Abstract
Snake venom disintegrins inhibit platelet aggregation and have anti-cancer activities. In this study, we report the cloning, expression, and functional activities of a recombinant disintegrin, r-viridistatin 2 (GenBank ID: JQ071899), from the Prairie rattlesnake. r-Viridistatin 2 was tested for anti-invasive and anti-adhesive activities against six different cancer cell lines (human urinary bladder carcinoma (T24), human fibrosarcoma (HT-1080), human skin melanoma (SK-Mel-28), human colorectal adenocarcinoma (CaCo-2), human breast adenocarcinoma (MDA-MB-231) and murine skin melanoma (B16F10)). r-Viridistatin 2 shares 96% and 64% amino acid identity with two other Prairie rattlesnake medium-sized disintegrins, viridin and viridistatin, respectively. r-Viridistatin 2 was able to inhibit adhesion of T24, SK-MEL-28, HT-1080, CaCo-2 and MDA-MB-231 to various extracellular matrix proteins with different affinities. r-Viridistatin 2 decreased the ability of T24 and SK-MEL-28 cells to migrate by 62 and 96% respectively, after 24 h of incubation and the invasion of T24, SK-MEL-28, HT-1080 and MDA-MB-231 cells were inhibited by 80, 85, 65 and 64% respectively, through a reconstituted basement membrane using a modified Boyden chamber. Finally, r-viridistatin 2 effectively inhibited lung colonization of murine melanoma cells in BALB/c mice by 71%, suggesting that r-viridistatin 2 could be a potent anti-cancer agent in vivo.
Keywords: Disintegrin, Recombinant snake toxins, Cancer cell lines, Anti-metastatic activity, Crotalus viridis viridis
1. Introduction
Cancer remains a major health problem worldwide, despite the efforts and progress of modern medicine in prevention, early detection, and treatment. Cancer is the second leading cause of mortality, surpassed only by cardiovascular disease (Peinado et al., 2011). A total of 1,596,670 new cancer cases and 571,950 deaths from cancer were projected to occur in the United States in 2011 (Siegel et al., 2011). The cause of death of most cancer patients is the development of metastases derived from primary tumors (Aokage et al., 2011). The metastatic process and colonization includes various complex steps that are a major challenge in clinical therapeutics (Gueron et al., 2011). Metastasis represents the final step in the progression of malignancy, the process is generally considered to follow a sequential cascade involving tumor cell intravasation, dissemination via the circulatory and/or lymphatic compartments, extravasation into a remote location, angiogenesis, and finally overt growth (Molloy and Van’t Veer, 2008).
Currently, the methods most often used to treat cancer are surgery, radio and chemotherapies, sometimes with limited success. Furthermore, these procedures also remove or destroy normal cells (Basanta et al., 2011; Navin and Hicks, 2011). Therefore, it is essential to search for new anti-cancer agents that have a different mode of action. The incorporation of biological molecules at much lower doses and with minimal side effects holds promise as a more effective anti-cancer treatment. Small molecule inhibitors and antibodies inhibiting growth factor receptors are currently being used to treat metastasis. For instance, tyrosine kinase inhibitors and antibodies against VEGF receptors are being used to reduce primary tumors and metastasis (Geiger and Peeper, 2009).
Snake venom contains components, some of which have found uses in the diagnosis and treatment of human diseases. In particular, disintegrins have already served as structural prototypes for the design and development of peptide and non-peptide mimetics for anti-thrombosis therapy (Sajevic et al., 2011; Markland, 1998). Disintegrins are a non-enzymatic group of small molecules (4–14 kDa) that bind selectively to integrin receptors. Most disintegrins have a “RGD” motif at the tip of a loop, which is responsible for binding to integrins. However, some disintegrins contain different functional motifs such as KGD, KTS, VGD, MGD, MLD, and MVD. Functionally, disintegrins are known to inhibit various biological activities, such as platelet aggregation, angiogenesis, metastasis, and tumor growth through their interaction with various integrins (Doley and Kini, 2009; McLane et al., 2004).
Disintegrins have previously shown good efficacy in the inhibition of different tumor cell lines in vitro and in animal cancer models. These small polypeptides hold a significant potential as anti-cancer agents based on their anti-angiogenic and anti-metastatic effects (Minea et al., 2012; Limam et al., 2010; Minea et al., 2010; Sánchez et al., 2009; Galán et al., 2008; Ramos et al., 2008; Tian et al., 2007; McLane et al., 2005; Minea et al., 2005; Selistre de Araujo et al., 2005). The purpose of this study was to test the anti-cancer activities of the recombinant disintegrin, r-viridistatin 2, in the presence of different tumoral cell lines.
2. Materials and methods
2.1. r-Viridistatin 2 subcloning
A full-length cDNA encoding a Crotalus viridis viridis venom metalloproteinase II was used (GenBank ID: GQ451440, Jia and Pérez, 2010) as a PCR template to subclone its disintegrin domain (designated as viridistatin 2). The forward primer was: 5′GGATCCGAGGCGGGAGAAGAATGTGACT3′ (a BamH I restriction site is underlined). The reverse primer was: 5′GAATTCTTAGGCATGGAAGCGATT3′ (an Eco R I restriction site is underlined). The PCR reaction was as follows: 94 °C for 1 min; 30 cycles of 94 °C for 30 s, 55 °C for 30 s, and 72 °C for 1 min. The amplified PCR product was digested with BamH I and EcoR I, and then ligated into a pGEX-4T-1 expression vector (GE Healthcare, NJ, and USA). The ligation was used to transform chemically competent E. coli cells BL21 Star (DE3) (Invitrogen, CA, USA) under ampicillin selection. Recombinant plasmids were isolated using a DNA miniprep kit (Sigma–Aldrich, MO, USA) and digested with BamH I and EcoR I to select plasmids containing inserts of the predicted size, and further sequenced to verify that the coding sequence was in-frame with the vector sequence that encodes the GST tag.
2.2. Expression and purification of r-viridistatin 2
r-Viridistatin 2 was expressed in E. coli and further purified by two-step chromatography, using the method of Sánchez et al. (2010). Briefly, E. coli BL21 cells were grown, induced by 0.5 mM of isopropyl β-D thiogalactoside (IPTG) and centrifuged. After bacterial cell disruption with a Branson Sonifier 450 (Danbury, CT), the cell debris was removed by centrifugation and the crude lysate was incubated with glutathione Sepharose 4B (GS4B) (Amersham Biosciences). r-Viridistatin 2 peptides were cleaved and eluted from glutathione S-transferase (GST) bound to GS4B by thrombin. Thrombin was removed from r-viridistatin 2 using a 1 mL HiTrap™ Benzamidine Sepharose 4 Fast Flow column (Amersham Biosciences). Purity of recombinant r-viridistatin 2 was determined by using a 10–20% Tricine gel (Schägger and von Jagow, 1987) in an Xcell SureLock Mini-Cell (Invitrogen Life Technologies, USA).
