Neoplastic cells must maintain telomeres in order to replicate extensively, often by inducing telomerase activity. However, some tumors use a telomerase-independent mechanism dependent upon homologous recombination known as alternative lengthening of telomeres (ALT) [1]. ALT has been identified in 11–24 % of glioma tissue samples [2–4], but has been demonstrated in only one glioma cell line (TG20) [5]. In this brief letter, we describe a second ALT-positive glioblastoma (GBM) cell line.
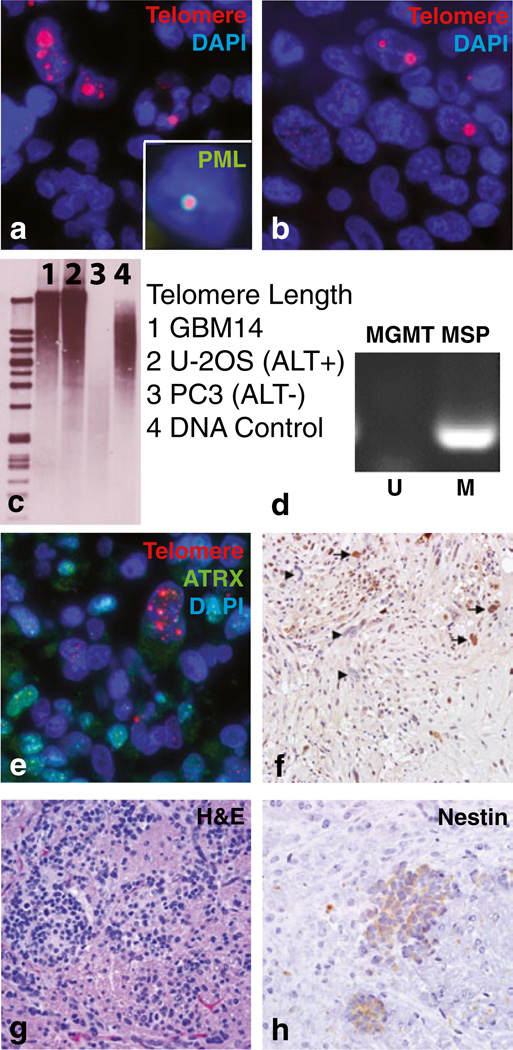
We examined five stem-like GBM neurosphere lines (HSR-GBM1, 040821, 040622, JHH-GBM10, and JHH-GBM14) by telomere-specific FISH and identified the ultra-bright telomeric DNA foci indicative of ALT [6] in JHH-GBM14 (Fig. 1a), which also contained ALT-associated PML bodies (APBs; inset). These neurospheres were isolated from an untreated primary frontal lobe glioblastoma in a 69-year-old male [7], and examination of the surgical specimen also revealed ALT (Fig. 1b). The percentage of cells displaying ultra-bright telomeric foci varied within the tumor, but was low (1–5 %) in both the surgical specimen and JHH-GBM14. In our prior study of 40 ALT-positive high-grade astrocytomas, the percentage of cells exhibiting the ALT phenotype varied significantly from case to case with the majority containing >30 % positive cells, while some displayed a smaller fraction as seen with JHH-GBM14 [2]. Southern blotting showed the highly heterogeneous telomere length distribution typical of ALT (Fig. 1c) [5]. PCR-based TRAP assays revealed low-level telomerase activity, possibly representing a mechanism concentrated in ALT-negative cells (data not shown).
Fig. 1.
ALT characterization in a glioblastoma neurosphere line. a Telomere-specific FISH analysis in JHH-GBM14 and b primary tumor, as well as concurrent telomere FISH and PML immunofluorescence (inset in a). c Highly heterogeneous telomere length distribution typical of ALT was seen by Southern blot analysis in JHH-GBM14 and U-2 OS ALT(+) cells, while ALT(−) PC3 cells lacked this distribution. d MGMT promoter analysis via methylation-specific PCR. e JHH-GBM14 cells showing loss of ATRX protein demonstrate the ALT phenotype. f Primary tumor, arrows: ATRX positive; arrowheads: ATRX negative tumor cells. g JHH-GBM14 intracranial xenografts, H&E. h Human-specific nestin immunohistochemistry
DNA sequencing revealed no mutations in TP53 exons 5–8, and PCR failed to detect EGFRvIII. A Y183C point mutation in IDH1 associated with familial osteoarthritis [8], but not with glioblastoma or ALT [9], was identified. Methylation-specific PCR analysis of the MGMT promoter revealed complete methylation (Fig. 1d), and treatment with temozolomide caused a significant (>75 %) decrease in culture growth.
