Abstract
Rituximab, a monoclonal antibody directed against the B-lymphocyte antigen CD20, has shown promise in several autoimmune disorders. Pulmonary Alveolar Proteinosis (PAP) is an autoimmune disorder characterized by autoantibodies to Granulocyte-Macrophage Colony Stimulating Factor (GM-CSF).
An open label proof-of-concept Phase II clinical trial was conducted in 10 PAP patients. Intervention consisted of two intravenous infusions of rituximab (1000mg), fifteen days apart. Bronchoalveolar lavage (BAL) and peripheral blood samples were collected.
The primary outcome was improvement in arterial blood oxygenation. Both PaO2 and A-a gradient on room air improved in 7/9 patients completing the study. Lung function and HRCT scans, secondary outcomes, also improved. Peripheral blood CD19+ B-lymphocytes decreased from 15±2% to <0.05% (n=10) fifteen days post therapy. This decrease persisted for 3 months in all patients; at six months CD19+ were detected in 4/7 patients (mean 5±2). Total anti-GM-CSF IgG levels from baseline to 6 months were decreased in BAL fluids (n=8), but unchanged in sera (n=9).
In this PAP cohort, (1) rituximab was well-tolerated and effectively ameliorated lung disease; (2) reduction in anti-GM-CSF IgG levels in the lung correlated with disease changes suggesting that disease pathogenesis is related to autoantibody levels in the target organ.
Keywords: PAP, rituximab, B Cells, BAL
INTRODUCTION
Rituximab, a chimeric murine-human monoclonal antibody directed against CD-20, a B lymphocyte-specific membrane antigen, has been shown to deplete human B cells in vivo [1]. B lymphocyte depletion in peripheral blood occurs rapidly, within days after rituximab administration. Proposed mechanisms of B cell depletion by rituximab include complement-mediated cytotoxicity, antibody-dependent cell-mediated cytotoxicity, and antibody-mediated apoptosis [2].
Rituximab was approved by the Food and Drug Administration in 1997 for treatment of CD20(+) B cell lymphoma and has since been in use for B cell malignancies. More recently, rituximab has been applied to the treatment of autoimmune disease, and has shown clinical benefit in systemic lupus erythematosus [2], rheumatoid arthritis [3], and Wegener’s granulomatosis [4] among others. Rituximab treatment has been found to reduce autoantibody levels to a greater extent than levels of total serum immunoglobulins or antimicrobial antibodies [5]. It is important to note that rituximab was added to other immunosuppressive therapies in these studies making it difficult to assess the direct effect of rituximab on human autoimmunity. CD20, the target of rituximab binding, is a 35 kD cell membrane protein considered to play a role in B cell cycling and differentiation [6]. CD20 first appears in the early pre-B cell stage and expression diminishes with terminal differentiation into the plasma cell stage. CD20 is thus detectable on most precursor B cells within the bone marrow, and on immature, mature-naive, and memory B cells [2].
Pulmonary alveolar proteinosis (PAP) is a rare idiopathic disease noted for deposition of extracellular lipoproteinaceous material within pulmonary alveoli. With the discovery that genetically altered mice, homozygous for a disrupted GM-CSF gene, developed excess lung surfactant and had a histologic resemblance to PAP [7;8], the pivotal role of GM-CSF in normal lung homeostasis was revealed. Interestingly, in adults with PAP, no mutations in GM-CSF receptor or surfactant coding sequences have been described [9]. Studies of GM-CSF knock-out mice initially suggested that PAP might be due to intrinsic defects in GM-CSF receptors or production. However, studies from our lab and others demonstrated that monocytes and alveolar macrophages from adult PAP patients were able to produce and respond to GM-CSF [10;11]. Evidence that adult PAP might be an autoimmune disease was first presented by Nakata et al, who noted that circulating anti-GM-CSF autoantibodies neutralized GM-CSF biological activity, and thus resulted in a virtual GM-CSF deficiency [12;13]. Subsequent studies in idiopathic adult PAP patients confirmed the existence of anti-GM-CSF antibodies and demonstrated that autoantibody levels were clinically useful for diagnosis [14–17].
Thus based upon current data indicating autoantibody involvement in PAP, we hypothesized that reduction of PAP B cells in the context of a rituximab clinical trial would diminish levels of anti-GM-CSF autoantibodies. Further, we hypothesized that decreasing autoantibody levels would allow restoration of surfactant catabolism and resolution of clinical disease.
METHODS
Study Design
This study was a prospective, open-label, proof-of-concept clinical trial of rituximab therapy in a group of 10 adult patients presenting with moderately symptomatic, idiopathic PAP. The study was approved by the Institutional Review Board at East Carolina University and informed consent was obtained from all patients. The trial was registered at clinicaltrials.gov (NCT00552461). The diagnosis of idiopathic PAP was confirmed by open lung biopsy (n=3) or bronchoscopy (n=7). All patients had moderately severe PAP and elevated anti-GM-CSF levels in sera (Table 1). A total of five visits were scheduled in which rituximab (1000 mg) was administered IV as an infusion during visits 1 and 2 (see supplement for monitoring during therapy and follow-up). Bronchoscopy was performed pre initial infusion and at 6 months after treatment. Serum samples were collected pre infusions #1 and 2 and at each subsequent visit (Figure 1).
