Abstract
Background
Phthalate contamination exists in the North coast karst aquifer system in Puerto Rico. In light of potential health impacts associated with phthalate exposure, targeted action for elimination of exposure sources may be warranted, especially for sensitive populations such as pregnant women. However, information on exposure to phthalates from a variety of sources in Puerto Rico is lacking. The objective of this study was to determine concentrations and predictors of urinary phthalate biomarkers measured at multiple times during pregnancy among women living in the Northern karst area of Puerto Rico.
Methods
We recruited 139 pregnant women in Northern Puerto Rico and collected urine samples and questionnaire data at three separate visits (18±2 weeks, 22±2 weeks, and 26±2 weeks of gestation). Urine samples were analyzed for eleven phthalate metabolites: mono-2-ethylhexyl phthalate (MEHP), mono-2-ethyl-5-hydroxyhexyl phthalate, mono-2-ethyl-5-oxohexyl phthalate, mono-2-ethyl-5-carboxypentyl phthalate, mono-ethyl phthalate (MEP), mono-n-butyl phthalate, mono-benzyl phthalate, mono-isobutyl phthalate, mono-3-carboxypropyl phthalate (MCPP), mono carboxyisononyl phthalate (MCNP), and mono carboxyisooctyl phthalate (MCOP).
Results
Detectable concentrations of phthalate metabolites among pregnant women living in Puerto Rico was prevalent, and metabolite concentrations tended to be higher than or similar to those measured in women of reproductive age from the general US population. Intraclass correlation coefficients ranged from very weak (MCNP; 0.05) to moderate (MEP; 0.44) reproducibility among all phthalate metabolites. We observed significant or suggestive positive associations between urinary phthalate metabolites concentrations and water usage/storage habits (MEP, MCNP, MCOP), use of personal care products (MEP), and consumption of certain food items (MCPP, MCNP, and MCOP).
Conclusions
To our knowledge this is the first study to report concentrations, temporal variability, and predictors of phthalate biomarkers among pregnant women in Puerto Rico. Preliminary results suggest several potentially important exposure sources to phthalates in this population and future analysis from this ongoing prospective cohort will help to inform targeted approaches to reduce exposure.
Keywords: biomarker, endocrine disruptor, phthalates, environment, epidemiology, exposure, pregnancy
1. Introduction
Endocrine disrupting chemicals (EDCs) are exogenous compounds with the potential to mimic or interfere with the normal actions of hormones in humans and animals (Tabb and Blumberg 2006). The widespread and increasing use of these compounds in chemical production and documented associations with a host of adverse health endpoints have spurred growing scientific concerns and public debate. One such group of EDCs are phthalate esters or phthalates. Phthalates are commonly utilized worldwide as additives providing flexibility to otherwise rigid plastics (Schettler 2006). Downstream applications of plastics containing high molecular weight phthalates, such as di-2-ethylhexyl phthalate (DEHP) and benzylbutyl phthalate (BBzP), include building materials (e.g. vinyl flooring), furnishings, medical devices, packaging, et al.; contamination is also found in a variety of food products (NRC 2008; Schecter et al.; Schettler 2006; Wormuth et al. 2006). Low molecular weight phthalates, such as dibutyl phthalate (DBP) and diethyl phthalate (DEP), are also used as plasticizers in a variety of applications, as solvents in many personal care products, as coatings for medications, and other uses (Duty et al. 2005; Hauser et al. 2004; NRC 2008; Sathyanarayana et al. 2008).
Phthalates are continuously released into the environment as a result of the absence of covalent bonding with the products in which they are used, and the rate of release can be altered by factors such as temperature (NRC 2008). Exposure in humans can occur through various routes, including ingestion of foods contaminated during processing and packaging, inhalation, and dermal absorption (Adibi et al. 2003; Rudel et al. 2003). Multiple studies worldwide, as well as in the United States, have demonstrated widespread exposure to phthalates, where detectable urinary concentrations of phthalate metabolites have been measured in nearly 100% of individuals (CDC 2012; Jonsson et al. 2005; Kolossa-Gehring et al. 2012). While exposure to phthalates has been commonly been reported within the general population, of particular concern is evidence of fetal exposure, where urinary levels of phthalate biomarkers have been detected in pregnant women around the world (Adibi et al. 2008; Casas et al. 2011; Huang et al. 2007; Irvin et al. 2010; Meeker et al. 2009; Philippat et al. 2012; Suzuki et al. 2010; Swan et al. 2005; Woodruff et al. 2011; Ye et al. 2008; Berman et al. 2009; Zeman et al. 2013).
In addition to the normal day to day dietary and product usage patterns, which may differ from other populations, Puerto Ricans may have higher than average phthalate exposures due to extensive environmental contamination of groundwater, especially along the North coast karst aquifer system (Padilla et al. 2011). Multiple hazardous waste sites in Puerto Rico, including four National Priority List sites: Barceloneta Landfill, Vega Baja Solid Waste Disposal, Scorpio Recycling, and Pesticide Warehouse III, have reported phthalate contamination (EPA 2013; Padilla et al. 2011). Phthalate exposure levels in humans and related sources are not well documented in Puerto Rico, although there is evidence of widespread endocrine disruption as Puerto Rico has had the highest rates of premature thelarche ever recorded (Larriuz-Serrano et al. 2001).
In light of potential health impacts of phthalate exposure, targeted action for elimination of exposure sources may be warranted, especially for sensitive populations like pregnant women. Given the wide range of products and environmental sources containing phthalate esters, variations in population geography, dietary practices, and lifestyle factors, designing effective strategies to reduce exposure will require culturally relevant information. Due to the lack of understanding on exposure to EDCs, including phthalates, in Puerto Rico, the objective of this study was to determine levels and predictors of urinary biomarkers of phthalates at multiple times during pregnancy of women living in the Northern karst area of Puerto Rico. Data on temporal variability in phthalate biomarker concentrations in pregnancy were also investigated to inform our ongoing epidemiology study on environmental risk factors for preterm birth, as Puerto Rico has the highest preterm birth rates of all US states and territories and phthalate exposure is a potential risk factor we aim to explore which has been previously associated with preterm birth (Blencowe et al. 2012; Meeker et al. 2009).
2. Materials and methods
2.1. Study participants
The data presented were collected from pregnant women participating in the Puerto Rico Testsite for Exploring Contamination Threats (PROTECT) project, an ongoing prospective birth cohort in the Northern Karst Region of Puerto Rico, which is designed to evaluate the potential relationship between environmental toxicants and risk of preterm delivery. Study participants were recruited around 14±2 weeks of gestation at seven prenatal clinics and hospitals throughout Northern Puerto Rico during 2010-2012. Subjects were eligible if they were between the ages of 18 to 40 years, resided in a municipality within the Northern karst region, received their first prenatal visit by the 20th week of pregnancy, did not use oral contraceptives three months prior to pregnancy or in vitro fertilization as a method of assisted reproductive technology, and were free of known medical/obstetrics complications. Questionnaires to collect demographic and water source data, in addition to select information on self-reported product use and dietary intake within the last 48 hours, were administered and urine samples were collected at three separate visits (18±2 weeks, 22±2 weeks, and 26±2 weeks of gestation.)
The current analysis reflects the N=139 mothers with urinary phthalate measures completed as of November 2012. The research protocol was approved by the Ethics and Research Committees of the University of Puerto Rico, the University of Michigan School of Public Health, Northeastern University, and the participating clinics. The involvement of the Centers for Disease Control and Prevention (CDC) laboratory was determined not to constitute engagement in human subjects research. The study was described in detail to all participating mothers and all study participants gave informed consent.
2.2. Urinary Phthalate Concentrations
Women provided spot urine samples at each study visit. Sample collection and processing procedures, as well as supplies used, were conducted according to CDC protocols developed for the National Health and Nutrition Evaluation Survey (NHANES) and other studies with only slight modifications. Briefly, urine was collected in polypropylene containers, divided into aliquots at the University of Puerto Rico, and frozen at −80°C until shipped in batches overnight to the CDC. All urine samples were analyzed at the National Center for Environmental Health of the CDC for eleven phthalate metabolites: mono-2-ethylhexyl phthalate (MEHP), mono-2-ethyl-5-hydroxyhexyl phthalate (MEHHP), mono-2-ethyl-5-oxohexyl phthalate (MEOHP), mono-2-ethyl-5-carboxypentyl phthalate (MECPP), monoethyl phthalate (MEP), mono-n-butyl phthalate (MnBP), monobenzyl phthalate (MBzP), mono-isobutyl phthalate (MiBP), mono-3-carboxypropyl phthalate (MCPP), mono carboxyisononyl phthalate (MCNP), and mono carboxyisooctyl phthalate (MCOP). The analytical approach used solid phase extraction high-performance liquid chromatography-isotope dilution tandem mass spectrometry methods described previously (Silva et al. 2007). To monitor for accuracy and precision, each analytical run included calibration standards, reagent blanks, and quality control materials of high and low concentrations. The limits of detection (LODs) varied for each phthalate metabolite, but were in the low ng/mL range. Concentrations below the LOD were assigned a value of LOD divided by the square root of 2. Specific gravity (SG) was measured at the University of Puerto Rico using a digital handheld refractometer (Atago Co., Ltd., Tokyo, Japan). For analyses utilizing SG-corrected metabolite concentrations, the following formula was used: Pc=P[(1.019-1)/(SG-1)] where Pc is the SG-adjusted phthalate metabolite concentration (ng/mL), P is the observed phthalate metabolite concentration, 1.019 was the SG population median, and SG is the specific gravity of the urine sample.
Several “biotransformation metrics” were calculated for the statistical analysis. First, we created a variable to represent the percentage of the total sum of measured DEHP metabolites made up by MEHP (%MEHP). This was done by converting the concentrations (ng/mL) of the four DEHP metabolites into nM, by dividing by their respective molar masses MEHP (278 g/mol), MEHHP (294 g/mol), MEOHP (292 g/mol), and MECPP (308 g/mol) , then dividing the molar mass of MEHP by the mass of the sum of all four metabolites, and then multiplying by 100. Because MEHP is bioactive, it is hypothesized that %MEHP may be a marker of individual phase I metabolic susceptibility to DEHP exposure since it could represent a person's relative efficiency to form the more hydrophilic and potentially less biologically active secondary DEHP metabolites (MEHHP, MEOHP, MECPP)(Meeker et al. 2012). Finally, the mass concentration ratio of MECPP to MEHHP was calculated as a hypothesized indicator of DEHP exposure timing, where a lower ratio is hypothesized to represent a more recent DEHP exposure due to the longer elimination half-life of MECPP compared to MEHHP (Lorber et al. 2010).
