Abstract
Objectives
To characterize the morphological features of plaque erosion and calcified nodule in patients with acute coronary syndrome (ACS) by optical coherence tomography (OCT).
Background
Plaque erosion and calcified nodule have not been systematically investigated in vivo.
Methods
One hundred and twenty-six patients with ACS who had undergone pre-intervention OCT imaging were included. The culprit lesions were classified as plaque rupture (PR), erosion (OCT-erosion), calcified nodule (OCT-CN), or others using a new set of diagnostic criteria for OCT.
Results
The incidences of PR, OCT-erosion, and OCT-CN were 43.7%, 31.0%, and 7.9%, respectively. Patients with OCT-erosion were the youngest compared with those with PR and OCT-CN (53.8±13.1 years vs. 60.6±11.5 years, 65.1±5.0 years, p=0.005). Compared with patients with PR, presentation with non-ST-segment elevation ACS (NSTE-ACS) was more common in patients with OCT-erosion (61.5% vs. 29.1%, p=0.008) and OCT-CN (100% vs. 29.1%, p<0.001). OCT-erosion had a lower frequency of lipid plaque (43.6% vs. 100%, p<0.001), thicker fibrous cap (169.3±99.1 μm vs. 60.4±16.6 μm, p<0.001), and smaller lipid arc (202.8±73.6° vs. 275.8±60.4°, p<0.001) than PR. The diameter stenosis was least severe in OCT-erosion followed by OCT-CN and PR (55.4±14.7% vs. 66.1±13.5% vs. 68.8±12.9%, p<0.001).
Conclusions
OCT is a promising modality for identifying OCT-erosion and OCT-CN in vivo. OCT-erosion is a frequent finding in patients with ACS, especially in those with NSTE-ACS and younger patients. OCT-CN is the least common etiology for ACS and is more common in older patients.
Keywords: plaque erosion, calcified nodule, plaque rupture, acute coronary syndrome, optical coherence tomography
Coronary thrombosis is the most frequent final event leading to an acute coronary syndrome (ACS). The three most common underlying mechanisms contributing to ACS are believed to be plaque rupture (PR), plaque erosion, and calcified nodule (1,2). PR is the most frequent finding in autopsy studies of patients with sudden cardiac death (SCD) (1,3,4). However, a significant portion of thrombotic lesions found on autopsy are not associated with an underlying PR. In a series of 20 cases with SCD, van der Wal et al found PR in only 60% of lesions; the remaining 40% showed only plaque erosion without rupture (5). Virmani et al studied over 200 cases of SCD. Only one-third of lesions could be described as PR and 35% of lesions with thrombi failed to show rupture (1). A more recent autopsy study reported that approximately two-thirds (69%) of SCD cases showed organizing or healing thrombi, of which 88% were caused by erosion (6). The least common pathologic finding associated with thrombosis is calcified nodules. Calcified nodules are pathologically defined as the presence of fracture of a calcified plate, interspersed fibrin, and a disrupted fibrous cap with an overlying thrombus (1,3). The frequency of erosion and calcified nodule may be underestimated in patients with ACS due to the lack of diagnostic modalities that readily identify them.
Optical coherence tomography (OCT) is an emerging intravascular imaging modality with a resolution of 10-20 μm. It can visualize the microstructure of atherosclerotic plaque (such as fibrous cap, thrombus, and calcification) and the OCT characteristics were validated by histology (7,8). Pathologically, plaque erosion is defined as a loss of endothelial lining with lacerations of the superficial intimal layers in the absence of “trans-cap” ruptures (1). However, OCT does not provide adequate resolution to identify the endothelial lining. Therefore, the pathological definition of erosion cannot simply be adapted for the OCT definition. In addition, calcified nodules have never been systematically studied by OCT.
The aim of our study was to evaluate the morphological characteristics of OCT-determined plaque erosion (OCT-erosion) and calcified nodules (OCT-CN) in patients with ACS (including ST-segment elevation myocardial infarction [STEMI] and non-ST-segment elevation acute coronary syndrome [NSTE-ACS]).
Methods
Study Population
The Massachusetts General Hospital (MGH) OCT Registry is a multicenter registry of patients undergoing OCT imaging of the coronary arteries and includes 20 sites across 6 countries. We selected patients with ACS who have undergone pre-intervention OCT imaging of culprit lesions from the registry. Out of 206 ACS patients, 126 were included for analysis. The remaining 80 cases were excluded for the following reasons: pre-dilatation (n = 38), previous stent implantation in the culprit vessel (n = 27), left main disease (n = 2), massive thrombus (n = 6), and poor image quality (n = 7).
The patients with ACS consisted of STEMI and NSTE-ACS. STEMI was defined as continuous chest pain that lasted > 30 minutes, arrival at the hospital within 12 hours from the onset of symptoms, ST-segment elevation > 0.1 mV in > 2 contiguous leads or new left bundle-branch block on the 12-lead electrocardiogram (ECG), and elevated cardiac markers (creatine kinase-MB or troponin T/I). NSTE-ACS included non-ST elevation myocardial infarction (NSTEMI) and unstable angina pectoris. NSTEMI was defined as ischemic symptoms in the absence of ST elevation on the ECG with elevated cardiac markers. Unstable angina pectoris was defined as having newly developed/accelerating chest symptoms on exertion or rest angina within 2 weeks. The culprit lesion was identified on the basis of coronary angiogram, stress test, ECG, left ventriculogram, or echocardiogram. The protocol for the registry was approved by each site’s Institutional Review Board, and all patients provided informed consent.
OCT Image Acquisition
OCT imaging of culprit lesions was acquired using either the commercially available time-domain (M2/M3 Cardiology Imaging System, Lightlab Imaging/St. Jude Medical, Westford, Massachusetts, USA) or frequency-domain OCT C7XR system and the Dragon Fly catheter (Lightlab Imaging/St. Jude Medical, Westford, Massachusetts, USA). Patients requiring pre-dilatation and aspiration thrombectomy prior to OCT imaging were excluded. In the M2/M3 system, an occlusion balloon (Helios, LightLab Imaging, Westford, Massachusetts, USA) was inflated proximal to the lesion at 0.4 to 0.6 atm during image acquisition. The optical probe was automatically pulled back from distal to proximal at a rate of 1.0 - 3.0 mm/s and saline was continuously infused from the tip of the occlusion balloon. In the C7XR system, a 2.7 F OCT imaging catheter was carefully advanced distal to the culprit lesion. The automated pullback was performed at 20 mm/sec, while blood was displaced by a short injection of contrast media or Dextran through the guiding catheter. The images were digitally stored for off-line analysis.
OCT Image Analysis
All OCT images were analyzed in the MGH OCT Core Laboratory by two experienced investigators (H.J. and F.A.) who were blinded to the angiographic data and clinical presentations. When there was discordance between the observers, a consensus reading was obtained from a third investigator.
