Abstract
Clusterin (CLU), in its cytoplasmic form, is abundant in many advanced cancers and has been established to be cytoprotective against chemotherapeutic agents including docetaxel. However, little is known of the mechanism of its induction. Here, we provide evidence that AKT plays a critical role in upregulating cytoplasmic/secretory sCLU, which is responsible for docetaxel resistance. Western blot analysis indicated that docetaxel-resistant sublines derived from DU145 and PC3 prostate tumor cell lines displayed a markedly increased phospho-AKT level closely accompanied by heightened sCLU expression when compared with parental cells. To examine if AKT has a role in sCLU expression, AKT blockade was done by treatment with a specific inhibitor, API-2, or dominant-negative AKT transduction before analysis of sCLU gene expression. Loss of AKT function resulted in loss of sCLU and was accompanied by chemosensitization to docetaxel and increased cell death via a caspase-3–dependent pathway. To confirm that AKT affected resistance to docetaxel through sCLU and not through other mediators, tumor cells were first transfected with full-length CLU for overexpression and then treated with the AKT inhibitor API-2. We found that once sCLU was overexpressed, API-2 could not chemosensitize the tumor cells to docetaxel. Thus, the chemoresistance to docetaxel is mediated by sCLU and it can be induced by AKT. Lastly, AKT was found to mediate sCLU induction via signal transducer and activator of transcription 1 activation, which we have earlier shown to drive sCLU gene expression. These results identify a previously unrecognized pathway linking AKT to cytoprotection by sCLU in tumor cells.
Introduction
Prostate cancer is the most common malignancy in men and complete cure is often impaired by resistance to docetaxel, which constitutes the current standard of care for castration-resistant prostate cancer (1, 2). Understanding the molecular basis for docetaxel resistance is pivotal for the identification of new therapeutic targets to achieve cure. In prostate cancer, of the changes associated with advanced tumor development and taxane resistance, clusterin (CLU) overexpression is one of the most prominent (3, 4). Recent data have shown that the CLU gene gives rise to at least two protein forms: a secreted heterodimeric isoform (sCLU) and an alternatively spliced isoform that mainly localizes in the nucleus (nCLU). Although mature sCLU is processed through the endoplasmic reticulum–Golgi secretory pathway, new evidence suggests that it may evade secretion and localize to the mitochondria (5) and cytosol (6, 7) as a cytoplasmic product. It is this cytoplasmic sCLU that is consistently reported to be associated with chemoresistance and it is present in a wide range of advanced cancers as shown in human tumor biopsies from prostate (8, 9), renal (10), breast (11, 12), ovarian (13), colon (14), lung (15), pancreas (16), cervical (17), melanoma (18), glioma (19), and anaplastic large cell lymphoma (20). Experimentally, sCLU overexpression in androgen-dependent prostate cancer cells has been shown to render them resistant to androgen starvation, radiation, and paclitaxel treatment (21-23). Moreover, antisense oligonucleotides or siRNA specific for CLU can resensitize resistant prostate tumor cells to docetaxel (24, 25). This effect was seen in both docetaxel-resistant (DR) DU145 and PC3 androgen-independent prostate tumor cells (24). Thus, sCLU can protect against both taxane compounds paclitaxel and docetaxel. Interestingly, sCLU also controls resistance to tumor necrosis factor (TNF)–related apoptosis-inducing ligand (TRAIL; ref. 26) and other chemotherapeutic agents, such as doxorubicin, camptothecin, cisplatin, 5-fluorouracil, dacarbazine, and etoposide, thus suggesting its central role in drug resistance (5, 18, 27-30). However, the molecular processes involved in CLU expression in tumor cells remain unclear. Some studies have focused on CLU gene induction, and its promoter region has revealed the participation of several transcription regulators, including Egr-1, API, heat shock factor 1/2, b-MYB, and c-MYC (4). In addition, it seems that signal transducer and activator of transcription 1 (STAT1), but not STAT3, is required for CLU gene expression in tumor cells (24). However, the signal processes up-stream of transcription factors involved in CLU induction are not well delineated. Because of consistent reports that AKT is highly activated in advancing prostate cancer (31) and other unrelated reports that CLU correlates with docetaxel resistance in prostate cancer (3, 4), we focused on whether AKT might be possibly linked to CLU expression. The present study thus sets out to analyze whether AKT is involved in sCLU gene expression and if this pathway is linked to docetaxel resistance in prostate tumor cells. In addition, because of our earlier report of STAT1 requirement for CLU induction (24), we examined if AKT could influence STAT1 function.
Materials and Methods
Antibodies and reagents
Mouse monoclonal antibodies to phospho-AKT (pAKT; Ser473), pAKT (Thr308), AKT, phospho–glycogen synthase kinase-3β (GSK-3β), GSK-3β, phospho-STAT1 (pSTAT1), and STAT1 were from Cell Signaling Technology. Mouse monoclonal anti-human CLU was from Up-state Biotechnology. Monoclonal anti–β-actin was from Sigma. The AKT specificity of the compound, API-2, was identified by Dr. Jin Cheng (H. Lee Moffitt Cancer Center, Tampa, FL; ref. 32), and he generously provided this reagent, together with constitutively active AKT (CA-AKT) and dominant-negative AKT (DN-AKT), which had triple mutations, including the threonine and serine sites required for AKT function (33).
Cell culture and selection of DR clones
Androgen-independent DU145 and PC3 prostate tumor cell lines (American Type Culture Collection) were maintained in RPMI 1640 containing 10% heat-inactivated fetal bovine serum with 100 units/mL penicillin and 100 μg/mL streptomycin. DR cell lines of DU145 and PC3 were developed as previously described (24). Briefly, the cells surviving initial culture in 1 nmol/L docetaxel were passaged four times before increase of docetaxel to 5.5 nmol/L and subsequently to 11 nmol/L in the culture medium. DU145 cells maintained continuously in 11 nmol/L docetaxel were labeled DU145-DR. PC3-DR tumor cells were significantly more resistant to docetaxel and were routinely maintained in 55 nmol/L docetaxel. CWR22Rv1 cells, a castration-resistant prostate tumor cell line that expresses the androgen receptor (34), were kindly provided by Dr. Carlos Casiano (Loma Linda University, Loma Linda, CA) and maintained in RPMI 1640 containing 10% heat-inactivated fetal bovine serum with 100 units/mL penicillin and 100 μg/mL streptomycin.
