Abstract
Alterations of inhibitory GABAergic neurons are implicated in multiple psychiatric and neurological disorders, including schizophrenia, autism and epilepsy. In particular, interneuron deficits in prefrontal areas, along with presumed decreased inhibition, have been reported in several human patients. The majority of forebrain GABAergic interneurons arise from a single subcortical source before migrating to their final regional destination. Factors that govern the interneuron populations have been identified, demonstrating that a single gene mutation may globally affect forebrain structures or a single area. In particular, mice lacking the urokinase plasminogen activator receptor (Plaur) gene have decreased GABAergic interneurons in frontal and parietal, but not caudal, cortical regions. Plaur assists in the activation of hepatocyte growth factor/scatter factor (HGF/SF), and several of the interneuron deficits are correlated with decreased levels of HGF/SF. In some cortical regions, the interneuron deficit can be remediated by endogenous overexpression of HGF/SF. In this study, we demonstrate decreased parvalbumin-expressing interneurons in the medial frontal cortex, but not in the hippocampus or basal lateral amygdala in the Plaur null mouse. The Plaur null mouse demonstrates impaired medial frontal cortical function in extinction of cued fear conditioning and the inability to form attentional sets. Endogenous HGF/SF overexpression increased the number of PV-expressing cells in medial frontal cortical areas to levels greater than found in wildtype mice, but did not remediate the behavioral deficits. These data suggest that proper medial frontal cortical function is dependent upon optimum levels of inhibition and that a deficit or excess of interneuron numbers impairs normal cognition.
Keywords: HGF/SF, urokinase plasminogen activator receptor, attentional set, Pavlovian fear-conditioning, cued extinction, Morris water maze, ID-ED shift, shift-cost
1. Introduction
Many psychiatric and neurological disorders present persistent neuroanatomical abnormalities that contribute to the dysfunction. Loss of forebrain GABAergic inhibition has been implicated in schizophrenia, epilepsy, and autism spectrum disorders [1–3]. The age of onset of these disorders suggests a developmental origin with disruptions in GABAergic neuron ontogeny as a possible cause. Many forebrain GABAergic interneurons arise subcortically in the developing basal ganglia and migrate to the cerebral cortex, hippocampus, amygdala, and olfactory bulbs [4–7] or remain in the striatum [8]. GABAergic neuronal ontogeny is regulated by multiple molecules [9]. Any alterations in a single factor have the potential to alter circuitry and function in multiple forebrain regions. However, very few studies have investigated the long-term consequences of early perturbations to GABAergic interneuron ontogeny. It is unknown whether loss of a single gene has global effects or selectively alters one or more interneuron populations. For example, global loss may lead to overall deficits in inhibition, a severe seizure disorder and possibly intellectual disability. On the other hand, specific loss of a cortical region, perhaps the hippocampus, may result in impaired learning and memory, in absence of other effects.
Hepatocyte growth factor/scatter factor (HGF/SF) and its sole receptor, the Met tyrosine kinase, are critical for mediating embryonic cerebral cortical GABAergic interneuron development [10]. Reduced embryonic levels of HGF/SF and Met lead to deficits in cerebral cortical GABAergic interneurons [11–14]. In particular, transgenic mice lacking urokinase plasminogen activator receptor (Plaur) have lower expression of HGF/SF and Met [10, 13]. The Plaur mice also show a selective loss of GABAergic interneurons in frontal and parietal cortical areas, with no alterations in piriform and occipital regions [10]. These defects in anterior cingulate and parietal cortex are specific for the parvalbumin-expressing (PV+) GABAergic interneuron subtype, whereas neurons expressing the somatostatin and calretinin markers are unaffected [15]. As the Plaur phenotype was hypothesized to be due to insufficient levels of HGF/SF, we designed a strategy to supplement HGF/SF in postnatal animals. The Gfap-HGF (abbreviated as HGF) mouse expresses HGF/SF in perinatal astrocytes, and when mated with the Plaur mouse, the Plaur/HGF mouse has near normal levels of HGF/SF and restored GABAergic interneuron numbers, especially PV+ cell numbers, in parietal regions [13]. We have shown that this strategy also rescues the PV+ interneuron losses in the orbital frontal cortex (OFC) and dorsal striatum and eliminates the impaired reversal learning observed in the Plaur mice [16].
In this study, we investigate the roles of interneurons in medial frontal cortex (MFC) and connected areas of the amygdala and hippocampus. All three areas were examined, as anatomical deficits in more than one area will alter the interpretation of the behavioral outcomes. We used three behavioral paradigms which test the functions of the hippocampus (Morris water maze and contextual fear conditioning), amygdala (cued fear conditioning), and MFC (set-shifting). Fear conditioning investigates rodent Pavlovian learning [17, 18]. Lesion studies demonstrate that the hippocampus (HC), basolateral amygdala (BLA) and medial prefrontal cortex (MFC) participate in the formation and extinction of the cued and contextual memory pairing [19–23]. The Morris water maze tests how animals navigate by spatial cues, using the HC and striatum [24–26]. Lastly, attentional set-shifting relies on intact MFC [27, 28]. By using these paradigms in concert, we predicted consistent deficits due to interneuron abnormalities in the MFC and restoration in the presence of HGF/SF.
2. Materials and methods
2.1 Subjects
The B6.129-Tg(Gfap-HGF)Ca (abbreviated as HGF) mouse line was a generous gift from our collaborators (C. Achim, University of San Diego and W. Mars and G. Michalopoulos, University of Pittsburgh). In the HGF line, human HGF is expressed under the control of the mouse glial fibrillary acidic protein (Gfap) promoter by astrocytes. Expression commences around birth and continues at low levels throughout adult life [13] (and unpublished results). The B6.129 – Plaurtm1/Mlg/Plaurtm1/Mlg mouse line (abbreviated as Plaur) lacks the gene that encodes the uPAR (urokinase plasminogen activator receptor) protein (a gift from P. Carmeliet (Center for Transgene Technology and Gene Therapy, Flanders Interuniversity Institute for Biotechnology, KU Leuven, Belgium)) [29]. All lines were maintained as heterozygotes on the C57BL/6 background (> 15 generations) using mice purchased from the Jackson Laboratory (Bar Harbor, ME). Mice used for these studies were generated from heterozygous matings of B6.129 – Plaurtm1/Mlg mice with B6.129 – Plaurtm1/Mlg/Tg(Gfap-HGF)Ca to produce: B6.129 (wildtype, WT), B6.129 – Plaurtm1/Mlg/Plaurtm1/Mlg (Plaur), B6.129-Tg(Gfap-HGF)Ca (HGF) and B6.129 – Plaurtm1/Mlg/Plaurtm1/Mlg/Tg(Gfap-HGF)Ca (Plaur/HGF) for experiments, along with B6.129 – Plaurtm1/Mlg/Tg(Gfap-HGF)Ca which were not used experimentally, but were maintained as breeders. Subjects were adult male littermates from at least 4 separate pedigrees. Littermates of multiple genotypes were housed together (4 to 5 per cage) unless undergoing food deprivation.
All research procedures using mice were approved by the Institutional Animal Care and Use Committee at University of Maryland and conformed to NIH Guide for the Care and Use of Laboratory Animals. The HGF mice were genotyped via PCR using the primer sets: 5’-ggC CAT gAA TTT gAC CTC TAT gAA-3’ and 5’-TTC AAC TTC TgA ACA CTg Agg AAT-3’ (250 bp) for HGF mice, and 5’-CCT CAT CCT ggg CCT ggT CTg gTC T-3’ and 5’- ggT TTT CCC CgC TgT ggT CAT CTg C-3’ (200 bp) for PAI-1 as a positive control. For genotyping Plaur mice, the primer sets were: 5’-gAT gAT AgA gAg CTg gAg gTg gTg AC-3’ and 5’- CAC Cgg gTC Tgg gCC TgT TgC AgA ggT-3’ (145 bp) for Plaur, 5’-ATT gAA gAA gAT ggA TTg CAC-3’ and 5’-TTC gTC CAg ATC ATC CTg ATC gAC-3’ (500 bp) for neomycin resistant gene.
