ABSTRACT
It is now well appreciated that bacterial cells are highly organized, which is far from the initial concept that they are merely bags of randomly distributed macromolecules and chemicals. Central to their spatial organization is the precise positioning of certain proteins in subcellular domains of the cell. In particular, the cell poles – the ends of rod-shaped cells – constitute important platforms for cellular regulation that underlie processes as essential as cell cycle progression, cellular differentiation, virulence, chemotaxis and growth of appendages. Thus, understanding how the polar localization of specific proteins is achieved and regulated is a crucial question in bacterial cell biology. Often, polarly localized proteins are recruited to the poles through their interaction with other proteins or protein complexes that were already located there, in a so-called diffusion-and-capture mechanism. Bacteria are also starting to reveal their secrets on how the initial pole ‘recognition’ can occur and how this event can be regulated to generate dynamic, reproducible patterns in time (for example, during the cell cycle) and space (for example, at a specific cell pole). Here, we review the major mechanisms that have been described in the literature, with an emphasis on the self-organizing principles. We also present regulation strategies adopted by bacterial cells to obtain complex spatiotemporal patterns of protein localization.
KEY WORDS: Bacterial cell cycle, Polar localization, Spatial organization
Introduction
Evidence for the elaborate spatial organization of bacterial cells started to accumulate more than two decades ago, challenging the antiquated idea that bacteria were simple vessels of randomly distributed macromolecules, far removed from organized, compartmentalized eukaryotic cells. This misconception naturally originated from the observation that the cytoplasm of these tiny cells generally lacks intracellular membrane-enclosed organelles in which biomolecules – hence cellular functions – can be sorted. It is now well appreciated that cellular components of various kinds (proteins, lipids, DNA, RNAs, ribosomes and metabolites) can display subcellular arrangements in bacterial cells. This spatial organization is critical for various aspects of bacterial physiology and adaptation to diverse environments (Matsumoto et al., 2006; Shapiro et al., 2009; Rudner and Losick, 2010; Campos and Jacobs-Wagner, 2013).
Within the bacterial cytoplasm, the cell poles – the rounded ends of rod-shaped cells that are generated by each division event – constitute a domain where a subset of proteins specifically localizes. Proteins that localize at the poles (referred to as ‘polar proteins’ hereafter) are numerous and vary widely in function. Localization of polar proteins, which is the focus of this Commentary, is essential for a large number of important cellular processes in bacteria, including cell cycle regulation, cell differentiation, virulence, chemotaxis, motility and adhesion (Bowman et al., 2011; Kirkpatrick and Viollier, 2011). Our goal here is not to provide a survey of the large number of polar proteins described so far, many of which have been reviewed elsewhere (Ebersbach and Jacobs-Wagner, 2007; Dworkin, 2009; Kirkpatrick and Viollier, 2011; Davis and Waldor, 2013). Instead, our objective is to review and illustrate the general principles used by bacterial cells to localize proteins at the poles.
The patterns displayed by proteins inside bacterial cells can be complex. Indeed, some proteins are known to change location over time, such as during the course of the cell cycle; others reproducibly accumulate at only one of the two cell poles. The dynamics and asymmetry of polar localization underlie central cellular processes in bacteria, yet the mechanisms that control polar localization in space and time are only starting to be uncovered. In this Commentary, we first summarize several polar localization mechanisms that have been selected during bacterial evolution. Then, we examine possible strategies that control these mechanisms to produce specific spatiotemporal patterns of protein localization.
How proteins localize at the cell poles: themes and variations
Diffusion and capture through protein–protein interaction
Most of the polar proteins identified so far are simply recruited to the cell poles through an interaction with a protein or protein complex already present at the pole (Fig. 1A). In this scenario, protein A diffuses in the cytoplasmic space until it encounters a polar protein B for which it has a binding affinity. Because of the transient nature of protein–protein interactions, protein A is continuously exchanged between the diffusing pool in the cell body and the localized pool at the pole. The concentration of protein B at the poles and the rates of association and dissociation between the interacting partners A and B will determine the extent of the polar accumulation of protein A at steady state. Note that ‘diffusion and capture’ is a general mechanism that also explains the subcellular localization of many non-polar proteins.
Fig. 1.
Localization of polar proteins through the recognition of polar features. (A) A protein (e.g. ParA1 in V. cholerae) diffusing in the cytoplasm (as indicated by single arrows) is trapped at the poles transiently (double arrows) through an affinity for a polar protein (e.g. HubP in V. cholerae) that is already localized at the cell poles. (B) Higher-order protein assemblies are favored in membrane regions of stronger curvature. Left: representation of a rod-shaped cell showing the radius of curvature (R) and the stronger negative curvature (C, curved arrows) at the cell poles (blue areas) compared with the sides of the cylinder (gray area), as described previously (Huang and Ramamurthi, 2010). Middle and right: formation of a higher-order protein assembly occurring preferentially in membrane regions of stronger negative curvature (e.g. DivIVA in B. subtilis). Arrows indicate the free diffusion of oligomers. (C) Formation of large protein assemblies such as higher-order structures (e.g. PopZ in C. crescentus) or protein aggregates (e.g. misfolded proteins in E. coli) is energetically favored outside the nucleoid region. (D) Differences in composition of the cytoplasmic membrane and peptidoglycan between cell poles and the rest of the cell envelope can serve as cues for localization of polar proteins. The particular case of a protein (e.g. ProP in E. coli) that preferentially binds anionic phospholipids enriched at the poles, such as cardiolipin, is depicted. E, extracellular space; OM, outer membrane; P, periplasmic space; CM, cytoplasmic membrane; C, cytoplasm. Note that the schematics in all figures are not to scale and do not reflect the structure of the illustrated proteins.
Remarkably, some proteins can serve as polar landmarks or hubs for multiple proteins, which can themselves recruit other proteins, and so forth. These hub proteins tend to play important roles in different pathways or cell cycle events. For example, DivIVA is involved in several cellular programs in Bacillus subtilis through the recruitment of multiple proteins: the competence regulator ComN (dos Santos et al., 2012), the division inhibitor Maf in competent cells (Briley et al., 2011) and the determinants of division site placement MinCD via MinJ (Bramkamp et al., 2008; Patrick and Kearns, 2008; Eswaramoorthy et al., 2011). Moreover, DivIVA attaches the chromosomal origin at the pole in the developing spore through an interaction with the DNA-binding protein RacA (Ben-Yehuda et al., 2003; Wu and Errington, 2003). In Caulobacter crescentus, PopZ, which shares no sequence or structural similarity with DivIVA, also anchors the chromosomal origin at the pole through an interaction with the chromosome partition complex, and recruits proteins that are involved in chromosome segregation and cell cycle control (Bowman et al., 2008; Ebersbach et al., 2008; Bowman et al., 2010; Schofield et al., 2010). The partition complex is composed of the parS sequences, located in the vicinity of the chromosomal origin, and the parS-binding protein ParB. Upon origin replication, one of the duplicated ParB–parS complexes remains at one pole while the other is segregated towards the opposite pole, followed by the rest of the sister chromosome. Because both ParB–parS complexes also carry a division inhibitor, their PopZ-dependent tight fastening at both poles after complete segregation is essential for the correct positioning of the division site near the midcell, where the inhibitor concentration is the lowest (Thanbichler and Shapiro, 2006; Ebersbach et al., 2008). In Vibrio cholerae, HubP provides a link between chromosome segregation, chemotaxis and flagellum synthesis by localizing three different ATPases that position elements of these machineries at the pole (Yamaichi et al., 2012).
But what is the ultimate positional information that allows these landmark proteins to ‘recognize’ the poles directly? To address this question, one must examine what makes the cell poles different from the rest of the cell. The poles have several distinctive features, and perhaps not surprisingly, bacteria can exploit several of them to efficiently position proteins at the poles through a diversity of mechanisms, as described below.
Direct sensing of enhanced curvature
A characteristic of the cell poles is their geometry. The total curvature (C) of the cell envelope is stronger at the poles because of their shape: for example, in bacilli, C is equal to 1/R along the cylindrical cell body (where R is the radius of the cylinder), whereas C equals 2/R at the hemispherical poles of the cell (Huang and Ramamurthi, 2010). An attractive hypothesis is that some proteins preferentially accumulate at the poles through an affinity for the more concave membrane curvature of the poles relative to the rest of the cell (Fig. 1B). An illustration of this mechanism is provided by the self-assembling protein DivIVA of B. subtilis, which preferentially localizes in the most concave regions of the cell (Lenarcic et al., 2009; Ramamurthi et al., 2009). After cell division, DivIVA remains localized at the poles newly generated from the division site (which are, at that time, the most curved regions in the cell), then mainly redistributes to a septal position when a new round of division is initiated (Ramamurthi et al., 2009; Eswaramoorthy et al., 2011). Whereas the polar localization of DivIVA in B. subtilis derives mostly from its curvature-dependent accumulation at the septum, DivIVA initially concentrates at the poles in outgrowing spores that are not yet engaged in cell division (Hamoen and Errington, 2003; Harry and Lewis, 2003). This is consistent with the idea that DivIVA localizes where negative curvature is the strongest, that is, primarily at the septum in actively dividing cells or at the poles in the absence of a septum. Experiments with B. subtilis cells in which cytokinesis is artificially blocked support this idea: as cells become filamentous and do not form new septa, DivIVA progressively relocalizes to the cell poles (Ramamurthi and Losick, 2009). In Streptomyces coelicolor hyphae, the DivIVA homolog mainly localizes at future and emerging hyphal tips, probably by sensing negatively curved surfaces (Flärdh, 2003; Hempel et al., 2008; Holmes et al., 2013). These hyphal tips are formed de novo along the cell without a division event.