Western blotting analysis
Tumor cells, seeded at 5 × 105 per well in a six-well plate, were untreated or treated with DMSO and 10, 20, or 40 μmol/L API-2 for 2 hours at 37°C. They were subsequently cultured for 48 hours at 37°C in 11 and 55 nmol/L docetaxel-containing medium for DU145-DR and PC3-DR tumor cells, respectively. Cells were then solubilized by incubation at 4°C for 30 minutes in 1% NP40, 10 mmol/L Tris, 140 mmol/L NaCl, 0.1 mmol/L phenylmethylsulfonyl fluoride, 10 mmol/L iodoacetamide, 50 mmol/L NaF, 1 mmol/L EDTA, 1 mmol/L sodium orthovanadate, 0.25% sodium deoxycholate, 100 μL aminopeptidase, leupeptin, aprotinin, and 100 μL of phosphatase inhibitor cocktails I and II (Sigma). Whole-cell lysates were centrifuged at 12,000 × g for 10 minutes to remove nuclei and cell debris. The protein concentration of the soluble extracts was determined by using the Bradford protein assay (Bio-Rad). Separation of 50 μg of total protein was done on 10% SDS-polyacrylamide gels and transferred to a nitrocellulose membrane before immunoblotting with primary antibodies specific for pAKT, AKT, or CLU. Equal loading controls were done by blotting with anti–β-actin.
DN-AKT, CA-AKT, or sCLU plasmid transfection
Transfection of DN-AKT (33) into DU145-DR or PC3-DR cells was done using the standard Lipofectamine protocol (Invitrogen–Life Technologies, Inc.). Briefly, 5 × 105cells were plated into each well of a six-well plate 24 hours before transfection with 4 μg of DN-AKT plasmid or PCDNA3 control plasmid. After culture for an additional 48 hours in 11 and 55 nmol/L docetaxel-containing medium for DU145-DR and PC3-DR, respectively, the transfected cells were washed twice with PBS. Cell lysates were prepared and loaded onto lanes of a 10% SDS-polyacrylamide gel. Effectiveness of DN-AKT transfection was first evaluated by immunoblotting for total AKT and pAKT. To ensure functional deletion of AKT, which normally directly phosphorylates GSK-3β, aliquots of the same cell lysates were also run in parallel and immunoblotted for pGSK-3β and total GSK-3β.
Transfection of CA-AKT (33) into parental DU145 cells was done using the same protocol as above. After effective transfection was checked by immunoblotting with anti-AKT, the lysates were probed for pAKT. Parallel aliquots were blotted for pSTAT1 and total STAT1, as well as CLU, to examine the effect of CA-AKT on STAT1 activation and sCLU induction.
Transfection of myc-tagged sCLU (35) or its control vector, PCDNA3, into DU145-DR tumor cells was done using the same Lipofectamine protocol. Transfected cells were lysed and probed with anti-myc as well as anti-CLU to visualize efficient transfection. The lysates were then analyzed by Western blotting for the presence of pAKT and total AKT. Equal loading for all Western blot experiments was monitored by β-actin.
Apoptosis assays
DU145-DR or PC3-DR tumor cells, seeded overnight in a six-well plate at 5 × 105 per well, were exposed to 10 to 40 μmol/L API-2 (or DMSO as control) for 2 hours at 37°C and further cultured in 11 or 55 nmol/L docetaxel-containing medium for 48 hours at 37°C. The cells were then analyzed by flow cytometry using the Annexin V-PI Apoptosis kit (BD Pharmingen). Each well was trypsinized and resuspended in 1× binding buffer at a concentration of 5 × 105 cells/mL and stained with Annexin-FITC and propidium iodide (PI).
To determine caspase-3 activation, 5 × 105 tumor cells seeded in each well of a six-well plate were treated with DMSO or 40 μmol/L API-2, with or without 50 μmol/L of the broad range caspase inhibitor zVAD.fmk (EMD Biosciences) or a caspase-1–specific inhibitor, zVAD.amc (Bachem). In addition, instead of API-2 treatment to suppress AKT function, DN-AKT–transfected tumor cells were also used and PCDNA3-transfected tumor cells served as the vector control. These transfected cells were treated with or without zVAD.fmk or zVAD.amc. The various groups of cells were then cultured 48 hours at 37°C in 11 or 55 nmol/L docetaxel, after which the cells were trypsinized and assayed by flow cytometry for active caspase-3 (Active Caspase-3 FITC MAb Apoptosis kit, BD Pharmingen).
Methylene blue staining
After AKT inhibition, via either API-2 treatment or DN-AKT expression, the cells were further cultured in docetaxel-containing medium for 72 hours at 37°C. To assess cell growth and survival, the medium was discarded before the cells were fixed with methanol for 3 minutes at room temperature, followed by staining with methylene blue for 2 minutes. The stained cells were washed twice with deionized water and allowed to dry overnight. Images were observed with a Leitz Orthoplan 2 microscope (Photometrics Ltd.), and pictures were captured by a charge-coupled device camera with the Smart Capture Program (Vysis).
Isolation of RNA and reverse transcription-PCR
Reverse transcription-PCR (RT-PCR) was used to determine mRNA expression for sCLU in DU145-DR and PC3-DR cells. Briefly, total RNA was prepared using Trizol reagent (Invitrogen). A total amount of 1 μg RNA was converted to cDNA by Omniscript reverse transcriptase in a solution containing random hexanucleotide, deoxynucleotide triphosphate, RNase inhibitor, and RT buffer (Qiagen). Aliquots of 1 μL DNA resulting from each RT reaction were then subjected to PCR. The temperature profiles of PCR were as follows: an initial denaturation step of 94°C for 5 minutes, followed by 25 cycles of 94°C for 15 seconds, 50°C for 15 seconds, 72°C for 30 seconds, and a final elongation step of 72°C for 7 minutes. PCR was done in reactions containing Taq DNA polymerase, deoxynucleotide triphosphate PCR buffer, and the sCLU primer (sense, 5′-CTTGATGCCCTTCTCTCCG-TA-3′; antisense, 5′-AACGTCCGAGTCAGAAGTGTG-3′). As a control, expression of human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA expression was evaluated using sense primer (5′-CAAAAGGGTCATCATCTCTGC-3′) and antisense primer (5′-GAGGGGCCATCCACAGTCTTC-3′). RT-PCR products were analyzed by agarose gel electrophoresis.
Results
Increased activated AKT and sCLU levels in DR prostate tumor cells
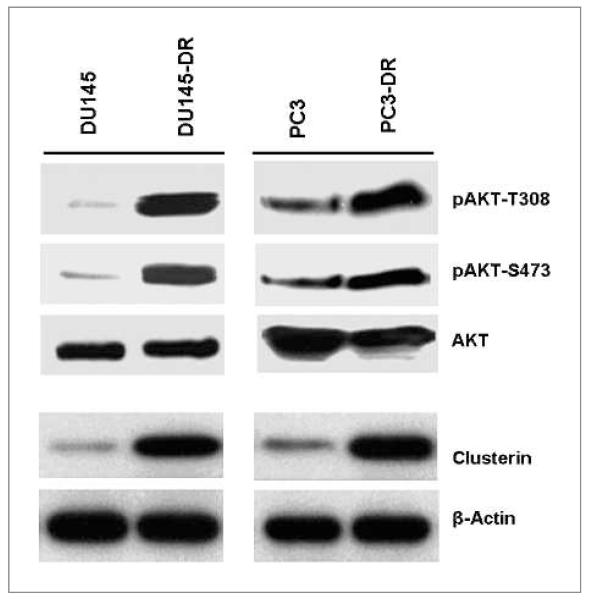
We first set out to examine if AKT levels were different in parental and DR paired prostate tumor cell lines that we had developed (24). Thus, DU145 and PC3 and their matched drug-resistant sublines were lysed and analyzed for the presence of both total and active pAKT. Western blot analysis showed that levels of AKT phosphorylated at amino acids T308 and S473 were strongly expressed in the DU145-DR and PC3-DR cell lines as opposed to the minimal expression in the respective parental lines, whereas levels of total AKT remained constant (Fig. 1). The expression of the sCLU isoform was similar to pAKT levels, being low or undetectable in parental cell lines and rising substantially in the drug-resistant cell lines. Parental PC3 cells already have moderate levels of pAKT because of a PTEN mutation in these cells (36) and likewise have a slightly higher background level of sCLU.
