ABSTRACT
We have previously shown that non-mammary and tumorigenic cells can respond to the signals of the mammary niche and alter their cell fate to that of mammary epithelial progenitor cells. Here we tested the hypothesis that paracrine signals from mammary epithelial cells expressing progesterone receptor (PR) are dispensable for redirection of testicular cells, and that re-directed wild-type testicular-derived mammary cells can rescue lobulogenesis of PR-null mammary epithelium by paracrine signaling during pregnancy. We injected PR-null epithelial cells mixed with testicular cells from wild-type adult male mice into cleared fat-pads of recipient mice. The testicular cells were redirected in vivo to mammary epithelial cell fate during regeneration of the mammary epithelium, and persisted in second-generation outgrowths. In the process, the redirected testicular cells rescued the developmentally deficient PR-null cells, signaling them through the paracrine factor RANKL to produce alveolar secretory structures during pregnancy. This is the first demonstration that paracrine signaling required for alveolar development is not required for cellular reprogramming in the mammary gland, and that reprogrammed testicular cells can provide paracrine signals to the surrounding mammary epithelium.
KEY WORDS: Progesterone receptor, RANKL, Mammary, Cellular reprogramming
INTRODUCTION
Previous studies have demonstrated the remarkable ability of the mouse mammary microenvironment to control cell fate determination and stem/progenitor cell function and identity. Exogenous cell types can compete to be incorporated into mammary niches during gland regeneration in vivo, resulting in the adoption of a mammary cell progenitor cell fate by the exogenous cell types (Boulanger et al., 2007; Booth et al., 2008; Bussard et al., 2010; Boulanger et al., 2012; Bruno and Smith, 2012; Boulanger et al., 2013). By co-inoculating non-mammary or tumorigenic cell types with normal mammary epithelial cells (MECs), we have demonstrated that testicular cells (Boulanger et al., 2007), neuronal stem cells (Booth et al., 2008), Lin– bone marrow cells (Boulanger et al., 2012), embryonic stem cells (Boulanger et al., 2013), as well as human (Bussard et al., 2010) and mouse (Booth et al., 2011) cancer cells can all be reprogrammed in the developing mammary gland microenvironment. In addition, a wild-type mammary microenvironment can restore the function of mammary epithelial lobule progenitors from Wap-Int3 transgenic glands, which do not develop secretory alveoli during pregnancy (Bruno et al., 2012). In all cases, reprogrammed and/or rescued cells were capable of contributing to lobules during pregnancy and were present in secondary outgrowths from chimeric mammary fragments. Together, these studies demonstrate that mammary epithelial cells in the context of the cleared mammary fat-pad are capable of producing the signals necessary to rescue and/or reprogram non-mammary and cancer cells that are otherwise unable to carryout normal mammary development on their own.
Ductal side-branching and alveolar development during pregnancy require activation of the progesterone receptor (PR) by the hormone progesterone, although PR is not required for the production of some milk proteins (Tsai and O'Malley, 1994; Lydon et al., 1995; Lydon et al., 1999). Brisken and co-workers demonstrated that when PR-knockout (PRKO) MECs are transplanted into the cleared mammary fat-pads of wild-type mice, the cells undergo normal ductal elongation and development, but fail to undergo complete alveolar development during pregnancy (Brisken et al., 1998). However, alveologenesis was rescued in PRKO cells when they were transplanted along with wild-type mammary epithelium into the cleared fat-pads of wild-type hosts. Both PRKO and wild-type cells contributed to lobular development during pregnancy, demonstrating that paracrine signals from the wild-type epithelium were sufficient to rescue alveologenesis in PR-null epithelium (Brisken et al., 1998).
Induction of alveologenesis by progesterone activation of PR is mediated by the ligand RANKL (receptor activator nuclear factor κ ligand) (Fata et al., 2000; Beleut et al., 2010). RANKL is a paracrine factor that induces the expression of the transcription factor Elf5 in nearby epithelium (Lee et al., 2013). This results in a mutually exclusive expression pattern of Elf5 and PR, with the former cells expanding and differentiating into mature secretory epithelium.