B. subtilis DivIVA spontaneously accumulates at the poles upon heterologous expression in very distant species that lack DivIVA homologs such as Escherichia coli and fission yeast (Edwards et al., 2000). This supports the idea that DivIVA directly senses stronger concavity without the need of a protein anchor. But how could a nanometer-sized protein sense a curvature difference that is negligible at the protein scale? Indeed, a mathematical model shows that the average size of a protein monomer is too small relative to the pole diameter to be able to detect any curvature change (Huang and Ramamurthi, 2010). Instead, the protein would need to assemble into large structures to achieve cooperative, long-range sensing of membrane curvature (Lenarcic et al., 2009; Huang and Ramamurthi, 2010) (Fig. 1B). Consistent with this notion, DivIVA oligomerizes in vitro and in vivo (Muchová et al., 2002; Stahlberg et al., 2004; Oliva et al., 2010), and these oligomers can further assemble into larger structures (Stahlberg et al., 2004; Wang et al., 2009). A ‘molecular-bridging’ model based on Monte-Carlo simulations argues that the clustering of DivIVA oligomers is favored at more-curved regions owing to the enhanced possibility of stabilizing interactions with the membrane (Lenarcic et al., 2009). A recent structural study showed that the intrinsic curvature of a DivIVA tetramer is distinct from the membrane curvature at the pole or septum (Oliva et al., 2010), further suggesting that a higher-order assembly is required for the curvature-mediated localization of DivIVA oligomers.
Self-assembly and nucleoid occlusion
Apart from their geometry, the cell poles are characterized by being largely devoid of chromosomal DNA (nucleoid). This distinguishing feature can also serve as a positional cue for protein localization. The concept is based on the notion that the formation of big structures through protein self-assembly is energetically more favorable outside bulky polymers such as the nucleoid because of volume-exclusion effects. Thus, large protein clusters can be passively sorted to the poles by a process called nucleoid occlusion (Fig. 1C). This polar localization mechanism was first proposed for the self-assembling hub protein PopZ in C. crescentus (Ebersbach et al., 2008). PopZ spontaneously interacts with itself and the resulting oligomers further assemble in vivo into a matrix that presumably enhances the availability of docking sites for multiple partners (Bowman et al., 2008; Ebersbach et al., 2008; Bowman et al., 2010). C. crescentus PopZ is also able to assemble into a polar matrix in the evolutionary divergent E. coli (Bowman et al., 2008; Ebersbach et al., 2008; Laloux and Jacobs-Wagner, 2013), even though E. coli lacks PopZ homologs or any known PopZ partners. PopZ multimerization is essential to its polar localization (Laloux and Jacobs-Wagner, 2013; Bowman et al., 2013). This was shown not only in C. crescentus, but also in E. coli, supporting the idea of a self-organizing localization mechanism that is based on PopZ assembly (Laloux and Jacobs-Wagner, 2013). A bona fide PopZ matrix can form not only at the poles but also in any non-polar DNA-free region, for example, in between segregated nucleoids along the filaments of a C. crescentus mutant or E. coli cells in which cell division is blocked (Ebersbach et al., 2008; Laloux and Jacobs-Wagner, 2013). Thus, the polar localization of PopZ is independent of curvature, and instead appears to be determined by its preferential self-assembly in regions of low DNA content (Ebersbach et al., 2008; Laloux and Jacobs-Wagner, 2013).
Misfolded proteins also passively aggregate at the cell poles in E. coli as a result of nucleoid occlusion (Winkler et al., 2010; Coquel et al., 2013). The principle is simple: misfolded proteins freely diffuse in the cytoplasm and tend to stick to each other owing to the exposure of hydrophobic patches on their surface. As the amorphous aggregates grow by the addition of more misfolded peptides, they are excluded from the nucleoid and accumulate at the cell poles where they can expand further.
Supporting this model, in silico simulations demonstrate that the passive diffusion of a particle, its intrinsic ability to multimerize and the absence of nucleoid at the poles are sufficient to obtain a polar-localization pattern by entropy alone (Saberi and Emberly, 2010; Coquel et al., 2013; Saberi and Emberly, 2013). Note that this process is spontaneous. However, as described in the second part of this Commentary, temporal and spatial control can be added to this process through regulation of protein self-assembly.
Affinity for pole-specific elements of the cell envelope
Another unique trait of the cell poles is the composition and maturation of their cell envelope. Therefore, another possible mechanism of polar localization relies on protein recognition of pole-specific features of the cell envelope (Fig. 1D). For example, some proteins interact preferentially with specific phospholipids such as cardiolipin (Mileykovskaya et al., 2003; Renner and Weibel, 2012) that are enriched in the cytoplasmic membrane surrounding the poles of several bacterial species (Mileykovskaya and Dowhan, 2000; Kawai et al., 2004; Bernal et al., 2007). The polar enrichment of cardiolipin is driven by its intrinsic curvature, which thermodynamically favors its insertion as clusters (‘microdomains’) in highly negatively curved membranes (Huang et al., 2006; Mukhopadhyay et al., 2008; Renner and Weibel, 2011). At least two E. coli proteins, ProP and MscS, localize to the poles in a cardiolipin-dependent manner (Romantsov et al., 2007; Romantsov et al., 2010). The frequency of polar localization in the cell population correlates with the overall content of cardiolipin, although it is unknown whether this is due to a direct protein–lipid interaction at the pole. It would be interesting to see whether the polar enrichments of lipids and proteins are also correlated at the single-cell level. This phenomenon is not unique: other proteins might also exploit the particular lipid content at the poles for their local accumulation (Renner and Weibel, 2012).
Bacteria could also exploit the particular composition and maturation of the peptidoglycan wall at the cell poles to localize proteins there. Indeed, another particularity of the cell envelope at the poles of most rod-shaped bacteria is the absence of new peptidoglycan synthesis. This is because insertion of peptidoglycan material in some bacteria such as E. coli is redirected to the sidewall following cell septation (de Pedro et al., 1997). Therefore, polar peptidoglycan is considered less ‘active’ in these species. It is conceivable that some proteins could directly bind pole-specific peptidoglycan features and thereby accumulate mainly at the cell poles. So far, a few proteins have been proposed to localize at the poles by this mechanism (Lawler et al., 2006; Radhakrishnan et al., 2008; Wirth et al., 2012; Yamaichi et al., 2012). However, at this stage, the evidence is mostly correlative and a difference in chemical composition between polar and non-polar peptidoglycan remains to be determined. The next challenge resides in advancing techniques of peptidoglycan composition analysis, which currently lack the spatial resolution required to identify the key chemical elements that serve as polar localization cues.
Growth-dependent transmission from the sidewall
Whereas specificities of the polar envelope can be exploited as a cue for protein localization (Fig. 1D), an alternative, less-direct strategy can use the lateral (non-polar) peptidoglycan wall as a ‘shuttle’ to passively direct proteins toward the poles (Fig. 2A). It has been proposed that the cell wall material is progressively pushed towards the poles through continuous lateral insertion of new ‘building blocks’ of peptidoglycan over several rounds of growth and division (Rafelski and Theriot, 2006). Accordingly, proteins that stably associate with cell wall constituents along the cell cylinder could be gradually guided toward the cell poles. This multi-step mechanism has been proposed to explain the slow polarization of the surface protein ActA of the Gram-positive pathogen Listeria monocytogenes (Rafelski and Theriot, 2006). When this pathogen is inside mammalian cells, ActA induces the polymerization of host actin at the cell pole, resulting in a comet actin tail that propels the bacterium inside the host cell and into neighboring cells (Kocks et al., 1992; Kocks et al., 1993; Lacayo et al., 2012). The prevailing model suggests that upon entry of the pathogen into a host cell, ActA is secreted at discrete spots along the bacterium membrane, then associates with the peptidoglycan (García-del Portillo et al., 2011) and eventually spreads uniformly along the entire cell length as a result of protein accumulation and cell wall growth. As the lateral wall grows, ActA is proposed to follow the older peptidoglycan towards the poles. The cell wall turnover slows down as the cell pole ages, so that the pool of ActA becomes ultimately trapped at the older pole after a few rounds of division (Rafelski and Theriot, 2006). Because polarization of ActA – and hence, the formation of a propelling actin tail – depends on the growth rate of the bacterium, such a localization mechanism might temporally connect the actin-powered motility of L. monocytogenes inside the host to the pathogen growth dynamics. Interestingly, IcsA, the functional counterpart of ActA in Shigella flexneri, appears to be directly targeted to one pole before its secretion into the periplasm and insertion in the outer membrane (Goldberg et al., 1993; Steinhauer et al., 1999; Charles et al., 2001). It remains unclear how this initial positioning occurs, but the maintenance of IcsA at the pole is achieved through specific proteolysis along the entire cell surface, preventing lateral diffusion of the protein away from the pole (Steinhauer et al., 1999).
