Abstract
Astrocytes have long been forgotten entities in our quest to understand brain function. Over the last few decades there has been an exponential increase in our knowledge of CNS function and consequently astrocytes have emerged as key figures in CNS physiology and disease. Indeed, several pediatric neurological disorders have recently been linked to astrocyte dysregulation including, leukodystrophies, autism spectrum disorders, and epilepsy. Given that pediatric disorders are rooted in developmental processes, the goal of this review is to catalog what we know about astrocyte development and function in the developing CNS. Moreover, we will highlight current challenges and questions that remain in the field of astrocyte development. Our hope is that this review will illuminate the potential of astrocytes and their associated developmental and physiological functions as potential therapeutic targets for the treatment of neurological disorders.
Introduction
In 1846, Rudolf Virchow, the father of modern pathology, described neuroglial cells as a homogenous population that generally supports neuronal function[1]. Since then astrocytes have emerged as the predominant cell type in the brain and are associated with a plethora of functions vital to CNS physiology, including blood brain barrier formation and maintenance, synaptogenesis, neurotransmission, metabolic regulation, and the latter functions forming leading the “tripartite synapse” model of neurotransmission (see below and [2, 3]). More recently, astrocytes have been directly associated with several neurological disorders, including ALS, MS, Alzheimers disease, Alexander disease, and Retts syndrome [4].
Because pediatric disorders are generally thought to originate through developmental dysregulation, an understanding of astrocyte development could provide new insight into the etiology and eventual treatment of pediatric neurological disorders. Importantly, unlike neurons, many aspects of astrocyte development occur postnatally [5], providing a potential therapeutic window to reverse their development dysregulation.
In spite of these recent advances, our knowledge of astrocyte development is light years behind that of neurons and oligodendrocytes, having been plagued by a lack of reliable markers, confounded by the challenge of performing gene manipulation in vivo without affecting neurogenesis, and the lack of reliable in vitro systems. As a result, several aspects of astrocyte development and biology remain undefined, and in turn, have hindered our understanding of neurological disorders. The goal of this review is to provide a summary of our current knowledge of astrocyte development and function. We will catalog the current knowledge of astrocyte development and discuss areas of research we believe need to be addressed.
Astrocyte Development
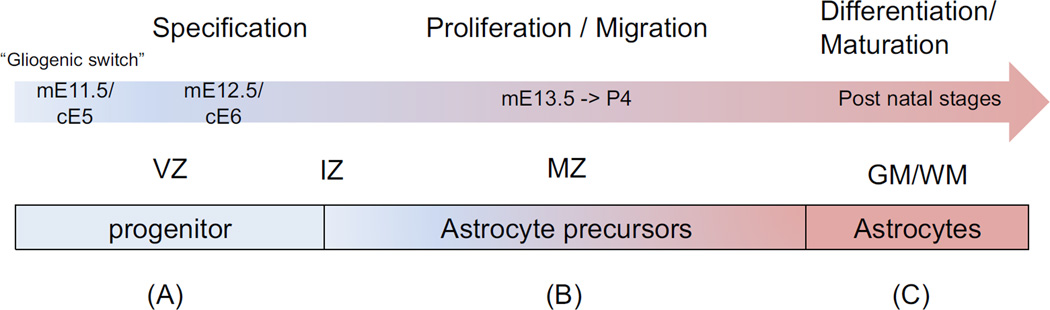
During CNS development, neurogenesis precedes gliogenesis, with radial glial serving as both the scaffolding for migration and the neural stem cell (NSC) substrate for both cell types [12, 14]. Neurons, and oligodendrocytes, develop following a step-wise process: A) stem cells are specified towards a given lineage, B) they migrate away from the germinal centers, C) exit cell cycle, and D) undergo terminal differentiation during which a given cell type initiates its physiological function (Figure 1) [6]. Whether astrocytes follow the same pattern of development has not been established. The main barriers to the study of astrocyte development reside from the lack of three essential tools: 1) Reliable markers to characterize precursors and astrocytes in vivo, 2) methods to specifically manipulate genes that do not affect neurogenesis and 3) reliable in vitro systems. Understanding astrocyte development is relevant to pediatric disorders because it occurs during the late stages of fetal development and postnatally, a similar time frame when pediatric and also adult neurological disorders manifest.