2.3. Statistical Analysis
Geometric means and selected percentiles were calculated to describe the distributions of urinary phthalate metabolites for each visit and for comparison with other published reports. Using geometric means and 95% CIs, we compared concentrations measured in the PROTECT participants with those measured in NHANES. We used publicly accessible urinary concentration data from NHANES 2009-2010 for the eleven phthalate metabolites in U.S. females between the ages of 18 and 40 years. Spearman's rank correlations were calculated and significance tests were performed to assess relationships between study visits, metabolites, and biotransformation metrics. To assess between- and within-person variability (i.e., temporal reliability) in urinary metabolite concentrations, intraclass correlation coefficients (ICCs) and their 95% confidence intervals were calculated (Hankinson et al. 1995). Geometric means were calculated for metabolite concentrations and compared between categories for maternal age, maternal education, marital status, household income, parity, prepregnancy body mass index (BMI), employment, time of day at urine sample collection, water usage/storage conditions, and 48 hour recall of food and product use characteristics. We examined the association between urinary phthalate metabolite concentrations and demographic, environmental, and 48 hour recall of food and product use factors using linear mixed models. Demographic factors were included as fixed effects in our mixed models. Environmental, time of sample collection, product use, and food consumption variables were modeled as time-dependent factors. Natural log-transformed specific gravity-adjusted urinary phthalate concentrations were the dependent variable in mixed models with separate models for each predictor. Finally, we included simultaneously in multivariate mixed effects models any demographic, water usage, and food/product usage variables that was associated (p<0.1) with concentrations of each urinary phthalate metabolite. Data were analyzed using SAS 9.2 (SAS Institute Inc. Cary, NC).
3. Results
Statistical analysis was conducted for both unadjusted and specific gravity-adjusted phthalate metabolite concentrations on a total of 373 urine samples from 139 women, and results were highly consistent between the two approaches. Of the total number of participants, 133 (95.7%) had two or more repeat urine samples for analysis. Demographic characteristics of our study sample are shown in Table 1. Most women in our study were highly educated, employed, married or in a domestic partnership, and had a household income below $40,000 per year. The mean age of the participants was 27.5 years and almost all were non-smokers.
Table 1.
Demographic characteristics of 139 pregnant women enrolled in the PROTECT study (2010-2012).
| Variable | Mean ± SD or n (%) |
|---|---|
| Maternal Age at enrollment (years) | 27.5 ± 5.2 |
| Gravity (# pregnancies) | 1.9 ± 1.0 |
| Parity (# live births) | 0.6 ± 0.7 |
| Years of Maternal Education | |
| < High school | 14 (10.7) |
| High school/equivalent | 10 (7.2) |
| College | 115 (82.7) |
| Household Income (US$) | |
| Missing | 21 (14.8) |
| < $20,000 | 57 (40.1) |
| ≥$20,000 to < $40,000 | 34 (24.0) |
| ≥ $40,000 | 30 (21.1) |
| Marital Status | |
| Single | 39 (28.6) |
| Married or living together | 100 (71.4) |
| Prepregnancy BMI (kg m−2) | |
| ≤ 25 | 80 (56.6) |
| > 25 to ≤ 30 | 42 (30.9) |
| > 30 | 17 (12.5) |
| Smoked During Pregnancy | |
| Missing | 7 (5.0) |
| Yes | 1 (0.8) |
| No | 131 (94.2) |
| Employment | |
| Unemployed | 55 (39.6) |
| Employed | 84 (60.4) |
The distributions of the eleven unadjusted urinary phthalate concentrations from PROTECT participants are presented in Table 2, along with distributions from women ages 18-40 from NHANES (2009-2010) for comparison. Phthalate metabolite concentrations were detectable for 93-100% of the PROTECT samples. When comparing phthalate distributions with women from NHANES 2009-2010, women in our study had somewhat higher geometric mean concentrations for most of the metabolites, except MBzP, MCPP and MCNP. MEHP concentrations were more than twice as high among PROTECT participants when compared to NHANES 2009-2010 (3.3 vs. 1.6 ng/mL), and MBzP concentrations were ~1.5 times lower (3.9 vs. 6.5 ng/mL). On average, PROTECT women had a higher %MEHP than the NHANES women (8.1% vs. 4.8%).
Table 2.
Urinary phthalate metabolite concentrations (ng/mL) in 139 pregnant women from Puerto Ricoa in 2010-2012 and comparison with a U.S. population–based sample of women ages 18-40 from NHANES 2009-10b.
| Percentiles |
||||||||
|---|---|---|---|---|---|---|---|---|
| %>LOD | GM (95% CI) | 25th | 50th | 75th | 95th | Max | ||
| MEHP | ||||||||
| PROTECT | 92.9 | 3.3 (3.0, 3.7) | 1.6 | 3.7 | 7.4 | 17.5 | 141 | |
| NHANES 09-10 | 78.6 | 1.6 (1.4, 1.9) | 0.5 | 1.6 | 3.2 | 11.0 | 119 | |
| MEHHP | ||||||||
| PROTECT | 100 | 10.7 (9.7, 11.8) | 6.1 | 11.5 | 20.6 | 46.3 | 361 | |
| NHANES 09-10 | 100 | 10.6 (8.6, 12.9) | 5.1 | 11.4 | 21.9 | 64.1 | 545 | |
| MEOHP | ||||||||
| PROTECT | 100 | 8.9 (8.1, 9.9) | 5.2 | 9.3 | 17.3 | 35.3 | 281 | |
| NHANES 09-10 | 100 | 7.1 (5.9, 8.6) | 3.5 | 7.7 | 14.9 | 37.7 | 237 | |
| MECPP | ||||||||
| PROTECT | 100 | 19.6 (18.0, 21.3) | 11.6 | 19.9 | 33.2 | 72.6 | 749 | |
| NHANES 09-10 | 100 | 17.9 (14.9, 21.5) | 9.5 | 17.5 | 32.3 | 95.3 | 663 | |
| % MEHP | ||||||||
| PROTECT | 8.1 (7.7, 8.6) | 6.0 | 8.5 | 11.8 | 16.2 | 24.2 | ||
| NHANES 09-10 | 4.8 (4.4, 5.2) | 3.0 | 4.9 | 7.5 | 13.5 | 25.5 | ||
| MECPP/MEHHP | ||||||||
| PROTECT | 1.7 (1.7, 1.8) | 1.4 | 1.7 | 2.1 | 3.2 | 5.4 | ||
| NHANES 09-10 | 1.6 (1.5, 1.7) | 1.2 | 1.6 | 2.1 | 3.2 | 9.9 | ||
| MBzP | ||||||||
| PROTECT | 98.4 | 3.9 (3.4, 4.4) | 1.6 | 4.0 | 8.1 | 30.2 | 305 | |
| NHANES 09-10 | 99.5 | 6.5 (5.6, 7.5) | 3.3 | 6.9 | 15.5 | 42.6 | 189 | |
| MiBP | ||||||||
| PROTECT | 100 | 10.9 (9.8, 12.1) | 5.9 | 11.0 | 20.3 | 63.5 | 964 | |
| NHANES 09-10 | 99.8 | 8.8 (7.4, 10.5) | 4.5 | 9.6 | 18.6 | 42.0 | 600 | |
| MEP | ||||||||
| PROTECT | 100 | 102.2 (85.4, 122.2) | 24.7 | 99.2 | 388 | 1880 | 12700 | |
| NHANES 09-10 | 100 | 76.5 (60.3, 97.0) | 24.4 | 72.5 | 205 | 1286 | 17257 | |
| MnBP | ||||||||
| PROTECT | 98.7 | 19.2 (17.0, 21.7) | 9.8 | 20.9 | 42.0 | 117 | 413 | |
| NHANES 09-10 | 99.8 | 15.7 (13.2, 18.6) | 7.8 | 18.5 | 32.9 | 83.0 | 1079 | |
| MCPP | ||||||||
| PROTECT | 98.9 | 2.3 (2.0, 2.5) | 1.1 | 2.2 | 4.1 | 20.7 | 109 | |
| NHANES 09-10 | 96.4 | 2.7 (2.2, 3.4) | 1.3 | 2.8 | 6.0 | 23.4 | 71.3 | |
| MCNP | ||||||||
| PROTECT | 99.7 | 2.3 (2.1, 2.5) | 1.2 | 2.2 | 3.6 | 12.1 | 59.8 | |
| NHANES 09-10 | 99.0 | 2.9 (2.6, 3.4) | 1.4 | 2.7 | 5.8 | 22.7 | 94.6 | |
| MCOP | ||||||||
| PROTECT | 100 | 16.4 (14.6, 18.5) | 7.1 | 14.2 | 34.1 | 137 | 1230 | |
| NHANES 09-10 | 99.8 | 12.9 (9.8, 17.0) | 4.9 | 11.9 | 34.2 | 164 | 809 | |
NHANES, National Health and Nutrition Examination Survey; PROTECT, Puerto Rico Testsite for Environmental Contamination Threats; GM, geometric mean
Includes biomarker concentrations for up to 3 repeated samples per woman (n=373 samples).