Definition and Classification
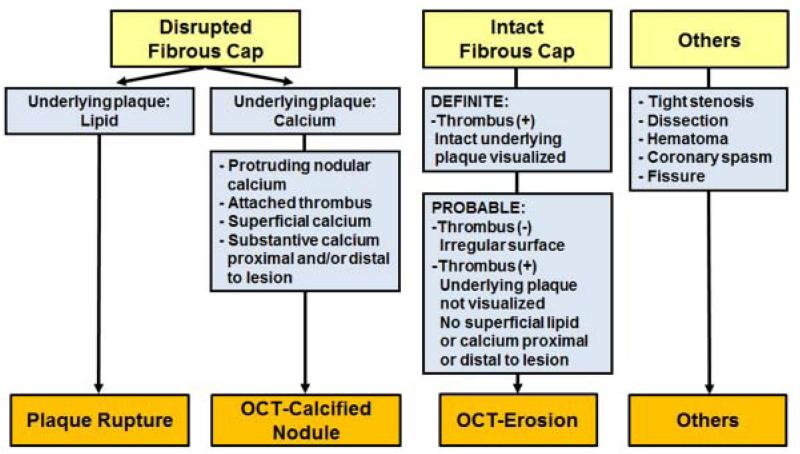
The plaque classification algorithm is shown in Figure 1. The current definitions of plaque erosion and calcified nodules have been well established by pathology studies. To establish OCT criteria of OCT-erosion and OCT-CN, the resolution limits of OCT and the effects of prior treatment of patients with antithrombotics and thrombolysis had to be considered. A new set of OCT diagnostic criteria for OCT-erosion and OCT-CN was developed that incorporated the key aspects of the pathological definitions that could be visualized by OCT in the context of live treated patients. Since the OCT metrics for erosion are different from the pathological definition, we used the term “OCT-erosion” instead of erosion. OCT-erosion was defined and categorized according to the absence of fibrous cap disruption and the presence of thrombus. Definite OCT-erosion was identified by the presence of attached thrombus overlying an intact and visualized plaque (Figure 2). Probable OCT-erosion was defined by: 1) luminal surface irregularity at the culprit lesion in the absence of thrombus; or 2) attenuation of underlying plaque by thrombus without superficial lipid or calcification immediately proximal or distal to the site of thrombus (Figure 3). This is in contrast to the pathologic definition of erosion, which requires the presence of attached thrombus. Distinct from autopsy studies of acute coronary events, these subjects survived the acute event and were treated with antithrombotic therapy. As a result, the thrombus overlying the lesion may have been dissolved before OCT imaging. OCT-CN was defined when fibrous cap disruption was detected over a calcified plaque characterized by protruding calcification, superficial calcium, and the presence of substantive calcium proximal and/or distal to the lesion (Figure 4). PR was identified by the presence of fibrous cap discontinuity with a clear cavity formed inside the plaque (Figure 5).
Figure 1.
Plaque Classification Algorithm by OCT
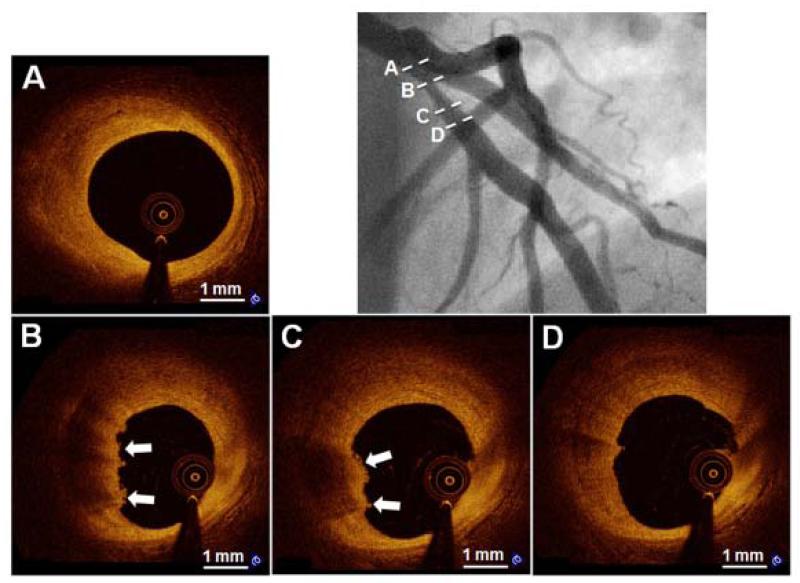
Figure 2. Representative Case of “Definite OCT-Erosion”.
A 31-year-old man presented with NSTEMI. Angiographic image (upper right) shows a moderate stenosis in the proximal LAD. Serial OCT cross-sectional images from proximal to distal of the culprit lesion indicate that no rupture is detected. Cross-sectional images indicate fibrous plaque (homogeneous high signal region) proximal (A) and distal (D) to thrombus. OCT-erosion is identified as an irregular lumen surface with attached mural thrombus (arrows) overlying a fibrous plaque (B and C). NSTEMI = non-ST-segment elevation myocardial infarction. LAD = left anterior descending coronary artery.
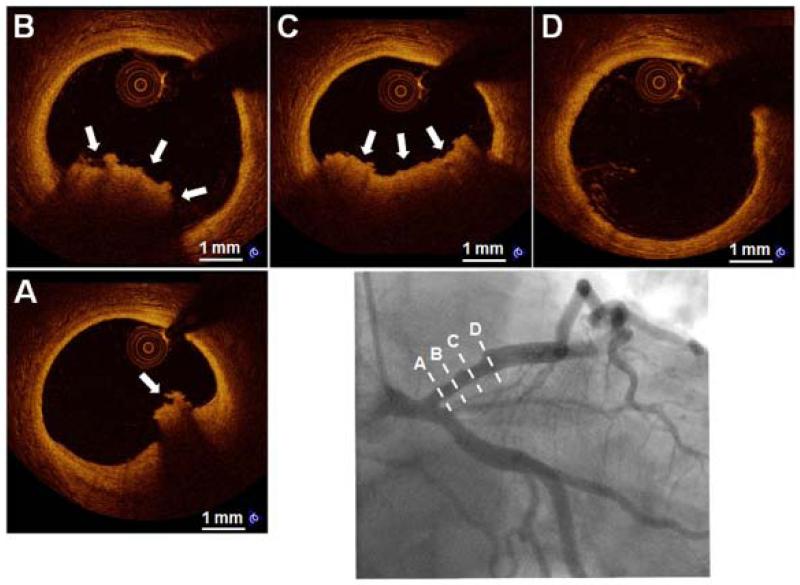
Figure 3. Representative Case of “Probable OCT-Erosion”.