Figure 1. Stages of astrocyte lineage progression.
(A) In the spinal cord, the NSCs located in the ventricular zone (VZ) transition fate from neurogenesis to gliogenesis in a process called the gliogenic switch. (B) Upon specification, astrocyte progenitor emerge from the VZ into the intermediate zone (IZ). Waves of proliferation from astrocyte precursors (ASPs) have been reported but this ability decreases as development progresses (20). A similar observation was reported in the cortex, although the timing is different (13). At the same time, ASPs migrate into the mantle zone (MZ) to reach their final location. While the mechanisms that regulate this process remain relatively unknown, it has been suggested that astrocytes have limited migratory capabilities (31). (C) Finally, astrocytes become mature and functional during the post natal stages of development.
Neural stem cells and specification
During early development, the neural tube is patterned throughout the dorso-ventral (D/V) axis by a combination of morphogens (Shh, BMPs and Wnts), which regulate the expression of homeodomain transcription factors that further cross-repress each other, forming tight boundaries or domains from which different sub-types of neurons will emerge [7]. This homeodomain patterning is conserved during gliogenesis [8–10] and regulates the generation of astrocyte sub-types; in particular Pax6 and Nkx6.1 are required for the generation of three subpopulations of white matter astrocytes [11]. However, these homeodomain transcription factors function to establish D/V patterning and are, therefore, not specific to glial cells, raising the question of whether astro-glial specific transcription factors exist. To dissect this question, we must first understand where and when astro-glia are generated and the developing spinal provides an ideal model for undetstanding these formative stages of astro-gliogenesis. The gliogenic switch is a tightly regulated developmental interval during which NSCs in the ventricular zone (VZ) transition from neurogenesis to gliogenesis; in the developing spinal cord this switch occurs around E12.5 and in the cortex around E16-18 (Figure 1A) [12, 13]. Many factors have been implicated, directly or indirectly, in gliogenesis, including Notch signaling, the transcriptional repressor N-CoR, the methylase Dnmt1 and histone methylation, all of which are permissive, but not instructive (reviewed in [6]). Recently the transcription factor, Nuclear Factor I-A was found to be both necessary and sufficient for embryonic gliogenesis, as well as astrocytogenesis [12]. While it is initially expressed in all glial precursors that occupy the spinal cord VZ, as development progresses, its expression is maintained in astrocytes. More recently, Sox9 was also shown to regulate early gliogenesis through its regulation of NFIA induction and its association with NFIA during subsequent gliogenic stages (Table I) [14].
Table I. Markers used in astrocyte development studies.
This table summarizes markers used in the literature and the stages they are expressed. Of note, only NFIA/B and Sox9 are nuclear proteins, all other markers are cytoplasmic or membrane-bound and usually result in diffuse labeling. Some markers have only been tested in the spinal cord or cortex and their use in other tissue needs to be validated. WM: white matter.
| Marker | Stages |
|---|---|
| NFIA/B | Gliogenic switch, precursor and some mature (Spinal cord) |
| Sox9 | Gliogenic switch, precursor and some mature (Spinal cord) |
| Glast | Gliogenic switch, precursor and some mature (Spinal cord) |
| FGFR3 | Precursor and some mature |
| FABP7/BLBP | Precursor and some mature |
| Aldh1l1 | Precursor and mature |
| Id3 | Precursor and possibly mature? |
| Reelin/Slit | WM astrocytes – subtypes of mature astrocytes (Spinal cord) |
| GFAP | Mature (fibrous or reactive) |
| S100b | Mature (protoplasmic) |
| Aquaporin 4 | Mature – end feet |
| Aldolase C | Precursor/mature |
| CD44 | Precursor and mature |
| Glutamine Synthase | Late precursor and mature |
| Acsbg1/Bubblegum | Mature (cortex) |
| Connexin 43 | Mature/functional |
| Glt-1 | Mature |
Intermediate stages and migration
Once specified, neuronal and oligodendrocyte lineages express a set of markers associated with post-specification, migratory populations [7, 15]. For the astrocyte lineage, three markers are currently used to demarcate astrocyte precursors; Glast, FABP7/BLBP and FGFR3 (Table I) [16–19]. Glast is a glutamate transporter functionally active in astrocytes and its expression coincides with the gliogenic switch, making it the most specific marker of astrocyte precursors [12, 17]. FABP7/BLBP and FGFR3 however are also expressed during the neurogenic stages, making them less specific [16, 18, 19]. One unique feature of the intermediate stages of astrocyte lineage development in both spinal cord and cortex is a wave of post-migratory proliferation of astrocyte precursors [13, 20]. BrdU pulsing experiments at different developmental time points and early post-natal stages demonstrated that astrocyte precursors remain proliferative after VZ specification (Figure 1B) [13, 20]. These data suggest that the shear number of astrocytes is not only due to NSC proliferation, but is also the result of their capacity to divide and generate astrocytes outside the VZ. This proliferative capacity decreases overtime and it is unclear whether all astrocytes retain proliferative capacity or if only a subset do.
