Abstract
Background
We report here the logistic modeling of the epidemiologic differences between a diagnostic and screening population recruited for the study of optical technologies for cervical cancer detection.
Methods
Epidemiologic data were obtained from a risk factor interview as a component of a multicenter Phase II clinical trial which employed fluorescence and reflectance point spectroscopy to diagnose cervical disease. Participants with a recent or past abnormal Papanicolaou smear were grouped into the diagnostic (high-risk) population, while those with a history of normal Papanicolaou smears and no cervical treatments were grouped into the screening (low-risk) population.
Results
Our model revealed that non-white race, greater than a high school education, and peri- and postmenopausal status were associated with the screening population. Meanwhile, a history of genital infections, current OC use, HPV positivity (by Hybrid Capture II and consensus PCR), and histology at clinic visit were important predictors of being in the diagnostic group.
Conclusions
We were successful in recruiting two distinctive populations, and we anticipate being able to use these results to more correctly classify women at higher risk for cervical lesions in our future studies of optical spectroscopy.
Keywords: cervical dysplasia, epidemiology, optical technologies, risk factors
Background
Cervical cancer is the second most common and third most deadly cancer among women worldwide, with an estimated 493,243 new cases and 273,505 deaths in 2002 alone1, and has been causally linked to human papillomavirus (HPV) infection2. Eighty-three percent of all cases occur in the developing world1, likely due to the absence of well-established screening programs to detect pre-cancerous dysplasia3. With early detection, death from cervical cancer is preventable and five-year survival is approximately 92%4. Yet, despite this life-saving advantage, such programs present a tremendous economic burden to society. Insinga, et al. estimated that the annual cost of cervical HPV-related disease amounted to 3.4 billion dollars in the United States in 19985. A noticeable portion of this expense (300 million dollars) of this was devoted to “treating” false positive Papanicolaou smears5. It would therefore be frugal, especially in the developing world (or other low-resource settings), to preferentially screen the populations of women who are most susceptible to the acquisition of this malignancy.
Additionally, more effective means of diagnosis and treatment (ideally simultaneously) would decrease not only the wait time associated with diagnosis, but would also reduce the overall number of healthcare visits required to treat pre-neoplastic lesions of the cervix. Our group has a keen interest in further developing optic technologies for the rapid diagnosis and treatment of cervical dysplasia. We have focused our attention on fluorescence and reflectance spectroscopy. We have been able to show differences between normal and abnormal cervical tissue in vitro and in vivo using these techniques. The spectroscopic system used to measure fluorescence excitation-emission matrices (EEMs) has been described in detail previously.6,7 Briefly, the system measures fluorescence emission spectra at 16 excitation wavelengths, ranging from 330 to 480 nm in 10 nm increments with a spectral resolution of 5 nm. The system incorporates a fiber optic probe, a xenon arc lamp coupled to a monochromator to provide excitation light, and a polychromator and thermoelectrically cooled charge coupled device camera to record fluorescence intensity as a function of emission wavelength.
Real-time diagnosis of cervical dysplasia using these optical techniques could greatly advance medical treatment of women diagnosed with these conditions. In developed countries this could greatly reduce the number of office visits and unnecessary biopsies. In developing countries, this could translate into the implementation of screening programs that do not rely on highly-trained medical professionals.
Several risk factors for cervical cancer have been repeatedly identified—specifically, smoking, genital infections, parity, oral contraceptive (OC) use, age of sexual debut, and number of lifetime partners8. However, the exact contribution of each to the natural history of disease is still unclear, as study results to date have been inconclusive. Important to this process, is identifying those factors that are more predictive of persistent HPV infection, i.e. those at high-risk of developing dysplasia or cancer. In this report, we attempt to address the controversies arising from conflicting studies through the logistic modeling of epidemiologic differences between a diagnostic and screening population recruited for the study of optical technologies for cervical cancer detection.
Methods
Data source
Epidemiologic data were obtained from a risk factor interview as a component of a multicenter Phase II clinical trial which employed fluorescence and reflectance point spectroscopy to diagnose cervical disease. The trial was conducted at The University of Texas M. D. Anderson Cancer Center and the Lyndon Baines Johnson Harris County Hospital in Houston, Texas, United States, as well as the British Columbia Cancer Agency in Vancouver, British Columbia, Canada. The trial began in 1999 and concluded in 2005, and enrolled women 18 years of age or older. Women who had had a hysterectomy or who were pregnant at the time of enrollment were ineligible. The institutional review board at each institution approved the protocols, and written consent was obtained from all participants.
Participants with a recent or past abnormal Papanicolaou smear were grouped into the diagnostic (high-risk) population, while those with a history of normal Papanicolaou smears and no cervical treatments were grouped into the screening (low-risk) population. Specific study procedures included: a risk factor interview, a complete medical history, a full physical and gynecologic exam, regular and ThinPrep (Cytyc, Corp., Marlborough, MA) Papanicolaou smears, cervical cultures, specimens for HPV typing, pan-colposcopy of the vulva, vagina, and cervix, spectroscopic measurements of the cervix, and biopsies. Each risk factor interview was conducted in a secluded area by a research nurse and qualified translator, if necessary. All other clinical procedures were performed by a nurse practitioner or study physician.
