Abstract
Purpose
Myeloid-derived suppressor cells (MDSC) accumulate in tumor-bearing hosts and are associated with immune suppression. To date, there have only been few studies that evaluate the direct effect of chemotherapeutic agents on MDSCs. Agents that inhibit MDSCs may be useful in the treatment of patients with various cancers.
Experimental Design
We investigated the in vivo effects of docetaxel on immune function in 4T1-Neu mammary tumor-bearing mice to examine if a favorable immunomodulatory effect accompanies tumor suppression. Primary focus was on the differentiation status of MDSCs and their ability to modulate T-cell responses.
Results
Docetaxel administration significantly inhibited tumor growth in 4T1-Neu tumor-bearing mice and considerably decreased MDSC proportion in the spleen. The treatment also selectively increased CTL responses. Docetaxel-pretreated MDSCs cocultured with OT-II splenocytes in the presence of OVA323–339 showed OT-II–specific CD4 activation and expansion in vitro. In characterizing the phenotype of MDSCs for M1 (CCR7) and M2 [mannose receptor (CD206)] markers, MDSCs from untreated tumor bearers were primarily MR+ with few CCR7+ cells. Docetaxel treatment polarized MDSCs toward an M1-like phenotype, resulting in 40% of MDSCs expressing CCR7 in vivo and in vitro, and macrophage differentiation markers such as MHC class II, CD11c, and CD86 were upregulated. Interestingly, docetaxel induced cell death selectively in MR+ MDSCs while sparing the M1-like phenotype. Finally, inhibition of signal transducer and activator of transcription 3 may in part be responsible for the observed results.
Conclusions
These findings suggest potential clinical benefit for the addition of docetaxel to current immunotherapeutic protocols.
Myeloid-derived suppressor cells (MDSC) are a phenotypically heterogeneous cell population that includes mature myeloid cells, such as granulocytes, monocytes/macrophages, and dendritic cells, as well as immature myelomonocytic precursors (1, 2). Effective antitumor immunity is frequently impeded by complicating factors such as tumor-induced immune suppression via the production of MDSCs (3). A significantly increased number of MDSCs have been observed in tumor-bearing mice (4–6) as well as in patients with squamous cell carcinoma, non–small cell lung carcinoma, breast cancer, and head and neck cancer (7). Importantly, increased levels of circulating MDSCs have recently been correlated with disease stage and extensive metastatic tumor burden in patients with breast cancer (8). In the murine system, MDSCs consist of a heterogeneous population of immature cells with myeloid lineage markers Gr-1 and CD11b. These Gr-1+ CD11b+ cells accumulate in the bone marrow, blood, spleen, lymph nodes, and at the tumor site (7–9). MDSCs have been shown to accumulate in mice and patients as tumor burden increases, resulting in immune suppression, including T-cell dysfunction (4, 10–12). Furthermore, MDSCs specifically reduce antigen-specific CD8+ T-cell proliferation and increase T-cell apoptosis. Given these immunosuppressive effects, it has been proposed that elimination of these MDSCs may significantly improve antitumor responses and enhance cancer immunotherapy efficacy (13–15). MDSCs can be directly eliminated in pathologic settings by using certain chemotherapeutic drugs. Administration of one such drug, gemcitabine, in mice bearing large tumors resulted in a dramatic reduction in the number of splenic MDSCs and a marked improvement in immunotherapeutic efficacy (16). A recent study showed that sunitinib malate, a receptor tyrosine kinase inhibitor, could reverse MDSC-mediated immune suppression and modulate the tumor microenvironment, thereby improving the efficacy of immune-based therapies (17). This effect was specific to MDSCs, as significant decreases in the number of B and T cells were not observed in these animals. In another study, Serafini et al. (18) reported showing that sildenafil downregulates arginase 1 and nitric oxide synthase-2 expression, thereby reducing the suppressive machinery of CD11b+Gr-1+ MDSCs recruited by growing tumors.
A recent study suggested that tumor-infiltrating MDSCs could be classified as alternatively activated monocyte/macrophages expressing some alternative macrophage activation (M2) markers while retaining other classic activation macrophage (M1) markers (19). Tumor-infiltrating MDSC populations accordingly expressed M1 (CCR2, CCR5, CX3CR1, and CCR7) and M2 [CCL17, mannose receptor (MR), interleukin-10 (IL-10), MCA38, and GL261] markers (19). M1 macrophages are generally considered potent effector cells, which kill microorganisms as well as tumor cells, generating copious amount of proinflammatory cytokines (e.g., IL-12 and tumor necrosis factor-α) and inducible nitric oxide synthase (iNOS). In contrast, various signals, such as IL-10, IL-4, and IL-13, and the expression of scavenger debris receptor MR portend M2 functions (i.e., by fine-tuning inflammatory responses) and promote tumor angiogenesis (20, 21). Moreover, a population of circulating Gr-1+CD11b+ inflammatory monocytes has been reported in tumor-bearing mice that express IL-4 receptor α and able to release both IL-13 and IFN-γ cytokines that are comparable with a function intermediate between those of M1 and M2 macrophages (22).
A promising approach in cancer immunotherapy would be to target MDSCs to promote their differentiation so that they no longer possess suppressive activity. Recent evidence suggests that antimicrotubule agents such as docetaxel might have immune-enhancing properties against tumors (23). Although immune changes have also been recorded in patients undergoing docetaxel therapy (24), thus far, it has not been used clinically for specific inhibition of immunosuppression caused by MDSCs. We therefore set out to address if docetaxel acts on MDSCs in a mouse mammary carcinoma model.
Materials and Methods
Reagents
The following antibodies were purchased from eBiosciences: rat anti-mouse Gr-1–phycoerythrin (PE), rat anti-mouse CD11b-PerCP5.5, rat anti-mouse CD4-FITC, rat anti-mouse CD8-PerCP5.5, rat anti-mouse IFN-γ–allophycocyanin (APC), rat anti-mouse CCR7-APC, and isotype control antibodies. Anti-Ly6G and anti-Ly6C biotinylated antibodies, FITC-conjugated anti-LY6C, APC-conjugated anti-LY6C, PE-conjugated anti-Ly6G, FITC-conjugated anti-mouse CD40, PE-conjugated anti-mouse CD86, FITC-conjugated anti-mouse CD80, APC-conjugated anti-mouse CD11c, and PE-conjugated anti–mouse I Ad were purchased from BD Biosciences; rat anti-mouse MR FITC (CD206) was from AbD Serotec; and streptavidin microbeads were purchased from Miltenyi Biotec. The Mouse Inflammation Kit for Cytokine Analysis [Cytometric Bead Array (CBA)] and Annexin V Staining kit were purchased from BD Biosciences. Carboxyfluorescein diacetate succinimidyl ester (CFSE) was purchased from Invitrogen Molecular Probes. Antibodies against total and active phosphorylated forms of AKT, signal transducer and activator of transcription 1 (STAT1), and STAT3 were purchased from Cell Signaling Technology, Inc.