2.2 Immunohistochemistry
The mice were transcardially perfused with buffered 4% paraformaldehyde and the brains were postfixed overnight at 4°C. Vibratome sections (50µm) were cut and stained using routine laboratory protocols [11, 13]. The sections were incubated with mouse anti-PV antibodies (1:1000, Sigma Chemical Co, St. Louis, MO) for 2 d at 4°C. Images were obtained by Leica DMRX microscope (Leica Microsystems GmbH, Wetzlar) with Phase One image capture software version 3.04 (Phase One A/S, Frederiksberg, Denmark).
2.3 Cell counting and analysis
For each region of interest, the numbers of immunoreactive cells were counted at several anatomical levels by a blinded observer, using the optical fractionator method with the Neurolucida system. An estimate of cell number in each region was calculated using unbiased stereology [30]. All structures were identified using the Paxinos and Franklin atlas [31]. In the hippocampus, cells in the dentate gyrus (DG), CA1, and CA3 regions were counted using at least 6 sections from each brain, each 400 µm apart, beginning at bregma level −1.58 mm and extending caudally to −4.00 mm [31]. The PV+ cells were also counted in the MFC with three sections between bregma level +2.46 and +1.46 mm. For counts in the basal lateral amygdala (BLA), bregma levels between −1.28 mm and −2.12 mm with at least 3 sections counted per mouse. Volume was estimated using the Cavalieri method. Data are provided as mean ± standard error of the mean (SEM), with at least 4 mice for each genotype. The statistical significance among four different genotypes was examined using two-way ANOVA followed by Student-Newman-Keuls (SNK) post-hoc analysis (SigmaStat, Systat, San Jose, CA).
2.4 Morris water maze
The task was performed in a 33” diameter rubber tub (Aquatic Systems) with the water temperature maintained 25°C, which has been reported as optimal for mice [32, 33]. Water level was 10 cm below the edge of the tub. A 10 cm diameter platform was submerged 0.5 cm below the water line and 15 cm from the edge of the tub. White tempera paint (Crayola) was added to the water to provide sufficient turbidity to obscure the platform. The maze was enclosed by walls and large shapes (circle, triangle, rhombus and star) were used as the cues. Animals were pre-trained using a 30 s free swim, 30 s on the submerged platform, and two trials to find and remain on the platform for 10 s [33]. If the mouse failed to find the platform within 60 s, it was placed on the hidden platform for 10 s. During training, the animals underwent four trials a day until the average latency for the mice to successfully find the platform was less than 10s. The day following training to criterion, the probe test was performed to measure spatial memory, in which the platform was removed and the quadrant location of the mouse was recorded for 60 s. Data are reported as the mean ± SEM, for groups of 7 mice per genotype. Latency data were analyzed using a three-way repeated measures ANOVA and probe test data (% time in each quadrant) were analyzed using a three-way ANOVA (SigmaStat). Statistical significance is at the p < 0.05 level.
2.5 Cued and contextual fear conditioning test
Separate cohorts of mice were used for the fear conditioning studies. Mice were habituated to the arena equipped with a shock grid floor (TruScan, Coulbourn Instruments, Allentown, PA) for at least 30 min prior to testing and then placed back in the home cage. At the beginning of testing, the mouse was placed in the arena and its movements were recorded for 5 min, in absence of external cues. For each trial, the mouse was exposed to the tone (800 Hz, 80 dB) for 30 s, with the final 2 s of the tone presentation accompanied by a 0.45 mA foot shock, with both tone and shock terminating simultaneously, followed by a 2 min inter-trial interval. After receiving three trials, the mouse was placed back in the home cage and returned to the animal facility. All animal movement was recorded via an automated photobeam tracking system and also visually with a video camcorder.
To measure contextual extinction, the mouse was placed in the exact context as the animal received the conditioning, in absence of the tone cue. For each extinction test, the percent rest time was calculated as a measure of freezing to context over a 5 min trial. Extinction testing occurred at 1, 7 and 14 d after conditioning [17, 34]. Data are reported as the mean ± SEM for percent time spent freezing for a group of 10 mice per genotype. Data were analyzed using a three-way repeated measures ANOVA followed by Holm-Sidak multiple comparisons, if warranted (SigmaStat).
For the cued extinction, animals received the same three tone presentations as in the habituation testing, however the environment was altered. The shock floor was removed, the pattern of visual cues (room view) on the sides of the area was changed to plain white paper, and the arena was washed with lemon scented dish detergent. The animal was placed in the altered arena for 5 min and its movement was recorded (pre-cue). After 5 min, the tone cues were presented for 30 s, followed by a 2 min inter-trial interval. No shock was given. Mice were tested for extinction at 1, 7, and 14 d following the initial conditioning. The percent of rest time was calculated as a measure of freezing to the cue due the 30 s cue presentation. Data are reported as the mean ± SEM for percent time spent freezing for 11 WT, 10 HGF, 10 Plaur, and 10 Plaur/HGF mice. Data were analyzed using a three-way repeated measures ANOVA followed by Holm-Sidak multiple comparisons, if warranted (SigmaStat).
2.6 Attentional set-shift task
A separate cohort of mice were used to measure the formation of an attentional set using the previously described lab paradigm [28]. Mice were individually housed and food restricted to reach 85% baseline free-feeding weight. Food deprived mice were trained to perform compound discriminations by digging in bowls of scented media. Intradimensional (ID) shifts required the mouse to learn a new rule with a new exemplar pair, but the same dimension (i.e. odor) was always the relevant cue. For the set-shift (or intradimensional – extradimensional (ID-ED) shift), the relevant dimension for the exemplar pair was switched to be irrelevant, and obtaining the reward then required information from the previously irrelevant dimension [28, 35]. The order of discriminations and exemplars was the same for all mice, but the direction of the extradimensional shift was counterbalanced within each experimental group. A criterion of eight consecutive correct trials was required to complete each task. For each genotype, a cohort of 11 WT, 13 HGF, 13 Plaur, and 11 Plaur/HGF mice was tested. The data required for learning the task were previously reported for the reversal learning portion of the test [16]. Here we report, for the first time, the extradimensional shift data. The data have been reanalyzed using a two-way repeated measures ANOVA (and multiple comparison testing using the Holm-Sidak method) for the extradimensional shift (EDS). A shift-cost was calculated as the difference between the trials to criterion for the EDS and the previous IDS task [36]. Shift-cost data were analyzed using a two-way ANOVA with Student-Newman-Keuls (SNK) post-hoc comparisons. Data are reported as the mean numbers of trials to criterion ± SEM. Statistical significance was considered as p < 0.05, and denoted by asterisks or ampersands, as described in each figure legend.
3. Results
3.1 HGF/SF levels alter forebrain interneuron numbers
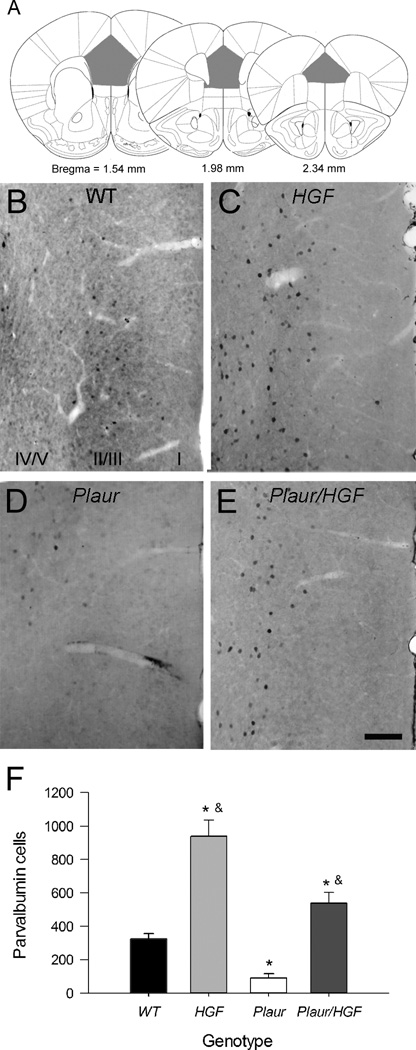
We have previously reported that the adult Plaur mice have decreased GABAergic interneurons and near complete loss of the PV-expressing (PV+) cells in anterior cingulate and parietal cortical areas as compared to wildtype (WT) littermates or C57BL/6 parent strain mice [12, 37]. Also, the Plaur mice have a dramatic specific decrease of PV+ cells in the OFC, which can be remediated by postnatal supplementation of HGF/SF in the Plaur/HGF mice. We therefore examined whether the interneurons were disrupted in the MFC. Immunohistochemistry for PV in the MFC (prelimbic and infralimbic regions, Fig 1a) shows many cells mainly in layers II/III and fewer in layers V/VI in WT mice (Fig. 1b). The increased expression of HGF/SF leads to more immunoreactive cells overall and a change in expression pattern, with the population equally distributed between layers II/III and V/VI (Fig. 1c). Plaur mice have a dramatic decrease in PV+ cells in all layers (Fig. 1d), and the addition of postnatal HGF/SF in the Plaur/HGF increases the number of PV+ cells (Fig. 1e), but in the aberrant distribution observed in the HGF mouse.
