Abstract
Objective
Mold in water-damaged homes has been linked to asthma. Our objective was to test a new metric to quantify mold exposures in asthmatic children’s homes in three widely dispersed cities in the United States.
Methods
The Environmental Relative Moldiness Index (ERMI) metric was created by the US Environmental Protection Agency, with assistance by the Department of Housing and Urban Development (HUD), to quantify mold contamination in US homes. The ERMI values in homes of asthmatic children were determined for the three widely dispersed cities of Boston, Kansas City, and San Diego.
Results
Asthmatic children in Boston (n = 76), Kansas City (n = 60), and San Diego (n = 93) were found to be living in homes with significantly higher ERMI values than were found in homes randomly selected during the 2006 HUD American Healthy Homes Survey (AHHS) from the same geographic areas (n = 34, 22, and 28, respectively). Taken together, the average ERMI value in the homes with an asthmatic child was 8.73 compared to 3.87 for the AHHS homes. In addition, Kansas City homes of children with “Mild, Moderate, or Severe Persistent Asthma” had average ERMI value of 12.4 compared to 7.9 for homes of children with only “Mild Intermittent Asthma.” Aspergillus niger was the only mold of the 36 tested which was measured in significantly greater concentration in the homes of asthmatic children in all three cities.
Conclusion
High ERMI values were associated with homes of asthmatic children in three widely dispersed cities in the United States.
Keywords: Aspergillus niger, dust, metric, mold, the United States
Introduction
Asthma is the most common chronic disease of children in the United States (1). Mold is one of the many triggers of asthma exacerbations in some individuals with atopic asthma (2, 3), although no asthma exacerbation threshold for mold exposures is known to exist. Since mold exposures cannot be totally avoided, a metric for quantifying mold contamination is needed.
The Environmental Relative Moldiness Index (ERMI) metric was created by the US Environmental Protection Agency (EPA), with assistance by the Department of Housing and Urban Development (HUD), to quantify mold contamination in US homes (4). During the 2006 HUD American Healthy Homes Survey (AHHS), standard dust samples were obtained from the living rooms and bedrooms in 1083 randomly selected homes across the United States (4). The dust samples from these homes were analyzed using a DNA-based technology called mold specific quantitative polymerase chain reaction (PCR) (MSQPCR) for 36 indicator mold species in each sample (5).
These 36 molds include 26 Group 1 molds that indicate water damage and 10 Group 2 species that are commonly found even without water damage (4). The ERMI scale ranges from approximately −10 to 20 (low to high). The upper quartile (highest mold contamination quartile) starts at an ERMI value of approximately 5 (4).
The ERMI metric was used previously to demonstrate significantly greater mold contamination in the homes of asthmatic children in Chapel Hill, NC and Detroit, MI (6, 7). Therefore, we wanted to determine whether higher ERMI values would be found in the homes of asthmatic children in the widely dispersed US cities of Boston, Kansas City, and San Diego.
Materials and Methods
General Methods of Recruitment and Dust Collection
The three cities selected for this study were each awarded a HUD demonstration grant in the same year. Although recruitment methods varied, each study confirmed the medical diagnosis of asthma for each child. Each city’s study and the comparison homes utilized dust collectors from Indoor Biotechnologies (Charlottesville, VA, USA) to collect the floor dust. Settled dust in the living room and bedroom were obtained from each home, except in Kansas City where only bedroom dust was obtained. Each dust sample was sieved through a 300 μm pore mesh before weighing and analysis.
The comparison homes were all selected at random during the 2006 HUD AHHS. It is likely that in some of the comparison homes, there were some with an asthmatic child. The number of households with children is 46% in the United States according to the most recent census. About 10% of children have asthma. Assuming the children with asthma are evenly distributed, the number of these comparison homes with an asthmatic child was probably around 5%.
Recruitment and Dust Collection in Boston
Participant children had doctor-diagnosed asthma and were recruited either through (1) participating in public health asthma programs; (2) enrolling through a study representative at the Boston Medical Center Pediatric Asthma and Allergy Clinic; (3) responding to ads in local newspapers; and (4) having the study referred to them by a previous participant. As a result, 76 households with zip codes starting with 01, 02, or 03 were enrolled. This study received approval from the Boston University/Boston Medical Center Institutional Review Board and this approval was renewed annually.