Mice
Six- to 8-week-old female BALB/c mice were purchased from the National Cancer Institute (Frederick, MD). The OT-II transgenic BALB/c mice were kindly provided by Dr. X.Z. Yu (Moffitt Cancer Center). All mice were kept in pathogen-free conditions in the animal facility of Moffitt Cancer Center. All experiments were done in accordance with preapproved institutional protocols within the guidelines of the Animal Care and Use Committee.
Cell lines
The mouse tumor cell line 4T1-Neu was grown in RPMI 1640 with 10% heat-inactivated fetal bovine serum with 100 units/mL penicillin, 100 μg/mL streptomycin, and 2 mmol/L glutamine.
Tumor establishment and docetaxel administration
To determine the in vivo efficacy of docetaxel (Taxotere, Sanofi-Aventis), tumors were established in BALB/c mice by injecting 4T1-Neu tumor cells (1 × 106) s.c. When tumors grew to a mean area of 8 mm2 on day 14, the mice were randomized into four groups of 10 mice per group as follows: (a) tumor bearers, (b) tumor bearers treated with docetaxel, (c) naive mice, and (d) naive mice treated with docetaxel. Two doses of docetaxel (33 mg/kg body weight; ref. 25) were given i.p. at a weekly interval at days 14 and 21. Mice weight and tumor size were measured every 2 days and recorded.
51Cr release cytotoxicity assay
A 24-hour 51Cr release assay was done as described previously (26) using 4T1-Neu tumor cells as targets and T cells, purified from spleens from three tumor bearers treated or untreated with docetaxel, as effector cells. Briefly, target tumor cells were labeled with 200 μCi of 51Cr (Amersham Corp.) in 0.2 mL of medium at 37°C in a 5% CO2 atmosphere for 1 hour. The labeled tumor cells were washed three times and added to the effector cells in triplicate wells of 96-well round-bottomed microplates at the 200:1, 100:1, and 50:1 E:T ratios. The percentage of specific 51Cr release was determined by the following equation: . All determinations were done in triplicate, and the SE of all assays was calculated and was typically 5% of the mean or less.
Splenic MDSCs
For purification of MDSCs, splenocytes pooled from three mice per group were depleted of RBCs using RBC lysing buffer and washed twice with degassed cold MACS buffer (1% bovine serum albumin in PBS with 2 mmol/L EDTA). Washed cells were resuspended at 2 × 108 cells in 1 mL of MACS buffer and incubated with 100 μL of biotinylated anti-LY6C and anti-Ly6G (Gr-1) antibody (Miltenyi Biotec) for 20 minutes at 4°C in MACS buffer. LY6C/Gr-1–labeled splenocytes (in 400 μL of MACS buffer in 50-mL tube) were then incubated at 4°C with 100 μL of streptavidin microbeads (Miltenyi Biotec) for 15 minutes followed by washing in MACS buffer. After centrifugation for 10 minutes at 290 × g, pelleted cells were resuspended in 5 mL of MACS buffer and sequentially applied to MACS column for positive selection according to the manufacturer’s instructions (Miltenyi Biotec). The resulting cells were assayed by flow cytometry and were routinely >90% Gr-1+CD11b+ cells. Purified MDSCs were then cultured 24 hours in the presence or absence of 11 nmol/L docetaxel in RPMI 1640 containing 20% fetal bovine serum and analyzed by flow cytometry.
Flow cytometry
One million cells (splenocytes or MDSCs) were incubated for 30 minutes on ice in staining medium with relevant antibodies for the surface expression analysis using standard protocols and antibodies from eBiosciences. For intracellular staining, cells were fixed and permeabilized with Cytofix/Cytoperm buffer (BD Biosciences) and washed with a 1× perm/wash solution (BD Biosciences) before incubation with relevant primary antibodies for 30 minutes. After washing, the samples were analyzed using a FACSCalibur flow cytometer (BD Biosciences) with the FlowJo software.
Apoptosis assay
MDSCs were cultured at a concentration of 1 × 106 cells per well. Cells were either untreated or treated with 11 nmol/L docetaxel for 24 hours at 37°C and analyzed for apoptosis using the Annexin V Apoptosis kit (BD Pharmingen). Next, cells were resuspended in 1× binding buffer at a concentration of 1 × 106/mL and stained for Annexin V–FITC or APC. Untreated and docetaxel-treated MDSCs were stained for M1 (CCR7) and M2 (MR) markers followed by Annexin V–FITC or APC staining.
T-cell proliferation assay
The suppressive activity of MDSCs was assessed in OVA peptide–mediated proliferation assay of T-cell receptor (TCR) transgenic H2-matched CD4+ T cells. MDSCs were isolated from spleens of tumor-bearing mice as described above. MDSCs were treated or untreated with 11 nmol/L docetaxel for 24 hours. For responder cells, we used total spleen cells from OT-II transgenic mice. CD4+ T cells from these mice have a TCR that recognizes OVA-derived peptide (OVA323–339). Splenocytes from OT-II BALB/c mice were labeled with CFSE. Briefly, the cells were suspended in serum-free RPMI 1640 and incubated with CFSE (5 μmol/L) at 37°C for 10 minutes followed by quenching with an equal volume of cold fetal bovine serum and washing three times with complete medium and then two times with cold PBS. These CFSE-labeled splenocytes from the TCR transgenic mice were cocultured with docetaxel-treated MDSCs or untreated MDSCs for 24 hours in the presence of OVA323–339 (1 μg/mL) and analyzed by flow cytometry for CD4+ T-cell proliferation in response to the peptide. MDSC alone or T cells with peptide alone served as controls.
CBA assay
Cultured supernatants from docetaxel-treated and untreated MDSCs were collected at 24 hours and analyzed for cytokines using the CBA Mouse Inflammation kit (27). The concentrations of IL-10 and IL-12 in the culture supernatants were measured by commercially available CBA Mouse Inflammation kit (BD Biosciences). Briefly, 50 μL of mixed capture beads were incubated with 50 μL of culture supernatant and 50 μL of PE detection reagent for 2 hours at room temperature. The immunocomplexes were then washed and analyzed using FACSCalibur affixed with a 488-nm laser, according to the manufacturer’s protocol.