A 37-year-old male smoker presented with STEMI. The angiographic image (bottom right) shows a mild stenosis in the proximal LAD. Serial OCT cross-sectional images from proximal to distal of the culprit lesion show absence of detectable rupture (A through D). Underlying plaque morphology is not well visualized due to the presence of residual red thrombus (A, B and C, arrows). OCT images in the distal and proximal segments of the thrombotic lesions show the absence of superficial lipid and calcification (A and D). Abbreviations as in Figure 2.
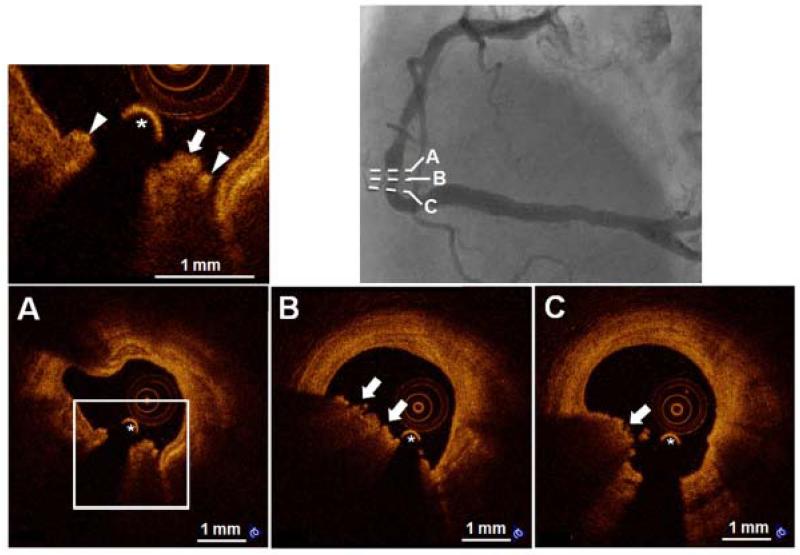
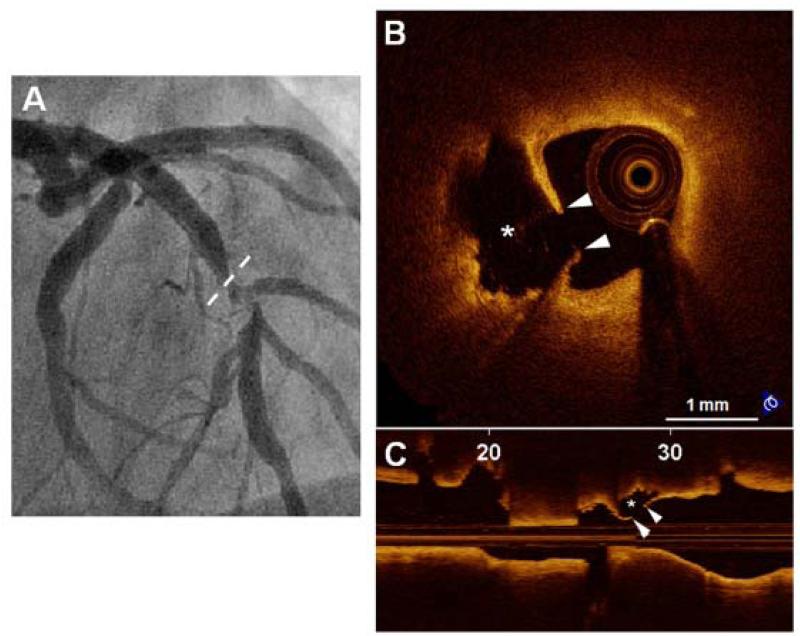
Figure 4. Representative Case of “OCT-Calcified Nodule”.
A 75-year-old male smoker with unstable angina. Coronary angiogram angiography (upper right) demonstrated a complex lesion in the distal right coronary artery. OCT-calcified nodule is identified as a nodular calcification (A) protruding into lumen through a disrupted fibrous cap (arrow heads) overlying superficial calcification with red thrombus (arrows) attached to the disrupted site. The asterisk (*) indicates the guide wire.
Figure 5. Representative Case of “Plaque Rupture”.
A 57-year-old man presenting with STEMI was treated with thrombolysis. (A) The coronary angiogram shows the culprit lesion in the mid LAD. Plaque rupture is identified on cross-sectional (B) and longitudinal (C) OCT images by the disrupted fibrous-cap (arrow heads) and a cavity (asterisk) formation inside the plaque. Abbreviations as in Figure 2.
The culprit lesions that did not meet the above criteria were classified as others which included tight stenosis (supplemental Figure 1) in the absence of any evidence of plaque rupture, OCT-erosion, or OCT-CN, spontaneous coronary artery dissection (SCAD) (supplemental Figure 2), coronary spasm (supplemental Figure 3), and fissure (supplemental Figure 4).
Tissue characteristics of underlying plaque were defined using previously established criteria (7-9). Plaques were classified as: (i) fibrous (homogeneous, high backscattering region) or (ii) lipid (low-signal region with diffuse border). For each lipid plaque, the maximal lipid arc was measured. Lipid length was recorded on a longitudinal view. Thin-cap fibroatheroma (TCFA) was defined as a plaque with lipid content in ≥ 2 quadrants and the thinnest part of the fibrous cap measuring < 65 μm. Intracoronary thrombus was defined as a mass (diameter > 250 μm) attached to the luminal surface or floating within the lumen, including red (red blood cell-rich) thrombus, defined by high backscattering and high attenuation, or white (platelet-rich) thrombus, defined by homogeneous backscattering with low attenuation. Calcification was defined as an area with low backscattering signal and a sharp border inside a plaque. Microchannels were defined as signal-poor voids that were sharply delineated in multiple contiguous frames (9). Inter-observer and intra-observer variability were assessed by the evaluation of all images by two independent observers and by the same observer at two separate time points, respectively. The inter-observer Kappa coefficients for thrombus, PR, definite OCT-erosion, probable OCT-erosion, and OCT-CN were 0.860, 0.885, 0.961, 0.877, and 0.927, respectively. The intra-observer Kappa coefficients for thrombus, PR, definite OCT-erosion, probable OCT-erosion, and OCT-CN were 0.953, 0.952, 0.970, 0.884, and 1.000, respectively.
Quantitative Coronary Angiography (QCA)
Coronary angiograms were analyzed with the Cardiovascular Angiography Analysis System (CAAS 5.10, Pie Medical Imaging B.V., Maastricht, The Netherlands). The reference diameter, minimum lumen diameter, diameter stenosis, area stenosis, and lesion length were measured.
Statistical Analysis
All statistical analyses were performed by an independent statistician at the Core Laboratory. Categorical variables were presented as counts and proportions, and the comparisons were performed using a Fisher’s exact test. Continuous variables were presented as mean ± standard deviation (SD). The means of the continuous measurements were examined using the independent samples t-test for two-group comparisons, and Analysis of Variance (ANOVA) for three-group comparisons (plaque rupture, OCT-erosion, and OCT-calcified nodule) followed by post-hoc test protected overall significance level of 0.05. A Bonferroni’s correction was used to control for multiple comparisons among the three groups (plaque rupture, OCT-erosion, and OCT-calcified nodule). All statistical analyses were performed with SPSS 17.0 (SPSS Inc., Chicago, IL). All p-values were two-sided.