Females 18-40 years of age; n=415 for NHANES 2009-2010
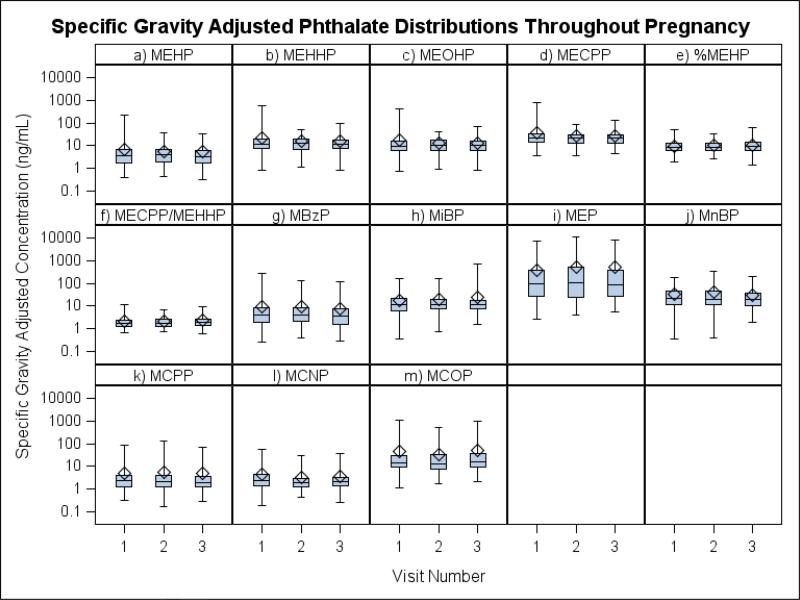
Comparisons of the concentration distributions between study visits (approximately 18±2, 22±2, and 26±2 weeks gestation, with a median duration of 35 days and range of 2-56 days elapsed between sample collection visits) are shown as box plots in Figure 1. There were no statistically significant differences for either the unadjusted or SG-adjusted geometric mean concentrations between the study visits for any of the metabolites. Correlations between visits for most metabolites of DEHP and low molecular weight phthalates were significant but modest (Spearman's r: 0.23-0.45), with the exception of MCPP and MCNP which were not significantly correlated between any study visits. As expected, because they derive from the same parent compound, there were strong correlations between all DEHP metabolites (Spearman's r>0.83) at any of the three time points. Correlations among the metabolites of low molecular weight phthalates, and between the metabolites of high and low molecular weight phthalates tended to be more variable (Spearman's 0.07>r<0.80) (not shown). The specific gravity adjusted concentrations of the DEHP metabolites, MiBP, MnBP, MCPP, MCNP, and MCOP showed considerable within-person variation with ICCs < 0.37 (Table 3). Concentrations of MBzP (ICC=0.41) and MEP (ICC=0.44) were slightly less variable throughout the collection period, and %MEHP (ICC=0.63) was the most stable.
Figure 1.
Comparisons of the specific gravity adjusted concentration distributions between study visits (approximately 18, 22, and 26 weeks gestation.)
Legend: Bottom and top edges of box indicate the interquartile range, while the line on the inside of the box represents the median value. The diamond marker represents the mean value and the whiskers represent the max and min values.
Table 3.
Intraclass correlation coefficients (ICCs) and 95% confidence intervals (95% CIs) for ln-transformed concentrations of urinary phthalate metabolites.
| Urinary biomarker | Unadjusteda | SG-adjustedb | ||
|---|---|---|---|---|
| ICC | 95% CI | ICC | 95% CI | |
| MEHP | 0.35 | 0.24, 0.47 | 0.36 | 0.26, 0.48 |
| MEHHP | 0.25 | 0.15, 0.38 | 0.24 | 0.14, 0.37 |
| MEOHP | 0.26 | 0.16, 0.39 | 0.25 | 0.15, 0.38 |
| MECPP | 0.20 | 0.11, 0.35 | 0.19 | 0.10, 0.33 |
| %MEHP | 0.62 | 0.52, 0.70 | 0.63 | 0.54, 0.71 |
| MECPP/MEHHP | 0.27 | 0.17, 0.40 | 0.31 | 0.21, 0.44 |
| MBzP | 0.37 | 0.27, 0.49 | 0.41 | 0.30, 0.52 |
| MiBP | 0.35 | 0.24, 0.47 | 0.34 | 0.24, 0.46 |
| MEP | 0.43 | 0.33, 0.54 | 0.44 | 0.34, 0.55 |
| MnBP | 0.41 | 0.30, 0.52 | 0.42 | 0.31, 0.53 |
| MCPP | 0.23 | 0.13, 0.37 | 0.20 | 0.11, 0.35 |
| MCNP | 0.09 | 0.03, 0.27 | 0.05 | 0.01, 0.33 |
| MCOP | 0.29 | 0.19, 0.41 | 0.28 | 0.18, 0.40 |
n=373 samples from 139 participants
n=369 samples from 138 participants
Urinary phthalate concentrations in relation to sampling, water usage, and demographic variable categories are presented in Tables 4 and 5. For many of the phthalate metabolites, with the notable exception of MEP, concentrations were the highest before 9:00AM, then dropped in the later morning hours (9:00AM to 11:59AM), and increased in the afternoon to reach higher levels late in the day (3:00 to 8:00 PM.). However, only MECPP and MiBP had significant (p<0.05) differences between time of day categories in mixed effects models. There were significant trends for increasing MCPP, MCNP and MCOP concentrations, and decreasing MnBP and MBzP concentrations, with increasing age categories. There were also suggestive increases (p<0.1) in MCPP concentrations with higher income and prepregnancy BMI categories. Women who were employed also had significantly higher concentrations of MCNP and MCOP compared to unemployed women. Finally, when interpreting the “biotransformation metrics,” a higher percentage of MEHP was associated with lower income and, though not significant, tended to increase throughout the day.
Table 4.
Geometric means of specific gravity adjusted urinary phthalate concentrations according to time of urine collection, demographic, and maternal factors.
| n (%)a | MEHP | MEHHP | MEOHP | MECPP | MiBP | MnBP | MBzP | MEP | MCPP | MCNP | MCOP | %MEHP | MECPP/MEHHP | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | 369 (100) | 3.5 | 11.3 | 9.5 | 20.6 | 11.7 | 20.5 | 4.1 | 110.3 | 2.3 | 2.5 | 17.7 | 8.1 | 1.7 |
| Time of day | ||||||||||||||
| 0600–0859 | 51 (10.1) | 3.8 | 13.7 | 12.0 | 26.9 | 14.0 | 22.1 | 4.5 | 95.0 | 2.9 | 2.8 | 22.0 | 7.0 | 1.9 |
| 0900–1159 | 151 (21.4) | 3.2 | 10.3 | 8.6 | 19.1 | 9.4 | 18.6 | 3.9 | 97.3 | 2.2 | 2.3 | 16.2 | 8.1 | 1.8 |
| 1200–1459 | 127 (48.3) | 3.8 | 11.9 | 9.7 | 21.4 | 13.4 | 23.1 | 4.2 | 114.6 | 2.4 | 2.5 | 17.3 | 8.4 | 1.7 |
| 1500–2000 | 41 (20.2) | 4.2 | 13.1 | 10.7 | 21.6 | 14.3 | 20.3 | 4.7 | 187.1 | 2.7 | 2.6 | 19.8 | 8.8 | 1.6 |
| p-valueb | 0.38 | 0.19 | 0.14 | 0.05 | 0.02 | 0.58 | 0.74 | 0.20 | 0.41 | 0.68 | 0.38 | 0.19 | 0.13 | |
| Maternal Age at Initial Visit (years) | ||||||||||||||
| <25 | 124 (33.6) | 3.6 | 12.2 | 10.5 | 21.6 | 11.9 | 27.3 | 5.3 | 105.3 | 2.2 | 2.2 | 14.0 | 7.9 | 1.7 |
| 25-30 | 112 (30.4) | 3.0 | 9.9 | 8.3 | 19.1 | 11.8 | 17.9 | 3.5 | 94.9 | 1.9 | 2.3 | 16.2 | 7.8 | 1.8 |
| >30 | 133 (36.0) | 4.0 | 11.7 | 9.7 | 21.0 | 11.4 | 17.7 | 3.7 | 130.8 | 3.0 | 2.9 | 23.7 | 8.6 | 1.7 |
| p-valueb | 0.33 | 0.28 | 0.20 | 0.51 | 0.87 | 0.01 | 0.05 | 0.61 | 0.003 | 0.02 | 0.006 | 0.74 | 0.29 | |
| Maternal Education (years) | ||||||||||||||
| <12 | 35 (9.5) | 2.9 | 10.9 | 9.7 | 23.0 | 9.8 | 24.4 | 3.6 | 49.5 | 2.2 | 1.9 | 13.4 | 6.5 | 2.0 |
| 12 | 23 (6.2) | 4.5 | 13.5 | 11.7 | 23.4 | 20.4 | 35.0 | 5.7 | 166.2 | 2.8 | 2.0 | 14.7 | 8.9 | 1.7 |
| >12 | 311 (84.3) | 3.6 | 11.2 | 9.3 | 20.2 | 11.4 | 19.4 | 4.1 | 117.1 | 2.3 | 2.6 | 18.5 | 8.2 | 1.7 |
| p-valueb | 0.51 | 0.71 | 0.61 | 0.47 | 0.05 | 0.08 | 0.57 | 0.11 | 0.65 | 0.08 | 0.38 | 0.24 | 0.09 | |
| Marital Status | ||||||||||||||
| Married/Civil Union | 300 (81.3) | 3.6 | 11.1 | 9.3 | 20.4 | 11.4 | 19.4 | 4.0 | 55.6 | 2.3 | 2.5 | 17.8 | 8.4 | 1.8 |
| Unmarried | 69 (18.7) | 3.4 | 12.7 | 10.5 | 22.4 | 13.2 | 28.0 | 4.6 | 101.5 | 2.6 | 2.3 | 16.4 | 7.2 | 1.7 |
| p-valueb | 0.57 | 0.34 | 0.45 | 0.42 | 0.40 | 0.04 | 0.57 | 0.62 | 0.36 | 0.46 | 0.73 | 0.09 | 0.49 | |
| Income (USD per year)c | ||||||||||||||
| < $20,000 | 149 (45.7) | 3.7 | 10.7 | 9.3 | 19.9 | 11.2 | 20.8 | 4.1 | 96.9 | 2.0 | 2.3 | 15.0 | 8.9 | 1.8 |
| ≥ $20,000 to < $40,000 | 95 (29.1) | 2.9 | 10.7 | 8.8 | 20.0 | 12.1 | 21.1 | 4.1 | 117.2 | 2.4 | 2.8 | 20.0 | 7.3 | 1.8 |
| ≥ $40,000 | 82 (25.2) | 3.8 | 11.8 | 9.5 | 20.4 | 10.2 | 15.9 | 4.0 | 157.0 | 2.8 | 2.6 | 21.8 | 8.2 | 1.6 |
| p-valueb | 0.22 | 0.74 | 0.85 | 0.97 | 0.52 | 0.23 | 0.76 | 0.37 | 0.06 | 0.25 | 0.08 | 0.03 | 0.37 | |
| Parity | ||||||||||||||
| 0 | 155 (42.0) | 3.4 | 10.7 | 9.3 | 19.5 | 10.7 | 18.6 | 3.7 | 118.6 | 2.1 | 2.3 | 19.0 | 8.1 | 1.7 |
| 1 | 140 (38.0) | 4.1 | 12.2 | 10.1 | 22.1 | 12.3 | 22.2 | 5.2 | 105.2 | 2.5 | 2.6 | 16.7 | 8.6 | 1.7 |
| >1 | 74 (20.0) | 3.1 | 10.9 | 8.9 | 20.3 | 12.8 | 22.0 | 3.3 | 103.8 | 2.6 | 2.5 | 16.8 | 7.4 | 1.8 |
| p-valueb | 0.18 | 0.47 | 0.57 | 0.40 | 0.41 | 0.29 | 0.02 | 0.92 | 0.25 | 0.47 | 0.56 | 0.25 | 0.83 | |
| Prepregnancy BMI (kg m−2) | ||||||||||||||
| ≤ 25 | 206 (55.8) | 3.6 | 11.4 | 9.5 | 20.7 | 11.5 | 18.9 | 4.0 | 111.2 | 2.1 | 2.4 | 16.1 | 8.3 | 1.7 |
| >25 to ≤ 30 | 110 (29.8) | 3.3 | 10.3 | 8.7 | 19.7 | 10.5 | 21.0 | 3.9 | 110.8 | 2.7 | 2.5 | 18.8 | 7.9 | 1.8 |
| > 30 | 47 (12.7) | 4.0 | 13.2 | 11.7 | 22.4 | 15.3 | 27.9 | 5.2 | 125.0 | 2.9 | 2.6 | 23.8 | 7.9 | 1.6 |
| p-valueb | 0.48 | 0.28 | 0.21 | 0.62 | 0.10 | 0.16 | 0.39 | 0.85 | 0.06 | 0.84 | 0.17 | 0.73 | 0.16 | |
| Occupation | ||||||||||||||
| Unemployed | 141 (38.2) | 3.8 | 12.4 | 10.5 | 22.4 | 13.0 | 23.0 | 4.2 | 86.6 | 2.4 | 2.1 | 14.8 | 8.1 | 1.7 |
| Employed | 228 (61.8) | 3.4 | 10.6 | 8.9 | 19.6 | 10.9 | 19.2 | 4.0 | 128.1 | 2.3 | 2.8 | 19.8 | 8.1 | 1.8 |
| p-valueb | 0.37 | 0.16 | 0.12 | 0.12 | 0.11 | 0.10 | 0.56 | 0.15 | 0.90 | 0.003 | 0.02 | 0.87 | 0.78 | |
n represents number of samples, not participants.
