Abstract
Persistent neuroinflammation and microglial activation play an integral role in the pathogenesis of many neurological disorders. We investigated the role of voltage-gated sodium channels (VGSC) and Na+/H+ exchangers (NHE) in the activation of immortalized microglial cells (BV-2) after lipopolysaccharide (LPS) exposure. LPS (10 and 100 ng/ml) caused a dose- and time-dependent accumulation of intracellular sodium [(Na+)i] in BV-2 cells. Pre-treatment of cells with the VGSC antagonist tetrodotoxin (TTX, 1 μM) abolished short-term Na+ influx, but was unable to prevent the accumulation of (Na+)i observed at 6 and 24 h after LPS exposure. The NHE inhibitor cariporide (1 μM) significantly reduced accumulation of (Na+)i 6 and 24 h after LPS exposure. Furthermore, LPS increased the mRNA expression and protein level of NHE-1 in a dose- and time-dependent manner, which was significantly reduced after co-treatment with TTX and/or cariporide. LPS increased production of TNF-α, ROS, and H2O2 and expression of gp91phox, an active subunit of NADPH oxidase, in a dose- and time-dependent manner, which was significantly reduced by TTX or TTX + cariporide. Collectively, these data demonstrate a closely-linked temporal relationship between VGSC and NHE-1 in regulating function in activated microglia, which may provide avenues for therapeutic interventions aimed at reducing neuroinflammation.
Keywords: BV-2, Lipopolysaccharide, Tetrodotoxin, Inflammation, Microglia, Neurodegeneration, NADPH oxidase, NHE-1, Nav 1.6, Sodium channel, Sodium hydrogen exchanger
Introduction
A growing body of evidence indicates that neuroinflammation and microglial activation contribute to the pathogenesis of a variety of neurodegenerative diseases, including brain ischemia, Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), and amyotrophic lateral sclerosis (Akiyama et al., 2000; Block and Hong, 2005; Fujita et al., 2012; Politis et al., 2011; Tansey and Goldberg, 2010). Microglia are resident macrophages in the brain and play an active role in host defense and tissue repair in the central nervous system by removing cellular debris and pathogens (Aloisi, 2001; Su et al., 2009). However, uncontrolled activation can lead to chronic inflammation resulting in neuronal damage. Indeed, several pro-inflammatory pathways are initiated following the activation of microglia, including the production of reactive oxygen species (ROS) and hydrogen peroxide (H2O2) and cytokine secretion by NADPH oxidase (Chao et al., 1992; Harrigan et al., 2008; Liu et al., 2010).
Regulation of microglial activation involves a number of signal transduction pathways, and microglia express several ion channels, including K+, Ca++, and Na+ channels, which may participate in this process (Black et al., 2009; Eder, 1998, 2005; Farber and Kettenmann, 2005, 2006; Schilling and Eder, 2007). Voltage-gated sodium channels (VGSC) are primarily responsible for generation of action potential in neurons. However, abnormal expression of VGSC in activated microglia was observed in multiple sclerosis cases (Black et al., 2012; Mantegazza et al., 2010; Waxman, 2008). The exact role of VGSC in the microglia is not well established and is not without controversy (Kettenmann et al., 2011). Recent studies by Black et al. (2009, 2012) reported that inhibition of VGSC significantly reduced the phagocytic activity and migration of activated microglia in culture, suggesting potential immunomodulatory properties of VGSC. Liu et al. (2010) reported that exposure of microglial cells to LPS, phorbol esters, or oxygen-glucose deprivation followed by reoxygenation leads to increased internal Na+ levels and microglial activation.
In addition to VGSC, Na+/H+ exchangers (NHE) regulate intracellular Na+ levels. The mammalian NHE family consists of 9 isoforms (NHE-1 to NHE-9), of which NHE-1 is the best characterized and most abundant isoform in the CNS (Liu et al., 2010; Malo and Fliegel, 2006). NHE-1 regulates intracellular pH by exchanging one intracellular H+ ion for one extracellular Na+ ion in an electroneutral manner (Fliegel, 2005; Malo and Fliegel, 2006). Although a significant amount of research has focused on the association of NHE-1 with heart and kidney diseases, only recently has NHE-1 been associated with microglial activation following brain injury and cerebral ischemia (Cengiz et al., 2011; Kauppinen et al., 2008).
Given the potential for VGSC and NHE to regulate microglia function through manipulation of intracellular Na+ levels, we sought to characterize the relative roles of VGSC and NHE following activation of microglia by LPS exposure and determine whether these two ion channels functionally interact. Our data demonstrate that activation of microglial cells with lipopolysaccharide (LPS) causes a rapid Na+ influx into microglial cells that is sensitive to the VGSC antagonist tetrodotoxin (TTX). Continued exposure to LPS for 6–24 h leads to a down-regulation of VGSC and an up-regulation of NHE-1. The combined actions of VGSC and NHE-1 contribute to activation of NADPH oxidase, generation of ROS and release of tumor necrosis factor alpha (TNF-α), which can be attenuated by combinations of TTX and the NHE-1 antagonist cariporide. These data demonstrate that VGSC and NHE-1 coordinately regulate important components of the inflammatory response in the microglia and may be viable targets for therapeutic interventions to reduce neuroinflammation.
Materials and methods
Cell culture
Immortalized mouse (C57Bl/6) microglial cells (BV-2) were cultured in minimum essential medium (MEM) containing 10% fetal bovine serum, 2 mM l-glutamine, 1 mM sodium pyruvate, 1 mM non-essential amino acids, 50 IU penicillin, and 50 μg/ml streptomycin, as described previously (Gibson et al., 2012). The cells were maintained at 37 °C in a humidified atmosphere of 5% CO2. A stock solution (1 mg/ml) of LPS (γ-irradiated Escherichia coli 0111:B4; Sigma Aldrich, St. Louis, MO) was prepared in sterile water. Dilutions were made in MEM medium and added to the cells in the presence or absence of the Na+ channel antagonist, TTX (1 μM) and the Na+/H+ exchanger (NHE) antagonist, cariporide (1 μM). Doses for TTX and cariporide were selected based on our (Hossain and Richardson, 2011) and other (Liu et al., 2010) studies.