Results
Baseline Demographics and Laboratory Results
The clinical characteristics of classified patients (PR, OCT-erosion or OCT-CN) and patients with other atypical lesion characteristics are summarized in Table 1. There were no significant differences in all of the clinical characteristic variables between the two groups. The comparison of patient characteristics among PR, OCT-erosion, and OCT-CN are summarized in Table 2. Patients with OCT-erosion were the youngest compared to those with PR and OCT-CN. Patients with OCT-CN had the highest incidence of hypertension and chronic kidney disease compared to the other two groups. STEMI was more common in patients with PR than in those with OCT-erosion and OCT-CN. In contrast, the presentation of NSTE-ACS was predominant in patients with OCT-erosion and OCT-CN. Other variables including gender, smoking, diabetes mellitus, hyperlipidemia, family history of coronary artery disease, prior MI, angiotensin-converting-enzyme inhibitor/ angiotensin II receptor blocker use, and statin treatment were comparable among the groups. Creatinine levels were highest in patients with OCT-CN followed by those with PR and OCT-erosion. Other laboratory variables were comparable among the groups (Table 2).
Table 1. Patient Characteristics of the Classified Group and Others.
| Classified (n = 104) |
Others (n = 22) |
p value | |
|---|---|---|---|
| Age, years | 58.5 ± 12.2 | 60.0 ± 10.8 | 0.593 |
| Male | 84 (80.8%) | 16 (72.7%) | 0.395 |
| Risk factors | |||
| Smoking | 54 (51.9%) | 7 (46.7%) | 0.103 |
| Diabetes mellitus | 24 (23.1%) | 4 (18.2%) | 0.781 |
| Hyperlipidemia | 67 (64.4%) | 13 (59.1%) | 0.635 |
| Hypertension | 60 (57.7%) | 12 (54.5%) | 0.816 |
| Family history of CAD |
12 (11.5%) | 2 (9.1%) | 1.000 |
| Chronic kidney disease | 5 (4.8%) | 0 (0.0%) | 0.586 |
| Prior MI | 16 (15.4%) | 2 (9.1%) | 0.737 |
| Medications | |||
| ACEI/ARB use | 31 (30.1%) | 9 (40.9%) | 0.323 |
| Statin use | 33 (32.0%) | 7 (31.8%) | 1.000 |
| Presentation | |||
| STEMI | 54 (51.9%) | 7 (31.8%) | 0.103 |
| NSTE-ACS | 50 (48.1%) | 15 (68.2%) |
Data are presented as mean ± SD, or n (%). The p-values were calculated with the use of t-test for continuous variables and Fisher’s exact test for categorical variables.
CAD = coronary artery disease; ACE-I = angiotensin-converting-enzyme inhibitor; ARB = angiotensin II receptor blocker; MI = myocardial infarction; STEMI = ST-segment elevation myocardial infarction; NSTE-ACS = non-ST-segment elevation acute coronary syndrome.
Table 2. Baseline Characteristics of the Patients with PR, OCT-erosion, and OCT-CN.
| PR (n = 55) |
OCT-erosion (n = 39) |
OCT-CN (n = 10) |
p value | p value* |
|||
|---|---|---|---|---|---|---|---|
| PR vs. OCT-erosion |
OCT-erosion vs. OCT-CN |
PR vs. OCT-CN |
|||||
| Age, years | 60.6 ± 11.5 | 53.8 ± 13.1 | 65.1 ± 5.0 | 0.005 | 0.019 | 0.023 | 0.802 |
| Male | 44 (80.0%) | 32 (82.1%) | 8 (80.0%) | 0.968 | N/A | N/A | N/A |
| Risk factors | |||||||
| Smoking | 26 (47.3%) | 22 (56.4%) | 6 (60.0%) | 0.591 | N/A | N/A | N/A |
| Diabetes mellitus | 11 (20.0%) | 9 (23.1%) | 4 (40.0%) | 0.385 | N/A | N/A | N/A |
| Hyperlipidemia | 41 (74.5%) | 21 (53.8%) | 5 (50.0%) | 0.072 | N/A | N/A | N/A |
| Hypertension | 34 (61.8%) | 17 (43.6%) | 9 (90.0%) | 0.020 | 0.285 | 0.036 | 0.435 |
| Family history of CAD |
7 (12.7%) | 3 (7.7%) | 2 (20.0%) | 0.511 | N/A | N/A | N/A |
| Chronic kidney disease |
3 (5.5%) | 0 (0.0%) | 2 (20.0%) | 0.029 | 0.792 | 0.115 | 0.498 |
| Prior MI | 7 (12.7%) | 5 (12.8%) | 4 (40.0%) | 0.076 | N/A | N/A | N/A |
| Medications | |||||||
| ACEI/ARB use | 13 (23.6%) | 12(31.6%) | 6 (60.0%) | 0.068 | N/A | N/A | N/A |
| Statin use | 19(34.5%) | 9 (23.7%) | 5 (50.0%) | 0.239 | N/A | N/A | N/A |
| Presentation | |||||||
| STEMI | 39 (70.9%) | 15 (38.5%) | 0 (0.0%) | <0.001 | 0.008 | 0.063 | <0.001 |
| NSTE-ACS | 16(29.1%) | 24 (61.5%) | 10 (100%) | ||||
|
Laboratory
variables |
|||||||
| TC, mg/dl | 178.9 ±43.5 | 172.2 ± 55.2 | 157.6 ±38.7 | 0.455 | N/A | N/A | N/A |
| HDL-C, mg/dl | 43.47 ± 17.2 | 50.92 ±25.3 | 40.9 ±5.2 | 0.165 | N/A | N/A | N/A |
| LDL-C, mg/dl | 102.5 ±38.2 | 95.5 ±36.7 | 93.7 ±40.9 | 0.621 | N/A | N/A | N/A |
| TG, mg/dl | 180.9 ± 123.3 | 155.3 ± 88.7 | 164.1 ± 121.0 | 0.551 | N/A | N/A | N/A |
| hs-CRP, mg/dl | 0.91 ± 2.1 | 0.22 ± 0.16 | 0.55 ± 0.52 | 0.246 | N/A | N/A | N/A |
| Creatinine, mg/dl | 0.95 ± 0.17 | 0.90 ± 0.16 | 1.55 ± 1.74 | 0.004 | 1.000 | 0.003 | 0.006 |
Data are presented as mean ± SD, or n (%).
Categorical variables were compared with the use of Fisher’s exact test. Continuous variables were compared with the use of ANOVA followed by post-hoc test only if the ANOVA p < 0.05.
p-values were adjusted by Bonferroni's correction for multiple comparisons among the three groups (PR, OCT-erosion, and OCT-CN).