Dust samples were collected from the living room floor using the Eureka Mighty Mite (EMM, model 3670, Electrolux Home Care Products, Inc., Peoria, IL, USA), which collects dust using the DUSTREAM™ Collector (Indoor Biotechnologies, Inc., Charlottesville, VA, USA) (8). After collection, the dust was immediately placed in a 76 mm × 20 mm tube with cap (Sarstedt Aktiengeselischaft & Co., Numbrecht, Germany).
In the 2006 HUD AHHS, dust samples were collected from the living rooms and bedrooms of 34 homes in the 01, 02, or 03 zip codes. The samples were then frozen before shipment to the analytical laboratory.
Recruitment and Dust Collection in Kansas City
The project in Kansas City was carried out in Children’s Mercy Hospital under the Kansas City Safe and Healthy Homes Partnership (KCSHHP). Dust samples were collected from the bedrooms of 60 asthmatic children in the greater Kansas City area, as previously described (9). Because no homes from Kansas City were included in the AHHS, the 22 homes from the State of Kansas were used for comparison. Pediatric subjects were enrolled in KCSHHP if they had a history of asthma. During the enrollment visit to the allergy clinic, subjects were assigned to either “Mild Intermittent Asthma” or “Mild, Moderate, or Severe Persistent Asthma” category (10).
Recruitment and Dust Collection in San Diego
The project recruitment in the City of San Diego was through outreach and referrals from many different agencies and entities in the area, including the American Lung Association, City of San Diego Housing Commission, and the San Diego Unified School District. Dust samples were collected from the living rooms and bedrooms of homes of 93 asthmatic children with a zip code starting with 92 using the 2006 HUD AHHS protocol (4). The 2006 HUD AHHS included 28 homes from the area of San Diego that had a zip code starting with 92 and were used for comparison.
Recruitment and Dust Collection for the 2006 HUD National Healthy Home Survey
The process of selecting homes for the 2006 HUD National Healthy Homes Survey has been described in detail at the website: http://portal.hud.gov/hudportal/documents/huddoc?id=AHHS_REPORT.pdf. The subsets of samples from Boston, Kansas City, and San Diego were from a total of 1083 obtained in the entire United States (4).
The 2006 HUD AHHS was reviewed for human subject involvement by the Westat Institutional Review Board and approved on 9 November 2004. A Confidentiality Certificate protecting the identity of the survey respondents was issued to QuanTech by the National Institute of Environmental Health Sciences on 22 February 2005.
Dust Sample Extraction and DNA Purification
Dust samples were sieved through a 300 μm pore size nylon mesh (Gilson Company, Inc., Lewis Center, OH, USA). Each dust sample (5.0 ± 0.1 mg) was spiked with 1 × 106 conidia of Geotrichum candidum as an external reference. Each extraction tube was shaken in the bead beater (Biospec Products, Bartlesville, OK, USA) for 1 min and the DNA was purified using the DNA-EZ extraction kit (GeneRite, Cherry Hill, NJ, USA).
Mold Quantification
Methods and assays have been reported previously for performing MSQPCR analyses (11, 12). Briefly, the standard reaction assays contained 12.5 μl of “Universal Master Mix” (Applied Biosystems Inc., Foster City, CA, USA), 1 μl of a mixture of forward and reverse primers at 25 μM each, 2.5 μl of a 400 nM TaqMan probe (Applied Biosystems Inc.), 2.5 μl of 2 mg/ml fraction V bovine serum albumin (Sigma Chemical, St. Louis, MO, USA), and 2.5 μl of DNA-free water (Cepheid, Sunnyvale, CA, USA). To this mix was added 5 μl of the DNA extract from the sample. All primer and probe sequences used in the assays, as well as known species comprising the assay groups, are available at the website: http://www.epa.gov/nerlcwww/moldtech.htm. Primers and probes were synthesized commercially (Applied Biosystems, Inc.).
Reactions were performed with thermal cycling conditions consisting of 2 min at 50°C, 10 min at 95°C, followed by 40 cycles of 15 s at 95°C for template denaturation and 1 min at 60°C for probe and primer annealing and primer extension. The cycle threshold determinations were automatically performed by the instrument ABI 7900 (Applied Biosystems, Inc.) using default parameters.