Test for fixed effects using log-transformed data in mixed models with random subject effects
n=326 for income
Table 5.
Geometric means of urinary specific gravity adjusted phthalate concentrations according to reported water usage and storage conditions.
| na | MEHP | MEHHP | MEOHP | MECPP | MiBP | MnBP | MBzP | MEP | MCPP | MCNP | MCOP | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | 369 | 3.5 | 11.3 | 9.5 | 20.6 | 11.7 | 20.5 | 4.1 | 110.3 | 2.3 | 2.5 | 17.7 |
| Water Source for Drinkingb | ||||||||||||
| AAAd | 232 | 3.3 | 10.7 | 9.1 | 20.3 | 11.5 | 22.1 | 4.0 | 97.5 | 2.3 | 2.4 | 17.3 |
| Bottled Water | 123 | 4.0 | 12.4 | 10.3 | 21.0 | 12.0 | 18.1 | 4.5 | 144.0 | 2.6 | 2.7 | 18.5 |
| Private Well | 3 | 9.7 | 19.5 | 19.1 | 42.6 | 8.9 | 23.4 | 3.3 | 57.5 | 0.6 | 2.0 | 6.7 |
| Community Well | 1 | 2.6 | 5.2 | 5.7 | 16.7 | 12.4 | 23.3 | 4.2 | 518.5 | 2.3 | 1.6 | 12.6 |
| Surface Water | 6 | 2.4 | 9.6 | 8.1 | 18.8 | 9.3 | 12.7 | 2.2 | 41.9 | 1.2 | 1.7 | 11.8 |
| Collected Rainwater | 4 | 5.4 | 12.4 | 11.2 | 19.4 | 16.5 | 26.9 | 4.6 | 182.9 | 3.2 | 6.0 | 68.4 |
| p-valueb | 0.21 | 0.23 | 0.33 | 0.75 | 0.80 | 0.16 | 0.63 | 0.14 | 0.40 | 0.14 | 0.63 | |
| Water Source for Cookingb | ||||||||||||
| AAAd | 337 | 3.5 | 11.0 | 9.4 | 20.4 | 11.7 | 20.4 | 4.0 | 103.8 | 2.3 | 2.4 | 17.5 |
| Bottled Water | 25 | 4.5 | 15.4 | 11.4 | 21.4 | 12.7 | 22.7 | 6.6 | 326.0 | 2.9 | 3.4 | 23.9 |
| Private Well | 3 | 9.7 | 19.5 | 19.1 | 42.6 | 8.9 | 23.4 | 3.3 | 57.5 | 0.6 | 2.0 | 6.7 |
| Community Well | 1 | 2.6 | 5.2 | 5.7 | 16.7 | 12.4 | 23.3 | 4.2 | 518.5 | 2.3 | 1.6 | 12.6 |
| Surface Water | 3 | 2.4 | 9.3 | 7.1 | 20.0 | 8.3 | 16.3 | 4.8 | 13.5 | 1.6 | 1.7 | 15.8 |
| p-valueb | 0.34 | 0.12 | 0.38 | 0.81 | 0.71 | 0.70 | 0.13 | 0.009 | 0.38 | 0.09 | 0.27 | |
| Store water in a Cistern (Cistern Material) | ||||||||||||
| Yes(Plastic) | 143 | 3.7 | 11.1 | 9.3 | 20.5 | 11.2 | 19.1 | 4.2 | 129.1 | 2.5 | 2.7 | 20.8 |
| Yes(Metal) | 17 | 2.9 | 14.6 | 12.4 | 24.9 | 14.5 | 32.1 | 3.9 | 73.8 | 2.8 | 1.6 | 12.0 |
| No | 209 | 3.5 | 11.2 | 9.4 | 20.4 | 11.8 | 20.9 | 4.0 | 102.4 | 2.2 | 2.4 | 16.4 |
| p-valuec | 0.66 | 0.56 | 0.54 | 0.60 | 0.67 | 0.30 | 0.95 | 0.48 | 0.45 | 0.08 | 0.10 | |
n represents number of samples, not participants
Test for fixed effects between AAA and bottle water (due to low number of response for other water usage sources) using log-transformed data in mixed models with random subject effects
Test for fixed effects between plastic and metal storage material using log-transformed data in mixed models with random subject effects
Autoridad de Acueductos y Alcantarillados de PR (AAA)
Water usage and storage conditions (Table 5) were mostly not associated with phthalate metabolite concentrations, though there were some notable exceptions. Participants who reported using bottled water for cooking had significantly (p=0.009) higher concentrations of MEP (326 vs. 104 ng/mL) compared to participants who used public supply water from the Autoridad de Acueductos y Alcantarillados of Puerto Rico. Finally, those participants who reportedly stored their water in plastic cisterns had higher concentrations of the metabolites of di-isodecyl phthalate (2.7 vs. 1.6 ng/mL; p=0.08) and di-isononyl phthalate (20.8 vs. 12.0 ng/mL; p=0.10) compared to those who stored water in metal cisterns.
Frequencies of selected product use and food item consumption in the 48 hours prior to urine sample collection and their associations with phthalate metabolites are reported in Table 6. Twelve women (9%) did not provide complete product use information. Reported use varied widely by product type with deodorant (100% of women reported using prior to collection of at least one of the repeated urine samples), bar soap (90%), liquid soap (86%), perfume/cologne (84%), hand/body lotion (84%), and colored cosmetics (79%) being the most commonly used in the 48 hours prior to urine collection. Sunscreen (4%), shaving cream (7%), and vinyl boots or gloves (7%) were the least likely products to be used. Use of perfume (132 vs. 43 ng/mL; p-value<0.001) and colored cosmetics (124 vs. 71 ng/mL; p-value=0.04) were associated with significantly higher MEP concentrations, while use of nail polish indicated a similar trend for MEP concentrations (136 vs. 101 ng/mL; p-value=0.10). Use of fabric softener (10.2 vs. 8.7 ng/mL; p-value=0.07) and lotion (11.6 vs. 9.2 ng/mL; p-value=0.09) resulted in higher concentrations of MEOHP and MEHHP, respectively. The use of laundry detergent was negatively associated with MiBP (p=0.02) and MBzP (p=0.06) concentrations, and use of general cleaners and perfume were both negatively associated with MiBP (p=0.08) and MEHP (p=0.02) concentrations, respectively. It is interesting to note that if participants responded that they always try to use of fragrance-free products their phthalate metabolite concentrations were generally lower, though the small number of respondents (N=17) limited our ability to detect statistically significant differences. In contrast, if participants reported heating plastic food/drink containers or wrappings in the microwave, concentrations of MCOP (20.5 vs. 15.5 ng/mL; p=0.02) were significantly higher. Participants who consumed ice cream or chicken within 48 hours of urine sample collection had higher concentrations of MCPP, MCNP, and MCOP compared to participants who didn't consume those products. Conversely, those who reported consuming cheese (MBzP: 3.8 vs. 6.2), fish (MiBP: 13.8 vs. 21.5; MCPP: 1.7 vs. 2.4), or sausages (MEOHP: 8.9 vs. 11.8; MEHHP 7.6 vs. 9.9; MECPP 17.3 vs. 21.3) had lower metabolite concentrations compared to participants who did not consume those products. When demographic and food/product use variables that were associated with concentrations of each biomarker were included simultaneously in multivariate models, results were similar for each food/product use variable, though effect estimates for the individual predictors were somewhat attenuated (not shown).
Table 6.
