Abstract
Objectives To determine the efficacy of intra-articular corticosteroid injections for osteoarthritis of the knee and to identify numbers needed to treat.
Data sources Cochrane controlled trials register, Medline (1966 to 2003), Embase (1980 to 2003), hand searches, and contact with authors.
Inclusion criteria Randomised controlled trial in which the efficacy of intra-articular corticosteroid injections for osteoarthritis of the knee could be ascertained.
Results In high quality studies, the pooled relative risk for improvement in symptoms of osteoarthritis of the knee at 16-24 weeks after intra-articular corticosteroid injections was 2.09 (95% confidence interval 1.2 to 3.7) and the number needed to treat was 4.4. The pooled relative risk for improvement up to two weeks after injections was 1.66 (1.37 to 2.0). The numbers needed to treat to get one improvement in the statistically significant studies was 1.3 to 3.5 patients.
Conclusion Evidence supports short term (up to two weeks) improvement in symptoms of osteoarthritis of the knee after intra-articular corticosteroid injection. Significant improvement was also shown in the only methodologically sound studies addressing longer term response (16-24 weeks). A dose equivalent to 50 mg of prednisone may be needed to show benefit at 16-24 weeks.
Introduction
Knee pain is relatively common. Around a quarter of people aged 55 years or more in the United Kingdom and the Netherlands have persistent pain, and one in six will consult their general practitioner.1 Osteoarthritis is the single most common cause of disability in older adults, with 10% of patients aged 55 or more having painful disabling osteoarthritis of the knee, a quarter of whom are severely disabled.1 With no cure (excluding joint replacement), treatment is directed at pain relief and improvement or maintenance of function.
Intra-articular injection of steroid is a common treatment for osteoarthritis of the knee. Clinical evidence suggests that benefit is short lived, usually one to four weeks.2 The short term effect of steroids shown by controlled trials and clinical experience vary, however, with some patients seen by rheumatologists achieving a significant and sustained response beyond a few weeks. This may be explained by only one injection usually being given in clinical trials and at a lower dose (20 mg) than the 40 mg triamcinolone recommended by the American College of Rheumatologists.3 Pain scores may also be an insensitive outcome measure.
Concern has been expressed that long term treatment could promote joint destruction and tissue atrophy.2 Studies of cartilage damage, however, tend to suggest that changes are more likely due to the underlying disease than the steroid injection.4
Three papers have reviewed the general management of osteoarthritis of the knee, one specifically on corticosteroid injections, but no meta-analysis has been undertaken.1,4-6 We therefore performed a meta-analysis to determine whether intra-articular injections of corticosteroid are more efficacious than placebo in improving the symptoms of osteoarthritis of the knee.
Methods
We searched the Cochrane controlled trials register, Medline (1966 to 2003), and Embase (1980 to 2003) using the MeSH terms triamcinolone; prednisolone; prednisone; hydrocortisone; adrenal cortex hormones; osteoarthritis; knee; injections, intra-articular; and randomized controlled trial, and the non-MeSH terms injections; randomised controlled trial; and corticosteroid and steroid. Authors of included studies were contacted for details of any further work. The reference lists were scrutinised for relevant papers.
Our selection criterion was randomised placebo controlled trials in which the efficacy of intra-articular corticosteroids for osteoarthritis of the knee, of any duration, could be assessed. We considered improvement as the most important patient oriented outcome. Terms used to determine the discrete outcomes were distinct improvement, subjective improvement, decreased pain, overall improvement, clinically relevant outcomes, and response to the osteoarthritis research scale.7-12 Numbers needed to treat were calculated from dichotomous outcomes.13
The two authors independently assessed the methodological quality using the Jadad scoring system.14 Consensus was reached through discussion. Data extraction was similarly achieved. Data were analysed with Review Manager 4.1 (Update Software, Oxford). We calculated the relative risk and number needed to treat for improvement. An a priori subgroup analysis was conducted for study quality, dose of drug, duration of effect, specialty of injector, and condition of the knee. The dose equivalents were obtained from elsewhere.15 The conduct of this review was undertaken according to the QUOROM statement.16
Results
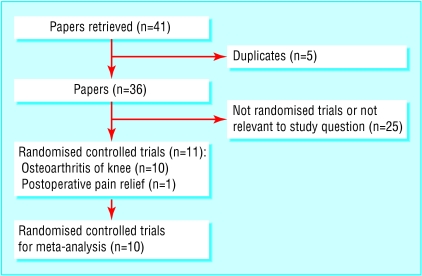
Ten trials met the inclusion criteria (fig 1).2,7-12,17-19 An additional paper examined intra-articular corticosteroid injections postoperatively, but we did not consider this paper in the review.20 Table 1 shows the quality scores of the included studies, and table 2 summarises details of the studies and improvements attained.
