Abstract
Objective
To identify the neuromuscular attributes that are associated with self reported mobility status among older primary care patients
Design
Cohort Study
Setting
Metropolitan based health care system
Participants
Community-dwelling primary care patients age ≥ 65 years (N=430), with self-reported modification of mobility tasks resulting from underlying health conditions.
Interventions
Not applicable
Main Outcome Measures
Basic and Advanced Lower Extremity Function as measured by the Late Life Function and Disability Instrument
Results
We constructed multivariable linear regression models evaluating both outcomes. For Basic-Lower Extremity Function, leg strength, leg velocity, trunk extensor muscle endurance and ankle range of motion were statistically significant predictors (p<.001 R2 = .21). For Advanced-Lower Extremity Function, leg strength, leg strength asymmetry, leg velocity, trunk extensor muscle endurance and knee flexion range of motion were statistically significant predictors (p<.001, R2 =.39). Sensitivity analyses conducted using multiple imputations to account for missing data confirmed these findings.
Conclusions
This analysis highlights the relevance and importance of 5 categories of neuromuscular attributes: strength, speed of movement, range of motion, asymmetry and trunk stability. It identifies novel attributes (leg velocity and trunk extensor muscle endurance) relevant to mobility and highlights that impairment profiles vary by the level of mobility assessed. These findings will inform the design of more thorough and potentially more effective disability prevention strategies.
Keywords: Aged, Rehabilitation, Mobility Limitation, Primary Health Care
Problems with mobility activities, such as walking, climbing stairs and getting up from a chair, are very common among older primary care patients. It has been estimated that over 25% of adults aged 70 year or older manifest mobility problems,1 which are recognized as a frequent consequence of the most common chronic diseases affecting older primary care patients.2 Performance on basic mobility tasks is recognized as an important screening tool that predicts such outcomes as mortality, nursing home placement and the development of disability3 and that can serve as part of health promotion strategies.2 For this reason, clinical trials prioritizing the prevention of disability have focused on mobility as the outcome of interest.
In spite of advances, there are significant knowledge gaps with regards to the body system impairments that contribute most to declining mobility in later life.4 Optimal mobility rehabilitation should be evidenced based, parsimonious in its approach and correct impairments within those body systems that most influence mobility and that are amenable to rehabilitative care.5 Unfortunately, there is inadequate evidence guiding mobility rehabilitation. While certain attributes such as strength, balance and endurance have been the primary targets of studies addressing mobility limitations, the literature does not include thorough investigations that simultaneously evaluated other attributes that may be relevant to mobility, including limb speed, reaction time, kyphosis, trunk muscle endurance and asymmetries of strength, power and range of motion (ROM).6 It is important to know which of these attributes are or are not relevant to mobility when the contributions of these different attributes are considered simultaneously. An ideal approach to maximizing late-life mobility should include an understanding of the combinations of attributes that most influence mobility skills and thus should be prioritized within disability prevention strategies.
The Boston Rehabilitative Impairment Study of the Elderly (Boston RISE) was designed to address these concerns and is conceptually based upon the International Classification of Functioning, Disability and Health.7,8 It is a longitudinal cohort study of 430 primary care patients who are at risk for mobility decline. The aim of Boston RISE is to identify the neuromuscular impairments that are most associated with mobility status at baseline and the neuromuscular impairments at baseline that are most responsible for mobility decline and disability over time. This manuscript will present the analysis of the first aim of the Boston RISE study: evaluating which neuromuscular attributes are most associated with mobility at baseline among this cohort of older primary care patients.
Methods
A detailed description of the methods has been previously published.7 Briefly, primary care patients ≥65 years and at risk for mobility decline were recruited from primary care practices at Massachusetts General Hospital (MGH) and Brigham and Women’s Hospital (BWH).9, 10 Potential participants were identified through a Partners Healthcare database, and potential eligibility was corroborated by their primary care physicians. Potential participants were screened via telephone by study staff and invited to an initial screening and assessment visit. Individuals meeting eligibility criteria completed this initial visit and a subsequent visit within 2 weeks. Eligibility criteria include age ≥65 years, ability to speak and understand English, currently receiving primary care at MGH or BWH, difficulty or task modification with walking ½ mile and/or climbing 1 flight of stairs,9 no planned major surgery and expectation of living in the area for at least 2 years. Exclusion criteria included significant visual impairment, uncontrolled hypertension, amputation of a lower extremity, use of supplemental oxygen, myocardial infarction or major surgery in the previous 6 months, Mini Mental Status score <18 and Short Physical Performance Battery (SPPB) score <4.11, 12 Recruitment was targeted to ensure accurate ethnic and racial representation of older adults residing within a 10 mile radius of our health care facility and functional diversity as defined by the SPPB. All of the methods of the Boston RISE study were approved by the Institutional Review Board of Spaulding Rehabilitation Hospital.
Among 7,403 primary care patients identified, 5,333 (72%) were approved by primary care providers to receive communication about the study. Study staff conducted phone screenings with 1,349 people, of whom 712 (56%) were eligible to participate in the final screening at the first baseline visit. Of the 523 people who gave informed consent at the first visit, 443 (85%) were eligible to continue in the study, and, of those, 430 (97%) completed both baseline visits.