PR = plaque rupture; OCT-CN = OCT-calcified nodules; CAD = coronary artery disease; MI = myocardial infarction; ACE-I = angiotensin-converting-enzyme inhibitor; ARB = angiotensin II receptor blocker; STEMI = ST-segment elevation myocardial infarction;
NSTE-ACS = non-ST-segment elevation acute coronary syndrome; TC = total cholesterol; HDL-C = high-density lipoprotein cholesterol;
LDL-C = low-density lipoprotein cholesterol; TG = triglyceride; hs-CRP = high-sensitivity C-reactive protein.
Incidences of PR, OCT-erosion, and OCT-CN in Patients with ACS
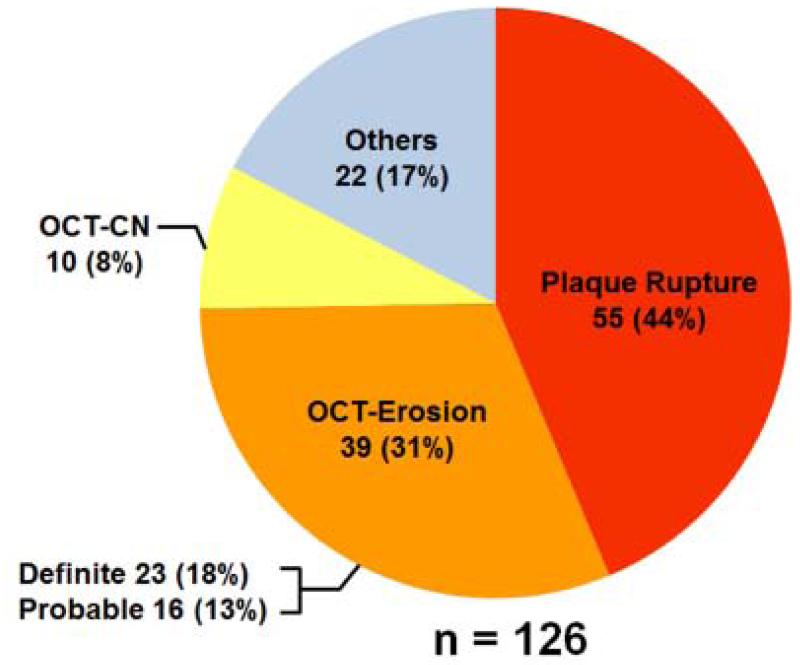
Among 126 culprit lesions studied, 55 (43.7%) lesions were classified as PR, 39 (31.0%) lesions as OCT-erosion, 10 (7.9%) lesions as OCT-CN, and 22 lesions (17.5%) were classified as others which consisted of 8 (6.3%) lesions with tight stenosis, 3 (2.4%) with dissection, 2 (1.6%) with coronary spasm, 1 (0.8%) with fissure, 1(0.8%) with Takotsubo, and the remaining 7 (5.6%) showing absence of any characteristics mentioned above. Among 39 OCT-erosion cases, definite OCT-erosion was detected in 23 (18.3%) patients and probable OCT-erosion in 16 (12.7%) patients (Figure 6).
Figure 6. Incidence of Plaque Rupture, OCT-Erosion, and OCT-Calcified Nodule in Patients with ACS.
Among the 126 culprit lesions, 55 (44%) lesions were classified as plaque rupture, 39 (31%) lesions were classified as OCT-erosion, 10 (8%) lesions were classified as OCT-calcified nodule, and 22 (17%) lesions were classified as others. OCT-CN = OCT-calcified nodule. ACS = acute coronary syndrome.
Angiographic Findings
The lesion distribution and QCA data are listed in Table 3. OCT-erosion was more frequently detected in the left anterior descending artery (LAD), followed by the right coronary artery (RCA), and least in the left circumflex artery (LCX). PR was equally distributed in the LAD and RCA. The reference diameter was comparable among the three groups. The minimum lumen diameter was largest in the OCT-erosion group followed by the OCT-CN and PR groups (p = 0.007). The diameter stenosis was least severe in the OCT-erosion group followed by the OCT-CN and PR groups (p < 0.001). No significant difference was seen in lesion length (p = 0.424).
Table 3. Angiographic Findings.
| PR (n = 55) |
OCT-erosion (n = 39) |
OCT-CN (n = 10) |
p value | p value* |
|||
|---|---|---|---|---|---|---|---|
| PR vs. OCT-erosion |
OCT-erosion vs. OCT-CN |
PR vs. OCT-CN |
|||||
| Lesion location | |||||||
| LAD | 27 (49.1%) | 28 (71.8%) | 6 (60.0%) | 0.012 | 0.006 | 1.000 | 1.000 |
| RCA | 20 (36.4%) | 6 (15.4%) | 3 (30%) | ||||
| LCX | 8 (14.5%) | 5 (12.8%) | 1 (10.0%) | ||||
| QCA data | |||||||
| RVD, mm | 3.6 ± 0.9 | 3.7 ± 1.0 | 3.2 ± 0.7 | 0.242 | N/A | N/A | N/A |
| MLD, mm | 1.0 ± 0.5 | 1.4 ± 0.6 | 1.2 ± 0.6 | 0.007 | 0.005 | 0.824 | 1.000 |
| Diameter stenosis, % | 68.8 ± 12.9 | 55.4 ± 14.7 | 66.1 ± 13.5 | < 0.001 | < 0.001 | 0.096 | 1.000 |
| Lesion length, mm | 16.4 ± 5.8 | 15.0 ± 5.0 | 17.1 ± 5.5 | 0.424 | N/A | N/A | N/A |
Data are presented as mean ± SD, or n (%).
Categorical variables were compared with the use of Fisher’s exact test. Continuous variables were compared with the use of ANOVA followed by post-hoc test only if the ANOVA p < 0.05.
p-values were adjusted by Bonferroni's correction for multiple comparisons among the three groups (PR, OCT-erosion, and OCT-CN) only if p-value for the overall three-group test < 0.05.
PR = plaque rupture; OCT-CN = OCT-calcified nodule; LAD = left anterior descending coronary artery; LCX = left circumflex artery; RCA = right coronary artery; QCA = Quantitative coronary angiogram analysis; RVD = reference vessel diameter; MLD = minimum lumen diameter.
Underlying Plaque Characteristics by OCT
The tissue characteristics of underlying plaque are shown in Table 4. In all rupture cases, the underlying plaques were lipid plaque. However, OCT-erosion was detected both in fibrous plaque and lipid plaque. Calcification was present in 22 of 55 (40.0%) PR compared with 5 of 39 (12.8%) OCT-erosion (p = 0.016). TCFA was observed in 67.3% of PR, 10.3% of OCT-erosion, and none of OCT-CN (p < 0.001). There was no significant difference in the presence of microchannels among the three groups. White thrombus was predominantly detected with OCT-erosion and OCT-CN, whereas red thrombus was found most frequently with PR (Table 4). Quantitative OCT analysis of lipid plaque is shown in Table 5. Lipid plaque detected underneath OCT-erosion had a thicker fibrous cap (p = 0.001), smaller lipid arc (p < 0.001), and shorter lipid length (p = 0.008), as compared to those underneath the PR.
