitory receptor for T cells by engaging the PD-L1 counter-receptor, expressed by antigen-presenting cells in a regulated manner and constitutively by many tumors (179). Antibodies that block the CTLA-4/CD80/CD86 or PD-1/PD-L1 axes therefore prevent the physiological contraction of the T-cell immune system. The CTLA-4 antibody ipilimumab has shown remarkable effects in a randomized clinical study in a subset of melanoma patients (176) and promising results have also been reported for antibodies blocking PD-1 or PD-L1 in patients with renal carcinoma (177). The combination of these antibodies with adoptive transfer of CART cells is a logical evolution of the current clinical protocols to prolong the effector function of CAR-T cells at sites of solid tumor. This combination does, however, have the potential for uncontrolled proliferation and activation of the CAR-T cells (180) and will need to be examined using careful dose escalation studies and - ideally - with other safety interventions in place (see ‘Increasing the safety of CAR-T cells’).
Instead of influencing receptors for inhibitory signals, it is also possible to directly silence genes that render T cells susceptible to inhibitory signals in the tumor microenvironment. For example, many tumor cells express FAS ligand (Fas-L), and silencing FAS expression in T cells prevents FAS-induced apoptosis (181).
Dominant negative and chimeric cytokine receptors
CAR-T cells can be engineered to be resistant to cytokines such as IL-4 and TGFβ that are widely used by tumors as an immune evasion strategy (Figs 4 and 5). TGFβ promotes tumor growth and limits effector T-cell function through SMAD-mediated pathways resulting in decreased expression of cytolytic gene products such as perforin, decreased cell proliferation, and increased apoptosis (182, 183). These detrimental effects can be negated by modifying T cells to express a dominant-negative TGFβ receptor type II (TGFβ-DNR), which lacks most of the cytoplasmic component including the kinase domain (184, 185). DNR expression interferes with TGFβ-signaling, thereby blocking TGFβ-induced SMAD2 phosphorylation so that T-cell effector function is sustained even in the presence of TGFβ. We have used the TGFβ-DNR to modify T cells directed to tumors through their native T-cell receptors. We then gave these TGFβ-resistant T cells specific for the EBV antigens LMP1/LMP2 into patients with EBV-positive Hodgkin’s lymphoma (HL) and non-Hodgkin’s lymphoma (NHL) and have obtained clinical benefit including complete responses (186) even in patients who failed with LMP1/LMP2-specific T cells expressing only wildtype TGFβ receptor type II. Other clinical studies are now in progress in which the TGFβ-DNR is expressed in CAR-T cells.
It is also possible to engineer T cells to actively benefit from the inhibitory signals generated by the tumor environment, by converting inhibitory into stimulatory signals. For example, linking the extracellular domain of the TGFβ RII to the endodomain of toll-like receptor (TLR) 4 results in a chimeric receptor that not only renders T cells resistant to TGFβ, but also induces T-cell activation and expansion (187). Chimeric IL-4 receptors are another example of these ‘signal converters’. Many tumors secrete IL-4 to create a Th2-polarized environment, and two groups of investigators have shown that expression of chimeric IL-4 receptors consisting of the ectodomain of the IL-4 receptor and the endodomain of the IL-7Rα (188) or the IL-2Rβ chain (189) enable T cells to proliferate in the presence of IL-4 and retain their effector function including Th1-polarization.
Targeting the cellular components of tumor stroma
Most solid tumors have a stromal compartment that supports tumor growth directly through paracrine secretion of cytokines, growth factors, and provision of nutrients and that also contributes to tumor-induced immunosuppression (190–193). This compartment may be a suitable target for CAR-T cell therapy. For example, we have shown in preclinical studies that T cells expressing CARs specific for fibroblast activation protein (FAP) expressed on cancer-associated fibroblasts (CAFs) have potent antitumor effects, which is enhanced when they were combined with tumor-specific CAR-T cells (89). Hence, targeting CAFs has the potential to improve the antitumor activity of adoptively transferred CAR T cells. Other investigators are targeting the tumor vasculature with CARs (108, 109). These studies initially targeted VEGFR-2 using a VEGF-based CAR, but more recent pre-clinical studies have used a VEGFR-2-specific scFv (108). The combination of CAR-T cells targeting vasculature and tumor cells was again more effective than CAR-T cells targeting either component alone. In addition combining VEGFR-2-specific CARs and IL-12 in T cells was sufficient to eradicate tumors, indicating another means of potentiating effects (194). Any approach that targets antigens present on normal tissue has to consider the inevitable safety concerns, but while these require consideration, clinical studies evaluating the safety and efficacy of the approach, for example with VEGFR-2-specific CAR-T cells, are in progress.
Increasing the safety of CAR-T cells
The efficacy of CAR-modified T cells has not been devoid of toxicities. These fall into three categories.
Toxicity from the gene delivery system
Up to now the most effective approaches to express CARs in human T lymphocytes have been based on γ-retroviral vectors and lentiviral vectors (41, 42, 44, 195). Both vectors allow robust and stable expression of CARs in T cells without requiring complex and long procedures of ex vivo selection. Genomic integration of viral vectors may, however, cause toxicities due to insertional mutagenesis. Unlike past experience with γ-retroviral vector-mediated gene transfer to CD34+ hematopoietic progenitor cells (196), insertional mutagenesis leading to lymphoproliferation of T lymphocytes (including CAR-T cells) has not yet occurred, perhaps because integration is occurring into more differentiated cells with fewer developmental pathways open to disruption by integration events.
On target toxicities
This second type of toxicity is directly attributable to T cells engaging the targeted antigen. For example, the infusion of long-term persisting CD19-specific CAR-T cells is followed by long-term elimination of all cells bearing the CD19 antigen, irrespective of whether they are malignant or normal and leads to profound and prolonged B-cell aplasia and ultimately hypogammaglobulinemia (37, 41–44). This particular toxicity may be ameliorated by infusing immunoglobulin preparations.
On biological target but off organ toxicities
Other on-target toxicities may be more severe and less amenable to correction, particularly if they are on target but ‘out of organ’. For example, targeting the carbonic anhydrase IX that is highly expressed by renal cell carcinoma also causes significant liver toxicity, since the targeted antigen is also expressed by cells of the biliary tree (61, 118). Similarly, a fatal adverse event occurred in a patient infused with HER2-specific CAR-T cells which may have been due to low level HER2 expression in the pulmonary parenchyma or vasculature (73).
Systemic inflammatory response syndrome (SIRS) or cytokine storm
This toxicity is attributable to rapid and extensive activation of infused CAR-T cells upon antigen engagement, with general perturbation of the immune system, and the associated release of high levels of proinflammatory cytokines, such as TNF-α and IL-6 (42, 44). This toxicity has now been observed after administration of CAR-T cells with several different specificities and, while often associated with a tumor response and potentially reversible, it remains a major concern for broader introduction of the approach.
Controlling toxicity from the gene delivery system
Although oncogenicity from retroviruses is currently only a hypothetical concern for CAR-T cells, there is considerable interest in developing alternative vector systems that retain significant genomic integration capacity, but are based on DNA plasmids such as the transposon/transposes system which may be less likely to selectively integrate in critical sites in the genome (197, 198). A phase I study in which T cells are engineered to express a CD19-specific CAR encoded in a Sleeping Beauty transposon system has been recently approved by the FDA and the clinical trial is ongoing at MD Anderson Cancer Center.
Controlling on target toxicity
To prevent on target toxicity requires accurate antigen selection, so that careful dose escalation of CAR-T cells currently remains a fundamental aspects of CAR-T-cell therapies and T-cell therapies in general. It may also be possible to reduce this type of toxicity by targeting antigens that are more restricted in their expression. In B-cell malignancies, for example, we generated CARs specific to κ-light chain of immunoglobulin, since unlike a CD19 CAR-T-cell which will target both normal and malignant B cells equally well, a κ+CAR-T cell will target all the cells of a κ+ malignancy (since the tumor is clonal) while sparing the subset of normal B cells that express λ (54). Other groups are targeting ROR1 in clinical trials (7). Nevertheless, to extend CAR-T-cell therapies beyond hematological malignancies into the arena of solid tumors will likely continue to require targeting of antigens that are inevitably expressed by normal tissues. Preclinical models may be insufficient or inadequate to predict the organ toxicity and in these circumstances alternative safety strategies are required. One approach is to infuse T cells with only transient expression of the CAR, for example after electroporation of mRNA encoding the receptor (199–201). Unlike T cells transduced with a (genome-integrating) vector, in which each daughter cell contains the same transgene, translated to the same level, mRNA transduced T cells express the transgene for a finite period of time (depending on the stability of the mRNA and the translated protein); moreover, levels of expression diminish as the cells divide, and the transcripts become progressively diluted. Since CAR-T cells may expand 1000–10,000-fold over 7–10 days, this dilutional effect may be rapid. The approach is being used at the University of Pennsylvania to test a CAR specific for mesothelin in patients with pancreatic cancer (145).