Fig 1.
Summary of search results
Table 1.
Jadad quality scores for 10 studies of intra-articular corticosteroid injections for osteoarthritis of the knee
| Study | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | Jadad score14 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cederlõf 19667 | + | + | ? | ? | + | ? | + | + | + | + | − | 3 |
| Dieppe 19808 | + | + | ? | + | ? | − | + | + | + | + | + | 3 |
| Friedman 19809 | + | + | ? | + | + | + | + | + | + | + | − | 5 |
| Gaffney 199510 | + | + | ? | + | − | − | + | + | − | + | + | 3 |
| Jones 199617 | + | + | ? | + | + | − | + | − | − | + | + | 3 |
| Miller 195818 | + | + | ? | + | − | ? | + | + | − | + | − | 2 |
| Ravaud 199911 | + | + | ? | + | + | + | + | + | + | + | + | 5 |
| Raynauld 20032 | + | + | ? | + | − | − | + | + | + | + | + | 3 |
| Smith 200312 | + | + | ? | − | + | + | + | + | + | + | + | 5 |
| Wright 196019 | + | + | ? | + | + | + | ? | + | − | + | − | 5 |
Numbers 1-11 follow Pedro format (www.cchs.usyd.edu.au/pedro/); Jadad score is calculated from different set of criteria14: 1=eligibility criteria specified; 2=patients randomised to groups; 3=concealment of allocation; 4=groups similar at baseline; 5=patients blinded; 6=practitioners administering intervention blinded; 7=assessors blinded; 8=measurements of key outcomes obtained from >85% of patients; 9=intention to treat analysis; 10=statistical comparisons between groups; 11=point measures and measures of variability provided.
+Criterion clearly satisfied.
−Criterion not clearly satisfied.
?Unclear whether criterion was satisfied.
Table 2.
Details of included studies with outcomes on improvement in osteoarthritis of knee
| Study, location | Condition | Details of patients | Injectors; nature of injection | Outcome | Jadad score |
|---|---|---|---|---|---|
| Cederlõf 1966,7 Sweden | History of aching after exertion, not trauma related and positive radiograph but no noticeable cartilage destruction | ≥40 years; no details on sex or duration of osteoarthritis | Surgeons; aspiration and intra-articular steroid injection (Meticortelone 2 ml) compared with placebo (saline); prednisone equivalent 50 mg | No significant difference between groups at 1, 3, and 8 weeks. Results reported as distinct improvement. At one week, 18/26 in experimental group, 14/25 in control group; eight weeks, 17/26 in experimental group, 19/25 in control group had continued improvement compared with baseline | ⅗ |
| Dieppe 1980,8 United Kingdom | Bilateral symptomatic osteoarthritis of knees | Mean 65 years; eight females, four males; most had grade 2-4 radiographic changes. Duration of osteoarthritis 7.5 years | Rheumatologist; aspiration and intra-articular steroid injection (triamcinolone hexacetonide 20 mg) compared with placebo (saline); prednisone equivalent 25 mg | Small, transient reduction in pain and tenderness compared with placebo. At one week, subjective improvement in 10/12 in experimental group, 1/12 in control group. Visual analogue scale at one week: mean 36 (SD 29) in experimental group, 70 (30) in control group | ⅗ |
| Friedman 1980,9 United States | Mild to moderate changes on radiograph | 42-75 years; mean duration of osteoarthritis 24 months for corticosteroid group and 36 months for placebo group | Rheumatologist; aspiration and intra-articular steroid injection (triamcinolone hexacetonide 20 mg) compared with placebo (saline); prednisone equivalent 25 mg | Steroid provided short term pain relief; at one week but not at 4, 6, 8 weeks. At one week described as decreased pain; 15/17 in experimental group, 12/17 in control group | 5/5 |
| Gaffney 1995,10 United Kingdom | 38% synovial fluid and knee pain for six months | Mean 67 years; 60 females, 24 males. Mean duration 6.7 years for corticosteroid group and 7.1 years for placebo group | Rhematologist; aspiration and intra-articular steroid injection (triamcinolone hexacetonide 20 mg) compared with placebo (saline); prednisone equivalent 25 mg | Steroid provided short term pain relief. Benefit at one week but not at six weeks. At one week overall improvement; 33/42 in experimental group, 21/42 in control group. Visual analogue scale: mean 21.7 (SD 20.7) in experimental group, 43.1 (28.7) in control group | ⅗ |