Table 4. OCT Findings of Underlying Plaque Characteristics.
| PR (n = 55) |
OCT-erosion (n = 39) |
OCT-CN (n = 10) |
p value | p value* |
|||
|---|---|---|---|---|---|---|---|
| PR vs. OCT-erosion |
OCT-erosion vs. OCT-CN |
PR vs. OCT-CN | |||||
| Fibrous plaque |
0 (0.0%) | 22 (56.4%) | 10 (100%) | < 0.001 | < 0.001 | 0.027 | < 0.001 |
| Lipid plaque | 55 (100%) | 17 (43.6%) | 0 (0.0%) | < 0.001 | < 0.001 | 0.027 | < 0.001 |
| TCFA | 37 (67.3%) | 3 (10.3%) | 0 (0.0%) | < 0.001 | < 0.001 | 1.000 | < 0.001 |
| Calcification | 22 (40.0%) | 5 (12.8%) | 10 (100%) | < 0.001 | 0.016 | < 0.001 | 0.001 |
| MicroChannel | 21 (38.2%) | 7 (17.9%) | 2 (6.7%) | 0.083 | N/A | N/A | N/A |
| Thrombus | 45 (81.8%) | 33 (84.6%) | 10 (100%) | 0.242 | N/A | N/A | N/A |
| Red thrombus | 39 (70.9%) | 6 (15.4%) | 4 (40.0%) | < 0.001 | < 0.001 | 0.541 | 0.226 |
| White thrombus |
6 (10.9%) | 27 (69.2%) | 6 (60.0%) | < 0.001 | < 0.001 | 1.000 | 0.005 |
Data are presented as n. (%).
p-values were calculated by Fisher’s exact test and adjusted by Bonferroni’s correction for multiple comparisons within 3 groups (PR, OCT-erosion, and OCT-CN) only if p-value for the overall three-group test < 0.05.
PR = plaque rupture; OCT-CN = OCT-calcified nodules; TCFA = thin-cap fibroatheroma.
Table 5. OCT Measurements of Lipid Plaque.
| PR (n = 55) |
OCT-erosion (n = 17) |
p value | |
|---|---|---|---|
| Fibrous cap thickness, μm | 60.4 ± 16.6 | 169.3 ± 99.1 | 0.001 |
| Maximal Lipid arc, ° | 275.8 ± 60.4 | 202.8 ± 73.6 | < 0.001 |
| Lipid length, mm | 13.1 ± 4.5 | 9.7 ± 23.2 | 0.008 |
Data are presented as mean ± SD.
Discussion
To our knowledge, this study represents the first systematic effort to utilize OCT to characterize the morphologies of the three most common causes of ACS. The major findings of the present study are: (i) OCT provides unique insights in patients with plaque erosion and calcified nodule in addition to plaque rupture; (ii) fibrous cap rupture was absent in more than half of culprit lesions; 31% of lesions were classified as OCT-erosion, 8% were classified as OCT-CN, and the remaining 17% were classified as others and did not meet the criteria of PR, OCT-erosion, or OCT-CN; (iii) patients with OCT-erosion were younger, had less severe stenosis, and less frequently presented with STEMI than those with PR. NSTE-ACS is the predominant presentation for the patients with OCT-erosion; (iv) lipid was less frequently detected in OCT-erosion than in PR. When lipid was present underneath OCT-erosion, overlying fibrous cap was thicker, lipid arc was smaller, and lipid length was shorter compared with those involved in PR.
In Vivo Detection of Plaque Erosion and Calcified Nodule Using Intravascular OCT
Coronary angiography is considered the gold standard diagnostic modality for the evaluation of patients presenting with ACS. However, angiography shows only the luminal outline and is not able to visualize intravascular structure. Although intravascular ultrasound (IVUS) is widely used to evaluate plaque morphology, including plaque burden and remodeling, the resolution is inadequate to characterize subtle changes in the vascular wall. For example, IVUS cannot be used to detect mural thrombus, thin fibrous cap, and irregular or eroded surface. OCT is a promising modality for in vivo identification of these characteristics, which are predominantly located on the superficial surface of plaques. A limited number of imaging studies have evaluated the role of plaque erosion and calcified nodule in the pathophysiology of ACS in vivo (10,11). Moreover, the definitions used in those studies were based purely on pathological findings (1) (loss of endothelial cell lines and/or dysfunction of endothelial cells) which are beyond the resolution of OCT. In the present study, we established new diagnostic criteria for OCT-erosion and OCT-CN based on pathologic findings but also taking into account the limitations of OCT and the differences between live patient and post-mortem evaluations. We utilized the proposed definitions to systematically classify the culprit lesions of patients with ACS. These definitions will be helpful for future OCT studies on investigating the underlying pathological mechanism of ACS.
Frequency of PR, OCT-erosion and OCT-CN in Patients with ACS
The most common underlying mechanisms responsible for acute coronary thrombosis are PR, plaque erosion, and calcified nodules (1). PR is a widely recognized cause of ACS and is the most common morphology associated with acute coronary thrombosis. A previous autopsy study reported that the prevalence of PR and erosion in postmortem subjects with AMI was 60% and 40%, respectively (5). Farb et al studied 50 consecutive SCD cases and found ruptures in 28 patients and erosions in 22 (12). Another autopsy study conducted by Hisaki et al reported 70 PR and 54 erosions in 124 lesions of 122 postmortem patients with ACS (13). These pathological studies indicate that coronary thrombosis results from PR and plaque erosions in about 55-60% and 33-44% of cases, respectively. The incidence of calcified nodules which represent the least frequent cause of luminal thrombosis in ACS, was reported 4-7% (1). Our study showed that the prevalence of PR in patients with ACS was 44%, while those of OCT-erosion and OCT-CN were 31% and 8%, respectively. One of reasons for the lower incidence of PR and OCT-erosion in the present study is likely due to a different population being studied. van der Wal et al studied only cases presenting with AMI, while Farb et al studied cases dying of SCD, and Hisaki et al studied cases dying of ACS. We studied typical patients presenting with the full range of ACS. Another reason is due to the selection of patients based on the ability to undergo OCT imaging. Patients with STEMI, large NSTEMI, and sicker patients would be less likely to undergo pre-intervention OCT imaging. This biases the study toward a patient population with more stable presentation and more NSTE-ACS. Given that PR is more common in STEMI the frequency of PR in our population might have been underestimated.
