Abstract
Introduction
In this study we address the challenging issue of potential use of muscle strength to predict function in clinical trials. This has immediate relevance to translational studies that attempt to improve quadriceps strength in sporadic inclusion-body myositis (sIBM).
Methods
Maximum voluntary isometric contraction testing as a measure of muscle strength and a battery of functional outcomes were tested in 85 ambulatory subjects with sIBM.
Results
Marked quadriceps weakness was noted in all patients. Strength was correlated with distance walked at 2 and 6 minutes. Additional correlations were found with time to get up from a chair, climb stairs, and step up on curbs.
Conclusions
Quadriceps (knee extensor) strength correlated with performance in this large cohort of sIBM subjects, which demonstrated its potential to predict function in this disease. These data provide initial support for use of muscle strength as a surrogate for function, although validation in a clinical trial is required.
Keywords: functional outcomes, maximum voluntary isometric contraction testing, sporadic inclusion-body myositis, strength
Sporadic inclusion-body myositis (sIBM) is the most common idiopathic inflammatory myopathy affecting those >50 years of age.1,2 Typically, onset is characterized by insidious and frequently asymmetrical weakness of the arms and legs, which is often erroneously attributed to aging and can therefore remain undiagnosed for years. Progressive weakness of the quadriceps muscles in sIBM is an early finding, followed by a decline in functional abilities, progressive loss of ambulation, and wheelchair dependence. The typical course of decline is 10–15 years after onset of the disease.3,4 Currently, no treatments have been found to slow or reverse the progression of muscle weakness and deterioration in sIBM.5–16 Recent opportunities for treatment of sIBM have emerged using strategies to inhibit the myostatin pathway to increase quadriceps muscle strength. Contrasting approaches include an inhibitory myostatin antibody that binds to the activin IIB receptor (BYM338; Novartis Pharmaceuticals; ClinicalTrials.gov) or an alternatively spliced product of the follistatin gene delivered by adeno-associated virus (AAV) directly into muscle.17,18
The quadriceps muscles are targets for these clinical trials because of their preferential weakness in sIBM, their suspected contribution to gait impairment, and their accessibility as a treatment target. In the BYM338 trial, the size of the quadriceps will be measured at 8 weeks and, in the follistatin gene therapy trial, quadriceps muscle strength is the intended primary outcome variable. Despite these laudatory goals, an important unaddressed issue is the absence of any previous study demonstrating a correlation between quadriceps muscle strength and function.
In this study our aim was to directly assess knee extensor strength using the highest standards of measurement recognized by academic and regulatory authorities,19 including the 2- and 6-minute walk tests (2MWT and 6MWT)20; the IBM Functional Rating Scale (IBMFRS)21; and timed tests22–24 of stair climbing, standing from a chair, and stepping up on curbs.
METHODS
Eighty-five subjects (64 men and 21 women; mean age 65.7 years, range 44–84 years) were recruited through the Neuromuscular Disease Clinic at Nationwide Children’s Hospital and through an advertisement on the Myositis Association website. Subjects with a confirmed diagnosis of definite or possible sIBM1 with the ability to walk 6 minutes20 with or without an assistive device were eligible for participation. The study was approved by the institutional review board of Nationwide Children’s Hospital, and all subjects provided informed consent. Muscle strength and functional performance were evaluated during a single study session.
Muscle Strength Testing
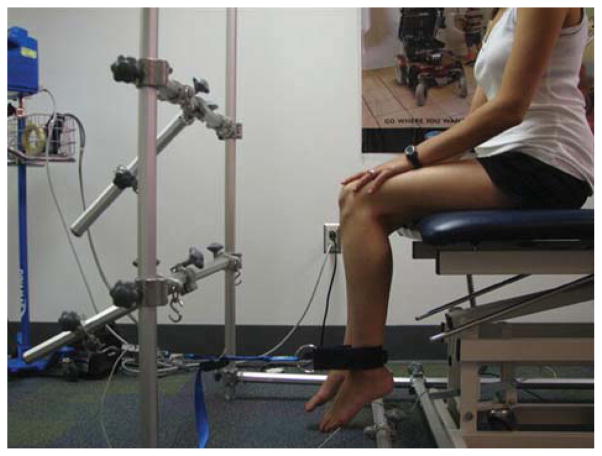
Maximum voluntary isometric contraction testing (MVICT) of bilateral knee extensors (quadriceps muscle) and knee flexors (hamstring muscles) was performed using the quantitative muscle testing system, originally described by Biomech Designs (PhysioTech, Inc., Richmond, Virginia).22–24 The system uses an adjustable cuff to attach the subject’s arm or leg to an inelastic strap linked to a force transducer with a load of 0.5–1000 N (0.05–100.0 kg). Subjects were tested on an adjustable examination table enclosed in a frame of octagonal tubing, which provides a rigid and adjustable anchor for the transducer. Isometric muscle strength was tested with the hips and knees positioned at 90° of flexion (Fig. 1). Each muscle group was tested a minimum of two consecutive times for 5 seconds, with 20 seconds of rest between trials. Muscles were tested until an error ≤10% was achieved between the two best trials. The maximum force generated (in kilograms) over 5 seconds was recorded for each trial. Subjects were stabilized by the examiner at the hips and trunk to prevent use of compensatory muscles. Two examiners were responsible for collecting data. Inter- and intrarater reliability studies on normal volunteers met standards of reproducibility showing a test–retest measurement error of ≤10% in muscle groups tested on consecutive days, as previously described.22–24
FIGURE 1.