Western blot analysis of AKT, STAT1, and STAT3
Freshly isolated MDSCs from tumor bearers were cultured in the presence or absence of 11 nmol/L docetaxel for 0, 3, and 6 hours. Cells were collected and solubilized by incubation at 4°C for 30 minutes in 1% NP40, 10 mmol/L Tris, 140 mmol/L NaCl, 0.1 mmol/L phenylmethylsulfonyl fluoride, 10 mmol/L iodoacetamide, 50 mmol/L NaF, 1 mmol/L EDTA, 1 mmol/L sodium orthovanadate, 0.25% sodium deoxycholate, 100 μL 4-(2-Aminoethyl) benzenesulfonyl fluoride hydrochloride (AEBSF), leupeptin, aprotonin (ALA), and 100 μL of phosphatase inhibitor cocktails I and II (Sigma). Whole-cell lysates were centrifuged at 12,000 × g for 10 minutes to remove nuclei and cell debris. The protein concentration of the soluble extracts was determined by using the Bradford protein assay (Bio-Rad). Separation of 20 μg of total protein was done on 10% SDS-polyacrylamide gels and transferred to a nitrocellulose membrane before immunoblotting with antibodies against total and active phosphorylated forms of AKT, STAT1, and STAT3. The specific proteins were detected by the enhanced chemiluminescence detection system.
Statistical analysis
Results are expressed as mean ± SE. The statistical significance of differences between groups was determined by the Student’s t test, and P < 0.05 was considered statistically significant. For all experiments, the graphs represent the mean of three separate experiments and the error bars represent the SE.
Results
Docetaxel reduces tumor burden and proportion of Gr-1+/CD11b+ cells in mice bearing 4T1-Neu mammary tumors
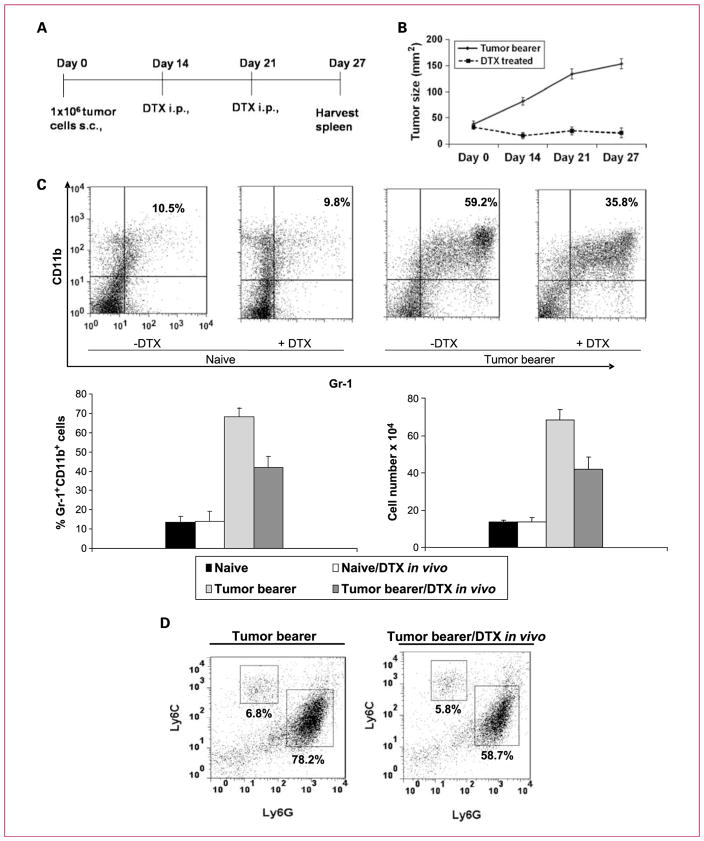
To evaluate the effect of docetaxel in our tumor model, BALB/c mice were injected s.c. with 4T1-Neu mammary tumor cells or PBS and randomly assigned to either of the study groups (n = 10): (a) tumor bearers receiving PBS, (b) tumor bearers receiving docetaxel treatment, (c) naive mice receiving PBS, and (d) naive mice receiving docetaxel. Two doses of docetaxel (33 mg/kg body weight) or PBS alone were given at weekly intervals on days 14 and 21 (Fig. 1A) after tumor inoculation when tumor size was ~8 mm2. We observed that docetaxel significantly delayed tumor progression at day 27. The untreated controls had tumors that were 153.8 ± 21.3 mm2 compared with 21.2 ± 10.2 mm2 in the docetaxel-treated group (P < 0.001; Fig. 1B).
Fig. 1.
Docetaxel treatment of BALB/c mice bearing established 4T1 mammary tumors reduces tumor growth. A, schematic representation of the docetaxel (DTX) treatment regimen. B, in vivo injection of docetaxel in tumor bearers retarded tumor growth (n = 10). C, docetaxel treatment (33 mg/kg body weight) reduced Gr-1+CD11b+ MDSCs in the spleens of mice bearing tumors. Spleen cells were stained with PE-conjugated anti–Gr-1 and APC-conjugated anti-CD11b antibodies. The percentages of double-positive Gr-1+CD11b+ cells are shown for spleens of three mice per group as follows: (a) naive mice, (b) docetaxel-treated naive mice, (c) untreated tumor bearers, and (d) docetaxel-treated tumor bearers. Columns, mean of three separate experiments; bars, SE. D, docetaxel treatment reduced granulocytic MDSCs. Monocytic MDSCs are Ly6ChiLy6Glo, whereas granulocytic MDSCs are Ly6CloLy6Ghi.
Using two-color flow cytometry, Gr-1+CD11b+ markers for MDSCs were analyzed in tumor bearers before and after docetaxel administration. On day 27, 6 days after the second treatment with docetaxel, spleens were harvested and stained for MDSCs bearing Gr-1+ and CD11b+ markers. Splenic MDSCs from naive mice, treated and untreated with docetaxel, were also analyzed. In untreated tumor-bearing mice, MDSCs made up to 59.2% of the spleen cells, whereas the docetaxel treatment reduced the proportion of Gr-1+CD11b+ cells to 35.8%, as shown in a representative experiment, and the mean ± SE of the percentage and the cell number of three identically conducted experiments reflected the same trend (Fig. 1C). Because MDSCs are phenotypically heterogeneous, containing both granulocytic and monocytic subsets, we evaluated which of these populations was affected by docetaxel treatment in tumor bearers in vivo (5). The use of Ly6C and Ly6G allows for their differentiation, and analysis of these markers indicated that docetaxel administration reduced the Ly6CloLy6Ghi granulocytic MDSC population from 78.2% to 58.7%, whereas the Ly6ChiLy6Glo monocytic MDSC population seemed to be unaffected (Fig. 1D). These data suggest that docetaxel suppresses the accumulation of granulocytic MDSCs in tumor-bearing mice.