Ismail and colleagues described the generation of the PR-LacZ knock-in mouse, in which the LacZ reporter gene is targeted in-frame into exon1 of the PR gene (Ismail et al., 2002). Mice homozygous for the transgene (referred to here as PRKO-LacZ) do not express PR protein, but do express a nuclear-localized β-galactosidase enzyme (β-Gal) in cells that have transactivated the PR promoter. Here, we mixed PRKO-LacZ MECs with wild-type testicular cells to determine if PR-null MECs were capable of reprogramming non-mammary cells in the absence of PR signaling. Furthermore, we hypothesized that reprogrammed wild-type testicular cells would differentiate to produce PR-positive epithelium and rescue lobulogenesis in the chimeric gland. Our results demonstrate that PR expression is not required to redirect wild-type testicular cells to mammary cell fates including secretory development, and further that the reprogrammed testis-derived cells are able to support lobular development in the PR-null epithelium.
RESULTS AND DISCUSSION
Progesterone receptor is not expressed in the seminiferous tubules
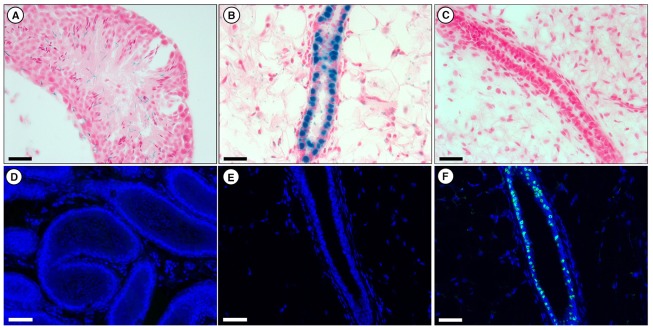
To determine whether PR is expressed in any cells within the seminiferous tubules, we examined both 5-bromo-4-chloro-3-indolyl-b-D-galactopyranoside (X-gal)-stained PRKO-LacZ as well as anti-PR-stained wild-type tissues (Fig. 1). PRKO-LacZ mice express a nuclear localized β-Gal from the endogenous PR promoter locus, so promoter activation results in positive (blue) X-gal-stained nuclei (Ismail et al., 2002). As shown in Fig. 1A, no PR activation was detected in the seminiferous tubules of PRKO-LacZ mice. Conversely, we confirmed previous findings (Ismail et al., 2002) that PRKO-LacZ mammary glands contain several evenly distributed β-Gal-positive luminal epithelial cells, demonstrating the transactivation of the PR promoter in the PR-null cell types (Fig. 1B). In addition, no reactivity with an anti-PR antibody was seen in cross-sections of wild-type seminiferous tubules or mammary outgrowths derived from PRKO-LacZ MECs (Fig. 1D,E). As expected, wild-type glands were negative for X-gal stain and contained several PR-positive epithelial cells identified by an anti-PR antibody (Fig. 1C,F). These results confirmed there was no PR expression</emph> in seminiferous tubule cells prior to reprogramming, and therefore any PR expression in subsequent experiments would be the result of de novo activation of the PR promoter.
Fig. 1.
PR expression in PRKO-LacZ and wild-type mammary and seminiferous tubules. (A–C) X-gal-stained (blue) cross sections of seminiferous tubules of PRKO-LacZ mouse (A), PRKO-LacZ mammary tissue (B) and wild-type mammary tissue (C). Sections are counterstained with Nuclear Fast Red. Scale bars: 100 µM. (D–F) Anti-PR-stained (green) cross-sections of wild-type seminiferous tubules (D), PRKO-LacZ mammary tissue (E) and wild-type mammary tissue (F). Sections are counterstained with DAPI. Scale bars: 200 µM.
