Abstract
Background
Hepatic lipoprotein production has been shown previously to be regulated by free fatty acid (FFA) flux to the liver, whereas intestinal lipoprotein production is stimulated mainly by ingested fat absorbed from the intestinal lumen. Emerging evidence indicates that intestinal lipoprotein production is increased in insulin resistance and type 2 diabetes mellitus, conditions that are associated with increased levels of circulating FFAs. Here we investigated whether short-term elevation of plasma FFAs stimulates intestinal apolipoprotein (apo) B-48– and hepatic apoB-100–containing triglyceride-rich lipoprotein (TRL) production in humans in the fed state.
Methods and Results
TRL apoB-48 and apoB-100 metabolism were examined in 12 healthy men during a constant fed state. The studies were as follows, respectively: (1) Intralipid/heparin was infused intravenously immediately before and during the kinetics study to induce an ≈3-fold difference in plasma FFA compared with the saline study; (2) saline was infused intravenously as a control. ApoB-48– and apoB-100–containing TRL production and clearance were determined with a 12-hour primed constant infusion of [D3]L-leucine and multicompartmental kinetic modeling. TRL apoB-48 production rate was 69% higher in the Intralipid/heparin study than in the saline control (5.95±1.13 versus 3.53±0.58 mg/kg per day; P=0.027), and there was no significant difference in TRL apoB-48 clearance. TRL apoB-100 concentrations were also increased (P<0.001) and TRL apoB-100 production rate was 35% higher in the Intralipid/ heparin study compared with saline (28±4 versus 21±3 mg/kg per day; P=0.020).
Conclusions
This is the first study to demonstrate that intestinal TRL apoB-48 production is increased after short-term elevation of plasma FFAs in humans in the fed state, similar to the well-described stimulation of hepatic TRL apoB100–containing particles by FFAs.
Keywords: fatty acids, lipoproteins, lipids
Dyslipidemia is a prominent feature of both insulin resistance and type 2 diabetes mellitus and contributes to the increased risk of cardiovascular events.1,2 Characteristic features of the typical dyslipidemia of insulin-resistant states and type 2 diabetes mellitus include elevated plasma triglycerides, increased plasma free fatty acid (FFA) levels, low plasma high-density lipoprotein cholesterol concentration, and an increased number of small, dense low-density lipoprotein particles.1 The elevation of triglyceride-rich lipoprotein (TRL) particles in insulin-resistant states is contributed to by both hepatic (apolipoprotein [apo] B-100–containing) and intestinal (apoB-48–containing) lipoproteins in fasted and postprandial states.3–7 We have shown recently that diet-induced insulin resistance in Syrian Golden hamsters is associated with a marked increase in intestinal lipoprotein production rate in both the fasting and the fed states.8–12 Because apoB-48–containing intestinally derived lipoproteins have been identified as proatherogenic13,14 and their secretion is increased in insulin-resistant states15 and type 2 diabetes mellitus,16 it is critical to understand the factors regulating their production and/or clearance.
Plasma FFAs are generally increased in insulin-resistant states and type 2 diabetes mellitus.17 This elevation is attributed to saturation of the adipose tissue storage capacity and diminished insulin suppression of adipose tissue lipolysis.18 This in turn leads to increased plasma FFA levels and their diversion toward nonadipose tissues, a phenomenon that can cause lipotoxicity. Defining the role of FFAs in stimulating apoB-containing lipoprotein secretion is crucial in view of the intricate relationship between insulin resistance and the accumulation of TRLs, particularly in the postprandial state. FFA flux to the liver has been shown to act as a driving force for very-low-density lipoprotein (VLDL) triglyceride secretion: Fatty acids enhance apoB-containing lipoprotein secretion in HepG2 cells or cultured hepatocytes,19–23 although some controversies exist.24–26 Elevation of FFA has been shown to stimulate VLDL assembly and secretion in mice.27 In humans, a short-term elevation of plasma FFA levels has been shown to acutely stimulate VLDL production in healthy fasted humans,28,29 although influence of acute elevation of FFA on hepatic TRL metabolism in the fed state has, to our knowledge, not been investigated previously and is of particular interest because the majority of the day is spent in the postprandial state.
The effect of plasma FFAs on intestinally derived TRL production, on the other hand, is poorly documented. Tracer studies in pancreatectomized dogs suggested that circulating FFA might enter the intestinal mucosal cells directly and be esterified and secreted in intestinal lipoproteins.30 We have recently demonstrated that acute elevation of plasma FFA by Intralipid/heparin (IH) infusion in chow-fed hamster increased intestinal apoB-48 production rate.31 Furthermore, oleic acid perfusion of primary hamster enterocytes has been shown to increase the number of secreted apoB-48–containing lipoprotein particles.10 However, although FFAs may stimulate production of intestinal lipoproteins and elevated FFAs may play an important role in the overproduction of intestinal lipoprotein in insulin-resistant states, this phenomenon has not been examined previously in humans. In the present study, we investigated the effect of acute elevation of FFAs on steady state fed TRL apoB-48 and TRL apoB-100 production and clearance rates in healthy men.
Methods
Subjects
The demographic characteristics and fasting biochemical profiles of the 12 healthy, male participants in the present study are outlined in Table 1. See the online-only Data Supplement for more details.
Table 1.
Demographic Characteristics and Fasting Biochemical Parameters of Subjects
| Subject | Age, y | Weight, kg |
BMI, kg/m2 |
Glucose, mmol/L |
Insulin, pmol/L |
Plasma FFA, mmol/L |
Plasma Triglycerides, mmol/L |
Plasma TC, mmol/L |
TRL ApoB-48, mg/L |
TRL ApoB-100, mg/L |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 52 | 68 | 22.6 | 5.2 | 37.6 | 0.21 | 1.54 | 4.90 | 9.53 | 67.5 |
| 2 | 53 | 67 | 25.5 | 5.2 | 27.2 | 0.47 | 1.60 | 4.63 | 24.77 | 63.5 |
| 3 | 30 | 80 | 24.7 | 4.3 | 50.1 | 0.23 | 0.72 | 4.42 | 3.70 | 63.5 |
| 4 | 49 | 89 | 31.7 | 4.7 | 54.3 | 0.47 | 1.19 | 4.27 | 9.00 | 39.5 |
| 5 | 54 | 78 | 29.9 | 5.1 | 52.8 | 0.42 | 1.32 | 4.73 | 23.50 | 92.0 |
| 6 | 43 | 89 | 30.0 | 4.9 | 48.4 | 0.39 | 0.72 | 4.57 | 4.40 | 16.2 |
| 7 | 44 | 95 | 27.0 | 5.3 | 28.0 | 0.53 | 1.15 | 4.34 | 3.90 | 60.1 |
| 8 | 32 | 79 | 25.6 | 4.9 | 89.5 | 1.00 | 2.87 | 5.11 | 20.76 | 65.7 |
| 9 | 52 | 80 | 27.2 | 5.5 | 52.5 | 0.67 | 1.42 | 3.40 | 11.77 | 41.2 |
| 10 | 54 | 101 | 31.2 | 5.1 | 42.5 | 0.36 | 1.05 | 4.99 | 7.92 | 20.7 |
| 11 | 57 | 88 | 28.5 | 4.4 | 75.6 | 0.52 | 0.99 | 5.25 | 6.47 | 61.6 |
| 12 | 55 | 70 | 22.8 | 4.7 | 73.8 | 0.47 | 1.45 | 4.34 | 8.45 | 74.2 |
| Mean±SEM | 47.92±2.39 | 82±3 | 27.23±0.89 | 5.0±0.1 | 52.7±5.5 | 0.48±0.06 | 1.33±0.16 | 4.58±0.14 | 11.18±2.0 | 55.5±6.4 |
BMI indicates body mass index; TC, total cholesterol.
