Abstract
Nipah virus (NiV) continues to cause outbreaks of fatal human encephalitis due to spillover from its bat reservoir. We determined that a single dose of replication-defective vesicular stomatitis virus (VSV)-based vaccine vectors expressing either the NiV fusion (F) or attachment (G) glycoproteins protected hamsters from over 1000 times LD50 NiV challenge. This highly effective single-dose protection coupled with an enhanced safety profile makes these candidates ideal for potential use in livestock and humans.
Keywords: Henipavirus, Nipah, vaccine, VSV, single-dose, hamster, glycoproteins
Nipah virus (NiV) is a highly pathogenic paramyxovirus responsible for causing fatal human encephalitis with high case fatality rates from 40%–75% (Lo and Rota, 2008). Since its initial outbreak in Malaysia from 1998–1999, NiV has caused smaller sporadic outbreaks of fatal encephalitis in Bangladesh on a near-annual basis (Luby and Gurley, 2012). A soluble subunit glycoprotein vaccine approved for animal use against the closely-related Hendra virus requiring a two-dose prime-boost regimen has shown protection against NiV in several animal models (Bossart et al., 2012; Broder et al., 2013; McEachern et al., 2008; Mungall et al., 2006; Pallister et al., 2013). Previous work demonstrated that a single dose of replication-defective single-cycle recombinant vesicular stomatitis viruses (VSV-ΔG) expressing either the NiV fusion (F) (VSV-ΔG-NiVF) or attachment (G) (VSV-ΔG-NiVG) glycoproteins induced neutralizing antibodies in mice against VSV-ΔG-particles pseudotyped with NiV F and G glycoproteins (VSV-ΔG-eGFP-NEUT) (Chattopadhyay and Rose, 2011). In order to evaluate the protective efficacy of the VSV-ΔG-NiVF and VSV-ΔG-NiVG vaccines against lethal NiV challenge in an animal model that mimics NiV disease, we tested these vaccines in the Syrian golden hamster (DeBuysscher et al., 2013; Guillaume et al., 2004; Rockx et al., 2011; Wong et al., 2003). We obtained approval for animal experiments from the Centers for Disease Control and Prevention (CDC) Institutional Animal Care and Use Committee (IACUC). All animal work was performed by certified staff in Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC)-approved biosafety level 2(BSL-2) (vaccination phase) or BSL-4 (challenge phase) facilities at CDC.
We produced stocks of the single-cycle viruses VSV-ΔG-NiVG, VSV-ΔG-NiVF, VSV-ΔG-eGFP pseudotyped with VSV G glycoprotein, and also the VSV-ΔG-eGFP-NEUT pseudotyped with NiV F and G as previously described (Chattopadhyay and Rose, 2011). For vaccination 6-week old female Syrian golden hamsters (Mesocricetus auratus, Charles River Laboratories, Wilmington, VA) were anesthetized (isoflurane) and inoculated intramuscularly in the right quadriceps with 1 x 106 infectious particles of either VSV-ΔG-NiVG (10 animals), VSV-ΔG-NiVF (10 animals), or VSV-ΔG-eGFP (10 animals). At 28 days post-vaccination, ~ 100 μl of blood was collected for determination of serum neutralizing antibody titers (SNT) as previously described (Chattopadhyay and Rose, 2011). The vaccinated hamsters, along with 3 additional unvaccinated hamsters (to serve as unvaccinated controls) were transferred into the BSL-4 lab and were given 3 days to adjust to their new surroundings. On day 32 post-vaccination (challenge day 0), all hamsters were inoculated via the intraperitoneal route with a previously described uniformly lethal challenge dose (105 TCID50/hamster, >1000 times LD50) of NiV Malaysia strain passaged 3 times on Vero E6 cells (Chua et al., 2000; DeBuysscher et al., 2013; Harcourt et al., 2000; Rockx et al., 2011). Animals were examined and scored daily for two weeks post-challenge for signs of clinical illness, neurologic disease, respiratory distress, and weight loss (weight evaluation for 3 vaccinated groups began on day 3 post-challenge). Animals showing significant weight loss (>25% of initial weight on challenge day 0) alongside any neurological or respiratory signs were humanely euthanized. Animals without clinical illness after 14 days post-infection (p.i.) continued to be monitored daily but were only weighed in 2–5 day intervals until day 32 p.i. in which all surviving animals were humanely euthanized. At time of euthanasia, ~ 3 ml of blood was collected by cardiac puncture for SNT determination. Necropsies were performed to collect lung, spleen, kidney, and brain tissues. Tissues were either inactivated in MAGMAX RNA lysis buffer (Life Technologies, Carlsbad, CA) for subsequent RNA extraction and real-time RT-PCR as previously described (Lo et al., 2012), or fixed in 10% formalin for histopathology and immunohistochemistry (IHC) analysis as previously described (Wong et al., 2003).