2.3. Inhibition of platelet aggregation
The inhibition of platelet aggregation study was done according to the Sánchez et al. (2010) method using a dual-channel Chronolog-Log Whole-Blood Aggregometer [Ca+2] model 560 (Havertown, USA). Briefly, different concentrations of r-viridistatin 2 (10 μL) were added to 10% citrated whole human blood, and pre-incubated at 37 °C for 2 min. Platelet aggregation was initiated by 10 μL of ADP (10 μM), and percentage of impedance reflecting percentage of aggregation was measured. The maximal aggregation in the absence of r-viridistatin 2 was given as 100% aggregation.
2.4. Cells lines and culture conditions
The human urinary bladder carcinoma cell line (T24), human fibrosarcoma (HT-1080), human skin melanoma (SK-Mel-28), human colorectal adenocarcinoma (CaCo-2), human breast adenocarcinoma (MDA-MB-231), and murine skin melanoma (B16F10) cell lines were obtained from the American Type Culture Collection (ATCC, Manassas, VA). T24 cells were maintained as a monolayer culture with McCoy’s 5A minimum essential medium, supplemented with 10% fetal calf serum (FBS) and 50 U/mL penicillin, 50 μg/mL streptomycin. HT-1080 and SK-Mel-28 cell lines were maintained with Eagle’s minimum essential medium, supplemented with 10% fetal calf serum and 50 U/mL penicillin, 50 μg/mL streptomycin. CaCo-2 cells were maintained with Eagle’s minimum essential medium, supplemented with 20% fetal calf serum and 50 U/mL penicillin, 50 μg/mL streptomycin. MDA-MB-231 cells were maintained with RPMI-1640 medium, supplemented with 10% fetal calf serum and 50 U/mL penicillin, 50 μg/mL streptomycin. B16F10 cells were maintained with Dulbecco’s modified Eagle’s medium, supplemented with 10% fetal calf serum and 50 U/mL penicillin, 50 μg/mL streptomycin. The cells were maintained in a humidified 5% CO2 air incubator at 37 °C.
2.5. Cellular adhesion inhibition assay
r-Viridistatin 2 was used to inhibit the binding of T24, SKMEL-28, HT-1080, CaCo-2 and MDA-MB-231 cells on different extracellular matrix proteins (fibronectin, laminin and collagen type IV at 10 μg/mL) coated plates (Sánchez et al., 2009). Commercial echistatin at 0.1 mg/mL (SIGMA, Lot. 023K12301), a disintegrin that blocks binding of tumor cells to fibronectin, was used as a positive control in some experiments (Sánchez et al., 2009). In the positive control, the tumor cells failed to bind to fibronectin. The negative control consisted of T24, SK-Mel-28, HT-1080, CaCo-2 and MDA-MB-231 cells incubated with PBS. The negative controls allowed binding of cells to extracellular matrix proteins. The percent inhibition was calculated by the following formula: [(absorbance of negative control–absorbance of cell/r-viridistatin 2 sample) ÷ absorbance of negative control] × 100.
2.6. Migration assays
Tumor cell migration was measured after scraping cells from the bottom of the well as described by Galán et al. (2008). Commercial echistatin at 2.7 μM, a disintegrin that blocks migration of tumor cells, was used as a positive control. The positive controls prevent tumor cells migration. The negative control consisted of T24, SK-Mel-28, HT-1080, and MDA-MB-231 cells incubated with PBS, which allowed cell migration to occur. Cells were then incubated in a CO2 chamber and were only removed from the incubator for microscopy images at times 0, 3, 6, 12, and 24 h after disintegrin incubation. The concentration of r-viridistatin 2 used was 6.3 μM. Percent of closure was calculated by the following equation: % Closure: [(C–E)/C] × 100, where C is the units of distance of cell edge (mm) at zero time for each sample, and E is the distance from the cell edge (mm) at each incubation time (3–24 h).
2.7. In vitro invasion assays
The T24, HT-1080, SK-ML-28 and MDA-MB-231 invasion assays were performed using a modified method of Yang et al. (2005). Transwells (8.0-μm pore size; Corning) containing polycarbonate filters (Transwell inserts) were coated with Matrigel, a basement membrane matrix extracted from Engelbreth-Holm-Swarm mouse sarcoma, which consists of collagen type IV, heparan sulfate proteoglycan, entactin and laminin (Becton Dickinson). Matrigel was diluted to 2 μg/mL using serum-free McCoy’s 5A minimum essential medium, Eagle’s minimum essential medium or RPMI-1640 medium at 4 °C and an aliquot (40 μL) of Matrigel (80 μg/well) was added to each filter insert and incubated for 30 min at 37 °C to form a uniform three-dimensional gel. Then, the lower chamber was filled with 0.6 mL of medium supplemented with 10% fetal calf serum. Tumor cells (7.5 × 105 cells/mL) were incubated with or without r-viridistatin 2 (6.3 μM) for 30 min and aliquots (200 μL) of cells were plated at the upper chamber of the Transwell. Previously, we showed that r-mojastin 1 inhibited invasion of T24 and SK-MEL-28 (Lucena et al., 2011), therefore, in this study, r-mojastin 1 at 6.3 μM was used as positive control. After 24-h incubation, all nonmigrant cells were removed from the upper membrane of the Transwell with a cotton swab, and the migrant cells were fixed and stained with 0.5% toluidine blue in 4% paraformaldehyde. Invasion was quantified by counting the number of stained cells on the lower membrane per 40 × field and photographed with a light microscope (Nixon, Japan).
2.8. In vivo inhibition of lung colonization of melanoma cells
Murine melanoma cells (B16F10), (5.0 × 106 cells/mL) were suspended in Dulbecco’s modified Eagle’s medium without FBS in the presence or absence of r-viridistatin 2 at 1000 μg/kg per mouse and incubated 1 h at 37 °C. Controls consisted of mice injected with B16F10 cells in Dulbecco’s modified Eagle’s medium without FBS and mice injected with medium alone. Two hundred microliters of cells/r-viridistatin 2 mixture were injected intravenously (IV) in the lateral tail vein of BALB/c mice (18–20 g). Mice were sacrificed 19 days post injection, and lungs were examined for the presence of tumors. Lungs were visualized with a 4× stereomicroscope. The tumors were counted for statistical analysis.