Controlling SIRS
To reduce the onset or severity of SIRS, investigators are modifying T-cell dose escalation and have introduced the prompt use of antibodies blocking the effects of TNF-α and IL-6.
General reduction in toxicity
While the specific measures outlined above may all be beneficial, the inherent potential of T cells to persist and expand means that the associated toxicities may show corresponding persistence and worsen with time. Thus, there is a strong incentive to use engineered T cells that also express a suicide or safety switch along with the CAR. These cells would then retain their long-term capacity for engraftment, expansion and expression but could be eliminated on demand by the activation of the suicide gene in the event of toxicity. We have selected this approach for our forthcoming clinical trial in which a 3rd CAR targeting the GD2 antigen has been coupled with the inducible caspase9 suicide gene (202, 203). This particular suicide gene can be selectively activated by an otherwise bioinert small molecule (chemical inducer of dimerization (CID). Compared to the more widely used herpes thymidine kinase/ganciclovir approach this system may act more rapidly by induction of apoptosis, and to have reduced immunogenicity since the sequences are all human derived (203, 204).
Although the possibility of rapidly eliminating CAR-T cells in the event of toxicity represents an important consideration for safety, the elimination of these cells also abrogates their anti-tumor effects. This may be problematic if, as seems likely, long term immune surveillance is necessary to prevent tumor relapse. Thus, the ultimate goal is to retain the beneficial antitumor effects of CAR-T cells even against antigens that are shared, with normal tissues. One means of accomplishing this is to preferentially express CARs on the T cells only at the tumor site, by exploiting metabolic conditions that are commonly developed within the tumor environment such as hypoxia (205). Targeting multiple antigens on a single cell may also reduce toxicity, by providing the engineered T cells with true pattern-recognition ability. For example, engineering T cells to express two CARs with complementary signaling domains restricts full T-cell activation only to tumor sites at which both antigens are expressed (206–208). These elegant models seem very promising in preclinical experiments, but it remains to be demonstrated whether the benefits can be recapitulated within heterogeneous human malignancies in which the levels and patterns of antigen expression may vary between one cell and another.
Conclusions
CAR-T cells are making the transition from merely ‘promising’ to being ‘effective’ treatments for hematological malignancies. As we continue to improve the functionality of the T cells that express chimeric tumor-targeting receptors and enable them to function in the tumor micro-environment, we can anticipate broader application beyond hematological cancers and into solid tumors. With increasing interest in the field from commercial entities we are hopeful that development and clinical implementation of this exciting approach will now accelerate.
Acknowledgments
We are all grateful for long-term support from the NIH (NCI, NHLBI), The Department of Defense, and The Leukemia and Lymphoma Research Fund. We are also grateful to the foundations who have supported these Center Investigators including Alex’s Lemonade Stand, Clayton Foundation for Research, V Foundation, Dana Foundation, James S McDonnell Foundation, Adrienne Helis Medical Research Foundation and Bear Necessities Pediatric Cancer Foundation. MKB is a Scientific Advisory Board Member of Jennerex and of bluebird bio. Current research in CAGT (of which he is Director) receives support from CellMedica and the Center has a Research Collaboration with Celgene and bluebird bio. All authors have patent applications in the field of T cell and gene-modified T-cell therapy for cancer.
References
- 1.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sadelain M, Brentjens R, Riviere I. The basic principles of chimeric antigen receptor design. Cancer Discov. 2013;3:388–398. doi: 10.1158/2159-8290.CD-12-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramos CA, Dotti G. Chimeric antigen receptor (CAR)-engineered lymphocytes for cancer therapy. Expert Opin Biol Ther. 2011;11:855–873. doi: 10.1517/14712598.2011.573476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Curran KJ, Pegram HJ, Brentjens RJ. Chimeric antigen receptors for T cell immunotherapy: current understanding and future directions. J Gene Med. 2012;14:405–415. doi: 10.1002/jgm.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maher J. Immunotherapy of malignant disease using chimeric antigen receptor engrafted T cells. ISRN Oncol. 2012;2012:278093. doi: 10.5402/2012/278093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuwana Y, Asakura Y, Utsunomiya N, Nakanishi M, Arata Y, Itoh S, et al. Expression of chimeric receptor composed of immunoglobulin-derived V regions and T-cell receptor-derived C regions. Biochem Biophys Res Commun. 1987;149:960–968. doi: 10.1016/0006-291x(87)90502-x. [DOI] [PubMed] [Google Scholar]
- 7.Hudecek M, Lupo Stanghellini MT, Kosasih PL, Sommermeyer D, Jensen M, Rader C, et al. Receptor affinity and extracellular domain modifications affect tumor recognition by ROR1-specific chimeric antigen receptor T-cells. Clin Cancer Res. 2013 doi: 10.1158/1078-0432.CCR-13-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chmielewski M, Hombach A, Heuser C, Adams GP, Abken H. T cell activation by antibody-like immunoreceptors: increase in affinity of the single-chain fragment domain above threshold does not increase T cell activation against antigen-positive target cells but decreases selectivity. J Immunol. 2004;173:7647–7653. doi: 10.4049/jimmunol.173.12.7647. [DOI] [PubMed] [Google Scholar]
- 9.Hombach AA, Schildgen V, Heuser C, Finnern R, Gilham DE, Abken H. T cell activation by antibody-like immunoreceptors: the position of the binding epitope within the target molecule determines the efficiency of activation of redirected T cells. J Immunol. 2007;178:4650–4657. doi: 10.4049/jimmunol.178.7.4650. [DOI] [PubMed] [Google Scholar]
- 10.Haso W, Lee DW, Shah NN, Stetler-Stevenson M, Yuan CM, Pastan IH, et al. Anti-CD22-chimeric antigen receptors targeting B-cell precursor acute lymphoblastic leukemia. Blood. 2013;121:1165–1174. doi: 10.1182/blood-2012-06-438002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grada Z, Hegde M, Byrd T, Shaffer DR, Ghazi A, Brawley VS, et al. TanCAR: A Novel Bispecific Chimeric Antigen Receptor for Cancer Immunotherapy. Mol Ther Nucleic Acids. 2013;2:e105. doi: 10.1038/mtna.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharifzadeh Z, Rahbarizadeh F, Shokrgozar MA, Ahmadvand D, Mahboudi F, Jamnani FR, et al. Genetically engineered T cells bearing chimeric nanoconstructed receptors harboring TAG-72-specific camelid single domain antibodies as targeting agents. Cancer Lett. 2013;334:237–244. doi: 10.1016/j.canlet.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 13.Ahmed N, Ratnayake M, Savoldo B, Perlaky L, Dotti G, Wels WS, et al. Regression of experimental medulloblastoma following transfer of HER2-specific T cells. Cancer Res. 2007;67:5957–5964. doi: 10.1158/0008-5472.CAN-06-4309. [DOI] [PubMed] [Google Scholar]
- 14.Chow KK, Naik S, Kakarla S, Brawley VS, Shaffer DR, Yi Z, et al. T Cells Redirected to EphA2 for the Immunotherapy of Glioblastoma. Mol Ther. 2013;21:629–637. doi: 10.1038/mt.2012.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lanitis E, Poussin M, Hagemann IS, Coukos G, Sandaltzopoulos R, Scholler N, et al. Redirected antitumor activity of primary human lymphocytes transduced with a fully human anti-mesothelin chimeric receptor. Mol Ther. 2012;20:633–643. doi: 10.1038/mt.2011.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kahlon KS, Brown C, Cooper LJ, Raubitschek A, Forman SJ, Jensen MC. Specific recognition and killing of glioblastoma multiforme by interleukin 13-zetakine redirected cytolytic T cells. Cancer Res. 2004;64:9160–9166. doi: 10.1158/0008-5472.CAN-04-0454. [DOI] [PubMed] [Google Scholar]