Redirected testicular cells rescue lobulogenesis of PRKO MECs
We next asked whether testicular cells could be reprogrammed by MECs that lacked PR signaling. To test this, wild-type testicular cells were mixed with PRKO-LacZ MECs in a 1∶1 ratio (5×104:5×104) and inoculated into cleared mammary fat-pads of athymic nude mice (Table 1; Fig. 2). After recovery from surgery, the mice were mated and glands were recovered at parturition. As expected, wild-type MECs underwent complete alveolar development (Fig. 2A,B), testicular cells failed to grow in the cleared fat-pad (Fig. 2C,D), and PRKO-LacZ MECs grew but failed to undergo complete lobular development (Fig. 2E,F). However, when 5×104 testicular cells were mixed with 5×104 PRKO-LacZ MECs, 50% of the resulting outgrowths demonstrated increased alveolar formation (Fig. 2G,H; Table 1). The rescue of alveologenesis in the chimeric glands was incomplete compared with that in wild-type controls, but was markedly increased above that seen with PRKO-LacZ cells alone, which failed to develop any mature lobules. The presence of male cells in the chimeric gland was confirmed by PCR detection of the Y chromosome (Fig. 2I).
Table 1. Summary of the transplantation results of inoculations of dispersed wild-type MECs, PRKO-LacZ MECs, wild-type testicular cells and PRKO-LacZ plus wild-type testicular cells.

Results are given as the number of mammary outgrowths observed in whole mounts over the number of total glands inoculated.
Numbers given are the number of glands observed to have extensive lobular development in whole mounts and sections of glands taken at parturition over the total number of glands observed at parturition.
Fig. 2.
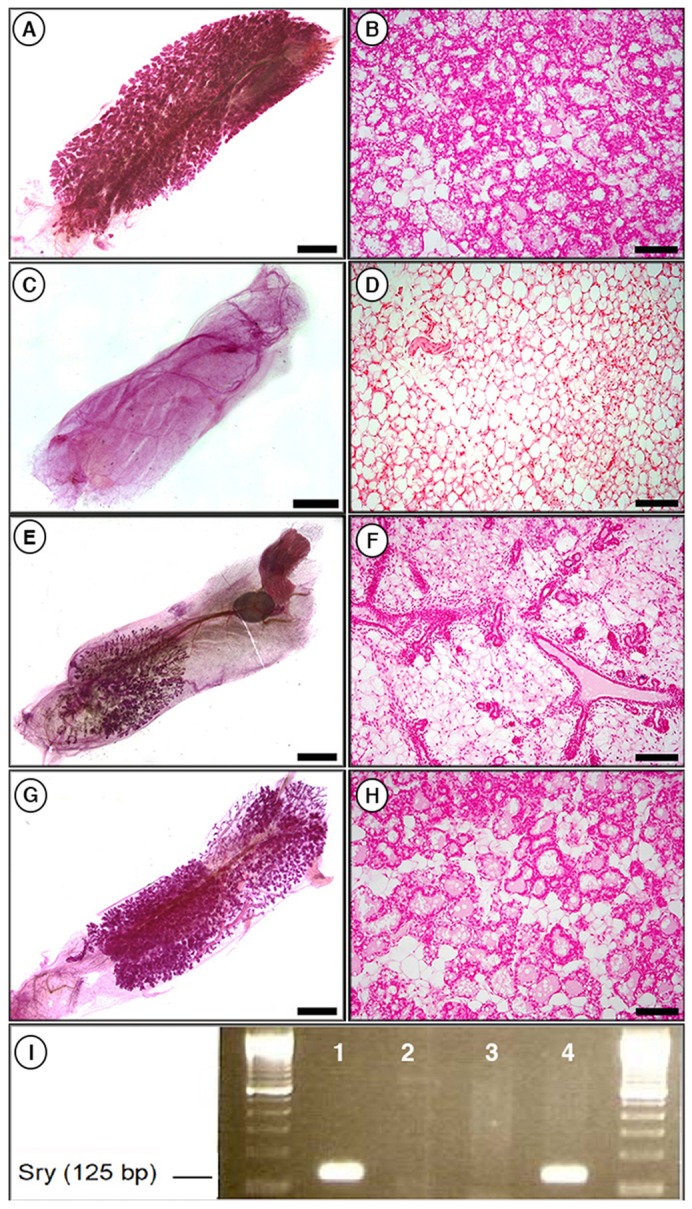