Fig. 2.
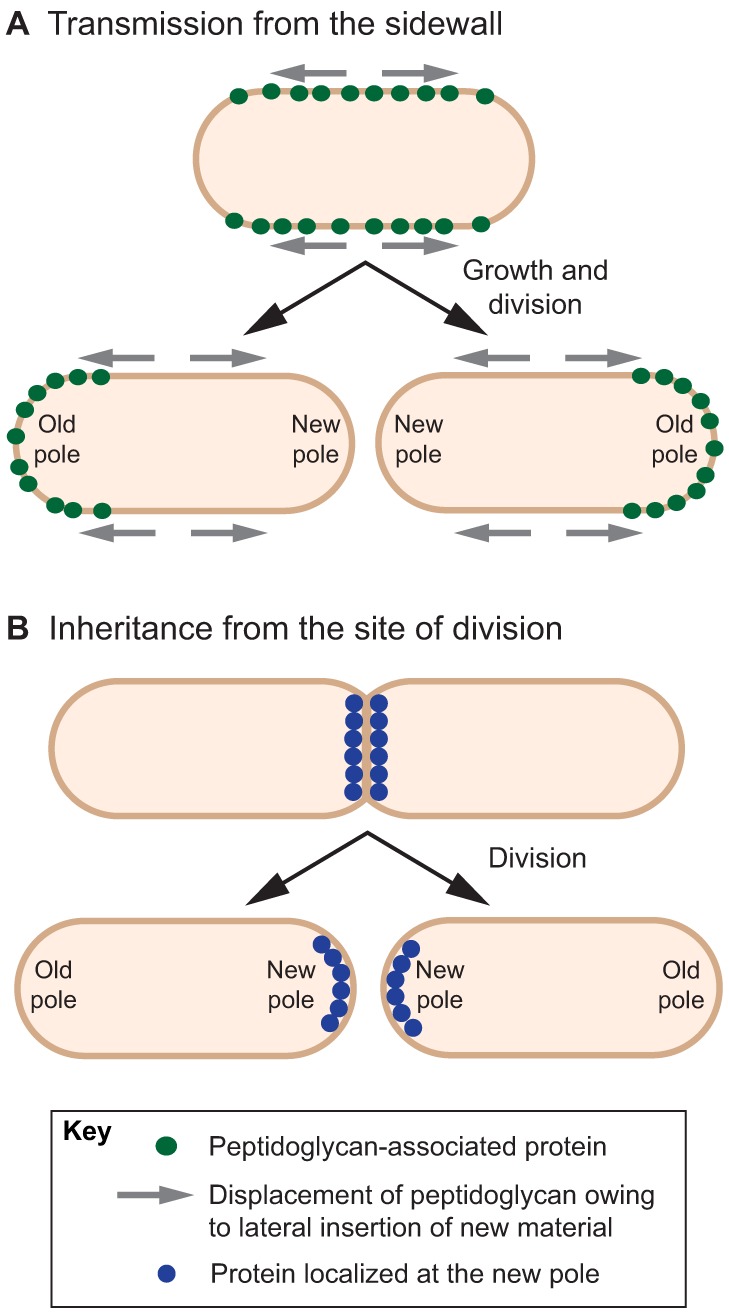
Localization of polar proteins resulting from cell growth and division. (A) A protein associated with the peptidoglycan is progressively directed towards the poles as the cell proceeds through cycles of growth and division, as new peptidoglycan is inserted laterally and becomes more and more inert during cell aging (e.g. ActA in L. monocytogenes). (B) Proteins stably localized at the midcell before cell division remain associated with the newly formed cell poles in the progeny (e.g. TipN in C. crescentus).
Inheritance from the division site
When rod-shaped bacteria divide, each daughter cell invariably inherits an ‘old’ pole from its mother and a ‘new’ pole freshly formed at the site of division. Therefore, a protein stably localized at the division site before cytokinesis could result in localization at a new pole after cell separation (Fig. 2B). The most straightforward illustration of this mechanism is given by components of the division machinery. In C. crescentus, the tubulin homolog FtsZ, along with interacting partners, remains localized at the new cell poles after division (Thanbichler and Shapiro, 2006; Goley et al., 2011). Proteins that are not directly involved in cell division can then couple their localization to division cues, thereby ensuring their localization at the new pole in the progeny. This is illustrated by the polar landmark TipN in C. crescentus (Huitema et al., 2006; Lam et al., 2006). At the beginning of the cell cycle, the membrane-bound coiled-coil protein TipN is present at the new pole where it acts as a beacon for developmental and cell cycle programs such as the polar biogenesis of a flagellum (Huitema et al., 2006; Lam et al., 2006). TipN also promotes the unidirectional segregation of the duplicated ParB–parS partition complex toward the new pole (Ptacin et al., 2010; Schofield et al., 2010). In late predivisional cells, TipN delocalizes from the new pole, possibly in a cell-size-dependent manner, and relocalizes at the midcell. This step is dependent on cell division and requires the Tol–Pal complex (Huitema et al., 2006; Lam et al., 2006; Yeh et al., 2010), a component of the divisome in Gram-negative bacteria that is responsible for outer membrane invagination during cytokinesis (Gerding et al., 2007). This way, each daughter cell inherits a TipN marker at its newborn pole.
In a variation of this mechanism, the midcell localization step can occur spontaneously, independently of any division-associated factor. This appears to be the case for chemoreceptors in E. coli. Arrays of chemoreceptors, which are among the largest signaling structures in bacterial cells, display a remarkable spatial organization at the membrane that is crucial for their function in chemotaxis. Chemoreceptor clusters are predominantly polar (Maddock and Shapiro, 1993), but a few are regularly positioned along the cell body (Sourjik and Armitage, 2010). This periodic arrangement is spontaneous: chemoreceptors stochastically self-assemble in a concentration-dependent manner to build new clusters or to expand old ones, and the formation of new clusters is entropically favored far away from pre-existing ones (Thiem et al., 2007; Thiem and Sourjik, 2008; Wang et al., 2008; Greenfield et al., 2009). As a consequence of this organization, larger clusters appear confined to future division sites (Thiem et al., 2007). This progressively leads to their polar accumulation and retention after several generations. Thus, two recurrent keys for protein localization, spontaneous self-assembly and inheritance at division, drive the polar clustering of chemoreceptors in E. coli. Interestingly, the localization of the membrane-associated chemoreceptor clusters in Rhodobacter spheroides has recently been shown to be independent of the cytokinetic FtsZ ring or the sites of future division. Instead, these clusters diffuse freely in the membrane, excluding the area of the FtsZ ring, and tend to accumulate at the poles possibly by a diffusion-and-capture process that could involve trapping of the clusters through negative-curvature sensing (Chiu et al., 2013).
Pole-to-pole oscillations through nucleotide switch and membrane binding
Remarkably, some proteins oscillate between the two cell poles (Lenz and Søgaard-Andersen, 2011). A well-studied case of pole-to-pole oscillation is the Min system in E. coli (Fig. 3), which contributes to the proper positioning of the division site (Raskin and de Boer, 1999) and might facilitate chromosome segregation (Di Ventura et al., 2013). In this system, a pool of MinD proteins rapidly oscillates between poles and carries along the division inhibitor MinC. Because of continuous pole-to-pole oscillations, the concentration of the MinC division inhibitor is, on time average, the lowest at the midcell, allowing division at that location only. Experimental and modeling studies reveal that the oscillatory behavior of Min is a multi-step self-organizing process (Shih and Zheng, 2013). Dimers of ATP-bound MinD cooperatively bind the membrane in a zone that extends from the pole towards the center of the cell (Hu et al., 2002; Hu et al., 2003; Lackner et al., 2003). The MinE protein binds to MinD at the edge of that zone and stimulates ATP hydrolysis (Hu and Lutkenhaus, 2001), thereby releasing MinD monomers from the membrane into the cytoplasm. MinE then interacts with and destabilizes the next MinD dimer at the membrane, and so on, in a so-called ‘Tarzan of the jungle’ model (Park et al., 2011; Park et al., 2012). MinE eventually reaches the pole, dissociates and diffuses freely in the cytoplasm before starting a new cycle on the other side of the cell where MinD has re-assembled. What triggers the formation of a new MinD zone at the pole? Experiments in mutant round cells indicate that MinD can localize anywhere on the membrane but preferentially re-assembles the furthest away from the MinE-destabilizing effect (Corbin et al., 2002). When cell poles are further away than normal (e.g. in filamentous cells), the Min system adopts a striped localization pattern (Raskin and de Boer, 1999), suggesting that no polar feature is needed to obtain cycles of collective membrane binding and unbinding. Thus, the cell geometry and the MinD–MinE interplay at the membrane might be sufficient to explain pole-to-pole oscillations in vivo (Meinhardt and de Boer, 2001; Howard and Kruse, 2005; Varma et al., 2008; Di Ventura and Sourjik, 2011; Loose et al., 2011; Halatek and Frey, 2012). Consistent with this idea, Min proteins form spontaneous waves on artificial lipid layers in vitro (Loose et al., 2008). The establishment of the MinD polar cap could be facilitated by a specific polar-nucleation protein such as cardiolipin, to which MinD preferentially binds (Mileykovskaya et al., 2003; Drew et al., 2005; Cytrynbaum and Marshall, 2007; Renner and Weibel, 2012), although the importance of such spatial cues is still under investigation (Halatek and Frey, 2012).