Primary microglia
Primary microglia were isolated from 1 day old C57BL/6 mice as described by Gordon et al. (2011). In brief, brain tissues were freed of meninges and dissociated in a nylon mesh sterile cell strainer after enzymatic digestion in 0.25% trypsin in a 37 °C water bath for 30 min. The cells were suspended in DMEM-F12 complete medium and seeded in T-75 tissue culture flasks. After 12–14 days of incubation, the microglia were separated from mixed glial cultures using EasySep® mouse CD11b positive selection kit (STEMCELL Technologies Inc., Vancouver, Canada) according to the manufacturer’s instructions and plated according to the cell number required for experiments.
Cytotoxicity assay
Cell viability was evaluated with AlamarBlue® cell viability reagent (Invitrogen, Grand Island, NY). Cells (2.5 × 104 cells/well) were seeded in 96 well plates and treated with LPS (0, 1, 10, 100, and 500 ng/ml) for 6 and 24 h. At the end of treatment, the medium was removed and the cells were incubated in 10 μl AlamarBlue containing 100 μl MEM medium for 3 h at 37 °C. Following incubation, formation of resorufin was measured with fluorescence excitation 570 nm and emission 585 nm in a Spectramax microplate reader (Molecular Devices, Sunnyvale, CA).
Measurement of Na+ influx and intracellular Na+ [(Na+)i]
Na+ influx and (Na+)i was measured as previously described (Hossain and Richardson, 2011). Briefly, after 12 h in culture, microglia cells were rinsed once with PBS and then incubated with 10 μM CoroNa™ Green-AM (Invitrogen, Grand Island, NY) in oxygenated Krebs–Ringer–HEPES buffer (KRHB) for 1 h at 37 °C. Cells were rinsed twice with KRHB and then test compounds were added after obtaining a stable baseline. Na+ influx was determined by measuring fluorescence intensity every 30 s for 30 min with excitation 492 nm and emission 516 nm. Accumulation of [(Na+)i] in BV-2 cells was measured 6 and 24 h after LPS exposure with CoroNa™ green indicator as described above.
Measurement of intracellular reactive oxygen species (ROS)
Production of intracellular ROS was measured using the fluorescent probe 2,7-dichlorofluorescin diacetate (H2DCFDA) (Molecular Probe, Grand Island, NY) as previously described (Hu et al., 2011) with some modifications. After 12 h culturing, BV-2 cells were incubated with 5 μM H2DCFDA prepared in 1% glucose containing KRHB (pH 7.4) for 30 min at 37 °C, and then rinsed twice with PBS. Test compounds were added to the cells and ROS levels were determined at 0 and 1 h by measuring the fluorescent intensity with excitation 530 nm and emission 570 nm using Spectramax microplate reader (Molecular Devices, Sunnyvale, CA).
Measurement of H2O2
Production of H2O2 from the cells into medium was measured with Amplex Red® (Molecular Probe) as previously described (Fussell et al., 2011). Cells (2.5 × 104 cells/well in 96-well plates) were treated with LPS (0–100 ng/ml) in serum- and phenol red-free MEM medium with or without 1 μM TTX and cariporide for 24 h at 37 °C. After the treatment, 100 μl of cell-free media was added to 25 μl of ice cold acetonitrile to stop the reaction. The fluorescence was then measured with 25 μM Amplex Red and 1 U/ml horseradish peroxidase at 540 nm excitation and 595 nm emission using a Spectramax microplate reader (Molecular Devices, Sunnyvale, CA).
Measurement of TNF-α release
Cells (2.5 × 104 cells/well) were seeded in 96-well plates and were treated with LPS (0–100 ng/ml) for 6 and 24 h. At the end of treatment, cultured media were collected and secretion of TNF-α into media was measured using a commercial enzyme-linked immunosorbent assay (ELISA) kit as per manufacturer’s instructions (Invitrogen, CA, USA). The absorbance at 450 nm was determined using a Spectramax microplate reader (Molecular Devices, Sunnyvale, CA).
RNA isolation and cDNA synthesis
Total cellular RNA was isolated using a Qiagen RNeasy® mini kit (Valencia, CA) according to the manufacturer’s instructions. RNA concentration was determined on a NanoDrop 2000 Spectrophotometer (Thermo Scientific) and then 1 μg of total RNA was reverse transcribed using Superscript II kit (Invitrogen, San Diego, CA) for cDNA synthesis according to the manufacturer’s protocol.
Quantitative reverse transcriptase polymerase chain reaction (qPCR)
The primer sequences (Table 1) were designed using the Primer Blast program (NCBI) and ordered through Integrated DNA Technology (Coralville, IA). Real-time qPCR was performed using an ABI 7900HT and SYBR Green (Applied Biosystems) detection system to determine mRNA expression, as described previously (Richardson et al., 2006). Melting curves were obtained to confirm the quality of product for each primer set. Each primer set was checked by agarose gel electrophoresis to confirm a single PCR product of the expected size. The expression of GAPDH was used as a house keeping gene to normalize the amount of mRNA in each sample. Data were analyzed using the ΔΔCt method and results are presented as relative levels of mRNA.
Table 1.
The primer sequences for qPCR designed with primer blast (NCBI).
| Mouse primers |
Forward | Reverse |
|---|---|---|
| NHE-1 | 5′-TGGGCGTGGTCTACGGGGTA-3′ | 5′-GCCTCCACATAGGGGCGCAT-3′ |
| gp91phox | 5′-TCCTGCTGCCAGTGTGTCGAAA-3′ | 5′-TGCAATTGTGTGGATGGCGGTG-3′ |
| GAPDH | 5′-GGGCTGGCATTGCTCTCAATGAC-3′ | 5′-TCTTGCTCAGTGTCCTTGCTGGG-3′ |
Western immunoblotting
After 6 and 24 h LPS exposure, cells were harvested in lysis buffer with protease inhibitors as previously described (Hossain and Richardson, 2011). Samples were centrifuged at 14,000 ×g for 10 min, and supernatants were collected. Protein concentrations were quantified using the bicinchoninic acid (BCA) assay (Smith et al., 1985), and 20 μg of protein sample was loaded per lane on 4–12% NuPAGE® Novex® Bis-Tris Mini Gels (Invitrogen, Carlsbad, CA). After electrophoresis, proteins from the gels were blotted to polyvinylidene difluoride (PVDF) membranes (Invitrogen, Carlsbad, CA). The membranes were incubated in 7.5% non fat milk in 0.1% Tween 20 containing Tris buffered saline (TTBS) for 1 h at room temperature to block non-specific protein binding sites. The membranes were then incubated overnight at 4 °C with 1:500 diluted anti-Nav1.6 (ASC-009, Alomone Labs, Israel), anti-NHE-1 (sc-16097, Santa Cruz, CA) or anti-gp91phox (sc-5827 Santa Cruz, CA) polyclonal primary antibody. After being washed three times with TTBS, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies for 1 h at room temperature. The specific antibody bound protein was detected by SuperSignal® West Dura Extended Duration Substrate (Thermo Scientific, Rockford, IL) using an Alpha Innotech Fluorchem (San Leandro, CA) imaging system and stored as a digital image. Membranes were then stripped for 15 min at room temperature with Pierce Stripping Buffer and re-probed with a monoclonal α-tubulin antibody to confirm equal protein loading in each lane.