Subject positioned for maximum voluntary isometric contraction testing of knee flexion (hamstrings). The position of the transducer is reversed and comes from behind the leg for knee extension (quadriceps) testing.
Functional Tests
Modified Timed Up and Go
The standard modified time up and go (mTUG) procedure requires subjects to rise from a chair without pushing with their arms, walk 3 meters, and return to sitting in the chair. The test was modified to allow subjects to use their arms, because most with sIBM cannot perform the task without pushing off. The height of the chair was positioned so that the hips and knees were at 90°. The use of nearby walls, equipment, or assistance from a caregiver was not allowed. Subjects performed two trials of the mTUG, and the fastest time was used in the data analysis.
Stair Climbing
Subjects were timed ascending and descending a set of four standard stairs, each 6 inches in height. Use of handrails was permitted. Subjects were encouraged to ascend and descend the stairs as quickly, but safely, as possible. They were able to use any technique or compensation required to successfully ascend or descend the stairs. However, participants had to remain in an upright position and were not permitted to use any outside assistance to complete the task. Timing was stopped when subjects were at the top of the stairs with arms at their sides.
Stepping Up on Curbs
Subjects were asked to step up a series of four curbs of increasing height (2, 4, 6, and 8 inches) without upper extremity support. Successful completion of this task was measured if subjects could place both feet on top of the curb and maintain their balance independently. They were given assistance to safely step off the curb if needed. The height of the highest curb completed was recorded.
Walking
A 25-meter track was placed in a quiet, non-crowded hallway to perform the 2MWT and 6MWT. The track was marked at 1-m increments to assist in distance measures. Pulse and blood pressure were recorded just prior to the walk, immediately after the walk, and after 3 minutes of recovery. Subjects were instructed to walk down one side of the track and back along the opposite side as quickly and safely as possible for 6 minutes. Subjects were allowed to take breaks as needed during the walking period, but timing continued during breaks. Time to complete each 50-meter lap and distance walked in meters was recorded after 2 minutes and 6 minutes.
IBM Functional Rating Scale
The Inclusion-Body Myositis Functional Rating Scale (IBMFRS) is a 10-question scale that includes items related to all areas of daily life (i.e., swallowing, fine motor tasks, dressing, bathing, walking, stair climbing, etc.).21 Performance on each item is rated on a 4-point scale with a maximum score of 40 total points. Participants rated themselves based on their typical performance of each activity.
RESULTS
Demographic Information
A description of the characteristics of our sample is presented in Table 1. Age, gender, height, and weight are reported along with the time between symptom onset and diagnosis and duration of symptoms.
Table 1.
Subject demographics upon study enrollment.
| N | Minimum | Maximum | Mean (SD) | ||
|---|---|---|---|---|---|
| Age (years) | All patients | 85 | 44.8 | 83.9 | 65.7 (±8.5) |
| Male | 64 | 44.8 | 83.9 | 66.3 (±8.1) | |
| Female | 21 | 49.4 | 80.8 | 63.9 (±9.6) | |
| Height (cm) | All patients | 81 | 152.4 | 195.6 | 176.2 (±8.8) |
| Male | 61 | 165.1 | 195.6 | 179.8 (±6.1) | |
| Female | 20 | 152.4 | 177.8 | 165.2 (±6.7) | |
| Weight (lbs.) | All patients | 81 | 98 | 270 | 184.0 (±36.9) |
| Male | 61 | 135 | 270 | 196.3 (±29.8) | |
| Female | 20 | 98 | 225 | 146.5 (±31.1) | |
| Onset of symptoms to diagnosis (years) | All patients | 77 | <1 | 16 | 3.2 (±3.1) |
| Male | 57 | <1 | 16 | 3.0 (±3.1) | |
| Female | 20 | <1 | 12 | 4.0 (± 32) | |
| Diagnosis to visit (years) | All patients | 78 | <1 | 16 | 5.1 (±3.5) |
| Male | 58 | <1 | 16 | 5.0 (±3.5) | |
| Female | 20 | 1 | 12 | 5.3 (±3.6) | |
| Onset of symptoms to visit (years) | All patients | 77 | 2 | 20 | 8.2 (±4.5) |
| Male | 57 | 2 | 20 | 7.9 (±4.3) | |
| Female | 20 | 3 | 20 | 9.1 (±5.0) |
Strength Data
MVICT of quadriceps (knee extension) ranged from <1 kg to 41.40 kg. Seven participants registered quadriceps strength of <1 kg. Comparing these values to normative data based on subject age, gender, and height represents a range of 0–85% (average 18%) of the expected MVICT measures.20 Ninety-three percent of subjects had a knee extensor strength <50% of predicted MVICT (Table 2).