2.9. Characterization of integrin expression on HT-1080 cells
The following antibodies were used to determine integrin expression on HT-1080 cells. Antibodies against αvβ3 (αvβ3 sc-7312-FITC), α1 (α1 sc-81733-FITC), α2 (α2 sc-53352-FITC), α3 (α3 sc-13545-FITC), α6 (α6 sc-19622-FITC), αv (αv-53360-FITC), β1 (β1 sc-9970-FITC), β3 (β3 sc-51738-FITC), and respective isotypes were purchased from Santa Cruz Biotechnology. Antibodies against αvβ5 (MAB1961F) were purchased from Millipore. Cells were cultured to 90–95% confluency in a 75 cm2 tissue culture flask and detached from the flask surface using 0.05% trypsin EDTA. Trypsin was neutralized with complete culture media. Cells were resuspended in 1× PBS to a final concentration of 106 cells/mL, and blocked with 1 μL/mL normal rabbit serum, for 25 min at room temperature. After blocking, cells were washed with 1 mL 1× PBS, centrifuged, and resuspended in FCM Wash Buffer (Santa Cruz Biotechnology) to 106 cells/mL. One hundred microliters of cells were distributed in three, 12 × 75 mm round bottom glass test tubes and fixed in 1% formaldehyde for 1 h. After fixation, cells were washed three times in 1× PBS. The first tube received 5–20 μL of a cocktail containing fluorochrome-conjugated monoclonal antibody. The second tube received the same volume of matched isotype control antibody. The third tube of cells received an equivalent volume of 1× PBS. This served as an auto-fluorescence control to establish appropriate flow cytometer settings. Cells were incubated with conjugated antibodies or control for 1 h, at room temperature, in the dark. Three washes with 1× PBS were performed to remove unbound antibodies. Four hundred microliters of 1× binding buffer were added and the samples analyzed using a Becton Dickinson FACScan flow cytometer and FACSCalibur software.
2.10. Apoptosis assay
Five hundred thousand SK-Mel-28 cells were seeded in six wells of a 12 well plate and allowed to grow for 24 h. Cells were treated for 18 h with 5 μM r-viridistatin 2, equal volume of PBS buffer, or no treatment. All cells were treated at the same final volume. Non-adherent cells were transferred to a flow cytometry tube, followed by detachment of the remaining cells with 0.05% trypsin EDTA. Cells were pelleted by centrifugation at 300 × g for 5 min and fixed in 1% paraformaldehyde for 1 h. Cells were washed twice with 1× PBS and resuspended in 70% ethanol. Cell suspensions were stored at −20 °C for 24 h. Cells were processed using an APO-BRDU™ Apoptosis Detection Kit (BD Biosciences) according to the manufacturer’s protocol. Cell suspensions were analyzed using a Becton Dickinson FACSCalibur flow cytometer with CellQuest software. Experiments were performed in triplicate.
2.11. Statistical analyses
Results of wound healing and invasion were expressed as the mean ± standard deviation (n = 3), and analyzed using the one way-Anova test followed by Newman–Keuls Multiple Comparison Test, using the software program Graph Pad Prism. A two tailed t-test followed by Mann Whitney test was used to determine the significance of r-viridistatin 2 and the control in inhibiting the number of tumors in vivo. Differences were statistically significant if p was less than 0.05.
3. Results
3.1. r-Viridistatin 2 expression and purification
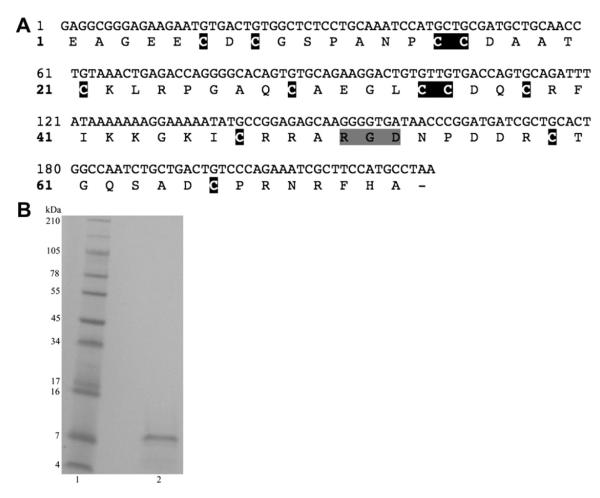
Fig. 1A shows the predicted amino acid sequence of the cloned r-viridistatin 2 cDNA. r-Viridistatin 2 contains 73 amino acids with a molecular weight of 7.85 kDa. It begins with a Glu amino acid and ends with an Ala and has a RGDNP motif. Purified r-viridistatin 2 peptide is shown on Fig. 1B (Lane 2). Fig. 2 shows the comparison of r-viridistatin 2 with two other C. v. viridis disintegrins identified in other studies. r-Viridistatin 2 shows a 64 and 96% homology with viridistatin (Soto et al., 2006) and viridin (Scarborough et al., 1993), respectively.
Fig. 1.
A) cDNA sequence and predicted amino acid sequence of r-viridistatin 2. The cDNA sequence is located on the upper line and the amino acid sequence on the lower line. The cysteine residues are shaded in black and the RGD motif is shaded in gray. B) SDS Gel electrophoresis. r-Viridistatin 2 was run reduced on a 10–20% Tricine gel. The gel was run with 1× Tricine SDS running buffer using an XCell SureLock Mini-cell at 125 V for 90 min. The gel was stained with Simply Blue Safe Stain for 1 h and destained with Milli-Q water. Lane 1) See Blue Plus 2 markers; Lane 2) r-viridistatin 2 (20 μg).
Fig. 2.

Amino acid sequence comparison of Crotalus viridis viridis disintegrins. Viridistatin 2 (Accession # JQ071899; this work); viridistatin (Soto et al., 2006); viridin (Scarborough et al., 1993). Viridistatin 2 has a 96% and a 64% amino acid sequence homology with viridin and viridistatin, respectively. Viridin was isolated from the snake venom and the viridistatins were cloned from venom glands. The gray shaded area indicates amino acid homology.
3.2. Inhibition of platelet aggregation
Disintegrins were first described as potent inhibitors of the platelet fibrinogen receptor, integrin αIIbβ3 (Calvete et al., 2005), thus, the inhibition of platelet aggregation is the test more frequently used for the initial evaluation of the disintegrin activity. r-viridistatin 2 inhibited ADP-induced platelet aggregation in whole human blood and this effect was concentration-dependent with an IC50 of 34 nM (Fig. 3).
Fig. 3.
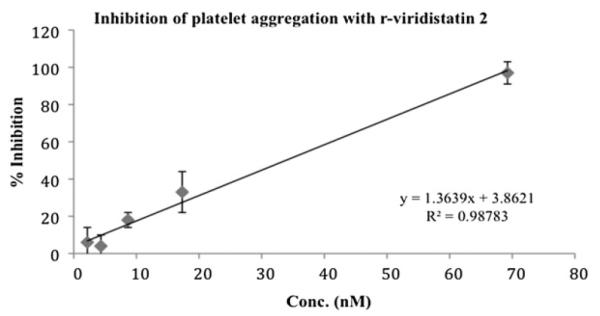
Inhibition of platelet aggregation using whole blood by r-viridistatin 2. A Chronolog aggregometer was used to measure ADP-induced platelet aggregation by impedance. A total of 10 μL of r-viridistatin 2 at varying concentrations was added to whole blood and incubated 2 min at 37 °C prior to adding 10 μM of ADP.
3.3. Inhibition of cell adhesion in presence of fibronectin, laminin and collagen
Adhesion, migration, and invasion of tumor cells to the vascular extracellular matrix are a major part of metastasis. We therefore, evaluated the effect of r-viridistatin 2 on these functions using different tumor cell lines. r-Viridistatin 2 inhibited T24, SK-Mel-28, HT-1080, CaCo-2 and MDA-MB-231 adhesion to fibronectin, laminin and collagen, in a dose-dependent manner, with different affinities depending of the extracellular matrix proteins used (Table 1). The greatest inhibition was observed in presence of fibronectin with T24 and SK-MEL-28 cells.