Table 1.
Patient demographics and clinical characteristics
| Characteristic | n | Median (range) or Mean ± SEM |
|---|---|---|
| Age (years) | 10 | 41 (22–68) |
| Gender (M/F) | 7/3 | |
| Race (Caucasian/African American) | 8/2 | |
| Current smoker/non-smoker/ex-smoker | 3/3/4 | |
| Median duration of PAP (months) | 10 | 15 (2–115) |
| Serum anti-GM-CSF (μg/ml) | 10 | 1227 ± 343 |
| Baseline home O2 | 8/10 | |
| Prior whole lung lavages | 8/10 | 5.1 (0–16) |
| Interval since last WLL* (months) | 8/10 | 5.8 (1–29) |
| Functional dyspnea grade Mahler’s BDI*, 0–4 | 10 | 1.0 (0–2.5) |
Abbrevations – WLL = whole lung lavage; BDI = baseline dyspnea index
Figure 1.
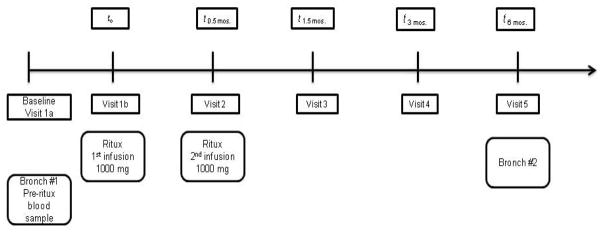
Schematic of study visits for rituximab trial
Study Eligibility
Eligibility criteria included the following: (1) newly diagnosed PAP or chronic longstanding disease in patients of age > 18 years; (2) moderately symptomatic disease as defined by the presence of symptoms attributable to PAP (e.g., dyspnea, cough), moderately impaired gas exchange (PaO2<70 and >55 mmHg on room air or up to supplemental O2 6 L/min NC), and radiographic abnormalities; and (3) presence of high titer anti-GM-CSF antibody (≥6400). Exclusion criteria included the following: (1) PAP resulting from another condition (e.g., occupational exposure to silica, underlying HIV, respiratory infections, myeloproliferative disorder or leukemia); (2) active cardiovascular disease (e.g., cardiogenic pulmonary edema); (3) pregnant and lactating women; (4) renal (serum creatinine >2 times normal) or liver (bilirubin >2, enzymes >2.5 times normal) disease.
Assessments
The main endpoint was an improvement in oxygenation as assessed by the alveolar-arterial oxygen gradient. A reduction in A-a gradient by 10 mmHg was considered significant. Other endpoints included: (1) improvement in patients’ symptoms as assessed by functional status with Mahler’s dyspnea questionnaire [18], forced expiratory volume in one second (FEV1), diffusing capacity (DLCO), six-minute walk distance/oxygen saturation and chest CT severity index, (2) overall tolerability of therapy, (3) requirement for whole lung lavage, (4) sustainability of response by follow-up phone interviews, (5) change in serum anti-GM-CSF level, as well as markers for B and T cells.
Radiographic Grading
De-identified chest high resolution computed tomography (HRCT) obtained prior to therapy and 6 months post-therapy was evaluated by a board certified radiologist [19;20]. Briefly, the extent of ground glass opacity (GGO) was quantified visually in CT slices representing the three lung regions: upper (above the aortic arch), middle (at the main carina), and lower (at the bifurcation of the linear and lower lobe bronchi). Scans were scored as follows: 0=no GGO, 1 = less than 5% GGO, 2 = 5–24% GGO, 3 = 25–49% GGO, 4 = 50–74% GGO and 5 = 75% or more GGO. The total score was calculated as the sum of all HRCT score values for each scan.
Laboratory Measurements
Bronchoscopic lavage (BAL) was carried out prior to and 6 months after therapy as described [10;21]. For autoantibody analyses of both BAL fluids and sera, anti GM-CSF IgG levels were determined by Luminex technology [22]; and GM-CSF neutralizing activity was evaluated by bioassay of TF-1 cells as previously described [10]. From peripheral blood white blood cells, hemoglobin, platelets, total immunoglobulin and IgG subclasses were all quantified by standard methods. Flow cytometry was used to quantify peripheral blood B cells using CD19, CD4 and CD8 T cells.
Statistical Analysis
Parametric data are presented as means (±SEM) and nonparametric data are presented as medians and ranges. Statistical comparisons of parametric data were made with Student’s t test. Nonparametric data were compared with Wilcoxon test. The correlation coefficient was obtained using Spearman’s correlation. P ≤ 0.05 was considered significant.
RESULTS
Patients
Ten patients were enrolled in the study and received rituximab infusions. One patient dropped out of the study after the second infusion for unknown reasons. The remaining 9 patients completed the 6-month study period. Table 2 summarizes the clinical parameters pre- and post-rituximab.
Table 2.
