Abstract
Serotonergic dysfunction has been hypothesized to play an important role in the pathophysiology of alcoholism. However, whether congenital serotonin (5-HT) deficiency leads to increased alcohol consumption or affects ethanol-related behaviors has not been established. Here, we use a transgenic mouse line that expresses a hypofunctional variant of the 5-HT synthesis enzyme, tryptophan hydroxylase 2, to examine the impact of 5-HT deficiency on responses to alcohol. We demonstrate that these 5-HT-deficient transgenic animals (Tph2KI mice) recover their righting reflex more rapidly than wild-type controls following a high dose of ethanol and exhibit blunted locomotor retardation in response to repeated ethanol administration. In addition, compared to WT controls, 5-HT-deficient animals consume significantly more ethanol and exhibit increased preference for ethanol in two-bottle choice tests. Our data also suggest that 5-HT plays a critical role in mediating the effects of ethanol on Akt/GSK3β signaling in the nucleus accumbens. Overall, our results corroborate previous theories regarding the importance of brain 5-HT levels in mediating responsiveness to alcohol and demonstrate, for the first time, that congenital 5-HT deficiency leads to increased ethanol consumption and decreased sensitivity to the sedative-like effects of ethanol, perhaps in part through modulating Akt/GSK3β signaling.
Keywords: serotonin, tryptophan hydroxylase 2, alcohol, behavior
1. INTRODUCTION
Numerous lines of research have suggested that deficient central serotonin (5-HT) neurotransmission plays a critical role in alcoholism. For example, clinical studies have reported putative biomarkers of 5-HT deficiency (e.g., reduced levels of the 5-HT metabolite, 5-HIAA) in alcoholic patients (Borg et al., 1985). Preclinical studies have shown that reduced levels of brain 5-HT are associated with increased ethanol (ETOH) intake in both rodents and primates (Murphy et al., 1982; Higley et al., 1996), and drugs that elevate extracellular levels of 5-HT have been reported to reduce ETOH intake in animal models (Lu et al., 1993; LeMarquand et al., 1994a; Lu et al., 1994; Maurel et al., 1999) and in humans (LeMarquand et al., 1994b; Naranjo et al., 1994). In addition, mutations in genes encoding components of the 5-HT system, such as the 5-HT1B receptor (Lappalainen et al., 1998) and the 5-HT transporter (5-HTT) (Feinn et al., 2005), have been associated with alcoholism. Previous preclinical research has also implicated many 5-HT-system components in ETOH-related behaviors, including the 5-HT1B receptor (Crabbe et al., 1996), the 5-HT2A receptor (Nakamura, Matsushita et al. 1999), the 5-HTT (Kelai et al., 2003) and the 5-HT3 receptor (Engel et al., 1998). However, the effects of congenital 5-HT deficiency on ETOH-related behaviors, including ETOH consumption, have not been established.
Here, we examined the effects of 5-HT deficiency on ETOH sensitivity and consumption using a knock-in mouse line that expresses a hypofunctional R439H mutation in the rate-limiting enzyme in 5-HT synthesis, tryptophan hydroxylase – 2, (Tph2). This Tph2(R439H) knock-in (Tph2KI) mouse line exhibits 60–80% reductions in the levels of brain 5-HT and has been shown to exhibit depression-, anxiety-, and aggression-like behaviors (Beaulieu et al., 2008; Jacobsen et al., 2012). To investigate the effects of congenital 5-HT deficiency on ETOH-related behaviors, we compared the behavior of WT and Tph2KI mice in the two-bottle free-choice paradigm, the recovery of righting reflex test and locomotor activity assays following repeated acute ETOH injections.
In addition to behavioral responses to ETOH, we also examined the effects of 5-HT deficiency on signaling pathways induced by ETOH in the nucleus accumbens (NAc) and the hippocampus, two brain areas that are known to be responsive to ETOH (Vilpoux et al., 2009). Previous research has indicated that Akt/GSK3β signaling in the NAc plays an important role in behavioral responses to ETOH (Neasta et al., 2011), and pharmacologic manipulation of 5-HT signaling in the NAc has been shown to modulate ETOH consumption and ETOH-seeking behaviors (Czachowski, 2005). Given that 5-HT signaling has been shown to modulate GSK3 signaling in the brain (Li et al., 2004) and ETOH is known to induce 5-HT release (Yoshimoto et al., 1992; Portas et al., 1994; Thielen et al., 2002), we hypothesized that Tph2KI mice would exhibit blunted GSK3β responses to acute ETOH administration.
2. MATERIALS AND METHODS
2.1. Animals
The generation of Tph2KI mice, which are on a mixed background (c57BL6/J – 129S6/SvEvTac), has been described previously (Beaulieu et al., 2008). Age-matched male WT and Tph2KI littermates derived from heterozygous breedings were used for all experiments. Mice were housed 4–5/cage except for alcohol drinking experiments, in which they were singly housed. Mice were maintained on a 12 hour light-dark cycle in a temperature-controlled facility and had ad libitum access to food and water. Experiments were conducted during the light phase. All experiments were performed in accordance with the National Institute of Health’s Guide for the Care and Use of Laboratory Animals and were covered by a protocol that had been approved by the Duke University Institutional Animal Care and Use Committee.
2.2. Drugs
ETOH for injections and drinking studies was purchased from Kopf. It was diluted to 12.5% in saline and administered to mice via intra-peritoneal (IP) injections.
2.3. Behavioral Testing
2.3.1. Two-bottle free-choice tests
Two separate ETOH preference tests were performed. For both, mice were allowed to acclimate to the presence of two water bottles (rather than the standard one water bottle) for a period of three days. After this time, in the first test, mice were given free access to a solution of 3% ETOH in 2% sucrose in one bottle for three days (with standard drinking water in the other bottle), followed by four days of access to a 6% ETOH/2% sucrose solution and four days of access to a 12% ETOH/2% sucrose solution. In the second test, mice were again given access to 3, 6 and 12% ETOH solutions in 2% sucrose, but the other bottle contained a 2% sucrose solution instead of standard drinking water. For the sucrose preference test, mice were acclimated to the presence of two water bottles for 3 days. Both water bottles were removed for four hours starting one hour before the start of the dark cycle. After this four-hour water deprivation period, mice were provided free access to a 2% or a 10% sucrose solution for two hours. The relative quantities from each bottle were recorded, and the preference for each was determined.