Table 2.
Distribution of strength testing for quadriceps and hamstrings muscles for all subjects tested.
| N | Minimum | Maximum | Mean (SD) | ||
|---|---|---|---|---|---|
| Quadriceps strength right (kg) | All patients | 85 | 0.21 | 41.40 | 7.7 (±7.3) |
| Male | 64 | 0.21 | 41.40 | 8.4 (±8.0) | |
| Female | 21 | 0.53 | 13.4 | 5.4 (±3.9) | |
| Quadriceps strength left (kg) | All patients | 85 | 0.1 | 39.8 | 7.3 (±6.8) |
| Male | 64 | 0.1 | 39.8 | 7.9 (±7.3) | |
| Female | 21 | 0.25 | 16.8 | 5.3 (±4.1) | |
| Percent of predicted quadriceps strength of both legs combined | All patients | 81 | 0.22% | 84.9% | 18.7 (±15.6) |
| Male | 60* | 0.22% | 84.9% | 17.9 (±16.0) | |
| Female | 21 | 1.78% | 50.4% | 20.9 (±14.6) | |
| Hamstring strength right (kg) | All patients | 85 | 0.1 | 20.2 | 9.2 (±4.1) |
| Male | 64 | 0.1 | 20.2 | 3.0 (±3.1) | |
| Female | 21 | 0.33 | 12.1 | 7.1 (±3.5) | |
| Hamstring strength left (kg) | All patients | 84 | 0.1 | 20.2 | 9.0 (±4.1) |
| Male | 64 | 0.1 | 20.2 | 9.6 (±4.2) | |
| Female | 21 | 1.3 | 16.0 | 7.4 (±3.6) |
N = 60 because height was not available for 4 subjects so percent predicted could not be calculated.
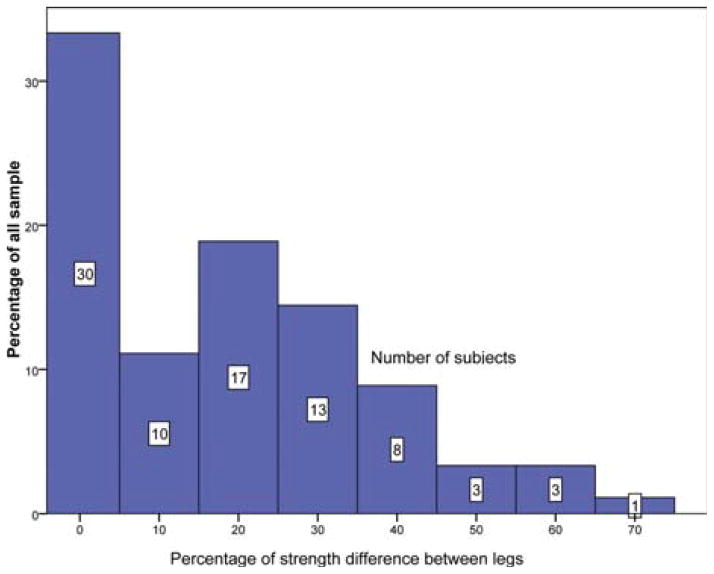
The distribution of weakness was also examined to assess asymmetrical patterns of weakness that typically occur in sIBM. The range of asymmetry ranged from 0% to 73%, with a mean of 23%. Fifty-three percent of our sample had a difference in quadriceps strength of ≥20% between sides (Fig. 2). In healthy adults, the ratio of quadriceps to hamstring muscle strength is 3:2.25 In this sIBM cohort we found an inverse ratio, as hamstring muscle strength was equal to or stronger than the quadriceps in 68% of subjects. The average ratio of quadriceps to hamstring strength in our study was 1:1, considerably lower than that of controls.25
FIGURE 2.