Summary of Clinical Parameters Pre- and Post-rituximab Therapy
| Characteristic | Pre-rituximab therapy (Baseline) Mean ± SEM or Median (range) | Post-rituximab therapy (6 Months) Mean ± SEM or Median (range) | P value |
|---|---|---|---|
| Arterial blood gas analysis | |||
| PaO2 mmHg | 54.7±3.4 | 74.3±4.6 | 0.002 |
| A-a | 50.0±3.4 | 32.9±4.6 | 0.006 |
| Lung Function | |||
| FVC (L) | 2.7±0.4 | 3.0±0.4 | 0.16 |
| %FVC | 61.0±7.8 | 63.7±10.2 | 0.66 |
| TLC | 3.7±0.6 | 4.2±0.5 | 0.041 |
| %TLC (L) | 59.9±7.2 | 68.7±6.5 | 0.050 |
| DLCO | 10.3±1.6 | 12.5±2.5 | 0.16 |
| %DLCO | 39.6±5.3 | 56.4±9.7 | 0.13 |
| Functional Status | |||
| FI Gradea | 1.1±0.4 | 2.0±0.3 | 0.063 |
| ME gradea | 1.3±0.4 | 2.4±0.3 | 0.038 |
| MT gradea | 1.0±0.3 | 2.4±0.3 | 0.014 |
| 6 min walk test (meters) | 376±43 | 395±38 | 0.64 |
| B Cells | |||
| CD19 cells/μl | 378(108–1518) | 73(0–216) | 0.0078 |
| GM-CSF autoantibody Serum | |||
| Anti-GM-CSF μg/ml | 1227 ± 343 | 949 ± 294 | 0.098 |
| IC 50 (dilution 1:X) | 3546 ± 712 | 2976 ± 590 | 0.50 |
| BAL | |||
| Anti-GM-CSF μg/ml | 15 ± 5 | 7 ± 2 | 0.016 |
| IC 50 (dilution 1:X) | 55 ± 23 | 22 ± 2 | 0.058 |
FI: Functional Impairment, ME: Magnitude of Effort, MT: Magnitude of Task
Safety of Rituximab
No major pulmonary or cardiac infusion-related reactions were noted. The majority of adverse events in 10 patients were minor (Table 3) with most occurring at the time of the rituximab infusions. Two patients reported an upper respiratory tract infection. No other infections were documented.
Table 3.
Adverse Events
|
Body General
| |
| Fatigue | 5/10 |
| Headache | 4/10 |
| Dizziness | 3/10 |
|
Digestive
| |
| Nausea | 3/10 |
| Anorexia | 2/10 |
| Weight gain | 3/10 |
| Skin reactions | 1/10 |
|
| |
| Respiratory system | |
| Nasal congestion | 5/10 |
| Upper respiratory infection | 2/10 |
| Chest pains | 1/10 |
Primary Outcome
Combined data from all patients indicated that oxygenation, the primary endpoint, significantly improved over baseline values. PaO2 was significantly improved from baseline to three and six month post-infusion [57(45–67) mmHg baseline to 69 (46–102) 3 months (p = 0.008, n=9) and 74(46–95) 6 months (p = 0.004, n=9)] (Figure 2A) while A-a gradient improved from 49(38–61) baseline to 33(5–39) 3 months (p =0.010) and 33(5–49) 6 months (p = 0.006) (Figure 2B).
Figure 2. Lung function parameters are improved with rituximab treatment.
(A) PaO2 was measured at baseline (before rituximab), 3 and 6 months post treatment in all patients except #1 where a 3 month sample was not obtained and #6 who refused clinical follow-up after visit 3. (B) A-a gradient was measured pre- and 3 and 6 months post-rituximab. (C) High resolution computed tomography (HRCT) demonstrated improvement with rituximab therapy. The effect of rituximab therapy on the severity of pulmonary alveolar proteinosis (PAP) lung disease was measured by the zonal HRCT score [19] as described in the supplement. (D) HRCT scan of a representative mid zone of patient #8 pre- and post-rituximab therapy.
Additional Pulmonary Outcomes
TLC and %TLC showed significant improvement overall (Table 2) but other lung function parameters (FVC, %FVC, DLCO, %DLCO) were unchanged from baseline. Assessment of functional status indicated improvement in both magnitude of effort (ME) and magnitude of task (MT) grades (Table 2) but not in functional impairment (FI) grade or in the 6 minute walk test. Chest HRCT scans improved after rituximab therapy (p = 0.027) [Figure 2C]. Marked improvement in ground glass infiltrates post therapy is noted in a representative mid zone of patient #8 as compared to the pre-therapy scan (Figure 2D). Furthermore, HRCT scores correlated with PaO2 (r2 = −0.696; p = 0.0019).
Peripheral Blood Studies
Total white blood cell counts did not change from baseline or at any time point during follow-up, nor did absolute numbers of neutrophils, eosinophils, platelets or hemoglobin (Table 2). Absolute numbers of monocytes showed a slight but significant decrease.