2.3.2. Locomotor activity
To investigate the effects of 5-HT deficiency on ETOH-induced hypolocomotion, WT and Tph2KI mice were injected with 2.0 mg/kg ETOH every day for fourteen days. This dose has been previously shown to reduce locomotor activity in mice (Kim, Kim et al. 2011). On days one, seven and fourteen, the locomotor activity of mice was measured using VersaMax activity monitors (AccuScan Instruments Inc., Columbus, OH) for the 20 min immediately prior to an acute injection with ETOH and for 20 min following ETOH administration. Mice were placed in an activity monitor, and total distance travelled was measured for 20 min. Mice were then injected with ETOH and returned to the activity monitor for another 20 min. Behavioral data were recorded using VersaMax software. These mice were injected again on day 15 with ETOH or saline, and sacrificed 20 min later for the Western blotting experiments described below.
2.3.3. Recovery of righting reflex
WT and Tph2KI mice were injected with a sedative dose of ETOH (4.0 g/kg, IP) and placed in a supine position, and the latency of each mouse to recover its righting reflex was recorded, as described previously (Kapfhamer, Taylor et al. 2013). The righting reflex was considered to have been recovered at the point at which a mouse was able to right itself successfully three times within 30 s after being placed in a supine position.
2.4.1. Ethanol-induced hypothermia
WT and Tph2KI mice were injected with 4.0 g/kg ETOH (IP), and their body temperature was monitored as described previously (Jacobsen et al., 2012).
2.4.2. Ethanol metabolism
WT and Tph2KI mice were injected with either 2.0 or 4.0 g/kg ETOH (IP), and tail vein blood samples were collected 20, 60 and 120 minutes later. Serum was collected, and levels of ETOH were determined using an Ethanol Assay Kit (Abcam, Cambridge, MA, USA) according to the manufacturer’s instructions.
2.5. Western blotting
Western blotting was performed essentially as described previously (Sachs, Rodriguiz et al. 2013). Briefly, mice were sacrificed by rapid cervical dislocation 20 min following an acute injection of either saline or 2.0 g/kg ETOH, and the brains were removed rapidly. Immediately upon excision, brains were submerged in liquid nitrogen for approximately 6 seconds to cool the tissue. Brains were then transferred onto an ice-cold 0.5 mm brain sectioning block (Braintree Scientific, Braintree, MA). Each brain was sectioned into 1 mm slices, and 2 mm diameter punches were obtained from the dorsal hippocampus (primarily dentate gyrus and some CA1), and the NAc (both core and shell). Bilateral punches were homogenized in a solution containing 0.32 M sucrose, 20 mM HEPES (pH 7.4), 1 mM EDTA with protease and phosphatase inhibitors. The homogenate was centrifuged for 10 min at 12,000 rpm at 4 °C, and the supernatants were collected for further analysis. The protein concentrations of the supernatants were determined using a BCA assay (Thermo Scientific, Rockford, IL), and 25 μg of each sample was loaded onto 10% Tris-glycine gels. Antibodies used were mouse anti-GAPDH (MAB374, Millipore; 1:1000, Billerica, MA), rabbit anti-phospho-GSK3β (#9323, Serine 9, Cell Signaling; 1:500, Danvers, MA), mouse anti-total GSK3α/β (sc-7291, Santa Cruz, 1:500, Santa Cruz, CA), rabbit anti-phospho-p44/p42 MAPK (Erk1/2) (#9101, Tyrosine 202/204, Cell Signaling; 1:500), mouse anti-total ERK (#9107, Cell Signaling, 1:500), anti-pAkt (Serine 473, #9271, Cell Signaling; 1:500) and anti-total Akt (#9272, Cell Signaling: 1:1000). The appropriate AlexaFluor 680- or AlexaFluor 800- conjugated secondary antibodies were used (Life Technologies/Molecular Probes; 1:10,000, Grand Island, NY), and blots were developed using an Odyssey LI-COR system (LI-COR Biosciences, Lincoln, NE).
2.6. Statistical Analyses
Statistical analyses were performed using JMP Pro 9 software (SAS, Cary, NC). Data were analyzed by t-test or by two-way ANOVA followed by Tukey’s post hoc tests, as appropriate. The significance threshold was set at p < 0.05.
3. RESULTS
3.1. Congenital brain 5-HT deficiency leads to increased ETOH consumption in mice
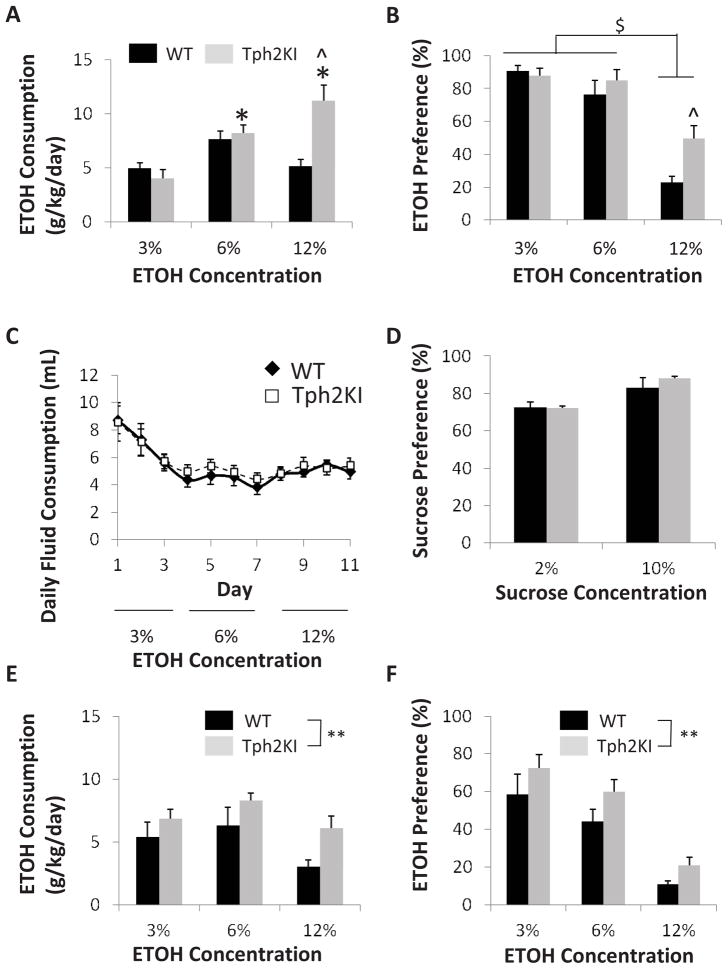
Because of the suggested link between low 5-HT function and increased ETOH consumption (Murphy et al., 1982; Borg et al., 1985; Higley et al., 1996), we hypothesized that Tph2KI mice would consume more ETOH than their wild-type (WT) littermates. Tph2KI mice exhibit an escalation of ETOH consumption in a two-bottle free-choice ETOH consumption paradigm, in which mice were given access to standard drinking water or to increasing concentrations of ETOH over an eleven-day period. A two-way ANOVA revealed significant main effects of genotype [F(1, 51) = 7.7139, p = 0.0076] and ETOH concentration [F(2, 51) = 11.7607, p < 0.0001] and a significant interaction between genotype and ETOH concentration [F(2, 51) = 9.4149, p = 0.0003]. Post hoc analyses revealed no significant genotype differences in the consumption of 3% or 6% ETOH, but Tph2KI mice consumed significantly more of a 12% ETOH solution than WT controls (Tukey’s post hoc test: p < 0.0001, Fig. 1A). Tph2KI mice consumed significantly more ETOH (in terms of total dose, not volume) when provided access to the 6% ETOH solution and the 12% ETOH solution compared to when they had access to the 3% solution (Tukey’s post hoc test: p = 0.0097 and p < 0.0001, respectively). In contrast, no significant escalation of ETOH consumption was observed in WT animals (Fig. 1A).