The primary outcomes of our analysis were the lower extremity mobility function scales of the Late Life Function and Disability Instrument (LLFDI).13 Physical functioning in the LLFDI consists of 48 questions addressing one functional difficulty dimension and three separate sub-domains: Advanced-Lower Extremity Function (activities that involve a high level of physical ability and endurance, such as walking several blocks or getting up from the floor), Basic-Lower Extremity Function (activities primarily involving standing, stooping, and fundamental walking activities such as walking around one floor of home) and Upper Extremity Function (activities of the hands and arms, such as holding a full glass of water or reaching behind your back). Given that the focus of our study was on mobility, our outcomes were both lower extremity function scales. Scores for all outcomes are scaled between 0 (lowest function) and 100 (highest function).
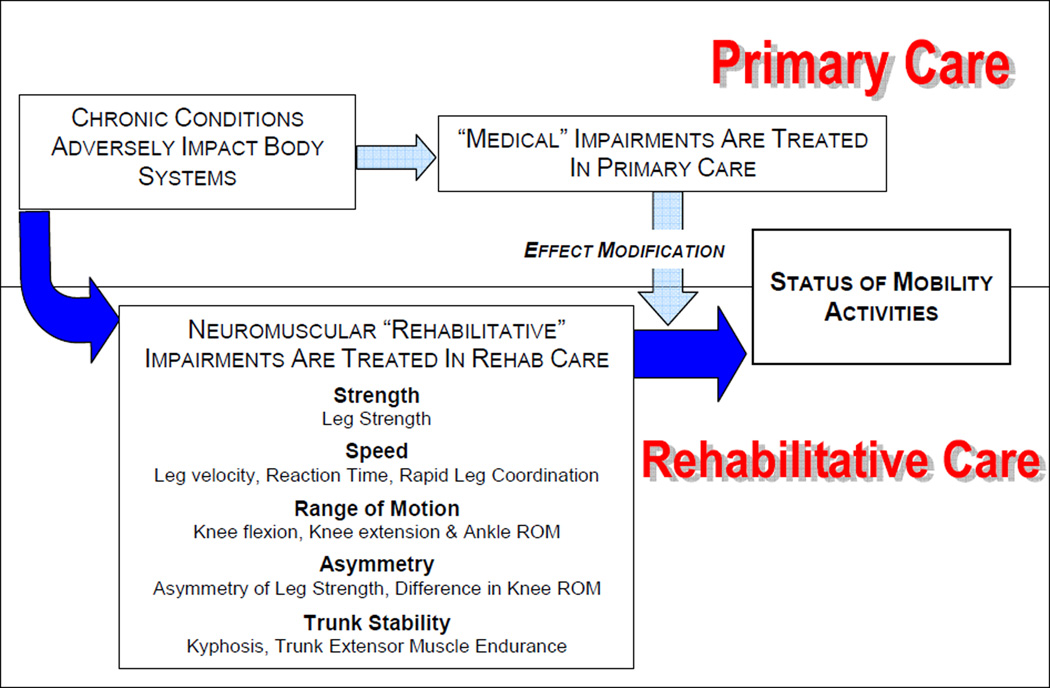
Our conceptual approach was based on our clinical and research experience and the existing literature, and we identified 5 categories of neuromuscular impairments that we hypothesized would be linked to poor mobility status (see figure 1): strength, speed of movement, ROM, asymmetry and trunk stability. We viewed impairments within these categories as the product of disease and comorbidity and as a potentially critical pathway to the development of restricted late-life mobility.
Figure 1.
Conceptual framework for the analyses of baseline data from the Boston RISE study
Neuromuscular attributes encompassing these 5 categories included Strength (leg strength); Speed of movement (leg velocity, reaction time, rapid leg coordination); ROM (knee flexion ROM, knee extension ROM, ankle ROM); Asymmetry (side to side asymmetry of leg strength, side to side asymmetry of leg power, side to side asymmetry of both knee ROM measures); and Trunk Stability (trunk extensor muscle endurance and kyphosis). All are attributes that have been linked to mobility status and are potentially amenable to rehabilitative interventions.14–17
Leg press strength was measured by determining the one repetition maximum (1RM) for each leg individually using a Keiser A420 electronic pneumatic leg press machine and applying a previously published protocol.18 The maximum value observed on the A420 graphical display of either side was recorded as the peak leg strength. Leg press power was measured as the peak power graphically recorded when the individual performed a single leg press repetition pushing out as quickly as possible at 40% or 70% of 1RM. Five repetitions were recorded for each leg at each resistance. The highest recorded power of all repetitions (either side, either resistance) was recorded as peak leg press power. Leg velocity was recorded by dividing the peak leg press power by the graphically displayed force simultaneously recorded during testing. The highest leg velocity recorded (either side, either resistance) was recorded as leg velocity. Reaction time was measured using a device developed and validated by Lord et al.19 Participants pressed a mouse button after the appearance of a bright light, which appeared at random intervals. Participants were given five practice trials, and reaction time was recorded as the mean of 10 subsequent measurement trials Rapid leg coordination time was measured using heel to floor time, which was measured in a seated position as the time to complete 10 repetitions in which the heel of one foot was placed just below the opposite knee and then back to the floor.20 Knee and ankle ROM were measured using a goniometer.21 Asymmetry measures were defined separately for leg strength, leg power and both knee ROM measures. They were defined as the higher value of the two sides divided by the lower value for the leg strength and leg power variables and as the difference (higher value minus the lower value) for the knee ROM measures. Trunk extensor muscle endurance was measured,17 while the participant was lying prone on a specialized plinth positioned 45° from vertical with feet fixed in position on a footplate and the body supported below the waist by the table. The participant maintained their trunk in a neutral position within the sagital plane in line with their pelvis and legs for as long as possible with arms across the chest. The test was terminated when the participant was no longer able to maintain the unsupported position. Kyphosis was measured using the reliable and valid flexicurve technique described by Milne and Lauder.22