Astrocytes populate all areas of the CNS and they must therefore migrate to colonize their final destination. Upon birth from NSCs, astrocytes migrate along radial glia processes [21], however lineage tracing studies in the spinal cord indicate that mature astrocyte populations are “tethered” to their VZ site of origin, suggesting limited migration during development [21]. However, during early post-natal stages, radial glial cells lose their processes suggesting that another, yet undefined, mode of migration may regulate the second wave of astrocyte precursors. Nevertheless, the molecular and cellular processes regulating astrocyte precursor migration remain poorly defined in vivo, though several groups have used in vitro astrocyte cultures as a model system to examine polarity and basic migratory mechanisms [22, 23]. Whether these processes actually contribute to development in vivo remains an open question.
Maturation and differentiation
There are several markers that label mature astrocytes, including: GFAP, S100 , Aldh1L1, AldoC, Ascgb1, Glt1, and aquaporin 4 (Table I) [4]. However, none of these markers labels all astrocyte populations; for example, GFAP preferentially labels white matter astrocytes, whereas S100 marks grey matter astrocytes and some oligodendrocyte populations [24, 25]. The regulation of GFAP expression has been widely used as a model in which to examine the mechanisms regulating astrocyte differentiation (Figure 1C). Its promoter has been extensively studied and its expression is regulated by the LIF/gp130/STAT3 signaling pathway [26]. While its expression coincides with white matter astrocytes and is upregulated in reactive astrocytes, it is not a universal marker. Moreover, it has been shown that the GFAP promoter has a serum responsive element, thus when astrocytes are cultured, GFAP expression is induced [27, 28], somewhat marginalizing in vitro studies using the GFAP promoter. It remains to be determined whether only a subset of astrocytes can be cultured in vitro (i.e. GFAP expressing astrocytes) or whether the cells in these systems can be derived from populations that normally do not express GFAP (ie. grey matter astrocytes). These examples highlight the limitations of GFAP and reinforce the need to identify additional markers that label mature astrocytes and new in vitro systems. Recently, Aldh1l1 was identified as a broad marker of all astrocytes and reporter mice and Cre-lines have confirmed its utility in this context [29, 30].
Remaining questions
While we are beginning to understand astrocyte development, many aspects remain to be studied. At the forefront is the identification of reliable markers that demarcate all or subtypes of astrocytes. Additional questions include: do all or only a subset of astrocyte precursors have the proliferative properties? What is the mode of migration of astrocytes born from the VZ vs. astrocytes born later? Are immature astrocytes present in adult tissue and if so, how do they remain “immature”? Finally, are astrocytes a homogeneous or a heterogeneous population of cells? The answers to these questions are likely to engage researchers for many years to come and have important implications in the understanding of developing CNS networks that would influence new treatment paradigms for pediatric neurological disorders, as well as forging efforts in promoting neuroplasticity for adult neurological disorders.
Astrocyte Heterogeneity
Astrocytes are receiving more attention as their diverse morphologies and functions are uncovered. In this section, the diversity of astrocytes and their contributions to nervous system function will be discussed.