Figure 1.
AKT activation accompanies docetaxel resistance in DU145 and PC3 human prostate tumor cells. Paired parental and DR DU145 and PC3 prostate tumor cells were lysed and analyzed by Western blotting with specific antibodies against CLU. Activated AKT was evaluated using antibodies directed against the phosphorylation site at Thr308 or Ser473. Equal loading was monitored by β-actin levels. Immunoblot analysis of pAKT and sCLU indicated that active AKT and sCLU were increased in DU145-DR and PC3-DR cells compared with their matched parental cells.
Inhibition of AKT function causes a reduction in sCLU expression and enhances chemosensitivity to docetaxel
To investigate if AKT phosphorylation was linked to the upregulation of sCLU seen in our drug-resistant cell lines, DU145-DR and PC3-DR tumor cells were treated for 2 hours with increasing concentrations of a recently identified specific AKT inhibitor, API-2, from 10 to 40 μmol/L (32) and then evaluated for the presence of sCLU after a further 48-hour incubation in docetaxel medium. Western blot analysis confirmed that API-2 treatment was highly efficient in suppressing AKT phosphorylation, as depicted by pAKT-T308 and pAKT-S473 (Fig. 2A). A dose-dependent loss of pAKT was obtained with increasing concentrations of API-2. Most importantly, analysis of the same cells showed that API-2 treatment also downregulated sCLU expression in a dose-dependent manner. Because reports about the heterogeneity of prostate cancer have shown that up to 60% of hormone-refractory prostate cancers still express the androgen receptor (37), we repeated the above experiment in CWR22Rv1 cells, which have androgen receptor expression but are an androgen-independent prostate tumor cell line (34). CWR22Rv1 cells have a robust expression of sCLU (Fig. 2B), which correlates with previous studies that have shown sCLU to play a critical role in androgen independence (22). Additionally, we found that AKT was constitutively activated in these cells. Thus, API-2 treatment resulted in complete inhibition of pAKT with subsequent downregulation of sCLU expression, thus confirming that AKT activation accompanies sCLU production.
Figure 2.
API-2 blockade of AKT function reduces sCLU expression and induces chemosensitivity to docetaxel in drug-resistant tumor cells. A, DU145-DR and PC3-DR tumor cells were pretreated 2 h with the indicated doses of API-2, a potent AKT inhibitor, and exposed to docetaxel-containing medium for 48 h. Cell lysates were then prepared and analyzed by Western blotting with antibodies against pAKT. The lysates were also probed with antibodies specific for total AKT and sCLU. Treatment with API-2 caused a dose-dependent inhibition of active AKT and sCLU protein expression. B, CWR22Rv1 tumor cells were pretreated 2 h with the indicated doses of API-2 and cultured for 48 h in complete medium. Cell lysates were made and Western blot analysis was done to detect pAKT, total AKT, sCLU, and β-actin. Treatment with API-2 caused a dose-dependent inhibition of active AKT and sCLU protein expression. C, DU145-DR and PC3-DR tumor cells, similarly treated with API-2 for 2 h, were analyzed for cell apoptosis by Annexin V/PI staining after a further 48-h culture in docetaxel medium. Both cell lines succumbed to docetaxel-induced cell death after API-2 treatment to deplete AKT function.
We next analyzed if inhibition of AKT activation, and subsequent loss of sCLU expression, would alter cell survival and resistance to docetaxel in our cell lines. The DR cell lines DU145-DR and PC3-DR were pretreated with either medium, DMSO, 20 μmol/L API-2, or 40 μmol/L of API-2, and subsequently, all treated groups were exposed to docetaxel-containing medium. After 48 hours, apoptosis levels were analyzed by Annexin V-PI staining (Fig. 2C). As indicated by the flow cytometry data, treatment with medium alone did not cause any significant apoptosis even when exposed to docetaxel because of the acquired resistance of the cells. However, API-2 plus docetaxel treatment dramatically increased the number of apoptotic (Annexin V+) and necrotic (Annexin V+ PI+ and PI+) cells in a dose-dependent manner. Thus, API-2 can decrease sCLU (Fig. 2A and B) and at the same time chemosensitize tumor cells to docetaxel (Fig. 2C). From these results, we can conclude that AKT is sufficient to induce sCLU, which confers cytoprotection against docetaxel.
DN-AKT downregulates sCLU expression
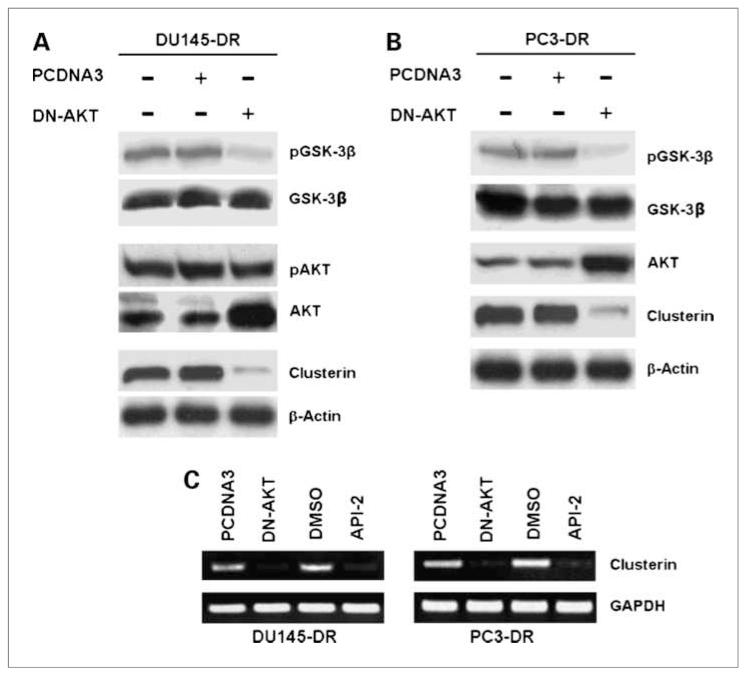
Another approach to investigate the contribution of AKT to sCLU expression is by depletion of AKT function via DN constructs. To confirm that AKT controls CLU expression, we transfected DN-AKT into our docetaxel-resistant cell lines and reanalyzed them for sCLU expression. The efficiency of functional AKT deletion was monitored by GSK-3β phosphorylation, which is the direct downstream target of AKT. Figure 3A shows that only DN-AKT–transfected, but not control PCDNA3 vector–transfected, DU145-DR tumor cells had markedly reduced pGSK-3β, whereas the total GSK-3β levels were constant and unaffected by these treatments. Western blotting with anti-AKT shows a darker band in DN-AKT–transfected cells because of the addition of the overexpressed construct to the constitutive levels of AKT. Although the level of total AKT was increased in DN-AKT–transfected cells, their pAKT levels remained constant as in the untransfected control cells because the construct itself has the critical phosphorylatable threonine and serine sites mutated to alanine (Fig. 3A). These analyses showed that DN-AKT was efficiently expressed and it markedly suppressed GSK-3β phosphorylation, confirming its function integrity. We then probed for the effect of DN-AKT expression on sCLU induction. Overexpression of DN-AKT, but not control PCDNA3 vector, in DU145-DR cells effectively suppressed CLU protein expression. These results were confirmed in PC3-DR cells that were transfected with DN-AKT, which exhibited loss of sCLU (Fig. 3B).