Table 1.
Adhesion of T24, SK-MEL-28, HT-1080, CaCo-2 and MDA-MB-231 cells to extracellular matrix proteins (ECM) in presence of recombinant r-viridistatin 2.
| ECM | Inhibition of cell adhesion T24 cellsa |
Inhibition of cell adhesion SK-Mel-28 cellsa |
Inhibition of cell adhesion HT-1080 cellsa |
Inhibition of cell adhesion CaCo-2 cellsa |
Inhibition of cell adhesion MDA-MB-231 cellsa |
|---|---|---|---|---|---|
| Fibronectin | 11 nM | 12 nM | 2406 nM | 996 nM | 4459 nM |
| Laminin | 305 nM | 228 nM | NB | 4813 nM | NB |
| Collagen | 1862 nM | 492 nM | NA | NA | NA |
NA: No activity.
NB: The HT-1080 and MDA-MB-231 cells did not bind to laminin.
The results are expressed as IC50.
3.4. Inhibition of cell migration
r-Viridistatin 2 at 6.3 μM allowed T24 and SK-MEL-28 cells to migrate by 38 and 4%, respectively after 24 h. This was in comparison to the negative control, PBS, which allowed both T24 and SK-MEL-28 cells to migrate by 100%. In contrast, r-viridistatin 2 at the same concentration allowed both HT-1080 and MDA-MB-231 cells to migrate by 100% after 24 h. Commercial echistatin, used as positive control of inhibition (2.7 μM), permitted cell migration by 71 and 16% with T24 and SK-MEL-28 cells, respectively, and allowed cell migration by 100 and 89% with HT-1080 and MDA-MB-231 cells, respectively (data not shown). In comparison to the negative control, a statistically significant difference with r-viridistatin 2 (p < 0.05) was observed with T24 and SK-MEL-28 cells (Table 2).
Table 2.
Migration of T24, SK-MEL-28, HT-1080 and MDA-MB-231 cells in presence of recombinant r-viridistatin 2.
| Time (h) | T24 | SK-Mel-28 | HT-1080 | MDA-MB-231 |
|---|---|---|---|---|
| 3 | 10 ± 9 | 0 ± 0 | 20 ± 0 | 3 ± 0 |
| 6 | 13 ± 6 | 4 ± 2 | 53 ± 8 | 9 ± 4 |
| 12 | 20 ± 5 | 7 ± 0 | 93 ± 9 | 16 ± 4 |
| 24 | 38 ± 3 | 4 ± 3 | 100 ± 0 | 100 ± 0 |
Data are expressed as mean ± SD (n = 3). The results are expressed in percentage migration and calculated by the equation shown in materials and methods section.
3.5. Inhibition of cell invasion
The ability of r-viridistatin 2 to block the invasion of T24, SK-MEL-28, HT-1080 and MDA-MB-231 cells through a reconstituted basement membrane was assessed using a modified Boyden chamber assay. T24, SK-MEL-28, HT-1080 and MDA-MB-231 cells migrated toward the medium containing fetal bovine serum used as chemoattractant (Fig. 4). r-Viridistatin 2 (6.3 μM) inhibited invasion of T24, SK-MEL-28, HT-1080 and MDA-MB-231 by 80%, 85%, 65%, and 64%, respectively. r-Mojastin 1 (6.3 μM) used as positive control inhibited invasion of T24, SK-MEL-28 and MDA-MB-231 by 85%, 69%, and 67%, respectively, but was not able to inhibit invasion of HT-1080 cells. r-Viridistatin 2 was more potent at inhibiting invasion of HT-1080 and SK-MEL-28 cells than r-mojastin 1(p < 0.05).
Fig. 4.
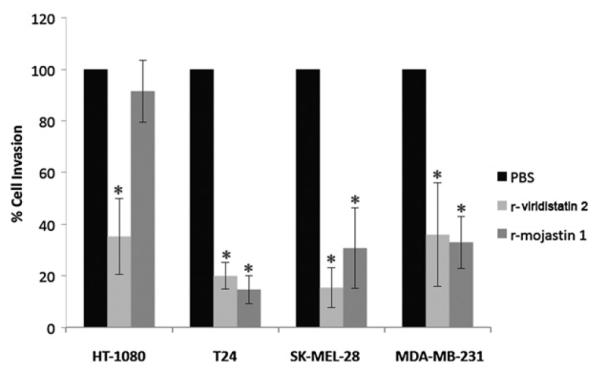
Inhibition of HT-1080, SK-MEL-28, T24 and MDA-MB-231 cells invasion in vitro by r-viridistatin 2. Tumor cells (7.5 × 105 cells/mL, 0.2 mL) were treated with 6.3 μM of r-viridistatin 2 for 30 min at 37 °C, and then placed in the upper Boyden chamber containing a matrigel-coated filter membrane. Invasion was induced by adding McCoy’s 5A minimum essential medium, Eagle’s minimum essential medium and RPMI-1640 medium with FBS to the lower chamber for 24 h. After fixation and removal of non-migrated cells, the cells that migrated to the underside of filter membrane were quantified by light microscopy (3 fields/40×). *p < 0.05 (r-disintegrins compared to negative control).
3.6. In vivo inhibition of lung colonization of melanoma cells
To investigate the inhibitory effect of r-viridistatin 2 on lung colonization in vivo, r-viridistatin 2 (19 μg) incubated with 200 μL of B16F10 melanoma cells (1 × 106) was injected IV in BALB/c mice. r-Viridistatin 2 inhibited lung colonization by 71.4% at a dose of 1000 μg/kg, compared to the control group (p-value = 0.0019, Table 3).
Table 3.
Comparative analysis of tumor foci per lung in BALB/c mice using B16F10 cells and r-viridistatin 2 at 1000 μg/kg compared to controls.
| Controlb | r-Viridistatin 2 | |
|---|---|---|
| # mice | 14 | 16 |
| Minimum tumors | 4 | 0 |
| Maximum tumors | 64 | 21 |
| Mean tumors | 28 | 8 |
| Standard deviation | 19.72 | 6.5 |
| p-Valuea | 0.0019 |
p-Value as compared to the control. p < 0.05 = significant difference.
The control consisted of B16F10 tumor cells in Dulbecco’s modified Eagle’s medium without FBS. Percent of lung colonization inhibition was calculated by the following equation: % Inhibition: [(Mean tumors (control)–Mean tumors (r-viridistatin 2))/Mean tumors (control)] × 100.
3.7. Characterization of integrin expression on HT-1080 cells
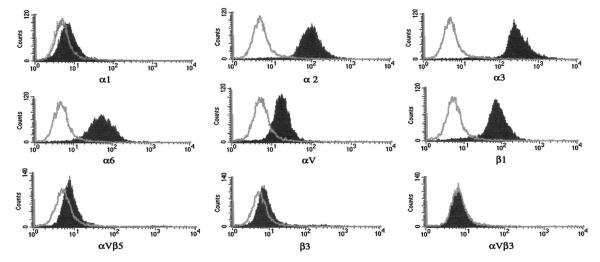
Flow cytometer was used to characterize integrin expression on HT-1080 cells, as the first step in determining which integrins bind to r-viridistatin 2. Our results showed that HT-1080 express α2, α3, α6, αv, β1, α1, β3 and αvβ5 (the last three at very low levels). The cells do not express αvβ3 (Fig. 5).