Wild-type testicular cells rescue alveologenesis when mixed with PRKO MECs. (A,B) Whole-mount (A) and cross-section (B) of a transplant of 5×104 wild-type MECs taken at parturition showing full normal lobule development. (C,D) Whole mount (C) and cross section (D) of a transplant of 5×104 testicular cells taken at parturition showing that testicular cells do not grow when transplanted into a cleared fat-pad on their own. (E,F) Whole mount (E) and cross section (F) of a transplant of 5×104 PRKO-LacZ MECs taken at parturition demonstrating a lack of alveolar development in the absence of PR. (G,H) Whole mount (G) and cross section (H) of a transplant of 5×104 PRKO-LacZ MECs and 5×104 wild-type testicular cells taken at parturition demonstrating partial rescue of alveologenesis in the chimeric gland. Whole mounts are stained with Carmine Alum; cross sections with Nuclear Fast Red. Scale bars: 2 mm (A,C,E,G); 400 µM (B,D,F,H). (I) PCR for the presence of Y chromosome (Sry) in DNA isolated from testicular cells (lane 1), wild-type MEC outgrowth (lane 2), PRKO MEC outgrowth (lane3) and chimeric outgrowth of 5×104 testicular cells and 5×104 PRKO MECs (lane 4), demonstrating the presence of male cells in the rescued chimeric outgrowth.
Cells derived from the testes produce PR-positive mammary epithelial cells
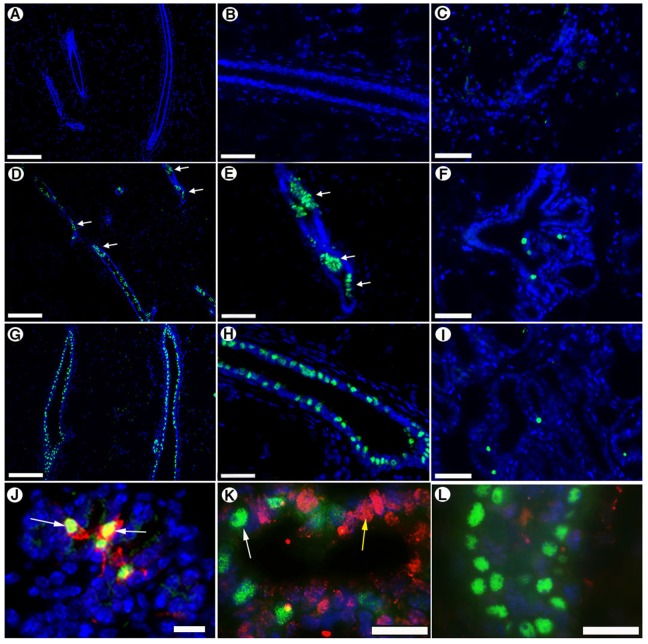
Next, we determined whether testicular-derived cells had differentiated into PR-positive epithelium. As expected, outgrowths derived from the inoculation of 5×104 PRKO-LacZ cells alone contained no PR-positive epithelium in virgin (Fig. 3A,B) or full-term pregnant glands (Fig. 3C). However, chimeric outgrowths derived from a mixture of 5×104 PRKO-LacZ MECs and 5×104 wild-type testicular cells contained PR-positive epithelium in both virgin (Fig. 3D,E) and lactating tissue (Fig. 3F). As the testicular-derived cells are the only cells with the capacity to express PR, these PR-positive cells must be derived from the redirected testicular cells.
Fig. 3.