Cellular Diversity
It is well established that neuronal development in the CNS is reliant upon patterning for subtype diversification[7] and that astrocytes employ similar mechanisms in the spinal cord and forebrain [11, 31]. Morphological classification is another form of heterogeneity that can be used to catalog astrocyte diversity. The morphological heterogeneity of astrocytes was originally recognized by Ramon y Cajal[32], and since that time the field has remained relatively static, as today astrocytes are broadly classified into two main categories: protoplasmic or fibrous. Protoplasmic astrocytes express S100β and are found in the gray matter. They are structurally complex, with multiple finely branching processes as well as large stem branch whose endfeet forms contact with blood vessels[33]. They function in part by regulating the extracellular concentration of various molecules as well as proper neuronal synaptic function[34, 35]. Fibrous astrocytes on the other hand, express GFAP and are located in the white matter. They have a relatively simple structure with long un-branched processes that form contacts with node of Ranvier; however, their exact function remain elusive[33, 36]. Although this simple classification is still widely used, it is clearly insufficient and outdated[33]. In fact, a recent study identified multiple morphologically distinct GFAP- or S100β- expressing astrocytes that demonstrate region-specific differences in morphology, as well as density and proliferation rate[37]. Hence regional specialization will likely add another layer to the complexity of astrocyte heterogeneity, as astrocytes from different regions may have different properties.
Functional Diversity
Blood Brain Barrier
The blood-brain barrier (BBB) is a highly specialized brain microvascular structure made up of endothelial cells coupled to astrocytes[33, 38]. The key function of the BBB is the blocking of harmful or toxic substances circulating in the blood stream from entering the brain. Another critical role of the BBB is the maintenance of ion gradients, which are essential for neurotransmission. These features are made possible by having capillaries that are many times tighter than the capillaries of the other regions[33], which is achieved by connecting each blood endothelial cell with tight junctions and adherens junctions [39]. Astrocytes contribute to BBB formation and function by using their endfeet to make physical contact with endothelial cells and secreting diffusible molecules (bFGF,GDNF and TGFβ) that regulate formation of tight junctions[40–42].
Synaptogenesis and neurotransmission
It is well established that astrocytes are required for synaptogenesis. Pfrieger and Barres first demonstrated that in an astrocyte-free culture retinal ganglion cells form synapses, but fail to generate spontaneous synaptic activity and demonstrated impaired neurotransmission. With the presence of astrocytes however, the normal synaptic functions as well as number of synapses of those neurons were being increased or restored[43] through the action of diffusible molecules synthesized by the astrocytes[44, 45]. These findings are the basis for the “tripartite synapse” model where pre- and post-synaptic connections are ensheathed by surrounding astrocytes that actively participate in neurotransmission. These are important functions in neuroplasticity and are a likely mode by which astrocytes influence behavioral disorders.
Communication
Unlike neurons in both the CNS and the PNS, astrocytes are not equipped with adequate ion channels to fire action potentials. Nonetheless, there are other methods besides the firing of an action potential to measure activity. Cytosolic calcium (Ca2+ ) concentration of astrocytes undergo changes in response to the release of neurotransmitters and this Ca2+ transient can be propagated through gap junctions formed by connexins, in a process termed “gliotransmission”. It is interesting to speculate that gliotransmission may serve as a new form of systems based networking between parallel neuronal circuits. It is also likely that such modalities influence neuroplasticity during development and disease. In addition, Takata and Hirase also demonstrated that astrocytes in each layer of cortex have distinct features in their Ca2+ activities, finding that the Ca2+ activity of astrocytes within layer 1 was significantly higher than layer 2/3 and most of these Ca2+ related activities were independent of neuronal activity [46]. Additionally, regional differences in astrocytic Ca2+ activities have also been reported[47].
Ion Balance
One of earliest known functional role for astrocytes is the regulation of ionic concentration in extracellular space[33]. The activation of neurons by firing action potential would result in the accumulation of K+ extracellularly and astrocytes that enwrap the synapse would take up excess amount of K+ then dilute it by passing it to other astrocytes through the gap junctions[48, 49] and failure of removing excess amount of K+ would result in neuronal hyperexcitibility and seizures. This is an important role in regional networking, and perhaps global networking.