Fig. 3.
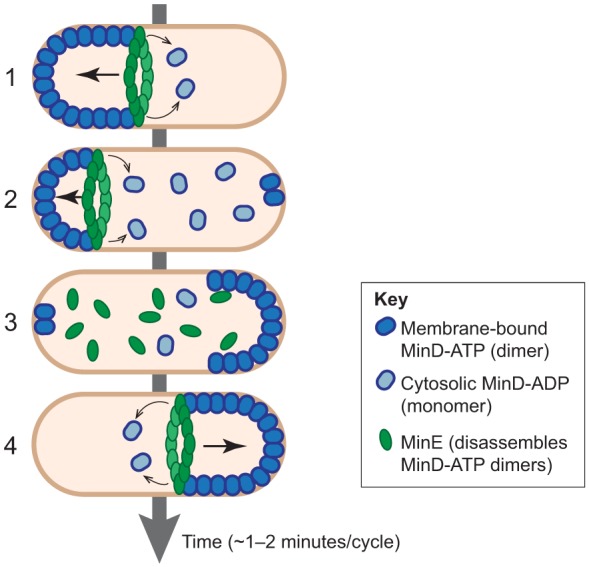
Pole-to-pole oscillation through nucleotide switch and membrane binding. In E. coli, MinE binds MinD-ATP dimers at the edge of a membrane-bound MinD-ATP dimer zone and triggers ATP hydrolysis, which releases MinD-ADP monomers in the cytosol (Step 1, thin arrows). MinE then binds to the next MinD-ATP. Iterations of this process lead to a progressive retraction of the MinD-ATP dimer zone at the membrane (Step 2, thick arrow). Following ADP/ATP nucleotide exchange, MinD-ATP redimerizes and dimers collectively reassociate with the membrane far from the MinE ring, i.e. at the opposite pole (Steps 2 and 3). After ‘running out’ of membrane-associated MinD-ATP dimers at one pole, MinE is released into the cytoplasm, diffuses until it associates with the edge of the new MinD-ATP dimer zone at the membrane (Steps 3 and 4).
Control of polar localization in space and time
Polar localization is often temporally and spatially regulated, which is essential for bacterial life and development (Shapiro et al., 2009). Some proteins accumulate at one pole only or change their localization (e.g. from diffuse to polar or from unipolar to bipolar) as the cell cycle proceeds or in response to environmental signals. The mechanistic basis underlying the various localization dynamics observed in bacteria is currently a hot topic of investigation. Below, we consider several regulatory strategies that can modulate protein localization in time or space. Some have concrete examples, whereas others are speculative.
Intrinsic temporal and spatial patterning through cell division
Some polar localization mechanisms intrinsically yield cell-cycle-regulated patterns. For example, TipN only marks the new pole of newborn C. crescentus cells because its polar position is acquired from the division plane of the mother cell (Fig. 2B), which forms the new pole of the progeny (Huitema et al., 2006; Lam et al., 2006). Along the same lines, cell-cycle-associated alterations of the intracellular geometry can provide spatio-temporal regulation to curvature-dependent polar localization. Provided that protein assemblies are dynamic (i.e. exchanges exist between the cytoplasmic pool and the polar cluster), the formation or disappearance of regions of stronger curvature could redeploy protein enrichments from one area of the cell to another. This is the case for DivIVA, which redistributes itself between the poles or septum depending on the initiation or block of cell division (Ramamurthi et al., 2009; Eswaramoorthy et al., 2011).
Cell division also inherently contributes to the transmission of asymmetric polar patterns. For example, PopZ, which decorates both poles of predivisional C. crescentus cells, is invariably transmitted to the old pole of each daughter cell (Fig. 4A) (Bowman et al., 2008; Ebersbach et al., 2008). This is particularly important to maintain the spatial organization of the cell, because each sibling receives a copy of the chromosome with a conserved configuration, held in place by the ParB–parS partition complex that is tethered at the old pole by a PopZ matrix. We describe below how PopZ reverts to a bipolar localization at a later stage of the cell cycle. In another scenario, proteins that are stably confined at one pole of the mother cell will be transmitted to the old pole of only one of the daughter cells. This is the case for large aggregates of misfolded proteins in E. coli (Fig. 4A) (Winkler et al., 2010). Hence, the propagation of asymmetric (i.e. unipolar) polar localization patterns can be ‘encoded’ in the mechanism that drives polar localization itself or can simply derive from cell division.
Fig. 4.
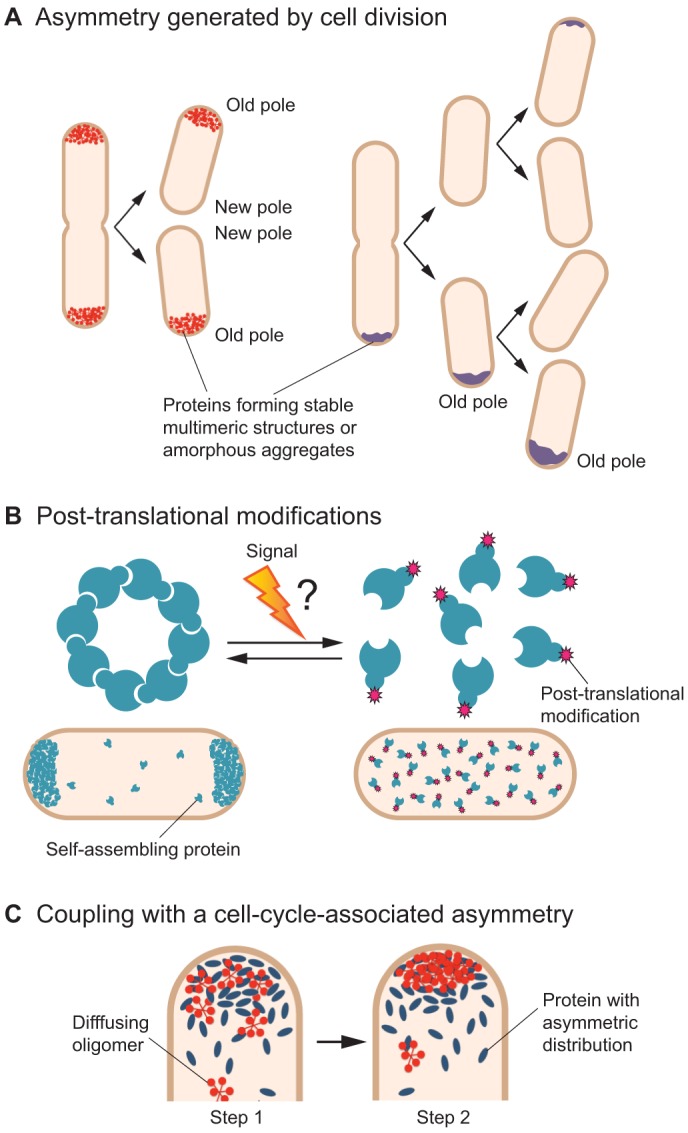
Possible strategies for spatial and temporal regulation of polar localization. (A) Asymmetric polar patterns can be naturally produced by a cell division event. Left: bipolar to old-pole localization (e.g. PopZ in C. crescentus). Right: propagation of an old-pole accumulation (e.g. protein aggregates in E. coli). In the case of protein aggregates, misfolded proteins produced in the progeny accumulate onto the existing polar aggregate. Eventually, de novo polar accretions can appear in progeny that did not acquire a polar focus (top cell), for example after new protein synthesis. (B) The ability of some proteins to self-assemble and thereby to localize at the poles (e.g. Wag31, the DivIVA homolog in M. tuberculosis) could be influenced by modifications such as phosphorylation upon a specific signal (e.g. expression of the kinases of Wag31 in exponential phase in M. tuberculosis may increase Wag31 phosphorylation). The question mark indicates a hypothetical step. (C) The concentration of a self-assembling protein or oligomer (e.g. PopZ oligomer in C. crescentus), and thereby its propensity to multimerize, can be modified locally through protein–protein interaction with a partner whose subcellular distribution is asymmetric (e.g. ParA during DNA segregation in C. crescentus). In Step 1, the protein (blue) has an asymmetric distribution inherent to a cell cycle event. Concentration of the diffusing protein oligomer (red) increases locally owing to interaction with the asymmetric protein. In Step 2, the self-assembly of a protein or oligomer leads to the formation of a large structure at the pole. This provides spatial and temporal regulation to a multimerization-dependent polar localization.
Temporal and spatial modulation of protein assembly
As described above, protein assembly into large complexes or structures emerges as a recurrent key process for self-organizing mechanisms of polar localization based, for example, on membrane curvature sensing (Fig. 1B) or nucleoid occlusion (Fig. 1C). Hence, a general strategy for modulating polar patterns might be to facilitate or prevent protein assembly at given locations and times.