Experimental Protocol for Lipoprotein Kinetics Studies
All 12 subjects underwent 2 separate lipoprotein kinetics studies (IH and saline), in random order, 4 to 6 weeks apart. Because the infusion of Intralipid (a synthetic triglyceride emulsion that provides a source of mainly polyunsaturated fatty acids) and heparin (to activate lipoprotein lipase) raises both FFAs and glycerol, 2 control studies were performed, 1 with saline and 1 with glycerol infusion (the latter only in a subset of 5 subjects).
After an overnight fast, kinetics studies were performed in a constant fed state because apoB-48 levels are extremely low in the fasted state, and in pilot studies we found that the mass of TRL-apoB48 was too low in fasted humans to accurately assess isotopic enrichment for calculation of kinetic parameters. To achieve this constant fed state, the subject was instructed to ingest 3 identical hourly volumes of a liquid food supplement for the first 3 hours starting at 4 AM (Hormel Great Shake Plus, Hormel Health Labs; 49% calories from fat, 38% from carbohydrates, 13% from proteins), each hourly aliquot equivalent to 1/17th of their total daily caloric needs. After the first 3 hours (after 7 am), the subjects ingested the same formula every half hour for the remainder of the study, ie, 1/34th of their daily caloric intake every half hour. At 4 am, at the same time as the start of the hourly formula ingestion, an intravenous infusion with either IH (Baxter, Mississauga, Canada) (Intralipid 20% at 20 mL/h plus heparin 250 U/h) or saline (65 mL/h) or glycerol (2.25 g/h) was started and was constantly infused for the full 17-hour duration of the study. Five hours later, all subjects received a primed constant infusion (10 µmol/kg bolus, 10 µmol/kg per hour for 12 hours) of deuterated leucine32 (L-[5,5,5-2H3]-leucine; 98%, Cambridge Isotope Laboratories, Andover, Mass), and apoB-containing lipoprotein particle production and clearance rates were calculated as described previously.33 See the online-only Data Supplement for details.
Laboratory Methods
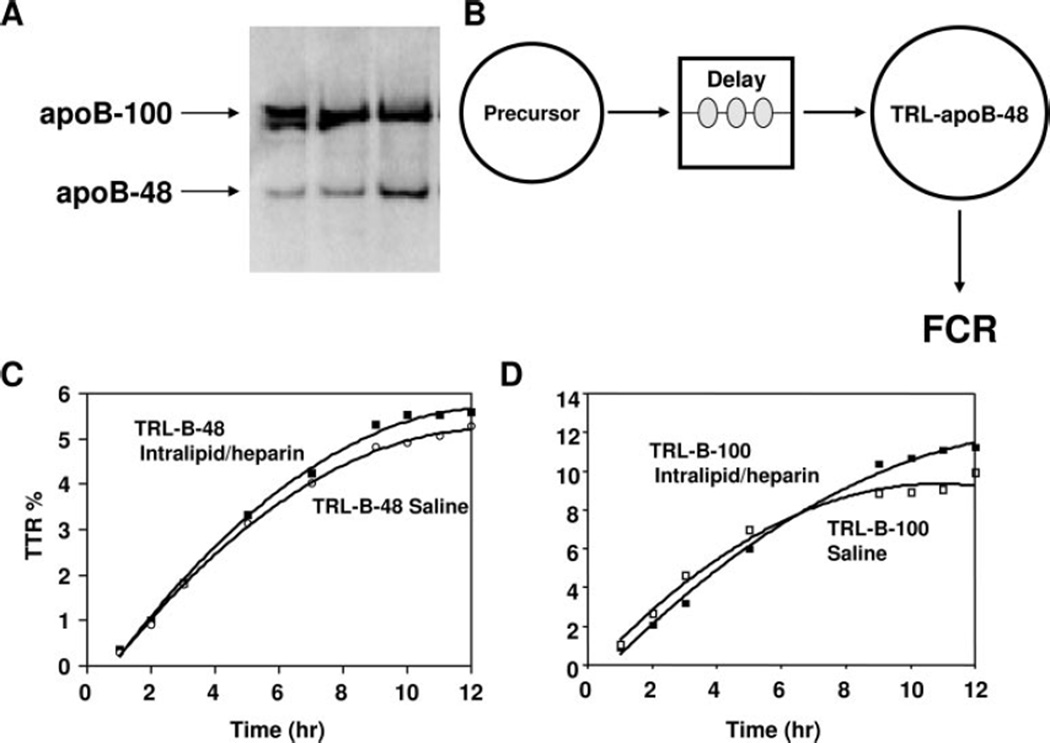
See the online-only Data Supplement for triglyceride, FFA, plasma insulin, and glucose measurements. Blood samples for FFA and triglyceride analyses were collected on ice into tubes containing the lipase inhibitor Orlistat.34 TRLs were isolated, and proteins in the TRL fraction were determined by Lowry’s method,35 delipidated, and subsequently separated by SDS-PAGE, with clear separation of the apoB-100 and apoB-48 bands, as shown in Figure 1. Gel bands containing apoB-48 and apoB-100 were excised, hydrolyzed, and derivatized to allow for the determination of plasma leucine isotopic enrichment by gas chromatography/mass spectrometry as described.36 Tracer-to-tracee ratios were calculated from isotopic ratios for each sample according to the formula derived by Cobelli et al.37 See the online-only Data Supplement for details.
Figure 1.
Assessment of TRL apoB-48/100 production and clearance rates by multicompartmental modeling. A, Separation of the apoB-100 and apoB-48 bands after SDS-PAGE. B, TRL apoB-48 and TRL apoB-100 multicompartmental kinetics model. Compartment 1 represents plasma deuterated-leucine enrichment; compartment 2 is an intracellular delay compartment, which accounts for the synthesis, assembly, and secretion of apolipoproteins; and compartment 3 is plasma lipoproteins. Fractional catabolic rate (FCR) was equivalent to the fractional synthetic rate, and production rate is derived from fractional catabolic rate of the TRL apoB-48/100 multiplied by pool size (ie, TRL apoB-48/100 concentration). C, D, Representative study performed during constant enteral feeding in 1 individual. Gel bands containing apoB-48 and apoB-100 were excised and processed for gas chromatography/mass spectrometry analysis of tracer/tracee ratios (TTR) for kinetic modeling. TRL apoB-48 (C) and TRL apoB-100 (D) tracer-to-tracee ratios vs time are depicted.