Adjustment variables included demographic characteristics (age, gender, race, level of education) and attributes commonly treated in primary care that can influence the course of rehabilitative care. These attributes included symptoms of depression as defined by a Patient Health Questionnaire-9 score of >5,23 executive function as defined by score on the Digit Symbol Substitution Test,24 sensory loss as defined by the Semmes-Weinstein monofilament test,25 visual impairment as defined by the inability to successfully read the 20/50 line of a Snellen eye chart,26 overweight and obese status as defined by body mass index categories27 and number of chronic illnesses as defined by a comorbidity questionnaire developed and validated by Sangha et al (heart disease, high blood pressure, lung disease, diabetes, ulcer or stomach disease, kidney disease, liver disease, anemia or other blood diseases, cancer, depression, osteoarthritis or degenerative arthritis, back pain, and rheumatoid arthritis).28 Disease specific status was not included as an adjustment variable, since this would represent an over-adjustment for impairment status within our conceptual model.
Initially, all variables were evaluated by descriptive statistics using means and standard deviations for continuous variables and by frequency and proportions for categorical variables. We inspected the correlation between all measured variables. Given concerns for high colinearity among predictors, selection of neuromuscular attributes and adjustment variables was based on ensuring a correlation coefficient between these variables of r < .40 and the strength of association with the outcomes. If the association within a particular attribute category was relatively high (r > .20), we utilized the attribute most highly associated with the outcome. Multivariable linear regression models were constructed to evaluate each outcome measure (Basic- and Advanced-Lower Extremity Function). This was done by a manual backwards elimination process. Age, gender and only those remaining attributes that were significant predictors were retained. Other adjustment variables were then added to these initial models and evaluated by the same manual backwards elimination process. We retained adjustment variables in the final model if they materially altered the relationships between individual neuromuscular attributes (standardized estimate of attribute changed by ≥ 20%) and the outcomes. Lastly, comorbidity was evaluated as a separate adjustment variable in order to evaluate if it modified our final models.
To validate our approach, we conducted a factor analysis of all available neuromuscular attributes to determine if the identified neuromuscular factors were statistically superior in predicting mobility limitations compared with our original approach. To address missing data, we performed a comparison of subjects excluded from our final analysis with those included. To confirm our findings, we conducted a sensitivity analysis imputing missing values and were guided by techniques advocated by Carpenter et al. that provide strategies to address data missing at random as well as missing not at random.29 We utilized SAS v9.1 in conducting all statistical analyses with the exception of the factor analysis, which was conducted using Mplus.30, 31
Results
Baseline characteristics are presented in Table 1, and baseline values for the neuromuscular attributes are shown in Table 2. The age, gender and racial distributions in our sample are consistent with the 2004 census for older adults living within our recruitment area. Among the neuromuscular attributes, the largest amount of missing data was observed with leg power asymmetry (15%), leg strength asymmetry (13.5%) and leg strength (10%). Given a correlation of r=.46 between leg strength asymmetry and leg power asymmetry and the greater magnitude of missing data for leg power asymmetry; it was not evaluated in subsequent analyses. As part of our initial validation of the conceptualized impairment groupings, our preplanned factor analysis did not identify any statistically relevant groupings of neuromuscular attributes that were statistically superior to the 5 clinical groupings we conceptualized initially (data not shown).
Table 1.
Baseline Characteristics of Boston RISE Participants
| Variable | N | Mean or % | SD | Min | Max | |
|---|---|---|---|---|---|---|
| Age | 430 | 76.6 | 7.0 | 65.00 | 96.00 | |
| 65–75 | 203 | 47.2 % | ||||
| 76–96 | 227 | 52.8 % | ||||
| Female Gender | 291 | 67.7 % | ||||
| Race | ||||||
| Non-white | 75 | 17.4 % | ||||
| White | 355 | 82.6 % | ||||
| Education | ||||||
| < High School | 54 | 12.6 % | ||||
| High School | 130 | 30.2 % | ||||
| College Graduate | 140 | 32.6 % | ||||
| Post Graduate | 106 | 24.7 % | ||||
| Medical Attributes | ||||||
| Chronic Conditions (#) | 430 | 4.0 | 1.9 | 0 | 11 | |
| Delayed Recall (#) | 430 | 5.71 | 3.1 | 0 | 12.0 | |
| Trails B (sec.) | 430 | 147.3 | 82.7 | 28.6 | 300.0 | |
| Vision | 424 | |||||
| Intact | 405 | 95.5 % | ||||
| Impairment | 19 | 4.5 % | ||||
| Sensory Loss | 422 | |||||
| Intact | 299 | 70.9 % | ||||
| Impaired | 123 | 29.2 % | ||||
| BMI | 429 | |||||
| BMI <25 | 102 | 23.8 % | ||||
| BMI = 25–30 | 170 | 39.6 % | ||||
| BMI >30 | 157 | 36.6 % | ||||
| Outcomes | ||||||
| LLFDI Advanced Function | 430 | 41.8 | 14.7 | 0.00 | 100.00 | |
| LLFDI Basic Function | 430 | 66.0 | 12.1 | 40.8 | 100.00 | |
Table 2.