Affecting self-assembly through post-translational modifications
A variety of post-translational modifications, such as changes of phosphorylation state or nucleotide binding, control the complex intracellular distribution of several proteins that are involved in cell cycle regulation, signal transduction, and polarized motility and adhesion (Bulyha et al., 2011; Kirkpatrick and Viollier, 2012). Although most examples known to date concern proteins that are at some point recruited to the poles through protein–protein interactions (Fig. 1A), similar modifications could also influence the ability of some proteins to multimerize, thereby impacting their spontaneous polar accumulation. If the presence or activity of the cognate kinases, phosphatases, GTPase-activating proteins (GAPs), among others, is under temporal regulation, this would provide a way to regulate an otherwise spontaneous polar localization in time (Fig. 4B). Recent studies suggest that this strategy can be exploited by bacteria. For example, in S. coelicolor, the increased phosphorylation of DivIVA by the eukaryotic-like Ser/Thr protein kinase AfsK, which can be activated artificially or upon arrest of cell wall synthesis, leads to the disassembly of DivIVA foci at the hyphal tips with dramatic effects on apical growth and hyphal branching (Hempel et al., 2012). Hence, phosphorylation of DivIVA could modify its oligomerization state. In fast-growing Mycobacterium tuberculosis cells, expression of kinases for the DivIVA homolog Wag31 is enhanced (Kang et al., 2005), which might result in an increase in phosphorylation of Wag31. Phosphorylation stimulates Wag31 oligomerization and thereby its polar localization (Jani et al., 2010). Polar localization, in turn, would promote Wag31-dependent synthesis of polar peptidoglycans (Jani et al., 2010).
Protein cleavage by specific proteases might also represent a strategy to modulate polar localization in space and time, as proposed for the polar beacon PodJ. Conversion of PodJ from a long (PodJL) to a shorter form (PodJS) by a cell-cycle-regulated proteolytic sequence that eventually degrades PodJS ensures proper localization and hence function of PodJ (Viollier et al., 2002; Chen et al., 2006). However, the precise mechanism whereby both forms of PodJ differentially localize at the poles remains to be determined.
Coupling to a cell cycle event
Temporal and spatial control of the localization of polar proteins can also occur through coupling to a particular cell cycle event (Bowman et al., 2010; Iniesta et al., 2010; Ditkowski et al., 2013; Fernandez-Fernandez et al., 2013; Laloux and Jacobs-Wagner, 2013), and C. crescentus PopZ provides an example. As mentioned above, daughter cells inherit a PopZ matrix at the old pole through division (Fig. 4A), but its chromosome-tethering function requires that a second PopZ matrix forms at the new pole. Evidence from our work suggests that this unipolar-to-bipolar transition is directly linked to the accumulation of the partitioning protein ParA at the new pole (Laloux and Jacobs-Wagner, 2013), which occurs during the segregation of the ParB–parS partition complex (Ptacin et al., 2010; Schofield et al., 2010; Shebelut et al., 2010). Because ParA interacts with PopZ (Schofield et al., 2010; Laloux and Jacobs-Wagner, 2013), our model (Fig. 4C) proposes that the accumulation of ParA at the new pole results in a local accumulation of diffusing PopZ oligomers at that location (Laloux and Jacobs-Wagner, 2013). A higher concentration of PopZ oligomers, in turn, promotes matrix formation at the right pole (new pole) and at the right time (when tethering of the segregated ParB–parS complex is needed). Consistent with this model, the unipolar-to-bipolar localization pattern of PopZ could be recapitulated in a ParA-dependent fashion in a heterologous E. coli system (Laloux and Jacobs-Wagner, 2013).
Other self-assembling proteins might similarly rely on an interacting factor that is present at a specific pole at a specific time during the cell cycle to modulate their concentration in time and space, thereby modulating their propensity to self-assemble and localize at the poles. Bactofilins, which self-assemble in vitro without any cofactor and whose low cytoplasmic concentration might not allow a spontaneous polymerization, could be good candidates for such a spatiotemporally regulated polar accumulation (Kühn et al., 2010).
Conclusion and perspectives
Bacteria use a diversity of mechanistic principles to drive protein localization at the cell poles (Figs 1–3). Molecular dynamics and asymmetries inherent to specific cell cycle events or physiological responses can be exploited in order to bring spatial and temporal regulation to otherwise spontaneous localization processes (Fig. 4). Although exciting progress has been made in the past few years, it will take a concerted effort from both experimentalists and theorists to understand the intricacies of each localization mechanism. We also anticipate that additional mechanisms await discovery as the list of identified polar proteins keeps increasing.
Essential to the investigation of protein localization is the tracking of proteins inside live bacterial cells. The development of less-bulky fluorescent tags and membrane-permeable organic dyes for protein visualization in bacteria (Griffin et al., 1998; Charbon et al., 2011), combined with near-native or adjustable expression levels, should ensure more physiologically relevant observations of subcellular distributions in the future. Currently, artefactual localization patterns – often polar accumulations – remain a concern as they can arise upon overexpression, misfolding or fusion to some fluorescent tags, depending on the protein being visualized (Winkler et al., 2010; Landgraf et al., 2012; Margolin, 2012; Swulius and Jensen, 2012). In addition, the quantification of subcellular patterns should become an integral part of any localization study. In that respect, recent post-imaging software packages are becoming essential tools to quantify protein localization dynamics – at both population and single-cell levels – that cannot be inferred from simple observations or manual measurements (Guberman et al., 2008; Sliusarenko et al., 2011; van Teeffelen et al., 2012). Importantly, their automation precludes the unconscious biases inherent to visual inspections. The field will also benefit from novel in vitro and in vivo assays based on the reconstruction of minimal cells with variable shape or composition (Renner and Weibel, 2011; Renner and Weibel, 2012).
Acknowledgments
We are grateful to the members of the Jacobs-Wagner lab for critical reading of the manuscript. We apologize to all authors whose work could not be cited owing to space limitations.
Footnotes
Competing interests
The authors declare no competing interests.
Funding
Work in the Jacobs-Wagner lab is funded by the National Institutes of Health [grant number RO1 GM065835]. G.L. was the recipient of a fellowship from the Research Department of the French-speaking Community of Belgium. C.J.-W is an investigator of the Howard Hughes Medical Institute. Deposited in PMC for release after 12 months.
References
- Ben-Yehuda S., Rudner D. Z., Losick R. (2003). RacA, a bacterial protein that anchors chromosomes to the cell poles. Science 299, 532–536 10.1126/science.1079914 [DOI] [PubMed] [Google Scholar]
- Bernal P., Muñoz-Rojas J., Hurtado A., Ramos J. L., Segura A. (2007). A Pseudomonas putida cardiolipin synthesis mutant exhibits increased sensitivity to drugs related to transport functionality. Environ. Microbiol. 9, 1135–1145 10.1126/science.1079914 [DOI] [PubMed] [Google Scholar]
- Bowman G. R., Comolli L. R., Zhu J., Eckart M., Koenig M., Downing K. H., Moerner W. E., Earnest T., Shapiro L. (2008). A polymeric protein anchors the chromosomal origin/ParB complex at a bacterial cell pole. Cell 134, 945–955 10.1016/j.cell.2008.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman G. R., Comolli L. R., Gaietta G. M., Fero M., Hong S-H., Jones Y., Lee J. H., Downing K. H., Ellisman M. H., McAdams H. H. et al. (2010). Caulobacter PopZ forms a polar subdomain dictating sequential changes in pole composition and function. Mol. Microbiol. 76, 173–189 10.1111/j.1365-2958.2010.07088.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman G. R., Lyuksyutova A. I., Shapiro L. (2011). Bacterial polarity. Curr. Opin. Cell Biol. 23, 71–77 10.1016/j.ceb.2010.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman G. R., Perez A. M., Ptacin J. L., Ighodaro E., Folta-Stogniew E., Comolli L. R., Shapiro L. (2013). Oligomerization and higher-order assembly contribute to sub-cellular localization of a bacterial scaffold. Mol. Microbiol 10.1111/mmi.12398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramkamp M., Emmins R., Weston L., Donovan C., Daniel R. A., Errington J. (2008). A novel component of the division-site selection system of Bacillus subtilis and a new mode of action for the division inhibitor MinCD. Mol. Microbiol. 70, 1556–1569 10.1111/j.1365-2958.2008.06501.x [DOI] [PubMed] [Google Scholar]