Immunofluorescent staining
BV-2 cells (1 × 105 cells/well) were seed ed in 8 well chamber slides and allowed to attach overnight. Cells were treated with 10 or 100 ng/ml LPS for 6 and 24 h with or without TTX. At the end of treatment, cells were washed with PBS and fixed with 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). Slides were washed and blocked with 1% BSA in PBS with 0.1% Triton X-100 for 1 h. Slides were then incubated overnight at 4 °C with anti-Nav1.6 (1:250 dilution, ASC-009, Alomone Labs, Israel). After being washed three times with PBS, slides were incubated with the appropriate, species specific secondary antibody-conjugated to Alexa Fluor 488 (Life Technologies, Grand Island, NY) for 1 h at room temperature. Primary microglia were incubated with anti-Iba1 (1:500) and labeled with Alexa Fluor 594 (Life technologies, Grand Island, NY). Slides were coverslipped with Prolong Gold mounting medium containing 4′,6-diamidino-2-phenylindole (DAPI) (Life technologies, Carlsbad, CA). Images were captured on a Zeiss Observer D1 microscope (Zeiss Inc., Thornwood, NY) with an X-Cite series 120Q fluorescent illuminator and a Jenoptik camera with ProgRes CapturePro 2.8 software (Jenoptik, Easthampton, MA). Negative controls without primary antibody were included to ensure minimal nonspecific staining (data not shown).
Statistical analysis
Statistical analysis was performed using GraphPad Prism 5.01 software (GraphPad Software, Inc., La Jolla, CA). All experiments were performed at least 3 separate times using different vials of frozen cell stocks. On individual days, experiments were performed in duplicate or triplicate and averaged to form a single experimental unit. Data are presented as mean ± SEM. All analyses were performed on raw data using analysis of variance (ANOVA) or Student’s t-test where appropriate. For ANOVA, Bonferroni’s or Tukey’s post hoc multiple comparison tests were performed where appropriate. Statistical significance was determined at level of p < 0.05.
Results
LPS causes a rapid and sustained influx of Na+ in BV-2 and primary microglia cells
We assessed the relative contributions of VGSC and NHE-1 to intracellular Na+ [(Na+)i] in LPS-stimulated BV-2 cells. Cells were treated with 10 and 100 ng/ml LPS and Na+ influx was measured every 30 s with CoroNa™ Green-AM for 30 min. LPS caused an immediate (48%–75%) increase in Na+ influx which was completely abolished by 1 μM TTX (Fig. 1A). Similar results were found with primary microglia (Fig. 1B).
Fig. 1.
LPS causes rapid Na+ influx in microglia that is sensitive to tetrodotoxin. LPS administration increases Na+ influx that is inhibited by the sodium channel antagonist (TTX). Representative traces of Na+ influx in BV-2 cells (A) and primary microglia (B) and changes in [Na+]i (C and D) in BV-2 cells. Data represent mean ± SEM of three to five individual experiments, each performed in triplicate and expressed as percentage of control. * indicates significant difference from control and # indicates significant differences compared to LPS-treated cells (p < 0.05).
(Na+)i was measured 6 and 24 h after LPS exposure with or without inhibition of VGSC and Na+/H+ exchanger (NHE) to determine effects on long-term accumulation of Na+. LPS caused accumulation of (Na+)i in BV-2 cells in a dose- and time-dependent manner (Figs. 1C; D). Intracellular Na+ was increased 145% relative to control at 6 h after exposure and increased 150% and 175% 24 h after exposure to 10 and 100 ng/ml LPS, respectively. In contrast to the short-term Na+ influx, TTX was unable to significantly prevent the accumulation of (Na+)i observed at 6 and 24 h after LPS exposure. However, inhibition of NHE with cariporide significantly reduced intracellular Na+ accumulation after 6 and 24 h of LPS treatment (Figs. 1C; D).
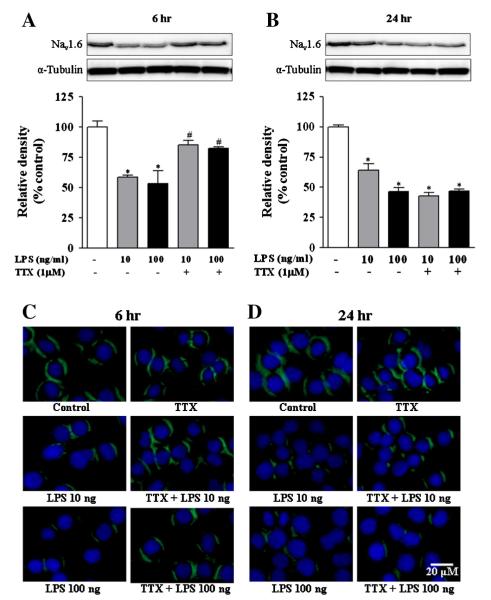
LPS down-regulates Nav 1.6 in BV2 cells
Based on the lack of efficacy of TTX at decreasing Na+ accumulation at 6 and 24 h, we hypothesized that the increased Na+ flux resulted in the down-regulation of VGSC, similar to that observed in cultured neurons (Dargent and Couraud, 1990). Because the Nav 1.6 isoform was reported to be specifically involved in microglial phagocytosis and migration (Black et al., 2009; Craner et al., 2005), we measured protein expression of Nav1.6 following LPS exposure by western immunoblot. LPS decreased Nav1.6 protein by 40% to 50% at 6 h (Fig. 2A) and 36% to 54% at 24 h (Fig. 2B) after exposure with 10 and 100 ng/ml LPS, respectively. The down-regulation of Nav1.6 could be blocked by TTX at the 6 h, but not the 24 h, time point. We also confirmed these effects by immunofluorescence in BV-2 cells (Figs. 2C; D) and primary microglia at 24 h (Fig. 3). These data demonstrate that the initial Na+ influx caused by LPS results in the down-regulation of VGSC and is TTX-sensitive at the early time point. However, additional stimulation resulted in down-regulation even in the presence of TTX, suggesting an alternative pathway, possibly involving NHE.