Frequencies of select product use and food type consumption reported in the 48-h recall questionnaire in relation to urinary phthalate metabolite concentrations.
| n=346 | MEHP MEHHP | MEOHP | MECPP | MiBP | MnBP | MBzP | MEP | MCPP | MCNP | MCOP | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| General Usage of Fragrance Free Products | ||||||||||||
| Always | 17 | 3.2 | 10.8 | 8.4 | 20.0 | 10.3 | 19.0 | 2.6 | 82.7 | 1.9 | 1.8 | 16.7 |
| Sometimes | 114 | 3.5 | 11.2 | 9.3 | 20.0 | 11.1 | 17.4 | 4.3 | 117.2 | 2.4 | 2.6 | 17.2 |
| Never | 215 | 3.6 | 11.3 | 9.6 | 20.9 | 11.9 | 22.2 | 4.1 | 108.7 | 2.3 | 2.4 | 17.6 |
| Cleaning Products | ||||||||||||
| Laundry Detergent | ||||||||||||
| Yes | 194 | 3.4 | 11.1 | 9.4 | 20.7 | 10.3 | 18.3 | 3.7 | 105.8 | 2.3 | 2.5 | 17.8 |
| No | 152 | 3.7 | 11.4 | 9.5 | 20.4 | 13.3** | 23.3* | 4.6 | 115.5 | 2.3 | 2.4 | 17.0 |
| Fabric Softener | ||||||||||||
| Yes | 169 | 3.6 | 11.9 | 10.2* | 21.6 | 11.2 | 20.4 | 4.0 | 102.5 | 2.2 | 2.4 | 16.7 |
| No | 177 | 3.4 | 10.6 | 8.7 | 19.6 | 11.8 | 20.3 | 4.1 | 117.5 | 2.3 | 2.5 | 18.1 |
| General Cleaners | ||||||||||||
| Yes | 203 | 3.4 | 10.7 | 8.9 | 19.6 | 10.7 | 20.2 | 3.8 | 108.9 | 2.3 | 2.5 | 16.5 |
| No | 143 | 3.8 | 12.0 | 10.2 | 21.9 | 12.9* | 20.6 | 4.4 | 111.4 | 2.3 | 2.4 | 18.8 |
| Creams and Lotions | ||||||||||||
| Hand/Body Lotion | ||||||||||||
| Yes | 291 | 3.6 | 11.6* | 9.6 | 20.8 | 11.7 | 20.7 | 4.2 | 111.7 | 2.4 | 2.5 | 17.8 |
| No | 55 | 3.3 | 9.2 | 8.8 | 19.3 | 10.6 | 18.5 | 3.3 | 100.8 | 2.0 | 2.3 | 15.3 |
| Shaving Cream | ||||||||||||
| Yes | 24 | 2.7 | 9.3 | 7.6 | 17.3 | 13.1 | 19.8 | 3.6 | 51.7 | 2.3 | 2.3 | 16.0 |
| No | 322 | 3.6 | 11.4 | 9.6 | 20.8 | 11.4 | 20.4 | 4.1 | 116.3* | 2.3 | 2.5 | 17.5 |
| Sunscreen | ||||||||||||
| Yes | 15 | 4.4 | 10.1 | 10.8 | 18.1 | 13.4 | 17.0 | 4.0 | 145.6 | 2.8 | 3.5 | 24.3 |
| No | 331 | 3.5 | 11.3 | 9.4 | 20.6 | 11.5 | 20.5 | 4.1 | 108.5 | 2.3 | 2.4 | 17.2 |
| Toiletries and Cosmetics | ||||||||||||
| Perfume/Cologne | ||||||||||||
| Yes | 290 | 3.3 | 10.9 | 9.2 | 20.1 | 11.3 | 20.6 | 3.9 | 132.0** | 2.3 | 2.4 | 16.9 |
| No | 56 | 5.0** | 12.8 | 10.6 | 23.2 | 12.7 | 19.1 | 5.0 | 42.7 | 2.4 | 2.5 | 20.2 |
| Colored Cosmetics | ||||||||||||
| Yes | 272 | 3.5 | 11.3 | 9.5 | 20.7 | 11.3 | 19.4 | 4.0 | 123.7** | 2.3 | 2.5 | 17.6 |
| No | 74 | 3.7 | 10.9 | 9.2 | 20.0 | 12.2 | 24.1 | 4.6 | 71.3 | 2.5 | 2.1 | 16.6 |
| Bar Soap | ||||||||||||
| Yes | 311 | 3.5 | 11.2 | 9.5 | 20.6 | 11.3 | 20.4 | 4.0 | 108.7 | 2.3 | 2.4 | 17.4 |
| No | 35 | 3.6 | 11.3 | 9.1 | 20.3 | 13.8 | 20.4 | 4.9 | 121.9 | 2.6 | 2.4 | 17.8 |
| Liquid Soap | ||||||||||||
| Yes | 296 | 3.6 | 11.1 | 9.4 | 20.5 | 11.6 | 20.2 | 3.9 | 108.4 | 2.3 | 2.4 | 17.6 |
| No | 49 | 3.2 | 11.9 | 9.9 | 21.1 | 11.1 | 21.6 | 4.9 | 120.6 | 2.6 | 2.5 | 16.6 |
| Mouthwash | ||||||||||||
| Yes | 185 | 3.5 | 11.0 | 9.2 | 21.0 | 11.1 | 19.6 | 4.0 | 124.7 | 2.3 | 2.5 | 16.6 |
| No | 161 | 3.6 | 11.5 | 9.7 | 20.0 | 12.0 | 21.2 | 4.2 | 95.1 | 2.3 | 2.4 | 18.4 |
| Hair and Nail Products | ||||||||||||
| Hairspray | ||||||||||||
| Yes | 121 | 3.5 | 11.1 | 9.1 | 20.6 | 11.1 | 19.5 | 3.9 | 124.2 | 2.2 | 2.3 | 15.2 |
| No | 225 | 3.6 | 11.3 | 9.6 | 20.5 | 11.7 | 20.9 | 4.2 | 103.0 | 2.3 | 2.5 | 18.7* |
| Conditioner | ||||||||||||
| Yes | 227 | 3.5 | 11.3 | 9.3 | 20.2 | 11.2 | 19.6 | 4.1 | 99.2 | 2.2 | 2.3 | 16.4 |
| No | 119 | 3.6 | 11.1 | 9.6 | 21.2 | 12.2 | 21.9 | 3.9 | 133.7 | 2.5 | 2.8** | 19.5 |
| Shampoo | ||||||||||||
| Yes | 227 | 3.6 | 11.4 | 9.5 | 20.3 | 11.3 | 19.4 | 4.1 | 101.6 | 2.3 | 2.3 | 16.9 |
| No | 119 | 3.4 | 11.0 | 9.3 | 20.9 | 12.1 | 22.3 | 4.0 | 127.9 | 2.4 | 2.7 | 18.4 |
| Other Hair Products | ||||||||||||
| Yes | 75 | 4.1 | 12.3 | 10.2 | 21.7 | 10.8 | 20.2 | 3.7 | 100.4 | 2.4 | 2.5 | 18.3 |
| No | 268 | 3.4 | 11.0 | 9.3 | 20.2 | 11.8 | 20.6 | 4.2 | 111.5 | 2.3 | 2.4 | 17.1 |
| Nail Polish | ||||||||||||
| Yes | 112 | 3.7 | 11.9 | 10.3 | 21.4 | 11.7 | 21.7 | 3.9 | 135.6* | 2.2 | 2.4 | 16.2 |
| No | 233 | 3.5 | 11.0 | 9.1 | 20.2 | 11.5 | 19.9 | 4.1 | 100.5 | 2.4 | 2.5 | 18.1 |
| Vinyl Products | ||||||||||||
| Vinyl Gloves/Boots | ||||||||||||
| Yes | 25 | 3.6 | 12.2 | 10.3 | 21.9 | 11.8 | 20.4 | 4.4 | 116.1 | 2.0 | 2.5 | 16.0 |
| No | 321 | 3.5 | 11.1 | 9.4 | 20.4 | 11.5 | 20.4 | 4.0 | 109.5 | 2.3 | 2.4 | 17.5 |
| Vinyl Shower Curtain | ||||||||||||
| Yes | 235 | 3.5 | 11.1 | 9.3 | 20.8 | 11.6 | 20.3 | 4.1 | 104.8 | 2.2 | 2.6 | 18.1 |
| No | 111 | 3.6 | 11.5 | 9.6 | 20.0 | 11.4 | 20.5 | 4.0 | 121.6 | 2.5 | 2.2 | 16.1 |
| Food Products and Storage | ||||||||||||
| Milk | ||||||||||||
| Yes | 292 | 3.6 | 11.6 | 9.5 | 20.9 | 11.4 | 20.1 | 4.1 | 107.9 | 2.3 | 2.4 | 17.2 |
| No | 54 | 3.5 | 9.6 | 8.9 | 18.6 | 12.2 | 21.7 | 3.7 | 121.8 | 2.4 | 2.5 | 18.8 |
| Cheese | ||||||||||||
| Yes | 291 | 3.4 | 11.0 | 9.3 | 20.3 | 11.3 | 19.4 | 3.8 | 109.1 | 2.3 | 2.5 | 17.6 |
| No | 55 | 4.2 | 12.7 | 10.4 | 21.7 | 12.7 | 26.2* | 6.2** | 114.6 | 2.2 | 2.4 | 16.5 |
| Ice Cream | ||||||||||||
| Yes | 110 | 4.0 | 10.9 | 9.5 | 20.6 | 12.4 | 19.8 | 4.4 | 97.5 | 2.8** | 2.9** | 20.7* |
| No | 236 | 3.4 | 11.4 | 9.4 | 20.5 | 11.1 | 20.6 | 3.9 | 116.3 | 2.1 | 2.3 | 16.1 |
| Meat | ||||||||||||
| Yes | 222 | 3.6 | 11.3 | 9.6 | 20.5 | 11.9 | 20.8 | 4.1 | 119.1 | 2.4 | 2.5 | 17.7 |
| No | 124 | 3.5 | 11.0 | 9.2 | 20.6 | 11.0 | 19.6 | 4.1 | 95.3 | 2.2 | 2.4 | 17.0 |
| Chicken | ||||||||||||
| Yes | 258 | 3.6 | 11.2 | 9.5 | 20.9 | 11.4 | 19.9 | 4.0 | 100.4 | 2.4* | 2.5 | 19.1** |
| No | 88 | 3.3 | 11.3 | 9.3 | 19.5 | 12.0 | 21.6 | 4.5 | 143.6 | 2.0 | 2.3 | 13.3 |
| Fish | ||||||||||||
| Yes | 43 | 3.2 | 9.9 | 7.9 | 18.3 | 10.2 | 13.8 | 3.6 | 136.2 | 1.7 | 2.2 | 13.0 |
| No | 303 | 3.6 | 11.4 | 9.7 | 20.9 | 11.7 | 21.5** | 4.1 | 106.7 | 2.4** | 2.5 | 18.2* |
| Cold Cuts | ||||||||||||
| Yes | 212 | 3.7 | 11.1 | 9.3 | 20.3 | 11.8 | 19.3 | 4.0 | 114.1 | 2.4 | 2.6 | 18.0 |
| No | 134 | 3.4 | 11.4 | 9.6 | 21.0 | 11.1 | 22.1 | 4.1 | 103.7 | 2.1 | 2.2 | 16.5 |
| Hot Dog | ||||||||||||
| Yes | 70 | 3.2 | 9.8 | 8.2 | 19.3 | 11.8 | 20.0 | 3.5 | 130.6 | 2.4 | 2.4 | 17.6 |
| No | 276 | 3.6 | 11.6 | 9.8 | 20.9 | 11.5 | 20.5 | 4.2 | 105.2 | 2.3 | 2.5 | 17.4 |
| Sausage | ||||||||||||
| Yes | 59 | 3.1 | 8.9 | 7.6 | 17.3 | 12.0 | 18.2 | 3.7 | 114.2 | 2.4 | 2.4 | 20.5 |
| No | 287 | 3.6 | 11.8** | 9.9** | 21.3** | 11.4 | 20.8 | 4.1 | 109.1 | 2.3 | 2.5 | 16.8 |
| Heated Food/Drink in Microwave | ||||||||||||
| Yes | 144 | 3.8 | 11.5 | 9.4 | 21.1 | 11.1 | 18.7 | 4.3 | 125.8 | 2.4 | 2.5 | 20.5** |
| No | 202 | 3.4 | 11.0 | 9.4 | 20.2 | 11.8 | 21.6 | 3.9 | 99.9 | 2.3 | 2.4 | 15.5 |
p-value <0.05 for association between questionnaire item and metabolite in mixed effects models.