Reprogrammed testicular cells express PR in first- and second-generation chimeric outgrowths. Cross sections stained with anti-PR antibody (green) of mammary outgrowths from inoculation of 5×104 PRKO-LacZ cells (A–C), 5×104 testicular cells and 50,000 PRKO-LacZ MECs (D–F), and 5×104 wild-type MECs (G–I). (A,B,D,E,G,H) non-pregnant glands; (C,F,I) glands taken at parturition. (B,E,H) Higher-magnification images of the same glands in A,D and G, respectively. Note the clumped expression pattern (white arrows) seen in the chimeric outgrowths (D,E) compared with the even distribution of PR cells seen in wild-type controls (G,H). (J) Second-generation outgrowths generated by transplantation of tissue fragments from chimeric sample shown in D and E excised at day 7 of pregnancy and stained for PR (green) and RANKL (red) demonstrating PR-expressing testicular-derived cells also express RANKL (white arrows). (K) The same second-generation outgrowth shown in J stained for PR (green) and Elf5 (red) demonstrating Elf5 expression in epithelial cells (yellow arrow) surrounding the PR-positive epithelium but not in the PR-positive epithelium themselves (white arrow). (L) PR (green) and Elf5 (red) staining in a nulliparous gland. All sections are counterstained with DAPI. Scale bars: 400 µM (A,D,G); 100 µM (B,C,E,F,H–J); 40 µM (K,L).
Interestingly, the testicular-derived PR-positive cells in virgin chimeric glands often appeared in a clumped pattern (Fig. 3D,E, white arrows) not typically seen in outgrowths from wild-type tissues (Fig. 3G,H), which display more evenly distributed PR-positive epithelium. We interpret this result to mean that testicular cells compete with cells within the PRKO-LacZ MEC population for the occupation of niches during gland regeneration, and that PR-positive cells only occur in regions where a testicular cell occupied a mammary progenitor niche. The abundant expression of nearly all the cells within that niche might be a compensation for the lack of PR signaling in the adjacent microenvironments, which are generated by PRKO-LacZ-derived cell(s).
To determine if reprogrammed testicular-derived cells would self-renew and contribute progeny to secondary outgrowths, we transplanted tissue fragments from chimeric outgrowths into cleared mammary fat-pads of 3-week-old nu/nu mice. PR-positive MECs were detected in secondary outgrowths in both nulliparous and pregnant hosts (Fig. 3J,K). We determined that 40–50% of luminal MECs in both primary and secondary outgrowths were PR-positive, suggesting that reprogrammed testicular-derived cells were capable of self-renewing and maintaining the same population numbers in secondary chimeric outgrowths.
Paracrine signaling through RANKL in PR-positive testicular-derived epithelium activates Elf5 expression in adjacent epithelium
To determine whether testicular-derived PR-positive cells induced secretory differentiation in adjacent cell types, we stained sections from secondary outgrowths taken from hosts that had been pregnant for 7 days for PR, RANKL and Elf5 expression (Fig. 3J–L). Consistent with previous reports in normal mammary tissues (Fata et al., 2000; Beleut et al., 2010), we found that essentially all PR-positive MECs expressed RANKL during pregnancy (Fig. 3J). Furthermore, Elf5 was expressed in adjacent epithelium that was mutually exclusive to PR-positive epithelium (Fig. 3K) at day 7 of pregnancy. Elf5 was barely detected in nulliparous mammary glands (Fig. 3L). Therefore, the PR-positive cells, which could only be derived from the wild-type testicular cells, expressed PR, resulting in the expression of the downstream effector RANKL. On the basis of previous reported findings in normal mouse mammary tissue (Lee et al., 2013) and the observed staining pattern, we infer that RANKL mediated the rescue of alveolar development through induction of Elf5 in adjacent epithelium. This provides direct evidence that testicular-derived mammary epithelium can express PR, and signal through the receptor in the normal paracrine signaling network to induce secretory differentiation and alveolar development.