Figure 3.
Expression of DN-AKT disrupts sCLU expression. A, DU145-DR tumor cells were transfected with DN-AKT and cultured for 48 h in docetaxel-containing medium. Control transfection was with the empty vector PCDNA3. Transfection efficiency was monitored by Western blot analysis for total AKT. pAKT assessment indicated that the overexpressed DN-AKT is not phosphorylated, as expected, due to mutation at the critical threonine and serine sites, thus showing equal levels of pAKT in control-transfected and DN-AKT–transfected cells. Downstream AKT function was assessed by analysis of GSK-3β phosphorylation, which is a known AKT target. DN-AKT effectively suppressed GSK-3β phosphorylation and correspondingly suppressed sCLU expression. B, the above experiment was repeated on PC3-DR cells and produced confirmation of the loss of AKT to result in loss of pGSK-3β and sCLU. C, analysis of sCLU gene expression was done by RT-PCR on DU145DR and PC3-DR cells that were either treated with DMSO or API-2, or transfected with control PCDNA3 vector or DN-AKT. Both API-2 treatment and DN-AKT transfection markedly suppressed CLU mRNA expression in DU145-DR and PC3-DR tumor cells compared with their respective controls. Equal loading was monitored by GAPDH mRNA levels.
The above data indicated that sCLU protein was downregulated by the loss of AKT function. To identify if the downregulation of CLU protein levels brought on by inhibiting AKT function is mediated at the level of transcription, we assayed for full-length sCLU mRNA levels in DMSO-treated and API-2–treated, or PCDNA3 contro–transfected and DN-AKT–transfected cells (Fig. 3C). RT-PCR analysis indicated that inhibition of AKT function by DN-AKT expression dramatically decreased sCLU gene expression levels compared with control PCDNA3 transfection. Confirmation was also provided by the observation that API-2–treated tumor cells expressed no sCLU mRNA in comparison with DMSO-treated control tumor cells. As a control for equal loading, GAPDH mRNA was also measured and showed a consistent level of expression in all groups.
DN-AKT chemosensitizes tumor cells to docetaxel via caspase-3–dependent apoptosis
To identify if the docetaxel sensitivity following API-2 treatment or transfection of DN-AKT was mediated via caspase-3–dependent apoptosis, we analyzed these cells using the Active Caspase-3 FITC MAb Apoptosis kit. All groups before and after API-2 treatment or DN-AKT transfection, accompanied by DMSO and PCDNA3 controls, were placed in docetaxel medium for 48 hours and subsequently evaluated for caspase-3 activation. Figure 4A indicated that DMSO-treated and control PCDNA3 vector–transfected DU145-DR and PC3-DR tumor cells did not express active caspase-3 when exposed to docetaxel, which is consistent with their acquired property of drug resistance. On the other hand, levels of active caspase-3 were extremely elevated following API-2 treatment or DN-AKT transfection in both DU145-DR and PC3-DR cell lines. These elevated levels returned to near basal levels when the cells were pretreated with the broad range caspase inhibitor zVAD. fmk (Fig. 4A). However, the caspase-1–specific inhibitor zVAD.amc could not diminish active caspase-3. Thus, caspase-3–dependent apoptosis is responsible for the detrimental effect resulting from loss of AKT function induced by API-2. Visual analysis of docetaxel sensitivity of these cells was monitored by methylene blue staining, and it confirmed that treatment with API-2 or transfection with DN-AKT restored docetaxel sensitivity and showed few cell survival, whereas DMSO control–transfected and vector-transfected cells remained resistant and showed unimpeded growth (Fig. 4B). Taken together, these data lend concrete evidence to the importance of activated AKT in mediating docetaxel resistance.
Figure 4.
AKT inhibition by API-2 treatment or DN-AKT transfection induces chemosensitivity to docetaxel via a caspase-3–dependent pathway. A, DU145-DR and PC3-DR tumor cells, pretreated with API-2 or DN-AKT transfection (including DMSO or PCDNA3 controls), were treated with zVAD.fmk, a general caspase inhibitor, and zVAD.amc, a caspase-1–specific inhibitor. These cells were then exposed to docetaxel for 48 h before analysis of active caspase-3. High levels of active caspase-3 were detected in API-treated and DN-AKT–expressing tumor cells. This activity was inhibited by zVAD.fmk but not by zVAD.amc. B, visual assessment of cell survival by methylene blue staining of the same cells showed few cells surviving in DU145DR and PC3-DR tumor cells treated with API-2 or transfected with DN-AKT, whereas the control DMSO–treated and PCDNA3-expressing cells showed a robust growth.
sCLU expression alone is sufficient to develop the DR phenotype
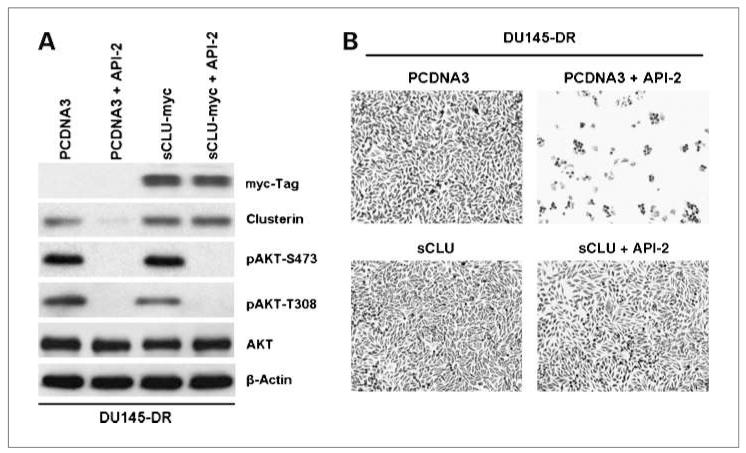
It is possible that AKT can induce other prosurvival proteins unrelated to sCLU to mediate chemoresistance in tumor cells. To identify if sCLU expression alone can produce docetaxel resistance in our cell lines, we transfected a full-length myc-tagged sCLU construct into DU145-DR cells before treatment with API-2. Then, the cells were exposed to docetaxel for 48 hours. Western blot analysis, first with anti-myc and then with anti-CLU, confirms myc-tagged sCLU overexpression in the transfected cells (Fig. 5A). On API-2 treatment, sCLU is lost in PCDNA3 control vector–transfected cells but not in sCLU-overexpressing cells. The effectiveness of API-2 was monitored by the presence of pAKT at S473 and T308, and it was confirmed that API-2 markedly reduced pAKT levels. These cells were then examined for growth in docetaxel-containing medium to determine if sCLU is overexpressed; the tumor cells should survive in docetaxel even under API-2 treatment (Fig. 5B). The results show that API-2 treatment can chemosensitize PCDNA3 control vector–transfected cells to docetaxel but cannot do so in sCLU-overexpressing tumor cells. Thus, once sCLU is present, AKT inhibition cannot induce cell death even in the presence of docetaxel. These results indicate that sCLU can overcome API-2–induced cell death and is a key mediator of AKT-mediated docetaxel resistance.