Flow cytometry indicated a dramatic depletion of CD19(+) B cells from a median of 378 cells/μl (108–1518 range) at baseline to 73 (0–216) (p = 0.008, n=10) after rituximab infusions (Figure 3). This depletion was maintained for 3 months. Increases in CD19(+) cells were not detectable until 6 months (4/7 patients). No significant changes in CD4(+) or CD8(+) T cells were observed (Supplement Table 1).
Figure 3. Rituximab therapy depletes B cells.
CD19+ cells were determined by flow cytometric analysis of blood samples at each study visit.
Immunoglobulins and Autoantibodies
Total levels of serum IgG, IgG1, IgG2, IgG3, and IgG4 were unchanged (Table 2). Serum IgA was also unchanged but significant decreases were noted in total IgM (p = 0.004) and IgE (p = 0.039) (Table 2).
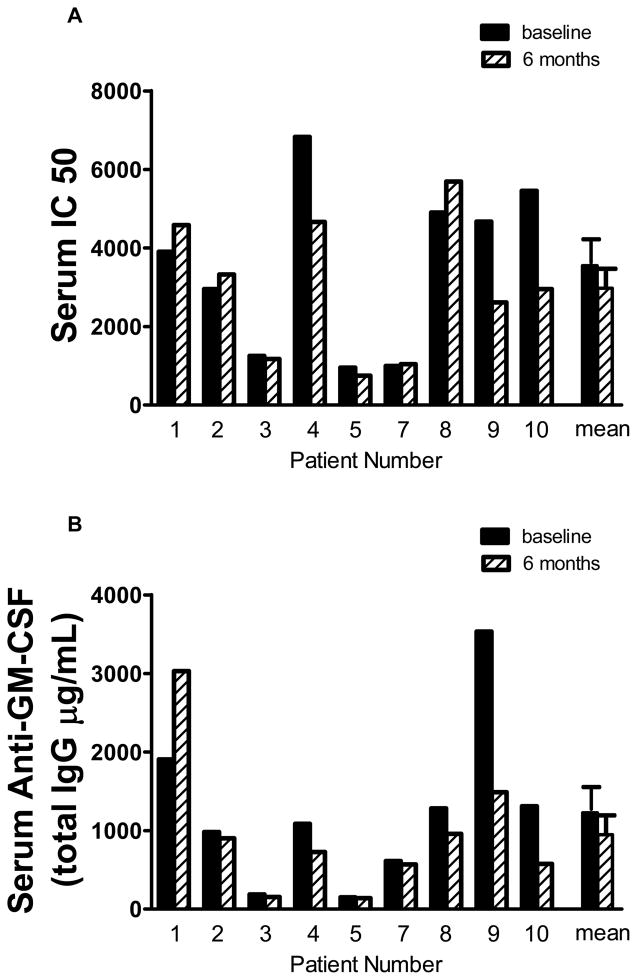
Overall, rituximab therapy did not significantly alter mean serum levels of specific anti-GM-CSF neutralizing activity (baseline = 3903(956–6826) IC50; 6 months post-treatment = 2950 (747–5692) IC50) (n=9) (Figure 4A). Similarly, anti-GM-CSF IgG autoantibody (baseline = 1086(150–3532) μg/ml) was also not significantly changed in serum post-treatment (726(139–3028) μg/ml) (Figure 4B). Analysis of BAL fluid (n=8) indicated a significant decrease in BAL fluid anti-GM-CSF IgG levels (baseline = 8.2(3.9–41.2) μg/ml) at 6 months post treatment (mean = 6.1(0.3–14.7) μg/ml; p = 0.016) (Figure 4D). A borderline reduction of BAL anti-GM-CSF neutralizing activity was detected (baseline = 30.5(15–210); 6 months post treatment = 22.5(5–50) IC50; p = 0.058) (Figure 4C). Of note, only patient #1 had an increase in neutralizing antibody in BAL and two patients (#3 and #7) showed no change. The remaining 5 patients demonstrated marked decreases in BAL autoantibody (range 33 to 87%) including patient #4 who’s PaO2 was unchanged but whose HRCT scan showed improvement. Therapy-associated changes in both BAL total anti-GM-CSF IgG and neutralizing anti-GM-CSF correlated with HRCT scores (r2 = 0.929, p =0.007); r2 = 0.991, p=0.0004, respectively). Anti-GM-CSF IgM and IgE were not detected at baseline or 6 month post-treatment.
Figure 4.
(A–B) Serum GM-CSF neutralizing capacity and total anti-GM-CSF immunoglobulin G (IgG) was not altered with rituximab therapy. Serum samples were obtained pre- and post-rituximab therapy. GM-CSF neutralizing activity was measured by the TF-1 assay as previously described [10] and anti-GM-CSF antibody was determined by a luminex based assay as described previously.[22] (C–D) Bronchial alveolar lavage (BAL) fluid GM-CSF neutralizing capacity and total anti-GM-CSF IgG was decreased with rituximab therapy. BAL fluid was obtained pre- and post-therapy and antibody levels were measured as described for serum.