ApoB-48 was separated by SDS-PAGE with the use of 3% to 8% Tris-acetate polyacrylamide gels, and it was detected with Western immunoblot techniques as described previously.38 ApoB-100 mass in the TRL fraction was quantified by analytical SDS-PAGE as described previously.39 See the online-only Data Supplement for details.
Calculation of Lipoprotein Production and Clearance Rates by Multicompartmental Modeling
Stable isotope enrichment curves for apoB-48 and apoB-100 were fitted to a 3-compartment model (Figure 1B).33 Each subject was in steady state with respect to apoB-48 and apoB-100 concentrations so that fractional catabolic rate was equivalent to fractional synthetic rate. Production rates were calculated with the use of the fractional catabolic rate of TRL apoB-48 or TRL apoB-100 multiplied by pool size measured over the 12 hours of the kinetics study. See the online-only Data Supplement for details.
Statistical Analysis
Results are presented as mean±SEM unless indicated otherwise. Paired t test was used to compare results from the IH and saline infusions with SPSS version 13. Sample size calculation was calculated with the use of gpower3 software. Two-factor ANOVA for repeated measures (Figure 2) was performed with the use of SAS software.
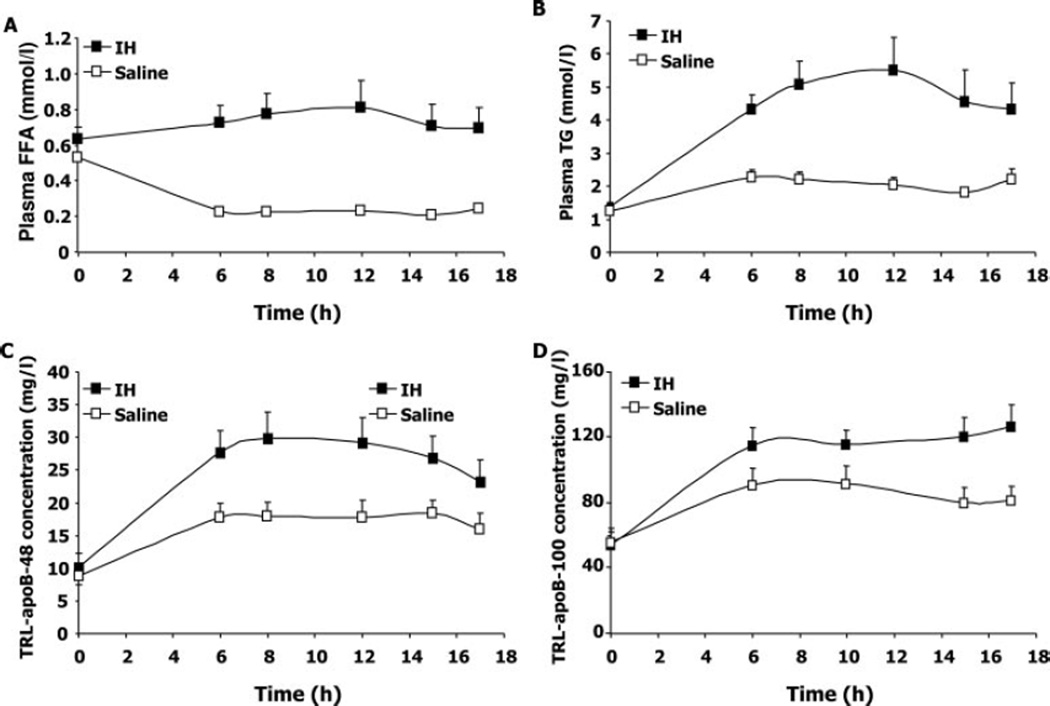
Figure 2.
Plasma FFA and triglycerides and TRL apoBs over the time course of the kinetics study. Plasma FFAs (A), plasma triglycerides (TG) (B), TRL apoB-48 (C), and TRL apoB-100 (D) were measured after an overnight fast and then throughout the 12-hour lipoprotein turnover study (from 5 to 17 hours) in subjects receiving either IH or saline (n=12). Between 0h and 17h, subjects ingested a liquid formula, as described in the Methods section. Values are mean±SEM for each group. The probability values for the difference between the 2 (IH vs saline) studies over the time course of the kinetics study analyzed by 2-factor ANOVA for repeated measures are as follows: for FFA plasma, P<0.0001; for plasma triglycerides, P<0.0001; for TRL apoB-48, P<0.005; and for TRL apoB-100, P<0.003.
The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Results
Demographic and Biochemical Characteristics, Fasted Plasma Lipids, and TRL Composition of Subjects
The clinical characteristics of subjects at baseline are provided in Table 1. All subjects were healthy men with no previous history of cardiovascular, gastrointestinal, or systemic illness or surgical intervention within 6 months before the studies. No subject was taking medications, and they were all normoglycemic. To exclude those with gross dyslipidemia from the study, individuals whose total cholesterol was >5.3 mmol/L, triglycerides >2 mmol/L, high-density lipoprotein cholesterol <0.9 mmol/L, or low-density lipoprotein cholesterol >3.2 mmol/L were excluded.
Glycerol Control Study
Glycerol infusion had no effect on either plasma FFA (saline 0.22±0.01 versus glycerol 0.19±0.03 mmol/L, P=0.454), plasma triglycerides (saline 2.00±0.36 versus glycerol 2.17±0.10 mmol/L, P=0.732), or TRL apoB-48 or TRL apo-B100 levels compared with saline (saline 23.66±12.9 versus glycerol 17.50±7.1 mg/L, P=0.597 and saline 80.3±1.71 versus glycerol 87.7±1.28 mg/L, P=0.259, respectively). In addition, apoB-48 and apoB-100 production and clearance rates did not differ between glycerol and saline infusion studies. For clarity and because power calculation estimated that n=64 subjects would be needed to detect a significance difference between saline and glycerol at P=0.05 with 80% power, all additional descriptions and illustrations of results will compare only the IH and saline control studies.
Effect of Acute Elevation of FFAs on Plasma and TRL Lipid and Apolipoprotein Concentrations in the Constant Fed State
IH infusion caused a significantly sustained rise in plasma FFA levels, whereas plasma FFA concentration progressively declined in the saline infusion study, resulting in a >3-fold difference in concentration during the kinetics study between IH and saline infusions (P<0.0001 by 2-factor ANOVA for repeated measures) (area under the curve [AUC] [mean±SD]: IH 12.31±5.66 mmol/L per hour versus saline 4.61± 1.29 mmol/L per hour; P=0.0006) (Table 2 and Figure 2A). IH infusion also caused a significant increase in plasma triglyceride levels (Table 2 and Figure 2B), much of it likely contributed to by the infusion of Intralipid itself. As expected, plasma and TRL/triglyceride levels increased in response to feeding and then remained stable during the 12-hour kinetics study. In the fed state, IH infusion significantly increased plasma triglycerides compared with the saline infusion (P<0.0001) (AUC [mean±SD]: IH 71.84± 35.85 mmol/L per hour versus saline 33.25±10.84 mmol/L per hour; P=0.004) (Table 2 and Figure 2B). We also found a significant increase in TRL/triglyceride concentration in subjects when they were infused with IH compared with the saline control (AUC [mean±SD]: IH 58.61±31.23 mmol/L per hour versus saline 24.95±10.26 mmol/L per hour; P=0.005) (Table 2). TRL apoB-48 concentration increased with feeding and then remained stable during the 12-hour kinetics study with saline infusion. During feeding, there was a significant increase in TRL apoB-48 concentration after IH infusion compared with saline (P<0.005) (AUC [mean±SD]: IH 410.54±165.87 mg/L per hour versus saline 275.22±110 mg/L per hour; P=0.006) (Table 2 and Figure 2C). Similarly, TRL apoB-100 concentrations increased to a greater extent during the 12-hour kinetics study with IH infusion compared with saline (P<0.003) (AUC [mean±SD]: IH 1.82±0.46 g/L per hour versus saline 1.40±0.51 g/L per hour; P=0.001) (Table 2 and Figure 2D).