Fig. 5.
Integrin expression of HT-1080 cells. Flow cytometry and human anti-integrin monoclonal antibodies were used to determine integrin expression. Isotype (negative control antibody) profile is indicated as a light gray line. Specific anti-human integrin monoclonal antibody profile is indicated as a gray shaded graph.
3.8. Apoptosis detection
A tunnel assay was used to determine if r-viridistatin (5 μM) has the ability to induce apoptosis of SK-Mel-28 cells after 24 h of treatment. r-Viridistatin 2 failed to induce apoptosis of SK-Mel-28 cells (data not shown).
4. Discussion
4.1. r-Viridistatin 2 expression and purification
The r-viridistatin 2 contained 73 amino acids showing 96 and 64% homology with viridin (Scarborough et al., 1993) and viridistatin (Soto et al., 2006), respectively (Fig. 2).
The participation of integrins in the inflammatory process, vascular diseases, and cancer is well known. Therefore the characterization of integrins antagonists is an interesting subject of study and disintegrins appear as putative candidates to be used as effective tools for cancer therapy (Higuchi et al., 2011). The expression of recombinant versions of interesting disintegrins has become essential, thus facilitating the maintenance of a continuous supply for drug development (Sánchez et al., 2010). When the recombinant technology is used, there is always the concern, when a bacterial expression is used, that these highly disulfide-bonded proteins will be misfolded, thus affecting activity (McLane et al., 2004). However, there is evidence showing that recombinant disintegrins can conserve their anti-cancer activity comparable to that of the native disintegrins. (Lucena et al., 2011; Minea et al., 2012, 2010, 2005).
Despite their enormous therapeutical potentials, to our knowledge none of these recombinant disintegrins have been used in human clinical trials for treating cancers. However, many of these polypeptides continue to be intensely investigated in various animal models in order to find a component that can someday be used therapeutically.
4.2. Role of integrins in metastasis
Metastasis consists of a number of different but intricately interrelated biological processes, all of which are required to work in synchrony for a tumor cell to successfully migrate from the primary tumor and grow in a distant organ (Molloy and Van’t Veer, 2008).
Integrins are heterodimeric transmembrane receptors composed of eighteen α subunits and eight β subunits that can be non-covalently assembled into 24 combinations. The integrin dimers bind to an array of different extracellular matrix protein (ECM) molecules with overlapping binding affinities. Therefore, the specific integrin expression patterns by a cell dictate which ECM substrate the cell can bind (Kim et al., 2011). Integrins play a pivotal role in normal homeostasis as well as oncogenic transformation. For the various types of cancers, different changes in integrin expression are further associated with tumor growth and metastasis. Tumor progression leading to metastasis appears to involve equipping cancer cells with the appropriate adhesive (integrin) phenotype for interaction with the ECM (Mizejewski, 1999).
Some toxins from snake venom specifically and potently inhibit integrin functions. Among these, several disintegrins have demonstrated their anti-cancer properties. For instance, r-contortrostatin (CN), a recombinant disintegrin derived from the venom of Agkistrodon contortrix contortrix, has been shown to possess potent anti-tumor and anti-angiogenic activities in an orthotopic, xenograft model of human breast cancer, with an inhibitory effect similar to the native contortrostatin (Minea et al., 2005). Vicrostatin (VCN), a chimeric recombinant disintegrin, has a direct effect on breast cancer cells inhibiting their in vitro motility. VCN delayed tumor growth and increased animal survival (Minea et al., 2012, 2010). Leucurogin, albolabrin, salmosin, accutin, r-mojastin-1 or rhodostomin have anti-metastatic and/or anti-angiogenic activities (Higuchi et al., 2011; Lucena et al., 2011; Yeh et al., 2001, 1998; Kang et al., 1999; Soszka et al., 1991).
Multimodal therapies with the ability to simultaneously inhibit the multiple biological steps required for metastatic spread will be highly beneficial. In this work, a recombinant disintegrin, r-viridistatin 2 inhibited different steps of the metastasis cascade of different tumor cell lines. r-Viridistatin 2 inhibited adhesion of T24, SK-MEL-28, HT-1080, CaCo-2 and MDA-MB-231 to various extracellular matrix proteins with different affinities. The greatest inhibition was shown with T24 and SK-MEL-28 cells in presence of fibronectin.
The cell type and composition of the surrounding matrix, along with tissue origin, determine which sets of integrins are critical in transducing downstream survival signals (Desiniotis and Kyprianou, 2011). Development of integrin cell expression profiles for individual tumors may have potential in identifying a cell surface signature for a specific tumor type and/or stage. The SK-Mel-28 cells express αvβ3, α6, αv, β1, α2 and β3 integrin receptors, while T24 cells express α1, α3, α6, αv, β1, β3, αvβ3 and αvβ5 (Teklemariam et al., 2011; Seoane et al., 2010). In this study, we demonstrated that the HT-1080 cells express α2, α3, α6, αv, β1, α1, β3 and αvβ5 integrins (the last three at very low levels).
HT-180 cells did not bind to laminin, although expressed integrins bind this ECM protein. Similar results were obtained with MDA-MB-231 cells. However, it should be noted that the laminin from Engelbreth-Holm-Swarm tumor used in this study is usually considered laminin-1 (Patarroyo et al., 2002). Numerous integrins, including α1β1, α2β1, α3β1, α6β1, α7β1, αvβ3, and α6β4, are known to recognize laminin isoforms, and in this recognition, distinct and overlapping ligand specificities are found. For example, laminin-5 is recognized by α3β1 but not by α7β1 integrins; whereas laminin-1 binds α7β1 but not α3β1 (Patarroyo et al., 2002). There are several possible explanations for the results obtained with HT-1080 and MDA-MB-231 and laminin. Perhaps these cells do not express α7, an integrin subunit important for binding laminin-1. Also, the binding specificity of an integrin extracellular domain is determined largely by the conformation adopted by the individual α and β chains, which comprise the complex. However, cellular context matters too; the same cDNA transfected into two different cells lines can result in the cells acquiring different binding activities (Belkin and Stepp, 2000). Thus, functional difference in integrins response may be due to cell type-specific modulation. Therefore, although the cells used in this study share some integrins that bind laminin, the response does not necessarily have to be similar.