The ERMI values were calculated by taking the sum of the logs of the concentrations of Group 1 molds (s1) and subtracting the sum of the logs of the concentrations of Group 2 molds (s2) (4) as follows:
| (Eq. 1) |
Statistical Analyses
Descriptive statistics were computed from log-transformed, left-censored species data using the nonparametric Kaplan–Meier procedure from the Non-detects and data analysis (NADA) (13) package for R (14). For consistency across species data and ERMI-related metrics, the Kaplan–Meier procedure was also used to compute descriptive statistics on sum logs Group 1, sum logs Group 2, and ERMI. Group differences were evaluated using the NADA implementation of the nonparametric Peto & Peto modification of the Gehan–Wilcoxon test. The results from the Peto & Peto were verified using the rank-based Brunner–Dette–Munk (15) permutation method in the R package asbio (16). The results of the mold species comparisons were adjusted for multicomparisons using the Holm methodology (17).
Results
Boston
The average ERMI in the homes of asthmatic children in Boston was statistically significantly (p = .03) greater than the average ERMI in the AHHS homes (8.73 and 5.16, respectively) from the same geographic areas matched by similar zip codes, i.e., starting with 01, 02, or 03 (Table 1). Three of the Group 1 molds (Aspergillus niger, Aureobasidium pullulans, and Trichoderma viride), but none of the Group 2 molds, were in statistically (p < .001) higher concentrations in the asthmatic children’s homes (Table 2). However, two Group 1 molds, Aspergillus penicillioides and the Eurotium group, and one Group 2 mold, Acremonium strictum, were in significantly (p < .001) greater concentrations in the AHHS homes than in the asthmatic children’s homes in Boston (Table 2).
Table 1.
Comparison of the means of the sum of the logs of Group 1 (SLG 1) molds, sum of the logs of Group 2 (SLG 2) molds, and ERMI values for homes of asthmatic children in Boston, Kansas City [also in Kansas City “Mild Intermittent Asthmatic” (Intermittent) children’s homes vs. “Mild, Moderate or Severe Persistent” (Persistent) asthmatic children’s homes], and San Diego compared to a random selection of homes from the same geographic areas obtained in the 2006 HUD AHHS.
| SLG 1 | SLG 2 | ERMI | ||||
|---|---|---|---|---|---|---|
| City or AHHS comparison | Mean | STD | Mean | STD | Mean | STD |
| Boston (n = 76) | 22.01 | 11.30 | 13.28 | 5.95 | 8.73 | 6.39 |
| AHHS (n = 34) | 16.77 | 6.53 | 11.61 | 3.86 | 5.16 | 2.95 |
| Student’s t-test (p-value) | .03 | .06 | .03 | |||
| Kansas City (n = 60) | 25.82 | 11.19 | 16.48 | 4.02 | 9.31 | 8.38 |
| AHHS (n = 22) | 17.29 | 7.48 | 13.79 | 3.02 | 3.49 | 6.27 |
| Student’s t-test (p-value) | < .01 | < .01 | < .01 | |||
| KC intermittent asthma (n = 42) | 23.58 | 10.74 | 15.65 | 3.53 | 7.90 | 8.59 |
| KC persistent asthma (n = 18) | 30.99 | 9.88 | 18.47 | 4.25 | 12.44 | 6.77 |
| Student’s t-test (p-value) | .03 | .04 | .05 | |||
| San Diego (n = 93) | 25.85 | 9.24 | 17.69 | 5.04 | 8.16 | 6.11 |
| AHHS (n = 28) | 9.96 | 5.66 | 7.88 | 4.58 | 2.08 | 3.28 |
| Student’s t-test (p-value) | < .001 | < .001 | < .001 | |||
Note: STD, standard deviation; KC, Kansas City.
Table 2.