Metabolism
Glutamate is the major neurotransmitter in the nervous system and maintenance of its concentration in the extracellular space is crucial for neuronal homeostasis and physiology. Astrocytes are the most important component in glutamate up-take and metabolism in the brain, and thus through this role are essential for neuronal function and are a key role of astrocytes in the “tripartite synapse” model. The up-take of glutamate in astrocytes is mainly accomplished by glutamate transporters GLAST or/and GLT-1[50], which are specifically expressed on astrocytes and their precursors. The subsequent conversion of glutamate to glutamine is an energy consuming process that requires the help of glutamine synthetase (GS), also an astrocyte-specific marker. Once glutamate is converted to glutamine, it is transported back to neurons for re-synthesis of glutamate.
In addition to glutamate metabolism, astrocytes also function as reservoir of energy substrates such as lactate for neurons. When glutamate is transported into astrocytes, Na+ is also transported, which increases the intracellular Na+ concentration leading to the activation Na+/K+ ATPase, requiring the consumption of ATP[51]. ATP is supplied by the glycolysis of glucose, ultimately resulting in the production of lactate, which is thought to be “shuttled” from astrocytes to neurons by monocarboxylate transporter MCT4[51, 52].
Conclusions
Astrocytes comprise at least 50% of the cellular constituency of the adult CNS, yet the molecular and cellular mechanisms controlling their genesis are only now starting to emerge. As has been the case for neurons and oligodendrocytes, a comprehensive understanding of the development processes controlling their generation will be critical in delineating the complexity of astrocyte function in the functioning CNS. In particular, an understanding of their cellular diversity, coupled with decoding the associated functional heterogeneity will be especially important in generating a comprehensive understanding of developing brain networks and circuits under normal and pathological conditions.
Acknowledgements
We apologize to those whose work we could not cite due to space limitations. This work was supported by funding from the Cancer Prevention and Research Institute of Texas RP101499 (LSC), Sontag Foundation (BD), and National Institutes of Health R01-NS071153 (BD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Virchow R. Uber das granulierte ansehen der Wandungen der Gerhirnventrikel. Allg Z Psychiatr. 1846;3:242–250. [Google Scholar]
- 2.Allen NJ, Barres BA. Neuroscience: Glia - more than just brain glue. Nature. 2009;457(7230):675–677. doi: 10.1038/457675a. Epub 2009/02/06. [DOI] [PubMed] [Google Scholar]
- 3.Matyash V, Kettenmann H. Heterogeneity in astrocyte morphology and physiology. Brain Res Rev. 63(1–2):2–10. doi: 10.1016/j.brainresrev.2009.12.001. Epub 2009/12/17. [DOI] [PubMed] [Google Scholar]
- 4.Molofsky AV, Krencik R, Ullian EM, Tsai HH, Deneen B, Richardson WD, et al. Astrocytes and disease: a neurodevelopmental perspective. Genes & development. 2012;26(9):891–907. doi: 10.1101/gad.188326.112. Epub 2012/05/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freeman MR. Specification and morphogenesis of astrocytes. Science. 330(6005):774–778. doi: 10.1126/science.1190928. Epub 2010/11/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Molofsky CH AV, Deneen B, Rowitch D. Mechanisms of astrocyte development. Patterning and cell type specification Developing CNS and PNS2013. :723–742. [Google Scholar]