Figure 1.
Brain 5-HT deficiency leads to increased ETOH consumption in mice. When provided a choice between standard drinking water and a solution of ETOH in 2% sucrose, Tph2KI mice, but not WT, exhibit a significant escalation of ETOH consumption with increasing concentrations of ETOH (A). Both WT and Tph2KI mice have a reduced preference for 12% ETOH compared to 3 and 6% ETOH, but WT mice display a marked aversion to 12% ETOH, whereas Tph2KI animals do not (B). No significant differences in total fluid consumption (C) or in the preference for 2% sucrose or 10% (D) were observed between the genotypes. When provided a choice between 2% sucrose and a solution of ETOH in 2% sucrose, Tph2KI mice consume significantly more ETOH (E) and exhibit an increased preference for ETOH (F). N = 9 per group for A–C. N = 6 per genotype for the 10% sucrose experiment and N = 9 WTs and 10 Tph2KIs for the 2% sucrose experiment for D. N = 8–10 mice per group for E and F. * indicates p < 0.05 compared to Tph2KI 3% ETOH, and ^ indicates p < 0.05 compared to WT 12% ETOH by Tukey’s post hoc tests. $ indicates a main effect of ETOH concentration, ** indicates a significant main effect of genotype, p < 0.05 by two way ANOVA.
When ETOH preference was measured (as a percentage of total fluid consumption), a significant genotype by dose interaction was observed [F(2, 51) = 3.7218, p = 0.0314]. A significant main effect of genotype [F(1, 51) = 8.3573, p = 0.0058] was observed, with Tph2KI mice exhibiting increased preference (Fig. 1B). A significant main effect of dose [F(2, 51) = 57.2307, p < 0.0001] was also observed, with higher doses of ETOH leading to reduced ETOH preference (Fig. 1B). Post hoc tests revealed no genotype differences in the preference for 3% or 6% ETOH, the preference for which ranged between 76% and 90% for both genotypes. However, WT mice developed a strong aversion to 12% ETOH (a 22.7% ‘preference’), whereas Tph2KI mice did not. Tph2KI animals consumed a significantly more of a 12% ETOH solution than WT controls (Tukey’s post hoc test: p = 0.0129) and showed neither a preference nor an aversion to this highest dose of ETOH (a 49.6% preference).
No significant differences in total fluid consumption were observed between the two genotypes during the course of the two-bottle free-choice test, but both genotypes drank less of the 12% ETOH solution than the 3% ETOH solution (Fig. 1C). Similarly, no differences in sucrose preference were observed between the genotypes (Fig. 1D).
ETOH consumption and preference were also measured in a second two-bottle choice test in which animals were given a choice between an ETOH solution (either 3%, 6% or 12% in 2% sucrose) in one bottle and a 2% sucrose solution in the other. No significant differences in total fluid consumption were observed between the genotypes, but animals of both genotypes consumed more liquid when given access to 3% ETOH when compared to 6 or 12% ETOH (data not shown). Overall, Tph2KI animals consumed significantly more ETOH than WT controls [main effect of genotype, F(1, 51) = 4.718, p = 0.0345, Fig. 1E]. A significant main effect of ETOH concentration was also observed [F(2, 51) = 33.6245, p < 0.0001], with animals of both genotypes consuming lower quantities of the higher concentrations of ETOH. Similarly, when ETOH preference was measured, significant main effects of genotype [F(1, 50) = 6.2223, p = 0.016] and ETOH concentration [F(2, 50) = 29.967, p < 0.0001, Fig. 1F] were observed. As in the previous ETOH preference test (Fig. 1B), Tph2KI animals exhibited a higher preference for ETOH than did WT animals, and ETOH preference decreased in both genotypes with increasing concentrations of ETOH.