Table 2.
Effect of Short-Term Intralipid Infusion on Plasma and TRL Lipids and ApoB Concentrations in the Fed State
| Subject | Plasma FFA, mmol/L |
Plasma Triglycerides, mmol/L |
Plasma TC, mmol/L |
TRL Triglycerides, mmol/L |
TRL Cholesterol, mmol/L |
TRL ApoB-48, mg/L |
TRL ApoB-100, mg/L |
|---|---|---|---|---|---|---|---|
| IH | |||||||
| 1 | 1.60 | 10.89 | 5.46 | 9.16 | 2.17 | 19.64 | 82.9 |
| 2 | 0.60 | 4.19 | 3.57 | 3.08 | 0.61 | 33.39 | 78.1 |
| 3 | 1.31 | 2.73 | 4.49 | 2.09 | 0.56 | 22.56 | 130.8 |
| 4 | 0.43 | 3.14 | 3.28 | 2.64 | 0.63 | 37.80 | 84.6 |
| 5 | 0.33 | 3.01 | 3.73 | 2.51 | 0.80 | 39.22 | 152.7 |
| 6 | 0.47 | 4.13 | 4.13 | 3.21 | 0.92 | 28.72 | 119.8 |
| 7 | 0.55 | 3.74 | 3.89 | 2.91 | 0.95 | 15.53 | 82.4 |
| 8 | 1.23 | 8.54 | 5.10 | 7.40 | 1.38 | 47.08 | 145.5 |
| 9 | 0.61 | 4.78 | 3.10 | 4.02 | 0.88 | 38.86 | 163.6 |
| 10 | 0.48 | 2.66 | 4.06 | 2.09 | 0.63 | 15.79 | 90.7 |
| 11 | 0.66 | 3.10 | 5.40 | 2.60 | 1.01 | 18.68 | 170.9 |
| 12 | 0.73 | 6.35 | 4.36 | 5.53 | 1.39 | 14.30 | 138.8 |
| Mean±SD | 0.75±0.40 | 4.77±2.58 | 4.21±0.78 | 3.94±2.27 | 1.00±0.46 | 27.63±11.32 | 120.1±34.3 |
| Saline | |||||||
| 1 | 0.25 | 1.23 | 4.69 | 0.53 | 0.40 | 11.08 | 55.0 |
| 2 | 0.18 | 1.54 | 4.28 | 1.15 | 0.47 | 10.69 | 83.0 |
| 3 | 0.19 | 1.93 | 4.49 | 1.16 | 0.56 | 10.09 | 109.4 |
| 4 | 0.23 | 1.97 | 3.96 | 1.52 | 0.54 | 15.19 | 45.3 |
| 5 | 0.23 | 3.35 | 4.29 | 2.66 | 0.77 | 20.26 | 143.5 |
| 6 | 0.21 | 1.93 | 4.08 | 1.69 | 0.61 | 30.35 | 72.5 |
| 7 | 0.27 | 2.80 | 3.93 | 2.13 | 0.57 | 14.59 | 74.3 |
| 8 | 0.41 | 3.06 | 4.61 | 2.53 | 0.70 | 23.95 | 70.4 |
| 9 | 0.20 | 2.46 | 3.08 | 1.89 | 0.63 | 31.71 | 106.5 |
| 10 | 0.14 | 1.22 | 4.47 | 0.95 | 0.37 | 13.47 | 61.3 |
| 11 | 0.23 | 1.61 | 4.73 | 1.11 | 0.57 | 16.00 | 128.9 |
| 12 | 0.20 | 2.05 | 4.66 | 1.46 | 0.57 | 13.48 | 89.8 |
| Mean±SD | 0.23±0.07 | 2.10±0.69 | 4.27±0.47 | 1.56±0.65 | 0.56±0.11 | 17.57±7.43 | 86.7±31.3 |
| P | 0.001 | 0.006 | 0.693 | 0.006 | 0.011 | 0.004 | 0.0001 |
Numbers are means for the duration of the 12-hour kinetics study, ie, from 5 to 17 hours. TC indicates total cholesterol.
Effects of Acute Elevation of FFAs on Hepatic and Intestinal Lipoprotein Production and Clearance Rates in the Fed State
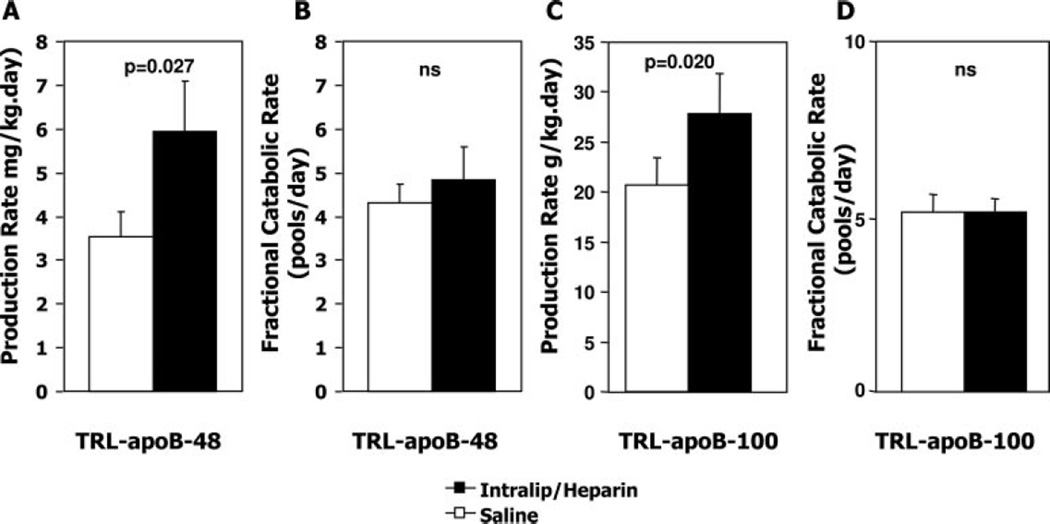
We next questioned whether the greater increase in TRL apoB-48 and TRL apoB-100 concentration in the IH study versus saline was due to a lower clearance rate of TRL apoB-48 and apoB-100 particles, higher production rate, or both. The fractional catabolic rate of TRL apoB-48 or TRL apoB-100 was not significantly different between saline and IH infusion studies (Figure 3). In contrast, the production rate of TRL apoB-48 was 69% higher in subjects infused with IH compared with saline (IH 5.95±1.13 mg/kg per day versus saline 3.53±0.58 mg/kg per day; P=0.027), demonstrating that an acute elevation of plasma FFAs stimulates intestinal lipoprotein particle production (Figure 3). Similarly, the production rate of TRL apoB-100 was 35% higher in subjects infused with IH compared with saline (IH 27.9±4.0 mg/kg per day versus saline 20.7±2.7 mg/kg per day; P=0.02).