| Jones 1996,17 United Kingdom | Clinical and radiological osteoarthritis of knee | Mean 71 years; 23 males, 37 females. No details on duration of osteoarthritis | Rheumatologist; aspiration and intra-articular steroid injection (methylprednisolone 40 mg) compared with placebo (saline); prednisone equivalent 40 mg | Steroid provided short term pain relief. Responders at eight weeks: 28/30 in experimental group, 9/30 in control group | ⅗ |
| Miller 1958,18 Scotland | Primary osteoarthritis | No details on age, sex, or duration of osteoarthritis | Unclear who injected; intra-articular steroid injection (hydrocortisone 50 mg) compared with lactic acid; local anaesthetic; saline; and mock injection. Injections given five times at two week intervals; prednisone equivalent 12.5 mg | Steroid did not provide improvement better than placebo at six weeks or six months follow up after completion of treatment. Term used was “improved.” At six months: 4/34 in experimental group, 2/34 in control group; at 16 weeks 6/37 in experimental group, 8/36 in control group | ⅖ |
| Ravaud 1999,11 France | Most had knee effusion; all had osteophytes and minimal joint space narrowing | Mean 63-67 years; 66 females, two males. No details on duration of osteoarthritis | Rheumatologist; intra-articular steroid injection (cortivazol 1.5 ml) with or without joint lavage compared with placebo (saline); prednisone equivalent 37.5 mg | Steroid provided short term pain relief up to four weeks but no effect at 24 weeks. At one week clinically relevant improvement in pain, 16/25 in experimental group and 7/28 in control group. At 24 weeks: 12/25 in experimental group and 6/28 in control group. Visual analogue scale at one week: (n=24) mean 23.7 (SD 26.2) in experimental group, (n=21) 45.7 (26.6) in control group | 5/5 |
| Raynauld 2003,2 Canada | Kellgren and Lawrence grade 2 or 3 | 63 years; 67.5% female. Mean duration of osteoarthritis 9.8 years for corticosteroid group and 8.7 years for placebo group | Rheumatologist; intra-articular steroid injection (triamcinolone 40 mg) and placebo (saline) every three months for two years; prednisone equivalent 50 mg | Area under curve showed benefit for night pain and stiffness: 34 in each of experimental and control groups. At one year patient visual analogue scale: 34.32 (SD 20.9) in experimental group, 31.1 (21.1) in control group | ⅗ |
| Smith 2003,12 Australia | Radiograph grade 2 or 3 | Mean 66-67 years; 44 males, 27 females | Orthopaedic surgeon and rheumatologist; intra-articular steroid injection (methylprednisolone acetate 120 mg) after joint lavage compared with placebo; prednisone equivalent 80 mg | Steroid better than placebo only at four week follow up but not at 8, 12, or 24 weeks. Osteoarthritis Research Society response occurred: at two weeks 25/38 in experimental group, 15/33 in control group; at 24 weeks 16/38 in experimental group, 7/33 in control group. Visual analogue scale at two weeks: mean 20.8 (SD 30) in experimental group, 24.7 (30) in control group | 5/5 |
| Wright 1960,19 United Kingdom | Denominator knees not pooled | No details on personal characteristics or duration of osteoarthritis | Internal medicine specialist; intra-articular steroid injections (hydrocortisone acetate 25 mg and hydrocortisone tertiary-butylacetate 25 mg) compared with placebo (injection vehicle). Four injections given at two weekly intervals; prednisone equivalent 6.25 mg | Both steroids provided transient pain relief at two weeks (25 patients, 38 knees) | 5/5 |
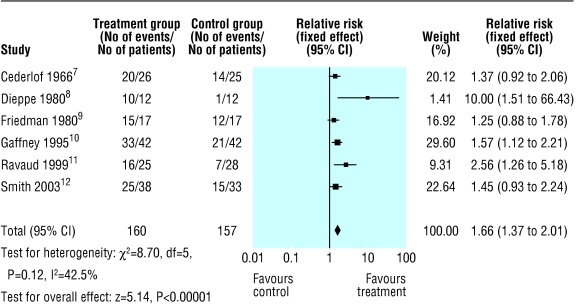
Six studies provided data on improvement of symptoms of osteoarthritis of the knee after intra-articular corticosteroid injections (fig 2). These showed a significant improvement (relative risk 1.66, 95% confidence interval 1.37 to 2.01). For the statistically significant studies the number needed to treat to obtain one improvement was between 1.3 and 3.5. No important harms were reported other than transient redness and discomfort. Only one study investigated potential loss of joint space and found no difference between corticosteroid and placebo up to two years.2
Fig 2.