Figure 5.
Overexpression of sCLU overcomes API-2-induced chemosensitivity to docetaxel. A, DU145-DR tumor cells were transfected with either control PCDNA3 or myc-tagged sCLU. These cells were then pretreated with API-2 for 2 h and incubated in docetaxel medium for 48 h. Western blot analysis with anti-myc was done to confirm efficient transfection, and further analysis of sCLU, pAKT, and total AKT was pursued. It is confirmed that API-2 can suppress AKT function in both PCDNA3- and sCLU-expressing tumor cells. B, these same cells were also analyzed for cell growth and survival by methylene blue staining. API-2 effectively induced docetaxel sensitivity and cell death in DU145-DR tumor cells but could not do so in the sCLU-overexpressing DU145-DR tumor cells.
Inhibition of AKT results in suppression of STAT1 activation
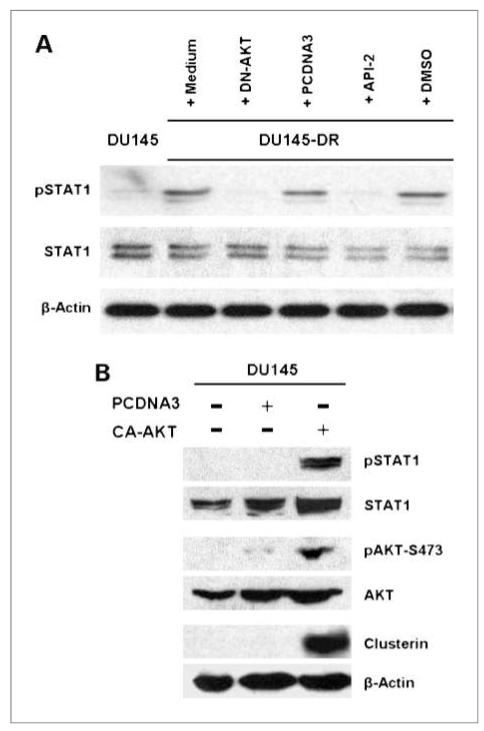
We have previously reported that STAT1 is critical for the upregulation of sCLU in our DR tumor cells (24). With the new knowledge that AKT is also required for sCLU induction, we asked the question whether there is a linkage between AKT and STAT1. Therefore, we analyzed for active STAT1 in DU145-DR tumor cells before and after suppression of AKT function (Fig. 6A). We found that DU145-DR expressed markedly higher pSTAT1 levels than in the parental DU145 tumor cells. Most importantly, suppression of AKT function via either DN-AKT transfection or API-2 treatment abolished the presence of pSTAT1, indicating that AKT does control STAT1 function. Thus, AKT seems to be upstream of STAT1, which we have proven earlier to be the transcription factor necessary for sCLU gene transcription (24).
Figure 6.
Inhibition of AKT function leads to STAT1 inactivation, whereas CA-AKT leads to STAT1 activation and CLU induction. A, DU145-DR tumor cells, pretreated with API-2 or DMSO in one set or transfected with DN-AKT or PCDNA3 control vector in a second set, were further cultured 48 h in docetaxel medium. Cells were then lysed and analyzed by Western blotting with anti-pSTAT1 or total STAT1. Equal loading was also monitored with anti–β-actin. B, parental DU145 tumor cells were transfected with CA-AKT or with the empty vector PCDNA3, followed by 48-h culture in medium. Transfection efficiency was monitored by Western blot analysis with anti-AKT, and functional activation was assessed by analysis of AKT phosphorylation. CA-AKT led to STAT1 activation and upregulation of CLU expression.
CA-AKT upregulates active STAT1 and sCLU expression in parental tumor cells
The above experiment indicated that DN-AKT could suppress STAT1 and sCLU expression in drug-resistant DU145-DR cells. To provide definitive proof that AKT drives STAT1-mediated sCLU expression, it is imperative to show that direct AKT activation in parental DU145 tumor cells, which naturally do not express sCLU, can lead to the upregulation of active STAT1 and sCLU. Thus, we transfected CA-AKT into parental DU145 tumor cells to investigate its effect on pSTAT1 and sCLU expression. Transfection efficiency was confirmed by the overexpression of pAKT and total AKT in CA-AKT–transfected cells in comparison with PCDNA3-transfected or medium control cells (Fig. 6B). As expected, overexpressed pAKT was accompanied by a robust induction of pSTAT1 and sCLU expression. Together, these results provide further evidence of the critical role of AKT in the regulation of sCLU expression.
Discussion
Our results, taken together, provide new insight into the development of docetaxel resistance in prostate cancer. Despite mounting evidence implicating CLU in paclitaxel/docetaxel resistance (21, 24, 38) and reports of AKT activation in prostate tumor cells (31), there has been no link between these two antiapoptotic proteins. We now identify a new pathway by which AKT can prevent cell death and it does so by acting as a key positive transcriptional regulator of sCLU. The evidence for this AKT-sCLU pathway was obtained by analysis of biological, biochemical, and molecular functions. We first showed that AKT was highly upregulated in the DR cell lines DU145-DR and PC3-DR in comparison with their wild-type counterparts. Second, we showed that AKT inhibition, either by a pharmacologic agent, API-2, or by overexpression of DN-AKT, could markedly suppress sCLU gene expression in the drug-resistant cells and resensitize these tumor cells to docetaxel. Finally, we were able to prevent DU145-DR cells from succumbing to API-2–induced docetaxel sensitivity by overexpressing sCLU, highlighting the importance of sCLU expression in docetaxel resistance and as a prosurvival factor. It is of importance that once sCLU was overexpressed, AKT inhibition had no ability to cause cell death even in the presence of docetaxel, suggesting that sCLU-expressing tumor cells are likely to be resistant not only to chemotherapeutic agents such as docetaxel but also to agents targeting AKT. It will require the use of both types of agents to induce cell death once the tumor cell expresses sCLU. Our findings that overexpression of sCLU alone is enough to overcome API suggest that other factors, such as bcl-2, survivin, or X-linked inhibitor of apoptosis protein (XIAP), are not involved. Indeed, it is well established that paclitaxel and docetaxel block bcl-2 function, and these taxanes do so by disrupting microtubule integrity that leads to bcl-2 phosphorylation and inactivation in tumor cells (39, 40). This property is the basis for the clinical use of docetaxel in patients with androgen-refractory prostate cancer who acquire abnormally activated bcl-2 in their tumors (2, 41). In addition to bcl-2, others have investigated the participation of survivin, XIAP, and sCLU in camptothecin resistance in prostate tumor cells (42). In analysis of camptothecin-resistant PC3 tumor cells, treatment with camptothecin was found to effectively reduce survivin and XIAP expression but, on the contrary, it markedly upregulated sCLU expression. Therefore, sCLU may be a common factor raised in tumor cells that develop resistance to chemotherapeutic agents.