- 17.Brown CE, Starr R, Aguilar B, Shami A, Martinez C, D’Apuzzo M, et al. Stem-like tumor initiating cells isolated from IL13Ralpha2-expressing gliomas are targeted and killed by IL13-zetakine redirected T cells. Clin Cancer Res. 2012 doi: 10.1158/1078-0432.CCR-11-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kong S, Sengupta S, Tyler B, Bais AJ, Ma Q, Doucette S, et al. Suppression of human glioma xenografts with second-generation IL13R-specific chimeric antigen receptor-modified T cells. Clin Cancer Res. 2012;18:5949–5960. doi: 10.1158/1078-0432.CCR-12-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang T, Lemoi BA, Sentman CL. Chimeric NK-receptor-bearing T cells mediate antitumor immunotherapy. Blood. 2005;106:1544–1551. doi: 10.1182/blood-2004-11-4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barber A, Zhang T, Megli CJ, Wu J, Meehan KR, Sentman CL. Chimeric NKG2D receptor-expressing T cells as an immunotherapy for multiple myeloma. Exp Hematol. 2008;36:1318–1328. doi: 10.1016/j.exphem.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shaffer DR, Savoldo B, Yi Z, Chow KK, Kakarla S, Spencer DM, et al. T cells redirected against CD70 for the immunotherapy of CD70-positive malignancies. Blood. 2011;117:4304–4314. doi: 10.1182/blood-2010-04-278218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davies DM, Foster J, van der Stegen SJ, Parente-Pereira AC, Chiapero-Stanke L, Delinassios GJ, et al. Flexible targeting of ErbB dimers that drive tumorigenesis by using genetically engineered T cells. Mol Med. 2012;18:565–576. doi: 10.2119/molmed.2011.00493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Urbanska K, Lanitis E, Poussin M, Lynn RC, Gavin BP, Kelderman S, et al. A universal strategy for adoptive immunotherapy of cancer through use of a novel T-cell antigen receptor. Cancer Res. 2012;72:1844–1852. doi: 10.1158/0008-5472.CAN-11-3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tamada K, Geng D, Sakoda Y, Bansal N, Srivastava R, Li Z, et al. Redirecting gene-modified T cells toward various cancer types using tagged antibodies. Clin Cancer Res. 2012;18:6436–6445. doi: 10.1158/1078-0432.CCR-12-1449. [DOI] [PubMed] [Google Scholar]
- 25.Yaghoubi SS, Jensen MC, Satyamurthy N, Budhiraja S, Paik D, Czernin J, et al. Noninvasive detection of therapeutic cytolytic T cells with 18F-FHBG PET in a patient with glioma. Nat Clin Pract Oncol. 2009;6:53–58. doi: 10.1038/ncponc1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown CE, Starr R, Naranjo A, Wright C, Bading J, Ressler J, et al. Adoptive Transfer of Autologous IL13-zetakine+ Engineered T Cell Clones for the Treatment of Recurrent Glioblastoma: Lessons from the Clinic. Molecular Therapy. 2011;19:S136–S137. [Google Scholar]
- 27.Guest RD, Hawkins RE, Kirillova N, Cheadle EJ, Arnold J, O’Neill A, et al. The role of extracellular spacer regions in the optimal design of chimeric immune receptors: evaluation of four different scFvs and antigens. J Immunother. 2005;28:203–211. doi: 10.1097/01.cji.0000161397.96582.59. [DOI] [PubMed] [Google Scholar]
- 28.Hombach A, Heuser C, Gerken M, Fischer B, Lewalter K, Diehl V, et al. T cell activation by recombinant FcepsilonRI gamma-chain immune receptors: an extracellular spacer domain impairs antigen-dependent T cell activation but not antigen recognition. Gene Ther. 2000;7:1067–1075. doi: 10.1038/sj.gt.3301195. [DOI] [PubMed] [Google Scholar]
- 29.Savoldo B, Ramos CA, Liu E, Mims MP, Keating MJ, Carrum G, et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Invest. 2011;121:1822–1826. doi: 10.1172/JCI46110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bridgeman JS, Hawkins RE, Bagley S, Blaylock M, Holland M, Gilham DE. The optimal antigen response of chimeric antigen receptors harboring the CD3zeta transmembrane domain is dependent upon incorporation of the receptor into the endogenous TCR/CD3 complex. J Immunol. 2010;184:6938–6949. doi: 10.4049/jimmunol.0901766. [DOI] [PubMed] [Google Scholar]
- 31.Irving BA, Weiss A. The cytoplasmic domain of the T cell receptor zeta chain is sufficient to couple to receptor-associated signal transduction pathways. Cell. 1991;64:891–901. doi: 10.1016/0092-8674(91)90314-o. [DOI] [PubMed] [Google Scholar]
- 32.Haynes NM, Snook MB, Trapani JA, Cerruti L, Jane SM, Smyth MJ, et al. Redirecting mouse CTL against colon carcinoma: superior signaling efficacy of single-chain variable domain chimeras containing TCR-zeta vs Fc epsilon RI-gamma. J Immunol. 2001;166:182–187. doi: 10.4049/jimmunol.166.1.182. [DOI] [PubMed] [Google Scholar]
- 33.Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM, Hecht TT, et al. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res. 2009;15:5323–5337. doi: 10.1158/1078-0432.CCR-09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sampson JH, Archer GE, Mitchell DA, Heimberger AB, Bigner DD. Tumor-specific immunotherapy targeting the EGFRvIII mutation in patients with malignant glioma. Semin Immunol. 2008 doi: 10.1016/j.smim.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cooper LJ, Al Kadhimi Z, Serrano LM, Pfeiffer T, Olivares S, Castro A, et al. Enhanced antilymphoma efficacy of CD19-redirected influenza MP1-specific CTLs by cotransfer of T cells modified to present influenza MP1. Blood. 2005;105:1622–1631. doi: 10.1182/blood-2004-03-1208. [DOI] [PubMed] [Google Scholar]
- 36.Jensen MC, Popplewell L, Cooper LJ, Digiusto D, Kalos M, Ostberg JR, et al. Anti-Transgene Rejection Responses Contribute to Attenuated Persistence of Adoptively Transferred CD20/CD19-Specific Chimeric Antigen Receptor Re-directed T Cells in Humans. Biol Blood Marrow Transplant. 2010 doi: 10.1016/j.bbmt.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kochenderfer JN, Dudley ME, Feldman SA, Wilson WH, Spaner DE, Maric I, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119:2709–2720. doi: 10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brentjens R, Riviere I, Hollyman D, Taylor C, Nikhamin Y, Stefanski J, et al. Unexpected Toxicity of Cyclophosphamide Followed by Adoptively Transferred CD19-Targeted T cells in a Patient with Bulky CLL. Molecular Therapy. 2009;17:S157. [Google Scholar]
- 39.Brentjens RJ, Latouche JB, Santos E, Marti F, Gong MC, Lyddane C, et al. Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nat Med. 2003;9:279–286. doi: 10.1038/nm827. [DOI] [PubMed] [Google Scholar]
- 40.Brentjens RJ, Riviere I, Park JH, Davila ML, Wang X, Stefanski J, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011;118:4817–4828. doi: 10.1182/blood-2011-04-348540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3:95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brentjens RJ, Davila ML, Riviere I, Park J, Wang X, Cowell LG, et al. CD19-Targeted T Cells Rapidly Induce Molecular Remissions in Adults with Chemotherapy-Refractory Acute Lymphoblastic Leukemia. Sci Transl Med. 2013;5:177ra38. doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, et al. Chimeric Antigen Receptor-Modified T Cells for Acute Lymphoid Leukemia. N Engl J Med. 2013 doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Till BG, Jensen MC, Wang J, Chen EY, Wood BL, Greisman HA, et al. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood. 2008;112:2261–2271. doi: 10.1182/blood-2007-12-128843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang J, Jensen M, Lin Y, Sui X, Chen E, Lindgren CG, et al. Optimizing adoptive polyclonal T cell immunotherapy of lymphomas, using a chimeric T cell receptor possessing CD28 and CD137 costimulatory domains. Hum Gene Ther. 2007;18:712–725. doi: 10.1089/hum.2007.028. [DOI] [PubMed] [Google Scholar]
- 47.Wang J, Press OW, Lindgren CG, Greenberg P, Riddell S, Qian X, et al. Cellular immunotherapy for follicular lymphoma using genetically modified CD20-specific CD8+ cytotoxic T lymphocytes. Mol Ther. 2004;9:577–586. doi: 10.1016/j.ymthe.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 48.Jensen M, Tan G, Forman S, Wu AM, Raubitschek A. CD20 is a molecular target for scFvFc:zeta receptor redirected T cells: implications for cellular immunotherapy of CD20+ malignancy. Biol Blood Marrow Transplant. 1998;4:75–83. doi: 10.1053/bbmt.1998.v4.pm9763110. [DOI] [PubMed] [Google Scholar]