Fig. 2.
Protein expression of Nav1.6 in activated microglia. (A–B) Relative expression of Nav1.6 protein was determined by densitometry from western blots after 6 and 24 h of BV-2 cell activation with LPS. Relative pixel densities from representative blots are presented in bar graphs. α-Tubulin was used as loading control. (C–D) Expression of Nav1.6 protein in BV-2 cells was visualized by immunolabeling (green) at basal and stimulated condition. Nuclei (blue) were stained with DAPI. There was no signal when the primary antibody was omitted (not shown). The values represent mean ± SEM from at least three individual experiments, each performed in triplicate. Data are expressed as percentage of control. * indicates significant difference from control and # indicates significant differences between LPS and LPS + TTX (p < 0.05). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 3.
Nav1.6 expression in primary mouse microglia after 24 h LPS (100 ng/ml) exposure with or without TTX. Microglia were labeled with anti-Iba1 antibody (red). Nav1.6 protein was visualized by immunolabeling (green) at basal and stimulated condition. Co-localization of Nav1.6 and Iba1 is indicated as yellow. Nuclei (blue) were stained with DAPI. There was no signal when the primary antibody was omitted (not shown). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
LPS increases NHE-1 expression in BV2 cells
Based on the effectiveness of cariporide in reducing intracellular Na+ accumulation at 6 and 24 h, we next determined the effect of LPS on NHE-1 expression. LPS exposure caused a dose- and time-dependent increase in the mRNA expression and protein levels of NHE-1 (Fig. 4). LPS (10 and 100 ng/ml) increased mRNA expression of NHE-1 by 30% to 60% at 6 h after exposure (Fig. 4A) and 50% to 70% at 24 h after exposure (Fig. 4B). Pre-treatment of BV-2 cells with cariporide significantly reduced LPS-induced mRNA expression of NHE-1. The protein level of NHE-1 was also increased by 32% to 46% at 6 h after exposure (Fig. 4C) and 70% to 86% at 24 h after exposure (Fig. 4D) with 10 and 100 ng/ml LPS, respectively. These effects were effectively blocked by TTX, cariporide, or their combination at 6 h. However, only the combination of the inhibitors was able to bring NHE-1 back to control levels at 24 h (Figs. 4B; D). These results indicate that increased expression of NHE-1 may be a secondary event to the initial Na+ influx through VGSC and may function to maintain Na+ influx during microglial activation by LPS.
Fig. 4.
LPS increases expression of NHE-1 in LPS stimulated BV-2 microglia. Relative expression of NHE-1 mRNA (A and B) and protein (C and D) were determined after 6 and 24 h incubation of BV-2 cells with LPS in the presence or absence of TTX, cariporide, or their combination. The expression of GAPDH was used to normalize the amount of mRNA in each sample. α-Tubulin was used as loading control for western blot analysis. Expression of protein was determined by densitometry from western blots. Relative pixel densities from representative blots are presented in bar graphs. The values represent mean ± SEM from at least three individual experiments, each performed in triplicate. * indicates significant difference from control and # indicates significant differences compared to LPS-treated cells (p < 0.05).
Inhibition of VGSC and NHE suppresses gp91phox mRNA and protein induction following LPS exposure
NADPH oxidase plays a crucial role in microglia-mediated neurodegeneration by catalyzing the formation of H2O2 and ROS in the brain (Bedard and Krause, 2007). The glycoprotein gp91phox is an essential catalytic subunit of NADPH oxidase that generates superoxide (O2•−) and has been shown to be reduced by NHE1 inhibition (Liu et al., 2010). Here, LPS significantly increased mRNA expression of gp91phox at 6 and 24 h after exposure (Fig. 5). The induction of gp91phox mRNA was about 4–5 fold higher than that of the control at 6 h (Fig. 5A) and remained increased 24 h after LPS exposure (Fig. 5B). Similarly, gp91phox protein was increased by 48% to 50% at 6 h after exposure (Fig. 5C) and 64% to 121% at 24 h after exposure (Fig. 5D) with 10 and 100 ng/ml LPS, respectively. The inhibition of VGSC (TTX), NHE (cariporide), or both significantly reduced the LPS-induced up-regulation of gp91phox mRNA and protein.
Fig. 5.
Expression of gp91phox in activated microglia following NHE and VGSC inhibition. BV-2 cells were pretreated with TTX, cariporide or their combination 20 min prior to the addition of LPS. Cells were collected at 6 and 24 h after LPS treatment and lysates were analyzed for mRNA (A–B) and protein expression (C–D). The expression of GAPDH was used to normalize the amount of mRNA in each sample. α-Tubulin was used as loading control for western blot analysis. Expression of protein was determined by densitometry from western blots. Relative pixel densities from representative blots are presented in bar graphs. The values represent mean ± SEM from at least three individual experiments, each performed in triplicate. * indicates significant difference from control and # indicates significant differences compared to LPS-treated cells (p < 0.05).
LPS-induced production of ROS and H2O2 is reduced by VGSC and NHE inhibition
LPS stimulated microglia have been shown to generate ROS and H2O2 through NADPH oxidase (Harrigan et al., 2008; Innamorato et al., 2009). Therefore, we determined whether inhibition of VGSC and NHE could prevent the production of LPS-induced ROS and H2O2. Following 1 and 24 h LPS exposure, ROS was measured using H2DCFDA. LPS (10 and 100 ng/ml) increased the production of ROS by 75% after 1 h (Fig. 6A) and ROS remained elevated up to 24 h after exposure (Fig. 6B). The production of ROS was significantly reduced after inhibition by TTX and cariporide, but the combination proved to be the most efficacious (Figs. 6A; B). The production of H2O2 was increased by 75% with 10 ng/ml and 150% with 100 ng/ml LPS 24 h after exposure (Fig. 6C). LPS-induced production of H2O2 was significantly reduced when cells were pretreated with TTX, cariporide, or their combination. This finding was confirmed in primary microglial cells (Figs. 6D and E).
Fig. 6.