We also investigated whether AKT was linked to STAT1 activation because of our earlier observations that sCLU expression is dependent on STAT1 (24). We were able to confirm that AKT does activate STAT1 by showing that API-2 treatment or DN-AKT expression was capable of suppressing STAT1 activation in DU145-DR tumor cells. Conversely, we documented that CA-AKT expression in parental DU145 tumor cells can induce STAT1 activation and sCLU production. Evidence of the involvement of AKT in STAT1 activation has also been seen by others in IFN-γ–treated cells (43). Our results may help explain the recent linkage of AKT to paclitaxel and TRAIL resistance (44, 45). Although AKT was found to mediate such resistance, the survival factor in both cases was unknown. Here, we have definitive data to identify sCLU as the link between AKT and paclitaxel/docetaxel resistance. Of interest, a recent study showed that extracellular CLU, on contact with tumor cells, could activate the phosphoinositide 3-kinase/AKT pathway, which led to resistance to TNF-α treatment, and this pathway implicated the megalin surface receptor for sCLU (46). Thus, sCLU may be able to autoregulate its own overexpression, leading to resistance to multiple apoptotic stimuli. We also recently showed that sCLU is responsible for TRAIL resistance in prostate tumor cells, thus providing an explanation for the reported AKT association with TRAIL resistance (26). Of note, we have shown that a tyrosine kinase inhibitor, resveratrol, was able to restore TRAIL sensitivity in resistant prostate tumor cells, adding the possibility of a Src kinase in the upregulation of sCLU. It is conceivable that Src activation leads to AKT activation, resulting in STAT1 upregulation of the CLU gene.
Emerging evidence, including data from this current report, indicates that in instances of cellular stress, there is an increase in the expression of the ~60-kDa full-length unprocessed sCLU form (4). This ~60-kDa sCLU has been reported to localize to the cytosol through an unknown mechanism by which it evades the endoplasmic reticulum–Golgi secretory pathway (6, 7). Once cytoplasmic, sCLU is thought to function in a prosurvival role during cell death and confer resistance to cytotoxic agents (24, 28, 38). Most importantly, sCLU expression is likely to lead to multidrug resistance, as it has been shown to confer protection against a number of clinically established chemotherapeutic agents, including cisplatin, doxorubicin, camptothecin, dacarbazine, etoposide, and 5-fluorouracil (5, 18, 27-30). In addition to these commonly used anticancer drugs, sCLU is a potent disruptor of targeted therapy involving the TNF family of proteins, including TNF (47), Fas (48), and TRAIL (26). It is thought that within the cell, sCLU exerts its cytoprotective effect by binding partially unfolded proteins to prevent stress-induced protein aggregation. Thus, its binding and stabilization of the Ku70-Bax complex is a key factor preventing mitochondria-mediated apoptosis (5, 7). Such sCLU binding prevents the release of Bax to the mitochondria to initiate cytochrome c release and the resultant caspase-3–dependent apoptotic pathway. It is to be noted that we have observed that the death of DU145-DR, on loss of sCLU, is mediated by caspase-3–induced cell death, confirming that sCLU is using the same pathway in our cells.
In contrast, the nuclear form of CLU, which is derived by alternative splicing and containing a nuclear localization sequence, has been reported to function as a prodeath protein in human cancer cells (30, 49). Its mechanism of action is also via stabilization of the Ku70-Bax complex. However, in the nucleus, Ku70 serves as a critical nuclear factor involved in DNA repair, and it is the sequestration of Ku70 by nCLU that impairs repair of DNA damage, thus leading to apoptosis. It is noteworthy that we only detected the sCLU form of CLU in our cell lines, which is consistent with other groups who also were unable to detect the nuclear form in prostate cancer cell lines (5).
How might our discovery of the AKT-CLU axis affect the current protocol to treat prostate cancer? It might well be that the acquisition of high pAKT levels, often seen in advanced cancer (50), may be associated with increased sCLU expression and become a barrier to successful docetaxel therapy for these patients. Positive screening of these molecular markers could divert treatment toward drugs that selectively target AKT and/or CLU. AKT inhibitors such as perifosine, API-2 (32, 51), and antisense oligos targeting CLU are already in early-phase clinical trials (52), and they could improve cancer sensitivity in combination with docetaxel or other antitumor therapeutics. This strategy would also benefit other cancers, including breast, ovarian, colon, lung, renal, pancreas, cervical and bladder carcinomas, melanoma, and gliomas, which are often treated with docetaxel and other anticancer drugs and are also reported to express high levels of CLU in late-stage tumors (10-19, 26).
Acknowledgments
We thank the Flow Cytometry, Analytical Microscopy, Molecular Biology, and Tissue Cores for their outstanding services.
Grant Support
NIH grant T32 CA115308 and Manuel and Adeline Garcia Endowment Fund (J.Y. Djeu).