3.2. Tph2KI mice exhibit reduced sensitivity to the sedative effects of ETOH
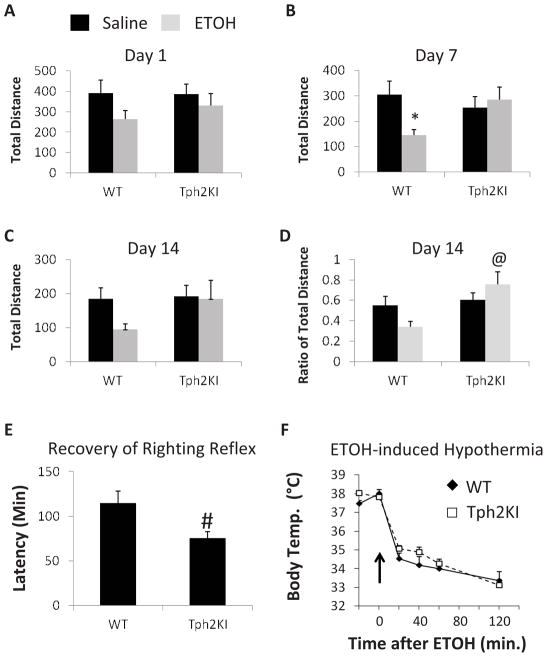
Because increased ETOH consumption (i.e., alcoholism) has been associated with decreased sensitivity to ETOH-induced sedation (Schuckit, 1994), we hypothesized that Tph2KI animals would exhibit a less severe inhibition of locomotor activity in response to repeated ETOH administration when compared to WT controls. To test this hypothesis, the effects of brain 5-HT deficiency on the sedative effects of ETOH were examined using locomotor activity assays in mice that were injected once daily with 2.0 g/kg ETOH for fourteen days. Although no significant differences in total distance travelled were observed between the genotypes on day one (Fig. 2A), a significant interaction between ETOH and genotype was observed on day seven [F(1, 67) = 5.3648, p = 0.0236] (Fig. 2B). ETOH induced a significant decrease in total distance travelled in WT mice (Tukey’s post hoc test: p = 0.0383), but this effect was not observed in Tph2KI animals. A similar overall pattern of responses was observed on day fourteen, but the interaction was not significant, likely due to the high degree of variability in the overall locomotor activity of animals on day fourteen, both before and after ETOH injections (Fig. 2C). Indeed, when we controlled for the initial locomotor activity of each animal and measured the ratio of distance travelled in the 20 min prior to ETOH administration to the distance travelled in the 20 min immediately following ETOH administration, we observed both a significant main effect of genotype [F(1, 67) = 7.9772, p = 0.0062] and a significant interaction between genotype and ETOH [F(1, 67) = 4.6797, p = 0.0341] (Fig. 2D). The pre-ETOH to post-ETOH ratio of distance travelled was significantly greater in Tph2KI animals than in WT controls (Tukey’s post hoc test: p = 0.0033, Fig. 2D), thus revealing that Tph2KI mice exhibit reduced sensitivity to the locomotor-inhibitory effects of ETOH. In contrast, there were no genotype differences in the behavioral responses to saline.
Figure 2.
The sedative and locomotor inhibitory effects of ETOH are reduced in Tph2KI mice. A single dose of ETOH did not have any significant effects on total distance travelled in either WT or Tph2KI mice (A). However, by the seventh day of repeated daily injections, ETOH significantly reduced the distance travelled in WT but not in Tph2KI animals (B). This effect on total distance was not quite significant on day 14 (C), but the ratio of the distance travelled in the 20 min following ETOH administration compared to the 20 min immediately before ETOH administration was higher in Tph2KI mice compared to WT controls (D). Tph2KI mice exhibit a more rapid recovery of righting reflex than WT animals (E). No genotype differences in ETOH-induced hypothermia were observed (F). N = 17–19 per group for A–D. N = 26 WTs and 24 Tph2KIs for E. N = 9 WTs and 8 Tph2KIs for F. * indicates p < 0.05 compared to WT saline, and @ indicates p < 0.05 compared to WT ETOH by Tukey’s post hoc test. # indicates p < 0.05 compared to WT by Student’s t test.
The sedative effects of ETOH were also examined using the recovery of righting reflex test. Tph2KI animals recovered their righting reflex more rapidly than did WT controls (Student’s t-test: p = 0.0129, degrees of freedom (DF) = 49, Fig. 2E). In contrast, no significant differences in ETOH-induced hypothermia were observed (Fig. 2F).
3.3. Signaling Effects of ETOH in WT and Tph2KI mice
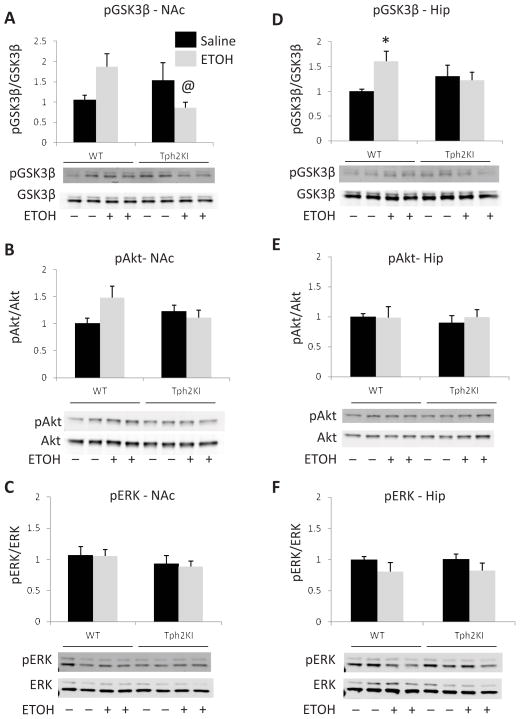
Prior work has shown that acute ETOH administration leads to increased GSK3 phosphorylation in the NAc (Neasta et al., 2011) and that 5-HT signaling regulates GSK3β activity in the brain (Li et al., 2004; Beaulieu et al., 2008). Thus, we next compared whether 5-HT deficiency impacts GSK3β responses to acute ETOH administration. Indeed, a significant genotype by treatment interaction was observed for pGSK3 in the NAc [F(1, 42) = 7.1449, p = 0.0107]. Tukey’s post hoc tests revealed that Tph2KI mice treated with ETOH exhibit significantly reduced levels of pGSKβ compared to ETOH-treated WT animals (p = 0.0426, Fig. 3A). A similar overall trend was observed for Akt phosphorylation in the NAc, which has also been previously shown to be induced by acute ETOH (Neasta et al., 2011), but the genotype by treatment interaction did not quite achieve statistical significance (p = 0.0518, Fig. 3B). In contrast to Akt and GSK3, prior work has shown that ETOH does not significantly affect ERK signaling in the NAc (Neasta et al., 2011). Our data are consistent with this prior finding, as we find no evidence of altered ERK signaling in the NAc following acute ETOH administration in either WT or Tph2KI animals (Fig. 3C).
Figure 3.
Tph2KI mice exhibit blunted GSK3β responses to ETOH. Tph2KI mice treated with ETOH exhibit reduced levels of pGSK3β in the NAc when compared to ETOH-treated WT mice (A). A similar trend was observed for pAkt in the NAc, but these results did not achieve statistical significance (B). No significant changes in pERK were observed in WT or Tph2KI mice in response to ETOH in the NAc (C). ETOH treatment led to a significant increase in pGSK3β in the Hip of WT mice, but not Tph2KI animals (D). No significant effects of ETOH or genotype were observed for pAkt (E) or pERK (F) in the Hip. N = 10–13 per group. * indicates p < 0.05 compared to WT saline, and @ indicates p < 0.05 compared to WT ETOH by Tukey’s post hoc test.