4.3. Cell migration and invasion inhibition
Cell migration is an essential process in organ homeostasis, in inflammation, and also in metastasis. The ECM serves as the molecular scaffold for cell adhesion and migration. To invade tissues and vessels, cells must acquire the ability to migrate (Geiger and Peeper, 2009). Integrins are emerging as important players in metastasis behavior. Many integrins mediate tumor cell migration and invasion of the basement membrane and its ECM. For example, over-expression of α3β1 integrin by tumor cells results in directed proteolysis for tumor cell invasion (Rathinam and Alahari, 2010). α6β4 integrin, which binds to laminin, forms signaling complexes with oncogenic receptor tyrosine kinases, including Met, HER2 and the EGFR. In addition, α6β4 over-expression in colon cancer cells correlates with their invasive capacity (Brooks et al., 2010). αvβ3 mediates vascular invasion, its binding to L1 endothelial cells promotes melanoma cell migration towards blood vessels and its binding to vitronectin upregulates MMP-2 expression leading to stromal degradation (Wang et al., 2004; Felding-Habermann et al., 2001). Furthermore, αvβ3 and α3β1 are involved in cancer cell adhesion to basement membrane underling the endothelium (Brooks et al., 2010).
αvβ3 is one of the best characterized integrins because it plays an essential role in angiogenesis and vascular remodeling (Janik et al., 2010). Normally its expression is minimal in endothelial cells, leukocytes and macrophages, but it has been shown to be up-regulated in many types of cancer, including melanoma, breast, lung, colon and pancreatic carcinomas (Janik et al., 2010). Integrin αvβ3 is involved in regulating migration of malignant cells through their appropriate binding with MMP-9 or urokinaseplasminogen receptor (uPAR) (Rathinam and Alahari, 2010). r-Viridistatin 2 decreased the ability of SK-MEL-28 and T24 cells to migrate after 24 h of incubation, but was unable to inhibit HT-1080 and MDA-MB-231 cell migration. A possible integrin target for r-viridistatin 2 binding and responsible for migration inhibition of SK-MEL-28 and T24 cells may be αvβ3, a receptor found on both cell lines but not on HT-1080. The αvβ3 integrin is expressed in very low levels on MDA-MB-231 cells (Taherian et al., 2011; Wong et al., 1998), this may explain the absence of migration inhibition observed in our study.
In addition, r-viridistatin 2 inhibited the invasion of SKMEL-28, T24 and MDA-MB-231 cells through a reconstituted basement membrane using a modified Boyden chamber. The activation, production, and release of various proteases by cancer cells lead to specifically modified, integrin-mediated signaling cascades. Metastatic cancer cells move through the basement membrane of the tumor, and integrins facilitate this movement by inducing the degradation of the basement membrane by proteolytic enzymes. For instance, αvβ3, αvβ5, αvβ6, α6β4, and α9β1 exhibit high expression in cancer, and they are involved in the degradation of the basement membrane through interaction with proteolytic enzymes like MMP-2 and MMP-9 (Rathinam and Alahari, 2010). Integrins α6β1 and α6β4 are laminin-binding receptors that are involved in regulating tumor cell invasion (Rathinam and Alahari, 2010). In the specific condition of the invasion assay where the studies were performed in presence of a reconstituted basal membrane, r-viridistatin 2 could possibly inhibit the integrin αvβ3 present in SK-MEL-28 and T24, without excluding other possible integrins that might be targeted by r-viridistatin 2, such as αvβ5 integrin, which is highly expressed in MDA-MB-231 cells and has been reported to mediate cancer cell invasion (Taherian et al., 2011; Zhou et al., 2000; Wong et al., 1998).
5. Conclusions
An important test to verify the effectiveness of the disintegrins as anti-cancer agents is the inhibition of lung tumor colonization in vivo. The anti-metastatic effects of three disintegrins, jarastatin from Bothrops jararaca, kistrin from Calloselasma rhodostoma and flavoridin from Trimeresurus flavoridis were evaluated on B16F10 melanoma cells through a metastatic assay, demonstrating that the three disintegrins significantly reduced tumor lung colonization in C57BL/6 mice (Oliva et al., 2007). DisBa-01 disintegrin from Bothrops alternatus, when injected intravenously in C57BL/6 mice together with B16F10 melanoma cells, time and dose dependently, inhibited lung metastasis monitored by bioluminescent imaging (Ramos et al., 2008). In our study, r-viridistatin 2 effectively inhibited lung colonization of murine melanoma cells in BALB/c mice by 71%, suggesting that r-viridistatin 2 could be a potent anti-cancer agent in vivo.
Our results suggest that r-viridistatin 2 may be helpful as a model in the design of new therapeutic strategies in cancers in terms of its anti-invasive and anti-adhesive activities such as the inhibition of adhesion, migration, invasion, and lung tumor colonization of different tumor cell lines. These results also demonstrated that the effect of r-viridistatin 2 was tumor cell line specific, confirming the heterogeneity of each cell line, and highlighting the importance of developing therapies that account for the different cellular and molecular mechanisms used in the invasion process used by different tumor cells.
Acknowledgments
Funding for this project was provided by NCRR/Viper # 5P40RR018300-09 (Texas A&M University-Kingsville), NIH/SCORE # 2SO6 GM008192, NIH Grant #R25GM071381 (San Jose State University), NSF/SUCCESS #DUE-07-03118 and USDA/STELLAR #2007-38422-18084 (Del Mar College). Thanks for the technical assistance by Nora Diaz Deleon, Angela Wyro and Mark Hockmuller (NNTRC serpentarium curator). We would also like to thank Drs. Daiyuan Zhang, Rob Hatherill and Jonda Halcomb from Del Mar College.
Footnotes
Conflict of interest statement The authors declare that there is no conflict of interest.