Blockade of VGSC and NHE reduces ROS and H2O2 in activated BV-2 cells and primary microglia. BV-2 cells were treated with TTX and cariporide for 20 min prior to addition of LPS. Production of intracellular ROS was measured using the fluorescent probe 2,7-dichlorofluorescin diacetate (H2DCFDA) at 1 and 24 h after LPS exposure (A, B, and D). Production of H2O2 in cell cultured medium was measured using Amplex Red® horse-radish peroxidase at 24 h of LPS exposure (C and E). The values represent mean ± SEM from at least three individual experiments, each performed in triplicate. Data are expressed as percentage of control. * indicates significant difference from control and # indicates significant differences compared to LPS-treated cells (p < 0.05).
Blockade of VGSC and NHE reduced the release of TNF-α following LPS stimulation
Because activation of NADPH oxidase can increase cytokine secretion from the microglia, we investigated whether inhibition of VGSC and NHE affected the release of the pro-inflammatory cytokine TNF-α. LPS stimulated BV-2 cells released TNF-α in a dose- and time-dependent manner (Fig. 7). Exposure to 10 ng/ml LPS increased the release of TNF-α about 200-fold at 6 h (Fig. 7A) and 700-fold at 24 h (Fig. 7B). LPS (100 ng/ml) increased TNF-α release by 300-fold at 6 h and 800-fold at 24 h. Release was significantly reduced by TTX or cariporide alone, but the combination was most efficacious, particularly at the highest dose of LPS. These data were confirmed in primary microglial cells (Figs. 7C and D).
Fig. 7.
Inhibition of VGSC and NHE reduces TNF-α secretion in response to LPS. BV-2 cells or primary microglia were treated with VGSC and NHE inhibitors for 20 min prior to stimulation with LPS. Cultured media were collected after 6 (A and C) and 24 h (B and D) LPS exposure for an ELISA to measure secretion of TNF-α into media. ND indicates that TNF-α concentration in medium was below the detection level. The values represent mean ± SEM from at least three individual experiments, each performed in triplicate. Data are expressed as percentage of control. * indicates significant difference from control and # indicates significant differences compared to LPS-treated cells (p < 0.05).
Discussion
The persistent activation of the microglia is thought to contribute to the pathogenesis of neurodegenerative diseases by increasing the secretion of inflammatory molecules, including cytokines, ROS and H2O2 in the brain. Recently, Black and co-workers reported that inhibition of VGSC significantly reduces the phagocytic activity and migration of activated microglia (Black et al., 2009). Another study revealed a role for NHE-1 in the microglia responses to inflammatory stimuli, including LPS, albeit at higher doses (Liu et al., 2010). Here, we demonstrate that stimulation of microglial cells with LPS causes a rapid influx of Na+ that is initially through VGSC and subsequently supported through the NHE. Following this influx, a series of inflammatory signaling events including activation of NADPH oxidase and release of pro-inflammatory cytokines such as TNF-α was initiated. Blockade of VGSC by tetrodotoxin (TTX), NHE-1 by cariporide, or the combination of TTX and cariporide effectively blocked or reduced these inflammatory events. Together, these data demonstrate a closely-linked relationship between VGSC and NHE-1 in microglial function during inflammation, which may provide novel targets for therapeutic interventions aimed at reducing neuroinflammation.
Although treatment of microglial cells with high doses (500 ng/ml) of LPS was reported to increase accumulation of intracellular Na+ 24 h after exposure (Liu et al., 2010), we found that lower doses of LPS (10 and 100 ng/ml) rapidly increased intracellular Na+ through VGSC, as evidenced by the ability of tetrodotoxin (TTX) to block Na+ influx. However, long-term accumulation (24 h) of intracellular Na+ inhibition was blocked by the NHE-1 inhibitor cariporide, similar to that observed by Liu et al. (2010), or the combination of TTX and cariporide, but not by TTX alone. These data suggest that LPS initiates a rapid Na+ influx through VGSC and, thereafter, this process is maintained through NHE-1.
We hypothesized that because LPS caused a rapid influx of Na+ through VGSC, down-regulation of VGSC may occur as a compensatory mechanism, as was shown for other compounds that cause increased Na+ influx in neurons (Dargent and Couraud, 1990). There are several VGSC isoforms present in the brain microglia (Black et al., 2009; Craner et al., 2005; Sontheimer et al., 1994), but we focused on the 1.6 isoform of VGSC (Nav1.6) based on reports of its ability to modulate microglial function (Black and Waxman, 2012). LPS exposure decreased Nav1.6 levels in both BV-2 cells and primary microglia following 6 h of LPS exposure, which was prevented by TTX. However, if the incubation time with LPS is extended to 24 h, TTX was no longer effective in preventing the down-regulation of Nav1.6. These data suggest that an additional mechanism, perhaps activation of NHE-1, may come into play with longer exposure as demonstrated for intracellular Na+.
Based on the results presented here and those of Liu et al. (2010), both NHE and VGSC appear to contribute to microglial activation through alteration of intracellular Na+. However, it is not clear whether there is a regulatory association between the two proteins. A previous study reported that mice lacking NHE-1 up-regulate Na+ channel mRNA expression in the brain, potentially as a mechanism to maintain Na+ homeostasis (Xia et al., 2003). There is also one report demonstrating that NHE inhibition by amiloride in trigeminal ganglion neurons reduces action potentials (Hwang et al., 2011), suggesting that there may be some common regulation. Here, LPS caused increased mRNA and protein expression of NHE-1. Importantly, these increases in NHE-1 could be reduced by TTX, demonstrating a role for VGSC in regulating NHE-1. To our knowledge, this is the first description that VGSC regulates NHE-1 in microglial cells. These data are in contrast to those of Liu et al. (2010), who observed elevated NHE-1 activity, but not protein, following LPS treatment of M4T.4 microglial cells. This difference may be the result of the lower dose of LPS used in the present study (10–100 ng/ml) or intrinsic differences between the two cell lines used (BV-2 and M4T.4).
Activation of NHE by LPS is associated with increased NADPH oxidase activity in the microglia, dendritic cells, and neutrophils (Demaurex et al., 1996; Rotte et al., 2012). NADPH oxidase is a multi-subunit enzyme consisting of six membrane-bound subunits, including gp91phox and P22phox (Cheng et al., 2001; Yu et al., 1998), with gp91phox serving as a primary catalytic subunit responsible for ROS and H2O2 production (Babior, 2000; Gorlach et al., 2000; Qin and Crews, 2012; Ushio-Fukai et al., 2002). Here, the LPS-induced increase of gp91phox mRNA and protein in BV-2 microglial cells was attenuated by TTX at the 6 h time point with both doses of LPS. In contrast, cariporide alone did not significantly impact the expression or levels of gp91phox at the 6 h time point, except for the highest dose of LPS. At the 24 h time point, TTX, cariporide, and their combination significantly decreased gp91phox expression and levels. Additionally, our data reveal that inhibition of VGSC and/or NHE-1 is most effective at reducing the protein expression of gp91phox, the active subunit of NADPH oxidase. These data support a role for VGSC in the regulation of gp91phox at the earliest time points, and a combination of NHE-1 and VGSC at the later time points, similar to that observed with intracellular Na+. Additional studies are required to determine the mechanism(s) by which gp91phox is regulated by VGSC, NHE-1, and intracellular Na+.