HPV typing and confirmation of disease status
HPV typing was completed through the use of Hybrid Capture II (Digene Corporation, Gaithersberg, Maryland) and quantitative PCR for the most common high-risk types (16 and 18). Cervical samples for PCR were placed into phosphate-buffered saline with 0.05% sodium azide and stored at -20°C. DNA was extracted using a commercially available kit (Qiagen, Valencia, CA). PCR for glycerol-3-phosphate dehydrogenase was performed to ensure the integrity of the DNA. Successful amplification was measured by the detection of a 450-base pair product. PCR for HPV DNA was performed with two L1 primers (MY-9 and MY-11) which recognize a 450-base pair region in the L1 open reading frame of at least 28 different types of HPV9. PCR products were transferred onto nylon membranes (Bio-Rad, Hercules, CA) and separately hybridized to 32P-labeled consensus and type specific HPV 16 and HPV 18 probes. Detection was performed via autoradiography after incubation for 24h at -80°C. DNA from HPV 18 positive HeLa cells, HPV 16 positive Caski cells, and a negative control without any DNA served as controls during PCR and hybridization procedures.
Biopsy specimens were fixed in buffered formalin and embedded in blocks of paraffin within an hour of their collection by the pathology laboratory at each institution using a standard protocol. For histopathologic diagnosis, four sets of three adjacent sections were cut at 4 μm and separately stained with hematoxylin and eosin (H&E) and thionin-Feulgen. Since each patient had two to four biopsies, it was possible for one patient to have multiple diagnoses. Therefore, we chose the worst diagnosis for each patient as the “true” disease status. In the present study though, classification into either the high- or low-risk population was based solely on each participant’s Papanicolaou smear history. Thus, biopsies here serve to demonstrate that the high-risk population truly has a greater prevalence of disease.
Statistical analysis
Differences in demographics by screening or diagnostic population were determined by Chi Square test for categorical variables or t-test for continuous variables using a commercially available statistical software package (SPSS, Inc., Chicago, IL). Prevalence ratios were used to assess the impact of each variable on the level of “risk”. Logistic regression modeling was performed utilizing the methods of Hosmer and Lemeshow10. A list of potential covariates was established based on the study hypothesis and suggestions from the literature.11,12 These included: race, age, annual household income, education level, employment status, marital status, ever smoking, alcohol use, history of genital infections, menopausal status, parity, current OC use, current use of condoms, HPV status by Hybrid Capture II, type of HPV DNA present, and the worst histologic diagnosis. Variables significant at the 0.20 alpha level in univariate analyses were considered for inclusion in the final multivariate model. Proper functional form (e.g., linear, dichotomous) was determined utilizing the Quartile Method10. Diagnostic graphs (residuals, leverage, and influence) were plotted to assess the fit of the final model for all covariate patterns. Any covariate patterns found to have unnecessary influence on the model were excluded from the analysis. Regression analyses were performed in Stata, v8.2 (Stata Corp, College Station, TX), and two-sided p-values are reported.
Results
In all, 850 diagnostic and 1,000 screening patients were recruited. As shown in Table 1, in every classification of disease considered abnormal, i.e., the histology diagnosis is greater than or equal to HPV Associated Changes, prevalence ratios were in fact greater in the diagnostic population, thus lending support to our classification scheme. It should be noted that biopsies showing Atypia were classified as normal. Recently, Atypia has represented a growing problem of being overcalled by pathologists. This may help explain the large percentages of potentially confounding Atypia results in our data. Any presence of disease in the screening population, as evidenced by a negative Papanicolaou smear history and positive biopsy, may be the development of a lesion after the smear was taken, a previously missed lesion, or a false negative. Likewise, any absence of disease in the diagnostic population, as evidenced by a positive Papanicolaou smear history and a negative biopsy, may represent the regression of a lesion, an overcalled smear, or a small lesion missed by the biopsy.
Table 1.
Histology and HPV infection, Diagnostic and Screening populations, Houston, TX and Vancouver, BC, 1999-2005.
| Diagnostic Population | Screening Population | Prev. Ratio | p Value | |
|---|---|---|---|---|
|
| ||||
| Worst Histology | 800 | 973 | <0.0001 | |
|
| ||||
| Normal | ||||
| Negative for Dysplasia | 227 (28.4%) | 638 (65.6%) | 0.43 | |
| Atypia | 145 (18.1%) | 207 (21.3%) | 0.85 | |
| Abnormal | ||||
| HPV Associated Changes | 96 (12.0%) | 90 (9.2%) | 1.30 | |
| CIN I (Mild Dysplasia) | 104 (13.0%) | 19 (2.0%) | 6.66 | |
| CIN II (Moderate Dysplasia) | 96 (12.0%) | 6 (0.6%) | 19.46 | |
| CIN III (Severe Dysplasia) | 81 (10.1%) | 10 (1.0%) | 9.85 | |
| CIS | 45 (5.6=%) | 0 (0.0%) | -- | |
| Cancer | 5 (0.6%) | 0 (0.0%) | -- | |
| No Diagnosis Possible | 1 (0.1%) | 3 (0.3%) | 0.41 | |
|
| ||||
| Hybrid Capture II | 831 | 981 | <0.0001 | |
|
| ||||
| Negative | 410 (49.3%) | 868 (88.5%) | 0.56 | |
| Low-risk | 24 (2.9%) | 17 (1.7%) | 1.67 | |
| High-risk | 337 (40.6%) | 83 (8.5%) | 4.79 | |
| Both | 60 (7.2%) | 13 (1.3%) | 5.45 | |
|
| ||||
| PCR | 759 | 773 | ||
|
| ||||
| Type-16 Positive | 226 (29.8%) | 140 (18.1%) | 1.64 | <0.0001 |
| Type-18 Positive | 123 (16.2%) | 115 (14.9%) | 1.09 | <0.473 |
| Consensus Positive | 650 (85.6%) | 533 (69.0%) | 1.24 | <0.0001 |
Prevalence of HPV infection
When considering the results from Hybrid Capture II, the diagnostic population was more positive for both low- and high-risk HPV types (PR=1.67 and 4.79 respectively, p<0.0001) when compared to the screening population. PCR results were similar, with the diagnostic group having a significantly higher prevalence of HPV 16 (PR=1.64, p<0.0001) when compared to the screening group. The diagnostic group was also more likely to be HPV consensus positive and HPV 18 positive, however the difference in HPV 18 positivity was not statistically significant (p<0.473).