Baseline Values of Neuromuscular Attributes
| Impairment Category |
Variable | N | Mean or % | SD | Min | Max | |
|---|---|---|---|---|---|---|---|
| Strength Speed of movement | Leg Strength (N/kg) | 387 | 9.45 | 2.54 | 3.10 | 20.25 | |
| Limb Velocity (m/s) | 381 | 1.00 | 0.25 | 0.23 | 1.92 | ||
| Average Reaction Time (msec.) | 430 | 248.7 | 51.5 | 172.1 | 648.4 | ||
| Rapid Leg Coordination (sec.) | 414 | 11.15 | 3.08 | 5.47 | 28.33 | ||
| Range of motion | Maximal Knee Flexion (degrees) | 425 | 124.59 | 13.61 | 58.00 | 149.00 | |
| Maximum Knee Extension (degrees) | 425 | 9.5 | 6.0 | −4.0 | 25.0 | ||
| Ankle Range | 429 | ||||||
| Intact | 307 | 71.6% | |||||
| Impaired | 122 | 28.4% | |||||
| Asymmetry (side to Side) | Leg Strength Ratio | 372 | 1.18 | 0.29 | 1.0 | 4.29 | |
| Leg Power Ratio Knee Flexion Difference (degrees) | 421 | 3.19 | 3.04 | 0.0 | 51.0 | ||
| Knee Extension Difference (degrees) | 421 | 3.20 | 3.0 | 0.0 | 16.00 | ||
| Trunk Stability | Kyphosis | 430 | 10.52 | 3.08 | 3.28 | 22.44 | |
| Trunk Extensor Muscle Endurance/ kg | 405 | 1.30 | 0.88 | 0 | 3.43 | ||
Table 3 presents the multivariable models predicting mobility function. For Basic-Lower Extremity Function, four of the 5 impairment categories were statistically significant predictors (model p<.001 R2 = .21), that being Strength (leg strength), Speed (leg velocity), ROM (ankle ROM) and Trunk stability (trunk extensor muscle endurance). The findings within the full adjusted model for Basic-Lower Extremity Function were similar, but ankle ROM achieved marginal significance (p=.05). For Advanced-Lower Extremity Function all five of the impairment categories were significantly associated within the initial (p<.001, R2 =.38) and fully adjusted model (p<.001, R2 =.39).The attributes that represented the five categories in the final models were as follows: Strength (leg strength), Speed (leg velocity), ROM (knee flexion ROM), Asymmetry (leg strength asymmetry) and Trunk Stability (trunk extensor muscle endurance).
Table 3.
Multivariable models evaluating the association between neuromuscular impairments and baseline mobility function among Boston RISE participants
| Outcome | Variables | Model 1 (R2 = .21) N=353 |
Model 2 (R2 = 21) N=348 |
||||
|---|---|---|---|---|---|---|---|
| LLFDI-Function Basic Lower Extremity | Estimate | S.E. | P-value | Estimate | S.E. | P-value | |
| Leg Strength | 1.22 | 0.27 | <.001 | 1.22 | .28 | <.001 | |
| Leg Velocity | 6.11 | 2.68 | .02 | 6.74 | 2.72 | .01 | |
| Ankle ROM | 3.24 | 1.34 | .02 | 2.70 | 1.38 | .05 | |
| Trunk Extensor Muscle Endurance | 2.45 | .71 | <.001 | 2.71 | .75 | <.001 | |
| Model 1 (R2 = .38) N=368 | Model 2 (R2 = .39) N=362 | ||||||
| LLFDI-Function Advanced Lower Extremity | Estimate | S.E. | P-value | Estimate | S.E. | P-value | |
| Leg Strength | 2.04 | 0.30 | <.001 | 2.04 | .31 | <.001 | |
| Leg Velocity | 5.86 | 2.93 | .04 | 5.91 | 2.96 | .04 | |
| Knee Flexion ROM | .22 | .05 | <.001 | .25 | .06 | <.001 | |
| Leg Strength Asymmetry | −5.07 | 2.08 | .02 | −4.51 | 2.10 | .03 | |
| Trunk Extensor Muscle Endurance | 3.22 | .77 | <.001 | 3.57 | .80 | <.001 | |
Note:
Model 1: adjusted for Age and Gender
Model 2: Model 1 + BMI categories and Sensory loss
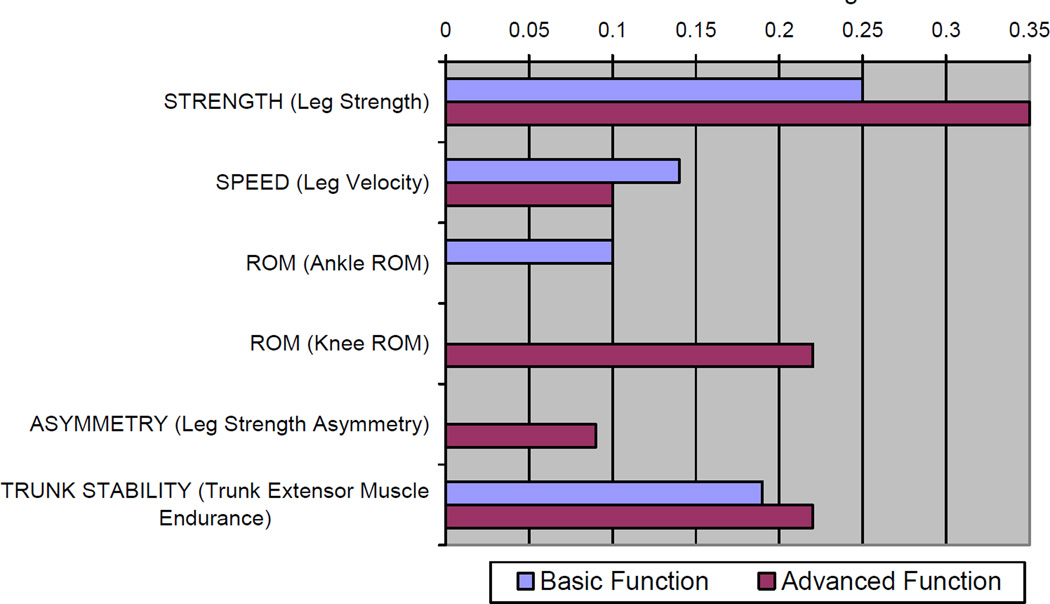