Activation of NADPH oxidase impacts the secretion of proinflammatory cytokines and production of ROS and H2O2 (Black et al., 2009; Moon et al., 2007; Rotte et al., 2010), which have been implicated as significant contributors to neurodegeneration (Block and Hong, 2007; Lucas et al., 2006). We observed production of ROS was evident at 1 h after LPS exposure, and H2O2 was significantly increased at 24 h following LPS exposure. We found that TTX and/or cariporide significantly decreased the production of ROS and H2O2. Additionally, the combination of TTX and cariporide was the most effective at reducing secretion of TNF-α in LPS-treated BV-2 cells. It was previously reported that the induction of pro-inflammatory cytokine expression was decreased in activated microglia after inhibition of VGSC (Black et al., 2007, 2009) and NHE-1 (Liu et al., 2010). Our data support and extend these previous results and provide additional support for the concept that VGSC and NHE-1 play a coordinated role in microglial function following LPS challenge.
In summary, our data demonstrate that the both VGSC and NHE contribute to microglial activation by LPS in a time- and dose-dependent manner by regulating intracellular Na+ levels. Na+ influx through VGSC appears to contribute significantly to initial activation of the respiratory burst via NADPH oxidase in the microglia, whereas elevation of [Na+]i through NHE-1 augments the ongoing activation. Previous studies reported that inhibition of NHE-1 was partially neuroprotective against ischemic brain injury, in part through dampening the microglial response (Cengiz et al., 2011; Lee and Jung, 2012; Shi et al., 2011). Likewise, anti-epileptic drug administration reduces microglial activation in experimental autoimmune encephalomyelitis, possibly through inhibition of VGSC (Craner et al., 2005). Our data suggest that coordinated inhibition of both VGSC and NHE may represent an effective method for reducing NADPH oxidase-mediated production of TNF-α, ROS, and H2O2 during inflammation.
Supplementary Material
Acknowledgments
This project was supported in part by R01ES015991, P30ES005022, and R21NS072097 grants from National Institutes of Health. We thank Sanofi-Aventis for the generous donation of cariporide. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of National Institute of Health. Neither NIEHS nor Sanofi-Aventis had any role in the design and analysis or writing of this manuscript.
Abbreviations
- VGSC
voltage-gated sodium channels
- NHE
Na+/H+ exchanger
- TTX
tetrodotoxin
- LPS
lipopolysaccharide
- MEM
minimum essential medium
- KRHB
Krebs–Ringer–HEPES buffer
- TTBS
Tween 20 Tris buffered saline
- PBS
phosphate-buffered saline
- qPCR
quantitative reverse transcriptase polymerase chain reaction
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- TNFα
tumor necrosis factor-α
- NADPH oxidase
nicotinamide adenine dinucleotide phosphate-oxidase
- ROS
reactive oxygen species
- H2O2
hydrogen peroxide
- (Na+)i
intracellular sodium
- H2DCFDA
2,7-Dichlorofluorescin diacetate
- DAPI
4′,6-Diamidino-2-phenylindole
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.taap.2013.09.011.
Conflict of interest The authors declare that they have no competing financial interests or conflicts of interest.
References
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, O’Banion MK, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T. Inflammation and Alzheimer’s disease. Neurobiol. Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen DG, Xiao XH. Role of the cardiac Na+/H+ exchanger during ischemia and reperfusion. Cardiovasc. Res. 2003;57:934–941. doi: 10.1016/s0008-6363(02)00836-2. [DOI] [PubMed] [Google Scholar]
- Aloisi F. Immune function of microglia. Glia. 2001;36:165–179. doi: 10.1002/glia.1106. [DOI] [PubMed] [Google Scholar]
- Anrather J, Racchumi G, Iadecola C. NF-kappaB regulates phagocytic NADPH oxidase by inducing the expression of gp91phox. J. Biol. Chem. 2006;281:5657–5667. doi: 10.1074/jbc.M506172200. [DOI] [PubMed] [Google Scholar]
- Babior BM. The NADPH oxidase of endothelial cells. IUBMB Life. 2000;50:267–269. doi: 10.1080/713803730. [DOI] [PubMed] [Google Scholar]
- Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- Black JA, Liu S, Hains BC, Saab CY, Waxman SG. Long-term protection of central axons with phenytoin in monophasic and chronic-relapsing EAE. Brain. 2006;129:3196–3208. doi: 10.1093/brain/awl216. [DOI] [PubMed] [Google Scholar]
- Black JA, Liu S, Carrithers M, Carrithers LM, Waxman SG. Exacerbation of experimental autoimmune encephalomyelitis after withdrawal of phenytoin and carbamazepine. Ann. Neurol. 2007;62:21–33. doi: 10.1002/ana.21172. [DOI] [PubMed] [Google Scholar]
- Black JA, Liu S, Waxman SG. Sodium channel activity modulates multiple functions in microglia. Glia. 2009;57:1072–1081. doi: 10.1002/glia.20830. [DOI] [PubMed] [Google Scholar]
- Black JA, Newcombe J, Waxman SG. Nav1.5 sodium channels in macrophages in multiple sclerosis lesions. Mult. Scler. 2012 doi: 10.1177/1352458512460417. [DOI] [PubMed] [Google Scholar]
- Block ML, Hong JS. Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Prog. Neurobiol. 2005;76:77–98. doi: 10.1016/j.pneurobio.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Block ML, Hong JS. Chronic microglial activation and progressive dopaminergic neurotoxicity. Biochem. Soc. Trans. 2007;35:1127–1132. doi: 10.1042/BST0351127. [DOI] [PubMed] [Google Scholar]