Comparison of the means of the concentrations (cells/mg dust) of each mold in Boston, Kansas City, and San Diego asthmatic children’s homes and homes from the same geographic areas obtained in the 2006 AHHS.
| Molds | Boston (n = 76) | AHHS (n = 34) | Kansas City (n = 60) | AHHS (n = 22) | San Diego (n = 93) | AHHS (n = 28) |
|---|---|---|---|---|---|---|
| Group 1 | Mean | Mean | Mean | Mean | Mean | Mean |
| Aspergillus flavus | 2 | 1 | 1 | 1 | 2 | 3 |
| Aspergillus fumigatus | 3 | 3 | 1 | 2 | 8 | 3 |
| Aspergillus niger | 22 | 2 | 27 | 2 | 46 | 4 |
| Aspergillus ochraceus | 7 | 4 | 6 | 0 | 12 | 4 |
| Aspergillus penicillioides | 17 | 201 | 36 | 26 | 10 | 7 |
| Aspergillus restrictus | 11 | 17 | 1 | 0 | 4 | 0 |
| Aspergillus sclerotiorum | 1 | 1 | 2 | 1 | 2 | 2 |
| Aspergillus sydowii | 4 | 14 | 5 | 0 | 3 | 18 |
| Aspergillus unguis | 2 | 6 | 2 | 0 | 1 | 2 |
| Aspergillus versicolor | 4 | 5 | 28 | 2 | 4 | 6 |
| Aureobasidium pullulans | 6671 | 431 | 1363 | 768 | 9243 | 145 |
| Chaetomium globosum | 3 | 3 | 42 | 4 | 3 | 2 |
| Cladosporium sphaerospermum | 38 | 21 | 56 | 11 | 144 | 2 |
| Eurotium group | 34 | 411 | 53 | 170 | 25 | 33 |
| Paecilomyces variotii | 3 | 3 | 2 | 1 | 3 | 2 |
| Penicillium brevicompactum | 13 | 5 | 5 | 2 | 100 | 7 |
| Penicillium corylophilum | 2 | 2 | 2 | 0 | 4 | 11 |
| Penicillium crustosum | 13 | 16 | 2 | 0 | 12 | 0 |
| Penicillium purpurogenum | 1 | 2 | 0 | 0 | 3 | 1 |
| Penicillium spinulosum | 2 | 2 | 0 | 0 | 2 | 2 |
| Penicillium variabile | 5 | 3 | 7 | 2 | 6 | 1 |
| Scopulariopsis brevicaulis | 2 | 2 | 2 | 3 | 2 | 1 |
| Scopulariopsis chartarum | 2 | 2 | 2 | 1 | 4 | 2 |
| Stachybotrys chartarum | 2 | 2 | 3 | 1 | 6 | 1 |
| Trichoderma viride | 4 | 1 | 4 | 1 | 6 | 2 |
| Wallemia sebi | 88 | 47 | 298 | 43 | 494 | 7 |
| Group 2 | ||||||
| Acremonium strictum | 2 | 5 | 2 | 3 | 2 | 2 |
| Alternaria alternata | 36 | 25 | 180 | 235 | 247 | 34 |
| Aspergillus ustus | 4 | 2 | 3 | 1 | 4 | 2 |
| Cladosporium cladosporioides type 1 | 548 | 386 | 1408 | 425 | 1840 | 70 |
| Cladosporium cladosporioides type 2 | 19 | 9 | 7 | 5 | 280 | 5 |
| Cladosporium herbarum | 95 | 20 | 362 | 26 | 292 | 24 |
| Epicoccum nigrum | 34 | 47 | 627 | 252 | 199 | 11 |
| Mucor group | 27 | 13 | 36 | 63 | 54 | 5 |
| Penicillium chrysogenum type 2 | 24 | 12 | 48 | 2 | 23 | 4 |
| Rhizopus stolonifer | 2 | 1 | 1 | 1 | 8 | 1 |
The means that were significantly different, after Holm adjustment for multiple comparisons (p < .001), are printed in bold type.
Kansas City
The average ERMI in the homes of asthmatic children in Kansas City (Table 1) was nearly 3 times higher than in the randomly selected homes (9.31 vs. 3.49) from the State of Kansas (p = .05). Only one Group 1 mold, A. niger, and three Group 2 molds, Cladosporium cladosporioides type 1, Cladosporium herbarum, and Penicillium chrysogenum type 2, were in significantly higher concentrations in the Kansas City asthmatic children’s homes (Table 2).
The average ERMI in the homes of “Mild, Moderate, or Severe Persistent Asthmatic” children was 12.44 compared to 7.90 in the “Mild Intermittent Asthmatic” children’s homes from Kansas City (Table 1). Only one Group 1 mold, A. niger, and one Group 2 mold, C. cladosporioides type 1, were in significantly higher concentrations in the mild, moderate, or severe persistent asthmatic children’s homes (Table 3).