Figure 3.
Effect of IH infusion on TRL apoB-48 and apoB-100 production and fractional catabolic rates in the constant fed state. ApoB-48 (A, B) and apoB-100 (C, D) production rate (A, C) and fractional catabolic rate (B, D) were determined at steady state in subjects receiving either IH or saline. There was no significant difference in fractional catabolic rate between the studies, whereas production rates of both TRL apoB-48 and TRL apoB-100 were significantly increased by IH infusion. Results are mean±SEM. Intralip indicates Intralipid.
Discussion
Insulin-resistant states and type 2 diabetes mellitus are accompanied by elevated plasma FFAs and increased plasma concentrations of TRLs, including intestinally derived apoB-48–containing TRLs in both fasted and fed states. FFA delivery to the liver has been shown previously to stimulate hepatic VLDL production in the fasted state,28 whereas the effect of plasma FFAs on intestinal lipoprotein production has not been examined previously in humans. In the present study, we examined whether an acute elevation of FFAs also stimulates the production of hepatic and intestinally derived TRL particles in healthy humans in the fed state. An infusion of IH was used to raise plasma FFA concentration in the constant fed state, and intestinal apoB-48– and apoB-100–containing TRL metabolism was examined with the use of stable isotope enrichment techniques. Plasma triglycerides, TRL triglycerides, TRL apoB-100, and TRL apoB-48 concentrations were significantly higher in the IH infusion study compared with the saline control study, and this increase was associated with a higher production rate of intestinal and hepatic TRL particles but no difference in their rate of clearance. Glycerol alone had no effect on triglycerides or apoB concentrations or their metabolism compared with saline, indicating that the effect of IH was due to the FFAs released by heparin-stimulated lipoprotein lipase lipolysis of the synthetic triglyceride emulsion and was not due to the release of glycerol. Thus, we have shown, for the first time in humans, that an acute elevation of plasma FFAs stimulates intestinal lipoprotein production.
We have shown previously that intestinal lipoprotein production is increased after acute elevation of plasma FFA by IH in fasted, insulin-resistant, chow-fed Syrian Golden hamsters.31 The present study extends these observations to humans. In humans, because plasma TRL apoB-48 mass is relatively low, it was necessary for technical reasons to study the metabolism of apoB-48–containing lipoproteins in the fed rather than in the fasted state. It is important to appreciate, therefore, that our observation of higher TRL apoB-48 production in the IH study occurred on a background of high-fat feeding, in which there is ongoing absorption of luminal fatty acids. We do not know whether we would have observed similar findings in the fasted human, were it technically possible to perform those studies, but extrapolation from our hamster studies suggests that this would indeed have been the case. It is also noteworthy that the carbohydrate in the formula used in this study (which constituted 38% of total calories) likely stimulated insulin secretion (equally in both the saline and IH studies), thereby contributing to suppression of plasma FFAs in the saline study. We deliberately chose to administer a mixed feed, albeit a solution with high fat content, to more closely mimic a real-life situation of mixed meal ingestion. The kinetic parameters were calculated with the use of the averaged apoB concentrations between 5 and 17 hours (steady state) 5 hours after the start of both the IH infusion and the ingestion of the liquid formula, a period in which FFA levels were stable.
Several mechanisms may be involved in the stimulation of intestinal lipoprotein production by elevated FFAs. Our previous experiments that examined this phenomenon in the Syrian Golden hamster demonstrated that IH infusion increased intracellular apoB-48 stability and reduced its degradation, a major step in apoB-48 assembly and secretion of intestinal TRL.31 Chylomicron assembly by intestinal cells is a 2-step process: (1) the production of a phospholipid-rich core and (2) its expansion as it acquires triglycerides, forming a large buoyant particle.41 Oleate treatment of primary cultured hamster enterocytes stimulates intracellular assembly as well as the secretion of apoB-48–containing lipoproteins.10 The effect of FFAs on apoB stability may also be affected by the intracellular fatty acid/triglyceride pool size, which might have increased because of simultaneous feeding and IH infusion used in our protocol. Interestingly, it has been reported that during lipid infusion in rats, incorporation of plasma FFA into intestinal lymphatic triglycerides was increased significantly in the fed state.42 Whether plasma FFAs are directly incorporated in newly synthesized lipoproteins, or stimulate mobilization and utilization of stored triglycerides, or increase lipid synthesis, as we previously demonstrated in the insulin-resistant hamster,8 is currently unknown.
Elevation of FFA in insulin resistance is due to combined resistance to insulin-mediated suppression of lipolysis in adipose tissue and reduced fatty acid trapping by insulin-resistant adipocytes.18 Ectopic fat deposition appears when the normal buffering capacity of adipose tissue is impaired or exceeded, especially during postprandial periods, and is characterized by diversion of FFAs from adipose depots and lipid deposition in nonadipose tissue (liver, muscle, heart, and pancreatic β-cells). In the insulin-responsive tissues (muscle, liver), FFA accumulation usually leads to altered insulin signaling and sensitivity.43 Although there was no way for us to measure it directly in the present study, it is highly likely that the FFA overload impaired insulin sensitivity of intestinal enterocytes. Indeed, a recent study has shown, using enterocytes from fructose-fed hamster, that intestinal apoB-48–containing lipoprotein overproduction was associated with impaired insulin signaling, that is, reduced Akt and IRS1 phosphorylation, and increased ERK signaling.11 Because insulin has been shown to acutely inhibit intestinal TRL secretion,11 it is plausible that the elevation of FFA may have indirectly stimulated intestinal TRL secretion by impairing the inhibitory effect of insulin.
We also report that TRL apo-B100 concentrations were increased by the infusion of IH because of increased TRL apoB-100 production rate, with no significant change in catabolic rate. We have previously reported that an acute elevation of plasma FFAs stimulates VLDL secretion in humans in the fasted state.28 In the present report, we demonstrate that this observation pertains to the fed state as well. Indeed, in the constant fed state, as is the case for the present studies, a large proportion of ingested fatty acids (up to 15%) are taken up by the liver and incorporated into hepatic VLDL triglyceride and contribute significantly to VLDL/triglyceride secretion from the liver.44–47 The time frame of our kinetics study, that is, beginning 5 hours after the start of hourly formula ingestion, is sufficient for this process to occur because it has been shown that dietary fatty acids appear in VLDL/triglycerides as early as 2 hours after fat ingestion.45,48 We believe that VLDL secretion was already stimulated in both the saline and the IH studies, as evidenced by increased TRL apoB-100 concentrations in the saline group in the fed state compared with the fasted value and by the higher rates of production of TRL apoB-100 that we observed compared with production rates reported previously by others in fasted humans.49,50 Nevertheless, elevation of plasma FFAs in a constant fed state further increases both hepatic and intestinal TRL production.