- 7.Ulloa F, Briscoe J. Morphogens and the control of cell proliferation and patterning in the spinal cord. Cell Cycle. 2007;6(21):2640–2649. doi: 10.4161/cc.6.21.4822. Epub 2007/10/04. [DOI] [PubMed] [Google Scholar]
- 8.Muroyama Y, Fujiwara Y, Orkin SH, Rowitch DH. Specification of astrocytes by bHLH protein SCL in a restricted region of the neural tube. Nature. 2005;438(7066):360–363. doi: 10.1038/nature04139. Epub 2005/11/18. [DOI] [PubMed] [Google Scholar]
- 9.Zhou Q, Anderson DJ. The bHLH transcription factors OLIG2 and OLIG1 couple neuronal and glial subtype specification. Cell. 2002;109(1):61–73. doi: 10.1016/s0092-8674(02)00677-3. Epub 2002/04/17. [DOI] [PubMed] [Google Scholar]
- 10.Lu QR, Sun T, Zhu Z, Ma N, Garcia M, Stiles CD, et al. Common developmental requirement for Olig function indicates a motor neuron/oligodendrocyte connection. Cell. 2002;109(1):75–86. doi: 10.1016/s0092-8674(02)00678-5. Epub 2002/04/17. [DOI] [PubMed] [Google Scholar]
- 11.Hochstim C, Deneen B, Lukaszewicz A, Zhou Q, Anderson DJ. Identification of positionally distinct astrocyte subtypes whose identities are specified by a homeodomain code. Cell. 2008;133(3):510–522. doi: 10.1016/j.cell.2008.02.046. Epub 2008/05/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deneen B, Ho R, Lukaszewicz A, Hochstim CJ, Gronostajski RM, Anderson DJ. The transcription factor NFIA controls the onset of gliogenesis in the developing spinal cord. Neuron. 2006;52(6):953–968. doi: 10.1016/j.neuron.2006.11.019. Epub 2006/12/21. [DOI] [PubMed] [Google Scholar]
- 13.Ge WP, Miyawaki A, Gage FH, Jan YN, Jan LY. Local generation of glia is a major astrocyte source in postnatal cortex. Nature. 484(7394):376–380. doi: 10.1038/nature10959. Epub 2012/03/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang P, Lee HK, Glasgow SM, Finley M, Donti T, Gaber ZB, et al. Sox9 and NFIA coordinate a transcriptional regulatory cascade during the initiation of gliogenesis. Neuron. 74(1):79–94. doi: 10.1016/j.neuron.2012.01.024. Epub 2012/04/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller RH. Regulation of oligodendrocyte development in the vertebrate CNS. Prog Neurobiol. 2002;67(6):451–467. doi: 10.1016/s0301-0082(02)00058-8. Epub 2002/10/19. [DOI] [PubMed] [Google Scholar]
- 16.Anthony TE, Klein C, Fishell G, Heintz N. Radial glia serve as neuronal progenitors in all regions of the central nervous system. Neuron. 2004;41(6):881–890. doi: 10.1016/s0896-6273(04)00140-0. Epub 2004/03/30. [DOI] [PubMed] [Google Scholar]
- 17.Shibata T, Yamada K, Watanabe M, Ikenaka K, Wada K, Tanaka K, et al. Glutamate transporter GLAST is expressed in the radial glia-astrocyte lineage of developing mouse spinal cord. J Neurosci. 1997;17(23):9212–9219. doi: 10.1523/JNEUROSCI.17-23-09212.1997. Epub 1997/12/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pringle NP, Yu WP, Howell M, Colvin JS, Ornitz DM, Richardson WD. Fgfr3 expression by astrocytes and their precursors: evidence that astrocytes and oligodendrocytes originate in distinct neuroepithelial domains. Development. 2003;130(1):93–102. doi: 10.1242/dev.00184. Epub 2002/11/21. [DOI] [PubMed] [Google Scholar]
- 19.Owada Y, Yoshimoto T, Kondo H. Spatio-temporally differential expression of genes for three members of fatty acid binding proteins in developing and mature rat brains. J Chem Neuroanat. 1996;12(2):113–122. doi: 10.1016/s0891-0618(96)00192-5. Epub 1996/12/01. [DOI] [PubMed] [Google Scholar]