at 0.05>P<0.1.
4. Discussion
In the present study, we found evidence for widespread exposure to phthalates among pregnant women living on the Northern coast of Puerto Rico, where all measured phthalate metabolites were detected for nearly 100% of the urine samples. Concentrations for a majority of the metabolites measured were higher in the Puerto Rican participants when compared to women of reproductive age in the mainland US population, especially MEHP which had a median and geometric mean concentrations that were more than twice as high. The two metabolites that were significantly lower in the PROTECT population when compared to the concentrations found in women of reproductive age from the 2009-2010 NHANES study were MBzP and MCNP.
As far as we are aware, the only other study of phthalate exposure conducted in Puerto Rico reported serum levels of DBP, DEP, DEHP, and MEHP in girls aged 1-10 years who were recruited in 1994-1998 (Colon et al. 2000). The authors reported DEHP concentrations that ranged from 187 to 2,098 μg/L (or ng/mL) and MEHP concentrations that ranged from 6.3 to 38 ng/mL in 41 girls with premature thelarche (Colon et al. 2000). In a follow-up commentary, McKee pointed out that measurement of parent phthalate compounds in biological media is difficult and prone to contamination, and that the concentrations of DEHP measured by Colon et al. were likely not plausible in the absence of contamination (McKee 2004). While Colon et al. also measured MEHP in serum, this may also result from contamination due to the possibility for DEHP to be transformed to MEHP by metabolizing enzymes present in the blood sample. The measurement of phthalate metabolites in urine, which was the approach used in the present study, aids in avoiding contamination concerns (Blount et al. 2000).
Other pregnancy cohort studies around the globe have also detected presence of phthalate metabolites in a majority of urine samples analyzed (Adibi et al. 2008; Braun et al. 2012; Casas et al. 2011; Huang et al. 2007; Irvin et al. 2010; Kim et al. 2011; Meeker et al. 2009; Philippat et al. 2012; Suzuki et al. 2010; Swan et al. 2005; Wolff et al. 2008; Ye et al. 2008). Urinary MnBP, MiBP, MBzP, and DEHP metabolite concentrations in this study were consistently lower when compared to pregnant women sampled in Europe between 2002-2008 (Casas et al. 2011; Philippat et al. 2012; Ye et al. 2008), though MEP, MCPP, MCNP, and MCOP concentrations were very similar or higher. Comparing to prenatal urine samples in Korea (Kim et al. 2011), Japan (Suzuki et al. 2010), and Taiwan (Huang et al. 2007) collected from 2005-2009, concentrations of MEP were consistently higher in Puerto Rico, while in general MEHP, MEHHP, and MEOHP were similar and MnBP was lower. In a majority of other US-based pregnancy cohorts, concentrations for nearly all phthalate metabolites were consistently higher when compared to Puerto Rico (Adibi et al. 2008; Braun et al. 2012; Swan et al. 2005; Wolff et al. 2008). However, participants in these studies were recruited in earlier years, and there is evidence for declining levels of DEHP metabolites when comparing more recent NHANES results with earlier years (CDC 2012). Thus, the most recent US-based population data available (NHANES 2009-2010) was selected as the most appropriate for comparison with PROTECT women, who were recruited from 2010-2012. Finally, when compared to studies in Mexico (Meeker et al. 2009) and Peru (Irvin et al. 2010), most phthalate metabolite concentrations were higher in our Puerto Rican population. It should be acknowledged that due to changing usage patterns of phthalate compounds in products and consumer habits, any comparison of previously reported concentrations to the PROTECT population need to take into account year of sample collection and other important differences in study design. Additionally, it has been found that certified standard solutions for phthalate metabolite analysis may be variable between different lots which may further limit the ability to compare phthalate metabolite concentrations between studies (Langlois et al. 2012).
Within-subject repeatability of all the phthalate metabolite concentrations measured at 18, 22, and 26 weeks among the pregnant women in our Puerto Rico study was generally low to moderate. The observation of weak to moderate ICC values in this population suggests that the collection of repeated samples should continue to be utilized in epidemiologic investigations in order to reduce exposure measurement error and attenuation of exposure-health outcome effect estimates. Other studies which assessed variability of phthalate metabolite concentrations during pregnancy were generally in accordance with our results, with some notable differences (Adibi et al. 2008; Braun et al. 2012; Irvin et al. 2010; Peck et al. 2010; Suzuki et al. 2010). A study of pregnant women in Boston reported slightly higher stability of MEP (ICC: 0.56 vs. 0.44) and lower stability of MEHP (ICC: 0.11 vs. 0.36) compared to PROTECT, while the ICCs for MnBP, MiBP, and MBzP were very similar to our study results (Braun et al. 2012). Conversely, a study of Dominican and African-American pregnant women in New York City reported higher stability for MnBP, MiBP, and MBzP, and lower stability of MEP, compared to this study (Adibi et al. 2008). Variations in exposure patterns, including consumer product usage and geographic location, and timing of repeated sample collection during pregnancy may explain some of these slight variations in reproducibility of phthalate metabolite concentrations in pregnant women.
In our study, %MEHP, a hypothesized marker of individual metabolic susceptibility to DEHP exposure, was nearly 2-fold higher when compared to women of child bearing age reported in NHANES 2009-2010 (GM: 8.1% vs. 4.8%) and slightly lower when compared to Dominican and African-American pregnant women in New York City (8.1% vs. 11.0%) (Adibi et al. 2008). Further, %MEHP was much more stable over our study collection period (ICC=0.63) compared with MEHP (ICC=0.36), which were very similar to patterns reported in the New York City study (%MEHP; ICC=0.64 vs. MEHP; ICC=0.35) (Adibi et al. 2008). In addition to inter-individual differences in metabolism, a higher %MEHP may also reflect more recent DEHP exposure (similar to a lower MECPP/MEHHP ratio) due to differences in the elimination half-lives between metabolites (Meeker et al. 2012). Interestingly, the geometric mean of the MECPP/MEHHP ratio was very similar in PROTECT women compared to women from NHANES 2009-2010 (1.7 vs. 1.6). If the higher %MEHP in PROTECT compared to NHANES were due to differences in timing of DEHP exposure, we would expect to also see a much lower MECPP/MEHHP ratio in PROTECT women. Results from our study suggest that pregnant women in Puerto Rico maybe less efficient in metabolizing DEHP and/or MEHP to less biologically active secondary metabolites. While other unknown factors may have influenced these findings (such as potential MEHP contamination), the sample collection and processing procedures were conducted according to CDC protocols developed for NHANES. Our study did not also control for additional information regarding potential timing of exposure (i.e. time of last meal or usage of personal care product) which would enhanced our understanding of variability in metabolism for this population. Since the elevated %MEHP in this study was similar to the study of pregnant women in New York City, more research on phthalate metabolism and the potential for increased susceptibility during pregnancy is needed.
Potential routes of human exposure to phthalates, including ingestion of contaminated food/water, inhalation, and dermal absorption through the use of personal care products, have not been well characterized, especially in the context of pregnancy. In our population, demographic information, water usage/storage habits, select dietary information, and personal care product usage were all explored for associations with phthalate biomarker concentrations. We observed generally higher urinary concentrations of most metabolites in samples collected before 9:00AM and after 3PM among pregnant women in Puerto Rico. While these patterns were significant in mixed models for MECPP and MiBP in this population, it is possible that our lack of association with time of day for the other metabolites may reflect more consistent, constant exposure sources compared to other studies reporting more drastic differences by time of day. Among demographic and anthropometric characteristics, increases in maternal age were significantly associated with higher concentrations of MCPP, MCNP, and MCOP and decreases in MnBP and MBzP in our population. Older women did not differ significantly from younger women in terms of water usage/storage characteristics, timing of urine collection, food item intake, or use of a majority of the personal care products (data not shown), suggesting exposure to these compounds may result from another source not captured in our study. We also observed some suggestive evidence for higher phthalate metabolite concentrations among women in the highest pre-pregnancy BMI category. While there are sparse data concerning demographic and anthropometric characteristics associated with phthalate metabolite concentrations in pregnant women, previous studies have reported similar relationships between phthalate metabolite concentrations, age, and BMI (Duty et al. 2005; Koo et al. 2002; Peck et al. 2010).