- 49.Di Stasi A, De Angelis B, Rooney CM, Zhang L, Mahendravada A, Foster AE, et al. T lymphocytes coexpressing CCR4 and a chimeric antigen receptor targeting CD30 have improved homing and antitumor activity in a Hodgkin tumor model. Blood. 2009;113:6392–6402. doi: 10.1182/blood-2009-03-209650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Savoldo B, Rooney CM, Di Stasi A, Abken H, Hombach A, Foster AE, et al. Epstein Barr virus specific cytotoxic T lymphocytes expressing the anti-CD30zeta artificial chimeric T-cell receptor for immunotherapy of Hodgkin disease. Blood. 2007;110:2620–2630. doi: 10.1182/blood-2006-11-059139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hombach A, Heuser C, Sircar R, Tillmann T, Diehl V, Pohl C, et al. An anti-CD30 chimeric receptor that mediates CD3-zeta-independent T-cell activation against Hodgkin’s lymphoma cells in the presence of soluble CD30. Cancer Res. 1998;58:1116–1119. [PubMed] [Google Scholar]
- 52.Finney HM, Lawson AD, Bebbington CR, Weir AN. Chimeric receptors providing both primary and costimulatory signaling in T cells from a single gene product. J Immunol. 1998;161:2791–2797. [PubMed] [Google Scholar]
- 53.Tettamanti S, Marin V, Pizzitola I, Magnani CF, Giordano Attianese GM, Cribioli E, et al. Targeting of acute myeloid leukaemia by cytokine-induced killer cells redirected with a novel CD123-specific chimeric antigen receptor. Br J Haematol. 2013;161:389–401. doi: 10.1111/bjh.12282. [DOI] [PubMed] [Google Scholar]
- 54.Vera J, Savoldo B, Vigouroux S, Biagi E, Pule M, Rossig C, et al. T lymphocytes redirected against the kappa light chain of human immunoglobulin efficiently kill mature B lymphocyte-derived malignant cells. Blood. 2006;108:3890–3897. doi: 10.1182/blood-2006-04-017061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peinert S, Prince HM, Guru PM, Kershaw MH, Smyth MJ, Trapani JA, et al. Gene-modified T cells as immunotherapy for multiple myeloma and acute myeloid leukemia expressing the Lewis Y antigen. Gene Ther. 2010;17:678–686. doi: 10.1038/gt.2010.21. [DOI] [PubMed] [Google Scholar]
- 56.Ritchie DS, Neeson PJ, Khot A, Peinert S, Tai T, Tainton K, et al. Persistence and efficacy of second generation CAR-T cell against the LeY antigen in acute myeloid leukemia. Mol Ther. 2013 doi: 10.1038/mt.2013.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lehner M, Gotz G, Proff J, Schaft N, Dorrie J, Full F, et al. Redirecting T cells to Ewing’s sarcoma family of tumors by a chimeric NKG2D receptor expressed by lentiviral transduction or mRNA transfection. PLoS One. 2012;7:e31210. doi: 10.1371/journal.pone.0031210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Song DG, Ye Q, Santoro S, Fang C, Best A, Powell DJ., Jr Chimeric NKG2D CAR-expressing T cell-mediated attack of human ovarian cancer is enhanced by histone deacetylase inhibition. Hum Gene Ther. 2013;24:295–305. doi: 10.1089/hum.2012.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Spear P, Barber A, Rynda-Apple A, Sentman CL. Chimeric antigen receptor T cells shape myeloid cell function within the tumor microenvironment through IFN-gamma and GM-CSF. J Immunol. 2012;188:6389–6398. doi: 10.4049/jimmunol.1103019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cheung NK, Guo HF, Modak S, Cheung IY. Anti-idiotypic antibody facilitates scFv chimeric immune receptor gene transduction and clonal expansion of human lymphocytes for tumor therapy. Hybrid Hybridomics. 2003;22:209–218. doi: 10.1089/153685903322328938. [DOI] [PubMed] [Google Scholar]
- 61.Lamers CH, Sleijfer S, Vulto AG, Kruit WH, Kliffen M, Debets R, et al. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. J Clin Oncol. 2006;24:e20–e22. doi: 10.1200/JCO.2006.05.9964. [DOI] [PubMed] [Google Scholar]
- 62.Weijtens ME, Willemsen RA, van Krimpen BA, Bolhuis RL. Chimeric scFv/gamma receptor-mediated T-cell lysis of tumor cells is coregulated by adhesion and accessory molecules. Int J Cancer. 1998;77:181–187. doi: 10.1002/(sici)1097-0215(19980717)77:2<181::aid-ijc2>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 63.Hekele A, Dall P, Moritz D, Wels W, Groner B, Herrlich P, et al. Growth retardation of tumors by adoptive transfer of cytotoxic T lymphocytes reprogrammed by CD44v6-specific scFv:zeta-chimera. Int J Cancer. 1996;68:232–238. doi: 10.1002/(SICI)1097-0215(19961009)68:2<232::AID-IJC16>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 64.Dall P, Herrmann I, Durst B, Stoff-Khalili MA, Bauerschmitz G, Hanstein B, et al. In vivo cervical cancer growth inhibition by genetically engineered cytotoxic T cells. Cancer Immunol Immunother. 2005;54:51–60. doi: 10.1007/s00262-004-0569-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Park JR, Digiusto DL, Slovak M, Wright C, Naranjo A, Wagner J, et al. Adoptive transfer of chimeric antigen receptor re-directed cytolytic T lymphocyte clones in patients with neuroblastoma. Mol Ther. 2007;15:825–833. doi: 10.1038/sj.mt.6300104. [DOI] [PubMed] [Google Scholar]
- 66.Nolan KF, Yun CO, Akamatsu Y, Murphy JC, Leung SO, Beecham EJ, et al. Bypassing immunization: optimized design of “designer T cells” against carcinoembryonic antigen (CEA)-expressing tumors, and lack of suppression by soluble CEA. Clin Cancer Res. 1999;5:3928–3941. [PubMed] [Google Scholar]
- 67.Bullain SS, Sahin A, Szentirmai O, Sanchez C, Lin N, Baratta E, et al. Genetically engineered T cells to target EGFRvIII expressing glioblastoma. J Neurooncol. 2009 doi: 10.1007/s11060-009-9889-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Morgan RA, Johnson LA, Davis JL, Zheng Z, Woolard KD, Reap EA, et al. Recognition of glioma stem cells by genetically modified T cells targeting EGFRvIII and development of adoptive cell therapy for glioma. Hum Gene Ther. 2012;23:1043–1053. doi: 10.1089/hum.2012.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Meier R, Golovko D, Tavri S, Henning TD, Knopp C, Piontek G, et al. Depicting adoptive immunotherapy for prostate cancer in an animal model with magnetic resonance imaging. Magn Reson Med. 2011;65:756–763. doi: 10.1002/mrm.22652. [DOI] [PubMed] [Google Scholar]
- 70.Ren-Heidenreich L, Mordini R, Hayman GT, Siebenlist R, LeFever A. Comparison of the TCR zeta-chain with the FcR gamma-chain in chimeric TCR constructs for T cell activation and apoptosis. Cancer Immunol Immunother. 2002;51:417–423. doi: 10.1007/s00262-002-0301-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Daly T, Royal RE, Kershaw MH, Treisman J, Wang G, Li W, et al. Recognition of human colon cancer by T cells transduced with a chimeric receptor gene. Cancer Gene Ther. 2000;7:284–291. doi: 10.1038/sj.cgt.7700121. [DOI] [PubMed] [Google Scholar]
- 72.Zhao Y, Wang QJ, Yang S, Kochenderfer JN, Zheng Z, Zhong X, et al. A herceptin-based chimeric antigen receptor with modified signaling domains leads to enhanced survival of transduced T lymphocytes and antitumor activity. J Immunol. 2009;183:5563–5574. doi: 10.4049/jimmunol.0900447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18:843–851. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pinthus JH, Waks T, Malina V, Kaufman-Francis K, Harmelin A, Aizenberg I, et al. Adoptive immunotherapy of prostate cancer bone lesions using redirected effector lymphocytes. J Clin Invest. 2004;114:1774–1781. doi: 10.1172/JCI22284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Teng MW, Kershaw MH, Moeller M, Smyth MJ, Darcy PK. Immunotherapy of cancer using systemically delivered gene-modified human T lymphocytes. Hum Gene Ther. 2004;15:699–708. doi: 10.1089/1043034041361235. [DOI] [PubMed] [Google Scholar]
- 76.Stancovski I, Schindler DG, Waks T, Yarden Y, Sela M, Eshhar Z. Targeting of T lymphocytes to Neu/HER2-expressing cells using chimeric single chain Fv receptors. J Immunol. 1993;151:6577–6582. [PubMed] [Google Scholar]