The results outlined here shed light on how reprogramming within the mammary gland occurs, and demonstrate for the first time that reprogrammed cells can be used to rescue an inhibited developmental function in mammary epithelium. We have previously shown that a functional mouse mammary microenvironment (niche) can rescue the function of Wap-Int3 alveolar progenitors (Bruno et al., 2012), as well as mammary progenitor function of MMTV-Erb2 tumor cells (Booth et al., 2011). However, here we show that redirected non-mammary cells can express PR despite its absence in the reprogramming mammary epithelial population. Paracrine signaling through RANKL by the reprogrammed testicular cells results in the development of secretory alveoli by the null epithelium. It is clear from these results that PR signaling and lobulogenesis are not required for testicular cell reprogramming.
Our results suggest that the occupation of mammary niches by testicular cells during end bud formation and ductal elongation (which does not require PR signaling) results in the cellular reprogramming of the testicular cells. Once in the niche, these cells are redirected to a mammary epithelial cell fate and can generate functional PR-positive epithelial cells, which, in turn, provide the signals necessary for lobulogenesis (e.g. RANKL) when progesterone is released during pregnancy. We hypothesize that the random occupation of niches by the testicular cells results in the uneven distribution of PR-positive epithelium in primary chimeric outgrowths (Fig. 3). This uneven distribution might account for the lack of complete alveologenesis seen in some chimeric glands (Fig. 2G,H). The presence of PR-positive cells throughout the chimeric glands suggests that some testicular cells are as efficient as wild-type epithelium at occupying reforming mammary niches. Previous studies showed that reprogrammed non-mammary cells contributed to lobulogenesis and milk protein production during pregnancy (Boulanger et al., 2007; Booth et al., 2008; Bussard et al., 2010). Milk protein production was not analyzed here because previous studies have shown expression of milk proteins in PRKO tissues during pregnancy (Lydon et al., 1999). The present results demonstrate that cellular reprogramming of testicular cells occurs during ductal elongation in the mammary gland and is sufficient to endow the reprogrammed cells with the capacity to support alveolar development.
MATERIALS AND METHODS
Mice
Female Nu/Nu/NCR mice were used as hosts for the transplantation studies. PRKO-lacZ mice have been described previously (Ismail et al., 2002; Ismail et al., 2003). All mice were housed in Association for Assessment and Accreditation of Laboratory Animal Care-accredited facilities in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The National Cancer Institute Animal Care and Use Committee approved all experimental procedures.
Mammary epithelial and testicular cell dissociation
Mammary glands were dissociated with 0.1% collagenase overnight at 37°C. The resulting organoids were cultured on plastic culture flasks in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10% fetal bovine serum, insulin (1.0 µg/ml), and epidermal growth factor (10 ng/ml). MECs were collected after 4–7 days; fibroblasts were reduced before collection of the epithelial cells by differential trypsinization. Testicular cells were isolated as described previously (Boulanger et al., 2007).
Mammary fat-pad clearing and cellular inoculation
The surgical techniques used to clear the mammary epithelium from the fat-pads of 3-week-old host mice and the subsequent transplantation of cell suspensions have been described in detail previously (Boulanger et al., 2007). In brief, the mice were anesthetized, and the clearing procedure was performed immediately before the insertion of transplanted tissue fragments or cell suspensions. Cell suspensions were implanted in 10 µl volumes of non-supplemented DMEM with a Hamilton syringe equipped with a 30 gauge needle.
X-gal staining of mammary and testicular tissues
Glands and testes were fixed in paraformaldehyde (4.0%) for 2 hours, permeabilized in 0.02% Nonidet P-40, 0.01% sodium deoxycholate and 0.002 M MgCl2 in phosphate-buffered saline overnight at 4°C, and then processed for X-gal as described previously (Wagner et al., 1997).