- Briley K., Jr, Prepiak P., Dias M. J., Hahn J., Dubnau D. (2011). Maf acts downstream of ComGA to arrest cell division in competent cells of B. subtilis. Mol. Microbiol. 81, 23–39 10.1111/j.1365-2958.2011.07695.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulyha I., Hot E., Huntley S., Søgaard-Andersen L. (2011). GTPases in bacterial cell polarity and signalling. Curr. Opin. Microbiol. 14, 726–733 10.1016/j.mib.2011.09.001 [DOI] [PubMed] [Google Scholar]
- Campos M., Jacobs-Wagner C. (2013). Cellular organization of the transfer of genetic information. Curr. Opin. Microbiol. 16, 171–176 10.1016/j.mib.2013.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charbon G., Brustad E., Scott K. A., Wang J., Løbner-Olesen A., Schultz P. G., Jacobs-Wagner C., Chapman E. (2011). Subcellular protein localization by using a genetically encoded fluorescent amino acid. ChemBioChem 12, 1818–1821 10.1002/cbic.201100282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles M., Pérez M., Kobil J. H., Goldberg M. B. (2001). Polar targeting of Shigella virulence factor IcsA in Enterobacteriacae and Vibrio. Proc. Natl. Acad. Sci. USA 98, 9871–9876 10.1073/pnas.171310498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. C., Hottes A. K., McAdams H. H., McGrath P. T., Viollier P. H., Shapiro L. (2006). Cytokinesis signals truncation of the PodJ polarity factor by a cell cycle-regulated protease. EMBO J. 25, 377–386 10.1038/sj.emboj.7600935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu S-W., Roberts M. A. J., Leake M. C., Armitage J. P. (2013). Positioning of chemosensory proteins and FtsZ through the Rhodobacter sphaeroides cell cycle. Mol. Microbiol. 90, 322–33710.1111/mmi.12366 10.1111/mmi.12366 [DOI] [PubMed] [Google Scholar]
- Coquel A-S., Jacob J-P., Primet M., Demarez A., Dimiccoli M., Julou T., Moisan L., Lindner A. B., Berry H. (2013). Localization of protein aggregation in Escherichia coli is governed by diffusion and nucleoid macromolecular crowding effect. PLOS Comput. Biol. 9, e1003038 10.1371/journal.pcbi.1003038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin B. D., Yu X-C., Margolin W. (2002). Exploring intracellular space: function of the Min system in round-shaped Escherichia coli. EMBO J. 21, 1998–2008 10.1093/emboj/21.8.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cytrynbaum E. N., Marshall B. D. L. (2007). A multistranded polymer model explains MinDE dynamics in E. coli cell division. Biophys. J. 93, 1134–1150 10.1529/biophysj.106.097162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis B. M., Waldor M. K. (2013). Establishing polar identity in gram-negative rods. Microbiol (in press) 10.1016/j.mib.2013.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Pedro M. A., Quintela J. C., Höltje J. V., Schwarz H. (1997). Murein segregation in Escherichia coli. J. Bacteriol. 179, 2823–2834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Ventura B., Sourjik V. (2011). Self-organized partitioning of dynamically localized proteins in bacterial cell division. Mol. Syst. Biol. 7, 457 10.1038/msb.2010.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Ventura B., Knecht B., Andreas H., Godinez W. J., Fritsche M., Rohr K., Nickel W., Heermann D. W., Sourjik V. (2013). Chromosome segregation by the Escherichia coli Min system. Mol. Syst. Biol. 9, 686 10.1038/msb.2013.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditkowski B., Holmes N., Rydzak J., Donczew M., Bezulska M., Ginda K., Kedzierski P., Zakrzewska-Czerwińska J., Kelemen G. H., Jakimowicz D. (2013). Dynamic interplay of ParA with the polarity protein, Scy, coordinates the growth with chromosome segregation in Streptomyces coelicolor. Open Biol. 3, 130006–130006 10.1098/rsob.130006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- dos Santos V. T., Bisson-Filho A. W., Gueiros-Filho F. J. (2012). DivIVA-mediated polar localization of ComN, a posttranscriptional regulator of Bacillus subtilis. J. Bacteriol. 194, 3661–3669 10.1128/JB.05879-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew D. A., Osborn M. J., Rothfield L. I. (2005). A polymerization-depolymerization model that accurately generates the self-sustained oscillatory system involved in bacterial division site placement. Proc. Natl. Acad. Sci. USA 102, 6114–6118 10.1073/pnas.0502037102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkin J. (2009). Cellular polarity in prokaryotic organisms. Cold Spring Harb. Perspect. Biol. 1, a003368 10.1101/cshperspect.a003368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebersbach G., Jacobs-Wagner C. (2007). Exploration into the spatial and temporal mechanisms of bacterial polarity. Trends Microbiol. 15, 101–108 10.1016/j.tim.2007.01.004 [DOI] [PubMed] [Google Scholar]
- Ebersbach G., Briegel A., Jensen G. J., Jacobs-Wagner C. (2008). A self-associating protein critical for chromosome attachment, division, and polar organization in caulobacter. Cell 134, 956–968 10.1016/j.cell.2008.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards D. H., Thomaides H. B., Errington J. (2000). Promiscuous targeting of Bacillus subtilis cell division protein DivIVA to division sites in Escherichia coli and fission yeast. EMBO J. 19, 2719–2727 10.1093/emboj/19.11.2719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eswaramoorthy P., Erb M. L., Gregory J. A., Silverman J., Pogliano K., Pogliano J., Ramamurthi K. S. (2011). Cellular architecture mediates DivIVA ultrastructure and regulates Min activity in Bacillus subtilis. mBio 2, e00257–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Fernandez C., Grosse K., Sourjik V., Collier J. (2013). The β-sliding clamp directs the localization of HdaA to the replisome in Caulobacter crescentus. Microbiology 10.1099/mic.0.068577-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flärdh K. (2003). Essential role of DivIVA in polar growth and morphogenesis in Streptomyces coelicolor A3(2). Mol. Microbiol. 49, 1523–1536 10.1046/j.1365-2958.2003.03660.x [DOI] [PubMed] [Google Scholar]
- García-del Portillo F., Calvo E., D'Orazio V., Pucciarelli M. G. (2011). Association of ActA to peptidoglycan revealed by cell wall proteomics of intracellular Listeria monocytogenes. J. Biol. Chem. 286, 34675–34689 10.1074/jbc.M111.230441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerding M. A., Ogata Y., Pecora N. D., Niki H., de Boer P. A. J. (2007). The trans-envelope Tol-Pal complex is part of the cell division machinery and required for proper outer-membrane invagination during cell constriction in E. coli. Mol. Microbiol. 63, 1008–1025 10.1111/j.1365-2958.2006.05571.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg M. B., Bârzu O., Parsot C., Sansonetti P. J. (1993). Unipolar localization and ATPase activity of IcsA, a Shigella flexneri protein involved in intracellular movement. J. Bacteriol. 175, 2189–2196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goley E. D., Yeh Y-C., Hong S-H., Fero M. J., Abeliuk E., McAdams H. H., Shapiro L. (2011). Assembly of the Caulobacter cell division machine. Mol. Microbiol. 80, 1680–1698 10.1111/j.1365-2958.2011.07677.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield D., McEvoy A. L., Shroff H., Crooks G. E., Wingreen N. S., Betzig E., Liphardt J. (2009). Self-organization of the Escherichia coli chemotaxis network imaged with super-resolution light microscopy. PLoS Biol. 7, e1000137 10.1371/journal.pbio.1000137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin B. A., Adams S. R., Tsien R. Y. (1998). Specific covalent labeling of recombinant protein molecules inside live cells. Science 281, 269–272 10.1126/science.281.5374.269 [DOI] [PubMed] [Google Scholar]
- Guberman J. M., Fay A., Dworkin J., Wingreen N. S., Gitai Z. (2008). PSICIC: noise and asymmetry in bacterial division revealed by computational image analysis at sub-pixel resolution. PLOS Comput. Biol. 4, e1000233 10.1371/journal.pcbi.1000233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halatek J., Frey E. (2012). Highly canalized MinD transfer and MinE sequestration explain the origin of robust MinCDE-protein dynamics. Cell Reports 1, 741–752 10.1016/j.celrep.2012.04.005 [DOI] [PubMed] [Google Scholar]
- Hamoen L. W., Errington J. (2003). Polar targeting of DivIVA in Bacillus subtilis is not directly dependent on FtsZ or PBP 2B. J. Bacteriol. 185, 693–697 10.1128/JB.185.2.693-697.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harry E. J., Lewis P. J. (2003). Early targeting of Min proteins to the cell poles in germinated spores of Bacillus subtilis: evidence for division apparatus-independent recruitment of Min proteins to the division site. Mol. Microbiol. 47, 37–48 10.1046/j.1365-2958.2003.03253.x [DOI] [PubMed] [Google Scholar]