Asymmetry in knee extension MVICT values between the legs. The y-axis represents the percentage of all subjects with the given degree of asymmetry indicated in the histogram. Each column represents a bin of 10% increments. The number of patients in each category is indicated above each column. Notably, over half of the subjects had >20% difference in strength between the two sides.
Functional Measures
2MWT and 6MWT
The ability to walk 6 minutes was a requirement for enrollment in this study, so data are available for all participants. The range of walking abilities in the group tested ranged between 25 and 219 meters (mean = 104.0 meters, SD = 38.2 meters) for the 2MWT and 61 to 620 m (mean = 304.2 meters, SD = 116.5 meters) for the 6MWT. The distances walked during the 2MWT and 6MWT were highly correlated (r = 0.965, P ≤ 0.001). Twenty subjects required the use of a cane for safety when walking, and 19 participants used a walker. None of the participants demonstrated a loss of balance or fell during testing.
mTUG
Sixty-three of 85 subjects (74%) were able to independently complete the mTUG. Those subjects who completed the test demonstrated consistent performance when comparing both trials of the mTUG (intraclass correlation coefficient = 0.966). Eighteen subjects needed >14 seconds to complete this task, and 22 participants were unable to complete the mTUG, which is predictive of a high risk of falls.26
Stair Climbing and Stepping Up on Curbs
Fifty-eight subjects (68%) successfully ascended and descended four stairs with use of one or both handrails placed 3 feet apart (ascending stairs: range 2.0–21.5 seconds, mean = 6.3 ± 3.7 seconds; descending stairs: range 1.6–22.9 seconds, mean = 6.1 ± 4.2 seconds). Seventy-five percent of subjects were able to successfully step onto at least a 2-inch step without support, 67% stepped onto a 4-inch step, 52% stepped onto a 6-inch step, and 35% stepped onto an 8-inch step.
IBMFRS
Eighty participants (94%) completed the IBMFRS (maximum score = 40), and scores ranged between 12 and 37 points (mean 26.3 ± 5.7 points). Fifty-two percent of respondents indicated no difficulty swallowing, 39% reported early swallowing problems, and 9% reported moderate to severe swallowing problems. Sixty-eight percent of participants reported difficulties with fine motor tasks requiring grip strength (i.e., handwriting, handling utensils, opening doors, and picking up small objects), and 75% indicated difficulty with tasks requiring upper extremity strength and flexibility (i.e., dressing, bathing). Ninety-nine percent of the subjects required use of compensatory movements, use of arms, or additional support to transition from sitting to standing from a standard chair. Ninety-six percent of participants reported changes in walking speed and balance, and 63% used a cane or walker at least part of the time. All subjects reported difficulty ascending stairs, and 60% were unable to climb stairs with one handrail only.
Relationship between Quadriceps Strength and Functional Measures
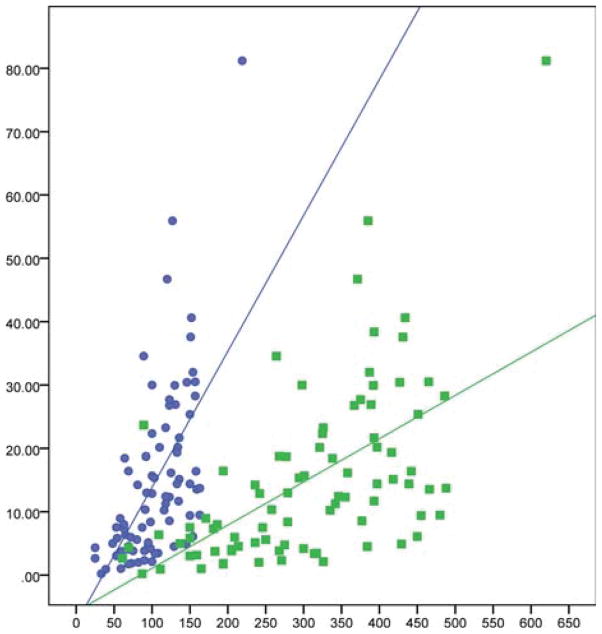
One-way Pearson’s correlation analysis revealed the strongest correlations between quadriceps strength and both the 2MWT (r = 0.603, P ≤ 0.001) and 6 MWT (r = 0.578, P ≤ 0.001) (Table 3 and Figs. 3 and 4). A correlation was also found between quadriceps strength and the mTUG (r = −0.419, P ≤ 0.001). The negative correlation indicates that the time required to complete the mTUG decreased when the subject had greater quadriceps strength. Although knee extensor strength showed a correlation between time to ascend (r = −0.307, P ≤ 0.019) and descend (r = −0.380, P ≤ 0.003), correlation coefficients were reduced compared with the 2MWT and 6MWT. Ability to step up curbs without extremity support was also strongly correlated with knee extensor strength (r = 0.506, P ≤ 0.001). The subject’s score on the IBMFRS also correlated with quadriceps strength (r = 0.375, P ≤ 0.001), although multiple factors influenced this finding and leg attributes could not be separated from other functions.