Docetaxel inhibits the suppressive effect of MDSCs and restores the functional activity of CD4+ and CD8+ T cells
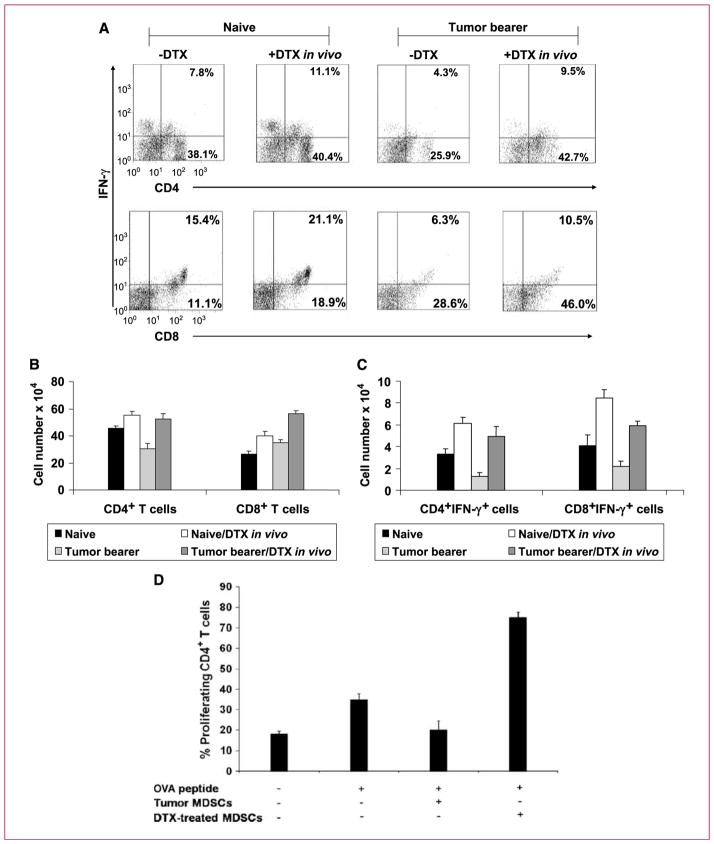
MDSCs inhibit T cells through various direct and indirect mechanisms (9, 12, 28). If docetaxel inhibits MDSCs, then T-cell function should recover in docetaxel-treated mice. To address this, we first analyzed CD4+ and CD8+ T-cell populations in the docetaxel-treated and untreated spleens of tumor-bearing mice by flow cytometry. Naive and docetaxel-treated naive mice were also analyzed as controls. In vivo administration of docetaxel enhanced the percentage of CD4+ T cells (P < 0.004) and CD8+ T cells (P < 0.002) compared with that of CD4+ T cells and CD8+ T cells in untreated tumor bearers (Fig. 2A). In an alternate analysis of total T cells per spleen, rather than percentage, Fig. 2B, which represents the mean ± SE of three experiments, shows that the same results can be obtained.
Fig. 2.
Docetaxel treatment of tumor-bearing mice upregulates CD4+ and CD8+ T cells and generates significant IFN-γ production. A, flow cytometric analysis of cells isolated from spleens of naive mice, docetaxel-treated naive mice, tumor bearers, and docetaxel-treated tumor bearers. Dual staining for surface CD4 or CD8 and intracellular IFN-γ showed that both CD4 and CD8 T cells had increased intracellular IFN-γ after docetaxel treatment. B, columns, mean cell number of CD4+ and CD8+ T cells (n = 3); bars, SE. C, columns, mean cell number of IFN-γ–producing cells (n = 3); bars, SE. D, docetaxel treatment causes MDSCs to lose their suppressive effect on CD4+ T cells and significantly increased T-cell–specific responses to antigen. Splenocytes from OT-II transgenic mice were stimulated with OVA323–329 peptide in the presence of docetaxel-treated or untreated MDSCs. In vitro proliferation of CD4+ T cells was measured by CFSE staining after 24 h. Columns, mean percentage of CD4+ T-cell–proliferating cells; bars, SE.
In an effort to determine if docetaxel treatment increased T-cell activation, we analyzed IFN-γ production as a measure of T-cell function. Spleens were immediately harvested from docetaxel-treated and untreated tumor-bearing mice and dually stained for intracellular IFN-γ and surface CD4 or CD8 to distinguish which cell might be the source of IFN-γ (Fig. 2C). Splenocytes from docetaxel-treated tumor bearers had significantly higher CD4+ T cells as well as CD8+ T cells expressing IFN-γ in comparison with untreated mice. Interestingly, this effect was observed in both naive and tumor-bearing mice treated with docetaxel. It should be noted that this effect is not due to binding to Toll-like receptor 4 (TLR4) because, although paclitaxel and docetaxel are related taxanes, docetaxel is a later product specifically designed not to bind TLR4 (29). Moreover, docetaxel in our study is a clinical-grade chemotherapeutic agent for cancer patients and has been tested to be free of bacterial lipopolysaccharide. Thus, these results indicate that docetaxel treatment can expand and augment the functional capability of CD4+ and CD8+ T cells within the spleen of tumor-bearing mice.
To investigate if enhanced T-cell function was due to loss of suppressive capacity in MDSCs, we next used an OVA antigen–specific syngeneic CD4+ T-cell model. Purified MDSCs from tumor bearers were untreated or pre-treated with docetaxel (11 nmol/L) for 24 hours. This dose of docetaxel has been defined by us to induce cytoplasmic clusterin in prostate tumor cells and mediate drug resistance (30, 31). Docetaxel-treated and untreated MDSCs were then cocultured with OVA323–339 peptide–pulsed splenocytes from OT-II transgenic mice (Fig. 2D). T-cell proliferation was then measured by CFSE staining. Our results showed that transgenic CD4+ T cells responded to OVA peptide–pulsed splenocytes by enhanced proliferation compared with CD4+ T cells without OVA. However, addition of untreated MDSCs to OVA-exposed CD4+ T cells caused the proliferative level to return to that of CD4+ T cells alone. What is most impressive is that once these same MDSCs were pretreated with docetaxel, they actually now markedly induced the proliferation of OVA-exposed CD4+ T cells, far beyond the level seen in the same cells without any MDSCs. Thus, MDSCs seemed to lose their normal immunosuppressive capacity and acquired stimulatory function.