Quantitatively, intestinal lipoprotein overproduction probably does not contribute greatly to the typical postabsorptive hypertriglyceridemia of insulin-resistant states and type 2 diabetes mellitus in humans because apoB-48 mass in fasted plasma is approximately one hundredth to one tenth of apoB100 mass.51 In the present study, the absolute production rate of hepatic (apoB-100) lipoproteins was 5- to 6-fold greater than the production rate of intestinal (apoB-48) lipoproteins in both the saline and the IH studies. Despite the fact that apoB-100–containing lipoprotein mass is quantitatively far greater than that of apoB-48, an increase in apoB-48 production in certain conditions may contribute to postprandial lipemia and could contribute significantly to atherogenesis if intestinal lipoproteins are shown to be highly atherogenic, as has been proposed by some.13,14 We speculate that chronic intestinal overproduction of apoB-48 primes the intestine to rapidly and efficiently facilitate the absorption of ingested fat, enhancing the assembly and secretion of intestinal TRL and hence contributing to postprandial lipemia. The prevailing concept is that postprandial lipemia in these conditions results in part from competition between ingested chylomicrons and the expanded pool of VLDL, with consequent delayed clearance of endogenous (B-100–containing) and exogenous (B-48–containing) TRL particles.52,53 Increased efficiency and rapidity of production of chylomicrons in conditions of insulin resistance and type 2 diabetes mellitus may also contribute to the typical postprandial lipemia of these conditions. This area requires extensive further investigation.
In conclusion, the present report provides evidence that intestinal apoB-48–containing TRL production is stimulated by a short-term elevation of plasma FFAs in healthy humans. We speculate that chronic elevation of plasma FFAs, as seen in insulin-resistant and diabetic individuals, is likely to play an important role in the overproduction of intestinal lipoprotein that has recently been observed in these conditions.15,16
Supplementary Material
CLINICAL PERSPECTIVE.
Dyslipidemia is a prominent feature of both insulin resistance and type 2 diabetes mellitus and contributes to the increased risk of cardiovascular events in these conditions. The typical hypertriglyceridemia of insulin-resistant states has been determined in numerous previous studies to be due predominantly to overproduction of hepatic (apolipoprotein B-100 – containing) very-low-density lipoproteins, as well as impairment in the clearance of these particles from the circulation. The mechanism of hepatic very-low-density lipoprotein overproduction in insulin resistance is complex, but elevated free fatty acid flux, predominantly from insulin-resistant adipose tissue to the liver, is an important mechanism accounting for this phenomenon. Recent studies in animal models of insulin resistance and in humans have demonstrated that the production rate of intestinal (apolipoprotein B-48 – containing) chylomicrons is also increased in insulin-resistant states. Intestinal chylomicron production is stimulated mainly by fat ingestion, which is efficiently absorbed from the intestinal lumen by the intestinal enterocyte and packaged into chylomicron particles, which enter the circulation via the lymphatic duct. Here we have shown, for the first time in humans, that an acute elevation of plasma free fatty acids stimulates not only hepatic but also intestinal triglyceride-rich lipoprotein production. We speculate that chronic elevation of plasma free fatty acids, as seen in insulin-resistant and type 2 diabetic individuals, is likely to play an important role in the overproduction of intestinal lipoproteins, thereby contributing to the dyslipidemia of insulin-resistant humans.
Acknowledgments
Sources of Funding
This work was supported by funding from the Canadian Institutes for Health Research (MOP-43839). Dr Lewis holds a Canada Research Chair in Diabetes and is a Career Investigator of the Heart and Stroke Foundation of Canada. Dr Lamarche holds a Canada Research Chair in Nutrition and Cardiovascular Health. Dr Duez is the recipient of a Postdoctoral Fellowship Award from the Heart and Stroke Foundation of Canada, and Dr Xiao is a recipient of a Postdoctoral Fellowship Award from the Canadian Diabetes Association. Dr Patterson was supported by National Institutes of Health grant P30 DK56341 (Clinical Nutrition Research Unit).
Footnotes
The online-only Data Supplement, which contains a supplemental Methods section, is available with this article at http://circ.ahajournals.org/cgi/content/full/CIRCULATIONAHA.107.739888/DC1.
Disclosures
None.
References
- 1.Ginsberg HN. Insulin resistance and cardiovascular disease. J Clin Invest. 2000;106:453–458. doi: 10.1172/JCI10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lewis GF, Steiner G. Hypertriglyceridemia and its metabolic consequences as a risk factor for atherosclerotic cardiovascular disease in non-insulin-dependent diabetes mellitus. Diabet Metab Rev. 1996;12:37–56. doi: 10.1002/(SICI)1099-0895(199603)12:1<37::AID-DMR154>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 3.Curtin A, Deegan P, Owens D, Collins P, Johnson A, Tomkin GH. Elevated triglyceride-rich lipoproteins in diabetes: a study of apoli-poprotein B-48. Acta Diabetol. 1996;33:205–210. doi: 10.1007/BF02048544. [DOI] [PubMed] [Google Scholar]
- 4.Schaefer EJ, McNamara JR, Shah PK, Nakajima K, Cupples LA, Ordovas JM, Wilson PW. Elevated remnant-like particle cholesterol and triglyceride levels in diabetic men and women in the Framingham Offspring Study. Diabetes Care. 2002;25:989–994. doi: 10.2337/diacare.25.6.989. [DOI] [PubMed] [Google Scholar]
- 5.Mero N, Syvanne M, Taskinen MR. Postprandial lipid metabolism in diabetes. Atherosclerosis. 1998;141(suppl 1):S53–S55. doi: 10.1016/s0021-9150(98)00218-4. [DOI] [PubMed] [Google Scholar]
- 6.Ohnishi H, Saitoh S, Takagi S, Ohata J, Isobe T, Kikuchi Y, Takeuchi H, Shimamoto K. Relationship between insulin-resistance and remnant-like particle cholesterol. Atherosclerosis. 2002;164:167–170. doi: 10.1016/s0021-9150(02)00057-6. [DOI] [PubMed] [Google Scholar]