Table 3.
Results of functional tests and correlation with quadriceps strength.
| N | Min | Max | Mean (SD) | r | P-value | |
|---|---|---|---|---|---|---|
| IBMFRS | 80 | 12 | 37 | 26.3 ± 5.7 | 0.375 | 0.001 |
| mTUG (s) | 63 | 5.6 | 35.2 | 12.2 ± 6.1 | −0.419 | 0.001 |
| Stairs-up (s) | 58 | 2.0 | 21.5 | 6.3 ± 3.7 | −0.307 | 0.019 |
| Stairs-down (s) | 58 | 1.6 | 22.9 | 6.1 ± 4.2 | −0.380 | 0.003 |
| Step height (inches) | 50 | 2 | 8 | 4.4 ± 3.3 | 0.506 | ≤0.001 |
| 2MWT distance (meters) | 84 | 25 | 219 | 104.0 ± 38.2 | 0.603 | ≤0.001 |
| 6MWT distance (meters) | 85 | 61 | 620 | 304.2 ± 116.5 | 0.578 | 0.001 |
N, number of subjects capable of performing task. All items significant at P ≤ 0.05. Min and Max indicate performance range for each item; Stairs-up = ascending 4 stairs; Stairs-down = descending 4 stairs; Step height = stepping up a series of four curbs of increasing height (2, 4, 6, 8 inches). IBMFRS, Inclusion-Body Myositis Functional Rating Scale; mTUG, modified timed up and go; 2MWT, 2-minute walk test; 6MWT, 6-minute walk test.
FIGURE 3.
Correlations shown between quadriceps strength and 2MWT (r = 0.603, P ≤ 0.001) and 6MWT (r = 0.578, P ≤ 0.001).
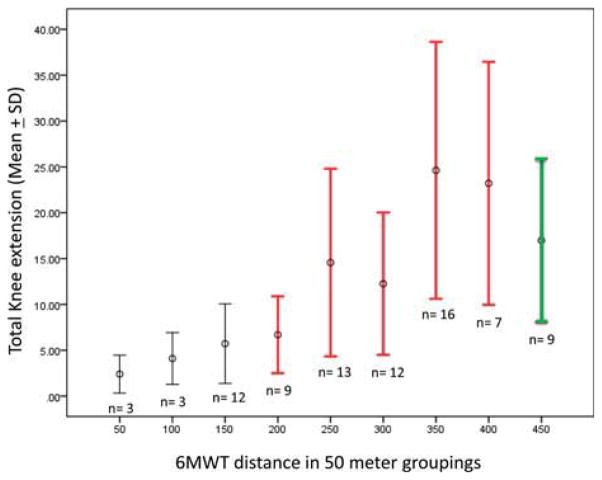
FIGURE 4.
Kilograms of force predict distance walked in 6MWT. Means and standard deviations of force generation by MVICT in kilograms of bilateral quadriceps muscles grouped by the number of complete laps (one lap = 50 meters) walked in 6 minutes. Red bars indicate a walking speed sufficient to safely cross a crosswalk. Green bar indicates typical community walking speed in the elderly (>1.3 meters/second). Variability reflects compensatory patterns used to complete functional tasks at every level of muscle strength.
DISCUSSION
It was not the intent of this study to demonstrate an independent role for quadriceps muscle strength as the predominant determinant of gait. However, a comparison of quadriceps and hamstring muscles showed an inverse strength ratio compared with normal (3.5:1 reduced to 1:1), which emphasizes that knee extensors are a targeted muscle group in sIBM. This quantitatively confirms what has been described repeatedly in the literature.1,3 Our study has addressed the challenging issue of the potential use of muscle strength to predict function in clinical trials. The findings demonstrate a strong correlation between knee extensor strength and distance walked at 2 and 6 minutes. The size of the sIBM cohort and the sensitivity of MVICT as a measure of strength support these conclusions. The results were reinforced by correlations in time tests, including rising from a chair, stepping up on a curb, and climbing stairs.