Interestingly, analyses of the BAL cell population revealed no significant changes in cell number or differential between baseline and 6 months (data not shown). CD19+ cells (2–3%) were detected in BAL by flow cytometry in 2/7 patients at baseline and none were detected at 6 months.
Follow-up
Post-therapy follow-up was obtained in 7/10 patients (Table 4). The follow-up (32 ± 6 months) showed 4/7 to be free of whole lung lavage (WLL) or home O2. Need for home O2 persisted in 1 patient (lives at high altitude) and two patients required one WLL. Interestingly, one of these patients requiring WLL had undergone monthly WLL for 32 months prior to therapy.
Table 4.
Patient Follow-up
| Patient # | Post Therapy (months) | Hospitalizations | WLLa | Home O2 |
|---|---|---|---|---|
| 1 | 30 | No | No | yesb |
| 2 | 42 | No | No | prna |
| 3 | 36 | No | No | No |
| 4c | ||||
| 5c | ||||
| 6d | 36 | No | No | No |
| 7 | 30 | No | No | No |
| 8 | 25 | 1 | 1 | No |
| 9 | 24 | No | 1 | No |
| 10c |
Abbrevations – WLL = whole lung lavage, prn = when necessary
patient lives at high altitude
lost to follow-up
patient refused clinical follow-up after 3rd visit
DISCUSSION
The present study reports the results from the first prospective, open label, proof of concept trial of rituximab in PAP and suggests that rituximab may be an effective primary therapy in this autoimmune disease. The most striking clinical finding from this study of rituximab in PAP was the significant improvement of oxygenation, the primary endpoint in 7/9 patients. Improvements were also noted in total lung capacity, HRCT scans and on a transitional dyspnea index. Importantly, neither the total serum anti-GM-CSF nor serum GM-CSF neutralizing capacity were reduced by following rituximab therapy. Interestingly, anti-GM-CSF levels in BAL fluid from the lung correlated with improvement in PaO2 and HRCT scans. Data also indicated that rituximab was well tolerated with no major adverse reactions in this PAP cohort.
Therapeutic WLL is considered the current standard of care [reviewed by Seymour and Presneill [23]] for PAP but has many limitations because of invasiveness, need for general anesthesia and requirement for hospitalization. A randomized trial or even a formal prospective study of WLL has not been conducted to measure magnitude or durability of response in PAP. Thus, the clinical impact on PAP is uncertain, although lavage is the first line therapy offered by clinicians and reimbursed by insurance companies because of perceived benefit. Furthermore, WLL procedures vary widely among institutions and there is a lack of established response criteria. Several clinical trials have used subcutaneously or inhaled GM-CSF on an experimental basis. We reported a prospective study of subcutaneous GM-CSF with a response rate of 48% and 4 of 12 responders required additional therapy within 39±17 months [24]. Most recently, a multicenter phase II trial of inhaled GM-CSF therapy for PAP had a response rate of 62% during a six-month treatment period [20] with 17% of patients requiring additional therapy within 1 year. Neither subcutaneous nor inhaled GM-CSF has been formally compared to conventional management (observation with episodic lung lavage) and neither is currently not FDA approved for use in PAP.
The role of autoantibodies in autoimmune diseases has not been clearly defined and may differ depending on disease stage and/or severity [25]. In PAP, studies have suggested a possible correlation of serum neutralizing anti-GM-CSF levels with disease activity [26]. Plasmapheresis has been tried with mixed results as reported in two separate case studies [27;28]. One patient showed marked improvement with reduction in antibody titer [27] while the other patient achieved only minor reduction in antibody and this was not reflected in clinical improvement [28]. In a case study of a rituximab-treated PAP patient, clinical improvement correlated with a decline in serum neutralizing antibody [29]. The present study did not show a significant correlation with serum activity, but direct comparison of other anti-GM-CSF assays [30] with the luminex assay used in the present study is not possible because of differences in standards. To eliminate assay-to-assay variability, all samples from an individual were run in the same assay and data were evaluated for the change (delta) in antibody levels. In BAL fluid, however, total anti-GM-CSF was significantly decreased and the neutralizing activity was near significance at p=0.058. Both the neutralizing activity and total anti-GM-CSF levels in BAL correlated with improvement in HRCT scans, suggesting that autoantibody levels in the target organ correlate with pathophysiology. Although the source of the antibody maybe from circulation, B cells are found in the lung [31] and our previous studies have noted a decrease in activin A, a negative regulator of B cell proliferation and activity, in PAP BAL fluid [32]. Our results support the concept that the pathology of PAP is mediated by GM-CSF autoantibody as suggested by results from passive transfer of a highly purified, PAP patient-derived, anti-GM-CSF preparation to primates [33;34]. PAP anti-GM-CSF antibody reproduced the biochemical, cellular and histopathologic features of PAP in treated primates [33;34].