- Hempel A. M., Wang S-B., Letek M., Gil J. A., Flärdh K. (2008). Assemblies of DivIVA mark sites for hyphal branching and can establish new zones of cell wall growth in Streptomyces coelicolor. J. Bacteriol. 190, 7579–7583 10.1128/JB.00839-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempel A. M., Cantlay S., Molle V., Wang S-B., Naldrett M. J., Parker J. L., Richards D. M., Jung Y-G., Buttner M. J., Flärdh K. (2012). The Ser/Thr protein kinase AfsK regulates polar growth and hyphal branching in the filamentous bacteria Streptomyces. Proc. Natl. Acad. Sci. USA 109, E2371–E2379 10.1073/pnas.1207409109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes N. A., Walshaw J., Leggett R. M., Thibessard A., Dalton K. A., Gillespie M. D., Hemmings A. M., Gust B., Kelemen G. H. (2013). Coiled-coil protein Scy is a key component of a multiprotein assembly controlling polarized growth in Streptomyces. Proc. Natl. Acad. Sci. USA 110, E397–E406 10.1073/pnas.1210657110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard M., Kruse K. (2005). Cellular organization by self-organization: mechanisms and models for Min protein dynamics. J. Cell Biol. 168, 533–536 10.1083/jcb.200411122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z., Lutkenhaus J. (2001). Topological regulation of cell division in E. coli. spatiotemporal oscillation of MinD requires stimulation of its ATPase by MinE and phospholipid. Mol. Cell 7, 1337–1343 10.1016/S1097-2765(01)00273-8 [DOI] [PubMed] [Google Scholar]
- Hu Z., Gogol E. P., Lutkenhaus J. (2002). Dynamic assembly of MinD on phospholipid vesicles regulated by ATP and MinE. Proc. Natl. Acad. Sci. USA 99, 6761–6766 10.1073/pnas.102059099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z., Saez C., Lutkenhaus J. (2003). Recruitment of MinC, an inhibitor of Z-ring formation, to the membrane in Escherichia coli: role of MinD and MinE. J. Bacteriol. 185, 196–203 10.1128/JB.185.1.196-203.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K. C., Ramamurthi K. S. (2010). Macromolecules that prefer their membranes curvy. Mol. Microbiol. 76, 822–832 10.1111/j.1365-2958.2010.07168.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K. C., Mukhopadhyay R., Wingreen N. S. (2006). A curvature-mediated mechanism for localization of lipids to bacterial poles. PLOS Comput. Biol. 2, e151 10.1371/journal.pcbi.0020151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huitema E., Pritchard S., Matteson D., Radhakrishnan S. K., Viollier P. H. (2006). Bacterial birth scar proteins mark future flagellum assembly site. Cell 124, 1025–1037 10.1016/j.cell.2006.01.019 [DOI] [PubMed] [Google Scholar]
- Iniesta A. A., Hillson N. J., Shapiro L. (2010). Polar remodeling and histidine kinase activation, which is essential for Caulobacter cell cycle progression, are dependent on DNA replication initiation. J. Bacteriol. 192, 3893–3902 10.1128/JB.00468-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jani C., Eoh H., Lee J. J., Hamasha K., Sahana M. B., Han J-S., Nyayapathy S., Lee J-Y., Suh J-W., Lee S. H. et al. (2010). Regulation of polar peptidoglycan biosynthesis by Wag31 phosphorylation in mycobacteria. BMC Microbiol. 10, 327 10.1186/1471-2180-10-327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C-M., Abbott D. W., Park S. T., Dascher C. C., Cantley L. C., Husson R. N. (2005). The Mycobacterium tuberculosis serine/threonine kinases PknA and PknB: substrate identification and regulation of cell shape. Genes Dev. 19, 1692–1704 10.1101/gad.1311105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai F., Shoda M., Harashima R., Sadaie Y., Hara H., Matsumoto K. (2004). Cardiolipin domains in Bacillus subtilis marburg membranes. J. Bacteriol. 186, 1475–1483 10.1101/cshperspect.a006809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick C. L., Viollier P. H. (2011). Poles apart: prokaryotic polar organelles and their spatial regulation. Cold Spring Harb. Perspect. Biol. 3, a006809 10.1101/cshperspect.a006809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick C. L., Viollier P. H. (2012). Decoding Caulobacter development. FEMS Microbiol. Rev. 36, 193–205 10.1111/j.1574-6976.2011.00309.x [DOI] [PubMed] [Google Scholar]
- Kocks C., Gouin E., Tabouret M., Berche P., Ohayon H., Cossart P. (1992). L. monocytogenes-induced actin assembly requires the actA gene product, a surface protein. Cell 68, 521–531 10.1016/0092-8674(92)90188-I [DOI] [PubMed] [Google Scholar]
- Kocks C., Hellio R., Gounon P., Ohayon H., Cossart P. (1993). Polarized distribution of Listeria monocytogenes surface protein ActA at the site of directional actin assembly. J. Cell Sci. 105, 699–710 [DOI] [PubMed] [Google Scholar]
- Kühn J., Briegel A., Mörschel E., Kahnt J., Leser K., Wick S., Jensen G. J., Thanbichler M. (2010). Bactofilins, a ubiquitous class of cytoskeletal proteins mediating polar localization of a cell wall synthase in Caulobacter crescentus. EMBO J. 29, 327–339 10.1038/emboj.2009.358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacayo C. I., Soneral P. A. G., Zhu J., Tsuchida M. A., Footer M. J., Soo F. S., Lu Y., Xia Y., Mogilner A., Theriot J. A. (2012). Choosing orientation: influence of cargo geometry and ActA polarization on actin comet tails. Mol. Biol. Cell 23, 614–629 10.1091/mbc.E11-06-0584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackner L. L., Raskin D. M., de Boer P. A. J. (2003). ATP-dependent interactions between Escherichia coli Min proteins and the phospholipid membrane in vitro. J. Bacteriol. 185, 735–749 10.1128/JB.185.3.735-749.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laloux G., Jacobs-Wagner C. (2013). Spatiotemporal control of PopZ localization through cell cycle-coupled multimerization. J. Cell Biol. 201, 827–841 10.1083/jcb.201303036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam H., Schofield W. B., Jacobs-Wagner C. (2006). A landmark protein essential for establishing and perpetuating the polarity of a bacterial cell. Cell 124, 1011–1023 10.1016/j.cell.2005.12.040 [DOI] [PubMed] [Google Scholar]
- Landgraf D., Okumus B., Chien P., Baker T. A., Paulsson J. (2012). Segregation of molecules at cell division reveals native protein localization. Nat. Methods 9, 480–482 10.1038/nmeth.1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler M. L., Larson D. E., Hinz A. J., Klein D., Brun Y. V. (2006). Dissection of functional domains of the polar localization factor PodJ in Caulobacter crescentus. Mol. Microbiol. 59, 301–316 10.1111/j.1365-2958.2005.04935.x [DOI] [PubMed] [Google Scholar]
- Lenarcic R., Halbedel S., Visser L., Shaw M., Wu L. J., Errington J., Marenduzzo D., Hamoen L. W. (2009). Localisation of DivIVA by targeting to negatively curved membranes. EMBO J. 28, 2272–2282 10.1038/emboj.2009.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz P., Søgaard-Andersen L. (2011). Temporal and spatial oscillations in bacteria. Nat. Rev. Microbiol. 9, 565–577 10.1038/nrmicro2612 [DOI] [PubMed] [Google Scholar]
- Loose M., Fischer-Friedrich E., Ries J., Kruse K., Schwille P. (2008). Spatial regulators for bacterial cell division self-organize into surface waves in vitro. Science 320, 789–792 10.1126/science.1154413 [DOI] [PubMed] [Google Scholar]
- Loose M., Kruse K., Schwille P. (2011). Protein self-organization: lessons from the min system. Annu. Rev. Biophys. 40, 315–336 10.1146/annurev-biophys-042910-155332 [DOI] [PubMed] [Google Scholar]
- Maddock J. R., Shapiro L. (1993). Polar location of the chemoreceptor complex in the Escherichia coli cell. Science 259, 1717–1723 10.1126/science.8456299 [DOI] [PubMed] [Google Scholar]
- Margolin W. (2012). The price of tags in protein localization studies. J. Bacteriol. 194, 6369–6371 10.1128/JB.01640-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K., Kusaka J., Nishibori A., Hara H. (2006). Lipid domains in bacterial membranes. Mol. Microbiol. 61, 1110–1117 10.1111/j.1365-2958.2006.05317.x [DOI] [PubMed] [Google Scholar]
- Meinhardt H., de Boer P. A. (2001). Pattern formation in Escherichia coli: a model for the pole-to-pole oscillations of Min proteins and the localization of the division site. Proc. Natl. Acad. Sci. USA 98, 14202–14207 10.1073/pnas.251216598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mileykovskaya E., Dowhan W. (2000). Visualization of phospholipid domains in Escherichia coli by using the cardiolipin-specific fluorescent dye 10-N-nonyl acridine orange. J. Bacteriol. 182, 1172–1175 10.1529/biophysj.107.126920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mileykovskaya E., Fishov I., Fu X., Corbin B. D., Margolin W., Dowhan W. (2003). Effects of phospholipid composition on MinD-membrane interactions in vitro and in vivo. J. Biol. Chem. 278, 22193–22198 10.1074/jbc.M302603200 [DOI] [PubMed] [Google Scholar]
- Muchová K., Kutejová E., Scott D. J., Brannigan J. A., Lewis R. J., Wilkinson A. J., Barák I. (2002). Oligomerization of the Bacillus subtilis division protein DivIVA. Microbiology 148, 807–813 [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay R., Huang K. C., Wingreen N. S. (2008). Lipid localization in bacterial cells through curvature-mediated microphase separation. Biophys. J. 95, 1034–1049 10.1529/biophysj.107.126920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva M. A., Halbedel S., Freund S. M., Dutow P., Leonard T. A., Veprintsev D. B., Hamoen L. W., Löwe J. (2010). Features critical for membrane binding revealed by DivIVA crystal structure. EMBO J. 29, 1988–2001 10.1038/emboj.2010.99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K-T., Wu W., Battaile K. P., Lovell S., Holyoak T., Lutkenhaus J. (2011). The Min oscillator uses MinD-dependent conformational changes in MinE to spatially regulate cytokinesis. Cell 146, 396–407 10.1016/j.cell.2011.06.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K-T., Wu W., Lovell S., Lutkenhaus J. (2012). Mechanism of the asymmetric activation of the MinD ATPase by MinE. Mol. Microbiol. 85, 271–281 10.1111/j.1365-2958.2012.08110.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick J. E., Kearns D. B. (2008). MinJ (YvjD) is a topological determinant of cell division in Bacillus subtilis. Mol. Microbiol. 70, 1166–1179 10.1111/j.1365-2958.2008.06469.x [DOI] [PubMed] [Google Scholar]