Improvements up to two weeks after steroid injection in knee
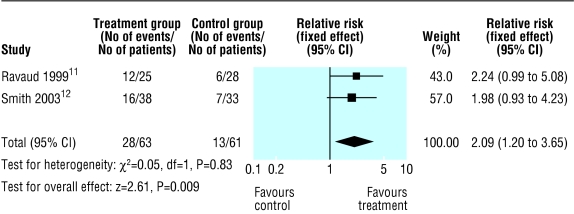
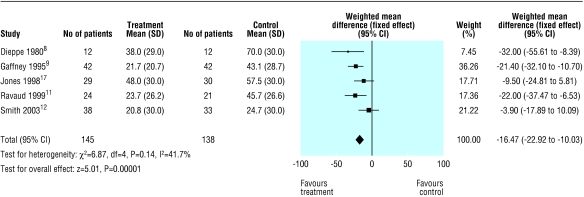
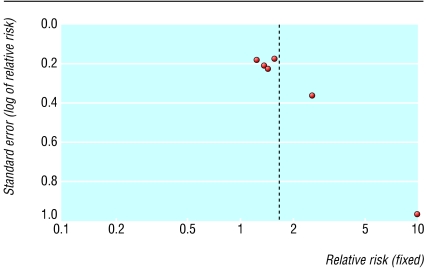
Neither of the two high quality studies were statistically significant for improvement at 16 to 24 weeks, but the pooled result gave a relative risk of 2.09 (1.20 to 3.65) with a number needed to treat of 4.4 based on this result (fig 3). Significant heterogeneity was found when the one low quality study was included. The result was non-significant by random effects analysis. Figure 4 shows the results of pooling the 100 mm visual analogue scale for five studies. When standard deviations were not reported, we assigned a value of 30, as this was the highest reported value and was taken as a conservative estimate. This result is statistically significant. We found no results for pain 16 weeks after injection. A funnel plot of the six studies suggested that there was an absence of small studies with small effects (fig 5). The smallest study had 12 patients and the largest 71.
Fig 3.
Improvements at 16-24 weeks after high dose steroid injection in knee for two high quality studies
Fig 4.
Visual analogue scale for pain up to two weeks after steroid injection in knee
Fig 5.
Funnel plot for corticosteroids compared with placebo
A similar result was found for improvement up to two weeks for the high dose studies. The effect at 16 to 24 weeks for these studies was the same as the two high quality studies. It was not possible to make a definitive analysis of the clinical conditions of the knee. The patients seemed to have mainly mild to moderate osteoarthritis. The dose equivalent to prednisone varied from 6.25 mg to 80 mg.
Discussion
Intra-articular injections of corticosteroid improve symptoms of osteoarthritis of the knee. Effects were beneficial up to two weeks and at 16 to 24 weeks. This is the first meta-analysis on this topic and the first review to show benefits of such injections in improvement of symptoms, which may extend beyond 16 weeks. We also report clinically significant numbers needed to treat, ranging between 1.3 and 3.5 patients. The one study that investigated potential loss of joint space found no difference between corticosteroid and placebo up to two years.2 This study also used a higher dose of triamcinolone (40 mg) than most of the other studies (20 mg) and gave repeated injections (every three months for two years).