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Hadaschik BA, Sowery RD, Gleave ME. Novel targets and approaches in advanced prostate cancer. Curr Opin Urol. 2007;17:182–7. doi: 10.1097/MOU.0b013e3280dd8a4f. [DOI] [PubMed] [Google Scholar]
- 2.Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–20. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 3.Shannan B, Seifert M, Leskov K, et al. Challenge and promise: roles for clusterin in pathogenesis, progression and therapy of cancer. Cell Death Differ. 2006;13:12–9. doi: 10.1038/sj.cdd.4401779. [DOI] [PubMed] [Google Scholar]
- 4.Trougakos IP, Djeu JY, Gonos ES, Boothman DA. Advances and challenges in basic and translational research on clusterin. Cancer Res. 2009;69:403–6. doi: 10.1158/0008-5472.CAN-08-2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang H, Kim JK, Edwards CA, Xu Z, Taichman R, Wang CY. Clusterin inhibits apoptosis by interacting with activated Bax. Nat Cell Biol. 2005;7:909–15. doi: 10.1038/ncb1291. [DOI] [PubMed] [Google Scholar]
- 6.Nizard P, Tetley S, Le Drean Y, et al. Stress-induced retrotranslocation of clusterin/ApoJ into the cytosol. Traffic. 2007;8:554–65. doi: 10.1111/j.1600-0854.2007.00549.x. [DOI] [PubMed] [Google Scholar]
- 7.Trougakos IP, Lourda M, Antonelou MH, et al. Intracellular clusterin inhibits mitochondrial apoptosis by suppressing p53-activating stress signals and stabilizing the cytosolic Ku70-Bax protein complex. Clin Cancer Res. 2009;15:48–59. doi: 10.1158/1078-0432.CCR-08-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miyake H, Muramaki M, Kurahashi T, et al. Expression of clusterin in prostate cancer correlates with Gleason score but not with prognosis in patients undergoing radical prostatectomy without neoadjuvant hormonal therapy. Urology. 2006;68:609–14. doi: 10.1016/j.urology.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 9.Steinberg J, Oyasu R, Lang S, et al. Intracellular levels of SGP-2 (clusterin) correlate with tumor grade in prostate cancer. Clin Cancer Res. 1997;3:1707–11. [PubMed] [Google Scholar]
- 10.Parczyk K, Pilarsky C, Rachel U, Koch-Brandt C. Gp80 (clusterin; TRPM-2) mRNA level is enhanced in human renal clear cell carcinomas. J Cancer Res Clin Oncol. 1994;120:186–8. doi: 10.1007/BF01202200. [DOI] [PubMed] [Google Scholar]
- 11.Redondo M, Villar E, Torres-Munoz J, Tellez T, Morell M, Petito CK. Overexpression of clusterin in human breast carcinoma. Am J Pathol. 2000;157:393–9. doi: 10.1016/S0002-9440(10)64552-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.So A, Sinnemann S, Huntsman D, Fazli L, Gleave M. Knockdown of the cytoprotective chaperone, clusterin, chemosensitizes human breast cancer cells both in vitro and in vivo. Mol Cancer Ther. 2005;4:1837–49. doi: 10.1158/1535-7163.MCT-05-0178. [DOI] [PubMed] [Google Scholar]
- 13.Xie D, Lau SH, Sham JS, et al. Up-regulated expression of cytoplasmic clusterin in human ovarian carcinoma. Cancer. 2005;103:277–83. doi: 10.1002/cncr.20765. [DOI] [PubMed] [Google Scholar]
- 14.Pucci S, Bonanno E, Pichiorri F, Angeloni C, Spagnoli LG. Modulation of different clusterin isoforms in human colon tumorigenesis. Oncogene. 2004;23:2298–304. doi: 10.1038/sj.onc.1207404. [DOI] [PubMed] [Google Scholar]
- 15.July LV, Beraldi E, So A, et al. Nucleotide-based therapies targeting clusterin chemosensitize human lung adenocarcinoma cells both in vitro and in vivo. Mol Cancer Ther. 2004;3:223–32. [PubMed] [Google Scholar]
- 16.Mourra N, Couvelard A, Tiret E, Olschwang S, Flejou JF. Clusterin is highly expressed in pancreatic endocrine tumours but not in solid pseudopapillary tumours. Histopathology. 2007;50:331–7. doi: 10.1111/j.1365-2559.2007.02608.x. [DOI] [PubMed] [Google Scholar]
- 17.Watari H, Ohta Y, Hassan MK, Xiong Y, Tanaka S, Sakuragi N. Clusterin expression predicts survival of invasive cervical cancer patients treated with radical hysterectomy and systematic lymphadenectomy. Gynecol Oncol. 2008;108:527–32. doi: 10.1016/j.ygyno.2007.11.026. [DOI] [PubMed] [Google Scholar]
- 18.Hoeller C, Pratscher B, Thallinger C, et al. Clusterin regulates drug-resistance in melanoma cells. J Invest Dermatol. 2005;124:1300–7. doi: 10.1111/j.0022-202X.2005.23720.x. [DOI] [PubMed] [Google Scholar]
- 19.Danik M, Chabot JG, Mercier C, et al. Human gliomas and epileptic foci express high levels of a mRNA related to rat testicular sulfated glycoprotein 2, a purported marker of cell death. Proc Natl Acad Sci U S A. 1991;88:8577–81. doi: 10.1073/pnas.88.19.8577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wellmann A, Thieblemont C, Pittaluga S, et al. Detection of differentially expressed genes in lymphomas using cDNA arrays: identification of clusterin as a new diagnostic marker for anaplastic large-cell lymphomas. Blood. 2000;96:398–404. [PubMed] [Google Scholar]
- 21.Miyake H, Nelson C, Rennie PS, Gleave ME. Acquisition of chemoresistant phenotype by overexpression of the antiapoptotic gene testosterone-repressed prostate message-2 in prostate cancer xenograft models. Cancer Res. 2000;60:2547–54. [PubMed] [Google Scholar]
- 22.Miyake H, Nelson C, Rennie PS, Gleave ME. Testosterone-repressed prostate message-2 is an antiapoptotic gene involved in progression to androgen independence in prostate cancer. Cancer Res. 2000;60:170–6. [PubMed] [Google Scholar]
- 23.Zellweger T, Chi K, Miyake H, et al. Enhanced radiation sensitivity in prostate cancer by inhibition of the cell survival protein clusterin. Clin Cancer Res. 2002;8:3276–84. [PubMed] [Google Scholar]
- 24.Patterson SG, Wei S, Chen X, et al. Novel role of Stat1 in the development of docetaxel resistance in prostate tumor cells. Oncogene. 2006;25:6113–22. doi: 10.1038/sj.onc.1209632. [DOI] [PubMed] [Google Scholar]
- 25.Sowery RD, Hadaschik BA, So AI, et al. Clusterin knockdown using the antisense oligonucleotide OGX-011 re-sensitizes docetaxel-refractory prostate cancer PC-3 cells to chemotherapy. BJU Int. 2008;102:389–97. doi: 10.1111/j.1464-410X.2008.07618.x. [DOI] [PubMed] [Google Scholar]
- 26.Sallman DA, Chen X, Zhong B, et al. Clusterin mediates TRAIL resistance in prostate tumor cells. Mol Cancer Ther. 2007;6:2938–47. doi: 10.1158/1535-7163.MCT-07-0345. [DOI] [PubMed] [Google Scholar]