- Ptacin J. L., Lee S. F., Garner E. C., Toro E., Eckart M., Comolli L. R., Moerner W. E., Shapiro L. (2010). A spindle-like apparatus guides bacterial chromosome segregation. Nat. Cell Biol. 12, 791–798 10.1038/ncb2083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishnan S. K., Thanbichler M., Viollier P. H. (2008). The dynamic interplay between a cell fate determinant and a lysozyme homolog drives the asymmetric division cycle of Caulobacter crescentus. Genes Dev. 22, 212–225 10.1101/gad.1601808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafelski S. M., Theriot J. A. (2006). Mechanism of polarization of Listeria monocytogenes surface protein ActA. Mol. Microbiol. 59, 1262–1279 10.1111/j.1365-2958.2006.05025.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamurthi K. S., Losick R. (2009). Negative membrane curvature as a cue for subcellular localization of a bacterial protein. Proc. Natl. Acad. Sci. USA 106, 13541–13545 10.1073/pnas.0906851106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamurthi K. S., Lecuyer S., Stone H. A., Losick R. (2009). Geometric cue for protein localization in a bacterium. Science 323, 1354–1357 10.1126/science.1169218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskin D. M., de Boer P. A. (1999). Rapid pole-to-pole oscillation of a protein required for directing division to the middle of Escherichia coli. Proc. Natl. Acad. Sci. USA 96, 4971–4976 10.1073/pnas.96.9.4971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner L. D., Weibel D. B. (2011). Cardiolipin microdomains localize to negatively curved regions of Escherichia coli membranes. Proc. Natl. Acad. Sci. USA 108, 6264–6269 10.1073/pnas.1015757108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner L. D., Weibel D. B. (2012). MinD and MinE interact with anionic phospholipids and regulate division plane formation in Escherichia coli. J. Biol. Chem. 287, 38835–38844 10.1074/jbc.M112.407817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romantsov T., Helbig S., Culham D. E., Gill C., Stalker L., Wood J. M. (2007). Cardiolipin promotes polar localization of osmosensory transporter ProP in Escherichia coli. Mol. Microbiol. 64, 1455–1465 10.1111/j.1365-2958.2007.05727.x [DOI] [PubMed] [Google Scholar]
- Romantsov T., Battle A. R., Hendel J. L., Martinac B., Wood J. M. (2010). Protein localization in Escherichia coli cells: comparison of the cytoplasmic membrane proteins ProP, LacY, ProW, AqpZ, MscS, and MscL. J. Bacteriol. 192, 912–924 10.1128/JB.00967-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudner D. Z., Losick R. (2010). Protein subcellular localization in bacteria. Cold Spring Harb. Perspect. Biol. 2, a000307 10.1101/cshperspect.a000307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saberi S., Emberly E. (2010). Chromosome driven spatial patterning of proteins in bacteria. PLOS Comput. Biol. 6, e1000986 10.1371/journal.pcbi.1000986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saberi S., Emberly E. (2013). Non-equilibrium polar localization of proteins in bacterial cells. PLoS ONE 8, e64075 10.1371/journal.pone.0064075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield W. B., Lim H. C., Jacobs-Wagner C. (2010). Cell cycle coordination and regulation of bacterial chromosome segregation dynamics by polarly localized proteins. EMBO J. 29, 3068–3081 10.1038/emboj.2010.207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro L., McAdams H. H., Losick R. (2009). Why and how bacteria localize proteins. Science 326, 1225–1228 10.1126/science.1175685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shebelut C. W., Guberman J. M., van Teeffelen S., Yakhnina A. A., Gitai Z. (2010). Caulobacter chromosome segregation is an ordered multistep process. Proc. Natl. Acad. Sci. USA 107, 14194–14198 10.1073/pnas.1005274107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih Y-L., Zheng M. (2013). Spatial control of the cell division site by the Min system in Escherichia coli. Environ. Microbiol 10.1111/1462-2920.12119 [DOI] [PubMed] [Google Scholar]
- Sliusarenko O., Heinritz J., Emonet T., Jacobs-Wagner C. (2011). High-throughput, subpixel precision analysis of bacterial morphogenesis and intracellular spatio-temporal dynamics. Mol. Microbiol. 80, 612–627 10.1111/j.1365-2958.2011.07579.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sourjik V., Armitage J. P. (2010). Spatial organization in bacterial chemotaxis. EMBO J. 29, 2724–2733 10.1038/emboj.2010.178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahlberg H., Kutejová E., Muchová K., Gregorini M., Lustig A., Müller S. A., Olivieri V., Engel A., Wilkinson A. J., Barák I. (2004). Oligomeric structure of the Bacillus subtilis cell division protein DivIVA determined by transmission electron microscopy. Mol. Microbiol. 52, 1281–1290 10.1111/j.1365-2958.2004.04074.x [DOI] [PubMed] [Google Scholar]
- Steinhauer J., Agha R., Pham T., Varga A. W., Goldberg M. B. (1999). The unipolar Shigella surface protein IcsA is targeted directly to the bacterial old pole: IcsP cleavage of IcsA occurs over the entire bacterial surface. Mol. Microbiol. 32, 367–377 10.1046/j.1365-2958.1999.01356.x [DOI] [PubMed] [Google Scholar]
- Swulius M. T., Jensen G. J. (2012). The helical MreB cytoskeleton in Escherichia coli MC1000/pLE7 is an artifact of the N-Terminal yellow fluorescent protein tag. J. Bacteriol. 194, 6382–6386 10.1128/JB.00505-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanbichler M., Shapiro L. (2006). MipZ, a spatial regulator coordinating chromosome segregation with cell division in Caulobacter. Cell 126, 147–162 10.1016/j.cell.2006.05.038 [DOI] [PubMed] [Google Scholar]
- Thiem S., Sourjik V. (2008). Stochastic assembly of chemoreceptor clusters in Escherichia coli. Mol. Microbiol. 68, 1228–1236 10.1111/j.1365-2958.2008.06227.x [DOI] [PubMed] [Google Scholar]
- Thiem S., Kentner D., Sourjik V. (2007). Positioning of chemosensory clusters in E. coli and its relation to cell division. EMBO J. 26, 1615–1623 10.1038/sj.emboj.7601610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Teeffelen S., Shaevitz J. W., Gitai Z. (2012). Image analysis in fluorescence microscopy: bacterial dynamics as a case study. Bioessays 34, 427–436 10.1002/bies.201100148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma A., Huang K. C., Young K. D. (2008). The Min system as a general cell geometry detection mechanism: branch lengths in Y-shaped Escherichia coli cells affect Min oscillation patterns and division dynamics. J. Bacteriol. 190, 2106–2117 10.1128/JB.00720-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viollier P. H., Sternheim N., Shapiro L. (2002). Identification of a localization factor for the polar positioning of bacterial structural and regulatory proteins. Proc. Natl. Acad. Sci. USA 99, 13831–13836 10.1073/pnas.182411999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Wingreen N. S., Mukhopadhyay R. (2008). Self-organized periodicity of protein clusters in growing bacteria. Phys. Rev. Lett. 101, 218101 10.1103/PhysRevLett.101.218101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S-B., Cantlay S., Nordberg N., Letek M., Gil J. A., Flärdh K. (2009). Domains involved in the in vivo function and oligomerization of apical growth determinant DivIVA in Streptomyces coelicolor. FEMS Microbiol. Lett. 297, 101–109 10.1111/j.1574-6968.2009.01678.x [DOI] [PubMed] [Google Scholar]
- Winkler J., Seybert A., König L., Pruggnaller S., Haselmann U., Sourjik V., Weiss M., Frangakis A. S., Mogk A., Bukau B. (2010). Quantitative and spatio-temporal features of protein aggregation in Escherichia coli and consequences on protein quality control and cellular ageing. EMBO J. 29, 910–923 10.1038/emboj.2009.412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth S. E., Krywy J. A., Aldridge B. B., Fortune S. M., Fernandez-Suarez M., Gray T. A., Derbyshire K. M. (2012). Polar assembly and scaffolding proteins of the virulence-associated ESX-1 secretory apparatus in mycobacteria. Mol. Microbiol. 83, 654–664 10.1111/j.1365-2958.2011.07958.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L. J., Errington J. (2003). RacA and the Soj-Spo0J system combine to effect polar chromosome segregation in sporulating Bacillus subtilis. Mol. Microbiol. 49, 1463–1475 10.1046/j.1365-2958.2003.03643.x [DOI] [PubMed] [Google Scholar]
- Yamaichi Y., Bruckner R., Ringgaard S., Möll A., Cameron D. E., Briegel A., Jensen G. J., Davis B. M., Waldor M. K. (2012). A multidomain hub anchors the chromosome segregation and chemotactic machinery to the bacterial pole. Genes Dev. 26, 2348–2360 10.1101/gad.199869.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh Y-C., Comolli L. R., Downing K. H., Shapiro L., McAdams H. H. (2010). The caulobacter Tol-Pal complex is essential for outer membrane integrity and the positioning of a polar localization factor. J. Bacteriol. 192, 4847–4858 10.1128/JB.00607-10 [DOI] [PMC free article] [PubMed] [Google Scholar]