Demographic and epidemiologic differences
Tables 2a and b summarize the results from the risk factor interview. A greater percentage of the diagnostic population was white, Asian, Native American, or of other race (p<0.0001) compared to the screening population. The diagnostic population was also younger, with a mean age of 36.6 years compared to 44.1 years, yielding a difference of 7.5 years (p<0.0001). The age range within the two groups was similar: 18-85 years for the diagnostic population, and 18-80 years for the screening population. Finally, women in the diagnostic population were more likely to be born in the United States, Canada, or China.
Table 2.
a. Demographic, socioeconomic, and health-related factors, Diagnostic and Screening populations, Houston, TX and Vancouver, BC, 1999-2005.
| Diagnostic Population | Screening Population | Prev. Ratio | p Value | |
|---|---|---|---|---|
|
| ||||
| Race | 850 | 1000 | <0.0001 | |
|
| ||||
| White | 543 (63.9%) | 491 (49.1%) | 1.3 | |
| Black | 94 (11.1%) | 154 (15.4%) | 0.72 | |
| Hispanic | 112 (13.2%) | 276 (27.6%) | 0.48 | |
| Asian | 63 (7.4%) | 67 (6.7%) | 1.11 | |
| Native American | 8 (0.9%) | 3 (0.3%) | 3.14 | |
| Other | 30 (3.5%) | 9 (0.9%) | 3.92 | |
|
| ||||
| Age | 850 | 1000 | <0.0001 | |
|
|
|
|||
| Mean ± Std Dev (Yrs) | 36.6 ± 11.8 | 44.1 ± 12.1 | ||
| Range (Yrs) | 18-85 | 18-80 | ||
|
|
|
|||
| Birthplace | 820 | 999 | <0.0001 | |
|
| ||||
| United States/Canada | 663 (80.9%) | 676 (67.7%) | 1.19 | |
| Mexico | 27 (3.3%) | 74 (7.4%) | 0.44 | |
| Central America | 11 (1.3%) | 33 (3.3%) | 0.41 | |
| South America | 12 (1.5%) | 78 (7.8%) | 0.19 | |
| Puerto Rico | 3 (0.4%) | 6 (0.6%) | 0.61 | |
| Vietnam | 4 (0.5%) | 5 (0.5%) | 0.97 | |
| China | 13 (1.6%) | 11 (1.1%) | 1.44 | |
| Other | 87 (10.6%) | 116 (11.6%) | 0.91 | |
|
| ||||
| Annual Household Income* | 816 | 998 | <0.0001 | |
|
| ||||
| Low ($0-$19,999) | 161 (19.7%) | 177 (17.7%) | 1.11 | |
| Medium ($20,000-$39,999) | 197 (24.1%) | 285 (28.6%) | 0.85 | |
| High (> $40,000) | 352 (43.1%) | 464 (46.5%) | 0.93 | |
| Don’t Know/Refused | 106 (13.0%) | 72 (7.2%) | 1.80 | |
|
| ||||
| Education Level | 844 | 999 | <0.131 | |
|
| ||||
| High School/GED or Less | 239 (28.3%) | 242 (24.2%) | 1.17 | |
| College | 501 (59.4%) | 622 (62.3%) | 0.95 | |
| Graduate School | 104 (12.3%) | 135 (13.5%) | 0.91 | |
|
| ||||
| Employment Status | 834 | 999 | <0.0001 | |
|
| ||||
| Full/Part Time | 565 (67.7%) | 679 (68%) | 1.00 | |
| Unemployed/Retired/Housewife | 185 (22.2%) | 293 (29.3%) | 0.76 | |
| Student | 83 (10.0%) | 25 (2.5%) | 3.98 | |
| Refused | 1 (0.1%) | 2 (0.2%) | 0.60 | |
|
| ||||
| Martial Status | 849 | 1000 | <0.0001 | |
|
| ||||
| Single (Never Married) | 244 (28.7%) | 198 (19.8%) | 1.45 | |
| Married/Married-Like Situation | 410 (48.3%) | 595 (59.5%) | 0.81 | |
| Divorced/Separated | 175 (20.6%) | 181 (18.1%) | 1.14 | |
| Widowed | 18 (2.1%) | 26 (2.6%) | 0.82 | |
| Refused | 2 (0.2%) | 0 (0.0%) | -- | |
|
| ||||
| b. Demographic, socioeconomic, and health-related factors, Diagnostic and Screening populations, Houston, TX and Vancouver, BC, 1999-2005. | ||||
|
| ||||
| Smoking | 848 | 1000 | <0.0001 | |
|
| ||||
| Ever, Yes | 374 (44.0%) | 337 (33.7%) | 1.31 | |
| Ever, No | 473 (55.9%) | 663 (66.3%) | 0.84 | |