Figure 2 presents the standardized estimates of the fully adjusted models predicting Basic- and Advanced-Lower Extremity Function. Leg strength, leg velocity and trunk extensor muscle endurance were the only attributes that were statistically significant predictors of both tests. Ankle ROM was only associated with Basic-Lower Extremity Function, and leg strength asymmetry and knee flexion ROM were uniquely associated with Advanced-Lower Extremity Function. The standardized estimate for association with Advanced-Lower Extremity Function was 40% greater (.35 vs. .25) and 29% lower (.10 vs. .14) for leg strength and leg velocity, respectively, when compared to Basic-Lower Extremity Function. The variation in magnitude of the association between trunk extensor muscle endurance was relatively lower between the two outcomes (16%, .19 vs. .22). Within our sensitivity analyses, no material differences were observed within the findings when imputing missing values. Also, additional adjustment for comorbidity (number of chronic conditions) did not materially alter our findings (data not shown).
Figure 2.
Standardized estimates for each impairment category within separate liner regression models predicting LLFDI Basic- and Advanced-lower extremity function. Standardized estimates are presented representing those attributes that were statistically significantly associated with the respective tests.
Note: Standardized estimates presented as absolute values. Both models were adjusted for age, gender, overweight status, obese status and the manifestation of sensory loss
Discussion
To our knowledge, this study is the most comprehensive analysis to date of neuromuscular attributes associated with late-life mobility. The novel findings of this study are: 1) the identification of 5 categories of neuromuscular attributes (strength, speed, ROM, asymmetry, and trunk stability) predictive of mobility function; 2) the identification of leg velocity and trunk extensor muscle endurance as importance attributes influencing mobility function; and 3) the differential association of neuromuscular attributes with mobility based on the complexity of the tasks involved.
We observed that all 5 impairment categories were relevant to Advanced Lower Extremity Function and that all, but asymmetry were relevant to Basic Lower Extremity Function. These findings have direct and practical relevance to the provision of rehabilitative exercise and physical therapy for mobility limited patients. While strength and ROM are commonly prioritized for patients with mobility problems, speed, trunk stability and asymmetry do not receive great emphasis32. Our findings suggest that, in the care of patients with mobility problems, all 5 categories of impairments should be prioritized within a prescription for exercise or rehabilitation.
While the relevance of leg velocity and trunk extensor muscle endurance were first established within preliminary studies leading to Boston RISE,33, 34 this analysis highlights their relevance in comparison to other more well established attributes. Leg velocity has been recognized as important within studies identifying associations between leg power and mobility.33, 35 Power is defined as the combination of force and velocity of movement. Whereas maximal strength refers to optimal force production, maximal leg velocity is the attribute that distinguishes optimal power from optimal strength. It is important to recognize that both leg strength and leg velocity are important attributes that impart independent contributions to mobility. Our findings support the need for rehabilitative approaches that address both attributes36.