We also examined ETOH-induced signaling changes in the hippocampus. Acute ETOH administration led to a significant increase in pGSK3 in the hippocampus of WT animals (Tukey’s post hoc test: p = 0.0475) but had no effect in Tph2KI animals [genotype by treatment interaction: F(1,43) = 4.5717, p = 0.0382, Fig. 3D]. However, no significant alterations in the hippocampal levels of pAkt were observed in any of the groups (Fig. 3E). Similarly, no significant effects of acute ETOH administration on ERK phosphorylation were observed in the hippocampus (Fig. 3F), although a trend towards reduced pERK in response to ETOH administration was observed [main effect of ETOH: F(1,42) = 3.4505, p = 0.0703].
3.4. Ethanol metabolism
To evaluate whether the differences observed above could result from differences in ETOH metabolism, we measured the serum levels of ETOH 20, 60 and 120 minutes following an acute dose of either 2.0 or 4.0 g/kg ETOH (IP). No significant genotype differences were observed at any time point following either dose (Fig. 4).
Figure 4.
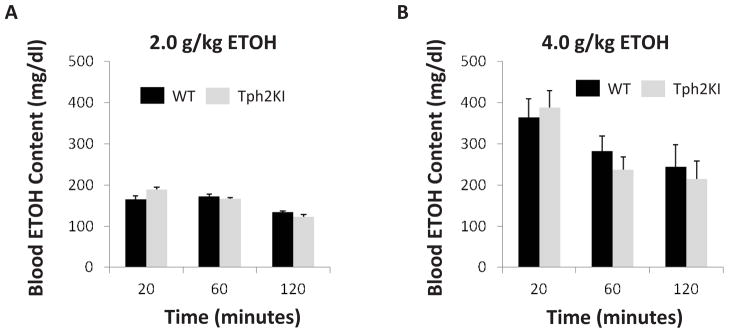
Brain 5-HT deficiency does not affect ETOH metabolism. No significant differences in blood ETOH concentration were observed between the genotypes at 20, 60 or 120 minutes following an acute injection of either 2.0 (A) or 4.0 (B) g/kg ETOH.
4. DISCUSSION
Our data indicate that brain 5-HT deficiency increases ETOH consumption and results in decreased sensitivity to the sedative and motor inhibitory effects of ETOH administration. However, neither genotype exhibited a strong preference for high concentrations of ETOH. Consequently, Tph2KI mice on this mixed genetic background should not be considered a strongly ETOH-preferring line of mice. Significant strain (and husbandry) differences have been well documented in ethanol consumption experiments in mice (Crabbe, Wahlsten et al. 1999), and it is likely that strain effects are responsible for the relatively low levels of ETOH consumption observed in our WT controls. It is possible that backcrossing Tph2KI animals to a more ETOH-preferring strain could lead to an even more robust ETOH-drinking behavioral phenotype.
Although our data suggest that 60–80% reductions in brain 5-HT can increase ETOH consumption, we do not suggest that the relationship between brain 5-HT levels and ETOH consumption is strictly linear. Rather, we hypothesize that multiple types of disturbances in 5-HT neurotransmission (either hyper- or hypo-activity) can contribute to psychiatric diseases and alcohol-related disorders. Indeed, it is likely that similar psychiatric symptoms or behavioral phenotypes (e.g., increased drinking behavior) can be induced via disparate neurobiological mechanisms in different genetic and/or environmental contexts. For example, individuals harboring the s allele of the 5-HTT promoter polymorphism (i.e., those with less 5-HTT expression) have been reported to be at an increased risk of developing alcoholism, despite the fact that they would be predicted to have elevated levels of extracellular 5-HT (Hallikainen, Saito et al. 1999). Further research will be required to determine the precise mechanisms whereby dysfunctions in 5-HT signaling can affect ETOH-related behaviors and alcoholism. It will be interesting to measure ETOH consumption in Tph2KI mice subjected to chronic stressors to determine if 5-HT deficiency exacerbates stress-induced alterations in alcohol-related behaviors.
Clinical studies have shown that decreased sensitivity to ETOH is a genetic trait that leads to increased risk of developing alcoholism (Holdstock et al., 2000; Schuckit, 2000; Heath et al., 2001). Similarly, preclinical reports have reported that decreased sensitivity to the sedative effects of ETOH is associated with increased alcohol consumption (Thiele et al., 2000; Corl et al., 2009). Thus, our data suggest that genetic mutations leading to brain 5-HT deficiency, including functional mutations in Tph2, could contribute to the development of alcoholism, at least in a subset of patients. It should be noted that several studies examining the associations between polymorphisms in Tph2 and alcoholism have yielded negative results (Zill et al., 2007; Gacek et al., 2008), but it is possible that further analyses examining additional polymorphisms or that specifically examine functional polymorphisms may reveal significant associations. In addition, 5-HT deficiency or impaired functioning of the 5-HT system could result from mutations in a variety of genes other than Tph2.
Given the association between low levels of brain 5-HT and increased ETOH consumption, elevating levels of 5-HT might be predicted to be effective in the treatment of alcoholism. Indeed, several clinical studies have suggested that selective serotonin reuptake inhibitors (SSRIs) can reduce ETOH consumption (LeMarquand et al., 1994b; Naranjo et al., 1994). However, other studies have reported that SSRIs exhibit limited efficacy in the treatment of alcoholism (Kranzler et al., 1995) or have suggested that SSRIs may exhibit differential effects in different subgroups of alcoholics (Pettinati, 2001). For example, it has been suggested that individuals with Type A alcoholism (lower risk/severity) may respond better to SSRIs than Type B alcoholics (higher risk/severity) (Pettinati et al., 2000). This finding was considered somewhat surprising given that Type B alcoholics have been considered more likely to exhibit 5-HT dysfunction, which SSRI therapy might have been expected to reverse. However, mutations in Tph2 have been associated with poor antidepressant treatment responses (Peters et al., 2004; Tzvetkov et al., 2008; Tsai et al., 2009), and thus, 5-HT deficiency may be associated with reduced sensitivity to SSRIs. Consistent with this, we have recently shown that 5-HT-deficient Tph2KI mice exhibit significantly blunted neurochemical, neurogenic and behavioral responses to chronic fluoxetine administration (Sachs et al., in press). These results highlight the need to identify novel therapeutic pathways for intervention in alcoholism.