Fig. 1.
HGF/SF levels affect the number of PV+ cells in the MFC. (a) Diagrams of locations of prefrontal areas for PV+ cell counts. Gray shaded areas denote counting regions. Figures are adapted from Paxinos and Watson [31]. (b–d) Immunohistochemistry of PV+ cells in MFC of adult WT (b), HGF (c), Plaur (d), and Plaur/HGF (e) mice. (f) The total numbers of PV+ cells were stereologically estimated in medial cortex. Each bar represents n ≥ 3, with error bars denoting the SEM. Asterisk (*) signifies a statistical significance as compared to WT mice (two-way ANOVA, SNK post-hoc test, p < 0.05), whereas the ampersand (&) denotes statistical significance as compared to Plaur mice. Bar =250 µm.
The numbers of PV+ cells in the MFC were estimated using unbiased stereology (Fig. 1f), and the comparison showed effects with respect to Plaur (F(1,11) = 32.41, p < 0.001) and HGF genes (F(1,11) = 91.29, p < 0.001). No interaction was found between Plaur and HGF genotypes (p = 0.171). The number of PV+ cells in the Plaur mice is about 30% of WT cells (p = 0.018). The number of PV+ cells is nearly tripled in the HGF mice (p < 0.001) as compared to WT. Increased postnatal HGF/SF expression in the Plaur/HGF mice eliminates the deficit observed in Plaur, but leads to abnormally high numbers of PV+ cells as compared to WT (p = 0.026). Volume estimation of prelimbic and infralimbic areas revealed no significant difference with respect to genotype (F(1,11) = 0.21). In summary, MFC PV+ interneuron number appears to be highly sensitive to postnatal HGF/SF levels.
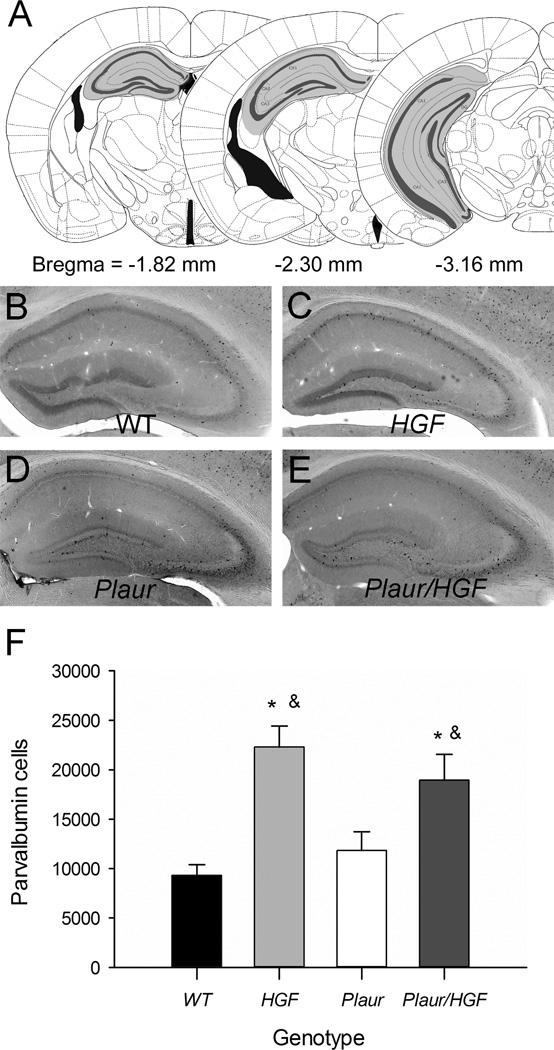
Immunohistochemistry for PV demonstrated that WT and Plaur mice had similar numbers and patterns of PV+ cells in all hippocampal regions (Fig. 2a, b, d). The HGF and Plaur/HGF mice had notable increases in PV+ cells, compared to WT mice, but the overall expression patterns of cell distribution were similar for all genotypes (Fig. 2b–e). Hippocampal volumes were similar for all mice (two-way ANOVA, F(1,17) = 1.55, p = 0.253). Comparison of the stereological estimates of total PV+ cells showed an effect of HGF genotype (two-way ANOVA, F(1,17) = 27.284, p < 0.001, Fig. 2f), but not Plaur genotype (F1,17) = 0.0457, p = 0.834). The expression of HGF in either the HGF or Plaur/HGF mice significantly increased the number of PV+ cells (p < 0.01). The number of PV+ cells in the Plaur/HGF mice was significantly different from Plaur mice (p = 0.021), but not different from HGF mice (p = 0.368). Overall, the loss of Plaur did not affect the hippocampal PV+ interneurons, but the addition of postnatal HGF/SF increased the number of interneurons by two-fold.
Fig. 2.
Hippocampal PV+ cells are increased HGF/SF. (a) Diagrams of counting locations of PV+ cells within the hippocampal formation. Gray shaded areas denote counting regions. Figures are adapted from Paxinos and Watson [31]. (b–d) Immunohistochemistry of PV+ cells in hippocampus of adult WT (b), HGF (c), Plaur (d), and Plaur/HGF (e) mice. Bar = 250 µm. (f) The total numbers of PV+ cells were stereologically estimated. Each bar represents n ≥ 3, with error bars denoting the SEM. Asterisk (*) signifies a statistical significance as compared to WT mice (two-way ANOVA, SNK post-hoc test, p < 0.05), whereas the ampersand (&) denotes statistical significance as compared to Plaur mice (p < 0.05).
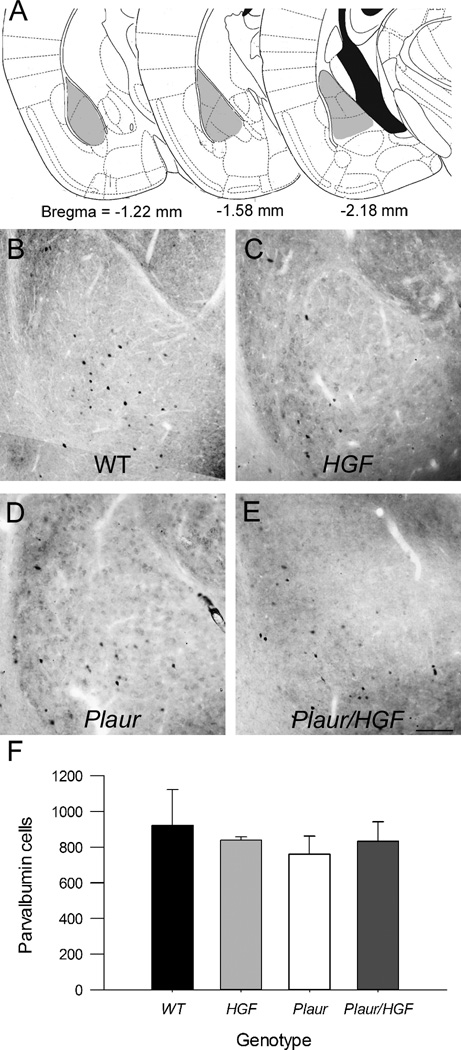
Immunohistochemistry for PV+ cells in the BLA was similar for all groups of mice (Fig. 3a–e). No effect of either Plaur (F1,12) = 0.334, p = 0.578) or HGF genotype (F(1,12) = 0.00179, p = 0.967) was found (Fig. 3f). There was no effect of genotype with regards to the BLA volume, F(3,9) = 0.18, p = 0.90. The numbers of PV+ interneurons in the BLA were not altered in the mutant mice.