Mammary whole mounts and immunofluorescence
For immunohistochemical examinations, glands were fixed for 24 hours in 4% paraformaldehyde, embedded in paraffin and sectioned at 6.0 µm. Primary antibodies used were rabbit anti-PR (1∶150; Dako, Carpinteria, CA) goat anti-RANKL antibody (1 µg/ml; R&D Systems, Minneapolis, MN), and goat anti-Elf5 (N-20) (1∶75; Santa Cruz Biotechnology, Dallas, TX). PR was imaged with an Alexa-Fluor-488-conjugated goat-anti rabbit IgG Ab (cat. no. A11008, Invitrogen, Carlsbad, CA). RANKL was imaged using the Vectastain ABC (rabbit anti-goat) kit (Vector Laboratories, Burlingame, CA) followed by incubation with an Alexa Fluor 568 tyramide substrate (Invitrogen). Elf5 was imaged using horse biotinylated anti-goat antibody (Vector Laboratories, Burlingame, CA) followed by incubation with Streptavidin–Alexa-Fluor-594 conjugate. For co-staining, the rabbit anti-goat secondary antibody used to detect RANKL was added prior to the addition of the goat-anti rabbit antibody used to detect PR to avoid cross-reaction. Antigen retrieval was performed by heating sections in boiling water bath for 20 minutes in pH 6.0 citrate buffer with 0.05% Tween 20 or pH 9.0 Tris-EDTA buffer with 0.05% Tween 20. All sections were counter-stained with DAPI. For whole mounts, glands were post-fixed in Carnoy's Fixative (60% methanol, 30% chloroform, 10% glacial acetic acid) for 6 hours, stained with carmine alum and dehydrated with 100% ethanol and xylenes. Sections from whole mounts were generated as above and counterstained with nuclear fast red.
DNA isolation and PCR detection of Y chromosomes
DNA was isolated from whole mounts using Qiagen DNeasy kit (cat. no. 69506; Valencia, CA). PCR analysis for detection of the Y-chromosome was performed as described by Boulanger and colleagues (Boulanger et al., 2007).
Footnotes
Competing interests
The authors declare no competing interests.
Author contributions
R.D.B. contributed to the animal experiments, immunochemistry, design of the experiments and wrote the manuscript. C.A.B. contributed to the animal experiments, design of the experimental procedures and contributed to the writing of the manuscript. S.M.R. contributed to the immunohistological detection of Elf5 and PR in second-generation outgrowths. L.H.A. contributed to the animal experiments and the PCR detection of the Y chromosome. J.P.L. contributed to the design of the experiment and the collection of the PRKO-LacZ tissues. G.H.S. conceived of the experiments, contributed to their design and to the writing of the manuscript.
Funding
This work was funded by the National Cancer Institute Center for Cancer Research and National Institutes of Health [grant numbers NICHD: U54 HD-0077495 and NCI: CA-77530 to J.P.L.]. Deposited in PMC for release after 12 months.
References
- Beleut M., Rajaram R. D., Caikovski M., Ayyanan A., Germano D., Choi Y., Schneider P., Brisken C. (2010). Two distinct mechanisms underlie progesterone-induced proliferation in the mammary gland. Proc. Natl. Acad. Sci. USA 107, 2989–2994 10.1073/pnas.0915148107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth B. W., Mack D. L., Androutsellis-Theotokis A., McKay R. D., Boulanger C. A., Smith G. H. (2008). The mammary microenvironment alters the differentiation repertoire of neural stem cells. Proc. Natl. Acad. Sci. USA 105, 14891–14896 10.1073/pnas.0803214105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth B., Boulanger C., Anderson L., Smith G. (2011). The normal mammary microenvironment suppresses the tumorigenic phenotype of mouse mammary tumor virus-neu-transformed mammary tumor cells. Oncogene 30, 679–689 10.1038/onc.2010.439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulanger C. A., Mack D. L., Booth B. W., Smith G. H. (2007). Interaction with the mammary microenvironment redirects spermatogenic cell fate in vivo. Proc. Natl. Acad. Sci. USA 104, 