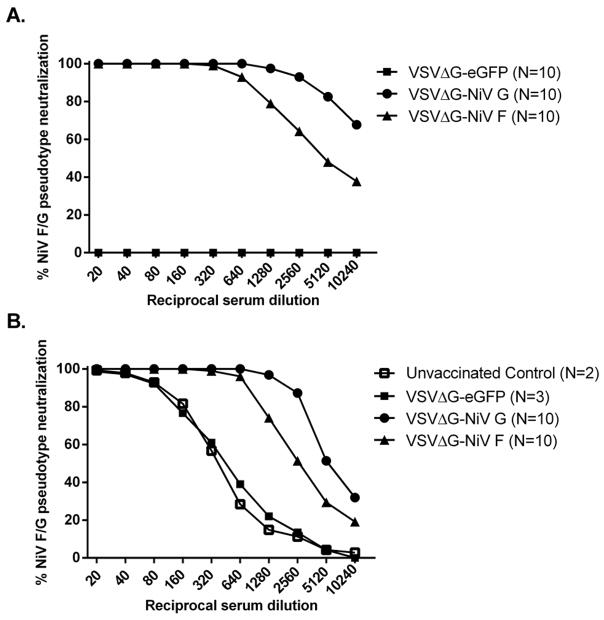
On day 6 post-challenge, all unvaccinated control hamsters either died or were euthanized due to the development of neurologic signs and respiratory distress. Similarly, 5 out of 10 (50%) backbone control VSV-ΔG-eGFP-vaccinated hamsters either died or were euthanized due to the onset of illness. By day 9 post-challenge, all VSV-ΔG-eGFP-vaccinated hamsters had either died or were euthanized (Figure 1A). In contrast, hamsters vaccinated with VSV-ΔG-NiVG or VSV-ΔG-NiVF did not develop any clinical illness nor weight loss throughout the course of infection, and were euthanized on day 32 p.i. (day 64 post-vaccination) (Figure 1A, 1B). SNTs against NiV were defined as the reciprocal of the highest serum dilution at which duplicate wells of each serum sample showed complete neutralization of 50 infectious particles of VSV-ΔG-eGFP-NEUT. Prior to NiV challenge, hamsters vaccinated with either VSV-ΔG-NiVG or VSV-ΔG-NiVF developed respective SNTs of 640 and 160, while hamsters vaccinated with VSV-ΔG-eGFP did not develop any detectable SNT (Figure 2A). Following NiV challenge, both unvaccinated control and VSV-ΔG-eGFP-vaccinated groups developed low levels of neutralizing antibodies (Figure 2B), but even at serum dilutions of 1:20 could not completely neutralize 50 particles of VSV-ΔG-eGFP-NEUT. The lack of an anamnestic immune response in the VSV-ΔG-NiVG or VSV-ΔG-NiVF-vaccinated groups following NiV challenge possibly indicates sterilizing immunity, as their respective post-challenge SNTs at the time of euthanasia remained similar to pre-challenge levels with comparatively lower percentages of neutralization at higher serum dilutions of 1:2560, 1:5120 and 1:10240 (Figure 2A, 2B). Histopathology and IHC results in unvaccinated and VSV-ΔG-eGFP-vaccinated hamsters were similar to those observed in a recent study of NiV pathogenesis in the hamster model (DeBuysscher et al., 2013), indicating bronchointerstitial pneumonia with vasculitis associated with virus replication (Figure 3). In the unvaccinated and eGFP control-vaccinated groups we detected both viral RNA and viral antigen in all tissues sampled from both groups except for the brain, in which we detected viral RNA but not viral antigen (Table 1, Figure 3, data not shown). In contrast, we did not observe any pathology and could neither detect the presence of viral RNA nor antigen in any tissues collected from VSV-ΔG-NiVG or VSV-ΔG-NiVF-vaccinated groups (Table 1, data not shown).