- 7.Taniguchi A, Fukushima M, Sakai M, Miwa K, Makita T, Nagata I, Nagasaka S, Doi K, Okumura T, Fukuda A, Kishimoto H, Fukuda T, Nakaishi S, Tokuyama K, Nakai Y. Remnant-like particle cholesterol, triglycerides, and insulin resistance in nonobese Japanese type 2 diabetic patients. Diabetes Care. 2000;23:1766–1769. doi: 10.2337/diacare.23.12.1766. [DOI] [PubMed] [Google Scholar]
- 8.Haidari M, Leung N, Mahbub F, Uffelman KD, Kohen-Avramoglu R, Lewis GF, Adeli K. Fasting and postprandial overproduction of intes-tinally derived lipoproteins in an animal model of insulin resistance: evidence that chronic fructose feeding in the hamster is accompanied by enhanced intestinal de novo lipogenesis and apoB48-containing lipoprotein overproduction. J Biol Chem. 2002;277:31646–31655. doi: 10.1074/jbc.M200544200. [DOI] [PubMed] [Google Scholar]
- 9.Leung N, Naples M, Uffelman K, Szeto L, Adeli K, Lewis GF. Rosiglitazone improves intestinal lipoprotein overproduction in the fat-fed Syrian Golden hamster, an animal model of nutritionally-induced insulin resistance. Atherosclerosis. 2004;174:235–241. doi: 10.1016/j.atherosclerosis.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 10.Guo Q, Avramoglu RK, Adeli K. Intestinal assembly and secretion of highly dense/lipid-poor apolipoprotein B48-containing lipoprotein particles in the fasting state: evidence for induction by insulin resistance and exogenous fatty acids. Metabolism. 2005;54:689–697. doi: 10.1016/j.metabol.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 11.Federico LM, Naples M, Taylor D, Adeli K. Intestinal insulin resistance and aberrant production of apolipoprotein b48 lipoproteins in an animal model of insulin resistance and metabolic dyslipidemia: evidence for activation of protein tyrosine phosphatase-1B, extracellular signal-related kinase, and sterol regulatory element-binding protein-1c in the fructose-fed hamster intestine. Diabetes. 2006;55:1316–1326. doi: 10.2337/db04-1084. [DOI] [PubMed] [Google Scholar]
- 12.Lewis GF, Uffelman K, Naples M, Szeto L, Haidari M, Adeli K. Intestinal lipoprotein overproduction, a newly recognized component of insulin resistance, is ameliorated by the insulin sensitizer rosiglitazone: studies in the fructose-fed Syrian golden hamster. Endocrinology. 2005;146:247–255. doi: 10.1210/en.2004-1143. [DOI] [PubMed] [Google Scholar]
- 13.McNamara JR, Shah PK, Nakajima K, Cupples LA, Wilson PW, Ordovas JM, Schaefer EJ. Remnant-like particle (RLP) cholesterol is an independent cardiovascular disease risk factor in women: results from the Framingham Heart Study. Atherosclerosis. 2001;154:229–236. doi: 10.1016/s0021-9150(00)00484-6. [DOI] [PubMed] [Google Scholar]
- 14.Mero N, Malmstrom R, Steiner G, Taskinen MR, Syvanne M. Postprandial metabolism of apolipoprotein B-48- and B-100-containing particles in type 2 diabetes mellitus: relations to angiographically verified severity of coronary artery disease. Atherosclerosis. 2000;150:167–177. doi: 10.1016/s0021-9150(99)00364-0. [DOI] [PubMed] [Google Scholar]
- 15.Duez H, Lamarche B, Uffelman KD, Valero R, Cohn JS, Lewis GF. Hyperinsulinemia is associated with increased production rate of intestinal apolipoprotein B-48-containing lipoproteins in humans. Arterioscler Thromb Vasc Biol. 2006;26:1357–1363. doi: 10.1161/01.ATV.0000222015.76038.14. [DOI] [PubMed] [Google Scholar]
- 16.Hogue JC, Lamarche B, Tremblay AJ, Bergeron J, Gagne C, Couture P. Evidence of increased secretion of apolipoprotein B-48-containing lipoproteins in subjects with type 2 diabetes. J Lipid Res. 2007;48:1336–1342. doi: 10.1194/jlr.M600548-JLR200. [DOI] [PubMed] [Google Scholar]
- 17.Kovacs P, Stumvoll M. Fatty acids and insulin resistance in muscle and liver. Best Pract Res Clin Endocrinol Metab. 2005;19:625–635. doi: 10.1016/j.beem.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 18.Lewis GF, Carpentier A, Adeli K, Giacca A. Disordered fat storage and mobilization in the pathogenesis of insulin resistance and type 2 diabetes. Endocr Rev. 2002;23:201–229. doi: 10.1210/edrv.23.2.0461. [DOI] [PubMed] [Google Scholar]
- 19.Byrne CD, Brindle NP, Wang, Hales CN. Interaction of non-esterified fatty acid and insulin in control of triacylglycerol secretion by Hep G2 cells. Biochem J. 1991;280(pt 1):99–104. doi: 10.1042/bj2800099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Byrne CD, Wang TW, Hales CN. Control of Hep G2-cell triacylglycerol and apolipoprotein B synthesis and secretion by polyunsaturated non-esterified fatty acids and insulin. Biochem J. 1992;288(pt 1):101–107. doi: 10.1042/bj2880101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cianflone KM, Yasruel Z, Rodriguez MA, Vas D, Sniderman AD. Regulation of apoB secretion from HepG2 cells: evidence for a critical role for cholesteryl ester synthesis in the response to a fatty acid challenge. J Lipid Res. 1990;31:2045–2055. [PubMed] [Google Scholar]
- 22.White AL, Graham DL, LeGros J, Pease RJ, Scott J. Oleate-mediated stimulation of apolipoprotein B secretion from rat hepatoma cells: a function of the ability of apolipoprotein B to direct lipoprotein assembly and escape presecretory degradation. J Biol Chem. 1992;267:15657–15664. [PubMed] [Google Scholar]
- 23.Dixon JL, Furukawa S, Ginsberg HN. Oleate stimulates secretion of apolipoprotein B-containing lipoproteins from Hep G2 cells by inhibiting early intracellular degradation of apolipoprotein B. J Biol Chem. 1991;266:5080–5086. [PubMed] [Google Scholar]
- 24.Arbeeny CM, Meyers DS, Bergquist KE, Gregg RE. Inhibition of fatty acid synthesis decreases very low density lipoprotein secretion in the hamster. J Lipid Res. 1992;33:843–851. [PubMed] [Google Scholar]
- 25.Sparks JD, Collins HL, Sabio I, Sowden MP, Smith HC, Cianci J, Sparks CE. Effects of fatty acids on apolipoprotein B secretion by McArdle RH-7777 rat hepatoma cells. Biochim Biophys Acta. 1997;1347:51–61. doi: 10.1016/s0005-2760(97)00050-7. [DOI] [PubMed] [Google Scholar]
- 26.Lin Y, Smit MJ, Havinga R, Verkade HJ, Vonk RJ, Kuipers F. Differential effects of eicosapentaenoic acid on glycerolipid and apolipoprotein B metabolism in primary human hepatocytes compared to HepG2 cells and primary rat hepatocytes. Biochim Biophys Acta. 1995;1256:88–96. doi: 10.1016/0005-2760(95)00006-x. [DOI] [PubMed] [Google Scholar]
- 27.Zhang YL, Hernandez-Ono A, Ko C, Huang LS, Ginsberg HN. Regulation of hepatic apolipoprotein B-lipoprotein assembly and secretion by the availability of fatty acids, I: differential response to the delivery of fatty acids via albumin or remnant-like emulsion particles. J Biol Chem. 2004;279:19362–19374. doi: 10.1074/jbc.M400220200. [DOI] [PubMed] [Google Scholar]