For self-reported water usage and storage habits, participants who reported using bottled water for cooking had significantly higher urinary MEP concentrations compared to those who utilized the public water supply system in Puerto Rico. A similar, though non-significant, pattern for MEP concentrations with usage of bottle water for drinking was also observed. Previous studies have reported the presence of DEP in a variety of plastic bottles, and that concentrations may be affected by storage conditions (Al-Saleh et al. 2011; Amiridou and Voutsa 2011). It is interesting to note that while very few participants reported using private well water for drinking and cooking, much higher concentrations of DEHP metabolites were found for those individuals when compared to other sources of water, which is of potential concern given groundwater contamination issues along the North Coast. Recruitment of pregnant women into PROTECT is ongoing, and we will be better equipped to test these relationships with greater statistical power in the future. While, much is still unknown regarding the extent of groundwater contamination by phthalates and what potential impacts this may pose to residents in the North Coast karst area,
Higher urinary MEP concentrations were also found for women who reported using perfume or colored cosmetics in the 48 hours prior sample collection. This finding is consistent with previous studies of men and women (Berman et al. 2009; Duty et al. 2005; Koo and Lee 2004; Parlett et al. 2012; Romero-Franco et al. 2011). DEP, the parent diester of MEP, has been shown to be an ingredient in many perfumes and fragranced products (Dodson et al. 2012; Koo and Lee 2004), and dermal absorption can occur (Api 2001; ATSDR 1995). Interestingly, the use of both perfume and laundry detergent were associated with lower levels of MEHP and MiBP, respectively. The reason for this inverse relationship is not known, though overall our results suggest sources of exposure other than personal care products for the parent compounds of these metabolites. We observed a non-significant trend for decreasing metabolite concentrations of several metabolites with increased usage of fragrance free products. This relationship was hypothesized given that DEP, DBP and BzBP are used in personal care products to hold scent and color (NRC 2008). However, more data and statistical power will be needed to adequately test this hypothesis.
It has also been reported that diet can represent a significant source of exposure to phthalates, especially DEHP and BzBP, due to contamination during processing, storage and transport (Rudel et al. 2003; Wormuth et al. 2006). An analysis from the 2003-2004 NHANES reported associations between consumption of poultry, fish, and meat products with increased urinary concentrations of DEHP metabolites, and increased urinary concentrations of both MCPP and DEHP metabolites in relation to consumption of dairy products (Colacino et al. 2010). In a follow-up analysis to a recent intervention study which aimed to reduce DEHP exposure by limiting plastic use in food procurement, storage, and preparation, elevated DEHP concentrations were found in dairy products and in particular spices (Sathyanarayana et al. 2013). In the present study, we found that consumption of ice cream and chicken in the 48 hours prior to urine collection was associated with higher concentrations of MCPP, MCNP, and MCOP, but not DEHP metabolites, when compared samples from participants who did not consume these items in the previous 48 hours. The parent compounds for MCNP and MCOP are beginning to replace DEHP in the global market (BASF 2011), but current evidence is sparse for their presence in food products (Bradley et al. 2013). Phthalate contamination may vary between food types because many food products undergo different processing, packaging, and storage conditions.
Strengths of this study include its focus on an understudied and potentially at-risk population, a fairly large sample size for assessing predictors of metabolite concentrations, important and original contributions for informing future exposure assessment and epidemiology studies, and the collection of repeated data on urinary biomarkers and self-reported product use (including dietary intake of selected food groups) which allowed for a statistically powerful analysis. Sparse cells for certain demographic, water usage, product use, and food use categories may have limited our ability to detect significant differences in phthalate metabolite concentrations between groups. As our sample size increases, future analysis will be able to address this potential limitation. In addition, we did not collect information regarding the amount, frequency, or brand/manufacturer/name of personal care product used which may have allowed for a more detailed analysis. However, adding this information for each type of product in the questionnaire would increase participant burden which, in turn, could adversely impact other aspects of the study (e.g., recruitment and retention). Finally, another potential limitation may be in our ability to compare our results with other studies or generalize our findings to other populations due to differences in populations (e.g., pregnant women), study (e.g., questionnaire) design, and differences in product formulation, availability and use by country/region.
5. Conclusions
To our knowledge this is the first study to report concentrations, temporal variability, and predictors of phthalate biomarkers among pregnant women in Puerto Rico. Concentrations for a majority of the metabolites measured were somewhat higher in the Puerto Rican participants when compared to women of reproductive age in the mainland US population. We found positive associations between MEP concentrations and self-reported use of perfume and colored cosmetics. Additionally, we observed increased concentrations of MCPP, MCNP, and MCOP in those participants who consumed ice cream and chicken. This information, coupled with data from studies measuring these chemicals in specific products, may help inform pregnant women et al. on how to reduce their exposure. Finally, the degree of temporal reliability for the three time point samples collected on the pregnant women in our Puerto Rico study was generally low to moderate. Most previous studies of health outcomes, including pregnancy outcomes, in relation to phthalates have relied on biomarker concentrations from a single point in time. Our observation of low ICCs in this population suggests that we should continue measuring phthalate metabolites at multiple times in pregnancy to reduce the attenuation of our power to detect exposure-outcome effects due to exposure misclassification in our ongoing work. Future epidemiological studies should also strive to include as many repeated measurements during pregnancy as is feasible to reduce measurement error and explore potential windows of susceptibility to adverse health outcomes.
Highlights.
Phthalate exposure is prevalent among pregnant women in Puerto Rico.
Compared to the US, urinary concentrations of some phthalate metabolites were higher while others were lower.
Within-subject repeatability of phthalate metabolites concentrations was generally low to moderate.
Monoethyl phthalate concentrations were higher among women who used perfume or cosmetics than among other women.
Acknowledgements
The project described was supported by P42ES017198 and P30ES017885 from the National Institute of Environmental Health Sciences (NIEHS). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Environmental Health Sciences, the National Institutes of Health, or the Centers for Disease Control and Prevention. We gratefully acknowledge Manori Silva, Ella Samandar, Jim Preau, and Tao Jia for technical assistance in measuring the urinary concentrations of the phthalate metabolites.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no competing financial interests.
References
- Adibi JJ, Perera FP, Jedrychowski W, Camann DE, Barr D, Jacek R, Whyatt RM. Prenatal exposures to phthalates among women in New York City and Krakow, Poland. Environ Health Perspect. 2003;111:1719–1722. doi: 10.1289/ehp.6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adibi JJ, Whyatt RM, Williams PL, Calafat AM, Camann D, Herrick R, Nelson H, Bhat HK, Perera FP, Silva MJ, Hauser R. Characterization of phthalate exposure among pregnant women assessed by repeat air and urine samples. Environ Health Perspect. 2008;116:467–473. doi: 10.1289/ehp.10749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Saleh I, Shinwari N, Alsabbaheen A. Phthalates residues in plastic bottled waters. J Toxicol Sci. 2011;36:469–478. doi: 10.2131/jts.36.469. [DOI] [PubMed] [Google Scholar]
- Amiridou D, Voutsa D. Alkylphenols and phthalates in bottled waters. J Hazard Mater. 2011;185:281–286. doi: 10.1016/j.jhazmat.2010.09.031. [DOI] [PubMed] [Google Scholar]
- Api AM. Toxicological profile of diethyl phthalate: a vehicle for fragrance and cosmetic ingredients. Food Chem Toxicol. 2001;39:97–108. doi: 10.1016/s0278-6915(00)00124-1. [DOI] [PubMed] [Google Scholar]
- ATSDR . Toxicological Profile for Diethyl Phthalate. Agency for Toxic Substances and Disease Registry; Atlanta, GA: 1995. [1 February 2013]. [ http://www.atsdr.cdc.gov/toxprofiles/tp73.html] [PubMed] [Google Scholar]
- BASF (SPI Vinyl Plastics Division) Plasticizers Market Update. [15 August 2013];Presented at the U.S. Consumer Products Safety Commission Chronic Hazard Advisory Panel (CHAP) on Phthalates Meeting. 2011 [ http://www.cpsc.gov/about/cpsia/chap0711.html]
- Berman T, Hochner-Celnikier D, Calafat AM, Needham LL, Amitai Y, Wormser U, Richter E. Phthalate exposure among pregnant women in Jerusalem, Israel: results of a pilot study. Environ Int. 2009;35:353–357. doi: 10.1016/j.envint.2008.08.010. [DOI] [PubMed] [Google Scholar]
- Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, Adler A, Vera- Garcia C, Rohde S, Say L, Lawn JE. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379:2162–2172. doi: 10.1016/S0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- Blount BC, Milgram KE, Silva MJ, Malek NA, Reidy JA, Needham LL, Brock JW. Quantitative detection of eight phthalate metabolites in human urine using HPLC-APCI-MS/MS. Anal Chem. 2000;72:4127–4134. doi: 10.1021/ac000422r. [DOI] [PubMed] [Google Scholar]
- Bradley EL, Burden RA, Leon I, Mortimer DN, Speck DR, Castle L. Determination of phthalate diesters in foods. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2013;30:722–734. doi: 10.1080/19440049.2013.781683. [DOI] [PubMed] [Google Scholar]
- Braun JM, Smith KW, Williams PL, Calafat AM, Berry K, Ehrlich S, Hauser R. Variability of urinary phthalate metabolite and bisphenol A concentrations before and during pregnancy. Environ Health Perspect. 2012;120:739–745. doi: 10.1289/ehp.1104139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casas L, Fernandez MF, Llop S, Guxens M, Ballester F, Olea N, Irurzun MB, Rodriguez LS, Riano I, Tardon A, Vrijheid M, Calafat AM, Sunyer J. Urinary concentrations of phthalates and phenols in a population of Spanish pregnant women and children. Environ Int. 2011;37:858–866. doi: 10.1016/j.envint.2011.02.012. [DOI] [PubMed] [Google Scholar]