- 27.Lee CH, Jin RJ, Kwak C, et al. Suppression of clusterin expression enhanced cisplatin-induced cytotoxicity on renal cell carcinoma cells. Urology. 2002;60:516–20. doi: 10.1016/s0090-4295(02)01806-x. [DOI] [PubMed] [Google Scholar]
- 28.Lourda M, Trougakos IP, Gonos ES. Development of resistance to chemotherapeutic drugs in human osteosarcoma cell lines largely depends on up-regulation of clusterin/apolipoprotein J. Int J Cancer. 2007;120:611–22. doi: 10.1002/ijc.22327. [DOI] [PubMed] [Google Scholar]
- 29.Miyake H, Hara I, Kamidono S, Gleave ME. Synergistic chemosensitization and inhibition of tumor growth and metastasis by the antisense oligodeoxynucleotide targeting clusterin gene in a human bladder cancer model. Clin Cancer Res. 2001;7:4245–52. [PubMed] [Google Scholar]
- 30.Wei L, Xue T, Wang J, et al. Roles of clusterin in progression, chemoresistance and metastasis of human ovarian cancer. Int J Cancer. 2009;125:791–806. doi: 10.1002/ijc.24316. [DOI] [PubMed] [Google Scholar]
- 31.Edwards J, Krishna NS, Witton CJ, Bartlett JM. Gene amplifications associated with the development of hormone-resistant prostate cancer. Clin Cancer Res. 2003;9:5271–81. [PubMed] [Google Scholar]
- 32.Yang L, Dan HC, Sun M, et al. Akt/protein kinase B signaling inhibitor-2, a selective small molecule inhibitor of Akt signaling with antitumor activity in cancer cells overexpressing Akt. Cancer Res. 2004;64:4394–9. doi: 10.1158/0008-5472.CAN-04-0343. [DOI] [PubMed] [Google Scholar]
- 33.Yuan ZQ, Feldman RI, Sussman GE, Coppola D, Nicosia SV, Cheng JQ. AKT2 inhibition of cisplatin-induced JNK/p38 and Bax activation by phosphorylation of ASK1: implication of AKT2 in chemoresistance. J Biol Chem. 2003;278:23432–40. doi: 10.1074/jbc.M302674200. [DOI] [PubMed] [Google Scholar]
- 34.Sramkoski RM, Pretlow TG, II, Giaconia JM, et al. A new human prostate carcinoma cell line, 22Rv1. In Vitro Cell Dev Biol Anim. 1999;35:403–9. doi: 10.1007/s11626-999-0115-4. [DOI] [PubMed] [Google Scholar]
- 35.Trougakos IP, Lourda M, Agiostratidou G, Kletsas D, Gonos ES. Differential effects of clusterin/apolipoprotein J on cellular growth and survival. Free Radic Biol Med. 2005;38:436–49. doi: 10.1016/j.freeradbiomed.2004.10.038. [DOI] [PubMed] [Google Scholar]
- 36.Vlietstra RJ, van Alewijk DC, Hermans KG, van Steenbrugge GJ, Trapman J. Frequent inactivation of PTEN in prostate cancer cell lines and xenografts. Cancer Res. 1998;58:2720–3. [PubMed] [Google Scholar]
- 37.Shah RB, Mehra R, Chinnaiyan AM, et al. Androgen-independent prostate cancer is a heterogeneous group of diseases: lessons from a rapid autopsy program. Cancer Res. 2004;64:9209–16. doi: 10.1158/0008-5472.CAN-04-2442. [DOI] [PubMed] [Google Scholar]
- 38.Trougakos IP, So A, Jansen B, Gleave ME, Gonos ES. Silencing expression of the clusterin/apolipoprotein J gene in human cancer cells using small interfering RNA induces spontaneous apoptosis, reduced growth ability, and cell sensitization to genotoxic and oxidative stress. Cancer Res. 2004;64:1834–42. doi: 10.1158/0008-5472.can-03-2664. [DOI] [PubMed] [Google Scholar]
- 39.Haldar S, Basu A, Croce CM. Bcl2 is the guardian of microtubule integrity. Cancer Res. 1997;57:229–33. [PubMed] [Google Scholar]
- 40.Scatena CD, Stewart ZA, Mays D, et al. Mitotic phosphorylation of Bcl-2 during normal cell cycle progression and Taxol-induced growth arrest. J Biol Chem. 1998;273:30777–84. doi: 10.1074/jbc.273.46.30777. [DOI] [PubMed] [Google Scholar]
- 41.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–12. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 42.Mizutani K, Matsumoto K, Hasegawa N, Deguchi T, Nozawa Y. Expression of clusterin, XIAP and survivin, and their changes by camptothecin (CPT) treatment in CPT-resistant PC-3 and CPT-sensitive LNCaP cells. Exp Oncol. 2006;28:209–15. [PubMed] [Google Scholar]
- 43.Nguyen H, Ramana CV, Bayes J, Stark GR. Roles of phosphatidylinositol 3-kinase in interferon-γ-dependent phosphorylation of STAT1on serine 727 and activation of gene expression. J Biol Chem. 2001;276:33361–8. doi: 10.1074/jbc.M105070200. [DOI] [PubMed] [Google Scholar]
- 44.Cheng GZ, Chan J, Wang Q, Zhang W, Sun CD, Wang LH. Twist transcriptionally up-regulates AKT2 in breast cancer cells leading to increased migration, invasion, and resistance to paclitaxel. Cancer Res. 2007;67:1979–87. doi: 10.1158/0008-5472.CAN-06-1479. [DOI] [PubMed] [Google Scholar]
- 45.Nesterov A, Lu X, Johnson M, Miller GJ, Ivashchenko Y, Kraft AS. Elevated AKT activity protects the prostate cancer cell line LNCaP from TRAIL-induced apoptosis. J Biol Chem. 2001;276:10767–74. doi: 10.1074/jbc.M005196200. [DOI] [PubMed] [Google Scholar]
- 46.Ammar H, Closset JL. Clusterin activates survival through the phosphatidylinositol 3-kinase/Akt pathway. J Biol Chem. 2008;283:12851–61. doi: 10.1074/jbc.M800403200. [DOI] [PubMed] [Google Scholar]
- 47.Sensibar JA, Sutkowski DM, Raffo A, et al. Prevention of cell death induced by tumor necrosis factor α in LNCaP cells by overexpression of sulfated glycoprotein-2 (clusterin) Cancer Res. 1995;55:2431–7. [PubMed] [Google Scholar]
- 48.Miyake H, Hara S, Zellweger T, Kamidono S, Gleave ME, Hara I. Acquisition of resistance to Fas-mediated apoptosis by overexpression of clusterin in human renal-cell carcinoma cells. Mol Urol. 2001;5:105–11. doi: 10.1089/10915360152559585. [DOI] [PubMed] [Google Scholar]
- 49.Yang CR, Leskov K, Hosley-Eberlein K, et al. Nuclear clusterin/XIP8, an x-ray-induced Ku70-binding protein that signals cell death. Proc Natl Acad Sci U S A. 2000;97:5907–12. doi: 10.1073/pnas.97.11.5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Luo J, Manning BD, Cantley LC. Targeting the PI3K-Akt pathway in human cancer: rationale and promise. Cancer Cell. 2003;4:257–62. doi: 10.1016/s1535-6108(03)00248-4. [DOI] [PubMed] [Google Scholar]
- 51.Chee KG, Longmate J, Quinn DI, et al. The AKT inhibitor perifosine in biochemically recurrent prostate cancer: a phase II California/Pittsburgh cancer consortium trial. Clin Genitourin Cancer. 2007;5:433–7. doi: 10.3816/CGC.2007.n.031. [DOI] [PubMed] [Google Scholar]
- 52.Chi KN, Siu LL, Hirte H, et al. A phase I study of OGX-011, a 2′-methoxyethyl phosphorothioate antisense to clusterin, in combination with docetaxel in patients with advanced cancer. Clin Cancer Res. 2008;14:833–9. doi: 10.1158/1078-0432.CCR-07-1310. [DOI] [PubMed] [Google Scholar]