Fig. 3.
PV+ cells in the BLA are not affected by changes in Plaur or HGF. (a) Diagrams of counted regions. Gray shaded areas denote counting regions. Figures are adapted from Paxinos and Watson [31]. (b–d) Immunohistochemistry of PV+ cells in BLA of adult WT (b), HGF (c), Plaur (d), and Plaur/HGF mice (e). Bar = 150 µm. (f) The numbers of PV+ cells were stereologically estimated in the BLA. Each bar represents n ≥ 3 mice, with error bars denoting the SEM.
3.2 Spatial learning is unchanged with increased levels of HGF/SF
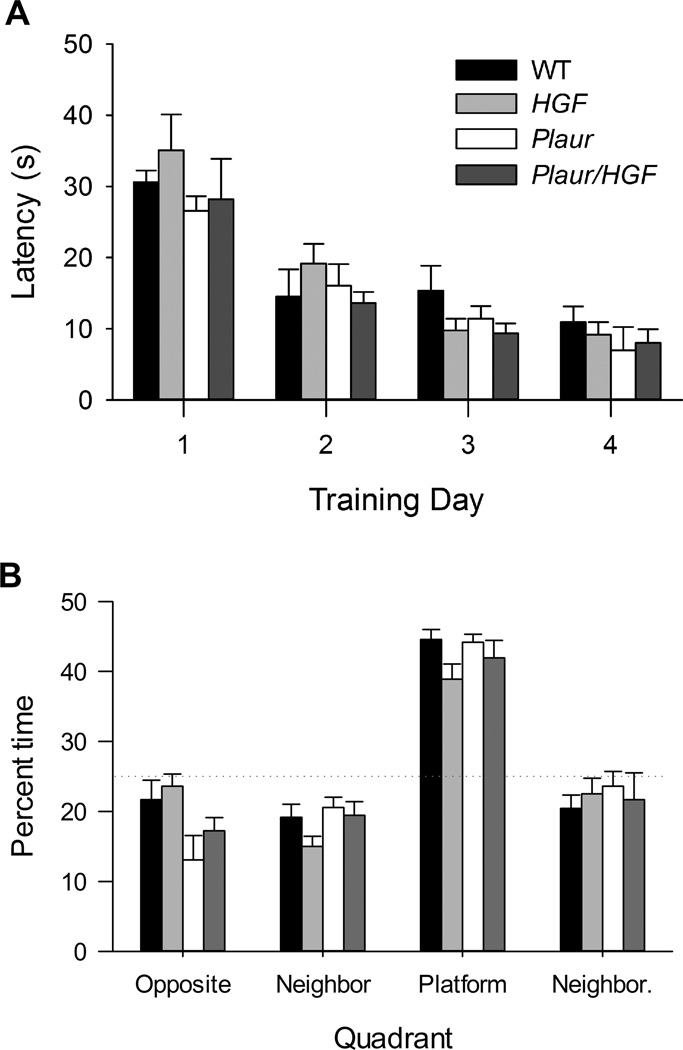
The Morris water maze was used to evaluate spatial memory in the four cohorts of mice. Previous analysis reported normal motor and sensory function and open field activity for all genotypes [12, 13, 29]. All mice reached criterion and were able to find the platform within the 10 s criteria by Day 4 (Fig. 4a). There was a main effect of training day, (F(3,114) = 39.971, p < 0.001), but no main effect of Plaur (F(1,114) = 1.752, p = 0.189) or HGF genotype, F(1,114) = 0.819, p = 0.368). In the probe test, all groups successfully swam to the quadrant that had previously contained the platform (Fig. 4b). A main effect of quadrant was observed, (F(3,115) = 28.995, p < 0.001), but no effect of Plaur or HGF genotype was found, (F(1,115) = 0.0173, p = 0.896, for each). In summary, all groups performed the spatial memory tasks similarly.
Fig. 4.
Spatial learning using the Morris water maze is normal for all genotypes. (a) All groups demonstrated similar learning in finding the hidden platform. (b) During the probe test, all groups spent more time in the quadrant that previously contained the hidden platform.
3.3 Abnormal interneuron densities impair extinction of fear conditioning
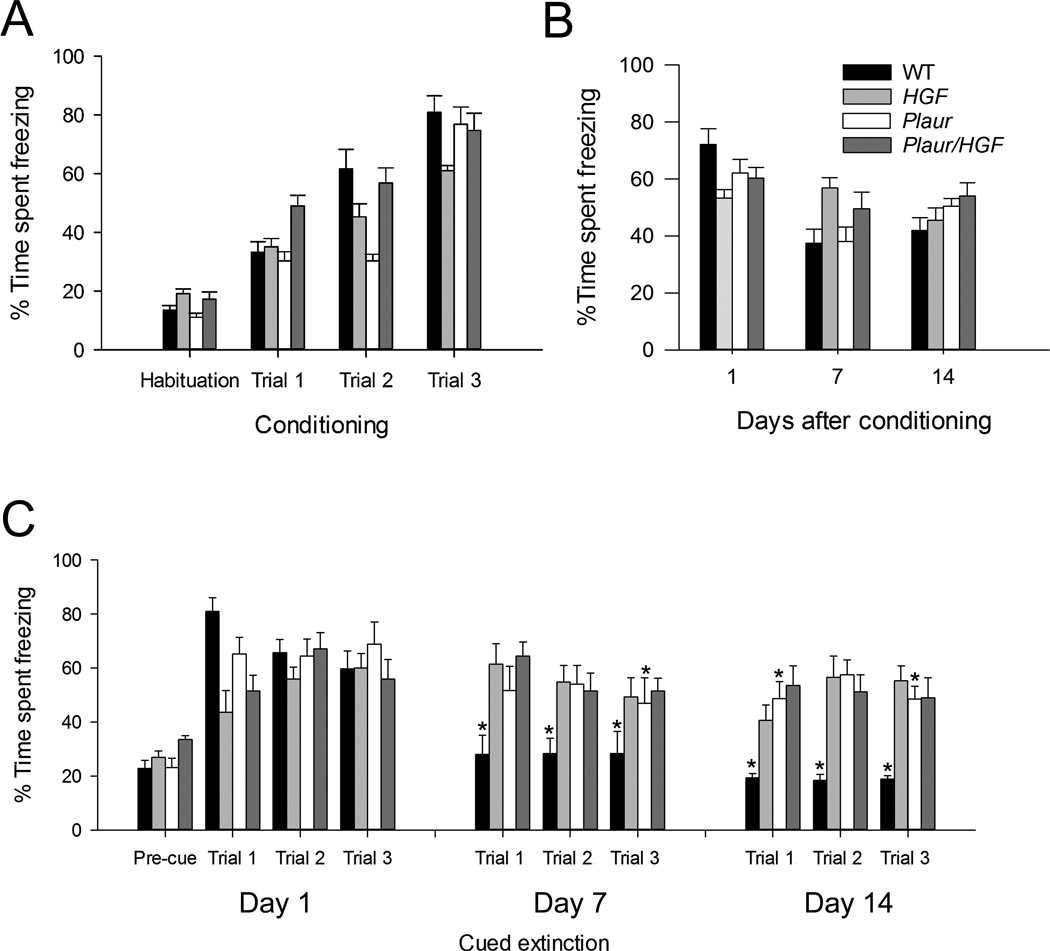
We investigated whether alterations in the GABAergic interneurons in these structures changed performance. All animals responded to the tone, as demonstrated by increased rest time (freezing time) with repeated paired tone and shock presentations during the conditioning (Fig. 5a). Three-way repeated measures ANOVA calculated a main effect of condition, F(3,163) = 143.652, p < 0.001), but no effect of either Plaur (F(1,163) – 0.0512, p = 0.821) or HGF genotype, F(1,163) = 1.581, p = 0.211. However, significant interactions were found between Plaur and HGF genotypes (F(1,163) = 23.371, p < 0.001), condition and Plaur genotype (F(3,163) = 3.164, p = 0.026), and condition and HGF genotype (F(3,163) = 5.308, p = 0.002), along with a three-way interaction between condition, Plaur and HGF genotype (F(3,163) = 4.648, p = 0.004). Post-hoc analysis of the interactions demonstrates that the outcomes are dependent on the HGF genotype (p < 0.001). Overall, all groups of mice increased the time spent freezing as they experienced more cued foot shocks, although not at the same rate or extent.