- 77.Ahmed N, Salsman VS, Yvon E, Louis CU, Perlaky L, Wels WS, et al. Immunotherapy for osteosarcoma: genetic modification of T cells overcomes low levels of tumor antigen expression. Mol Ther. 2009;17:1779–1787. doi: 10.1038/mt.2009.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ahmed N, Salsman VS, Kew Y, Shaffer D, Powell S, Zhang YJ, et al. HER2-specific T cells target primary glioblastoma stem cells and induce regression of autologous experimental tumors. Clin Cancer Res. 2010;16:474–485. doi: 10.1158/1078-0432.CCR-09-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Moritz D, Wels W, Mattern J, Groner B. Cytotoxic T lymphocytes with a grafted recognition specificity for ERBB2-expressing tumor cells. Proc Natl Acad Sci U S A. 1994;91:4318–4322. doi: 10.1073/pnas.91.10.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Muniappan A, Banapour B, Lebkowski J, Talib S. Ligand-mediated cytolysis of tumor cells: use of heregulin-zeta chimeras to redirect cytotoxic T lymphocytes. Cancer Gene Ther. 2000;7:128–134. doi: 10.1038/sj.cgt.7700100. [DOI] [PubMed] [Google Scholar]
- 81.Altenschmidt U, Kahl R, Moritz D, Schnierle BS, Gerstmayer B, Wels W, et al. Cytolysis of tumor cells expressing the Neu/erbB-2, erbB-3, and erbB-4 receptors by genetically targeted naive T lymphocytes. Clin Cancer Res. 1996;2:1001–1008. [PubMed] [Google Scholar]
- 82.Willemsen RA, Debets R, Hart E, Hoogenboom HR, Bolhuis RL, Chames P. A phage display selected fab fragment with MHC class I-restricted specificity for MAGE-A1 allows for retargeting of primary human T lymphocytes. Gene Ther. 2001;8:1601–1608. doi: 10.1038/sj.gt.3301570. [DOI] [PubMed] [Google Scholar]
- 83.Willemsen RA, Ronteltap C, Chames P, Debets R, Bolhuis RL. T cell retargeting with MHC class I-restricted antibodies: the CD28 costimulatory domain enhances antigen-specific cytotoxicity and cytokine production. J Immunol. 2005;174:7853–7858. doi: 10.4049/jimmunol.174.12.7853. [DOI] [PubMed] [Google Scholar]
- 84.Schuberth PC, Jakka G, Jensen SM, Wadle A, Gautschi F, Haley D, et al. Effector memory and central memory NY-ESO-1-specific re-directed T cells for treatment of multiple myeloma. Gene Ther. 2013;20:386–395. doi: 10.1038/gt.2012.48. [DOI] [PubMed] [Google Scholar]
- 85.Hwu P, Shafer GE, Treisman J, Schindler DG, Gross G, Cowherd R, et al. Lysis of ovarian cancer cells by human lymphocytes redirected with a chimeric gene composed of an antibody variable region and the Fc receptor gamma chain. J Exp Med. 1993;178:361–366. doi: 10.1084/jem.178.1.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kershaw MH, Westwood JA, Hwu P. Dual-specific T cells combine proliferation and antitumor activity. Nat Biotechnol. 2002;20:1221–1227. doi: 10.1038/nbt756. [DOI] [PubMed] [Google Scholar]
- 87.Kershaw MH, Westwood JA, Parker LL, Wang G, Eshhar Z, Mavroukakis SA, et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin Cancer Res. 2006;12:6106–6115. doi: 10.1158/1078-0432.CCR-06-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hwu P, Yang JC, Cowherd R, Treisman J, Shafer GE, Eshhar Z, et al. In vivo antitumor activity of T cells redirected with chimeric antibody/T cell receptor genes. Cancer Res. 1995;55:3369–3373. [PubMed] [Google Scholar]
- 89.Kakarla S, Chow KK, Mata M, Shaffer DR, Song XT, Wu MF, et al. Antitumor Effects of Chimeric Receptor Engineered Human T Cells Directed to Tumor Stroma. Mol Ther. 2013 doi: 10.1038/mt.2013.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gattenlohner S, Marx A, Markfort B, Pscherer S, Landmeier S, Juergens H, et al. Rhabdomyosarcoma lysis by T cells expressing a human autoantibody-based chimeric receptor targeting the fetal acetylcholine receptor. Cancer Res. 2006;66:24–28. doi: 10.1158/0008-5472.CAN-05-0542. [DOI] [PubMed] [Google Scholar]
- 91.Pule MA, Savoldo B, Myers GD, Rossig C, Russell HV, Dotti G, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med. 2008;14:1264–1270. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Louis CU, Savoldo B, Dotti G, Pule M, Yvon E, Myers GD, et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood. 2011;118:6050–6056. doi: 10.1182/blood-2011-05-354449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rossig C, Bollard CM, Nuchtern JG, Merchant DA, Brenner MK. Targeting of G(D2)-positive tumor cells by human T lymphocytes engineered to express chimeric T-cell receptor genes. Int J Cancer. 2001;94:228–236. doi: 10.1002/ijc.1457. [DOI] [PubMed] [Google Scholar]
- 94.Yun CO, Nolan KF, Beecham EJ, Reisfeld RA, Junghans RP. Targeting of T lymphocytes to melanoma cells through chimeric anti-GD3 immunoglobulin T-cell receptors. Neoplasia. 2000;2:449–459. doi: 10.1038/sj.neo.7900108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Burns WR, Zhao Y, Frankel TL, Hinrichs CS, Zheng Z, Xu H, et al. A high molecular weight melanoma-associated antigen-specific chimeric antigen receptor redirects lymphocytes to target human melanomas. Cancer Res. 2010;70:3027–3033. doi: 10.1158/0008-5472.CAN-09-2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Huang G, Yu L, Cooper LJ, Hollomon M, Huls H, Kleinerman ES. Genetically modified T cells targeting interleukin-11 receptor alpha-chain kill human osteosarcoma cells and induce the regression of established osteosarcoma lung metastases. Cancer Res. 2012;72:271–281. doi: 10.1158/0008-5472.CAN-11-2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Westwood JA, Smyth MJ, Teng MW, Moeller M, Trapani JA, Scott AM, et al. Adoptive transfer of T cells modified with a humanized chimeric receptor gene inhibits growth of Lewis-Y-expressing tumors in mice. Proc Natl Acad Sci U S A. 2005;102:19051–19056. doi: 10.1073/pnas.0504312102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mezzanzanica D, Canevari S, Mazzoni A, Figini M, Colnaghi MI, Waks T, et al. Transfer of chimeric receptor gene made of variable regions of tumor-specific antibody confers anticarbohydrate specificity on T cells. Cancer Gene Ther. 1998;5:401–407. [PubMed] [Google Scholar]
- 99.Moon EK, Carpenito C, Sun J, Wang LC, Kapoor V, Predina J, et al. Expression of a functional CCR2 receptor enhances tumor localization and tumor eradication by retargeted human T cells expressing a mesothelin-specific chimeric antibody receptor. Clin Cancer Res. 2011;17:4719–4730. doi: 10.1158/1078-0432.CCR-11-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wilkie S, Picco G, Foster J, Davies DM, Julien S, Cooper L, et al. Retargeting of human T cells to tumor-associated MUC1: the evolution of a chimeric antigen receptor. J Immunol. 2008;180:4901–4909. doi: 10.4049/jimmunol.180.7.4901. [DOI] [PubMed] [Google Scholar]
- 101.Gilham DE, O’Neil A, Hughes C, Guest RD, Kirillova N, Lehane M, et al. Primary polyclonal human T lymphocytes targeted to carcino-embryonic antigens and neural cell adhesion molecule tumor antigens by CD3zeta-based chimeric immune receptors. J Immunother. 2002;25:139–151. doi: 10.1097/00002371-200203000-00002. [DOI] [PubMed] [Google Scholar]
- 102.Morgenroth A, Cartellieri M, Schmitz M, Gunes S, Weigle B, Bachmann M, et al. Targeting of tumor cells expressing the prostate stem cell antigen (PSCA) using genetically engineered T-cells. Prostate. 2007;67:1121–1131. doi: 10.1002/pros.20608. [DOI] [PubMed] [Google Scholar]
- 103.Katari UL, Keirnan JM, Worth AC, Hodges SE, Leen AM, Fisher WE, et al. Engineered T cells for pancreatic cancer treatment. HPB (Oxford) 2011;13:643–650. doi: 10.1111/j.1477-2574.2011.00344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Maher J, Brentjens RJ, Gunset G, Riviere I, Sadelain M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta/CD28 receptor. Nat Biotechnol. 2002;20:70–75. doi: 10.1038/nbt0102-70. [DOI] [PubMed] [Google Scholar]
- 105.Gong MC, Latouche JB, Krause A, Heston WD, Bander NH, Sadelain M. Cancer patient T cells genetically targeted to prostate-specific membrane antigen specifically lyse prostate cancer cells and release cytokines in response to prostate-specific membrane antigen. Neoplasia. 1999;1:123–127. doi: 10.1038/sj.neo.7900018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hombach A, Heuser C, Sircar R, Tillmann T, Diehl V, Kruis W, et al. T cell targeting of TAG72+ tumor cells by a chimeric receptor with antibody-like specificity for a carbohydrate epitope. Gastroenterology. 1997;113:1163–1170. doi: 10.1053/gast.1997.v113.pm9322511. [DOI] [PubMed] [Google Scholar]