The mechanisms responsible for rituximab-mediated improvement in PAP disease activity are unclear. While rituximab effectively depleted circulating B lymphocytes for a period of 3 months in all 9 patients completing the study, total serum IgG levels were unaffected by treatment as has been reported previously in rituximab-treated systemic lupus erythematosis patients [35]. There were decreases in total IgM and E but autoantibody studies did not discern any anti-GM-CSF activity in either class of immunoglobulin (data not shown), in contrast to IgG autoantibody. Vallerskog and co-investigators also noted decreased IgM and IgE and speculated that both might be produced by short lived plasmablasts in contrast to IgG, and therefore would be more susceptible to rituximab [35].
Analysis of rituximab effects in other autoimmune disease is confounded by co-existent use of immunosuppressives. PAP, although a rare disease, serves as an outstanding model of human autoimmunity, and these patients are typically not on systemic immunomodulators (none of the 10 subjects were on other therapies). The potential role of B cells in PAP, however is not well defined. B cells participate in antigen presentation, regulatory signaling to T cells and provide regulatory cytokine signals to dendritic cells [36;37]. The majority of antibody-forming B cells are plasma cells which do not express CD20 and are therefore not susceptible to rituximab depletion [25]. Animal models suggest that reductions in autoantibody levels may be due to removal of short-lived antibody-forming cells or depletion of plasmablast precursors [38]. Additional studies are needed to explore the mechanisms by which rituximab mediates clinical improvement in PAP.
In conclusion, a single course of rituximab therapy for PAP is safe, well tolerated and efficacious. Rituximab may constitute an appropriate therapeutic alternative to WLL, the current standard of therapy. Furthermore, reduction in anti-GM-CSF IgG levels in the lung correlated with disease changes supporting the concept that disease pathogenesis is related to autoantibody levels in the target organ. Limitations of the present study include the open-label design and the absence of a placebo group. Questions remain concerning the durability of responses and the need for longer term therapy. Nevertheless, given the pilot nature of the study, the rarity of the disease and a single site design, this trial achieved its objective of laying the foundation for a larger multicenter, randomized, and controlled trial design of rituximab to establish frequency and duration of therapy for optimal treatment effects.
Supplementary Material
Acknowledgments
FUNDING SOURCE(S): Supported by Genentech and the National Institute of Health in part by grant RO1-AI064153.
Footnotes
AUTHOR CONTRIBUTIONS: Authors contributed to the conception and design (MSK, MJT), acquisition of data, analysis and interpretation of data (MSK, MJT, BPB, IH, IM, MM, RK, AM, HD) and the drafting and revising for critically for important intellectual content (MSK, MJT, BPB, IH, IM, AM). All authors are in accordance with the final version to be published.
This article has on online data supplement.
Reference List
- 1.Grillo-Lopez AJ, White CA, Varns C, Shen D, Wei A, McClure A, Dallaire BK. Overview of the clinical development of rituximab: first monoclonal antibody approved for the treatment of lymphoma. Semin Oncol. 1999;26:66–73. [PubMed] [Google Scholar]
- 2.Anolik J, Sanz I, Looney RJ. B cell depletion therapy in systemic lupus erythematosus. Curr Rheumatol Rep. 2003;5:350–356. doi: 10.1007/s11926-003-0020-x. [DOI] [PubMed] [Google Scholar]
- 3.Edwards JC, Szczepanski L, Szechinski J, Filipowicz-Sosnowska A, Emery P, Close DR, Stevens RM, Shaw T. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med. 2004;350:2572–2581. doi: 10.1056/NEJMoa032534. [DOI] [PubMed] [Google Scholar]
- 4.Stone JH, Merkel PA, Spiera R, Seo P, Langford CA, Hoffman GS, Kallenberg CGM, StClair EW, Turkiewicz A, Tchao NK, et al. Rituximab versus Cyclophosphamide for ANCA-Associated Vasculitis. N Engl J Med. 2010;363:221–232. doi: 10.1056/NEJMoa0909905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cambridge G, Leandro MJ, Edwards JC, Ehrenstein MR, Salden M, Bodman-Smith M, Webster AD. Serologic changes following B lymphocyte depletion therapy for rheumatoid arthritis. Arthritis Rheum. 2003;48:2146–2154. doi: 10.1002/art.11181. [DOI] [PubMed] [Google Scholar]