- CDC (Centers for Disease Control) National report on human exposure to environmental chemicals. Centers for Disease Control; Atlanta, GA: 2012. [1 February 2013]. [ http://www.cdc.gov/exposurereport/pdf/FourthReport_UpdatedTables_Sep2012.pdf] [Google Scholar]
- Colacino JA, Harris TR, Schecter A. Dietary intake is associated with phthalate body burden in a nationally representative sample. Environ Health Perspect. 2010;118:998–1003. doi: 10.1289/ehp.0901712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colon I, Caro D, Bourdony CJ, Rosario O. Identification of phthalate esters in the serum of young Puerto Rican girls with premature breast development. Environ Health Perspect. 2000;108:895–900. doi: 10.1289/ehp.108-2556932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson RE, Nishioka M, Standley LJ, Perovich LJ, Brody JG, Rudel RA. Endocrine disruptors and asthma-associated chemicals in consumer products. Environ Health Perspect. 2012;120:935–943. doi: 10.1289/ehp.1104052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duty SM, Ackerman RM, Calafat AM, Hauser R. Personal care product use predicts urinary concentrations of some phthalate monoesters. Environ Health Perspect. 2005;113:1530–1535. doi: 10.1289/ehp.8083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPA (Environmental Protection Agency) [1 February 2013];Superfund Information System: Record of Decision System. 2013 [ http://cumulis.epa.gov/superrods/index.cfm?fuseaction=data.RODSList]
- Hankinson SE, Manson JE, Spiegelman D, Willett WC, Longcope C, Speizer FE. Reproducibility of plasma hormone levels in postmenopausal women over a 2-3-year period. Cancer Epidemiol Biomarkers Prev. 1995;4:649–654. [PubMed] [Google Scholar]
- Hauser R, Meeker JD, Park S, Silva MJ, Calafat AM. Temporal variability of urinary phthalate metabolite levels in men of reproductive age. Environ Health Perspect. 2004;112:1734–1740. doi: 10.1289/ehp.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang PC, Kuo PL, Guo YL, Liao PC, Lee CC. Associations between urinary phthalate monoesters and thyroid hormones in pregnant women. Hum Reprod. 2007;22:2715–2722. doi: 10.1093/humrep/dem205. [DOI] [PubMed] [Google Scholar]
- Irvin EA, Calafat AM, Silva MJ, Aguilar-Villalobos M, Needham LL, Hall DB, Cassidy B, Naeher LP. An estimate of phthalate exposure among pregnant women living in Trujillo, Peru. Chemosphere. 2010;80:1301–1307. doi: 10.1016/j.chemosphere.2010.06.048. [DOI] [PubMed] [Google Scholar]
- Jonsson BA, Richthoff J, Rylander L, Giwercman A, Hagmar L. Urinary phthalate metabolites and biomarkers of reproductive function in young men. Epidemiology. 2005;16:487–493. doi: 10.1097/01.ede.0000164555.19041.01. [DOI] [PubMed] [Google Scholar]
- Kim Y, Ha EH, Kim EJ, Park H, Ha M, Kim JH, Hong YC, Chang N, Kim BN. Prenatal exposure to phthalates and infant development at 6 months: prospective Mothers and Children's Environmental Health (MOCEH) study. Environ Health Perspect. 2011;119:1495–1500. doi: 10.1289/ehp.1003178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolossa-Gehring M, Becker K, Conrad A, Schroter-Kermani C, Schulz C, Seiwert M. Environmental surveys, specimen bank and health related environmental monitoring in Germany. Int J Hyg Environ Health. 2012;215:120–126. doi: 10.1016/j.ijheh.2011.10.013. [DOI] [PubMed] [Google Scholar]
- Koo HJ, Lee BM. Estimated exposure to phthalates in cosmetics and risk assessment. J Toxicol Environ Health A. 2004;67:1901–1914. doi: 10.1080/15287390490513300. [DOI] [PubMed] [Google Scholar]
- Koo JW, Parham F, Kohn MC, Masten SA, Brock JW, Needham LL, Portier CJ. The association between biomarker-based exposure estimates for phthalates and demographic factors in a human reference population. Environ Health Perspect. 2002;110:405–410. doi: 10.1289/ehp.02110405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois E, Leblanc A, Simard Y, Thellen C. Accuracy investigation of phthalate metabolite standards. J Anal Toxicol. 2012;36:270–279. doi: 10.1093/jat/bks016. [DOI] [PubMed] [Google Scholar]
- Larriuz-Serrano MC, Perez-Cardona CM, Ramos-Valencia G, Bourdony CJ. Natural history and incidence of premature thelarche in Puerto Rican girls aged 6 months to 8 years diagnosed between 1990 and 1995. PR Health Sci. 2001;20:13–18. [PubMed] [Google Scholar]
- Lorber M, Koch HM, Angerer J. A critical evaluation of the creatinine correction approach: can it underestimate intakes of phthalates? A case study with di-2-ethylhexyl phthalate. J Expo Sci Environ Epidemiol. 2010;21:576–586. doi: 10.1038/jes.2010.43. [DOI] [PubMed] [Google Scholar]
- McKee RH. Phthalate exposure and early thelarche. Environ Health Perspect. 2004;112:A541–543. doi: 10.1289/ehp.112-a541b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Calafat AM, Hauser R. Urinary phthalate metabolites and their biotransformation products: predictors and temporal variability among men and women. J Expo Sci Environ Epidemiol. 2012;22:376–385. doi: 10.1038/jes.2012.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Hu H, Cantonwine DE, Lamadrid-Figueroa H, Calafat AM, Ettinger AS, Hernandez-Avila M, Loch-Caruso R, Tellez-Rojo MM. Urinary phthalate metabolites in relation to preterm birth in Mexico city. Environ Health Perspect. 2009;117:1587–1592. doi: 10.1289/ehp.0800522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NRC (National Research Council) Phthalates and Cumulative Risk Assessment; The Task Ahead. National Academies Press (U.S.).; Washington, DC: 2008. [PubMed] [Google Scholar]
- Padilla IY, Irizarry C, Steele K. Historical contamination of groundwater resources in the north coast karst aquifer of Puerto Rico. Dimension. 2011;25:7–12. [PMC free article] [PubMed] [Google Scholar]
- Parlett LE, Calafat AM, Swan SH. Women's exposure to phthalates in relation to use of personal care products. J Expo Sci Environ Epidemiol. 2012;23:197–206. doi: 10.1038/jes.2012.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck JD, Sweeney AM, Symanski E, Gardiner J, Silva MJ, Calafat AM, Schantz SL. Intra- and inter-individual variability of urinary phthalate metabolite concentrations in Hmong women of reproductive age. J Expo Sci Environ Epidemiol. 2010;20:90–100. doi: 10.1038/jes.2009.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippat C, Mortamais M, Chevrier C, Petit C, Calafat AM, Ye X, Silva MJ, Brambilla C, Pin I, Charles MA, Cordier S, Slama R. Exposure to phthalates and phenols during pregnancy and offspring size at birth. Environ Health Perspect. 2012;120:464–470. doi: 10.1289/ehp.1103634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Franco M, Hernandez-Ramirez RU, Calafat AM, Cebrian ME, Needham LL, Teitelbaum S, Wolff MS, Lopez-Carrillo L. Personal care product use and urinary levels of phthalate metabolites in Mexican women. Environ Int. 2011;37:867–871. doi: 10.1016/j.envint.2011.02.014. [DOI] [PubMed] [Google Scholar]
- Rudel RA, Gray JM, Engel CL, Rawsthorne TW, Dodson RE, Ackerman JM, Rizzo J, Nudelman JL, Brody JG. Food packaging and bisphenol A and bis(2-ethyhexyl) phthalate exposure: findings from a dietary intervention. Environ Health Perspect. 2003;119:914–920. doi: 10.1289/ehp.1003170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathyanarayana S, Alcedo G, Saelens BE, Zhou C, Dills RL, Yu J, Lanphear B. Unexpected results in a randomized dietary trial to reduce phthalate and bisphenol A exposures. J Expo Sci Environ Epidemiol Feb 27. 2013 doi: 10.1038/jes.2013.9. doi: 10.1038/jes.2013.9. [DOI] [PubMed] [Google Scholar]
- Sathyanarayana S, Karr CJ, Lozano P, Brown E, Calafat AM, Liu F, Swan SH. Baby care products: possible sources of infant phthalate exposure. Pediatrics. 2008;121:e260–268. doi: 10.1542/peds.2006-3766. [DOI] [PubMed] [Google Scholar]
- Schecter A, Lorber M, Guo Y, Wu Q, Yun SH, Kannan K, Hommel M, Imran N, Hynan LS, Cheng D, Colacino JA, Birnbaum LS. Phthalate concentrations and dietary exposure from food purchased in New York State. Environ Health Perspect. 2013;121:473–494. doi: 10.1289/ehp.1206367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schettler T. Human exposure to phthalates via consumer products. Int J Androl. 2006;29:134–139. doi: 10.1111/j.1365-2605.2005.00567.x. [DOI] [PubMed] [Google Scholar]
- Silva MJ, Samandar E, Preau JL, Reidy JA, Needham LL, Calafat AM. Quantification of 22 phthalate metabolites in human urine. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;860:106–112. doi: 10.1016/j.jchromb.2007.10.023. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Niwa M, Yoshinaga J, Mizumoto Y, Serizawa S, Shiraishi H. Prenatal exposure to phthalate esters and PAHs and birth outcomes. Environ Int. 2010;36:699–704. doi: 10.1016/j.envint.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Swan SH, Main KM, Liu F, Stewart SL, Kruse RL, Calafat AM, Mao CS, Redmon JB, Ternand CL, Sullivan S, Teague JL. Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environ Health Perspect. 2005;113:1056–1061. doi: 10.1289/ehp.8100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabb MM, Blumberg B. New modes of action for endocrine-disrupting chemicals. Mol Endocrinol. 2006;20:475–482. doi: 10.1210/me.2004-0513. [DOI] [PubMed] [Google Scholar]
- Wolff MS, Engel SM, Berkowitz GS, Ye X, Silva MJ, Zhu C, Wetmur J, Calafat AM. Prenatal phenol and phthalate exposures and birth outcomes. Environ Health Perspect. 2008;116:1092–1097. doi: 10.1289/ehp.11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff TJ, Zota AR, Schwartz JM. Environmental chemicals in pregnant women in the United States: NHANES 2003-2004. Environ Health Perspect. 2011;119:878–885. doi: 10.1289/ehp.1002727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wormuth M, Scheringer M, Vollenweider M, Hungerbuhler K. What are the sources of exposure to eight frequently used phthalic acid esters in Europeans? Risk Anal. 2006;26:803–824. doi: 10.1111/j.1539-6924.2006.00770.x. [DOI] [PubMed] [Google Scholar]
- Ye X, Pierik FH, Hauser R, Duty S, Angerer J, Park MM, Burdorf A, Hofman A, Jaddoe VW, Mackenbach JP, Steegers EA, Tiemeier H, Longnecker MP. Urinary metabolite concentrations of organophosphorous pesticides, bisphenol A, and phthalates among pregnant women in Rotterdam, the Netherlands: the Generation R study. Environ Res. 2008;108:260–267. doi: 10.1016/j.envres.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeman FA, Boudet C, Tack K, Floch Barneaud A, Brochot C, Péry ARR, Oleko A, Vandentorren S. Exposure assessment of phthalates in French pregnant women: Results of the ELFE pilot study. Int J Hyg Environ Health. 2013;21:271–279. doi: 10.1016/j.ijheh.2012.12.005. [DOI] [PubMed] [Google Scholar]