Docetaxel induces potent tumoricidal activity in T cells
We had earlier seen a rise in CD8+ T cells with docetaxel treatment in vivo (Fig. 2A and B). To examine if docetaxel could also modulate CTL function, we evaluated docetaxel-treated and untreated spleen cells directly harvested from tumor bearers for their ability to kill 4T1-Neu tumor cells in vitro. Purified splenic T cells were cocultured with 51Cr-labeled 4T1-Neu tumor cells at an E:T ratio of 200:1, 100:1, or 50:1 for 24 hours. T cells from docetaxel-treated tumor bearers showed substantially higher cytotoxicity against 4T1-Neu tumor cells than spleen cells of untreated tumor bearers (P < 0.002; Fig. 3). These data suggest that docetaxel treatment enhanced CTL responses against the tumor cells, which may be due to the inactivation of suppressive MDSCs.
Fig. 3.
Spleen cells from docetaxel-treated tumor-bearing mice have cytotoxic effect on 4T1 tumor cells. A 24-h 51Cr release assay was done using 4T1 tumor cells as targets and T cells from tumor bearers, either treated or untreated with docetaxel in vivo. The T cells were purified using a commercial affinity column and were >95% pure. The percentage of specific 51Cr release was determined by the following equation: . All determinations were done in triplicate, and the SEM of all assays was calculated and was typically 5% of the mean or less.
Docetaxel polarizes MDSCs toward an M1-like phenotype
The effect of docetaxel on MDSCs could be due to one of two pathways. Docetaxel can either induce MDSC differentiation or have a direct effect in killing them. To explore the first premise that differentiation is affected, we searched for the presence of MI (CCR7 and iNOS) or M2 (MR) markers on MDSCs before and after docetaxel treatment in vivo. CD11b+Gr-1+ MDSCs were gated for CCR7, iNOS, and MR expression. In a representative experiment, Fig. 4A shows that MDSCs from tumor-bearing mice showed high levels of MR expression but little CCR7, and docetaxel treatment significantly reduced MR expression and raised CCR7 expression. Analysis of three sets of identical experiments confirmed that docetaxel effectively upregulated CCR7 (Fig. 4B). MDSCs from docetaxel-treated mice also had higher levels of iNOS that were also positive for CCR7 expression, whereas in untreated mice, although we observed iNOS expression, they were not positive for CCR7 expression. More significantly, docetaxel seemed to downregulate MR in tumor-bearing MDSCs that represent the M2 phenotype. Docetaxel had little effect on either CCR7, iNOS, or MR expression in naive mice (Fig. 4A and B). These findings suggest that the effect of docetaxel is specific for tumor-bearing mice and polarizes the MDSC population to an M1-like phenotype. To confirm that such differentiation is taking place, we analyzed the effect of docetaxel on MDSCs in vitro for acquisition of various macrophage cell surface markers (i.e., MHC class II I Ad, CD11c, CD40, CD86, and CD80). Purified MDSCs from tumor bearers, cultured in the presence of docetaxel (11 nmol/L) for 24 hours, were found to express higher levels of CD11c, MHC class II, and CD86, whereas CD40 or CD80 expression remained the same as in untreated MDSCs (Fig. 4C).
Fig. 4.
A, MDSCs from docetaxel-treated mice express M1 markers. MDSCs were affinity column purified from three mice per group representing naive mice, docetaxel-treated naive mice, tumor bearers, and docetaxel-treated tumor bearers. They were immunostained for CCR7 and iNOS to represent M1 markers or MR to represent a specific M2 marker before flow cytometric analysis. A, histogram representation of MR, CCR7, and iNOS levels in naive mice, docetaxel-treated naive mice, tumor bearers, and docetaxel-treated tumor bearers. B, columns, mean fluorescence intensity units of CCR7, MR, and iNOS from three independent experiments; bars, SE. C, in vivo docetaxel-treated MDSCs have increased differentiation markers. MACS column–purified MDSCs from the above treatment groups were analyzed by flow cytometry for MHC class II, CD11c, CD40, CD80, and CD85 expression. Gray filled histograms are isotype controls, dotted lines represent the docetaxel-treated MDSCs, and black solid lines represent untreated MDSCs.
Next, we analyzed whether docetaxel had a direct apoptotic effect on MDSC in vitro. Purified Gr-1+CD11b+ MDSCs from tumor bearers were cultured in the presence or absence of docetaxel (11 nmol/L) for 24 hours and then stained for CCR7, MR, and Annexin V to analyze if M1 or M2 cells were preferentially sensitive to docetaxel. Docetaxel treatment in vitro significantly increased CCR7 expression on MDSCs from 3.7% to 41.1% (Fig. 5A), similar to its effect in vivo, as shown in Fig. 4B. Additionally, there was a reduction of MR+ cells from 79.3% to 58.1% with docetaxel treatment, similar to our in vivo observations in Fig. 4B. Next, docetaxel-treated or untreated MDSCs were gated for Annexin V+ and Annexin V− cells (Fig. 5B) and subsequently analyzed for CCR7 and MR expression within the above-gated populations. After docetaxel treatment, 68.2% of MDSCs were Annexin V+. Interestingly, in the docetaxel-treated group, Annexin V+ cells expressed high levels of MR and minimal CCR7 (Fig. 5C), whereas the converse was observed in the Annexin V− population (Fig. 5D). These data suggest that docetaxel selectively induces apoptosis of Gr-1+CD11b+MR+ cells while sparing the differentiated Gr-1+CD11b+CCR7+ cells in vitro.
Fig. 5.
Docetaxel differentiates MDSC into M1-like phenotype in vitro and selectively inhibits M2-like phenotype of MDSCs. A, MACS column–purified MDSCs were assayed by flow cytometry, confirmed to be >90% Gr-1+CD11b+ cells, and further used for in vitro assays. MDSCs were cultured in the presence or absence of docetaxel at 11 nmol/L for 24 h followed by analysis for M1 (CCR7) and M2 (MR) markers. A, flow cytometric analysis of the percentage of CCR7- and MR-expressing MDSCs before and after docetaxel culture. B, flow cytometric analysis of Annexin V+ MDSCs before and after docetaxel culture. C and D, percentage of Annexin V+ and Annexin V− cells in CCR7+ or MR+ MDSCs. Docetaxel induced apoptosis on MDSCs, and the cells that were differentiated into M1-like phenotype were negative for Annexin V but positive for CCR7. E, cytokine analysis of supernatants of MDSCs cultured in the presence or absence of docetaxel for 24 h. Docetaxel-treated MDSCs enhance IL-12 and suppress IL-10 production.
To further characterize the effect of docetaxel in modulating the MDSC population, we analyzed cytokine secretion in supernatants of MDSCs cultured with docetaxel. We found that MDSCs treated with docetaxel had significant upregulation of IL-12 (P < 0.0003) but downregulation of IL-10 production (P < 0.007) compared with untreated MDSCs. Taken together, our results suggest that docetaxel polarizes MDSCs to the M1-like phenotype, in turn downregulating IL-10 production and increasing IL-12 production.