Clinical Characteristics of Patients with PR, OCT-erosion or OCT-CN
Autopsy studies have shown a significantly increased prevalence of plaque erosion in younger patients (< 50 years old), especially in younger females (12). Burke et al reported that smoking was associated with plaque erosion among female victims of sudden death (14). In the present study, we also found that patients with OCT-erosion are younger (< 55 years old) than those with rupture. However, OCT-erosions were not found more frequently in women than in men. This discrepancy could be due to the difference in populations studied (cases of SCD versus patients with ACS). Specifically, subjects evaluated in the postmortem studies were significantly younger than typical patients with a history of CAD and/or ACS. Furthermore, sudden cardiac death is dependent not only on the plaque pathology but also the relative thrombotic state of the patient and their propensity to develop a fatal arrhythmia. This raises the possibility of selection bias in evaluating the clinical characteristics of these patients. The population in this study was more representative of patients who are seen in clinical practice. Alternatively, we may be classifying lesions as plaque erosions by OCT that would not be diagnosed as such by pathology. However, we found that the frequency of STEMI was significantly higher in the patients with PR than others. In contrast, NSTE-ACS was predominant in patients with OCT-erosion and OCT-CN. These differences were consistent with the previous study, which reported that patients with plaque erosion had less STEMI on admission and less Q-wave MI than those with ruptures (15). Pathologically, calcified nodules are heavily calcified lesions consisting of calcified plates and overlying disrupted thin fibrous cap and thrombus, and are more common in older individuals (1,16). Recent studies showed that coronary calcification was more frequent and severe in patients with chronic kidney disease compared to those with normal renal function (17,18). These results support our findings that OCT-CN was observed more frequently in older patients (> 65 years old) with hypertension, chronic renal disease, and higher level of creatinine.
Underlying Plaque Characteristics of ACS
Previous work showed that plaque erosion occurred over lesions rich in smooth muscle cells and proteoglycans. The deep intima of the eroded plaque often showed extracellular lipid pools, but necrotic cores were uncommon (1). In the present study, all PR were detected in the context of lipid plaques. In contrast, 44% of OCT-erosions were detected in lipid plaques and 56% in fibrous plaques. This finding is consistent with pathological results that necrotic core was detected in 100% of PR and 47% of plaque erosion (6). Autopsy studies have shown that more than 88% of coronary thrombi overlying plaque erosions exhibited late stages of healing characterized by invasion of organized layers of smooth muscle cells, endothelial cells with varying degrees of platelet/fibrin layering. In patients with PR, only 50% of thrombi showed evidence of healing (6). In our study, fibrin rich red thrombus was frequently found over ruptured plaque, whereas platelet rich white thrombus was the predominant type of thrombus formed over OCT-erosion and OCT-CN.
Clinical Implication
The distinct pathologic features and clinical characteristics associated with PR, OCT-erosion, and OCT-CN suggest that they may be caused by different pathophysiologic processes, and therefore may merit tailored treatment. The present study also showed that the presentation with STEMI was more common in patients with PR, whereas NSTE-ACS was more frequent in those with OCT-erosion and OCT-CN. PR induces massive thrombus formation at the culprit site. In contrast, OCT-erosion appears to result in less thrombus burden, preserved vascular structure and larger lumen (6,12). Given these features, it is conceivable that patients with OCT-erosion may be stabilized by effective antithrombotic treatment without stent implantation, thereby avoiding both early and late complications associated with stent. However, further evidence is needed to support our findings to guide clinical practice.
Study Limitations
There are several limitations in our study. First, the present study involves a small cohort with ACS and is highly-selected based on the ability to undergo OCT imaging. However, this is the first in vivo study to systematically investigate and classify the underlying plaque characteristics of ACS lesions using intravascular imaging. Second, the definitions of plaque erosion and calcified nodule as detected by OCT were not validated by pathology in these patients. True pathologic validation is impossible because of the fundamental difference in analyzing patients who died from ACS, and those who survived and have been treated with antithrombotics. Specifically, intracoronary thrombus burden in patients treated for ACS would have been altered by treatment. Therefore, the diagnostic criteria utilized were established in collaboration with pathologist (RV), imaging specialist (JN), and clinicians. Third, the presence of thrombus overlying the culprit lesion may reduce the ability to assess the underlying plaque characteristics by OCT. Therefore, patients with massive occlusive thrombosis were excluded from our study. In addition, the pathologic definition of calcified nodules requires a fracture of the underlying calcified plate. OCT is not an ideal tool to visualize a deep fractured calcified plate. Finally, the absence of endothelial cells is a key pathological criterion for erosion. Despite its high resolution, current OCT technique cannot detect individual endothelial cells. As a result, the OCT definition of plaque erosion was based primarily on a diagnosis of exclusion requiring the absence of a fibrous cap rupture.
Conclusions
This study demonstrates that OCT is a promising modality for in vivo diagnosis of PR, OCT-erosion and OCT-CN. OCT-erosion is a frequent finding in patients with ACS, which accounts for 31% of cases in the present study. OCT-erosion is more frequent in younger patients with NSTE-ACS and has less severe luminal stenosis compared to PR. In addition, OCT-erosion has higher incidence of platelet-rich thrombus. OCT-CN is the least common etiology for ACS and is more common in older patients.
Supplementary Material
Acknowledgements
The MGH OCT Registry
Executive committee: William Dec (Massachusetts General Hospital and Harvard Medical School, Boston, MA); Ik-Kyung Jang (Massachusetts General Hospital and Harvard Medical School, Boston, MA), Kyoichi Mizuno (Nippon Medical School, Tokyo, Japan); Yang-Soo Jang (Severance Cardiovascular Hospital, Yonsei University, Seoul, Korea); Abhiram Prasad (Mayo Clinic, Rochester, MN).
Advisors: Valentin Fuster (The Mt. Sinai Medical Center, New York, NY); James Fujimoto (Massachusetts Institute of Technology).
Publications Committee: Jagat Narula (Mount Sinai Medical Center, New York, NY); Shiro Uemura (Nara Medical University, Nara, Japan); Jingbo Hou (The Second Affiliated Hospital of Harbin Medical University); Owen C. Raffel (Prince Charles Hospital, Brisbane, Australia)
Statistician: Hang Lee (Biostatistics, Massachusetts General Hospital, Harvard Medical School, Boston, MA)
Data Manager: Christina M. Kratlian (Massachusetts General Hospital, Boston, MA)
Investigators: Owen C. Raffel; Harry Lowe (Concord Repatriation General Hospital, Sydney, Australia); Peter Barlis (The Northern Hospital, Melbourne, Australia); Bo Yu (The 2nd Affiliated Hospital of Harbin Medical University); Stephen Lee (Queen Mary Hospital, Hong Kong University, Hong Kong); Tsunekazu Kakuta (Tsuchiura Kyodo General Hospital, Tsuchiura, Japan); Kyoichi Mizuno; ShiroUemura; Tomonori Itoh (Iwate Medical School, Morioka, Japan); Soo-Joong Kim (Kyung Hee Medical Center, Seoul, Korea); Chang-Bum Park (Kyung Hee University, Seoul, Korea); Yang-Soo Jang; So-Yeon Choi (Ajou University Hospital, Suwon, Korea); Seung-Jung Park (Asan Medical Center, Seoul, Korea); Stanley Chia (National Heart Centre Singapore, Singapore); Harold L. Dauerman (University of Vermont, Burlington, VT); Abhiram Prasad; Catalin Toma (University of Pittsburgh, Pittsburgh, PA) and Ik-Kyung Jang. The authors thank Christina M. Kratlian for her editorial expertise on the manuscript.