Table 3.
Comparison of the means of the concentrations (cells/mg dust) of each mold in Kansas City “Mild Intermittent Asthmatic” (Intermittent) children’s homes (n = 42) versus “Mild, Moderate, or Severe Persistent” (Persistent) asthmatic children’s homes (n = 18).
| Molds | Intermittent asthmatic (n = 42) | Persistent asthmatic (n = 18) | |
|---|---|---|---|
| Group 1 | Mean | Mean | p-Value |
| Aspergillus flavus | 1 | 2 | .231 |
| Aspergillus fumigatus | 1 | 3 | .134 |
| Aspergillus niger | 21 | 48 | < .001 |
| Aspergillus ochraceus | 4 | 12 | .035 |
| Aspergillus penicillioides | 24 | 97 | .306 |
| Aspergillus restrictus | 0 | 2 | .011 |
| Aspergillus sclerotiorum | 2 | 2 | .019 |
| Aspergillus sydowii | 5 | 8 | .047 |
| Aspergillus unguis | 1 | 3 | .022 |
| Aspergillus versicolor | 25 | 33 | .003 |
| Aureobasidium pullulans | 996 | 2863 | .757 |
| Chaetomium globosum | 44 | 36 | .004 |
| Cladosporium sphaerospermum | 42 | 110 | .044 |
| Eurotium group | 40 | 107 | .003 |
| Paecilomyces variotii | 2 | 5 | .127 |
| Penicillium brevicompactum | 3 | 15 | .571 |
| Penicillium corylophilum | 2 | 2 | .002 |
| Penicillium crustosum | 1 | 5 | .017 |
| Penicillium purpurogenum | 0 | 1 | .296 |
| Penicillium spinulosum | 0 | 1 | .414 |
| Penicillium variabile | 5 | 19 | .325 |
| Scopulariopsis brevicaulis | 2 | 2 | .082 |
| Scopulariopsis chartarum | 1 | 3 | .433 |
| Stachybotrys chartarum | 2 | 11 | .723 |
| Trichoderma viride | 4 | 6 | .005 |
| Wallemia sebi | 143 | 1822 | .187 |
| Group 2 | |||
| Acremonium strictum | 2 | 2 | .076 |
| Alternaria alternata | 168 | 221 | .440 |
| Aspergillus ustus | 3 | 7 | .230 |
| Cladosporium cladosporioides type 1 | 1204 | 1979 | < .001 |
| Cladosporium cladosporioides type 2 | 5 | 14 | .690 |
| Cladosporium herbarum | 319 | 492 | .0013 |
| Epicoccum nigrum | 561 | 791 | .039 |
| Mucor group | 26 | 78 | .126 |
| Penicillium chrysogenum type 2 | 33 | 115 | .002 |
| Rhizopus stolonifer | 1 | 2 | .659 |
The means that were significantly different, after Holm adjustment for multiple comparisons (p < .001), are printed in bold type.
San Diego
The average ERMI value in the homes of asthmatic children in San Diego was more than twice as high as the randomly selected AHHS homes (8.11 vs. 3.28, respectively) from the same geographic areas with zip codes starting with 92 (Table 1). Eight Group 1 molds, including A. niger, A. pullulans, and T. viride, and seven Group 2 molds, including C. cladosporioides type 1, were in statistically significantly higher concentrations (Table 2).
Combined Cities Analysis
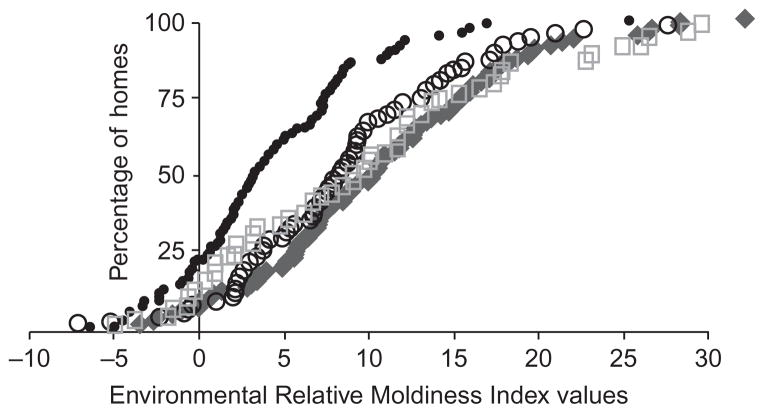
Significantly higher average ERMI values were measured in dust samples collected from the homes of asthmatic children in all three cities compared to the homes randomly selected in the AHHS. Taking all three projects together, the average ERMI value in the asthmatic’s homes was 8.73 compared to 3.87 in the AHHS homes sampled at essentially the same time. Figure 1 shows the ERMI values from each home in each study (including the AHHS samples) assembled on the x-axis from lowest to highest. Because there are different numbers of samples in each study, the y-axis values are located on the scale based on each total set of samples. For example, for Boston 76 equals 100%, for Kansas City 100% was based on 60, for San Diego 100% was based on 93, and AHHS with a total of 84 equals 100%.