Docetaxel inhibits MDSCs via STAT3 signaling
To investigate possible molecular mechanisms in docetaxel-mediated MDSC suppression, we examined the expression of STAT3, which is constitutively activated in MDSCs (32). Analysis of STAT1 and AKT was included for comparison. Western blot analysis of phosphorylated STAT3 (pSTAT3) in MDSCs isolated from tumor-bearing mice confirmed that basal levels of STAT3 activity were elevated. Although docetaxel had no effect on total STAT3 protein levels in MDSCs, inhibition of pSTAT3 was observed as early as 6 hours after docetaxel treatment (Fig. 6A). However, docetaxel did not have notable inhibitory effects on pAKT and pSTAT1 or their total proteins. In an effort to determine if docetaxel acts through STAT3 to inhibit MDSCs, we cultured these cells in the presence or absence of JSI 124, a specific STAT3 inhibitor, at a concentration of 0.125 μmol/L for 24 hours. As a control, these cells were monitored for STAT3 activation. We confirmed that JSI 124 efficiently suppressed STAT3 phosphorylation as early as 6 hours, and this effect was specific because STAT1 and AKT phosphorylation was unimpeded (Fig. 6B). These cells were further analyzed for the M1 (CCR7) and M2 (MR) markers as well as Annexin V. Here, we observed a similar effect to docetaxel in the JSI 124–treated MDSCs, including polarization to an M1-like phenotype by upregulation of CCR7, downregulation of MR, and selective induction of apoptosis in MR+ MDSCs (Fig. 6C and D). We further analyzed cytokine secretion in supernatants of MDSCs cultured with JSI 124 (Fig. 6E). We found that MDSCs treated with JSI 124 had significant upregulation of IL-12 (P < 0.001) and downregulation of IL-10 production (P < 0.003).
Fig. 6.
Docetaxel selectively inhibits MDSCs via STAT3 signaling. A and B, lysates from MACS column–purified MDSCs were immunoblotted (IB) for both phosphorylated and total AKT, STAT1, and STAT3. MDSCs were treated or untreated with docetaxel (11 nmol/L) or JSI 124 (0.125 μmol/L) for 0, 3, and 6 h before analysis of phosphoproteins. C and D, MDSCs, treated or untreated with JSI 124, were immunostained for CCR7 and MR markers followed by Annexin V staining. Columns, mean fluorescence intensity of the Annexin V+ and Annexin V− CCR7- and MR-expressing cells. E, cytokine analysis of supernatants of MDSCs cultured in the presence or absence of JSI 124 for 24 h. JSI 124–treated MDSCs enhanced IL-12 and suppressed IL-10 production.
Discussion
Docetaxel is a known antimitotic chemotherapeutic reagent, which has shown direct cytotoxicity in prior studies (33). Previous studies have evaluated the effect of taxanes (paclitaxel and docetaxel) for their ability in altering important immunologic parameters in breast cancer patients. However, the effects of docetaxel in breast cancer patients were more pronounced than those of paclitaxel (24) Here, we confirm the antitumor properties of docetaxel shown by the abrogation of tumor progression in a murine mammary carcinoma model. Furthermore, the classic cytotoxic property of docetaxel extended to MDSCs with an increase in apoptosis in vitro and a general decrease in splenic MDSCs in vivo. Moreover, because a significant MDSC population persisted in vivo, we sought to determine any modulations therein.
Similar to the system evolved after the Th1/Th2 concept, it was proposed that macrophages can be divided into classically activated M1 and alternatively activated M2 macrophages (34, 35). M1 macrophages are activated/differentiated by the Th1 cytokine IFN-γ and have tumor cytotoxicity, whereas M2 macrophages are activated/differentiated by Th2 cytokines such as IL-10 and favor tumor progression (21). Splenic leukocytes from docetaxel-treated tumor bearers stained for higher levels of the Th1 cytokines, IFN-γ and IL-12, and lower levels of the Th2 cytokine, IL-10, compared with untreated tumor bearers. This observed shift in polarization from Th2 to Th1 led us to investigate whether the in vivo cytokine milieu was sufficient to influence MDSC phenotype and function.
A recent study has shown that tumor-infiltrating MDSCs are pleiotropic inflammatory monocytes/macrophages that bear M1 and M2 characteristics (19). M1 and M2 macrophages can be differentiated based on surface protein expression and cytokine production, as reviewed elsewhere (36). Furthermore, iNOS is upregulated in response to inflammatory stimuli, as macrophages shift toward the M1 phenotype (24). Several studies have shown that macrophages are a heterogeneous population of cells whose phenotypic characteristics and functions are determined by the cytokine milieu in which they reside. M1 macrophages produce NO and reduce tumor growth, whereas M2 macrophages produce arginase and facilitate tumor progression (37).
We show a novel finding here that docetaxel polarizes MDSCs to an M1-like phenotype both in an in vitro and in an in vivo model. This observation is evidenced by an increase in the expression of classic M1 markers CCR7 and iNOS, and also the acquisition of higher levels of CD11c, MHC class II, and CD86 expression with a concomitant decrease in expression of the M2-associated receptor MR. Functionally, docetaxel selectively induces apoptosis of Gr-1+CD11b+MR+ cells while sparing the differentiated Gr-1+CD11b+CCR7+ cells in vitro, shifting them from predominantly secreting IL-10 to IL-12. It is plausible that docetaxel polarizes MDSCs to Gr-1+CD11b+CCR7+ MRlow cells, which in turn either directly or indirectly contributes to tumoricidal activity similar to classic M1 macrophages. Polarization of MDSCs to an M1-like phenotype may be an important component of immune surveillance against 4T1 mammary tumors, leading to enhanced antitumor immunity.
It is not clear whether all the MDSCs in a population respond similarly (and synchronously) to polarizing conditions such as T-cell–mediated activation or whether MDSC populations are heterogeneous, with disparate populations committed to M1 and M2 phenotypes. Alternatively, some plasticity might exist. For example, MDSCs might be able to alternate between M1 and M2 phenotypes depending on the stimulatory molecules they interact with (22). To examine this, we compared Annexin V+ and Annexin V− MDSC populations after docetaxel treatment. Interestingly, most Annexin V+ cells were positive for MR, whereas viable cells predominantly expressed CCR7. Because the parental, untreated MDSC population was not 100% MR positive, the findings suggest that M2-like MDSCs were more sensitive to the cytotoxic effects of docetaxel, whereas the undifferentiated MDSC phenotype was modulated by docetaxel toward M1-like.