This is the first large investigation to evaluate the relevance of trunk extensor muscle endurance among older adults. It is a generally well tolerated measure, and, next to leg strength, it has the highest association with LLFDI function as evidenced by the magnitude of the standardized estimates in Figure 2. Another important aspect of the findings regarding trunk extensor muscle endurance was the absence of an association between kyphosis and mobility. Kyphosis is identified as a factor influencing mobility and fall related injuries,15 but prior investigations evaluating the relevance of kyphosis have not included trunk extensor muscle endurance measures. These two attributes were not correlated in our study, yet trunk muscle force production has been theorized to be the mechanism by which kyphosis influences these functional outcomes.15 Our findings are not inconsistent with this contention and suggest that trunk extensor muscle endurance may be a better therapeutic target than kyphosis when mobility is a concern.
Another important observation from our study is that the attributes within the 5 impairment categories that were predictive of the basic and advanced lower extremity function varied in magnitude and significance of association with these outcomes. This is well illustrated in Figure 2. The LLFDI is unique in comparison to other functional measures in that it encompasses tasks that capture a very broad range of function. Our findings suggest that if a particular level of functioning is targeted, that treatment might be designed differently. For example, while leg velocity, leg strength and trunk endurance are important for all patients, our findings suggest that individuals at higher levels of functioning should prioritize correcting asymmetries in limb strength and loss of knee ROM, while those at lower levels of functioning might need to focus more on ankle ROM deficits. These findings, in combination with our prior work ,36 might support the argument that a more individualized approach to rehabilitative care may optimize the results of strategies to prevent mobility restriction. Hence, the value of our findings is not to advise a “one size fits all” approach, but rather to clarify that several impairments may be key targets in disability prevention and that an expanded assessment may be key to developing effective intervention programs. We believe that these initial findings from Boston RISE lay the ground work to design such individualized programs, which can be evaluated within the context of comparative effectiveness research.
Other major longitudinal cohort studies have attempted to identify impairments associated with mobility status among older adults. Perhaps the most prominent of these studies are the Health Aging and Body Composition Study (Health ABC) and the InCHIANTI study.37, 38 While they continue to provide important discoveries for Aging science, their relevance to rehabilitative care is more limited. Neither study includes as an extensive array of neuromuscular impairments that can be targeted within rehabilitative care as Boston RISE. Health ABC recruited a cohort without significant mobility problems and focused primarily on successful aging. InCHIANTI, conceptualized impairments that comprise subsystems that underlie mobility and that represent a variety of body systems, but it included a smaller set of neuromuscular attributes that can be treated in rehabilitative care settings. Other prominent research has been conducted by Lord and colleagues, who have identified specific neuromuscular impairments that are associated with falls.19 While falls are a result of poor mobility status, the relevance of these impairments to overall mobility has not been elucidated. Despite these differences, all three of these investigations informed the design and methods of Boston RISE and helped provide the rationale for the neuromuscular impairments we studied.
Study Limitations
Our study has certain limitations. Since some of our frail participants found some tests too challenging, we had a limited amount of missing data. Missing data is common within cohort studies among older adults, and the proportion of missing data is generally low in Boston RISE. However, since these values may not be missing at random, we performed a sensitivity analysis using an approach that has been advocated to address such methodological concerns and did not find material differences. Also, since this is not a population-based study, the findings may not be relevant to older adults residing in the community at large or in different geographic regions.
An important strength of our investigation is its conceptual and clinical basis within primary and rehabilitative care. We defined neuromuscular impairments and outcomes within the contexts of care in which they are treated. Furthermore, Boston RISE represents the most extensive measurement of attributes targeted within rehabilitative care among a relatively large sample of older primary care patients.
Our study’s findings may have important implications for future comparative effectiveness trials. To date, most intervention studies focusing on the prevention or amelioration of mobility related disability among older adults have focused on a more restricted group of neuromuscular attributes.39, 40 Additionally, these studies have focused on a single unified approach for all patients regardless of baseline impairment status or level of functioning. Our findings suggest that more optimal benefits may be achieved if interventions consider the impairments manifested by individual patients and the complexity of the mobility skills targeted. The forthcoming longitudinal analyses from Boston RISE will shed more light on these issues.
Conclusions
This baseline analysis of the Boston RISE study highlights the relevance and importance of 5 categories of rehabilitative attributes: strength, speed of movement, ROM, asymmetry and trunk stability. The identification of novel attributes (leg velocity and trunk extensor muscle endurance) and the recognition that impairment profiles vary by the level of mobility assessed will inform the design of more thorough and potentially more effective disability prevention strategies.
Acknowledgements
The authors would like to acknowledge Julia Thomas, Paige Landry, Braidie Campbell, and the staff of the Massachusetts General Hospital Clinical Research Center for their assistance with baseline data collection.
Boston RISE is funded by Grant Number 5 R01 AG032052-03 from the National Institute on Aging. This study is also supported by Grant Number 1 UL1 RR025758-01, Harvard Clinical and Translational Science Center, from the National Center for Research Resources.
Abbreviations
- LLFDI
Late Life Function and Disability Instrument
- SPPB
Short Physical Performance Battery
- ROM
Range of motion
- MGH
Massachusetts General Hospital
- BWH
Brigham and Women’s Hospital
- 1RM
One repetition maximum
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Portions of this material were presented at the annual meeting of the Gerontological Society of America in November 2012.