Responses to intra-articular corticosteroids injections vary between the clinical experience of rheumatologists, where some patients have a significant and sustained response, to the short term benefit shown by randomised controlled trials.4 Trials tend to use one injection only and at lower doses than the recommended 20 mg triamcinolone.3 Subjective pain scales may also be an insensitive outcome measure in this condition.4
One limitation of our review is possible publication bias, in that by missing unpublished trials or those that showed negative effects we may have overestimated the benefits of corticosteroid injections. We believe, however, that our comprehensive, systematic search strategy enabled us to identify most research in this discipline. Another limitation of our study was the small size of the included studies.
Unlike other reviews we report improvement in symptoms, as we believe this is a more important patient oriented outcome than increases in range of movement or pain reduction.21 Only the review by Pendleton and coworkers attempted to pool the results of papers, but they did not perform a meta-analysis, rather they reported the number of studies that showed benefits compared with those that did not and a median effect size.5 Apart from the fact that other reviewers did not pool their data, we had the benefit of access to an article that was in press.12 When this was added to the other two studies, the pooled result was statistically significant for the two high quality studies.12 Larger studies are needed to confirm these findings.
The dose of corticosteroid required to improve symptoms is not clear from our review. The equivalent dose of prednisone varied from 6.25 mg to 80 mg.12,19 A dose of 20 mg triamcinolone (equivalent to 25 mg of prednisone) seems to be efficacious for pain control at two weeks. Only one study used 40 mg triamcinolone, and this found a benefit at 24 months for night pain and stiffness on one scale but not on another.2 This study also gave repeated injections and monitored loss of joint space (reporting no difference). The three studies that reported improvement at 16 weeks used different cortisones. The two studies using high doses showed a statistically significant difference suggesting that higher dose steroids may give a longer benefit.2,12 It is not clear to whom the results of this study would apply.11,12 All the studies were done in hospital settings.
One study found that predicting benefit was not possible.17 In contrast to another study, those who had synovial fluid aspirated had a better response.10 This only occurred in the intervention group, ruling out that aspiration was associated with accurate placement of the needle. Another explanation is that the presence of knee effusion is correlated with the presence of synovitis and that intra-articular steroids my be effective against the inflammation.4 One study recommended joint lavage combined with steroid injection if a knee effusion persisted after one or two steroid injections eight to 10 days apart.4 Joint lavage was either efficacious (at two weeks) or nearly efficacious (efficacious when controlled for severity from radiographic evidence at 24 weeks) for more than 16 weeks.11,12
Evidence supports short term (up to two weeks) improvement of symptoms from intra-articular corticosteroid injection for osteoarthritis of the knee, and the only methodologically-sound studies addressing longer term response (16-24 weeks) also show significant improvement. Doses of 50 mg equivalent of prednisone may be needed to obtain benefits at 16 to 24 weeks. Corticosteroid injection in addition to lavage needs further investigation. Currently no evidence supports the promotion of disease progression by steroid injections. Repeat injections seem to be safe over two years but needs confirmation from other studies.
What is already known on this topic
Intra-articular corticosteroids provide short term (two weeks) relief of symptoms of osteoarthritis of the knee
Concerns are that multiple injections may damage articular cartilage
What this study adds
Intra-articular corticosteroids are probably effective in improving symptoms of osteoarthritis of the knee for 16 to 24 weeks
The number needed to treat is 4.4
Higher doses of cortisone (equivalent to 50 mg prednisone) may be more effective than lower doses, especially after 16 or more weeks
Contributors: BA and FG-S were involved in extracting the data, appraising the article, and writing the paper. BA did the mathematical pooling; he will act as guarantor for the paper. The guarantor accepts full responsibility for the conduct of the study, had access to the data, and controlled the decision to publish.
Funding. This study was funded by the New Zealand Accident Rehabilitation and Compensation Insurance Corporation. Their role was limited to commissioning the work.
Competing interests: None declared.
Ethical approval: Not required.