| Refused | 1 (0.1%) | 0 (0.0%) | -- | |
|
|
||||
| Length of Time (Yrs) | 371 | 337 | <0.003 | |
|
|
|
|||
| Mean ± Std Dev (Yrs) | 13.44 ± 9.61 | 15.81 ± 11.54 | ||
| Range (Yrs) | 0-57 | 0-51 | ||
|
|
|
|||
| Alcohol Use | 849 | 999 | <0.001 | |
|
| ||||
| Yes | 615 (72.4%) | 645 (64.6%) | 1.12 | |
| No | 234 (27.6%) | 353 (35.3%) | 0.78 | |
| Refused | 0 (0.0%) | 1 (0.1%) | 0.00 | |
|
| ||||
| Genital Infections | 827 | 986 | <0.0001 | |
|
| ||||
| Any, Yes | 277 (33.5%) | 204 (20.7%) | 1.62 | |
| Any, No | 550 (66.5%) | 782 (79.3%) | 0.84 | |
|
| ||||
|
Reproductive History
| ||||
| Status | 850 | 999 | <0.0001 | |
|
| ||||
| Premenopausal | 707 (83.2%) | 568 (56.9%) | 1.46 | |
| Perimenopausal | 24 (2.8%) | 131 (13.1%) | 0.22 | |
| Postmenopausal | 119 (14.0%) | 300 (30.0%) | 0.47 | |
|
|
||||
| Menarche | 847 | 1000 | <0.224 | |
|
|
|
|||
| Mean ± Std Dev (Yrs) | 12.63 ± 1.54 | 12.72 ± 1.62 | ||
| Range (Yrs) | 8-19 | 8-19 | ||
|
|
|
|||
| Parity | 850 | 993 | <0.0001 | |
|
|
|
|||
| Mean ± Std Dev (Yrs) | 1.31 ± 1.42 | 1.67 ± 1.55 | ||
| Range (Yrs) | 0-9 | 0-14 | ||
|
|
||||
| Current Oral Contraceptive Use | 850 | 993 | <0.0001 | |
|
| ||||
| Yes | 214 (25.2%) | 71 (7.2%) | 3.52 | |
| No | 636 (74.8%) | 922 (92.8%) | 0.81 | |
|
|
||||
| Current Condom Use | 824 | 978 | <0.001 | |
|
|
||||
| Yes | 180 (21.8%) | 151 (15.4%) | 0.71 | |
| No | 644 (78.2%) | 827 (84.6%) | 1.08 | |
|
| ||||
|
Sexual History
| ||||
| Age of Sexual Debut | 841 | 987 | <0.0001 | |
|
|
|
|||
| Mean ± Std Dev (Yrs) | 17.47 ± 3.27 | 18.95 ± 3.94 | ||
| Range (Yrs) | 2-32 | 4-50 | ||
|
|
|
|||
| Number of Lifetime Partners | 795 | 955 | <0.122 | |
|
|
|
|||
| Mean ± Std Dev (Yrs) | 9.05 ± 21.74 | 6.91 ± 33.47 | ||
| Range (Yrs) | 0-500 | 0-1000 | ||
Stratification into Low, Medium, and High is with respect to the currency of the country where each participant was seen (United States or Canada).
Annual household income was analyzed using equivalent income brackets in the currency of the participant’s home country (USD or CAD). Women in the diagnostic population were more like to report an annual income in the lowest income bracket ($0-$19,999), whereas women in the screening population were more likely to report a higher annual income. There was no significant difference in the educational level of the two populations; however, more women in the screening population tended to have a college or advanced degree. Employment status was similar for both groups, except for a significantly higher proportion of students in the diagnostic population. This seems to account for the overall significant difference in employment status between the two populations. Women in the diagnostic population were also more likely to be single or divorced/separated, when compared to the screening population (p<0.0001).
Table 3 summarizes the proportional risks as calculated. A higher proportion of the diagnostic population reported having ever smoked (PR=1.31, p<0.0001). Among smokers, the screening population reported a longer period of smoking; however, this may be due to the screening population being older than the diagnostic group. More women in the diagnostic population also reported any alcohol use (72.4% versus 64.6% in the screening group).