3871–3876 10.1073/pnas.0611637104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulanger C. A., Bruno R. D., Rosu-Myles M., Smith G. H. (2012). The mouse mammary microenvironment redirects mesoderm-derived bone marrow cells to a mammary epithelial progenitor cell fate. Stem Cells Dev. 21, 948–954 10.1089/scd.2011.0148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulanger C. A., Bruno R. D., Mack D. L., Gonzales M., Castro N. P., Salomon D. S., Smith G. H. (2013). Embryonic stem cells are redirected to non-tumorigenic epithelial cell fate by interaction with the mammary microenvironment. PLoS ONE 8, e62019 10.1371/journal.pone.0062019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisken C., Park S., Vass T., Lydon J. P., O'Malley B. W., Weinberg R. A. (1998). A paracrine role for the epithelial progesterone receptor in mammary gland development. Proc. Natl. Acad. Sci. USA 95, 5076–5081 10.1073/pnas.95.9.5076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno R. D., Smith G. H. (2012). Reprogramming non-mammary and cancer cells in the developing mouse mammary gland. Semin. Cell Dev. Biol. 23, 591–598 10.1016/j.semcdb.2012.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno R. D., Boulanger C. A., Smith G. H. (2012). Notch-induced mammary tumorigenesis does not involve the lobule-limited epithelial progenitor. Oncogene 31, 60–67 10.1038/onc.2011.215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussard K. M., Boulanger C. A., Booth B. W., Bruno R. D., Smith G. H. (2010). Reprogramming human cancer cells in the mouse mammary gland. Cancer Res. 70, 6336–6343 10.1158/0008-5472.CAN-10-0591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fata J. E., Kong Y. Y., Li J., Sasaki T., Irie-Sasaki J., Moorehead R. A., Elliott R., Scully S., Voura E. B., Lacey D. L. et al. (2000). The osteoclast differentiation factor osteoprotegerin-ligand is essential for mammary gland development. Cell 103, 41–50 10.1016/S0092-8674(00)00103-3 [DOI] [PubMed] [Google Scholar]
- Ismail P. M., Li J., DeMayo F. J., O'Malley B. W., Lydon J. P. (2002). A novel LacZ reporter mouse reveals complex regulation of the progesterone receptor promoter during mammary gland development. Mol. Endocrinol. 16, 2475–2489 10.1210/me.2002-0169 [DOI] [PubMed] [Google Scholar]
- Ismail P. M., Amato P., Soyal S. M., DeMayo F. J., Conneely O. M., O'Malley B. W., Lydon J. P. (2003). Progesterone involvement in breast development and tumorigenesis – as revealed by progesterone receptor ‘knockout’ and ‘knockin’ mouse models. Steroids 68, 779–787 10.1016/S0039-128X(03)00133-8 [DOI] [PubMed] [Google Scholar]
- Lee H. J., Gallego-Ortega D., Ledger A., Schramek D., Joshi P., Szwarc M. M., Cho C., Lydon J. P., Khokha R., Penninger J. M. et al. (2013). Progesterone drives mammary secretory differentiation via RankL-mediated induction of Elf5 in luminal progenitor cells. Development 140, 1397–1401 10.1242/dev.088948 [DOI] [PubMed] [Google Scholar]
- Lydon J. P., DeMayo F. J., Funk C. R., Mani S. K., Hughes A. R., Montgomery C. A., Jr, Shyamala G., Conneely O. M., O'Malley B. W. (1995). Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 9, 2266–2278 10.1101/gad.9.18.2266 [DOI] [PubMed] [Google Scholar]
- Lydon J. P., Ge G., Kittrell F. S., Medina D., O'Malley B. W. (1999). Murine mammary gland carcinogenesis is critically dependent on progesterone receptor function. Cancer Res. 59, 4276–4284 [PubMed] [Google Scholar]
- Tsai M. J., O'Malley B. W. (1994). Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu. Rev. Biochem. 63, 451–486 10.1146/annurev.bi.63.070194.002315 [DOI] [PubMed] [Google Scholar]
- Wagner K. U., Wall R. J., St-Onge L., Gruss P., Wynshaw-Boris A., Garrett L., Li M., Furth P. A., Hennighausen L. (1997). Cre-mediated gene deletion in the mammary gland. Nucleic Acids Res. 25, 4323–4330 10.1093/nar/25.21.4323 [DOI] [PMC free article] [PubMed] [Google Scholar]