- Cengiz P, Kleman N, Uluc K, Kendigelen P, Hagemann T, Akture E, Messing A, Ferrazzano P, Sun D. Inhibition of Na+/H+ exchanger isoform 1 is neuroprotective in neonatal hypoxic ischemic brain injury. Antioxid. Redox Signal. 2011;14:1803–1813. doi: 10.1089/ars.2010.3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao CC, Hu S, Molitor TW, Shaskan EG, Peterson PK. Activated microglia mediate neuronal cell injury via a nitric oxide mechanism. J. Immunol. 1992;149:2736–2741. [PubMed] [Google Scholar]
- Cheng G, Cao Z, Xu X, van Meir EG, Lambeth JD. Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene. 2001;269:131–140. doi: 10.1016/s0378-1119(01)00449-8. [DOI] [PubMed] [Google Scholar]
- Craner MJ, Damarjian TG, Liu S, Hains BC, Lo AC, Black JA, Newcombe J, Cuzner ML, Waxman SG. Sodium channels contribute to microglia/macrophage activation and function in EAE and MS. Glia. 2005;49:220–229. doi: 10.1002/glia.20112. [DOI] [PubMed] [Google Scholar]
- Dargent B, Couraud F. Down-regulation of voltage-dependent sodium channels initiated by sodium influx in developing neurons. Proc. Natl. Acad. Sci. U. S. A. 1990;87:5907–5911. doi: 10.1073/pnas.87.15.5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaurex N, Downey GP, Waddell TK, Grinstein S. Intracellular pH regulation during spreading of human neutrophils. J. Cell Biol. 1996;133:1391–1402. doi: 10.1083/jcb.133.6.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eder C. Ion channels in microglia (brain macrophages) Am. J. Physiol. 1998;275:C327–C342. doi: 10.1152/ajpcell.1998.275.2.C327. [DOI] [PubMed] [Google Scholar]
- Eder C. Regulation of microglial behavior by ion channel activity. J. Neurosci. Res. 2005;81:314–321. doi: 10.1002/jnr.20476. [DOI] [PubMed] [Google Scholar]
- Farber K, Kettenmann H. Physiology of microglial cells. Brain Res. Brain Res. Rev. 2005;48:133–143. doi: 10.1016/j.brainresrev.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Farber K, Kettenmann H. Functional role of calcium signals for microglial function. Glia. 2006;54:656–665. doi: 10.1002/glia.20412. [DOI] [PubMed] [Google Scholar]
- Fliegel L. The Na+/H+ exchanger isoform 1. Int. J. Biochem. Cell Biol. 2005;37:33–37. doi: 10.1016/j.biocel.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Fujita K, Izumi Y, Kaji R. Inflammatory mechanisms in amyotrophic lateral sclerosis. Brain Nerve. 2012;64:273–278. [PubMed] [Google Scholar]
- Fussell KC, Udasin RG, Gray JP, Mishin V, Smith PJ, Heck DE, Laskin JD. Redox cycling and increased oxygen utilization contribute to diquat-induced oxidative stress and cytotoxicity in Chinese hamster ovary cells overexpressing NADPH-cytochrome P450 reductase. Free Radic. Biol. Med. 2011;50:874–882. doi: 10.1016/j.freeradbiomed.2010.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon R, Hogan CE, Neal ML, Anantharam V, Kanthasamy AG, Kanthasamy A. A simple magnetic separation method for high-yield isolation of pure primary microglia. J. Neurosci. Methods. 2011;194:287–296. doi: 10.1016/j.jneumeth.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorlach A, Brandes RP, Nguyen K, Amidi M, Dehghani F, Busse R. A gp91phox containing NADPH oxidase selectively expressed in endothelial cells is a major source of oxygen radical generation in the arterial wall. Circ. Res. 2000;87:26–32. doi: 10.1161/01.res.87.1.26. [DOI] [PubMed] [Google Scholar]
- Harrigan TJ, Abdullaev IF, Jourd’heuil D, Mongin AA. Activation of microglia with zymosan promotes excitatory amino acid release via volume-regulated anion channels: the role of NADPH oxidases. J. Neurochem. 2008;106:2449–2462. doi: 10.1111/j.1471-4159.2008.05553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain MM, Richardson JR. Mechanism of pyrethroid pesticide-induced apoptosis: role of calpain and the ER stress pathway. Toxicol. Sci. 2011;122:512–525. doi: 10.1093/toxsci/kfr111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Sheng WS, Schachtele SJ, Lokensgard JR. Reactive oxygen species drive herpes simplex virus (HSV)-1-induced proinflammatory cytokine production by murine microglia. J. Neuroinflammation. 2011;8:123. doi: 10.1186/1742-2094-8-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innamorato NG, Lastres-Becker I, Cuadrado A. Role of microglial redox balance in modulation of neuroinflammation. Curr. Opin. Neurol. 2009;22:308–314. doi: 10.1097/WCO.0b013e32832a3225. [DOI] [PubMed] [Google Scholar]
- Kauppinen TM, Higashi Y, Suh SW, Escartin C, Nagasawa K, Swanson RA. Zinc triggers microglial activation. J. Neurosci. 2008;28:5827–5835. doi: 10.1523/JNEUROSCI.1236-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiol. Rev. 2011;91:461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- Lambeth JD, Kawahara T, Diebold B. Regulation of Nox and Duox enzymatic activity and expression. Free Radic. Biol. Med. 2007;43:319–331. doi: 10.1016/j.freeradbiomed.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BK, Jung YS. The Na+/H+ exchanger-1 inhibitor cariporide prevents glutamate-induced necrotic neuronal death by inhibiting mitochondrial Ca2+ overload. J. Neurosci. Res. 2012;90:860–869. doi: 10.1002/jnr.22818. [DOI] [PubMed] [Google Scholar]
- Liu B, Hong JS. Role of microglia in inflammation-mediated neurodegenerative diseases: mechanisms and strategies for therapeutic intervention. J. Pharmacol. Exp. Ther. 2003;304:1–7. doi: 10.1124/jpet.102.035048. [DOI] [PubMed] [Google Scholar]
- Liu Y, Kintner DB, Chanana V, Algharabli J, Chen X, Gao Y, Chen J, Ferrazzano P, Olson JK, Sun D. Activation of microglia depends on Na+/H+ exchange-mediated H+ homeostasis. J. Neurosci. 2010;30:15210–15220. doi: 10.1523/JNEUROSCI.3950-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas SM, Rothwell NJ, Gibson RM. The role of inflammation in CNS injury and disease. Br. J. Pharmacol. 2006;147(Suppl. 1):S232–S240. doi: 10.1038/sj.bjp.0706400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malo ME, Fliegel L. Physiological role and regulation of the Na+/H+ exchanger. Can. J. Physiol. Pharmacol. 2006;84:1081–1095. doi: 10.1139/y06-065. [DOI] [PubMed] [Google Scholar]