To determine a possible molecular mechanism by which docetaxel inhibits MDSC function, we examined several major downstream signaling molecules such as AKT, STAT1, and STAT3. MDSCs from tumor-bearing mice have markedly increased levels of pSTAT3, arguably the main transcription factor that regulates the expansion of MDSCs (1, 32). Supporting this premise, recent studies have shown that selective STAT3 inhibitors strikingly reduced the expansion of MDSCs, resulting in suppressed regulatory T cells and increased dendritic cell and CD8+ T-cell responses in tumor-bearing mice (38–40). STAT3 is required for biological functions such as gene transcription of IL-10, and therefore, its inhibition results in restored expression of proinflammatory mediators, including IL-12 and tumor necrosis factor-α (40), by infiltrating leukocytes, leading to antitumor immunity. Moreover, members of the STAT family transcription factors play a pivotal role in polarization of myeloid cell functions, as well as in tumor progression and the altered immune response to cancer. In particular, STAT3 has been shown to be selectively activated by M2 macrophage–polarizing cytokines such as IL-10, IL-4, and IL-13 (21).
In this study, we found that docetaxel treatment results in diminished pSTAT3 in MDSCs; however, docetaxel did not have any notable inhibitory effects on AKT or STAT1 phosphorylation. Interestingly, when we treated MDSCs with a specific STAT3 inhibitor, JSI 124, we observed effects similar to docetaxel treatment. Of note, JSI 124–treated MDSCs were polarized to an M1-like phenotype, showing elevated expression of CCR7. Furthermore, JSI 124 selectively induced apoptosis in MR-expressing MDSCs. These results suggest that the immunomodulatory effect of docetaxel is mediated by STAT3 inhibition in MDSCs, leading to their selective differentiation of MDSCs into M1-like phenotype.
These studies show the novel finding that docetaxel directly modulates MDSC signaling and therefore phenotype and function (i.e., cytokine) in vitro. Moreover, as evidenced by the MDSC splenic leukocyte coculture, MDSCs may be able to influence polarization of T cells as well. The in vivo circumstances may be more complex, but it can be inferred that docetaxel can directly induce an M1-like polarization of MDSCs and contribute to an overall shift toward M1/Th1 antitumor responses. However, it does not rule out the possibility of paracrine contribution of T cells and other leukocytes. Until now, in vivo docetaxel treatment is thought to reduce tumor burden through direct cytotoxicity (41); however, in this study, we show an indirect immunomodulatory mechanism as well. As previously mentioned, MDSC accumulation has been correlated directly with tumor burden (8). Thus, there may be synergism between multiple mechanisms of action of docetaxel (i.e., direct cytotoxicity to tumors and a specific subset of MDSCs and indirect antitumor responses via immunomodulation). Our findings provide a mechanistic explanation for the observed in vivo enhancement of T-cell function by others (23). Further studies are warranted to dissect the relative contributions of these components, which may lead to a better understanding of tumor-mediated immune suppression and to the development of more efficacious immunomodulatory strategies. Moreover, this study supports the novel paradigm of studying MDSC biology in relation to M1- and M2-like subpopulations being discrete population and tending away from plasticity of committed M2-like MDSCs.
Translational Relevance.
A promising approach in cancer immunotherapy is targeting/inhibiting myeloid-derived suppressor cell (MDSC) to promote differentiation to disable their ability to possess suppressive activity. This study is specifically designed to target the MDSC population to improve the antitumor immune responses through immunomodulation, therefore enhancing the effect of docetaxel. Although docetaxel is a standard antimitotic agent in cancer therapy, with established treatment modality, we present here the preclinical results of docetaxel in reducing MDSC recruitment. We also elucidate in a mouse model that interference with the molecular pathways of MDSC differentiation/expansion or blocking their suppressive functions may multifacet into a more effective approach in therapy of cancer.
Acknowledgments
Grant Support
This research was funded in part by the Garcia Endowed Chair (J.Y. Djeu) and facilitated by the Flow Cytometry and Mouse Models Core facilities at the Moffitt Cancer Center.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–74. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kusmartsev S, Gabrilovich DI. Role of immature myeloid cells in mechanisms of immune evasion in cancer. Cancer Immunol Immunother. 2006;55:237–45. doi: 10.1007/s00262-005-0048-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Serafini P, De Santo C, Marigo I, et al. Derangement of immune responses by myeloid suppressor cells. Cancer Immunol Immunother. 2004;53:64–72. doi: 10.1007/s00262-003-0443-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Terabe M, Matsui S, Park JM, et al. Transforming growth factor-β production and myeloid cells are an effector mechanism through which CD1d-restricted T cells block cytotoxic T lymphocyte-mediated tumor immunosurveillance: abrogation prevents tumor recurrence. J Exp Med. 2003;198:1741–52. doi: 10.1084/jem.20022227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181:5791–802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Young MR, Newby M, Wepsic HT. Hematopoiesis and suppressor bone marrow cells in mice bearing large metastatic Lewis lung carcinoma tumors. Cancer Res. 1987;47:100–5. [PubMed] [Google Scholar]
- 7.Almand B, Clark JI, Nikitina E, et al. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol. 2001;166:678–89. doi: 10.4049/jimmunol.166.1.678. [DOI] [PubMed] [Google Scholar]
- 8.Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother. 2009;58:49–59. doi: 10.1007/s00262-008-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bunt SK, Yang L, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Reduced inflammation in the tumor microenvironment delays the accumulation of myeloid-derived suppressor cells and limits tumor progression. Cancer Res. 2007;67:10019–26. doi: 10.1158/0008-5472.CAN-07-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kusmartsev SA, Li Y, Chen SH. Gr-1+ myeloid cells derived from tumor-bearing mice inhibit primary T cell activation induced through CD3/CD28 costimulation. J Immunol. 2000;165:779–85. doi: 10.4049/jimmunol.165.2.779. [DOI] [PubMed] [Google Scholar]
- 11.Mazzoni A, Bronte V, Visintin A, et al. Myeloid suppressor lines inhibit T cell responses by an NO-dependent mechanism. J Immunol. 2002;168:689–95. doi: 10.4049/jimmunol.168.2.689. [DOI] [PubMed] [Google Scholar]