- 20.Tien AC, Tsai HH, Molofsky AV, McMahon M, Foo LC, Kaul A, et al. Regulated temporalspatial astrocyte precursor cell proliferation involves BRAF signalling in mammalian spinal cord. Development. 139(14):2477–2487. doi: 10.1242/dev.077214. Epub 2012/06/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacobsen CT, Miller RH. Control of astrocyte migration in the developing cerebral cortex. Dev Neurosci. 2003;25(2–4):207–216. doi: 10.1159/000072269. Epub 2003/09/11. [DOI] [PubMed] [Google Scholar]
- 22.Etienne-Manneville S. In vitro assay of primary astrocyte migration as a tool to study Rho GTPase function in cell polarization. Methods Enzymol. 2006;406:565–578. doi: 10.1016/S0076-6879(06)06044-7. Epub 2006/02/14. [DOI] [PubMed] [Google Scholar]
- 23.Osmani N, Vitale N, Borg JP, Etienne-Manneville S. Scrib controls Cdc42 localization and activity to promote cell polarization during astrocyte migration. Curr Biol. 2006;16(24):2395–2405. doi: 10.1016/j.cub.2006.10.026. Epub 2006/11/04. [DOI] [PubMed] [Google Scholar]
- 24.Bignami A, Eng LF, Dahl D, Uyeda CT. Localization of the glial fibrillary acidic protein in astrocytes by immunofluorescence. Brain Res. 1972;43(2):429–435. doi: 10.1016/0006-8993(72)90398-8. Epub 1972/08/25. [DOI] [PubMed] [Google Scholar]
- 25.Miller RH, Raff MC. Fibrous and protoplasmic astrocytes are biochemically and developmentally distinct. J Neurosci. 1984;4(2):585–592. doi: 10.1523/JNEUROSCI.04-02-00585.1984. Epub 1984/02/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gomes FC, Paulin D, Moura Neto V. Glial fibrillary acidic protein (GFAP): modulation by growth factors and its implication in astrocyte differentiation. Braz J Med Biol Res. 1999;32(5):619–631. doi: 10.1590/s0100-879x1999000500016. Epub 1999/07/21. [DOI] [PubMed] [Google Scholar]
- 27.Eng LF. GaVJJ. A study of proteins in old multiple sclerosis plaques. Trans Am Soc Neurochem. 1970;1(42) [Google Scholar]
- 28.Eng LF, Ghirnikar RS, Lee YL. Glial fibrillary acidic protein: GFAP-thirty-one years (1969–2000) Neurochem Res. 2000;25(9–10):1439–1451. doi: 10.1023/a:1007677003387. Epub 2000/11/04. [DOI] [PubMed] [Google Scholar]
- 29.Anthony TE, Heintz N. The folate metabolic enzyme ALDH1L1 is restricted to the midline of the early CNS, suggesting a role in human neural tube defects. The Journal of comparative neurology. 2007;500(2):368–383. doi: 10.1002/cne.21179. Epub 2006/11/18. [DOI] [PubMed] [Google Scholar]
- 30.Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, et al. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci. 2008;28(1):264–278. doi: 10.1523/JNEUROSCI.4178-07.2008. Epub 2008/01/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsai HH, Li H, Fuentealba LC, Molofsky AV, Taveira-Marques R, Zhuang H, et al. Regional astrocyte allocation regulates CNS synaptogenesis and repair. Science. 2012;337(6092):358–362. doi: 10.1126/science.1222381. Epub 2012/06/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oberheim NA, Goldman SA, Nedergaard M. Heterogeneity of astrocytic form and function. Methods Mol Biol. 2012;814:23–45. doi: 10.1007/978-1-61779-452-0_3. Epub 2011/12/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang DD, Bordey A. The astrocyte odyssey. Progress in neurobiology. 2008;86(4):342–367. doi: 10.1016/j.pneurobio.2008.09.015. Epub 2008/10/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chaboub LS, Deneen B. Developmental origins of astrocyte heterogeneity: the final frontier of CNS development. Dev Neurosci. 2012;34(5):379–388. doi: 10.1159/000343723. Epub 2012/11/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bushong EA, Martone ME, Jones YZ, Ellisman MH. Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J Neurosci. 2002;22(1):183–192. doi: 10.1523/JNEUROSCI.22-01-00183.2002. Epub 2002/01/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta neuropathologica. 2010;119(1):7–35. doi: 10.1007/s00401-009-0619-8. Epub 2009/12/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Emsley JG, Macklis JD. Astroglial heterogeneity closely reflects the neuronal-defined anatomy of the adult murine CNS. Neuron Glia Biol. 2006;2(3):175–186. doi: 10.1017/S1740925X06000202. Epub 2007/03/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57(2):178–201. doi: 10.1016/j.neuron.2008.01.003. Epub 2008/01/25. [DOI] [PubMed] [Google Scholar]