- 28.Lewis GF, Uffelman KD, Szeto LW, Weller B, Steiner G. Interaction between free fatty acids and insulin in the acute control of very low density lipoprotein production in humans. J Clin Invest. 1995;95:158–166. doi: 10.1172/JCI117633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lewis GF. Fatty acid regulation of very low density lipoprotein production. Curr Opin Lipidol. 1997;8:146–153. doi: 10.1097/00041433-199706000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Steiner G, Poapst ME, Davidson JK. Production of chylomicron-like lipoproteins from endogenous lipid by the intestine and liver of diabetic dogs. Diabetes. 1975;24:263–271. doi: 10.2337/diab.24.3.263. [DOI] [PubMed] [Google Scholar]
- 31.Lewis GF, Naples M, Uffelman K, Leung N, Szeto L, Adeli K. Intestinal lipoprotein production is stimulated by an acute elevation of plasma free fatty acids in the fasting state: studies in insulin-resistant and insulin-sensitized Syrian golden hamsters. Endocrinology. 2004;145:5006–5012. doi: 10.1210/en.2003-1559. [DOI] [PubMed] [Google Scholar]
- 32.Cohn JS, Wagner DA, Cohn SD, Millar JS, Schaefer EJ. Measurement of very low density and low density lipoprotein apolipoprotein (Apo) B-100 and high density lipoprotein Apo A-I production in human subjects using deuterated leucine: effect of fasting and feeding. J Clin Invest. 1990;85:804–811. doi: 10.1172/JCI114507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Batal R, Tremblay M, Barrett PH, Jacques H, Fredenrich A, Mamer O, Davignon J, Cohn JS. Plasma kinetics of apoC-III and apoE in normo-lipidemic and hypertriglyceridemic subjects. J Lipid Res. 2000;41:706–718. [PubMed] [Google Scholar]
- 34.Lookene A, Skottova N, Olivecrona G. Interactions of lipoprotein-lipase with the active-site inhibitor tetrahydrolipstatin (Orlistat)(R) Eur J Biochem. 1994;222:395–403. doi: 10.1111/j.1432-1033.1994.tb18878.x. [DOI] [PubMed] [Google Scholar]
- 35.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 36.Dwyer KP, Barrett PHR, Chan D, Foo JI, Watts GF, Croft KD. Oxazo-linone derivative of leucine for GC-MS: a sensitive and robust method for stable isotope kinetic studies of lipoproteins. J Lipid Res. 2002;43:344–349. [PubMed] [Google Scholar]
- 37.Cobelli C, Toffolo G, Foster DM. Tracer-to-tracee ratio for analysis of stable isotope tracer data: link with radioactive kinetic formalism. Am J Physiol. 1992;262:E968–E975. doi: 10.1152/ajpendo.1992.262.6.E968. [DOI] [PubMed] [Google Scholar]
- 38.Vine DF, Takechi R, Russell JC, Proctor SD. Impaired postprandial apolipoprotein-B48 metabolism in the obese, insulin-resistant JCR:LA-cp rat: increased atherogenicity for the metabolic syndrome. Atherosclerosis. 2007;190:282–290. doi: 10.1016/j.atherosclerosis.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 39.Karpe F, Hamsten A, Uffelma, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Meth. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 40.Deleted in proof
- 41.Hussain MM, Fatma S, Pan X, Iqbal J. Intestinal lipoprotein assembly. Curr Opin Lipidol. 2005;16:281–285. doi: 10.1097/01.mol.0000169347.53568.5a. [DOI] [PubMed] [Google Scholar]
- 42.Gangl A, Ockner RK. Intestinal metabolism of plasma free fatty acids: intracellular compartmentation and mechanisms of control. J Clin Invest. 1975;55:803–813. doi: 10.1172/JCI107991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Savage DB, Petersen KF, Shulman GI. Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol Rev. 2007;87:507–520. doi: 10.1152/physrev.00024.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heath RB, Karpe F, Milne RW, Burdge GC, Wootton SA, Frayn KN. Selective partitioning of dietary fatty acids into the VLDL TG pool in the early postprandial period. J Lipid Res. 2003;44:2065–2072. doi: 10.1194/jlr.M300167-JLR200. [DOI] [PubMed] [Google Scholar]
- 45.Heath RB, Karpe F, Milne RW, Burdge GC, Wootton SA, Frayn KN. Dietary fatty acids make a rapid and substantial contribution to VLDL-triacylglycerol in the fed state. Am J Physiol. 2007;292:E732–E739. doi: 10.1152/ajpendo.00409.2006. [DOI] [PubMed] [Google Scholar]
- 46.Barrows BR, Parks E. Contributions of different fatty acid sources to very low-density lipoprotein-triacylglycerol in the fasted and fed states. J Clin Endocrinol Metab. 2006;91:1446–1452. doi: 10.1210/jc.2005-1709. [DOI] [PubMed] [Google Scholar]
- 47.Hodson L, Bickerton AST, McQuaid SE, Roberts R, Karpe F, Frayn KN, Fielding BA. The contribution of splanchnic fat to VLDL triglyceride is greater in insulin-resistant than insulin-sensitive men and women: studies in the postprandial state. Diabetes. 2007;56:2433–2441. doi: 10.2337/db07-0654. [DOI] [PubMed] [Google Scholar]
- 48.Parks E, Hellerstein MK. Recent advances in liver triacylglycerol and fatty acid metabolism using stable isotope labeling techniques. J Lipid Res. 2006;47:1651–1660. doi: 10.1194/jlr.R600018-JLR200. [DOI] [PubMed] [Google Scholar]
- 49.Marsh JB, Welty FK, Lichtenstein AH, Lamon-Fava S, Schaefer EJ. Apolipoprotein B metabolism in humans: studies with stable isotope-labeled amino acid precursors. Atherosclerosis. 2002;162:227–244. doi: 10.1016/s0021-9150(01)00709-2. [DOI] [PubMed] [Google Scholar]
- 50.Chan DC, Watts GF, Ng TW, Uchida Y, Sakai N, Yamashita S, Barrett PH. Adiponectin and other adipocytokines as predictors of markers of triglyceride-rich lipoprotein metabolism. Clin Chem. 2005;51:578–585. doi: 10.1373/clinchem.2004.045120. [DOI] [PubMed] [Google Scholar]
- 51.Karpe F. Postprandial lipoprotein metabolism and atherosclerosis. J Intern Med. 1999;246:341–355. doi: 10.1046/j.1365-2796.1999.00548.x. [DOI] [PubMed] [Google Scholar]
- 52.Brunzell JD, Hazzard WR, Porte D, Jr, Bierman EL. Evidence for a common, saturable, triglyceride removal mechanism for chylomicrons and very low density lipoproteins in man. J Clin Invest. 1973;52:1578–1585. doi: 10.1172/JCI107334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bjorkegren J, Packard CJ, Hamsten A, Bedford D, Caslake M, Foster L, Shepherd J, Stewart P, Karpe F. Accumulation of large very low density lipoprotein in plasma during intravenous infusion of a chylomicron-like triglyceride emulsion reflects competition for a common lipolytic pathway. J Lipid Res. 1996;37:76–86. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.