Fig. 5.
Contextual and cued fear conditioning test demonstrates impaired cued extinction. (a) Conditioning to the context and tone cued shock. (b) Contextual extinction. (c) Cued extinction for three trials per session. Asterisks denote significant difference from the initial % time spent freezing (Conditioning, Trial 3) for each genotype (p < 0.05).
Hippocampal function was measured using contextual recall and extinction (Fig. 5b). There was an effect of day (F(3,160) = 31.368, p < 0.001) but no effect of either Plaur (F(1,160) = 0.834, p = 0.363) or HGF genotype (F(1,160) = 0.0560, p = 0.813). However, a day × HGF genotype interaction was found (F(3,160) = 7.436, p < 0.001). On Day 1, all groups demonstrated percent freezing times that reflected the conditioning trials, demonstrating contextual recall. On Days 7 and 14, the percent-time spent freezing for WT, Plaur and Plaur/HGF mice significantly decreased from the last conditioning trial (p < 0.011 for all comparisons). Only the HGF mice did not demonstrate significant decrease in percent-time spent freezing (p > 0.118 for all comparisons).
Cued recall and extinction were altered in all mutant groups (Fig. 5c). Three-way repeated measures ANOVA demonstrated main effects of trial, (F(9,409) = 11.814, p < 0.001), Plaur (F(1,409) = 23.339, p < 0.001) and HGF genotype, F(1,409) = 6.867, p = 0.009). WT mice extinguished the freezing response on Days 7 and 14 (p < 0.001 for all trials), and Plaur mice showed significant reductions in freezing on Day 7 (Trial 3) and Day 14 (p < 0.05). The HGF and Plaur/HGF groups never demonstrated significant decreases in freezing behaviors, even after 14 days (p > 0.05). In summary, all groups learned the task, WT mice demonstrated extinction by Day 7, and Plaur mice exhibited a delayed response, but groups with an HGF allele never extinguished the fear response.
3.4 Formation of an attentional set is impaired in mice with a PV+ interneuron deficit
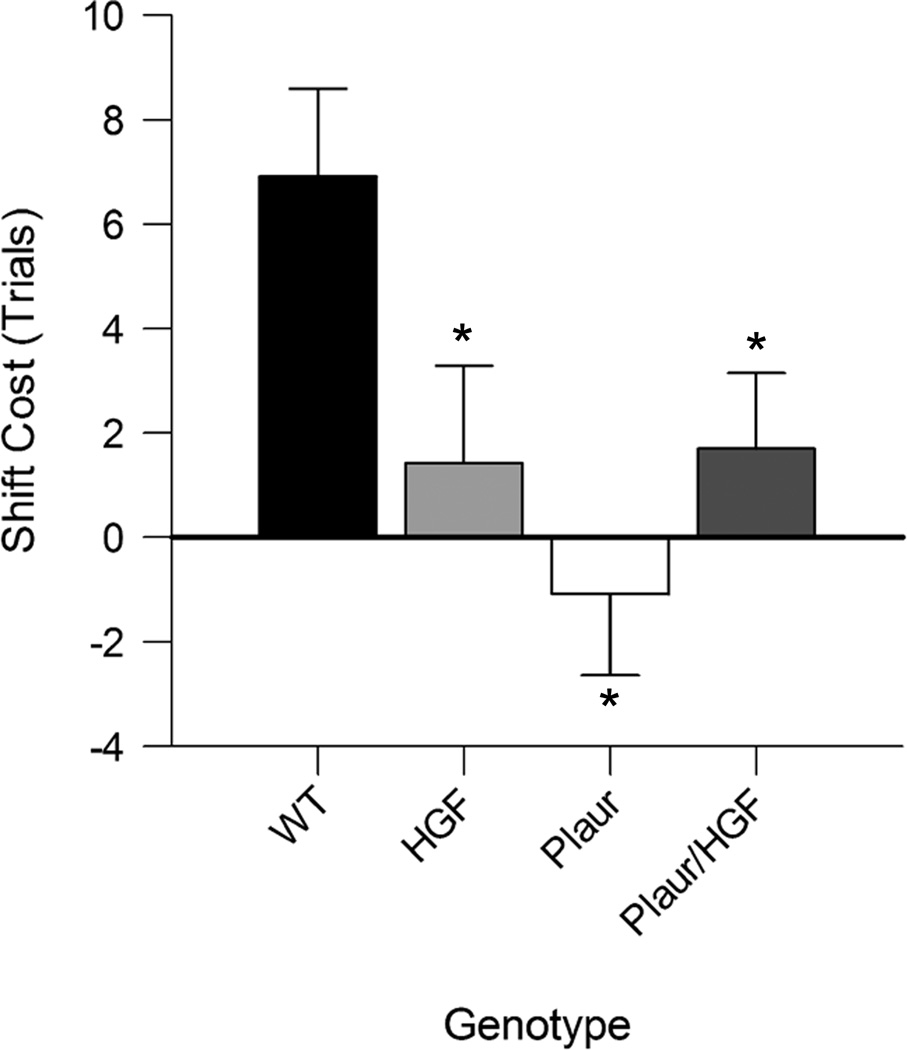
Previously, we have shown that, in mice, reversal learning is mediated through the OFC and is impaired in animals with decreased PV+ interneurons [14, 16] and rescued with HGF/SF supplementation [16]. Our lesion studies indicated that the ability to form and shift between attentional sets is dependent upon an intact MFC [28]. The formation of an attentional set was measured by an increase in trials to criterion from the intradimensional shift discrimination to the extradimensional shift discrimination, in which the previously irrelevant dimension was shifted to become relevant (also referred to as the ID-ED shift). The shift-cost measures the difference in trials required for the ID-ED shift [38, 39]. For WT mice, the shift-cost is 6.9 ± 1.7 trials, whereas for the other groups the shift-costs are between 2 and −2. A two-way ANOVA indicated an effect of Plaur genotype, (F(1,46) = 3.942, p = 0.027), and an interaction between Plaur and HGF genotypes (F(2,46) = 7.175, p = 0.002). Post-hoc analysis demonstrated that shift-cost in WT mice was significantly different than HGF (p = 0.012), Plaur (p < 0.001), or Plaur/HGF (p = 0.029). No difference was found between the other groups (p > 0.394). While all mouse groups were able to learn the compound discriminations, only WT mice were able to respond to rule changes by set-shifting.
4. Discussion
Understanding developmental perturbations of the GABA system have far reaching implications for human mental disorders. Disorders ranging from schizophrenia and autism to epilepsy all have overlapping similarities, especially regarding loss of GABAergic inhibition [1, 40]. In particular, fast-spiking PV+ neurons are often decreased [41–45]. The resulting agreement of behavioral alterations with changes in GABAergic tone in specific forebrain structures indicates any shift away from an optimized normal level of GABA yields cognitive impairments.
Forebrain GABAergic interneurons arise from the ganglionic eminences and migrate to distant targets during the prenatal period [6–9]. The PV+ interneurons that populate the cerebral cortex, hippocampus and dorsal striatum originate from the medial ganglionic eminence during embryonic days 11 through 17 [5, 7, 8]. During the same period, the amygdalar interneurons are born in the caudal ganglionic eminence [6]. Perturbations in the molecules that regulate interneuron number (proliferation or survival), migration or maturation, can have far-reaching and possibly diverse effects on the forebrain populations. The behavioral phenotypes of the perturbations may be mild or severe, depending on the extent each interneuron population is altered.
The Plaur mouse has a delay in embryonic cortical interneuron migration, likely due to diminished levels of HGF/SF in the embryo and at birth [12, 13]. The loss of Plaur leads to fewer PV+ interneurons in the rostral cortical regions and striatum [10, 13, 16]. Postnatal replenishing of HGF/SF, with the HGF mouse, restores the interneuron numbers in the striatum and orbital frontal and somatosensory cortical areas in the Plaur/HGF mouse. However, in this study, the addition of HGF/SF yields aberrant PV+ interneurons in the MFC (prelimbic and infralimbic areas, Fig. 1). We have previously shown that the OFC in the HGF and Plaur/HGF mouse is nearly normal [16]. In this study, the altered anatomy in the MFC provides supporting evidence for MFC and OFC as unique cortical areas in the mouse brain with separate interneuronal ontogenies.