- 107.McGuinness RP, Ge Y, Patel SD, Kashmiri SV, Lee HS, Hand PH, et al. Anti-tumor activity of human T cells expressing the CC49-zeta chimeric immune receptor [see comments] Hum Gene Ther. 1999;10:165–173. doi: 10.1089/10430349950018968. [DOI] [PubMed] [Google Scholar]
- 108.Chinnasamy D, Yu Z, Theoret MR, Zhao Y, Shrimali RK, Morgan RA, et al. Gene therapy using genetically modified lymphocytes targeting VEGFR-2 inhibits the growth of vascularized syngenic tumors in mice. J Clin Invest. 2010;120:3953–3968. doi: 10.1172/JCI43490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Niederman TM, Ghogawala Z, Carter BS, Tompkins HS, Russell MM, Mulligan RC. Antitumor activity of cytotoxic T lymphocytes engineered to target vascular endothelial growth factor receptors. Proc Natl Acad Sci U S A. 2002;99:7009–7014. doi: 10.1073/pnas.092562399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ocvirk J, Heeger S, McCloud P, Hofheinz RD. A review of the treatment options for skin rash induced by EGFR-targeted therapies: Evidence from randomized clinical trials and a meta-analysis. Radiol Oncol. 2013;47:166–175. doi: 10.2478/raon-2013-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ahmed N, Brawley V, Diouf O, Anderson P, Hicks MJ, Wang LL, et al. T cells redirected against HER2 for the adoptive immunotherapy for HER2-positive osteosarcoma. Cancer Res. 2012;72:Abstract 3500. [Google Scholar]
- 112.Cooper LJ, Topp MS, Serrano LM, Gonzalez S, Chang WC, Naranjo A, et al. T-cell clones can be rendered specific for CD19: toward the selective augmentation of the graft-versus-B-lineage leukemia effect. Blood. 2003;101:1637–1644. doi: 10.1182/blood-2002-07-1989. [DOI] [PubMed] [Google Scholar]
- 113.Gottschalk S, Ng CYC, Smith CA, Perez M, Sample C, Brenner MK, et al. An Epstein-Barr virus deletion mutant that causes fatal lymphoproliferative disease unresponsive to virus-specific T cell therapy. Blood. 2001;97:835–843. doi: 10.1182/blood.v97.4.835. [DOI] [PubMed] [Google Scholar]
- 114.Yee C, Thompson JA, Byrd D, Riddell SR, Roche P, Celis E, et al. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci U S A. 2002;99:16168–16173. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329–360. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- 116.Hedge M, Ahmed N, Yvon E, Corder A, Gottschalk S, Chow K, et al. Targeting Tumor Heterogeneity in childhood high grade glioma: Bispecific T cells exhibit enhanced effector functions and offset antigen loss variants. Pediatr Blood Cancer. 2012;58:1016–1017. [Google Scholar]
- 117.Janssen A, Medema RH. Genetic instability: tipping the balance. Oncogene. 2012 doi: 10.1038/onc.2012.576. [DOI] [PubMed] [Google Scholar]
- 118.Lamers CH, Sleijfer S, van SS, van EP, van KB, Groot C, et al. Treatment of metastatic renal cell carcinoma with CAIX CAR-engineered T cells: clinical evaluation and management of on-target toxicity. Mol Ther. 2013;21:904–912. doi: 10.1038/mt.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Klebanoff CA, Gattinoni L, Restifo NP. Sorting through subsets: which T-cell populations mediate highly effective adoptive immunotherapy? J Immunother. 2012;35:651–660. doi: 10.1097/CJI.0b013e31827806e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol. 2012;12:269–281. doi: 10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Brownlie RJ, Zamoyska R. T cell receptor signalling networks: branched, diversified and bounded. Nat Rev Immunol. 2013;13:257–269. doi: 10.1038/nri3403. [DOI] [PubMed] [Google Scholar]
- 122.Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13:227–242. doi: 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.King CG, Koehli S, Hausmann B, Schmaler M, Zehn D, Palmer E. T cell affinity regulates asymmetric division, effector cell differentiation, and tissue pathology. Immunity. 2012;37:709–720. doi: 10.1016/j.immuni.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Perret R, Ronchese F. Memory T cells in cancer immunotherapy: which CD8 T-cell population provides the best protection against tumours? Tissue Antigens. 2008;72:187–194. doi: 10.1111/j.1399-0039.2008.01088.x. [DOI] [PubMed] [Google Scholar]
- 125.Lanzavecchia A, Sallusto F. Understanding the generation and function of memory T cell subsets. Curr Opin Immunol. 2005;17:326–332. doi: 10.1016/j.coi.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 126.Mueller SN, Gebhardt T, Carbone FR, Heath WR. Memory T cell subsets, migration patterns, and tissue residence. Annu Rev Immunol. 2013;31:137–161. doi: 10.1146/annurev-immunol-032712-095954. [DOI] [PubMed] [Google Scholar]
- 127.Gebhardt T, Mueller SN, Heath WR, Carbone FR. Peripheral tissue surveillance and residency by memory T cells. Trends Immunol. 2013;34:27–32. doi: 10.1016/j.it.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 128.Terakura S, Yamamoto TN, Gardner RA, Turtle CJ, Jensen MC, Riddell SR. Generation of CD19-chimeric antigen receptor modified CD8+ T cells derived from virus-specific central memory T cells. Blood. 2012;119:72–82. doi: 10.1182/blood-2011-07-366419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gattinoni L, Klebanoff CA, Restifo NP. Paths to stemness: building the ultimate antitumour T cell. Nat Rev Cancer. 2012;12:671–684. doi: 10.1038/nrc3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rooney CM, Smith CA, Ng CYC, Loftin SK, Sixbey JW, Gan Y-J, et al. Infusion of cytotoxic T cells for the prevention and treatment of Epstein-Barr virus-induced lymphoma in allogeneic transplant recipients. Blood. 1998;92:1549–1555. [PubMed] [Google Scholar]
- 131.Heslop HE, Slobod KS, Pule MA, Hale GA, Rousseau A, Smith CA, et al. Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood. 2010;115:925–935. doi: 10.1182/blood-2009-08-239186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hislop AD, Taylor GS, Sauce D, Rickinson AB. Cellular responses to viral infection in humans: lessons from Epstein-Barr virus. Annu Rev Immunol. 2007;25:587–617. doi: 10.1146/annurev.immunol.25.022106.141553. [DOI] [PubMed] [Google Scholar]
- 133.Gajewski TF, Meng Y, Blank C, Brown I, Kacha A, Kline J, et al. Immune resistance orchestrated by the tumor microenvironment. Immunol Rev. 2006;213:131–145. doi: 10.1111/j.1600-065X.2006.00442.x. [DOI] [PubMed] [Google Scholar]
- 134.Pule MA, Straathof KC, Dotti G, Heslop HE, Rooney CM, Brenner MK. A chimeric T cell antigen receptor that augments cytokine release and supports clonal expansion of primary human T cells. Mol Ther. 2005;12:933–941. doi: 10.1016/j.ymthe.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 135.Imai C, Mihara K, Andreansky M, Nicholson IC, Pui CH, Geiger TL, et al. Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia. 2004;18:676–684. doi: 10.1038/sj.leu.2403302. [DOI] [PubMed] [Google Scholar]
- 136.Carpenito C, Milone MC, Hassan R, Simonet JC, Lakhal M, Suhoski MM, et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc Natl Acad Sci U S A. 2009;106:3360–3365. doi: 10.1073/pnas.0813101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Song DG, Ye Q, Poussin M, Harms GM, Figini M, Powell DJ., Jr CD27 costimulation augments the survival and antitumor activity of redirected human T cells in vivo. Blood. 2012;119:696–706. doi: 10.1182/blood-2011-03-344275. [DOI] [PubMed] [Google Scholar]
- 138.Stephan MT, Ponomarev V, Brentjens RJ, Chang AH, Dobrenkov KV, Heller G, et al. T cell-encoded CD80 and 4-1BBL induce auto- and transcostimulation, resulting in potent tumor rejection. Nat Med. 2007;13:1440–1449. doi: 10.1038/nm1676. [DOI] [PubMed] [Google Scholar]