Financial disclosure/conflicts of interest: None
Suppliers
Keiser A420 electronic pneumatic leg press machine, Keiser, 2470 S. Cherry Ave, Fresno, CA 93706
Reaction timer, Neuroscience Research Australia, Hospital Road, Randwic NSW 2031, Australia
SAS, SAS Institute Inc., 100 SAS Campus Drive, Cary, NC 27513
MPlus, Muthén & Muthén, 3463 Stoner Avenue, Los Angeles, CA 90066
References
- 1.Liao Y, McGee DL, Cao G, Cooper RS. Recent changes in the health status of the older U.S. population: findings from the 1984 and 1994 supplement on aging. J Am Geriatr Soc. 2001;49:443–449. doi: 10.1046/j.1532-5415.2001.49089.x. [DOI] [PubMed] [Google Scholar]
- 2.Freedman VA, Hodgson N, Lynn J, et al. Promoting Declines in the Prevalence of Late-Life Disability: Comparisons of Three Potentially High-Impact Interventions. The Milbank Quarterly. 2006;84:493–520. doi: 10.1111/j.1468-0009.2006.00456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morley JE. Mobility performance: a high-tech test for geriatricians. J Gerontol A Biol Sci Med Sci. 2003;58:712–714. doi: 10.1093/gerona/58.8.m712. [DOI] [PubMed] [Google Scholar]
- 4.Lee L, Siebens H. Geriatric Rehabilitation. In: LoCicero J, Rosenthal RA, Katlic MR, Pompei P, editors. A Supplement to New Frontiers in Geriatrics Research: An Agenda for Surgical and Related Medical Specialties. New York: American Geriatrics Society; 2007. [Google Scholar]
- 5.Boyd CM, Boult C, Shadmi E, et al. Guided care for multimorbid older adults. Gerontologist. 2007;47:697–704. doi: 10.1093/geront/47.5.697. [DOI] [PubMed] [Google Scholar]
- 6.Bean JF, Vora A, Frontera WR. Benefits of exercise for community-dwelling older adults. Arch Phys Med Rehabil. 2004;85:S31–S42. doi: 10.1016/j.apmr.2004.03.010. quiz S3–4. [DOI] [PubMed] [Google Scholar]
- 7.Holt NE, Percac-Lima S, Kurlinski LA, et al. The Boston Rehabilitative Impairment Study of the Elderly (Boston RISE): A description of methods. Arch Phys Med Rehabil. doi: 10.1016/j.apmr.2012.08.217. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO. Geneva: World Health Organization; 2001. International classification of functioning, disability, and health. [Google Scholar]
- 9.Fried LP, Bandeen-Roche K, Chaves PH, Johnson BA. Preclinical mobility disability predicts incident mobility disability in older women. J Gerontol A Biol Sci Med Sci. 2000;55:M43–M52. doi: 10.1093/gerona/55.1.m43. [DOI] [PubMed] [Google Scholar]
- 10.Fried LP, Young Y, Rubin G, Bandeen-Roche K. Self-reported preclinical disability identifies older women with early declines in performance and early disease. J Clin Epidemiol. 2001;54:889–901. doi: 10.1016/s0895-4356(01)00357-2. [DOI] [PubMed] [Google Scholar]
- 11.Folstein MF, Folstein SF, McHugh PR. "Mini-mental state": a practical method for grading the cognitive state of patients for the clinician. Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 12.Guralnik JM, Simonsick EM, Ferucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol Med Sci. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 13.Haley SM, Jette AM, Coster WJ, et al. Late Life Function and Disability Instrument: II. Development and evaluation of the function component. J Gerontol A Biol Sci Med Sci. 2002;57:M217–M222. doi: 10.1093/gerona/57.4.m217. [DOI] [PubMed] [Google Scholar]
- 14.Bean JF, Vora A, Frontera WR. Benefits of exercise for community-dwelling older adults. Arch Phys Med Rehabil. 2004;85:S31–S42. doi: 10.1016/j.apmr.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 15.Kado DM. The Rehabilitation of Hyperkyphotic Posture in the Elderly. Eur J Phys Rehabil Med. 2009;45:583–593. [PubMed] [Google Scholar]
- 16.Skelton DA, Kennedy J, Rutherford OM. Explosive power and asymmetry in leg muscle function in frequent fallers and non-fallers aged over 65. Age Ageing. 2002;31:119–125. doi: 10.1093/ageing/31.2.119. [DOI] [PubMed] [Google Scholar]
- 17.Suri P, Kiely DK, Leveille SG, Frontera WR, Bean JF. Trunk muscle attributes are associated with balance and mobility in older adults: a pilot study. PM R. 2009;1:916–924. doi: 10.1016/j.pmrj.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Callahan D, Phillips E, Carabello R, Frontera WR, Fielding RA. Assessment of lower extremity muscle power in functionally-limited elders. Aging Clin Exp Res. 2007;19:194–199. doi: 10.1007/BF03324689. [DOI] [PubMed] [Google Scholar]
- 19.Lord SR, Menz HB, Tiedemann A. A physiological profile approach to falls risk assessment and prevention. Phys Ther. 2003;83:237–252. [PubMed] [Google Scholar]
- 20.Shahar A, Patel KV, Semba RD, et al. Plasma selenium is positively related to performance in neurological tasks assessing coordination and motor speed. Mov Disord. 25:1909–1915. doi: 10.1002/mds.23218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watkins MA, Riddle DL, Lamb RL, Personius WJ. Reliability of goniometric measurements and visual estimates of knee range of motion obtained in a clinical setting. Phys Ther. 1991;71:90–96. doi: 10.1093/ptj/71.2.90. discussion 6–7. [DOI] [PubMed] [Google Scholar]