Table 3.
a. Univariate and multivariate logistic results, Diagnostic and Screening populations, Houston, TX and Vancouver, BC, 1999-2005.
| Univariate POR (95% CI) | p Value | Multivariate POR (95% CI) | p Value | |
|---|---|---|---|---|
|
| ||||
| Race | ||||
|
| ||||
| White | 1.00 | -- | 1.00 | -- |
|
| ||||
| Black | 0.55 (0.42-0.73) | <0.001 | 0.29 (0.19-0.43) | <0.0001 |
|
| ||||
| Hispanic | 0.37 (0.29-0.47) | <0.001 | 0.24 (0.17-0.35) | <0.0001 |
|
| ||||
| Other | 1.16 (0.84-1.59) | <0.372 | 1.14 (0.71-1.83) | <0.597 |
|
| ||||
| Age* | ||||
|
| ||||
| Years | 0.95 (0.94-0.96) | <0.001 | 0.99 (0.97-1.01) | <0.210 |
|
| ||||
|
Annual Household Income
|
||||
| Low ($0-$19,999) | 1.00 | -- | ||
| Medium ($20,000-$49,999) | 0.80 (0.61-1.04) | <0.094 | ||
| High (> $50,000) | 0.81 (0.62-1.06) | <0.127 | ||
| Don’t Know/Refused | 1.61 (1.12-2.34) | <0.010 | ||
|
| ||||
| Education Level | ||||
|
| ||||
| < High School | 1.00 | -- | 1.00 | -- |
|
| ||||
| HS Graduate/Some College | 0.66 (0.47-0.93) | <0.016 | 0.46 (0.28-0.77) | <0.003 |
|
| ||||
| College Graduate | 0.70 (0.49-1.01) | <0.056 | 0.43 (0.25-0.76) | <0.003 |
|
| ||||
| Advanced Degree | 0.53 (0.35-0.82) | <0.004 | 0.49 (0.26-0.92) | <0.026 |
|
| ||||
|
Employment Status
|
||||
| Full Time | 1.00 | -- | ||
| Part Time | 1.01 (0.76-1.33) | <0.969 | ||
| Retired/Housewife/Student | 0.99 (0.79-1.24) | <0.938 | ||
| Unemployed | 1.09 (0.77-1.55) | <0.635 | ||
|
| ||||
|
Martial Status
| ||||
| Not Married | 1.00 | -- | ||
| Married | 0.86 (0.75-0.98) | <0.028 | ||
|
| ||||
|
Smoking
| ||||
| Ever, No | 1.00 | -- | ||
| Ever, Yes | 1.56 (1.29-1.88) | <0.001 | ||
|
| ||||
|
Alcohol Use
| ||||
| No | 1.00 | -- | ||
| Yes | 1.44 (1.18-1.75) | <0.001 | ||
|
| ||||
|
b. Univariate and multivariate logistic results, Diagnostic and Screening populations, Houston, TX and Vancouver, BC, 1999-2005.
| ||||
| Genital Infections | ||||
|
| ||||
| Any, No | 1.00 | -- | 1.00 | -- |
|
| ||||
| Any, Yes | 1.93 (1.56-2.38) | <0.001 | 2.17 (1.61-2.92) | <0.0001 |
|
| ||||
| Menopausal Status | ||||
|
| ||||
| Premenopausal | 1.00 | -- | 1.00 | -- |
|
| ||||
| Perimenopausal | 0.15 (0.09-0.23) | <0.001 | 0.22 (0.12-0.41) | <0.0001 |
|
| ||||
| Postmenopausal | 0.32 (0.25-0.40) | <0.001 | 0.68 (0.42-1.09) | <0.106 |
|
| ||||
|
Parity
|
||||
| ≤ 2 | 1.00 | -- | ||
| 3+ | 0.73 (0.61-0.89) | <0.001 | ||
|
|
|
|||
| Current Oral Contraceptive Use | ||||
|
| ||||
| No | 1.00 | -- | 1.00 | -- |
|
| ||||
| Yes | 4.37 (3.28-5.82) | <0.001 | 2.31 (1.53-3.47) | <0.0001 |
|
| ||||
|
Current Condom Use
|
||||
| No | 1.00 | -- | ||
| Yes | 1.53 (1.20-1.95) | <0.001 | ||
|
|
|
|||
| HPV Status (as detected by HCII) | ||||
|
| ||||
| Negative | 1.00 | -- | 1.00 | -- |
|
| ||||
| Low-risk Type(s) | 2.99 (1.59-5.62) | <0.001 | 3.43(1.33-8.84) | <0.011 |
|
| ||||
| High-risk Type(s) | 8.60 (6.58-11.23) | <0.001 | 2.08 (1.44-3.03) | <0.0001 |
|
| ||||
| Consensus | 9.77 (5.30-18.00) | <0.001 | 3.46 (1.39-8.60) | <0.008 |
|
| ||||
|
HPV 16 DNA
|
||||
| Absent | 1.00 | -- | ||
| Present | 1.92 (1.51-2.44) | <0.001 | ||
|
| ||||
|
HPV 18 DNA
| ||||
| Absent | 1.00 | -- | ||
| Present | 1.11 (0.84-1.46) | <0.473 | ||
|
| ||||
| Consensus HPV DNA | ||||
|
| ||||
| Absent | 1.00 | -- | 1.00 | -- |
|
| ||||
| Present | 2.69 (2.08-3.46) | <0.001 | 1.66 (1.21-2.28) | <0.002 |
|
| ||||
| Worst Histology | ||||
|
| ||||
| Negative | 1.00 | -- | 1.00 | -- |
|
| ||||
| Atypia/HPV Assoc. Changes | 2.28 (1.82-2.86) | <0.001 | 1.86 (1.41-2.44) | <0.0001 |
|
| ||||
| LSIL | 15.38 (9.22-25.67) | <0.001 | 6.39 (3.48-11.75) | <0.0001 |
|
| ||||
| HSIL/CIS/SCC | 39.88 (23.49-67.68) | <0.001 | 12.08 (6.25-23.32) | <0.0001 |
Although age was statistically insignificant, its exclusion results in marked influence on the other variables, and thus was included.