There are many potential points of intersection between ETOH and 5-HT signaling. For example, ETOH is known to affect 5-HTT expression (Burnett, Davenport et al. 2012) and to inhibit 5-HT clearance in a 5-HTT-independent manner (Daws, Montanez et al. 2006). We have previously shown that Tph2KI mice do not have significant alterations in 5-HTT expression in the brainstem, hippocampus, striatum or frontal cortex (Beaulieu, Zhang et al. 2008), and thus, we do not believe that differences in 5-HTT function are responsible for any of the observed phenotypes of these animals. However, we have not examined 5-HTT expression in these animals following ETOH administration and thus have not completely ruled out a potential role of 5-HTT. We have previously shown that Tph2KI mice exhibit increased numbers of 5-HT2A receptors in the frontal cortex but no changes in hippocampal 5-HT1A receptors compared to WT controls (Jacobsen, Siesser et al. 2012), thus demonstrating that 5-HT deficiency can lead to specific regional alterations in 5-HT-signaling components. We hypothesize that at least some of the effects of 5-HT deficiency on ETOH-related behaviors are due to compensatory changes in the expression of 5-HT receptors, many of which are known to regulate alcohol-related behaviors (Sari, Johnson et al. 2011), but future research will be required to evaluate this.
Previous research has suggested that inhibiting Akt signaling in the NAc is sufficient to reduce ETOH consumption in rodents (Neasta et al., 2011). The results of our study are consistent with the idea that Akt-GSK3 signaling may play an important role in ETOH-related behaviors. Indeed, 5-HT deficiency blunts the effects of ETOH on behavior and on the Akt-GSK3 signaling pathway. However, the current results are correlative, and additional research would be required to evaluate the functional significance of changes in ETOH-induced Akt-GSK3 signaling resulting from 5-HT deficiency. Although previous work has shown that inhibiting Akt in the NAc, which would be predicted to potentiate GSK3 signaling, results in decreased ETOH consumption, our data suggest that Tph2KI mice, which exhibit increased GSK3 activity in the NAc following ETOH, consume more ETOH than their WT counterparts. Several areas other than the NAc are also important mediators of ETOH-related behaviors, and thus, some of the effects of 5-HT deficiency on ETOH-related behaviors may be mediated by signaling changes in other brain regions. It is also possible that differences in cell type specificity within the NAc could partially explain this observed difference. For example, using pharmacological inhibitors of Akt is likely to inhibit Akt and promote GSK3 signaling in all cell types, while 5-HT deficiency may only affect Akt and GSK3 signaling in the subset of cells that expresses certain 5-HT receptors. Indeed, given that 5-HT1 and 5-HT2 receptors have been shown to exhibit opposing effects on GSK3 signaling (Li et al., 2004), it is possible that 5-HT deficiency may lead to opposing effects on GSK3 activity in different cell populations depending upon their relative 5-HT receptor expression. Thus, additional research is required to determine the precise cell types through which Akt and GSK3 signaling mediate their effects on ETOH-related behaviors. It will also be important to identify novel ways to target Akt and/or GSK3 signaling in a cell-specific manner as a strategy to reduce ETOH consumption.
5. CONCLUSION
Overall, our data demonstrate that 5-HT deficiency modifies ETOH-related behaviors and increases ETOH consumption. Our results also suggest that the observed effects of 5-HT deficiency on ETOH-related behaviors may be mediated, in part, through alterations in Akt or GSK3 signaling in response to repeated ETOH exposure. These findings highlight the potential importance of 5-HT and Akt/GSK3 signaling in mediating the effects of ETOH and provide additional rationale for further study into the therapeutic efficacy of targeting Akt/GSK3 signaling for the treatment of alcohol-related disorders.
Highlights.
Brain serotonin deficiency decreases sensitivity to ethanol-induced sedation
Brain serotonin deficiency increases ethanol consumption in mice
Brain serotonin deficiency reduces ethanol-induced GSK3 phosphorylation
Acknowledgments
This work was supported in part by grants from the National Institutes of Health (MH79201 and MH60451) to MGC. Support from the Lennon Family Foundation to MGC for the initial part of this work is also greatly appreciated. BDS was the recipient of a Minority Supplement award from the National Institutes of Health (MH79201-03S1) and is currently the recipient of an NRSA postdoctoral fellowship (F32-MH093092). We would like to thank Wendy Roberts for husbandry and care of the mice and Trevor Thomas for technical assistance.
Abbreviations
- NAc
nucleus accumbens
- ETOH
ethanol
- IP
intra-peritoneal
- Tph2
tryptophan hydroxylase 2
- Tph2KI
tryptophan hydroxylase 2 R439H knock-in
- WT
wild-type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Benjamin D. Sachs, Email: Benjamin.sachs@dm.duke.edu.