This study was supported by research grants from St. Jude Medical, the Cardiology Division of Massachusetts General Hospital. Dr. Jang has received research grants and consulting fees from LightLab Imaging/St. Jude Medical. Dr. Jia has received a grant from the National Natural Science Foundation of China (grant contract number: 81200076) and Open Foundation of Key Laboratory of Myocardial Ischemia (Harbin Medical University), Chinese Ministry of Education (KF201205). Dr. Aguirre is funded by NIH T32HL094301. Dr. Vergallo has received a grant from the Enrico ed Enrica Sovena Foundation, Italy. Dr. Yu received a grant from the National Natural Science Foundation of China (grant contract number: 30871064/C140401).
Abbreviations and Acronyms
- ACS
acute coronary syndrome
- AMI
acute myocardial infarction
- DM
diabetes mellitus
- IVUS
intravascular ultrasound
- NSTE-ACS
non-ST-segment elevation acute coronary syndrome
- OCT
optical coherence tomography
- STEMI
ST-segment elevation myocardial infarction
- SCD
sudden cardiac death
- TCFA
thin-cap fibroatheroma
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical trial info: Massachusetts General Hospital Optical Coherence Tomography (OCT) Registry Clinical Trial Registration; NCT01110538
References
- 1.Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000;20:1262–75. doi: 10.1161/01.atv.20.5.1262. [DOI] [PubMed] [Google Scholar]
- 2.Naghavi M, Libby P, Falk E, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part I. Circulation. 2003;108:1664–72. doi: 10.1161/01.CIR.0000087480.94275.97. [DOI] [PubMed] [Google Scholar]
- 3.Virmani R, Burke AP, Farb A, Kolodgie FD. Pathology of the vulnerable plaque. J Am Coll Cardiol. 2006;47:C13–8. doi: 10.1016/j.jacc.2005.10.065. [DOI] [PubMed] [Google Scholar]
- 4.Davies MJ. Anatomic features in victims of sudden coronary death. Coronary artery pathology. Circulation. 1992;85:I19–24. [PubMed] [Google Scholar]
- 5.van der Wal AC, Becker AE, van der Loos CM, Das PK. Site of intimal rupture or erosion of thrombosed coronary atherosclerotic plaques is characterized by an inflammatory process irrespective of the dominant plaque morphology. Circulation. 1994;89:36–44. doi: 10.1161/01.cir.89.1.36. [DOI] [PubMed] [Google Scholar]
- 6.Kramer MC, Rittersma SZ, de Winter RJ, et al. Relationship of thrombus healing to underlying plaque morphology in sudden coronary death. J Am Coll Cardiol. 2010;55:122–32. doi: 10.1016/j.jacc.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 7.Jang IK, Bouma BE, Kang DH, et al. Visualization of coronary atherosclerotic plaques in patients using optical coherence tomography: comparison with intravascular ultrasound. J Am Coll Cardiol. 2002;39:604–9. doi: 10.1016/s0735-1097(01)01799-5. [DOI] [PubMed] [Google Scholar]
- 8.Yabushita H, Bouma BE, Houser SL, et al. Characterization of human atherosclerosis by optical coherence tomography. Circulation. 2002;106:1640–5. doi: 10.1161/01.cir.0000029927.92825.f6. [DOI] [PubMed] [Google Scholar]
- 9.Tearney GJ, Regar E, Akasaka T, et al. Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: a report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J Am Coll Cardiol. 2012;59:1058–72. doi: 10.1016/j.jacc.2011.09.079. [DOI] [PubMed] [Google Scholar]
- 10.Kubo T, Imanishi T, Takarada S, et al. Assessment of culprit lesion morphology in acute myocardial infarction: ability of optical coherence tomography compared with intravascular ultrasound and coronary angioscopy. J Am Coll Cardiol. 2007;50:933–9. doi: 10.1016/j.jacc.2007.04.082. [DOI] [PubMed] [Google Scholar]
- 11.Rathore S, Terashima M, Matsuo H, et al. Association of coronary plaque composition and arterial remodelling: a optical coherence tomography study. Atherosclerosis. 2012;221:405–15. doi: 10.1016/j.atherosclerosis.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 12.Farb A, Burke AP, Tang AL, et al. Coronary Plaque Erosion Without Rupture Into a Lipid Core: A Frequent Cause of Coronary Thrombosis in Sudden Coronary Death. Circulation. 1996;93:1354–1363. doi: 10.1161/01.cir.93.7.1354. [DOI] [PubMed] [Google Scholar]
- 13.Hisaki R, Yutani C. Plaque morphology of acute coronary syndrome. J Atheroscler Thromb. 1998;4:156–161. [Google Scholar]
- 14.Burke AP, Farb A, Malcom GT, Liang Y, Smialek J, Virmani R. Effect of risk factors on the mechanism of acute thrombosis and sudden coronary death in women. Circulation. 1998;97:2110–6. doi: 10.1161/01.cir.97.21.2110. [DOI] [PubMed] [Google Scholar]
- 15.Hayashi T, Kiyoshima T, Matsuura M, et al. Plaque erosion in the culprit lesion is prone to develop a smaller myocardial infarction size compared with plaque rupture. Am Heart J. 2005;149:284–90. doi: 10.1016/j.ahj.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 16.Xu Y, Mintz GS, Tam A, et al. Prevalence, Distribution, Predictors, and Outcomes of Patients With Calcified Nodules in Native Coronary Arteries: A 3-Vessel Intravascular Ultrasound Analysis From Providing Regional Observations to Study Predictors of Events in the Coronary Tree (PROSPECT) Circulation. 2012;126:537–45. doi: 10.1161/CIRCULATIONAHA.111.055004. [DOI] [PubMed] [Google Scholar]
- 17.Budoff MJ, Rader DJ, Reilly MP, et al. Relationship of estimated GFR and coronary artery calcification in the CRIC (Chronic Renal Insufficiency Cohort) Study. Am J Kidney Dis. 2011;58:519–26. doi: 10.1053/j.ajkd.2011.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koukoulaki M, Papachristou E, Kalogeropoulou C, et al. Increased Prevalence and Severity of Coronary Artery Calcification in Patients with Chronic Kidney Disease Stage III and IV. Nephron Extra. 2012;2:192–204. doi: 10.1159/000339786. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.