Women in the diagnostic group were also more likely to report a history of genital infections (PR=1.62, p<0.0001) when compared to the screening group. The infections included were: trichomoniasis, yeast infection, bacterial vaginosis, Gardinella vaginalis, gonorrhea, syphilis, genital herpes, genital warts, and Chlamydia trachomatis. Women in the diagnostic population were more likely premenopausal, while women in the screening population tended to be either perimenopausal or postmenopausal (p<0.0001). There was no statistically significant difference in the mean age at menarche between the two groups (12.6 years in the diagnostic population compared to 12.7 years in the screening population).
In our population, parity appeared to have a protective effect, as the diagnostic population reported a lower average number of births (1.3 versus 1.7 in the screening population, p<0.0001). This seems to be opposite of the effects of parity reported in other studies. Bosch, et al. reported that high parity (seven or more births) actually increased the risk of squamous cell carcinoma of the cervix, after controlling for HPV-positivity13. Given that parity decreases as society progresses, this result may be a consequence of the screening population being on average 7.5 years older than the diagnostic population, the urban setting, or the inclusion of multinational participants. Current oral contraceptive (OC) use was more prevalent in the diagnostic population (PR=3.52, p<0.0001). Reports from previous studies are inconclusive, although the general consensus is that OC use increases the risk of cervical carcinoma14-17. However, these studies have been unable to control for other factors, such as an avoidance of other types of contraceptives (e.g. condoms) and a correlation of OC users with more lifetime sexual partners18, 19. Franceschi, et al. showed a positive association between OC use and cervical cancer risk when controlling for HPV status17; however, Dillner, et al. found that a similar correlation lost significance when HPV status was taken into account16.
Women in the diagnostic population reported an earlier age of sexual debut (mean: 17.47 years versus 18.95 years in the screening group; p<0.0001), and more lifetime sexual partners (mean: 9.05 versus 6.91, respectively). However, this result was statistically insignificant (p < 0.122), most likely due to the large range of sexual partners reported in each group (0-500 and 0-1000 for the diagnostic and screening populations, respectively).
Modeling the high-risk woman
Table 3 presents both univariate and multivariate logistic regression results. This model revealed that non-white race, greater than a high school education, and peri- or postmenopausal status were associated with the screening population. Meanwhile history of genital infections, current OC use, HPV positivity (by Hybrid Capture II and consensus PCR), and histology at clinic visit were important predictors of being in the diagnostic group. Interestingly, pre- and postmenopausal women were the most likely to be in the diagnostic group. This phenomenon likely represents the bimodal distribution of cancer cases that are seen in young women and older women. Also, we saw a similar two-fold increase in the likelihood of being in the diagnostic group for women who reported a history of genital infections or current OC use. Both of which have previously been reported to increase a woman’s risk of cervical cancer. We also saw an increase in the odds of being in the diagnostic group with increasing levels of dysplasia. This lends strength to our hypothesis that women in the diagnostic group were more at “risk” for dysplasia than women in the screening group. Taken together, our results suggest that, within our study population, pre-menopausal urban white women who have less than a high school education, have a history of genital infections, currently use OCs, are HPV positive, and have an abnormal level of disease by histology are the most likely to require additional diagnostic services. An overall goodness-of-fit test was performed and a Receiver Operating Characteristic (ROC) curve plotted (Figure 1) of the most parsimonious model.
Figure 1.
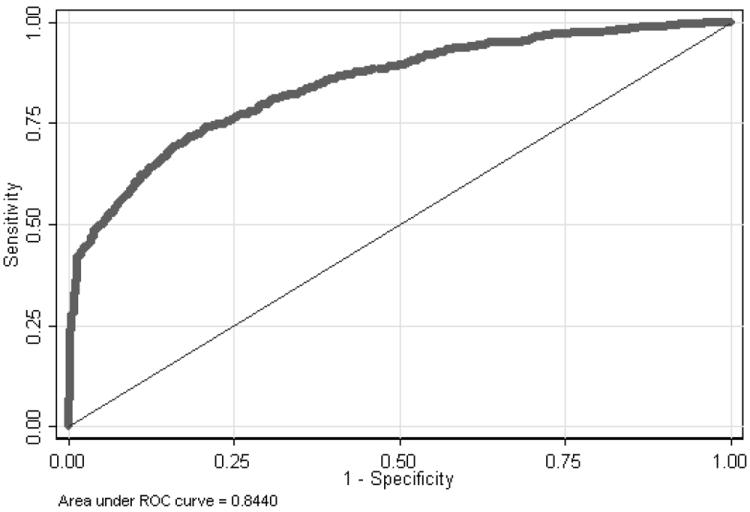
ROC curve for the final logistic model.