Figure 1.
A single-dose vaccination of hamsters with single-cycle replication-deficient VSV viral vectors (VSV-ΔG) expressing either NiV G or NiV F confers complete protection from lethal NiV challenge. (A) Survival curves of unvaccinated hamsters and hamsters vaccinated with VSV-ΔG-NiVG, VSV-ΔG-NiVF, or VSV-ΔG-eGFP, and challenged 32 days later with virulent NiV. (B) Weight curves of vaccinated hamsters challenged with lethal dose of NiV. Weight changes are expressed as the mean percentage changes for NiV challenged animals relative to their weights at day zero.
Figure 2.
A single-dose vaccination of hamsters with either VSV-ΔG-NiVG or VSV-ΔG-NiVF induces protective serum neutralizing antibody titers (SNT). Percent neutralization curves of pooled serum from each group of hamsters at (A) 28 days post vaccination pre-NiV challenge, and (B) 64 days post-vaccination, 32 days post-NiV challenge for VSV-ΔG-NiVG or VSV-ΔG-NiVF-vaccinated hamsters, 6 days post-challenge for unvaccinated hamsters, and at 6, 7, and 9 days post-challenge for VSV-ΔG-eGFP-vaccinated hamsters. SNT for each group was determined as the highest reciprocal serum dilution in which 50 particles of VSV-ΔG-eGFP virus pseudotyped with NiV F and G glycoproteins were completely (100%) neutralized.
Figure 3.

Lungs from unvaccinated hamsters show vasculitis and abundant Nipah virus antigen associated with bronchial arteries. (A) Vasculitis characterized by acute inflammation and necrotic cellular debris that obscures the arterial wall (arrows). The adjacent parenchyma shows interstitial pneumonia (asterisks). (B) Nipah virus IHC shows abundant antigen (red) in endothelial cells (arrow heads) and smooth muscle cells of artery (arrows). Inset: Nipah virus antigen within endothelial cell (arrow head). Original magnification: 100x, 400x (inset).
TABLE 1.
Mean NiV nucleoprotein gene RNA copy numbers/μg RNA from tissue
| Tissue | ||||
|---|---|---|---|---|
| Unvaccinated Control (N=3) | VSV-ΔG-eGFP (N=10) | VSV-ΔG-NiVG (N=10) | VSV-ΔG-NiVF (N=10) | |
| Spleen | 4.26E+06 | 1.66E+06 | ND | ND |
| Kidney | 3.56E+06 | 1.63E+06 | ND | ND |
| Lung | 9.68E+05 | 7.35E+06 | ND | ND |
| Brain | 2.70E+05 | 2.70E+05 | ND | ND |
ND= not detected
In summary, we have demonstrated that a single dose of these single-cycle vaccine candidates expressing either NiV G or F conferred complete protection from lethal NiV challenge in the Syrian golden hamster animal model. This is in marked contrast with all other previously evaluated viral vaccine vectors expressing NiV G or F that required multi-dose prime-boost regimens (Guillaume et al., 2004; Weingartl et al., 2006; Yoneda et al., 2013) with the exception of an adenovirus-associated virus (AAV) vector which required an extremely high dose of vaccine (6 x 1011 genome particles) for protection (Ploquin et al., 2013). Furthermore, when compared to these other vectors expressing NiV G, the VSV-ΔG-NiVG induced higher levels of SNT at 28 days post-vaccination, and was the only vaccine administered in which no anamnestic immune response was detected. Additionally, the NiV challenge dose used in this study was at least 10 times greater than that used by any other NiV vaccine protection study in the hamster model, which highlights the extremely robust protection conferred by the VSV-ΔG NiV vaccines. While replication-competent VSV-based vectors expressing appropriate foreign antigens have shown to be highly effective vaccines against a number of viral and bacterial pathogens (Cobleigh et al., 2010; Jones et al., 2005; Kahn et al., 2001; Kapadia et al., 2005; Liao et al., 2008; Palin et al., 2007; Roberts et al., 1998; Roberts et al., 2004; Rose et al., 2001), regulatory approval of these vaccine vectors in humans has been slow due to concerns regarding potential pathogenesis. On the other hand, replication-deficient VSV-ΔG vectors have shown to induce equivalent or even greater levels of humoral and cell-mediated