The correlation of knee extensor strength with functional outcome measures is important for several reasons. One of the most significant challenges for clinician-scientists is validating the significance of muscle strength in the setting of a clinical trial and extrapolating the findings to the long-term outcome of patients with neuromuscular disease. The need is exemplified by the use of glucocorticoids in Duchenne muscular dystrophy (DMD). In a double-blind, controlled trial, prednisone given for 6 months increased strength by 6.7% when manual muscle testing was used as the outcome measure.27 The placebo group showed a decline of 4.8% during the same period. Despite this evidence of unequivocal improvement generated using the “gold standard” of clinical design (randomized, controlled, double-blind), the data had no intrinsic meaning in the absence of correlation with a clinically meaningful outcome for patients. It has taken several decades and multiple studies to demonstrate that glucocorti-coid treatment prolongs ambulation and increases both survival and quality of life,28–32 with greater benefit accompanying earlier intervention.33
To avoid the mistakes of the past, the FDA has strongly urged clinicians to identify surrogate outcomes that are predictive of long-term efficacy for use in clinical trials.19 Standards for surrogate measures have been identified in the Federal Registry (21 CFR 314.510) and are available online from the FDA website. A surrogate endpoint is defined (57 FR13234 at 13235, April 15, 1992) as a “laboratory or physical sign that is used in therapeutic trials as a substitute for clinically meaningful endpoint that is a direct measure of how a patient feels, functions, or survives and that is expected to predict the effect of a therapy.” Thus, the surrogate is a short-term outcome that can predict a clinically meaningful result. Our study was designed to confront this challenge for trials of myostatin inhibition that target the quadriceps muscle. We found that the strength of the knee extensors (quadriceps), as measured by MVICT, showed a strong correlation with the distance walked in both the 2MWT (r = 0.603, P ≤ 0.001) and the 6MWT (r = 0.578, P ≤ 0.001). Correlations with knee extensor strength were also found in the mTUG, ability to step up on curbs, and ascend and descend stairs.
These findings lay the foundation for using quadriceps strength as a meaningful short-term surrogate outcome with the potential to predict increase in function in the clinical trial. It goes without saying, however, that the findings from this study must be validated in a treatment trial in sIBM, after which there will be explicit proof of principle corroborating the relationship of muscle strength to function. As we introduce molecular-based phase I/II clinical trials that include small numbers of patients with multiple dosing levels, the support of a large, pretrial database will add decisive credibility to the findings.
Caveats for the conclusions reached herein also need emphasis. For example, it is important to stress that the correlations of strength and function in sIBM cannot be extrapolated to other diseases without appropriate testing prior to a clinical trial. It is also essential to add that the testing conditions used to predict functional outcome may not always strictly apply to the environmental circumstances of the patient. For example, take the conclusion that quadriceps strength predicts reduced time for stair climbing. This may only apply to staircases with handrails that exactly simulate those used in the test environment. With regard to predicting walking distance from quadriceps muscle strength, it is naive to discount individual patient differences in compensatory gait patterns that may influence the results of an individual subject enrolled in a clinical trial. This underscores the importance of a quality-of-life assessment like the IBMFRS that must also be used in clinical trial design. It was reassuring that the potential use of quadriceps strength to predict function was reinforced in this study, because the subjects with the best-preserved quadriceps strength exhibited the highest IBMFRS scores.
Acknowledgments
This study was supported by the Myositis Association and Parent Project Muscular Dystrophy.