Discussion
Here we show that women who present with a history of abnormal Papanicolaou testing for diagnostic purposes, are more likely to be urban white, less educated, have a history of genital infections, be pre-menopausal, be current users of OCs, and, as would be expected, HPV positive and have cervical dysplasia compared to women who present for cervical screening and have no prior history of abnormal Papanicolaou tests. This is among the first reports, to our knowledge, to show meaningful comparisons between two clinical populations for screening and diagnosis. This is a great strength of our analysis because we show important differences in the characteristics of these two populations: those who present for screening purposes and those who present for diagnostic and treatment purposes. Most studies reported to date have compared a histologically “normal” population to one with dysplasia, at varying levels. In order to make the biggest difference in screening women for cervical dysplasia, we must be able to identify those women who could be screened versus those who might need extra scrutiny.
Our current results suggest that simple demographic characteristics could be used to identify women at increased risk for the development of dysplasia who might need further evaluation and immediate care. Current cervical cancer screening recommendations in the US are universally based on age; but have been recently updated to include HPV DNA testing.20 The inclusion of HPV DNA testing into routine screening will hopefully lower the number of false positive women unnecessarily treated; however, the expense of the assay still leaves some room for improvement. In addition, the currently approved HPV DNA test does not provide viral type-specific results, and it has been shown that there are differences in prognosis of cervical lesions based on the infecting HPV type.21 Therefore, the identification of factors related to individual screening need could help better triage women for standard screening methods versus emerging real-time screening techniques.
The introduction of the Bethesda system for cervical cytology was intended to reduce the variation in classifying cervical abnormalities; however, the correct classification of Atypia is still questioned and often revised22. The term “atypical squamous cells of undetermined significance” (ASCUS) was established to categorize abnormalities that were more than “reactive” but markedly less than a diagnosis of squamous intraepithelial lesion. It is also recognized that the categorization of ASCUS does lead to more-intensive follow up of patients. Two new categories, atypical squamous cells, cannot exclude high-grade lesion (ASC-H) and atypical glandular cells of undetermined significance (AGCUS), have also been established and are treated as high-grade disease. It is hoped that ASC-H will prompt even more rapid determination of more severe disease, if it exists. A recent report showed that more than 50% of patients with ASC-H developed HSIL on follow up, most within 1 year of the ASC-H diagnosis23. Given the constant desire to better our classification schemes, studies such as the one we present here become important in identifying women who deserve and require more intensive screening and potential therapeutic intervention.
A strength of our current study is the large number of women in each arm, screening (n=1000) and diagnostic (n=850). The current study was also conducted at multiple centers in the United States and Canada, allowing for easier generalization of results. Our team was also able to recruit races in proportion with the demographics of the United States. Another major strength of our study is the systematic way in which pathology was determined. Overall disease status was based upon a consensus diagnosis based on multiple biopsies graded by several expert pathologists, if necessary. A study of our interpathologist and intrapathologist readings found high levels of agreement24.
A limitation in our current study is the inability to analyze specific HPV types other than HPV 16 and HPV 18. However, these two types represent the majority of lesions in our population, and, as technologies advance to analyze more types in a cost-effective manner, we will adapt our protocol accordingly. We are very interested in type-specific differences in disease progression, especially as this might apply to our international population. While our population may be representative of the general population in terms of race, we have an unusually large number of urban women, likely due to the location of our clinics. Another limitation of our study is that we have 2-year follow-up data for all of our Canadian patients, while we have this data for only 50% of our American patients.
Acknowledgments
The authors wish to acknowledge the contributions of Nan Earle and Laura M. Dillon.
Financial disclosures, funding considerations: This study was funded by the National Cancer Institute (grant number: P01-CA82710).
Footnotes
conflicts of interest: The authors have no conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Parkin D, Bray F, Ferlay J, Pisani P. Global Cancer Statistics, 2002. CA: A Cancer Journal for Clinicians. 2005;55(2):74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Walboomers J, Jacobs M, Manos M, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. The Journal of Pathology. 1999;189(1):12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 3.Gustafsson L, Pontén J, Zack M, Adami H. International incidence rates of invasive cervical cancer after introduction of cytological screening. Cancer Causes and Control. 1997;8(5):755–763. doi: 10.1023/a:1018435522475. [DOI] [PubMed] [Google Scholar]