- 139.Milone MC, Fish JD, Carpenito C, Carroll RG, Binder GK, Teachey D, et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol Ther. 2009;17:1453–1464. doi: 10.1038/mt.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhong XS, Matsushita M, Plotkin J, Riviere I, Sadelain M. Chimeric antigen receptors combining 4-1BB and CD28 signaling domains augment PI3kinase/AKT/Bcl-XL activation and CD8+ T cell-mediated tumor eradication. Mol Ther. 2010;18:413–420. doi: 10.1038/mt.2009.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17:4550–4557. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Xu XJ, Zhao HZ, Tang YM. Efficacy and safety of adoptive immunotherapy using anti-CD19 chimeric antigen receptor transduced T-cells: a systematic review of phase I clinical trials. Leuk Lymphoma. 2013;54:255–260. doi: 10.3109/10428194.2012.715350. [DOI] [PubMed] [Google Scholar]
- 143.Till BG, Jensen MC, Wang J, Qian X, Gopal AK, Maloney DG, et al. CD20-specific adoptive immunotherapy for lymphoma using a chimeric antigen receptor with both CD28 and 4-1BB domains: pilot clinical trial results. Blood. 2012;119:3940–3950. doi: 10.1182/blood-2011-10-387969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ramos C, Savoldo B, Liu E, Gee A, Mei Z, Grilley B, et al. Clinical Responses in Patients Infused with T Lymphocytes Redirected To Target ? Light Immunoglobulin Chain Mol Ther. 2013;22:S114. [Google Scholar]
- 145.Maus MV, Haas AR, Beatty GL, Albelda SM, Levine BL, Liu X, et al. T Cells Expressing Chimeric Antigen Receptors Can Cause Anaphylaxis in Humans. Cancer Immunol Res. 2013 doi: 10.1158/2326-6066.CIR-13-0006. OnlineFirst April 7, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rossig C, Bollard CM, Nuchtern JG, Rooney CM, Brenner MK. Epstein-Barr virus-specific human T lymphocytes expressing antitumor chimeric T-cell receptors: potential for improved immunotherapy. Blood. 2002;99:2009–2016. doi: 10.1182/blood.v99.6.2009. [DOI] [PubMed] [Google Scholar]
- 147.Savoldo B, Rooney CM, Di Stasi A, Abken H, Hombach A, Foster AE, et al. Epstein Barr virus specific cytotoxic T lymphocytes expressing the anti-CD30zeta artificial chimeric T-cell receptor for immunotherapy of Hodgkin disease. Blood. 2007;110:2620–2630. doi: 10.1182/blood-2006-11-059139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Cruz C, Micklethwaite KP, Savoldo B, Ku S, Krance R, Diouf O, et al. Infsuion of CD19-directed/multivirus-specific CTLs post HSCT for B cell malignancies. Mol Ther. 2012;20:S207. [Google Scholar]
- 149.Myers D, Jun J, Rooney C, Lapteva N, Gee A, Dotti G, et al. Administration of GD2 Chimeric Antigen Receptor Modified, Tri-Virus Specific Cytotoxic T Lymphocytes after HLA Mismatched Allogeneic Stem Cell Transplantation for Relapsed, Refractory Neuroblastoma. Mol Ther. 2013;22:S5–S6. [Google Scholar]
- 150.Cobbs CS, Harkins L, Samanta M, Gillespie GY, Bharara S, King PH, et al. Human cytomegalovirus infection and expression in human malignant glioma. Cancer Res. 2002;62:3347–3350. [PubMed] [Google Scholar]
- 151.Mitchell DA, Xie W, Schmittling R, Learn C, Friedman A, McLendon RE, et al. Sensitive detection of human cytomegalovirus in tumors and peripheral blood of patients diagnosed with glioblastoma. Neuro Oncol. 2008;10:10–18. doi: 10.1215/15228517-2007-035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Scheurer ME, Bondy ML, Aldape KD, Albrecht T, El Zein R. Detection of human cytomegalovirus in different histological types of gliomas. Acta Neuropathol. 2008;116:79–86. doi: 10.1007/s00401-008-0359-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ghazi A, Ashoori A, Hanley PJ, Brawley VS, Shaffer DR, Kew Y, et al. Generation of polyclonal CMV-specific T cells for the adoptive immunotherapy of glioblastoma. J Immunother. 2012;35:159–168. doi: 10.1097/CJI.0b013e318247642f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Leen AM, Rooney CM, Foster AE. Improving T cell therapy for cancer. Annu Rev Immunol. 2007;25:243–265. doi: 10.1146/annurev.immunol.25.022106.141527. [DOI] [PubMed] [Google Scholar]
- 155.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007;25:267–296. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Li XC, Demirci G, Ferrari-Lacraz S, Groves C, Coyle A, Malek TR, et al. IL-15 and IL-2: a matter of life and death for T cells in vivo. Nat Med. 2001;7:114–118. doi: 10.1038/83253. [DOI] [PubMed] [Google Scholar]
- 157.Perna SK, De AB, Pagliara D, Hasan ST, Zhang L, Mahendravada A, et al. Interleukin 15 provides relief to CTLs from regulatory T cell-mediated inhibition: implications for adoptive T cell-based therapies for lymphoma. Clin Cancer Res. 2013;19:106–117. doi: 10.1158/1078-0432.CCR-12-2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Nelson BH. IL-2, regulatory T cells, and tolerance. J Immunol. 2004;172:3983–3988. doi: 10.4049/jimmunol.172.7.3983. [DOI] [PubMed] [Google Scholar]
- 159.Waldmann TA. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol. 2006;6:595–601. doi: 10.1038/nri1901. [DOI] [PubMed] [Google Scholar]
- 160.Stonier SW, Schluns KS. Trans-presentation: a novel mechanism regulating IL-15 delivery and responses. Immunol Lett. 2010;127:85–92. doi: 10.1016/j.imlet.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Mueller K, Schweier O, Pircher H. Efficacy of IL-2- versus IL-15-stimulated CD8 T cells in adoptive immunotherapy. Eur J Immunol. 2008;38:2874–2885. doi: 10.1002/eji.200838426. [DOI] [PubMed] [Google Scholar]
- 162.Ochoa MC, Mazzolini G, Hervas-Stubbs S, de Sanmamed MF, Berraondo P, Melero I. Interleukin-15 in gene therapy of cancer. Curr Gene Ther. 2013;13:15–30. doi: 10.2174/156652313804806561. [DOI] [PubMed] [Google Scholar]
- 163.Quintarelli C, Vera JF, Savoldo B, Giordano Attianese GM, Pule M, Foster AE, et al. Co-expression of cytokine and suicide genes to enhance the activity and safety of tumor-specific cytotoxic T lymphocytes. Blood. 2007;110:2793–2802. doi: 10.1182/blood-2007-02-072843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hoyos V, Savoldo B, Quintarelli C, Mahendravada A, Zhang M, Vera J, et al. Engineering CD19-specific T lymphocytes with interleukin-15 and a suicide gene to enhance their anti-lymphoma/leukemia effects and safety. Leukemia. 2010;24:1160–1170. doi: 10.1038/leu.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hsu C, Hughes MS, Zheng Z, Bray RB, Rosenberg SA, Morgan RA. Primary human T lymphocytes engineered with a codon-optimized IL-15 gene resist cytokine withdrawal-induced apoptosis and persist long-term in the absence of exogenous cytokine. J Immunol. 2005;175:7226–7234. doi: 10.4049/jimmunol.175.11.7226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Klebanoff CA, Finkelstein SE, Surman DR, Lichtman MK, Gattinoni L, Theoret MR, et al. IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8+ T cells. Proc Natl Acad Sci U S A. 2004;101:1969–1974. doi: 10.1073/pnas.0307298101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Carrette F, Surh CD. IL-7 signaling and CD127 receptor regulation in the control of T cell homeostasis. Semin Immunol. 2012;24:209–217. doi: 10.1016/j.smim.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sportes C, Hakim FT, Memon SA, Zhang H, Chua KS, Brown MR, et al. Administration of rhIL-7 in humans increases in vivo TCR repertoire diversity by preferential expansion of naive T cell subsets. J Exp Med. 2008;205:1701–1714. doi: 10.1084/jem.20071681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Vera J, Hoyos V, Savoldo B, Quintarelli C, Girodano-Attianese G, Foster A, et al. Gene Transfer of IL-7R alpha (IL-7R) on Antigen-Specific Cytotoxic T Cells (CTLs) Restores Their Ability To Respond to IL-7 Cytokine. Blood. 2007;110(11):2756. (Abstract) [Google Scholar]
- 170.Xu M, Mizoguchi I, Morishima N, Chiba Y, Mizuguchi J, Yoshimoto T. Regulation of antitumor immune responses by the IL-12 family cytokines, IL-12, IL-23, and IL-27. Clin Dev Immunol. 2010 doi: 10.1155/2010/832454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Pegram HJ, Lee JC, Hayman EG, Imperato GH, Tedder TF, Sadelain M, et al. Tumor-targeted T cells modified to secrete IL-12 eradicate systemic tumors without need for prior conditioning. Blood. 2012;119:4133–4141. doi: 10.1182/blood-2011-12-400044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zhang L, Kerkar SP, Yu Z, Zheng Z, Yang S, Restifo NP, et al. Improving adoptive T cell therapy by targeting and controlling IL-12 expression to the tumor environment. Mol Ther. 2011;19:751–759. doi: 10.1038/mt.2010.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wagner HJ, Bollard CM, Vigouroux S, Huls MH, Anderson R, Prentice HG, et al. A strategy for treatment of Epstein-Barr virus-positive Hodgkin’s disease by targeting interleukin 12 to the tumor environment using tumor antigen-specific T cells. Cancer Gene Ther. 2004;11:81–91. doi: 10.1038/sj.cgt.7700664. [DOI] [PubMed] [Google Scholar]