Abbreviations
- AAV
adeno-associated virus
- DMD
Duchenne muscular dystrophy
- FDA
Food and Drug Administration
- IBMFRS
Inclusion-Body Myositis Functional Rating Scale
- mTUG
modified timed up and go
- 2MWT
2-minute walk test
- 6MWT
6-minute walk test
- MVICT
maximum voluntary isometric contraction testing
- sIBM
sporadic inclusion-body myositis
References
- 1.Griggs RC, Askanas V, DiMauro S, Engel A, Karpati G, Mendell JR, et al. Inclusion body myositis and myopathies. Ann Neurol. 1995;38:705–713. doi: 10.1002/ana.410380504. [DOI] [PubMed] [Google Scholar]
- 2.Griggs RC. The current status of treatment for inclusion-body myositis. Neurology. 2006;66(suppl):S30–32. doi: 10.1212/01.wnl.0000192262.29924.9e. [DOI] [PubMed] [Google Scholar]
- 3.Peng A, Koffman BM, Malley JD, Dalakas MC. Disease progression in sporadic inclusion body myositis: observations in 78 patients. Neurology. 2000;55:296–298. doi: 10.1212/wnl.55.2.296. [DOI] [PubMed] [Google Scholar]
- 4.Rose MR, McDermott MP, Thornton CA, Palenski C, Martens WB, Griggs RC. A prospective natural history study of inclusion body myositis: implications for clinical trials. Neurology. 2001;57:548–550. doi: 10.1212/wnl.57.3.548. [DOI] [PubMed] [Google Scholar]
- 5.Amato AA, Barohn RJ, Jackson CE, Pappert EJ, Sahenk Z, Kissel JT. Inclusion body myositis: treatment with intravenous immunoglobulin. Neurology. 1994;44:1516–1518. doi: 10.1212/wnl.44.8.1516. [DOI] [PubMed] [Google Scholar]
- 6.Greenberg SA. Proposed immunologic models of the inflammatory myopathies and potential therapeutic implications. Neurology. 2007;69:2008–2019. doi: 10.1212/01.WNL.0000291619.17160.b8. [DOI] [PubMed] [Google Scholar]
- 7.Needham M, Mastaglia FL. Inclusion body myositis: current pathogenetic concepts and diagnostic and therapeutic approaches. Lancet Neurol. 2007;6:620–631. doi: 10.1016/S1474-4422(07)70171-0. [DOI] [PubMed] [Google Scholar]
- 8.Muscle Study Group. Randomized pilot trial of betaINF1a (Avonex) in patients with inclusion body myositis. Neurology. 2001;57:1566–1570. doi: 10.1212/wnl.57.9.1566. [DOI] [PubMed] [Google Scholar]
- 9.Muscle Study Group. Randomized pilot trial of high-dose betaINF-1a in patients with inclusion body myositis. Neurology. 2004;63:718–720. doi: 10.1212/01.wnl.0000134675.98525.79. [DOI] [PubMed] [Google Scholar]
- 10.Dalakas MC, Koffman B, Fujii M, Spector S, Sivakumar K, Cupler E. A controlled study of intravenous immunoglobulin combined with prednisone in the treatment of IBM. Neurology. 2001;56:323–327. doi: 10.1212/wnl.56.3.323. [DOI] [PubMed] [Google Scholar]
- 11.Dalakas MC, Sonies B, Dambrosia J, Sekul E, Cupler E, Sivakumar K. Treatment of inclusion-body myositis with IVIg: a double-blind, placebo-controlled study. Neurology. 1997;48:712–716. doi: 10.1212/wnl.48.3.712. [DOI] [PubMed] [Google Scholar]
- 12.Badrising UA, Maat-Schieman ML, Ferrari MD, Zwinderman AH, Wessels JA, Breedveld FC, et al. Comparison of weakness progression in inclusion body myositis during treatment with methotrexate or placebo. Ann Neurol. 2002;51:369–372. doi: 10.1002/ana.10121. [DOI] [PubMed] [Google Scholar]
- 13.Lindberg C, Trysberg E, Tarkowski A, Oldfors A. Anti-T-lymphocyte globulin treatment in inclusion body myositis: a randomized pilot study. Neurology. 2003;61:260–262. doi: 10.1212/01.wnl.0000071852.27182.c7. [DOI] [PubMed] [Google Scholar]
- 14.Rutkove SB, Parker RA, Nardin RA, Connolly CE, Felice KJ, Raynor EM. A pilot randomized trial of oxandrolone in inclusion body myositis. Neurology. 2002;58:1081–1087. doi: 10.1212/wnl.58.7.1081. [DOI] [PubMed] [Google Scholar]
- 15.Barohn RJ, Herbelin L, Kissel JT, King W, McVey AL, Saperstein DS, Mendell JR. Pilot trial of etanercept in the treatment of inclusion-body myositis. Neurology. 2006;66(suppl 1):S123–124. doi: 10.1212/01.wnl.0000192258.32408.54. [DOI] [PubMed] [Google Scholar]
- 16.Sancricca C, Mora M, Ricci E, Tonali PA, Mantegazza R, Mirabella M. Pilot trial of simvastatin in the treatment of sporadic inclusion-body myositis. Neurol Sci. 2011;32:841–847. doi: 10.1007/s10072-011-0657-6. [DOI] [PubMed] [Google Scholar]