Figure 1.
Distribution of ERMI values of asthmatic children’s homes in Boston (circle), Kansas City (square), and San Diego (diamond) and in the combined, comparison AHHS homes (black dots). The ERMI values from each home in each study (including the AHHS samples) were assembled on the x-axis from lowest to highest. Because there are different numbers of samples in each study, the y-axis values are located on the scale based on each total set of samples. In Boston 76 equals 100%, for Kansas City 100% was based on 60, for San Diego 100% was based on 93, and AHHS with a total of 84 equals 100%.
Discussion
The average asthmatic child in these cities was living in a home with an ERMI value in the highest quartile (>5) of the ERMI scale which was based on a nationally representative survey of homes. In Kansas City, homes of “Mild, Moderate, or Severe Persistent Asthmatic” children were found to have an even higher average ERMI values compared to those with “Mild Intermittent Asthma.” This suggests that differences in inhalation exposure might be important in the expression of the disease.
These results are consistent with other studies of childhood asthma which utilized the ERMI metric to quantify mold contamination. For example, Reponen et al. (18) found that infants exposed to homes with an ERMI value above 5 were significantly more likely to develop physician-diagnosed asthma by age 7. In the Detroit, MI, homes of “severe” asthmatic children, the average ERMI was 8.2 (7) and in Chapel Hill, NC, asthmatics’ homes, the average ERMI was 16.4 (6). Based on the studies to date, homes with ERMI values in the highest ERMI quartile (>5) are more likely to be associated with childhood asthma than lower ERMI homes.
These results bolster the growing evidence linking water damage and mold exposures to asthma (3). However, it seems likely that all molds are not equally relevant to the disease. Identifying specific molds that are potentially associated with asthma could narrow down our search for the most important molds.
As a possible example of this approach, we found that A. niger was the only mold significantly associated with asthmatic children’s homes in all three cities. In laboratory studies, A. niger, specifically the secreted “active proteases,” caused the development of asthma-like symptoms in an animal model of asthma (19). When production of these proteases was genetically eliminated from a strain of A. niger, it no longer elicited these asthma-like symptoms in the same animal model (20).
Other Group 1 molds significantly associated with asthmatic children’s homes in two of these cities (Boston and San Diego) were A. pullulans and T. viride. Only one Group 2 mold (C. cladosporioides type 1) was in significantly higher concentrations in these two cities. However, since only 36 molds were measured, it is likely that there are other relevant molds that were not measured in these homes.
Future refinements of the ERMI scale will likely include some new species and eliminate others as more is learned about the development of asthma. In a prospective study of asthma development, Reponen et al. (21) found that three Group 1 molds were just as accurate in predicting the development of asthma as the entire 36 ERMI species. However, this study was limited to one geographic area and many additional studies are needed before generalizations can be made about the role of specific molds in asthma.
In spite of any limitations of the ERMI scale that currently exist, the application of the ERMI metric was useful in detecting 50% of fourth quartile ERMI homes in which the home owner was unaware of any moisture or mold problem and the trained inspector did not detect in the investigation (22). Therefore, the ERMI analysis might be useful in homes of children with poorly controlled asthma and homes of pregnant women where one of the parents is atopic and there is reason to suspect a possible problem.
There are several limitations to this study. Since this is a cross-sectional study, no causality should be attributed. There was also no adjustment for asthma severity in subjects except for the substudy in Kansas City. Although mold can make people symptomatic through an irritation pathway (not just an allergic pathway), mold allergy in subjects should be noted. The sample size is also fairly small in each study. Lastly, zip codes may not be a perfect substitute for the selection of homes based on specific characteristics, including multifamily versus single family, age of housing, etc.