References
- Aokage K, Ishii G, Ohtaki Y, Yamaguchi Y, Hishida T, Yoshida J, Nishimura M, Nagai K, Ochiai A. Dynamic molecular changes associated with epithelial-mesenchymal transition and subsequent mesenchymal-epithelial transition in the early phase of metastatic tumor formation. Int. J. Cancer. 2011;128:1585–1595. doi: 10.1002/ijc.25500. [DOI] [PubMed] [Google Scholar]
- Basanta AM, Chapman C, Dorsey JF, Rengan R, Hahn SM. A glimpse of the future: where will new combinations of diagnosis and therapies take us? Cancer J. 2011;17:190–194. doi: 10.1097/PPO.0b013e3182216bc0. [DOI] [PubMed] [Google Scholar]
- Belkin AM, Stepp MA. Integrins as receptors for laminins. Microsc. Res. Tech. 2000;51:280–301. doi: 10.1002/1097-0029(20001101)51:3<280::AID-JEMT7>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Brooks SA, Lomax-Browne HJ, Carter TM, Kinch CE, Hall DMS. Molecular interactions in cancer cell metastasis. Acta Histochem. 2010;112:3–25. doi: 10.1016/j.acthis.2008.11.022. [DOI] [PubMed] [Google Scholar]
- Calvete JJ, Marcinkiewicz C, Monleón D, Esteve V, Celda B, Juárez P, Sanz L. Snake venom disintegrins: evolution of structure and function. Toxicon. 2005;45:1063–1074. doi: 10.1016/j.toxicon.2005.02.024. [DOI] [PubMed] [Google Scholar]
- Desiniotis A, Kyprianou N. Significance of talin in cancer progression and metastasis. Int. Rev. Cell Mol. Biol. 2011;289:117–147. doi: 10.1016/B978-0-12-386039-2.00004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doley R, Kini RM. Protein complexes in snake venom. Cell Mol. Life Sci. 2009;66:2851–2871. doi: 10.1007/s00018-009-0050-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felding-Habermann B, O’Toole TE, Smith JW, Fransvea E, Ruggeri ZM, Ginsberg MH, Hughes PE, Pampori N, Shattil SJ, Saven A, Mueller BM. Integrin activation controls metastasis in human breast cancer. Proc. Natl. Acad. Sci. USA. 2001;98:1853–1858. doi: 10.1073/pnas.98.4.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galán JA, Sánchez EE, Rodríguez-Acosta A, Soto JG, Bashir S, McLane MA, Paquette-Straub C, Pérez JC. Inhibition of lung tumor colonization and cell migration with the disintegrin crotatroxin 2 isolated from the venom of Crotalus atrox. Toxicon. 2008;51:1186–1196. doi: 10.1016/j.toxicon.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger TR, Peeper DS. Metastasis mechanisms. Biochim. Biophys. Acta. 2009;2:293–308. doi: 10.1016/j.bbcan.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Gueron G, De Siervi A, Vazquez E. Key questions in metastasis: new insights in molecular pathways and therapeutic implications. Curr. Pharm. Biotechnol. 2011;12:1–14. doi: 10.2174/138920111798376996. [DOI] [PubMed] [Google Scholar]
- Higuchi DA, Almeida MC, Barros CC, Sanchez EF, Pesquero PR, Lang EAS, Samaan M, Araujo RC, Pesquero JB, Pesquero JL. Leucurogin, a new recombinant disintegrin cloned form Bothrops leucurus (white-tailed-jararaca) with potent activity upon platelet aggregation and tumor growth. Toxicon. 2011;58:123–129. doi: 10.1016/j.toxicon.2011.05.013. [DOI] [PubMed] [Google Scholar]
- Janik ME, Litynska A, Vereecken P. Cell migration-The role of integrin glycosylation. Boichim. Biophys. Acta. 2010;1800:545–555. doi: 10.1016/j.bbagen.2010.03.013. [DOI] [PubMed] [Google Scholar]
- Kang IC, Lee YD, Kim DS. A novel disintegrin salmosin inhibits tumor angiogenesis. Cancer Res. 1999;59:3754–3760. [PubMed] [Google Scholar]
- Kim SH, Turnbull J, Guimond S. Extracellular matrix and cell signaling: the dynamic cooperation of integrin, proteoglycan and growth factor receptor. J. Endocrinol. 2011;209:139–151. doi: 10.1530/JOE-10-0377. [DOI] [PubMed] [Google Scholar]
- Limam I, Bazaa A, Srairi-Abid N, Taboubi S, Jebali J, Zouari-Kessentini R, Kallech-Ziri O, Mejdoub H, Hammami A, El Ayeb M, Luis J, Marrakchi N. Leberagin-C, a disintegrin-like/cysteine-rich protein from Macrovipera lebetina transmediterranea venom, inhibits alphavbeta3 integrin-mediated cell adhesion. Matrix Biol. 2010;29:117–126. doi: 10.1016/j.matbio.2009.09.009. [DOI] [PubMed] [Google Scholar]
- Lucena S, Sanchez EE, Perez JC. Anti-metastatic activity of the recombinant disintegrin, r-mojastin-1, from the Mohave rattlesnake. Toxicon. 2011;5:794–802. doi: 10.1016/j.toxicon.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markland FS. Snake venoms and the hemostatic system. Toxicon. 1998;36:1749–1800. doi: 10.1016/s0041-0101(98)00126-3. [DOI] [PubMed] [Google Scholar]
- McLane MA, Sánchez EE, Wong A, Paquette-Straub C, Pérez JC. Disintegrins. Curr. Drug Targets-Cardiovasc. Haematol. Dis. 2004;4:327–355. doi: 10.2174/1568006043335880. [DOI] [PubMed] [Google Scholar]
- McLane MA, Zhang X, Tian J, Zelinskas C, Srivastava A, Hensley B, Paquette-Straub C. Scratching below the surface: wounding healing and alanine mutagenesis provide unique insights into interactions between eristostatin, platelets and melanoma cells. Pathophysiol. Haemost. Thromb. 2005;34:164–168. doi: 10.1159/000092417. [DOI] [PubMed] [Google Scholar]
- Minea R, Swenson S, Costa F, Chen T, Markland FS. Development of a novel recombinant disintegrin, Contortrostatin, as a effective anti-tumor and anti-angiogenic agent. Pathophysiol. Haemost. Thromb. 2005;34:177–183. doi: 10.1159/000092419. [DOI] [PubMed] [Google Scholar]
- Minea R, Helchowski CM, Zidovetzki SJ, Costa FK, Swenson S, Markland FS. Vicrostatin-An anti-invasive multi-integrin targeting chimeric disintegrin with tumor anti-angiogenic and proapoptotic activities. PLoS ONE. 2010;5(6):e10929. doi: 10.1371/journal.pone.0010929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minea R, Helchowski C, Rubino B, Brodmann K, Swenson S, Markland F., Jr. Development of a chimeric recombinant disintegrin as a cost-effective anti-cancer agent with promising translational potential. Toxicon. 2012;59:472–486. doi: 10.1016/j.toxicon.2011.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizejewski GJ. Role of integrins in cancer: survey of expression patterns. Proc. Soc. Exp. Biol. Med. 1999;2:124–138. doi: 10.1177/153537029922200203. [DOI] [PubMed] [Google Scholar]
- Molloy T, Van ’t Veer L. Recent advances in metastasis research. Curr. Opin. Genet. Dev. 2008;18:35–41. doi: 10.1016/j.gde.2008.01.019. [DOI] [PubMed] [Google Scholar]
- Navin N, Hicks J. Future medical applications of single-cell sequencing in cancer. Genome Med. 2011;3:51. doi: 10.1186/gm247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva IB, Coelho RM, Barcellos GG, Saldanha-Gama R, Wermelinger LS, Marcinkiewicz C, Benedeta ZR, Barja-Fidalgo C. Effect of RGD-disintegrins on melanoma cell growth and metastasis: involvement of the actin cytoskeleton, FAK and c-Fos. Toxicon. 2007;15:1053–1063. doi: 10.1016/j.toxicon.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Patarroyo M, Tryggvason K, Virtanen I. Laminin isoforms in tumor invasion, angiogenesis and metastasis. Semin. Cancer Biol. 2002;12:197–207. doi: 10.1016/S1044-579X(02)00023-8. [DOI] [PubMed] [Google Scholar]