A. Ayten Salahi, Email: Aylin.salahi@duke.edu.
References
- Beaulieu JM, Zhang X, Rodriguiz RM, Sotnikova TD, Cools MJ, Wetsel WC, Gainetdinov RR, Caron MG. Role of GSK3 beta in behavioral abnormalities induced by serotonin deficiency. Proc Natl Acad Sci U S A. 2008;105:1333–1338. doi: 10.1073/pnas.0711496105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg S, Kvande H, Liljeberg P, Mossberg D, Valverius P. 5-Hydroxyindoleacetic acid in cerebrospinal fluid in alcoholic patients under different clinical conditions. Alcohol. 1985;2:415–418. doi: 10.1016/0741-8329(85)90106-5. [DOI] [PubMed] [Google Scholar]
- Burnett EJ, Davenport AT, et al. The effects of chronic ethanol self-administration on hippocampal serotonin transporter density in monkeys. Front Psychiatry. 2012;3:38. doi: 10.3389/fpsyt.2012.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corl AB, Berger KH, Ophir-Shohat G, Gesch J, Simms JA, Bartlett SE, Heberlein U. Happyhour, a Ste20 family kinase, implicates EGFR signaling in ethanol-induced behaviors. Cell. 2009;137:949–960. doi: 10.1016/j.cell.2009.03.020. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Phillips TJ, Feller DJ, Hen R, Wenger CD, Lessov CN, Schafer GL. Elevated alcohol consumption in null mutant mice lacking 5-HT1B serotonin receptors. Nat Genet. 1996;14:98–101. doi: 10.1038/ng0996-98. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Wahlsten D, et al. Genetics of mouse behavior: interactions with laboratory environment. Science. 1999;284(5420):1670–1672. doi: 10.1126/science.284.5420.1670. [DOI] [PubMed] [Google Scholar]
- Czachowski CL. Manipulations of serotonin function in the nucleus accumbens core produce differential effects on ethanol and sucrose seeking and intake. Alcohol Clin Exp Res. 2005;29:1146–1155. doi: 10.1097/01.alc.0000171944.50381.86. [DOI] [PubMed] [Google Scholar]
- Daws LC, Montanez S, et al. Ethanol inhibits clearance of brain serotonin by a serotonin transporter-independent mechanism. J Neurosci. 2006;26(24):6431–6438. doi: 10.1523/JNEUROSCI.4050-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel SR, Lyons CR, Allan AM. 5-HT3 receptor over-expression decreases ethanol self administration in transgenic mice. Psychopharmacology. 1998;140:243–248. doi: 10.1007/s002130050763. [DOI] [PubMed] [Google Scholar]
- Feinn R, Nellissery M, Kranzler HR. Meta-analysis of the association of a functional serotonin transporter promoter polymorphism with alcohol dependence. Am J Med Genet B Neuropsychiatr Genet. 2005;133B:79–84. doi: 10.1002/ajmg.b.30132. [DOI] [PubMed] [Google Scholar]
- Gacek P, Conner TS, Tennen H, Kranzler HR, Covault J. Tryptophan hydroxylase 2 gene and alcohol use among college students. Addict Biol. 2008;13:440–448. doi: 10.1111/j.1369-1600.2008.00118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallikainen T, Saito T, et al. Association between low activity serotonin transporter promoter genotype and early onset alcoholism with habitual impulsive violent behavior. Mol psychiatry. 1999;4(4):385–388. doi: 10.1038/sj.mp.4000526. [DOI] [PubMed] [Google Scholar]
- Heath AC, Whitfield JB, Madden PA, Bucholz KK, Dinwiddie SH, Slutske WS, Bierut LJ, Statham DB, Martin NG. Towards a molecular epidemiology of alcohol dependence: analysing the interplay of genetic and environmental risk factors. Br J Psychiatry Suppl. 2001;40:s33–40. doi: 10.1192/bjp.178.40.s33. [DOI] [PubMed] [Google Scholar]
- Higley JD, Suomi SJ, Linnoila M. A nonhuman primate model of type II excessive alcohol consumption? Part 1. Low cerebrospinal fluid 5-hydroxyindoleacetic acid concentrations and diminished social competence correlate with excessive alcohol consumption. Alcohol Clin Exp Res. 1996;20:629–642. doi: 10.1111/j.1530-0277.1996.tb01665.x. [DOI] [PubMed] [Google Scholar]
- Holdstock L, King AC, de Wit H. Subjective and objective responses to ethanol in moderate/heavy and light social drinkers. Alcohol Clin Exp Res. 2000;24:789–794. [PubMed] [Google Scholar]
- Jacobsen JP, Siesser WB, Sachs BD, Peterson S, Cools MJ, Setola V, Folgering JH, Flik G, Caron MG. Deficient serotonin neurotransmission and depression-like serotonin biomarker alterations in tryptophan hydroxylase 2 (Tph2) loss-of-function mice. Mol psychiatry. 2012;17:694–704. doi: 10.1038/mp.2011.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapfhamer D, Taylor S, et al. Taok2 controls behavioral response to ethanol in mice. Genes, brain, and behavior. 2013;12(1):87–97. doi: 10.1111/j.1601-183X.2012.00834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelai S, Aissi F, Lesch KP, Cohen-Salmon C, Hamon M, Lanfumey L. Alcohol intake after serotonin transporter inactivation in mice. Alcohol Alcohol. 2003;38:386–389. doi: 10.1093/alcalc/agg095. [DOI] [PubMed] [Google Scholar]
- Kim KS, Kim H, et al. Mice lacking adenylyl cyclase type 5 (AC5) show increased ethanol consumption and reduced ethanol sensitivity. Psychopharmacology. 2011;215(2):391–398. doi: 10.1007/s00213-010-2143-x. [DOI] [PubMed] [Google Scholar]
- Kranzler HR, Burleson JA, Korner P, Del Boca FK, Bohn MJ, Brown J, Liebowitz N. Placebo-controlled trial of fluoxetine as an adjunct to relapse prevention in alcoholics. Am J Psychiatry. 1995;152:391–397. doi: 10.1176/ajp.152.3.391. [DOI] [PubMed] [Google Scholar]
- Lappalainen J, Long JC, Eggert M, Ozaki N, Robin RW, Brown GL, Naukkarinen H, Virkkunen M, Linnoila M, Goldman D. Linkage of antisocial alcoholism to the serotonin 5-HT1B receptor gene in 2 populations. Arch General Psychiatry. 1998;55:989–994. doi: 10.1001/archpsyc.55.11.989. [DOI] [PubMed] [Google Scholar]
- LeMarquand D, Pihl RO, Benkelfat C. Serotonin and alcohol intake, abuse, and dependence: findings of animal studies. Biol Psychiatry. 1994a;36:395–421. doi: 10.1016/0006-3223(94)91215-7. [DOI] [PubMed] [Google Scholar]
- LeMarquand D, Pihl RO, Benkelfat C. Serotonin and alcohol intake, abuse, and dependence: clinical evidence. Biol Psychiatry. 1994b;36:326–337. doi: 10.1016/0006-3223(94)90630-0. [DOI] [PubMed] [Google Scholar]