- 4.Jemal A. Cancer Facts & Figures 2007. Atlanta, GA: American Cancer Society; 2007. press. [Google Scholar]
- 5.Insinga R, Glass A, Rush B. The health care costs of cervical human papillomavirus–related disease. American Journal of Obstetrics and Gynecology. 2004;191(1):114–120. doi: 10.1016/j.ajog.2004.01.042. [DOI] [PubMed] [Google Scholar]
- 6.Brewer M, Utzinger U, Silva E, Gershenson D, Bast RC, Jr, Follen M, Richards-Kortum R. Fluorescence spectroscopy for in vivo characterization of ovarian tissue. Lasers Surg Med. 2001;29(2):128–35. doi: 10.1002/lsm.1098. [DOI] [PubMed] [Google Scholar]
- 7.Mirabal YN, Chang SK, Atkinson EN, Malpica A, Follen M, Richards-Kortum R. Reflectance spectroscopy for in vivo detection of cervical precancer. J Biomed Opt. 2002 Oct;7(4):587–94. doi: 10.1117/1.1502675. [DOI] [PubMed] [Google Scholar]
- 8.Bosch F, Munoz N, de Sanjose S. Human papillomavirus and other risk factors for cervical cancer. Biomed Pharmacother. 1997;51(6-7):268–275. doi: 10.1016/s0753-3322(97)83542-1. [DOI] [PubMed] [Google Scholar]
- 9.Resnick R, Cornelissen M, Wright D, et al. Detection and Typing of Human Papillomavirus in Archival Cervical Cancer Specimens by DNA Amplification With Consensus Primers. jnci. 2018;82(18):1477–1484. doi: 10.1093/jnci/82.18.1477. [DOI] [PubMed] [Google Scholar]
- 10.Hosmer D, Lemeshow S. Applied Logistic Regression. 1989 [Google Scholar]
- 11.Castellsague X, Munoz N. Chapter 3: Cofactors in Human Papillomavirus Carcinogenesis-Role of Parity, Oral Contraceptives, and Tobacco Smoking. jncimono. 2003;2003(31):20–28. [PubMed] [Google Scholar]
- 12.Castle P, Giuliano A. Chapter 4: Genital Tract Infections, Cervical Inflammation, and Antioxidant Nutrients-Assessing Their Roles as Human Papillomavirus Cofactors. jncimono. 2003;2003(31):29–34. doi: 10.1093/oxfordjournals.jncimonographs.a003478. [DOI] [PubMed] [Google Scholar]
- 13.Muñoz N, Franceschi S, Bosetti C, et al. Role of parity and human papillomavirus in cervical cancer: the IARC multicentric case-control study. The Lancet. 2002;359(9312):1093–1101. doi: 10.1016/S0140-6736(02)08151-5. [DOI] [PubMed] [Google Scholar]
- 14.Brinton L, Reeves W, Brenes M, et al. Oral contraceptive use and risk of invasive cervical cancer. Int J Epidemiol. 1990;19(1):4–11. doi: 10.1093/ije/19.1.4. [DOI] [PubMed] [Google Scholar]
- 15.Daling J, Madeleine M, McKnight B, et al. The Relationship of Human Papillomavirus-related Cervical Tumors to Cigarette Smoking, Oral Contraceptive Use, and Prior Herpes Simplex Virus Type 2 Infection’. Cancer Epidemiology Biomarkers & Prevention. [PubMed] [Google Scholar]
- 16.Kjellberg L, Hallmans G, Ahren A, et al. Smoking, diet, pregnancy and oral contraceptive use as risk factors for cervical intra-epithelial neoplasia in relation to human papillomavirus infection. Br J Cancer. 2000;82(7):1332–1338. doi: 10.1054/bjoc.1999.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moreno V, Bosch F, Muñoz N, et al. Effect of oral contraceptives on risk of cervical cancer in women with human papillomavirus infection: the IARC multicentric case-control study. The Lancet. 2002;359(9312):1085–1092. doi: 10.1016/S0140-6736(02)08150-3. [DOI] [PubMed] [Google Scholar]
- 18.Franco E, Duarte-Franco E, Ferenczy A. Cervical cancer: epidemiology, prevention and the role of human papillomavirus infection. Canadian Medical Association Journal. 2001;164(7):1017–1025. [PMC free article] [PubMed] [Google Scholar]
- 19.Schoell W, Janicek M, Mirhashemi R. Epidemiology and biology of cervical cancer. Seminars in Surgical Oncology. 1999;16(3):203–211. doi: 10.1002/(sici)1098-2388(199904/05)16:3<203::aid-ssu2>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 20.Saslow D, Runowicz CD, Solomon D, et al. American Cancer Society guideline for the early detection of cervical neoplasia and cancer. CA Cancer J Clin. 2002;52:342–362. doi: 10.3322/canjclin.52.6.342. [DOI] [PubMed] [Google Scholar]
- 21.Gaarenstroom KN, Melkert P, Walboomers JMM, Van Den Brule AJC, Van Bommel PFJ, Meyer CJLM, Voorhorst FJ, Kenemans P, Helmerhorst ThJM. Human papillomavirus DNA and genotypes: prognostic factors for progression of cervical intraepithelial neoplasia International. J Gynecol Cancer. 1994;4:73–78. doi: 10.1046/j.1525-1438.1994.04020073.x. [DOI] [PubMed] [Google Scholar]
- 22.Solomon D. The 1988 Bethesda system for reporting cervical/vaginal cytologic diagnoses: developed and approved at a National Cancer Institute workshop, Bethesda MD, U. S. A., December 12-13, 1988. J Reproduct Med. 1989;34(10):779–785. [PubMed] [Google Scholar]
- 23.Lee C, Ng W. A Follow-up Study of Atypical Squamous Cells in Gynecologic Cytology Using Conventional Papanicolaou Smears and Liquid-Based Preparations: The Impact of the Bethesda System 2001. Am J Clin Pathol. 2007;127(4):548–555. doi: 10.1309/21U34K8YW053F21E. [DOI] [PubMed] [Google Scholar]
- 24.Malpica A, Matisic J, Niekirk D, et al. Kappa statistics to measure interrater and intrarater agreement for 1790 cervical biopsy specimens among twelve pathologists: Qualitative histopathologic analysis and methodologic issues. Gynecol Oncol. 2005 doi: 10.1016/j.ygyno.2005.07.040. [DOI] [PubMed] [Google Scholar]