References
- 1.Peat G, McCarney R, Croft P. Knee pain and osteoarthritis in older adults: a review of community burden and current use of primary health care. Ann Rheum Dis 2001;60: 91-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raynauld J, Buckland-Wright C, Ward R, Choquette D, Haraoui B, Martel-Pelletier J, et al. Safety and efficacy of long-term intraarticular steroid injections in osteoarthritis of the knee. Arth Rheum 2003;48: 370-7. [DOI] [PubMed] [Google Scholar]
- 3.American College of Rheumatology subcommittee on osteoarthritis guidelines. Recommendations for the medical management of osteoarthritis of the hip and knee. Arth Rheum 2000;43: 1905-15. [DOI] [PubMed] [Google Scholar]
- 4.Ayral X. Injections in the treatment of osteoarthritis. Best Pract Res Clin Rehumatol 2001;15: 609-26. [DOI] [PubMed] [Google Scholar]
- 5.Pendleton A, Arden N, Dougados M, Doherty M, Bannwarth B, Bijlsma JW, et al. EULAR recommendations for the management of knee osteoarthritis: report of a task force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis 2000;59: 936-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mazieres B, Masquelier AM, Capron MH. A French controlled multicenter study of intraarticular orgotein versus intraarticular corticosteroids in the treatment of knee osteoarthritis: a one-year follow up. J Rheumatol Suppl 1991;27: 134-7. [PubMed] [Google Scholar]
- 7.Cederlof S, Jonson G. Intraarticular prednisolone injection for osteoarthritis of the knee. A double blind test with placebo. Acta Chir Scand 1966;132: 532-7. [PubMed] [Google Scholar]
- 8.Dieppe PA, Sathapatayavongs B, Jones HE, Bacon PA, Ring EF. Intra-articular steroids in osteoarthritis. Rheumatol Rehabil 1980;19: 212-7. [DOI] [PubMed] [Google Scholar]
- 9.Friedman DM, Moore ME. The efficacy of intraarticular steroids in osteoarthritis: a double-blind study. J Rheumatol 1980;7: 850-6. [PubMed] [Google Scholar]
- 10.Gaffney K, Ledingham J, Perry JD. Intra-articular triamcinolone hexacetonide in knee osteoarthritis: factors influencing the clinical response. Ann Rheum Dis 1995;54: 379-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ravaud P, Moulinier L, Giraudeau B, Ayral X, Guerin C, Noel E, et al. Effects of joint lavage and steroid injection in patients with osteoarthritis of the knee: results of a multicenter, randomized, controlled trial. Arth Rheum 1999;42: 475-82. [DOI] [PubMed] [Google Scholar]
- 12.Smith MD, Wetherall M, Darby T, Esterman A, Slavotinek J, Robert-Thomson P, et al. A randomized placebo-controlled trial of arthroscopic lavage versus lavage plus intra-articular corticosteroids in the management of symptomatic osteoarthritis of the knee. Rheumatol 2003;42: 1477-85. [DOI] [PubMed] [Google Scholar]
- 13.Guyatt G, Juniper EF, Walter SD, Griffith LE, Goldstein RS. Interpreting treatment effects in randomised trials. BMJ 1998;316: 690-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds JM, Gavaghan DJ, et al. Assessing the quality of reports on randomised clinical trials: is blinding necessary? Control Clin Trials 1996;17: 1-12. [DOI] [PubMed] [Google Scholar]
- 15.Lane NE, Lukert B. The science and therapy of glucocorticoid-induced bone loss. Endocrinol Metab Clin North Am 1998;27: 465-83. [DOI] [PubMed] [Google Scholar]
- 16.Moher D, Cook D, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomized controlled trials: the QUOROM statement. Quality of reporting of meta-analyses. Lancet 1999;354: 1896-900. [DOI] [PubMed] [Google Scholar]
- 17.Jones A, Doherty M. Intra-articular corticosteroids are effective in osteoarthritis but there are no clinical predictors of response. Ann Rheum Dis 1996;55: 829-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller J, White J, Norton T. The value of intra-articular injections in osteoarthritis of the knee. J Bone Joint Surg 1958;4013: 636-43. [DOI] [PubMed] [Google Scholar]
- 19.Wright V, Chandler G, Morison R, Hartfall S. Intra-articular therapy in osteoarthritis. Comparison of hydrocortisone acetate and hydrocortisone teriary-butylacetate. Ann Rheum Dis 1960;19: 257-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang JJ, Ho ST, Lee SC, Tang JJ, Liaw WJ. Intraarticular triamcinolone acetonide for pain control after arthroscopic knee surgery. Anesth Analgesia 1998;87: 1113-6. [DOI] [PubMed] [Google Scholar]
- 21.Liang MH, Lew RA, Stucki G, Fortin PR, Daltroy L. Measuring clinically important changes with patient-oriented questionnaires. Med Care 2002;40: II45-51. [DOI] [PubMed] [Google Scholar]