- Li X, Zhu W, Roh MS, Friedman AB, Rosborough K, Jope RS. In vivo regulation of glycogen synthase kinase-3beta (GSK3beta) by serotonergic activity in mouse brain. Neuropsychopharmacology. 2004;29:1426–1431. doi: 10.1038/sj.npp.1300439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu MR, Wagner GC, Fisher H. Ethanol consumption following acute fenfluramine, fluoxetine, and dietary tryptophan. Pharmacol Biochem Behav. 1993;44:931–937. doi: 10.1016/0091-3057(93)90027-q. [DOI] [PubMed] [Google Scholar]
- Lu MR, Wagner GC, Fisher H. Ethanol consumption following acute treatment with methysergide, fluoxetine, fenfluramine, and their combination. Alcohol Clin Exp Res. 1994;18:60–63. doi: 10.1111/j.1530-0277.1994.tb00881.x. [DOI] [PubMed] [Google Scholar]
- Maurel S, De Vry J, Schreiber R. Comparison of the effects of the selective serotonin-reuptake inhibitors fluoxetine, paroxetine, citalopram and fluvoxamine in alcohol-preferring cAA rats. Alcohol. 1999;17:195–201. doi: 10.1016/s0741-8329(98)00046-9. [DOI] [PubMed] [Google Scholar]
- Murphy JM, McBride WJ, Lumeng L, Li TK. Regional brain levels of monoamines in alcohol-preferring and -nonpreferring lines of rats. Pharmacol Biochem Behav. 1982;16:145–149. doi: 10.1016/0091-3057(82)90026-0. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Matsushita S, et al. Association of a polymorphism of the 5HT2A receptor gene promoter region with alcohol dependence. Mol psychiatry. 1999;4(1):85–88. doi: 10.1038/sj.mp.4000474. [DOI] [PubMed] [Google Scholar]
- Naranjo CA, Poulos CX, Bremner KE, Lanctot KL. Fluoxetine attenuates alcohol intake and desire to drink. Int Clin Psychopharmacol. 1994;9:163–172. doi: 10.1097/00004850-199409000-00004. [DOI] [PubMed] [Google Scholar]
- Neasta J, Ben Hamida S, Yowell QV, Carnicella S, Ron D. AKT signaling pathway in the nucleus accumbens mediates excessive alcohol drinking behaviors. Biol Psychiatry. 2011;70:575–582. doi: 10.1016/j.biopsych.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters EJ, Slager SL, McGrath PJ, Knowles JA, Hamilton SP. Investigation of serotonin-related genes in antidepressant response. Mol Psychiatry. 2004;9:879–889. doi: 10.1038/sj.mp.4001502. [DOI] [PubMed] [Google Scholar]
- Pettinati HM. The use of selective serotonin reuptake inhibitors in treating alcoholic subtypes. J Clin Psychiatry. 2001;62(Suppl 20):26–31. [PubMed] [Google Scholar]
- Pettinati HM, Volpicelli JR, Kranzler HR, Luck G, Rukstalis MR, Cnaan A. Sertraline treatment for alcohol dependence: interactive effects of medication and alcoholic subtype. Alcohol Clin Exp Res. 2000;24:1041–1049. [PubMed] [Google Scholar]
- Portas CM, Devoto P, Gessa GL. Effect of ethanol on extracellular 5-hydroxytryptamine output in rat frontal cortex. Eur J Pharmacol. 1994;270:123–125. doi: 10.1016/0926-6917(94)90089-2. [DOI] [PubMed] [Google Scholar]
- Sachs BD, Rodriguiz RM, et al. The effects of brain serotonin deficiency on behavioural disinhibition and anxiety-like behaviour following mild early life stress. Int J Neuropsychopharmacol. 2013:1–14. doi: 10.1017/S1461145713000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs BD, Jacobsen JP, Thomas TL, Siesser WB, Roberts WL, Caron MG. The effects of congenital brain serotonin deficiency on responses to chronic fluoxetine. Transl Psychiatry. doi: 10.1038/tp.2013.65. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y, V, Johnson R, et al. Role of the serotonergic system in alcohol dependence: from animal models to clinics. Progress in molecular biology and translational science. 2011;98:401–443. doi: 10.1016/B978-0-12-385506-0.00010-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA. Low level of response to alcohol as a predictor of future alcoholism. Am J Psychiatry. 1994;151:184–189. doi: 10.1176/ajp.151.2.184. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Genetics of the risk for alcoholism. Am J Addict. 2000;9:103–112. doi: 10.1080/10550490050173172. [DOI] [PubMed] [Google Scholar]
- Thiele TE, Willis B, Stadler J, Reynolds JG, Bernstein IL, McKnight GS. High ethanol consumption and low sensitivity to ethanol-induced sedation in protein kinase A-mutant mice. J Neurosci. 2000;20:RC75. doi: 10.1523/JNEUROSCI.20-10-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thielen RJ, Bare DJ, McBride WJ, Lumeng L, Li TK. Ethanol-stimulated serotonin release in the ventral hippocampus: an absence of rapid tolerance for the alcohol-preferring P rat and insensitivity in the alcohol-nonpreferring NP rat. Pharmacol Biochem Behav. 2002;71:111–117. doi: 10.1016/s0091-3057(01)00633-5. [DOI] [PubMed] [Google Scholar]
- Tsai SJ, Hong CJ, Liou YJ, Yu YW, Chen TJ, Hou SJ, Yen FC. Tryptophan hydroxylase 2 gene is associated with major depression and antidepressant treatment response. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:637–641. doi: 10.1016/j.pnpbp.2009.02.020. [DOI] [PubMed] [Google Scholar]
- Tzvetkov MV, Brockmoller J, Roots I, Kirchheiner J. Common genetic variations in human brain-specific tryptophan hydroxylase-2 and response to antidepressant treatment. Pharmacogenet Genomics. 2008;18:495–506. doi: 10.1097/FPC.0b013e3282fb02cb. [DOI] [PubMed] [Google Scholar]
- Vilpoux C, Warnault V, Pierrefiche O, Daoust M, Naassila M. Ethanol-sensitive brain regions in rat and mouse: a cartographic review, using immediate early gene expression. Alcohol Clin Exp Res. 2009;33:945–969. doi: 10.1111/j.1530-0277.2009.00916.x. [DOI] [PubMed] [Google Scholar]
- Yoshimoto K, McBride WJ, Lumeng L, Li TK. Alcohol stimulates the release of dopamine and serotonin in the nucleus accumbens. Alcohol. 1992;9:17–22. doi: 10.1016/0741-8329(92)90004-t. [DOI] [PubMed] [Google Scholar]
- Zill P, Preuss UW, Koller G, Bondy B, Soyka M. SNP- and haplotype analysis of the tryptophan hydroxylase 2 gene in alcohol-dependent patients and alcohol-related suicide. Neuropsychopharmacology. 2007;32:1687–1694. doi: 10.1038/sj.npp.1301318. [DOI] [PubMed] [Google Scholar]