Mutations in ATRX and DAXX have been implicated in ALT [6, 10]. Both were sequenced in JHH-GBM14 cells but no mutations were found, consistent with a number of previously documented adult GBM cases [6]. Interestingly, immunostaining revealed that approximately 30 % of the JHH-GBM14 cell population was ATRX negative, and ATRX expression was absent in ALT-positive cells (Fig. 1e). ATRX protein expression was also lost in a significant proportion of the glioma cells in the surgical specimen (Fig. 1f). Nuclear DAXX protein expression was conserved in the JHH-GBM14 line (data not shown).
Having characterized the line in vitro, we injected cells into the flanks and brains of athymic mice to evaluate the potential for xenograft formation. Tumors developed in the majority within 6 months. Intracranial tumors were small but diffusely infiltrative and expressing human-specific nestin (Fig. 1g, h), with a Ki67 proliferation index of over 20 % (data not shown).
In summary, ALT is a telomere maintenance mechanism common in gliomas, but to date only one ALT-positive glioma cell line has been documented. Here, we describe a second ALT-positive GBM-derived neurosphere line with intact ATRX and DAXX genetic loci and focal ATRX protein loss corresponding to the characteristic telomere changes. The neurosphere line generates intracranial xenografts, and represents a valuable research tool for investigating ALT in the subset of GBM with loss of ATRX protein but no ATRX/DAXX mutation.
Contributor Information
Ping An, Email: pan4@jhmi.edu.
Alan K. Meeker, Email: ameeker1@jhmi.edu.
Charles G. Eberhart, Email: ceberha@jhmi.edu.
References
- 1.Bryan TM, Englezou A, Gupta J, Bacchetti S, Reddel RR. Telomere elongation in immortal human cells without detectable telomerase activity. EMBO J. 1995;14:4240–4248. doi: 10.1002/j.1460-2075.1995.tb00098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nguyen DN, Heaphy CM, de Wilde RF, Orr BA, Odia Y, Eberhart CG, et al. Molecular and morphologic correlates of the alternative lengthening of telomeres phenotype in high-grade astrocytomas. Brain Pathol. 2012 doi: 10.1111/j.1750-3639.2012.00630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heaphy CM, Subhawong AP, Hong SM, Goggins MG, Montgomery EA, Gabrielson E, et al. Prevalence of the alternative lengthening of telomeres telomere maintenance mechanism in human cancer subtypes. Am J Pathol. 2011;179:1608–1615. doi: 10.1016/j.ajpath.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McDonald KL, McDonnell J, Muntoni A, Henson JD, Hegi ME, von Deimling A, et al. Presence of alternative lengthening of telomeres mechanism in patients with glioblastoma identifies a less aggressive tumor type with longer survival. J Neuropathol Exp Neurol. 2010;69:729–736. doi: 10.1097/NEN.0b013e3181e576cf. [DOI] [PubMed] [Google Scholar]
- 5.Silvestre DC, Pineda JR, Hoffschir F, Studler JM, Mouthon MA, Pflumio F, et al. Alternative lengthening of telomeres in human glioma stem cells. Stem Cells. 2011;29:440–451. doi: 10.1002/stem.600. [DOI] [PubMed] [Google Scholar]
- 6.Heaphy CM, de Wilde RF, Jiao Y, Klein AP, Edil BH, Shi C, et al. Altered telomeres in tumors with ATRX and DAXX mutations. Science. 2011;333:425. doi: 10.1126/science.1207313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schreck KC, Taylor P, Marchionni L, Gopalakrishnan V, Bar EE, Gaiano N, et al. The Notch target Hes1 directly modulates Gli1 expression and Hedgehog signaling: a potential mechanism of therapeutic resistance. Clin Cancer Res. 2010;16:6060–6070. doi: 10.1158/1078-0432.CCR-10-1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Min JL, Meulenbelt I, Kloppenburg M, van Duijn CM, Slagboom PE. Mutation analysis of candidate genes within the 2q33.3 linkage area for familial early-onset generalised osteoarthritis. Eur J Hum Genet. 2007;15:791–799. doi: 10.1038/sj.ejhg.5201829. [DOI] [PubMed] [Google Scholar]
- 9.Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360:765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiao Y, Killela PJ, Reitman ZJ, Rasheed AB, Heaphy CM, de Wilde RF, et al. Frequent ATRX, CIC, and FUBP1 mutations refine the classification of malignant gliomas. Oncotarget. 2012;3:709–722. doi: 10.18632/oncotarget.588. [DOI] [PMC free article] [PubMed] [Google Scholar]