- 22.Milne JS, Williamson J. A longitudinal study of kyphosis in older people. Age Ageing. 1983;12:225–233. doi: 10.1093/ageing/12.3.225. [DOI] [PubMed] [Google Scholar]
- 23.Zuithoff NP, Vergouwe Y, King M, et al. The Patient Health Questionnaire-9 for detection of major depressive disorder in primary care: consequences of current thresholds in a crosssectional study. BMC family practice. 2010;11:98. doi: 10.1186/1471-2296-11-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karp JF, Reynolds CF, 3rd, Butters MA, et al. The relationship between pain and mental flexibility in older adult pain clinic patients. Pain Med. 2006;7:444–452. doi: 10.1111/j.1526-4637.2006.00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olaleye D, Perkins BA, Bril V. Evaluation of three screening tests and a risk assessment model for diagnosing peripheral neuropathy in the diabetes clinic. Diabetes research and clinical practice. 2001;54:115–128. doi: 10.1016/s0168-8227(01)00278-9. [DOI] [PubMed] [Google Scholar]
- 26.Kaiser PK. Prospective evaluation of visual acuity assessment: a comparison of snellen versus ETDRS charts in clinical practice (An AOS Thesis) Transactions of the American Ophthalmological Society. 2009;107:311–324. [PMC free article] [PubMed] [Google Scholar]
- 27.Lamon-Fava S, Wilson PW, Schaefer EJ. Impact of body mass index on coronary heart disease risk factors in men and women. The Framingham Offspring Study. Arterioscler Thromb Vasc Biol. 1996;16:1509–1515. doi: 10.1161/01.atv.16.12.1509. [DOI] [PubMed] [Google Scholar]
- 28.Sangha O, Stucki G, Liang MH, Fossel AH, Katz JN. The Self-Administered Comorbidity Questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis Rheum. 2003;49:156–163. doi: 10.1002/art.10993. [DOI] [PubMed] [Google Scholar]
- 29.Carpenter JR, Kenward MG, White IR. Sensitivity analysis after multiple imputation under missing at random: a weighting approach. Statistical Methods in Medical Research 16: 259–275. 2007;16:259–275. doi: 10.1177/0962280206075303. [DOI] [PubMed] [Google Scholar]
- 30.Muthen B, Muthen L. Mplus Statistical Analysis with Latent Variables User’s Guide. Los Angeles, CA: Muthén & Muthén; 2007. [Google Scholar]
- 31.SAS. SAS/STAT User's Guide, Version 9.0. Cary, NC: SAS Institute Inc.; 2005. [Google Scholar]
- 32.Aging NIo. Exercise and Physical Activity: Your everyday guide from the National Institute on Aging. 2009 [Google Scholar]
- 33.Bean JF, Kiely DK, LaRose S, Leveille SG. Which impairments are most associated with high mobility performance in older adults? Implications for a rehabilitation prescription. Arch Phys Med Rehabil. 2008;89:2278–84. doi: 10.1016/j.apmr.2008.04.029. [DOI] [PubMed] [Google Scholar]
- 34.Suri P, Kiely DK, Leveille SG, Frontera WR, Bean JF. Increased trunk extension endurance is associated with meaningful improvement in balance among older adults with mobility problems. Arch Phys Med Rehabil. 92:1038–1043. doi: 10.1016/j.apmr.2010.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bean JF, Olveczky DD, Kiely DK, LaRose SI, Jette AM. Performance-based versus patient-reported physical function: what are the underlying predictors? Phys Ther. 2011;91:1804–1811. doi: 10.2522/ptj.20100417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bean JF, Kiely DK, LaRose S, O'Neill E, Goldstein R, Frontera WR. Increased velocity exercise specific to task training versus the National Institute on Aging's strength training program: changes in limb power and mobility. J Gerontol A Biol Sci Med Sci. 2009;64:983–991. doi: 10.1093/gerona/glp056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferrucci L, Bandinelli S, Benvenuti E, et al. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48:1618–1625. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- 38.Hicks GE, Shardell M, Alley DE, et al. Absolute strength and loss of strength as predictors of mobility decline in older adults: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 67:66–73. doi: 10.1093/gerona/glr055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gill TM, Baker DI, Gottschalk M, Peduzzi PN, Allore H, Byers A. A program to prevent functional decline in physically frail, elderly persons who live at home. N Engl J Med. 2002;347:1068–1074. doi: 10.1056/NEJMoa020423. [DOI] [PubMed] [Google Scholar]
- 40.Pahor M, Blair SN, Espeland M, et al. Effects of a physical activity intervention on measures of physical performance: Results of the lifestyle interventions and independence for Elders Pilot (LIFE-P) study. J Gerontol A Biol Sci Med Sci. 2006;61:1157–1165. doi: 10.1093/gerona/61.11.1157. [DOI] [PubMed] [Google Scholar]