- 12.Sinha P, Okoro C, Foell D, Freeze HH, Ostrand-Rosenberg S, Srikrishna G. Proinflammatory S100 proteins regulate the accumulation of myeloid-derived suppressor cells. J Immunol. 2008;181:4666–75. doi: 10.4049/jimmunol.181.7.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chalmin F, Ladoire S, Mignot G, et al. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J Clin Invest. 120:457–71. doi: 10.1172/JCI40483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kusmartsev S, Cheng F, Yu B, et al. All-trans-retinoic acid eliminates immature myeloid cells from tumor-bearing mice and improves the effect of vaccination. Cancer Res. 2003;63:4441–9. [PubMed] [Google Scholar]
- 15.Kusmartsev S, Nefedova Y, Yoder D, Gabrilovich DI. Antigen-specific inhibition of CD8+ T cell response by immature myeloid cells in cancer is mediated by reactive oxygen species. J Immunol. 2004;172:989–99. doi: 10.4049/jimmunol.172.2.989. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res. 2005;11:6713–21. doi: 10.1158/1078-0432.CCR-05-0883. [DOI] [PubMed] [Google Scholar]
- 17.Ozao-Choy J, Ma G, Kao J, et al. The novel role of tyrosine kinase inhibitor in the reversal of immune suppression and modulation of tumor microenvironment for immune-based cancer therapies. Cancer Res. 2009;69:2514–22. doi: 10.1158/0008-5472.CAN-08-4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Serafini P, Meckel K, Kelso M, et al. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med. 2006;203:2691–702. doi: 10.1084/jem.20061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Umemura N, Saio M, Suwa T, et al. Tumor-infiltrating myeloid-derived suppressor cells are pleiotropic-inflamed monocytes/macrophages that bear M1- and M2-type characteristics. J Leukoc Biol. 2008;83:1136–44. doi: 10.1189/jlb.0907611. [DOI] [PubMed] [Google Scholar]
- 20.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–86. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 21.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–55. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 22.Sica A, Bronte V. Altered macrophage differentiation and immune dysfunction in tumor development. J Clin Invest. 2007;117:1155–66. doi: 10.1172/JCI31422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garnett CT, Schlom J, Hodge JW. Combination of docetaxel and recombinant vaccine enhances T-cell responses and antitumor activity: effects of docetaxel on immune enhancement. Clin Cancer Res. 2008;14:3536–44. doi: 10.1158/1078-0432.CCR-07-4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsavaris N, Kosmas C, Vadiaka M, Kanelopoulos P, Boulamatsis D. Immune changes in patients with advanced breast cancer undergoing chemotherapy with taxanes. Br J Cancer. 2002;87:21–7. doi: 10.1038/sj.bjc.6600347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vicari AP, Luu R, Zhang N, et al. Paclitaxel reduces regulatory T cell numbers and inhibitory function and enhances the anti-tumor effects of the TLR9 agonist PF-3512676 in the mouse. Cancer Immunol Immunother. 2009;58:615–28. doi: 10.1007/s00262-008-0586-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei S, Gamero AM, Liu JH, et al. Control of lytic function by mitogen-activated protein kinase/extracellular regulatory kinase 2 (ERK2) in a human natural killer cell line: identification of perforin and granzyme B mobilization by functional ERK2. J Exp Med. 1998;187:1753–65. doi: 10.1084/jem.187.11.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richens JL, Urbanowicz RA, Metcalf R, Corne J, O’Shea P, Fairclough L. Quantitative validation and comparison of multiplex cytokine kits. J Biomol Screen. 15:562–8. doi: 10.1177/1087057110362099. [DOI] [PubMed] [Google Scholar]
- 28.Gallina G, Dolcetti L, Serafini P, et al. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J Clin Invest. 2006;116:2777–90. doi: 10.1172/JCI28828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burkhart CA, Berman JW, Swindell CS, Horwitz SB. Relationship between the structure of taxol and other taxanes on induction of tumor necrosis factor-α gene expression and cytotoxicity. Cancer Res. 1994;54:5779–82. [PubMed] [Google Scholar]
- 30.Patterson SG, Wei S, Chen X, et al. Novel role of Stat1 in the development of docetaxel resistance in prostate tumor cells. Oncogene. 2006;25:6113–22. doi: 10.1038/sj.onc.1209632. [DOI] [PubMed] [Google Scholar]
- 31.Zhong B, Sallman DA, Gilvary DL, et al. Induction of clusterin by AKT-role in cytoprotection against docetaxel in prostate tumor cells. Mol Cancer Ther. 2010;9:1831–41. doi: 10.1158/1535-7163.MCT-09-0880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nefedova Y, Nagaraj S, Rosenbauer A, Muro-Cacho C, Sebti SM, Gabrilovich DI. Regulation of dendritic cell differentiation and anti-tumor immune response in cancer by pharmacologic-selective inhibition of the janus-activated kinase 2/signal transducers and activators of transcription 3 pathway. Cancer Res. 2005;65:9525–35. doi: 10.1158/0008-5472.CAN-05-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schiff PB, Horwitz SB. Taxol stabilizes microtubules in mouse fibroblast cells. Proc Natl Acad Sci U S A. 1980;77:1561–5. doi: 10.1073/pnas.77.3.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mantovani A. From phagocyte diversity and activation to probiotics: back to Metchnikoff. Eur J Immunol. 2008;38:3269–73. doi: 10.1002/eji.200838918. [DOI] [PubMed] [Google Scholar]
- 35.Mantovani A, Sica A, Locati M. New vistas on macrophage differentiation and activation. Eur J Immunol. 2007;37:14–6. doi: 10.1002/eji.200636910. [DOI] [PubMed] [Google Scholar]
- 36.Mantovani A, Sica A, Allavena P, Garlanda C, Locati M. Tumor-associated macrophages and the related myeloid-derived suppressor cells as a paradigm of the diversity of macrophage activation. Hum Immunol. 2009;70:325–30. doi: 10.1016/j.humimm.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 37.Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164:6166–73. doi: 10.4049/jimmunol.164.12.6166. [DOI] [PubMed] [Google Scholar]
- 38.Kortylewski M, Kujawski M, Wang T, et al. Inhibiting Stat3 signaling in the hematopoietic system elicits multicomponent antitumor immunity. Nat Med. 2005;11:1314–21. doi: 10.1038/nm1325. [DOI] [PubMed] [Google Scholar]
- 39.Nefedova Y, Huang M, Kusmartsev S, et al. Hyperactivation of STAT3 is involved in abnormal differentiation of dendritic cells in cancer. J Immunol. 2004;172:464–74. doi: 10.4049/jimmunol.172.1.464. [DOI] [PubMed] [Google Scholar]
- 40.Ugel S, Delpozzo F, Desantis G, et al. Therapeutic targeting of myeloid-derived suppressor cells. Curr Opin Pharmacol. 2009;9:470–81. doi: 10.1016/j.coph.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 41.Yoo GH, Subramanian G, Boinpally RR, et al. An in vivo evaluation of docetaxel delivered intratumorally in head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2005;131:418–29. doi: 10.1001/archotol.131.5.418. [DOI] [PubMed] [Google Scholar]