The PV+ profiles in the Plaur mouse hippocampus were similar to WT animals, in agreement with previous reports [37]. Mice with the HGF allele had nearly two-fold increase in PV+ interneurons in all hippocampal subfields (Fig. 2). HGF/SF has been widely reported in the hippocampus [46–48]. The increased peri- and postnatal HGF/SF expression in the HGF and Plaur/HGF mice may have recruited addition PV+ interneurons into the hippocampal region. Alternatively, more PV+ interneurons may have survived into adulthood. Finally, HGF/SF has been shown to elicit the expression of interneuron markers, such as calbindin, in cultured hippocampal neurons [49]. PV+ expression may have been increased in the presence of elevated HGF/SF levels, hence the observed aberrant numbers of immunoreactive cells. In summary, the interneuron profiles in the Plaur forebrain varied based on anatomical structure, and the addition of HGF/SF often increased the numbers of immunoreactive PV+ cells.
The neural substrates that mediate the different features of fear conditioning are well-established in the literature [50]. In the tested cohort, cued learning was similar in all genotypes, as expected, given the similar amygdala anatomy. Contextual memory did not differ within the groups, even though the HGF and Plaur/HGF mice presented with nearly two-fold more PV+ hippocampal interneurons. Previous studies have indicated that loss of PV+ interneuron activity leads to impaired spatial memory [51, 52]. However, cued extinction was impaired in all the mutant genotypes, implying dysfunction of the MFC.
The Plaur mice, with an MFC interneuron deficit, show behavioral responses similar to animals with MFC lesions [53, 54]. By contrast, the overexpression of HGF/SF leads to increased numbers of PV+ neurons in MFC and mice that were unable to extinguish the cued responses, even after 2 weeks. The performances of the HGF and Plaur/HGF mice are reminiscent of previous research showing inactivation of MFC on fear conditioning task [55]. It is also noted that the HGF mice had reducing freezing during conditioning, similar to rats with inactivated MFC areas. Mice harboring the HGF allele presented increased aberrant PV+ cells, suggesting that both increased inhibition and defective neural connectivity are responsible for the failure to extinguish the fear response. The lack of fear extinction is consistent with MFC dysfunction; however another possibility is that the HGF allele enhanced the retention of the original learning. Taken together, the behavioral responses for the cued learning and extinction were sensitive to developmental manipulations of interneuron profiles.
Lesion and pharmacological studies have indicated the MFC as an integral structure for the shifting of attentional sets (intradimensional/extradimensional, ID-ED shift) and that disruptions lead to increased trials to criterion to perform the task [27, 28, 56]. Only the WT mice were able to demonstrate the ID-ED shift, or a positive shift-cost, whereas mice with fewer PV+ interneurons in the MFC (Plaur) or with more PV+ cells (HGF and Plaur/HGF) did not require significantly more trials to criterion to solve the problem, and therefore had a very low or negative shift-cost. Lack of shift-cost is interpreted as the inability to form the attentional set, as the animals perform similarly on the ED and ID shifts, indicating that the animals did not attend to the relative dimension [36, 57–59]. In rat, lesions to the OFC or dorsal medial striatum prevented the formation of the attentional set [36, 57]. In mice, no ID-ED shift was observed in the presence of phencyclidine [60], or the acetylcholinesterase inhibitor diisopropyl fluorophosphate [61], or ketamine [62]. Transgenic mice lacking the dopamine D2 receptor or dopamine D3 receptor [63] or expressing COMT-Val variant of catechol-O-methyltransferase [64] also reported lack of formation of the attentional set. Our data with the Plaur, HGF, and Plaur/HGF mice are consistent with other rodent models of compromised behavioral flexibility.
In the studies with excitotoxic lesions and pharmacological perturbations, the underlying anatomy and circuitry are similar between control and experimental groups prior to the manipulations. When considering transgenic mice, in particular those with developmental genetic disruptions, the circuitry may be unique between groups. The changes in interneuron numbers with the combinations of Plaur and HGF alleles likely altered connectivity and overall neurotransmission, as shown by the reported variations of electroencephalogram recordings [13]. Similar differences of circuitry and cellular numbers occur in human patients with neurodevelopmental disorders [41, 42, 65]. These findings indicate that an optimum number of interneurons, or inhibition, is required to form the attentional set, providing a possible explanation of the observed sensitivity to medication in schizophrenic patients’ performance on set-shifting tasks.
The inability of the HGF allele to correct the set-shifting deficit in the Plaur mouse is in contrast to the successful strategy which dramatically reduced seizures and anxiety and corrected the anatomical and reversal learning deficits in the Plaur/HGF mouse [13, 16]. The significant anatomical differences in PV+ interneuron numbers and locations in the MFC and OFC regions in the transgenic mice provides additional support that unique frontal cortical areas are present in the mouse [66].
Fig. 6.
Attentional set-shifting is impaired. (a) All mice were trained on a naturalistic foraging task and the difference in trials needed to reach criterion to complete the extradimensional shift compared to the intradimensional shift was calculated as the shiftcost. Comparisons of the shift-cost for each group demonstrates significant shift-cost for WT mice, the other genotypes are significantly different from WT, as shown by the asterisks (two-way ANOVA, p < 0.05).
Highlights.
Loss of Plaur leads to reduced numbers of medial frontal cortical interneurons
Excess HGF/SF increases hippocampal and medial frontal cortical interneurons
Amygdalar interneurons are not affected by Plaur loss or excess HGF/SF
Fear extinction and attentional set formation are dependent on interneuron number
Acknowledgements
We thank Dr. Gabriela Martins for all her technical support and scientific discussions. This work was supported by NIH R01 DA018826, R01 MH57689 and by a NARSAD Young Investigator Award.
Abbreviations
- EDS
Extradimensional shift
- GABA
gamma-aminobutyric acid
- HGF
Gene for hepatocyte growth factor/scatter factor
- HGF/SF
Hepatocyte growth factor/scatter factor
- ID-ED
Intradimensional –extradimensional (shift)
- IDS
Intradimensional shift
- MFC
Medial frontal cortex
- OFC
Orbital frontal cortex
- PCR
Polymerase chain reaction
- PV
Parvalbumin
- Plaur
Gene that encodes urokinase plasminogen activator receptor
- SEM
Standard error of the mean
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hashimoto T, Arion D, Unger T, Maldonado-Aviles JG, Morris HM, Volk DW, et al. Alterations in GABA-related transcriptome in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2008;13:147–161. doi: 10.1038/sj.mp.4002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benes FM, McSparren J, Bird ED, SanGiovanni JP, Vincent SL. Deficits in small interneurons in prefrontal and cingulate cortices of schizophrenic and schizoaffective patients. Arch Gen Psychiatry. 1991;48:996–1001. doi: 10.1001/archpsyc.1991.01810350036005. [DOI] [PubMed] [Google Scholar]
- 3.Wills S, Rossi CC, Bennett J, Cerdeno VM, Ashwood P, Amaral DG, et al. Further characterization of autoantibodies to GABAergic neurons in the central nervous system produced by a subset of children with autism. Mol Autism. 2011;2:5. doi: 10.1186/2040-2392-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson SA, Qiu M, Bulfone A, Eisenstat DD, Meneses J, Pedersen R, et al. Mutations of the homeobox genes Dlx-1 and Dlx-2 disrupt the striatal subventricular zone and differentiation of late born striatal neurons. Neuron. 1997;19:27–37. doi: 10.1016/s0896-6273(00)80345-1. [DOI] [PubMed] [Google Scholar]