The cost of treating asthma in the United States is around $15 billion per year (23). Since the costs continue to escalate, methods to prevent asthma development or exacerbation are needed. One important step might be remediating water-damaged homes. In Cleveland, such remediation resulted in a 10-fold reduction in emergency room visits or hospitalizations for the asthmatic children (24).
Conclusion
The human and financial costs of asthma in the United States continue to increase. The ERMI metric appears to be applicable to studies of childhood asthma across a large geographic area of the United States.
Acknowledgments
These three projects were supported by HUD grants. The Boston study was supported by US Department of Housing and Urban Development, Office of Healthy Homes and Lead Hazard Control, Grant No. MALHH0163-07; the Kansas City Study was supported by MOLHH0159-07 awarded to Children’s Mercy Hospital; and the City of San Diego project was supported by Grant No. CALHH0158-07.
Footnotes
Declaration of Interest
The synthesis of all three investigations was undertaken with a Cooperative Research and Development Agreement No. RW86922858-01-0 between HUD and the US EPA. The US EPA through its Office of Research and Development collaborated in the research described here. Although this work was reviewed by EPA and approved for publication, it may not necessarily reflect official EPA policy. Mention of trade names or commercial products does not constitute endorsement or recommendation by the EPA for use. Since MSQPCR technology is patented by the US EPA, the Agency has a financial interest in its commercial use.
References
- 1.Rudd RA, Moorman JE. Asthma incidence: data from the National Health Interview Survey, 1980–1996. J Asthma. 2007;44:65–70. doi: 10.1080/02770900601125896. [DOI] [PubMed] [Google Scholar]
- 2.Institute of Medicine (IOM), National Academies of Science. Damp Indoor Spaces and Health. Washington, DC: The National Academies Press; 2004. [PubMed] [Google Scholar]
- 3.World Health Organization (WHO) Europe. WHO Guidelines for Indoor Air Quality: Dampness and Mould. Copenhagen: WHO; 2009. [PubMed] [Google Scholar]
- 4.Vesper SJ, McKinstry C, Haugland RA, Wymer L, Ashley P, Cox D, DeWalt G, Friedman W. Development of an environmental relative moldiness index for homes in the U.S. J Occup Environ Med. 2007;49:987–990. doi: 10.1097/JOM.0b013e3181255e98. [DOI] [PubMed] [Google Scholar]
- 5.Vesper S. Traditional mould analysis compared to a DNA-based method of mould analysis. Crit Rev Micro. 2012;37:15–24. doi: 10.3109/1040841X.2010.506177. [DOI] [PubMed] [Google Scholar]
- 6.Vesper SJ, McKinstry C, Ashley P, Haugland RA, Yeatts K, Bradham K, Svendsen E. Quantitative PCR analysis of molds in the dust from homes of asthmatic children in North Carolina. J Environ Monitor. 2007;9:826–830. doi: 10.1039/b704359g. [DOI] [PubMed] [Google Scholar]
- 7.Vesper S, McKinstry C, Haugland R, Neas L, Hudgens E, Heidenfelder B, Gallagher J. Higher Environmental Relative Moldiness Index (ERMIsm) values measured in Detroit homes of severely asthmatic children. Sci Total Environ. 2008;394:192–196. doi: 10.1016/j.scitotenv.2008.01.031. [DOI] [PubMed] [Google Scholar]
- 8.Vojta PJ, Friedman W, Marker DA, Clickner R, Rogers JW, Viet SM, Muilenberg ML, Thorne PS, Arbes SJ, Jr, Zeldin DC. First national survey of lead and allergens in housing, survey design and methods for the allergen and endotoxin components. Environ Health Perspect. 2002;110:527–532. doi: 10.1289/ehp.02110527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barnes CS, Kennedy K, Gard L, Forrest E, Johnson L, Pacheco F, Hu F, Amado M, Portnoy JM. The impact of home cleaning on quality of life for homes with asthmatic children. Allergy Asthma Proc. 2008;29:197–204. doi: 10.2500/aap.2008.29.3099. [DOI] [PubMed] [Google Scholar]