- 39.Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacological reviews. 2005;57(2):173–185. doi: 10.1124/pr.57.2.4. Epub 2005/05/26. [DOI] [PubMed] [Google Scholar]
- 40.Hayashi Y, Nomura M, Yamagishi S, Harada S, Yamashita J, Yamamoto H. Induction of various blood-brain barrier properties in non-neural endothelial cells by close apposition to co-cultured astrocytes. Glia. 1997;19(1):13–26. Epub 1997/01/01. [PubMed] [Google Scholar]
- 41.Sobue K, Yamamoto N, Yoneda K, Hodgson ME, Yamashiro K, Tsuruoka N, et al. Induction of blood-brain barrier properties in immortalized bovine brain endothelial cells by astrocytic factors. Neurosci Res. 1999;35(2):155–164. doi: 10.1016/s0168-0102(99)00079-6. Epub 2000/01/05. [DOI] [PubMed] [Google Scholar]
- 42.Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7(1):41–53. doi: 10.1038/nrn1824. Epub 2005/12/24. [DOI] [PubMed] [Google Scholar]
- 43.Pfrieger FW, Barres BA. Synaptic efficacy enhanced by glial cells in vitro. Science. 1997;277(5332):1684–1687. doi: 10.1126/science.277.5332.1684. Epub 1997/09/12. [DOI] [PubMed] [Google Scholar]
- 44.Christopherson KS, Ullian EM, Stokes CC, Mullowney CE, Hell JW, Agah A, et al. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell. 2005;120(3):421–433. doi: 10.1016/j.cell.2004.12.020. Epub 2005/02/15. [DOI] [PubMed] [Google Scholar]
- 45.Mauch DH, Nagler K, Schumacher S, Goritz C, Muller EC, Otto A, et al. CNS synaptogenesis promoted by glia-derived cholesterol. Science. 2001;294(5545):1354–1357. doi: 10.1126/science.294.5545.1354. Epub 2001/11/10. [DOI] [PubMed] [Google Scholar]
- 46.Takata N, Hirase H. Cortical layer 1 and layer 2/3 astrocytes exhibit distinct calcium dynamics in vivo. PLoS One. 2008;3(6):e2525. doi: 10.1371/journal.pone.0002525. Epub 2008/06/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nimmerjahn A, Mukamel EA, Schnitzer MJ. Motor behavior activates Bergmann glial networks. Neuron. 2009;62(3):400–412. doi: 10.1016/j.neuron.2009.03.019. Epub 2009/05/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Holthoff K, Witte OW. Directed spatial potassium redistribution in rat neocortex. Glia. 2000;29(3):288–292. doi: 10.1002/(sici)1098-1136(20000201)29:3<288::aid-glia10>3.0.co;2-8. Epub 2000/01/22. [DOI] [PubMed] [Google Scholar]
- 49.Kuffler SW, Nicholls JG. The physiology of neuroglial cells. Ergebnisse der Physiologie, biologischen Chemie und experimentellen Pharmakologie. 1966;57:1–90. Epub 1966/01/01. [PubMed] [Google Scholar]
- 50.Sonnewald U, Westergaard N, Schousboe A. Glutamate transport and metabolism in astrocytes. Glia. 1997;21(1):56–63. doi: 10.1002/(sici)1098-1136(199709)21:1<56::aid-glia6>3.0.co;2-#. Epub 1997/09/23. [DOI] [PubMed] [Google Scholar]
- 51.Pellerin L, Magistretti PJ. Sweet sixteen for ANLS. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2012;32(7):1152–1166. doi: 10.1038/jcbfm.2011.149. Epub 2011/10/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee Y, Morrison BM, Li Y, Lengacher S, Farah MH, Hoffman PN, et al. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature. 2012;487(7408):443–448. doi: 10.1038/nature11314. Epub 2012/07/18. [DOI] [PMC free article] [PubMed] [Google Scholar]