- 5.Pleasure SJ, Anderson S, Hevner R, Bagri A, Marin O, Lowenstein DH, et al. Cell migration from the ganglionic eminences is required for the development of hippocampal GABAergic interneurons. Neuron. 2000;28:727–740. doi: 10.1016/s0896-6273(00)00149-5. [DOI] [PubMed] [Google Scholar]
- 6.Nery S, Fishell G, Corbin JG. The caudal ganglionic eminence is a source of distinct cortical and subcortical cell populations. Nat Neurosci. 2002;5:1279–1287. doi: 10.1038/nn971. [DOI] [PubMed] [Google Scholar]
- 7.Anderson SA, Marin O, Horn C, Jennings K, Rubenstein JL. Distinct cortical migrations from the medial and lateral ganglionic eminences. Development. 2001;128:353–363. doi: 10.1242/dev.128.3.353. [DOI] [PubMed] [Google Scholar]
- 8.Marin O, Anderson SA, Rubenstein JL. Origin and molecular specification of striatal interneurons. J Neurosci. 2000;20:6063–6076. doi: 10.1523/JNEUROSCI.20-16-06063.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wonders CP, Anderson SA. The origin and specification of cortical interneurons. Nat Rev Neurosci. 2006;7:687–696. doi: 10.1038/nrn1954. [DOI] [PubMed] [Google Scholar]
- 10.Powell EM, Mars WM, Levitt P. Hepatocyte growth factor/scatter factor is a motogen for interneurons migrating from the ventral to dorsal telencephalon. Neuron. 2001;30:79–89. doi: 10.1016/s0896-6273(01)00264-1. [DOI] [PubMed] [Google Scholar]
- 11.Martins GJ, Plachez C, Powell EM. Loss of embryonic MET signaling alters profiles of hippocampal interneurons. Dev Neurosci. 2007;29:143–158. doi: 10.1159/000096219. [DOI] [PubMed] [Google Scholar]
- 12.Powell EM, Campbell DB, Stanwood GD, C D, Noebels JL, Levitt P. Genetic disruption of cortical interneuron development causes region- and GABA cell type-specific deficits, epilepsy, and behavioral dysfunction. J Neurosci. 2003;23:622–631. doi: 10.1523/JNEUROSCI.23-02-00622.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bae MH, Bissonette GB, Mars WM, Michalopoulos GK, Achim CL, Depireux DA, et al. Hepatocyte growth factor (HGF) modulates GABAergic inhibition and seizure susceptibility. Exp Neurol. 2010;221:129–135. doi: 10.1016/j.expneurol.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martins GJ, Shahrohk M, Powell EM. Genetic disruption of Met signaling impairs GABAergic striatal development and cognition. Neuroscience. 2011;176:199–209. doi: 10.1016/j.neuroscience.2010.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Powell EM, Muhlfriedel S, Bolz J, Levitt P. Differential regulation of thalamic and cortical axonal growth by hepatocyte growth factor/scatter factor. Dev Neurosci. 2003;25:197–206. doi: 10.1159/000072268. [DOI] [PubMed] [Google Scholar]
- 16.Bissonette GB, Bae MH, Suresh T, Jaffe DE, Powell EM. Astrocyte-mediated hepatocyte growth factor/scatter factor supplementation restores GABAergic interneurons and corrects reversal learning deficits in mice. J Neurosci. 2010;30:2918–2923. doi: 10.1523/JNEUROSCI.5268-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crawley JN. Behavioral phenotyping of transgenic and knockout mice: experimental design and evaluation of general health, sensory functions, motor abilities, and specific behavioral tests. Brain Res. 1999;835:18–26. doi: 10.1016/s0006-8993(98)01258-x. [DOI] [PubMed] [Google Scholar]
- 18.Nie T, Abel T. Fear conditioning in inbred mouse strains: an analysis of the time course of memory. Behav Neurosci. 2001;115:951–956. doi: 10.1037//0735-7044.115.4.951. [DOI] [PubMed] [Google Scholar]
- 19.Gerlai R. Contextual learning and cue association in fear conditioning in mice: a strain comparison and a lesion study. Behav Brain Res. 1998;95:191–203. doi: 10.1016/s0166-4328(97)00144-7. [DOI] [PubMed] [Google Scholar]
- 20.Bardgett ME, Boeckman R, Krochmal D, Fernando H, Ahrens R, Csernansky JG. NMDA receptor blockade and hippocampal neuronal loss impair fear conditioning and position habit reversal in C57Bl/6 mice. Brain Res Bull. 2003;60:131–142. doi: 10.1016/s0361-9230(03)00023-6. [DOI] [PubMed] [Google Scholar]
- 21.Cahill L, Vazdarjanova A, Setlow B. The basolateral amygdala complex is involved with, but is not necessary for, rapid acquisition of Pavlovian 'fear conditioning'. Eur J Neurosci. 2000;12:3044–3050. doi: 10.1046/j.1460-9568.2000.00187.x. [DOI] [PubMed] [Google Scholar]
- 22.Sangha S, Narayanan RT, Bergado-Acosta JR, Stork O, Seidenbecher T, Pape HC. Deficiency of the 65 kDa isoform of glutamic acid decarboxylase impairs extinction of cued but not contextual fear memory. J Neurosci. 2009;29:15713–15720. doi: 10.1523/JNEUROSCI.2620-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hefner K, Whittle N, Juhasz J, Norcross M, Karlsson RM, Saksida LM, et al. Impaired fear extinction learning and cortico-amygdala circuit abnormalities in a common genetic mouse strain. J Neurosci. 2008;28:8074–8085. doi: 10.1523/JNEUROSCI.4904-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Devan BD, McDonald RJ, White NM. Effects of medial and lateral caudate-putamen lesions on place- and cue-guided behaviors in the water maze: relation to thigmotaxis. Behav Brain Res. 1999;100:5–14. doi: 10.1016/s0166-4328(98)00107-7. [DOI] [PubMed] [Google Scholar]
- 25.Owen EH, Logue SF, Rasmussen DL, Wehner JM. Assessment of learning by the Morris water task and fear conditioning in inbred mouse strains and F1 hybrids: implications of genetic background for single gene mutations and quantitative trait loci analyses. Neuroscience. 1997;80:1087–1099. doi: 10.1016/s0306-4522(97)00165-6. [DOI] [PubMed] [Google Scholar]
- 26.Morris RG, Garrud P, Rawlins JN, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 27.Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bissonette GB, Martins GJ, Franz TM, Harper ES, Schoenbaum G, Powell EM. Double dissociation of the effects of medial and orbital prefrontal cortical lesions on attentional and affective shifts in mice. J Neurosci. 2008;28:11124–11130. doi: 10.1523/JNEUROSCI.2820-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dewerchin M, Nuffelen AV, Wallays G, Bouche A, Moons L, Carmeliet P, et al. Generation and characterization of urokinase receptor-deficient mice. J Clin Invest. 1996;97:870–878. doi: 10.1172/JCI118489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.West MJ. Stereological methods for estimating the total number of neurons and synapses: issues of precision and bias. Trends Neurosci. 1999;22:51–61. doi: 10.1016/s0166-2236(98)01362-9. [DOI] [PubMed] [Google Scholar]
- 31.Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. 2nd ed. San Diego: Academic Press; 2001. [Google Scholar]
- 32.Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1:848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wahlsten D, Cooper SF, Crabbe JC. Different rankings of inbred mouse strains on the Morris maze and a refined 4-arm water escape task. Behav Brain Res. 2005;165:36–51. doi: 10.1016/j.bbr.2005.06.047. [DOI] [PubMed] [Google Scholar]
- 34.Kinney JW, Starosta G, Holmes A, Wrenn CC, Yang RJ, Harris AP, et al. Deficits in trace cued fear conditioning in galanin-treated rats and galanin-overexpressing transgenic mice. Learn Mem. 2002;9:178–190. doi: 10.1101/m.49502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Colacicco G, Welzl H, Lipp HP, Wurbel H. Attentional set-shifting in mice: modification of a rat paradigm, and evidence for strain-dependent variation. Behav Brain Res. 2002;132:95–102. doi: 10.1016/s0166-4328(01)00391-6. [DOI] [PubMed] [Google Scholar]
- 36.Chase EA, Tait DS, Brown VJ. Lesions of the orbital prefrontal cortex impair the formation of attentional set in rats. Eur J Neurosci. 2012;36:2368–2375. doi: 10.1111/j.1460-9568.2012.08141.x. [DOI] [PubMed] [Google Scholar]