- Peinado H, Lavotshkin S, Lyden D. The secreted factors responsible for pre-metastatic niche formation: old sayings and new thoughts. Semin. Cancer Biol. 2011;21:139–146. doi: 10.1016/j.semcancer.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Ramos O, Kauskot A, Cominetti MR, Bechyne I, Salla CL, Chareyre F, Manent J, Vassy R, Giovannini M, Legrand C, Selistre de Araujo H, Crépin M, Bonnefoy A. A novel avb3 blocking disintegrin containing the RGD motive, DisBa-01, inhibits bFGF-induced angio-genesis and melanoma metastasis. Clin. Exp. Metastasis. 2008;25:53–64. doi: 10.1007/s10585-007-9101-y. [DOI] [PubMed] [Google Scholar]
- Rathinam R, Alahari SK. Important role of integrins in the cancer biology. Cancer Metastasis Rev. 2010;29:223–237. doi: 10.1007/s10555-010-9211-x. [DOI] [PubMed] [Google Scholar]
- Sajevic T, Leonardi A, Krizaj I. Haemostatically active proteins in snake venoms. Toxicon. 2011;57:627–645. doi: 10.1016/j.toxicon.2011.01.006. [DOI] [PubMed] [Google Scholar]
- Sánchez EE, Rodriguez AA, Palomar R, Lucena SE, Bashir S, Soto JG, Pérez JC. Colombistatin: a disintegrin isolated from the venom of the South American snake (Bothrops colombiensis) that effectively inhibits platelet aggregation and SK-MEL-28 cell adhesion. Arch. Toxicol. 2009;83:27–327. doi: 10.1007/s00204-008-0358-y. [DOI] [PubMed] [Google Scholar]
- Sánchez EE, Lucena SE, Reyes S, Soto JG, Cantu E, Lopez-Johnston JC, Guerrero B, Salazar AM, Rodríguez-Acosta A, Galán JA, Tao WA, Pérez JC. Cloning, expression, and hemostatic activities of a disintegrin, r-mojastin 1, from the Mohave rattlesnake (Crotalus scutulatus scutulatus) Thromb. Res. 2010;126:211–219. doi: 10.1016/j.thromres.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarborough RM, Rose JW, Naughton MA, Phillips DR, Nannizzi L, Arfsten A, Campbell AM, Charo IF. Characterization of the integrin specificities of disintegrins isolated from American pit viper venoms. J. Biol. Chem. 1993;268:1058–1065. [PubMed] [Google Scholar]
- Schägger H, von Jagow G. Tricine-sodium dodecyl sulfatepolyacrylamide gel electrophoresis for the separation of proteins in the range of 1 to 100 kDa. Anal. Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Selistre de Araujo HS, Cominetti MR, Terruggi CHB, Mariano-Oliveira A, De Freites MS, Crepin M, Figueiredo CC, Morandi V. Alternagin-C, a disintegrin-like protein from the venom of Bothrops alternatus, modulates a2b1 integrin-mediated cell adhesion, migration and proliferation. Braz. J. Med. Biol. Res. 2005;38:1505–1511. doi: 10.1590/s0100-879x2005001000007. [DOI] [PubMed] [Google Scholar]
- Seoane AI, Tran VL, Sánchez EE, White SA, Choi JL, Gaytan B, Chavez N, Reyes SR, Ramos CJ, Tran LH, Lucena SE, Sugarek M, Perez JC, Mandal SA, Ghorab S, Rodriguez-Acosta A, Fung BK, Soto JG. The mojastin mutant Moj-DM induces apoptosis of the human melanoma SK-MEL-28, but not the mutant Moj-NN nor the non-mutated recombinant Moj-WN. Toxicon. 2010;56:391–401. doi: 10.1016/j.toxicon.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011. CA: Cancer J. Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- Soszka T, Knudsen KA, Bevigilia L, Rossi C, Poggi A, Niewiarowski S. Inhibition of murine melanoma cell-matrix adhesion and experimental metastasis by albolabrin, and RGD-containing peptide isolated from the venom of Trimeresurus albolabris. Exp. Cell Res. 1991;196:6–12. doi: 10.1016/0014-4827(91)90449-5. [DOI] [PubMed] [Google Scholar]
- Soto JG, White SA, Reyes SR, Regalado R, Sanchez EE, Perez JP. Molecular evolution of PIII-SVMP and RGD disintegrin genes from the genus Crotalus. Gene. 2006;389:66–72. doi: 10.1016/j.gene.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Taherian A, Li X, Liu Y, Haas TA. Differences in integrin expression and signaling within human breast cancer cells. BMC Cancer. 2011;11:293. doi: 10.1186/1471-2407-11-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teklemariam T, Seoane AI, Ramos CJ, Sánchez EE, Lucena SE, Perez JC, Mandal SA, Soto JG. Functional analysis of a recombinant PIII-SVMP, GST-acocostatin; an apoptotic inducer of HUVEC and HeLa, but not SK-MEL-28 cells. Toxicon. 2011;57:646–656. doi: 10.1016/j.toxicon.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian J, Paquette-Straub C, Sage E, Funk SE, Patel V, Galileo D, McLane MA. Inhibition of melanoma cell motility by the snake venom disintegrin eristostatin. Toxicon. 2007;49:899–908. doi: 10.1016/j.toxicon.2006.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Fu W, Im JH, Zhou Z, Santoro SA, Iyer V, DiPersio CM, Yu QC, Quaranta V, Al-Mehdi A, Muschel RJ. Tumor cell alpha3beta1 integrin and vascular laminin-5 mediate pulmonary arrest and metastasis. J. Cell Biol. 2004;164:935–941. doi: 10.1083/jcb.200309112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong NC, Mueller BM, Barbas CF, Ruminski P, Quaranta V, Lin ECK, Smith JW. αv Integrins mediate adhesion and migration of breast carcinoma cell lines. Clin. Exp. Metastasis. 1998;16:50–61. doi: 10.1023/a:1006512018609. [DOI] [PubMed] [Google Scholar]
- Yang R, Tang C, Chuang W, Huang T, Peng H, Huang T, Fu W. Inhibition of tumor formation by snake venom disintegrin. Toxicon. 2005;45:661–669. doi: 10.1016/j.toxicon.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Yeh CH, Peng HC, Huang TF. Accutin, a new disintegrin, inhibits angiogenesis in vitro and in vivo by acting as integrin alphavbeta3 antagonist and inducing apoptosis. Blood. 1998;92:3268–3276. [PubMed] [Google Scholar]
- Yeh CH, Peng HC, Yang RS, Huang TF. Rhodostomin, a snake venom disintegrin, inhibits angiogenesis elicited by basic fibroblast growth factor and suppresses tumor growth by a selective alpha(v) beta(3) blockade of endothelial cells. Mol. Pharmacol. 2001;59:1333–1342. doi: 10.1124/mol.59.5.1333. [DOI] [PubMed] [Google Scholar]
- Jia Y, Pérez JC. Molecular cloning and characterization of cDNAs encoding metalloproteinases from snake venom glands. Toxicon. 2010;55:462–469. doi: 10.1016/j.toxicon.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Nakada MT, Brooks PC, Swenson SD, Ritter MR, Argounova S, Arnold C, Markland FS. Contortrostatin, a homodimeric disintegrin, binds to integrin avb5. Biochem. Biophys. Res. Commun. 2000;267:350–355. doi: 10.1006/bbrc.1999.1965. [DOI] [PubMed] [Google Scholar]