- Mantegazza M, Curia G, Biagini G, Ragsdale DS, Avoli M. Voltage-gated sodium channels as therapeutic targets in epilepsy and other neurological disorders. Lancet Neurol. 2010;9:413–424. doi: 10.1016/S1474-4422(10)70059-4. [DOI] [PubMed] [Google Scholar]
- Moon DO, Park SY, Lee KJ, Heo MS, Kim KC, Kim MO, Lee JD, Choi YH, Kim GY. Bee venom and melittin reduce proinflammatory mediators in lipopolysaccharide-stimulated BV2 microglia. Int. Immunopharmacol. 2007;7:1092–1101. doi: 10.1016/j.intimp.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Phillis JW, Estevez AY, Guyot LL, O’Regan MH. 5-(N-ethyl-N-isopropyl)-amiloride, an Na(+)-H(+) exchange inhibitor, protects gerbil hippocampal neurons from ischemic injury. Brain Res. 1999;839:199–202. doi: 10.1016/s0006-8993(99)01705-9. [DOI] [PubMed] [Google Scholar]
- Politis M, Pavese N, Tai YF, Kiferle L, Mason SL, Brooks DJ, Tabrizi SJ, Barker RA, Piccini P. Microglial activation in regions related to cognitive function predicts disease onset in Huntington’s disease: a multimodal imaging study. Hum. Brain Mapp. 2011;32:258–270. doi: 10.1002/hbm.21008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, Crews FT. NADPH oxidase and reactive oxygen species contribute to alcohol-induced microglial activation and neurodegeneration. J. Neuroinflammation. 2012;9:5. doi: 10.1186/1742-2094-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, Liu Y, Wang T, Wei SJ, Block ML, Wilson B, Liu B, Hong JS. NADPH oxidase mediates lipopolysaccharide-induced neurotoxicity and proinflammatory gene expression in activated microglia. J. Biol. Chem. 2004;279:1415–1421. doi: 10.1074/jbc.M307657200. [DOI] [PubMed] [Google Scholar]
- Richardson JR, Caudle WM, Wang M, Dean ED, Pennell KD, Miller GW. Developmental exposure to the pesticide dieldrin alters the dopamine system and increases neurotoxicity in an animal model of Parkinson’s disease. FASEB J. 2006;20:1695–1697. doi: 10.1096/fj.06-5864fje. [DOI] [PubMed] [Google Scholar]
- Rotte A, Pasham V, Eichenmuller M, Mahmud H, Xuan NT, Shumilina E, Gotz F, Lang F. Effect of bacterial lipopolysaccharide on Na(+)/H(+) exchanger activity in dendritic cells. Cell. Physiol. Biochem. 2010;26:553–562. doi: 10.1159/000322323. [DOI] [PubMed] [Google Scholar]
- Rotte A, Pasham V, Bhandaru M, Bobbala D, Zelenak C, Lang F. Rapamycin sensitive ROS formation and Na(+)/H(+) exchanger activity in dendritic cells. Cell. Physiol. Biochem. 2012;29:543–550. doi: 10.1159/000338508. [DOI] [PubMed] [Google Scholar]
- Schilling T, Eder C. Ion channel expression in resting and activated microglia of hippocampal slices from juvenile mice. Brain Res. 2007;1186:21–28. doi: 10.1016/j.brainres.2007.10.027. [DOI] [PubMed] [Google Scholar]
- Shi Y, Chanana V, Watters JJ, Ferrazzano P, Sun D. Role of sodium/hydrogen exchanger isoform 1 in microglial activation and proinflammatory responses in ischemic brains. J. Neurochem. 2011;119:124–135. doi: 10.1111/j.1471-4159.2011.07403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Sontheimer H, Fernandez-Marques E, Ullrich N, Pappas CA, Waxman SG. Astrocyte Na+ channels are required for maintenance of Na+/K(+)-ATPase activity. J. Neurosci. 1994;14:2464–2475. doi: 10.1523/JNEUROSCI.14-05-02464.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X, Federoff HJ, Maguire-Zeiss KA. Mutant alpha-synuclein overexpression mediates early proinflammatory activity. Neurotox. Res. 2009;16:238–254. doi: 10.1007/s12640-009-9053-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surace MJ, Block ML. Targeting microglia-mediated neurotoxicity: the potential of NOX2 inhibitors. Cell. Mol. Life Sci. 2012;69:2409–2427. doi: 10.1007/s00018-012-1015-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tansey MG, Goldberg MS. Neuroinflammation in Parkinson’s disease: its role in neuronal death and implications for therapeutic intervention. Neurobiol. Dis. 2010;37:510–518. doi: 10.1016/j.nbd.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ten Hove M, Nederhoff MG, Van Echteld CJ. Relative contributions of Na+/H+ exchange and cotransport to ischemic Nai+ overload in isolated rat hearts. Am. J. Physiol. Heart Circ. Physiol. 2005;288:H287–H292. doi: 10.1152/ajpheart.01102.2003. [DOI] [PubMed] [Google Scholar]
- Ushio-Fukai M, Tang Y, Fukai T, Dikalov SI, Ma Y, Fujimoto M, Quinn MT, Pagano PJ, Johnson C, Alexander RW. Novel role of gp91(phox)-containing NAD(P) H oxidase in vascular endothelial growth factor-induced signaling and angiogenesis. Circ. Res. 2002;91:1160–1167. doi: 10.1161/01.res.0000046227.65158.f8. [DOI] [PubMed] [Google Scholar]
- Waxman SG. Mechanisms of disease: sodium channels and neuroprotection in multiple sclerosis-current status. Nat. Clin. Pract. Neurol. 2008;4:159–169. doi: 10.1038/ncpneuro0735. [DOI] [PubMed] [Google Scholar]
- Xia Y, Zhao P, Xue J, Gu XQ, Sun X, Yao H, Haddad GG. Na+ channel expression and neuronal function in the Na+/H+ exchanger 1 null mutant mouse. J. Neurophysiol. 2003;89:229–236. doi: 10.1152/jn.00488.2002. [DOI] [PubMed] [Google Scholar]
- Yu L, Quinn MT, Cross AR, Dinauer MC. Gp91(phox) is the heme binding subunit of the superoxide-generating NADPH oxidase. Proc. Natl. Acad. Sci. U. S. A. 1998;95:7993–7998. doi: 10.1073/pnas.95.14.7993. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.