- 10.US Department of Health and Human Services (DHS); National Institutesof Health (NIH) National asthma education and prevention program: Expert Panel Report 3: guidelines for the diagnosis and management of asthma. Bethesda, MD: National Institutes of Health, National Heart Lung and Blood Institute; 2007. [Accessed February 3, 2011.]. NIH publication No: 97-4051. Available at: http://www.ncbi.nlm.nih.gov/books/NBK2358/ [Google Scholar]
- 11.Haugland RA, Brinkman NE, Vesper SJ. Evaluation of rapid DNA extraction methods for the quantitative detection of fungal cells using real time PCR analysis. J Microbiol Meth. 2002;50:319–323. doi: 10.1016/s0167-7012(02)00037-4. [DOI] [PubMed] [Google Scholar]
- 12.Haugland RA, Varma M, Wymer LJ, Vesper SJ. Quantitative PCR of selected Aspergillus, Penicillium and Paecilomyces species. Sys Appl Microbiol. 2004;27:198–210. doi: 10.1078/072320204322881826. [DOI] [PubMed] [Google Scholar]
- 13.Lee L. NADA: Nondetects and Data Analysis for Environmental Data. R Package Version 1.5–3. 2010 http://www.R-project.org/
- 14.R Development Core Team. Vienna: 2012. R: A Language and Environment for Statistical Computing. Version 2.14. 2. http://www.R-project.org/ [Google Scholar]
- 15.Brunner E, Dette H, Munk A. Box-type approximations in nonparametric factorial designs. J Amer Stat Assoc. 1997;92:1494–1502. [Google Scholar]
- 16.Aho K. Asbio: A Collection of Statistical Tools for Biologists. R Package Version 0.3–40. 2011 http://www.R-project.org/
- 17.Holm S. A simple sequentially rejective multiple test procedure. Scan J Stat. 1979;6:65–70. [Google Scholar]
- 18.Reponen T, Vesper S, Levin L, Johansson E, Ryan P, Burkle J, Grinspun SA, Zheng S, Berstein DI, Lockey J, Villareal M, Hershey GKK, LeMasters G. High Environmental Relative Moldiness Index during infancy as predictor of age seven asthma. Ann Allergy Asthma Immunol. 2011;107:120–126. doi: 10.1016/j.anai.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Porter P, Susarla SC, Polikepahad S, Qian Y, Hampton J, Kiss A, Vaidya S, Sur S, Ongeri V, Yang T, Delclos GL, Abramson S, Kheradmand F, Corry DB. Link between allergic asthma and airway mucosal infection suggested by proteinase-secreting household fungi. Mucosal Immunol. 2009;2:504–517. doi: 10.1038/mi.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Porter P, Polikepahad S, Qian Y, Knight JM, Lu W, Tai WM, Roberts L, Ongeri V, Yang T, Seryshev A, Abramson S, Delclos GL, Kheradmand F, Corry DB. Respiratory tract allergic disease and atopy: experimental evidence for a fungal infectious etiology. Med Mycol. 2011;49(Suppl 1):S158–S163. doi: 10.3109/13693786.2010.509743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reponen T, Lockey J, Berstein DI, Vesper SJ, Levin L, Zheng S, Ryan P, Grinspun SA, Villareal M, Hershey GKK, LeMasters G. Infants exposed to specific molds correlated with age seven asthma. J Allergy Clin Immunol. 2012;130:639–644. doi: 10.1016/j.jaci.2012.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vesper S, McKinstry C, David C, Gary D. Correlation between ERMI values and other moisture and mold assessments of homes in the American Healthy Homes Survey. J Urban Health. 2009;86:850–860. doi: 10.1007/s11524-009-9384-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mudarri D, Fisk WJ. Public health and economic impact of dampness and mold. Indoor Air. 2007;17:226–235. doi: 10.1111/j.1600-0668.2007.00474.x. [DOI] [PubMed] [Google Scholar]
- 24.Kercsmar CM, Dearborn DG, Schluchter MD, Xue L, Kirchner HL, Sobolewski J, Greenberg SJ, Vesper SJ, Allan TM. Reduction in asthma morbidity in children as a result of home remediation aimed at moisture sources. Environ Health Perspec. 2006;114:1574–1580. doi: 10.1289/ehp.8742. [DOI] [PMC free article] [PubMed] [Google Scholar]