- 6.Tedder TF, Engel P. CD20: a regulator of cell-cycle progression of B lymphocytes. Immunol Today. 1994;15:450–454. doi: 10.1016/0167-5699(94)90276-3. [DOI] [PubMed] [Google Scholar]
- 7.Dranoff G, Crawford AD, Sadelain M, Ream B, Rashid A, Bronson RT, Dickersin GR, Bachurski CJ, Mark EL, Whitsett JA, et al. Involvement of granulocyte-macrophage colony-stimulating factor in pulmonary homeostasis. Science. 1994;264:713–716. doi: 10.1126/science.8171324. [DOI] [PubMed] [Google Scholar]
- 8.Stanley E, Lieschke GJ, Grail D, Metcalf D, Hodgson G, Gall JAM, Maher DW, Cebon J, Sinickas V, Dunn AR. Granulocyte/macrophage colony-stimulating factor-deficient mice show no major perturbation of hematopoiesis but develop a characteristic pulmonary pathology. Proc Natl Acad Sci USA. 1994;91:5592–5596. doi: 10.1073/pnas.91.12.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huizar I, Kavuru MS. Alveolar proteinosis syndrome: pathogenesis, diagnosis, and management. Curr Opin Pulm Med. 2009;15:491–498. doi: 10.1097/MCP.0b013e32832ea51c. [DOI] [PubMed] [Google Scholar]
- 10.Thomassen MJ, Yi T, Raychaudhuri B, Malur A, Kavuru MS. Pulmonary alveolar proteinosis is a disease of decreased availability of GM-CSF rather than an intrinsic cellular defect. Clin Immunol. 2000;95:85–92. doi: 10.1006/clim.2000.4859. [DOI] [PubMed] [Google Scholar]
- 11.Carraway MS, Ghio AJ, Carter JD, Piantadosi CA. Detection of granulocyte-macrophage colony-stimulating factor in patients with pulmonary alveolar proteinosis. Am J Respir Crit Care Med. 2000;161:1294–1299. doi: 10.1164/ajrccm.161.4.9906080. [DOI] [PubMed] [Google Scholar]
- 12.Kitamura T, Tanaka N, Watanabe J, Uchida K, Kanegasaki S, Yamada Y, Nakata K. Idiopathic pulmonary alveolar proteinosis as an autoimmune disease with neutralizing antibody against granulocyte/macrophage colony-stimulating factor. J Exp Med. 1999;190:875–880. doi: 10.1084/jem.190.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanaka N, Watanabe J, Kitamura T, Yamada Y, Kanegasaki S, Nakata K. Lungs of patients with idiopathic pulmonary alveolar proteinosis express a factor which neutralizes granulocyte-macrophage colony-stimulating factor. FEBS Lett. 1999;442:246–250. doi: 10.1016/s0014-5793(98)01668-8. [DOI] [PubMed] [Google Scholar]
- 14.Bonfield TL, Kavuru MS, Thomassen MJ. Anti-GM-CSF titer predicts response to GM-CSF therapy in pulmonary alveolar proteinosis. Clin Immunol. 2002;105:342–350. doi: 10.1006/clim.2002.5301. [DOI] [PubMed] [Google Scholar]
- 15.Bonfield TL, Russell D, Burgess S, Malur A, Kavuru MS, Thomassen MJ. Autoantibodies against granulocyte macrophage colony-stimulating factor are diagnostic for pulmonary alveolar proteinosis. Am J Respir Cell Mol Biol. 2002;27:481–486. doi: 10.1165/rcmb.2002-0023OC. [DOI] [PubMed] [Google Scholar]
- 16.Seymour JF, I, Doyle R, Nakata K, Presneill JJ, Schoch OD, Hamano E, Uchida K, Fisher R, Dunn AR. Relationship of anti-GM-CSF antibody concentration, surfactant protein A and B levels, and serum LDH to pulmonary parameters and response to GM-CSF therapy in patients with idiopathic alveolar proteinosis. Thorax. 2003;58:252–257. doi: 10.1136/thorax.58.3.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kitamura T, Uchida K, Tanaka N, Tsuchiya T, Watanabe J, Yamada Y, Hanaoka K, Seymour JF, Schoch OD, Doyle I, et al. Serological diagnosis of idiopathic pulmonary alveolar proteinosis. Am J Respir Crit Care Med. 2000;162:658–662. doi: 10.1164/ajrccm.162.2.9910032. [DOI] [PubMed] [Google Scholar]
- 18.Mahler DA, Waterman LA, Ward J, McCusker C, ZuWallack R, Baird JC. Validity and responsiveness of the self-administered computerized versions of the baseline and transition dyspnea indexes. Chest. 2007;132:1283–1290. doi: 10.1378/chest.07-0703. [DOI] [PubMed] [Google Scholar]
- 19.Akira M, Inoue G, Yamamoto S, Sakatani M. Non-specific interstitial pneumonia: findings on sequential CT scans of nine patients. Thorax. 2000;55:854–859. doi: 10.1136/thorax.55.10.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tazawa R, Trapnell BC, Inoue Y, Arai T, Takada T, Nasuhara Y, Hizawa N, Kasahara Y, Tatsumi K, Hojo M, et al. Inhaled granulocyte/macrophage-colony stimulating factor as therapy for pulmonary alveolar proteinosis. Am J Respir Crit Care Med. 2010;181:1345–1354. doi: 10.1164/rccm.200906-0978OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomassen MJ, Buhrow LT, Connors MJ, Kaneko FT, Erzurum SC, Kavuru MS. Nitric oxide inhibits inflammatory cytokine production by human alveolar macrophages. Am J Respir Cell Mol Biol. 1997;17:279–283. doi: 10.1165/ajrcmb.17.3.2998m. [DOI] [PubMed] [Google Scholar]