- 17.Haidet AM, Rizo L, Handy C, Umapathi P, Eagle A, Shilling C, et al. Long-term enhancement of skeletal muscle mass and strength by single gene administration of myostatin inhibitors. Proc Natl Acad Sci USA. 2008;105:4318–4322. doi: 10.1073/pnas.0709144105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kota J, Handy CR, Haidet AM, Montgomery CL, Eagle A, Rodino-Klapac LR, et al. Follistatin gene delivery enhances muscle growth and strength in nonhuman primates. Sci Transl Med. 2009;1:6ra15. doi: 10.1126/scitranslmed.3000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mendell JR, Csimma C, McDonald CM, Escolar DM, Janis S, Porter JD, et al. Challenges in drug development for muscle disease: a stakeholders’ meeting. Muscle Nerve. 2007;35:8–16. doi: 10.1002/mus.20686. [DOI] [PubMed] [Google Scholar]
- 20.McDonald CM, Henricson EK, Han JJ, Abresch RT, Nicorici A, Atkinson L, et al. The 6-minute walk test in Duchenne/Becker muscular dystrophy: longitudinal observations. Muscle Nerve. 2010;42:966–974. doi: 10.1002/mus.21808. [DOI] [PubMed] [Google Scholar]
- 21.Jackson CE, Barohn RJ, Gronseth G, Pandya S, Herbelin L Muscle Study Group. Inclusion body myositis functional rating scale: a reliable and valid measure of disease severity. Muscle Nerve. 2008;37:473–476. doi: 10.1002/mus.20958. [DOI] [PubMed] [Google Scholar]
- 22.The FSH-DY Group. A prospective, quantitative study of the natural history of facioscapulohumeral muscular dystrophy (FSHD): implications for therapeutic trials. Neurology. 1997;48:38–46. doi: 10.1212/wnl.48.1.38. [DOI] [PubMed] [Google Scholar]
- 23.Tawil R, McDermott MP, Pandya S, King W, Kissel J, Mendell JR, et al. A pilot trial of prednisone in facioscapulohumeral muscular dystrophy. Neurology. 1997;48:46–49. doi: 10.1212/wnl.48.1.46. [DOI] [PubMed] [Google Scholar]
- 24.Kissel JT, McDermott MP, Mendell JR, King WM, Pandya S, Griggs RC, et al. Randomized, double-blind, placebo-controlled trial of albuterol in facioscapulohumeral dystrophy. Neurology. 21;57:1434–1440. doi: 10.1212/wnl.57.8.1434. [DOI] [PubMed] [Google Scholar]
- 25.Hewett TE, Myer GD, Zazulak BT. Hamstrings to quadriceps peak torque ratios diverge between sexes with increasing isokinetic angular velocity. J Sci Med Sport. 2007;11:452–459. doi: 10.1016/j.jsams.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bohannon RW. Reference values for the timed up and go test: a descriptive meta-analysis. J Geriatr Phys Ther. 2006;29:64–68. doi: 10.1519/00139143-200608000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Mendell JR, Moxley RT, Griggs RC, Brooke MH, Fenichel GM, Miller JP, et al. Randomized, double-blind six-month trial of prednisone in Duchenne’s muscular dystrophy. N Engl J Med. 1989;320:1592–1597. doi: 10.1056/NEJM198906153202405. [DOI] [PubMed] [Google Scholar]
- 28.Moxley RT, III, Pandya S, Ciafaloni E, Fox DJ, Campbell K. Change in natural history of Duchenne muscular dystrophy with long-term corticosteroid treatment: implications for management. J Child Neurol. 2010;25:1116–1129. doi: 10.1177/0883073810371004. [DOI] [PubMed] [Google Scholar]
- 29.Houde S, Filiatrault M, Fournier A, Dubé J, D’Arcy S, Bérubé D, et al. Deflazacort use in Duchenne muscular dystrophy: an 8-year follow-up. Pediat Neurol. 2008;38:2000–2006. doi: 10.1016/j.pediatrneurol.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 30.King WM, Ruttencutter R, Nagaraja HN, Matkovic V, Landoll J, Hoyle C, et al. Orthopedic outcomes of long-term daily corticosteroid treatment in Duchenne muscular dystrophy. Neurology. 2007;68:1607–1613. doi: 10.1212/01.wnl.0000260974.41514.83. [DOI] [PubMed] [Google Scholar]
- 31.Biggar WD, Harris VA, Eliasoph L, Alman B. Long-term benefits of deflazacort treatment for boys with Duchenne muscular dystrophy in their second decade. Neuromuscul Disord. 2006;16:249–255. doi: 10.1016/j.nmd.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 32.Balaban B, Matthews DJ, Clayton GH, Carry T. Corticosteroid treatment and functional improvement in Duchenne muscular dystrophy: long-term effect. Am J Phys Med Rehabil. 2005;84:843–850. doi: 10.1097/01.phm.0000184156.98671.d0. [DOI] [PubMed] [Google Scholar]
- 33.Manzur AY, Kuntzer T, Pike M, Swan A. Glucocorticoid corticosteroids for Duchenne muscular dystrophy. Cochrane Database Syst Rev. 2008;(1):CD003725. doi: 10.1002/14651858.CD003725.pub3. [DOI] [PubMed] [Google Scholar]