- 37.Eagleson KL, Bonnin A, Levitt P. Region- and age-specific deficits in gammaaminobutyric acidergic neuron development in the telencephalon of the uPAR(−/−) mouse. J Comp Neurol. 2005;489:449–466. doi: 10.1002/cne.20647. [DOI] [PubMed] [Google Scholar]
- 38.McGaughy J, Ross RS, Eichenbaum H. Noradrenergic, but not cholinergic, deafferentation of prefrontal cortex impairs attentional set-shifting. Neuroscience. 2008;153:63–71. doi: 10.1016/j.neuroscience.2008.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bissonette GB, Powell EM. Reversal learning and attentional set-shifting in mice. Neuropharmacology. 2011 doi: 10.1016/j.neuropharm.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gluck MR, Thomas RG, Davis KL, Haroutunian V. Implications for altered glutamate and GABA metabolism in the dorsolateral prefrontal cortex of aged schizophrenic patients. Am J Psychiatry. 2002;159:1165–1173. doi: 10.1176/appi.ajp.159.7.1165. [DOI] [PubMed] [Google Scholar]
- 41.Oblak AL, Rosene DL, Kemper TL, Bauman ML, Blatt GJ. Altered posterior cingulate cortical cyctoarchitecture, but normal density of neurons and interneurons in the posterior cingulate cortex and fusiform gyrus in autism. Autism Res. 2011;4:200–211. doi: 10.1002/aur.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lewis DA, Volk DW, Hashimoto T. Selective alterations in prefrontal cortical GABA neurotransmission in schizophrenia: a novel target for the treatment of working memory dysfunction. Psychopharmacology (Berl) 2003 doi: 10.1007/s00213-003-1673-x. [DOI] [PubMed] [Google Scholar]
- 43.Lawrence YA, Kemper TL, Bauman ML, Blatt GJ. Parvalbumin-, calbindin-, and calretinin-immunoreactive hippocampal interneuron density in autism. Acta Neurol Scand. 2010;121:99–108. doi: 10.1111/j.1600-0404.2009.01234.x. [DOI] [PubMed] [Google Scholar]
- 44.Andrioli A, Alonso-Nanclares L, Arellano JI, DeFelipe J. Quantitative analysis of parvalbumin-immunoreactive cells in the human epileptic hippocampus. Neuroscience. 2007;149:131–143. doi: 10.1016/j.neuroscience.2007.07.029. [DOI] [PubMed] [Google Scholar]
- 45.Aronica E, Redeker S, Boer K, Spliet WG, van Rijen PC, Gorter JA, et al. Inhibitory networks in epilepsy-associated gangliogliomas and in the perilesional epileptic cortex. Epilepsy Res. 2007;74:33–44. doi: 10.1016/j.eplepsyres.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 46.Jung W, Castren E, Odenthal M, Vande Woude GF, Ishii T, Dienes HP, et al. Expression and functional interaction of hepatocyte growth factor-scatter factor and its receptor c-met in mammalian brain. J Cell Biol. 1994;126:485–494. doi: 10.1083/jcb.126.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Honda S, Kagoshima M, Wanaka A, Tohyama M, Matsumoto K, Nakamura T. Localization and functional coupling of HGF and c-Met/HGF receptor in rat brain: implication as neurotrophic factor. Brain Res Mol Brain Res. 1995;32:197–210. doi: 10.1016/0169-328x(95)00075-4. [DOI] [PubMed] [Google Scholar]
- 48.Achim CL, Katyal S, Wiley CA, Shiratori M, Wang G, Oshika E, et al. Expression of HGF and cMet in the developing and adult brain. Brain Res Dev Brain Res. 1997;102:299–303. doi: 10.1016/s0165-3806(97)00108-9. [DOI] [PubMed] [Google Scholar]
- 49.Korhonen L, Sjoholm U, Takei N, Kern MA, Schirmacher P, Castren E, et al. Expression of c-Met in developing rat hippocampus: evidence for HGF as a neurotrophic factor for calbindin D-expressing neurons. Eur J Neurosci. 2000;12:3453–3461. doi: 10.1046/j.1460-9568.2000.00260.x. [DOI] [PubMed] [Google Scholar]
- 50.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 51.Korotkova T, Fuchs EC, Ponomarenko A, von Engelhardt J, Monyer H. NMDA receptor ablation on parvalbumin-positive interneurons impairs hippocampal synchrony, spatial representations, and working memory. Neuron. 2010;68:557–569. doi: 10.1016/j.neuron.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 52.Nguyen PT, Nakamura T, Hori E, Urakawa S, Uwano T, Zhao J, et al. Cognitive and socio-emotional deficits in platelet-derived growth factor receptor-beta gene knockout mice. PLoS One. 2011;6:e18004. doi: 10.1371/journal.pone.0018004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Quirk GJ, Russo GK, Barron JL, Lebron K. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. J Neurosci. 2000;20:6225–6231. doi: 10.1523/JNEUROSCI.20-16-06225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Santini E, Ge H, Ren K, Pena de Ortiz S, Quirk GJ. Consolidation of fear extinction requires protein synthesis in the medial prefrontal cortex. J Neurosci. 2004;24:5704–5710. doi: 10.1523/JNEUROSCI.0786-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sierra-Mercado D, Jr, Corcoran KA, Lebron-Milad K, Quirk GJ. Inactivation of the ventromedial prefrontal cortex reduces expression of conditioned fear and impairs subsequent recall of extinction. Eur J Neurosci. 2006;24:1751–1758. doi: 10.1111/j.1460-9568.2006.05014.x. [DOI] [PubMed] [Google Scholar]
- 56.Dias R, Robbins TW, Roberts AC. Dissociation in prefrontal cortex of affective and attentional shifts. Nature. 1996;380:69–72. doi: 10.1038/380069a0. [DOI] [PubMed] [Google Scholar]
- 57.Lindgren HS, Wickens R, Tait DS, Brown VJ, Dunnett SB. Lesions of the dorsomedial striatum impair formation of attentional set in rats. Neuropharmacology. 2013 doi: 10.1016/j.neuropharm.2013.03.034. [DOI] [PubMed] [Google Scholar]
- 58.Tait DS, Brown VJ. Lesions of the basal forebrain impair reversal learning but not shifting of attentional set in rats. Behav Brain Res. 2008;187:100–108. doi: 10.1016/j.bbr.2007.08.035. [DOI] [PubMed] [Google Scholar]
- 59.Bissonette GB, Powell EM. Reversal learning and attentional set-shifting in mice. Neuropharmacology. 2012;62:1168–1174. doi: 10.1016/j.neuropharm.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Laurent V, Podhorna J. Subchronic phencyclidine treatment impairs performance of C57BL/6 mice in the attentional set-shifting task. Behav Pharmacol. 2004;15:141–148. doi: 10.1097/00008877-200403000-00006. [DOI] [PubMed] [Google Scholar]
- 61.Levi Y, Kofman O, Schwebel M, Shaldubina A. Discrimination and avoidance learning in adult mice following developmental exposure to diisopropylfluorophosphate. Pharmacol Biochem Behav. 2008;88:438–445. doi: 10.1016/j.pbb.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 62.Kos T, Nikiforuk A, Rafa D, Popik P. The effects of NMDA receptor antagonists on attentional set-shifting task performance in mice. Psychopharmacology (Berl) 2010;214:911–921. doi: 10.1007/s00213-010-2102-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Glickstein SB, Desteno DA, Hof PR, Schmauss C. Mice lacking dopamine D2 and D3 receptors exhibit differential activation of prefrontal cortical neurons during tasks requiring attention. Cereb Cortex. 2005;15:1016–1024. doi: 10.1093/cercor/bhh202. [DOI] [PubMed] [Google Scholar]
- 64.Papaleo F, Crawley JN, Song J, Lipska BK, Pickel J, Weinberger DR, et al. Genetic dissection of the role of catechol-O-methyltransferase in cognition and stress reactivity in mice. J Neurosci. 2008;28:8709–8723. doi: 10.1523/JNEUROSCI.2077-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Leeson VC, Robbins TW, Matheson E, Hutton SB, Ron MA, Barnes TR, et al. Discrimination learning, reversal, and set-shifting in first-episode schizophrenia: stability over six years and specific associations with medication type and disorganization syndrome. Biol Psychiatry. 2009;66:586–593. doi: 10.1016/j.biopsych.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Van De Werd HJ, Rajkowska G, Evers P, Uylings HB. Cytoarchitectonic and chemoarchitectonic characterization of the prefrontal cortical areas in the mouse. Brain Struct Funct. 2010;214:339–353. doi: 10.1007/s00429-010-0247-z. [DOI] [PMC free article] [PubMed] [Google Scholar]