- 22.Bonfield TL, John N, Barna BP, Kavuru MS, Thomassen MJ, Yen-Lieberman B. Multiplexed particle-based anti-granulocyte macrophage colony stimulating factor (GM-CSF) assay used as pulmonary diagnostic test. Clin Diagn Lab Immunol. 2005;12:821–824. doi: 10.1128/CDLI.12.7.821-824.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seymour JF, Presneill JJ. Pulmonary alveolar proteinosis (Progress in the first 44 years) Am J Respir Crit Care Med. 2002;166:215–235. doi: 10.1164/rccm.2109105. [DOI] [PubMed] [Google Scholar]
- 24.Venkateshiah SB, Yan TD, Bonfield TL, Thomassen MJ, Meziane M, Czich C, Kavuru MS. An open-label trial of granulocyte macrophage colony stimulating factor therapy for moderate symptomatic pulmonary alveolar proteinosis. Chest. 2006;130:227–237. doi: 10.1378/chest.130.1.227. [DOI] [PubMed] [Google Scholar]
- 25.Sanz I. Indications of rituximab in autoimmune diseases. Drug Discov Today Ther Strateg. 2009;6:13–19. doi: 10.1016/j.ddstr.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uchida K, Nakata K, Suzuki T, Luisetti M, Watanabe M, Koch DE, Stevens CA, Beck DC, Denson LA, Carey BC, et al. Granulocyte/macrophage-colony-stimulating factor autoantibodies and myeloid cell immune functions in healthy subjects. Blood. 2009;113:2547–2556. doi: 10.1182/blood-2009-05-155689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kavuru MS, Bonfield TL, Thomassen MJ. Plasmapheresis, GM-CSF, and alveolar proteinosis. Am J Respir Crit Care Med. 2003;167:1036. doi: 10.1164/ajrccm.167.7.950. [DOI] [PubMed] [Google Scholar]
- 28.Luisetti M, Rodi G, Perotti C, Campo I, Mariani F, Pozzi E, Trapnell BC. Plasmapheresis for treatment of pulmonary alveolar proteinosis. Eur Respir J. 2009;33:1220–1222. doi: 10.1183/09031936.00097508. [DOI] [PubMed] [Google Scholar]
- 29.Borie R, Debray MP, Laine C, Aubier M, Crestani B. Rituximab therapy in autoimmune pulmonary alveolar proteinosis. Eur Respir J. 2009;33:1503–1506. doi: 10.1183/09031936.00160908. [DOI] [PubMed] [Google Scholar]
- 30.Inoue Y, Trapnell BC, Tazawa R, Arai T, Takada T, Hizawa N, Kasahara Y, Tatsumi K, Hojo M, Ichiwata T, et al. Characteristics of a large cohort of patients with autoimmune pulmonary alveolar proteinosis in Japan. Am J Respir Crit Care Med. 2008;177:752–762. doi: 10.1164/rccm.200708-1271OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zeimpekoglou K. The role of B-lymphocytes in lung disease. Pneumon. 2008:196–199. [Google Scholar]
- 32.Bonfield TL, Barna BP, John N, Malur A, Culver DA, Kavuru MS, Thomassen MJ. Suppression of activin A in autoimmune lung disease associated with anti-GM-CSF. Journal of Autoimmunity. 2006;26:37–41. doi: 10.1016/j.jaut.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 33.Sakagami T, Beck D, Uchida K, Suzuki T, Carey BC, Nakata K, Keller G, Wood RE, Wert SE, Ikegami M, et al. Patient-derived Granulocyte/Macrophage Colony-Stimulating Factor Autoantibodies Reproduce Pulmonary Alveolar Proteinosis in Nonhuman Primates. Am J Respir Crit Care Med. 2010;182:49–61. doi: 10.1164/rccm.201001-0008OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sakagami T, Uchida K, Suzuki T, Carey BC, Wood RE, Wert SE, Whitsett JA, Trapnell BC, Luisetti M. Human GM-CSF Autoantibodies and Reproduction of Pulmonary Alveolar Proteinosis. N Engl J Med. 2010;361:2679–2681. doi: 10.1056/NEJMc0904077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vallerskog T, Gunnarsson I, Widhe M, Risselada A, Klareskog L, van VR, Malmstrom V, Trollmo C. Treatment with rituximab affects both the cellular and the humoral arm of the immune system in patients with SLE. Clin Immunol. 2007;122:62–74. doi: 10.1016/j.clim.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 36.Perosa F, Prete M, Racanelli V, Dammacco F. CD20-depleting therapy in autoimmune diseases: from basic research to the clinic. J Intern Med. 2010;267:260–277. doi: 10.1111/j.1365-2796.2009.02207.x. [DOI] [PubMed] [Google Scholar]
- 37.Leandro MJ, de I T l. Translational Mini-Review Series on B Cell-Directed Therapies: The pathogenic role of B cells in autoantibody-associated autoimmune diseases--lessons from B cell-depletion therapy. Clin Exp Immunol. 2009;157:191–197. doi: 10.1111/j.1365-2249.2009.03978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahuja A, Shupe J, Dunn R, Kashgarian M, Kehry MR, Shlomchik MJ. Depletion of B Cells in Murine Lupus: Efficacy and Resistance. J Immunol. 2007;179:3351–3361. doi: 10.4049/jimmunol.179.5.3351. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.