- 174.Kerkar SP, Leonardi AJ, van PN, Zhang L, Yu Z, Crompton JG, et al. Collapse of the Tumor Stroma is Triggered by IL-12 Induction of Fas. Mol Ther. 2013;21:1369–1377. doi: 10.1038/mt.2013.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sun J, Dotti G, Huye LE, Foster AE, Savoldo B, Gramatges MM, et al. T cells expressing constitutively active Akt resist multiple tumor-associated inhibitory mechanisms. Mol Ther. 2010;18:2006–2017. doi: 10.1038/mt.2010.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Peggs KS, Quezada SA, Allison JP. Cancer immunotherapy: co-stimulatory agonists and co-inhibitory antagonists. Clin Exp Immunol. 2009;157:9–19. doi: 10.1111/j.1365-2249.2009.03912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Suntharalingam G, Perry MR, Ward S, Brett SJ, Castello-Cortes A, Brunner MD, et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med. 2006;355:1018–1028. doi: 10.1056/NEJMoa063842. [DOI] [PubMed] [Google Scholar]
- 181.Dotti G, Savoldo B, Pule M, Straathof KC, Biagi E, Yvon E, et al. Human cytotoxic T lymphocytes with reduced sensitivity to Fas-induced apoptosis. Blood. 2005;105:4677–4684. doi: 10.1182/blood-2004-08-3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Thomas DA, Massague J. TGF-beta directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell. 2005;8:369–380. doi: 10.1016/j.ccr.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 183.Akhurst RJ. TGF beta signaling in health and disease. Nat Genet. 2004;36:790–792. doi: 10.1038/ng0804-790. [DOI] [PubMed] [Google Scholar]
- 184.Bollard CM, Rossig C, Calonge MJ, Huls MH, Wagner HJ, Massague J, et al. Adapting a transforming growth factor beta-related tumor protection strategy to enhance antitumor immunity. Blood. 2002;99:3179–3187. doi: 10.1182/blood.v99.9.3179. [DOI] [PubMed] [Google Scholar]
- 185.Foster AE, Dotti G, Lu A, Khalil M, Brenner MK, Heslop HE, et al. Antitumor activity of EBV-specific T lymphocytes transduced with a dominant negative TGF-beta receptor. J Immunother. 2008;31:500–505. doi: 10.1097/CJI.0b013e318177092b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Bollard CM, Dotti G, Gottschalk S, Mims MP, Liu H, Gee AP, et al. Administration of TGF-beta resistant tumor-specific CTL to patienst with EBV-associated HL and NHL. Molecular Therapy. 2012;20:S22. [Google Scholar]
- 187.Watanabe N, Anurathapan U, Brenner M, Heslop H, Leen A, Rooney C, et al. Transgenic Expression of a Novel Immunosuppressive Signal Converter on T Cells. Mol Ther. 2013;22:S153. [Google Scholar]
- 188.Leen A, Katari U, Keiman J, Rooney C, Brenner M, Vera J. Improved Expansion and Anti-Tumor Activity of Tumor-Specific CTLs Using a Transgenic Chimeric Cytokine Receptor. Mol Ther. 2011;19:S194. [Google Scholar]
- 189.Wilkie S, Burbridge SE, Chiapero-Stanke L, Pereira AC, Cleary S, van der Stegen SJ, et al. Selective expansion of chimeric antigen receptor-targeted T-cells with potent effector function using interleukin-4. J Biol Chem. 2010;285:25538–25544. doi: 10.1074/jbc.M110.127951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Mueller MM, Fusenig NE. Friends or foes - bipolar effects of the tumour stroma in cancer. Nat Rev Cancer. 2004;4:839–849. doi: 10.1038/nrc1477. [DOI] [PubMed] [Google Scholar]
- 191.Marx J. Cancer biology. All in the stroma: cancer’s Cosa Nostra. Science. 2008;320:38–41. doi: 10.1126/science.320.5872.38. [DOI] [PubMed] [Google Scholar]
- 192.Cirri P, Chiarugi P. Cancer associated fibroblasts: the dark side of the coin. Am J Cancer Res. 2011;1:482–497. [PMC free article] [PubMed] [Google Scholar]
- 193.Kakarla S, Song XT, Gottschalk S. Cancer-associated fibroblasts as targets for immunotherapy. Immunotherapy. 2012;4:1129–1138. doi: 10.2217/imt.12.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Chinnasamy D, Yu Z, Kerkar SP, Zhang L, Morgan RA, Restifo NP, et al. Local delivery of interleukin-12 using T cells targeting VEGF receptor-2 eradicates multiple vascularized tumors in mice. Clin Cancer Res. 2012;18:1672–1683. doi: 10.1158/1078-0432.CCR-11-3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Bear AS, Morgan RA, Cornetta K, June CH, Binder-Scholl G, Dudley ME, et al. Replication-competent retroviruses in gene-modified T cells used in clinical trials: is it time to revise the testing requirements? Mol Ther. 2012;20:246–249. doi: 10.1038/mt.2011.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Cavazzana-Calvo M, Fischer A, Hacein-Bey-Abina S, Aiuti A. Gene therapy for primary immunodeficiencies: Part 1. Curr Opin Immunol. 2012;24:580–584. doi: 10.1016/j.coi.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 197.Hackett PB, Largaespada DA, Switzer KC, Cooper LJ. Evaluating risks of insertional mutagenesis by DNA transposons in gene therapy. Transl Res. 2013;161:265–283. doi: 10.1016/j.trsl.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Nakazawa Y, Huye LE, Salsman VS, Leen AM, Ahmed N, Rollins L, et al. PiggyBac-mediated Cancer Immunotherapy Using EBV-specific Cytotoxic T-cells Expressing HER2-specific Chimeric Antigen Receptor. Mol Ther. 2011;19:2133–2143. doi: 10.1038/mt.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Zhao Y, Moon E, Carpenito C, Paulos CM, Liu X, Brennan AL, et al. Multiple injections of electroporated autologous T cells expressing a chimeric antigen receptor mediate regression of human disseminated tumor. Cancer Res. 2010;70:9053–9061. doi: 10.1158/0008-5472.CAN-10-2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Barrett DM, Zhao Y, Liu X, Jiang S, Carpenito C, Kalos M, et al. Treatment of advanced leukemia in mice with mRNA engineered T cells. Hum Gene Ther. 2011;22:1575–1586. doi: 10.1089/hum.2011.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Almasbak H, Rian E, Hoel HJ, Pule M, Walchli S, Kvalheim G, et al. Transiently redirected T cells for adoptive transfer. Cytotherapy. 2011;13:629–640. doi: 10.3109/14653249.2010.542461. [DOI] [PubMed] [Google Scholar]
- 202.Straathof KC, Pule MA, Yotnda P, Dotti G, Vanin EF, Brenner MK, et al. An inducible caspase 9 safety switch for T-cell therapy. Blood. 2005;105:4247–4254. doi: 10.1182/blood-2004-11-4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Di Stasi A, Tey SK, Dotti G, Fujita Y, Kennedy-Nasser A, Martinez C, et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N Engl J Med. 2011;365:1673–1683. doi: 10.1056/NEJMoa1106152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Arber C, Abhyankar H, Heslop HE, Brenner MK, Liu H, Dotti G, et al. The immunogenicity of virus-derived 2A sequences in immunocompetent individuals. Gene Ther. 2013 doi: 10.1038/gt.2013.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Allen M, Louise JJ. Jekyll and Hyde: the role of the microenvironment on the progression of cancer. J Pathol. 2011;223:162–176. doi: 10.1002/path.2803. [DOI] [PubMed] [Google Scholar]
- 206.Wilkie S, van Schalkwyk MC, Hobbs S, Davies DM, van der Stegen SJ, Pereira AC, et al. Dual targeting of ErbB2 and MUC1 in breast cancer using chimeric antigen receptors engineered to provide complementary signaling. J Clin Immunol. 2012;32:1059–1070. doi: 10.1007/s10875-012-9689-9. [DOI] [PubMed] [Google Scholar]
- 207.Lanitis E, Poussin M, Klattenhof A, Song D, Sandaltzopoulos R, June CH, et al. Chimeric Antigen Receptor T Cells with Dissociated Signaling Domains Exhibit Focused Antitumor Activity with Reduced Potential for Toxicity In Vivo. Cancer Immunol Res. 2013 doi: 10.1158/2326-6066.CIR-13-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Kloss CC, Condomines M, Cartellieri M, Bachmann M, Sadelain M. Combinatorial antigen recognition with balanced signaling promotes selective tumor eradication by engineered T cells. Nat Biotechnol. 2013;31:71–75. doi: 10.1038/nbt.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]