immunity when compared with its corresponding replication-competent vector, particularly when vaccinating via the intramuscular route (Kapadia et al., 2008; Publicover et al., 2005). In comparison to the soluble HeV G subunit vaccine (HeVsG) currently in animal trials (Broder et al., 2013), the VSV-ΔG vector provides some advantages. The first and foremost advantage is the single-dose requirement for protection, and the second advantage is the lack of any adjuvants required to generate robust humoral and cell-mediated T-helper 1 (Th1) type immunity (Publicover et al., 2005). The two-dose HeVsG vaccine formulation requires the action of two adjuvants, Allhydrogel and CpG oligodeoxynucleotide (ODN) 2006 (Bossart et al., 2012) in order to generate a Th1 immune response, since Allhydrogel alone typically induces a Th2 response which is not appropriate against viral infections (Coffman et al., 2010; Steinhagen et al., 2011; Weeratna et al., 2001). The HeVsG vaccine however holds an advantage over the VSV-ΔG vector in that the vaccine antigen is more easily produced on a large scale versus the production of infectious VSV-ΔG particles which require multiple plasmid transfections (Pallister et al., 2011; Witko et al., 2010). The results reported in this study underscore the safety profile and robust protection conferred by a single dose of these replication-deficient single-cycle vectors against NiV. Future studies will establish the minimum protective dose and early protection kinetics of the VSV-ΔG NiV vaccines as potential tools for prophylaxis and reduction of NiV disease severity and/or mortality.
Highlights.
A single dose of VSVΔG-based vaccines expressing Nipah glycoproteins protected hamsters.
VSVΔG-based NiV vaccines induced high levels of serum neutralizing antibodies with one dose.
Lack of detectable anamnestic immune responses indicates potential sterilizing immunity.
Acknowledgments
We thank Mr. Eddie Jackson for assistance with implanting temperature/identification transponders in the hamsters, and Peter J. Eworonsky, Abiola Aminu, and Lester E. Slough for assistance in animal care and husbandry. The findings and conclusions in this report are those of the authors and do not necessarily represent those of the Centers for Disease Control and Prevention. This work was supported in part by NIH Northeast Biodefense Center grant U54-AI057158.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bossart KN, Rockx B, Feldmann F, Brining D, Scott D, LaCasse R, Geisbert JB, Feng YR, Chan YP, Hickey AC, Broder CC, Feldmann H, Geisbert TW. A Hendra virus G glycoprotein subunit vaccine protects African green monkeys from Nipah virus challenge. Science translational medicine. 2012;4:146ra107. doi: 10.1126/scitranslmed.3004241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broder CC, Xu K, Nikolov DB, Zhu Z, Dimitrov DS, Middleton D, Pallister J, Geisbert TW, Bossart KN, Wang LF. A treatment for and vaccine against the deadly Hendra and Nipah viruses. Antiviral research. 2013 doi: 10.1016/j.antiviral.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay A, Rose JK. Complementing defective viruses that express separate paramyxovirus glycoproteins provide a new vaccine vector approach. Journal of virology. 2011;85:2004–2011. doi: 10.1128/JVI.01852-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua KB, Bellini WJ, Rota PA, Harcourt BH, Tamin A, Lam SK, Ksiazek TG, Rollin PE, Zaki SR, Shieh W, Goldsmith CS, Gubler DJ, Roehrig JT, Eaton B, Gould AR, Olson J, Field H, Daniels P, Ling AE, Peters CJ, Anderson LJ, Mahy BW. Nipah virus: a recently emergent deadly paramyxovirus. Science. 2000;288:1432–1435. doi: 10.1126/science.288.5470.1432. [DOI] [PubMed] [Google Scholar]
- Cobleigh MA, Buonocore L, Uprichard SL, Rose JK, Robek MD. A vesicular stomatitis virus-based hepatitis B virus vaccine vector provides protection against challenge in a single dose. Journal of virology. 2010;84:7513–7522. doi: 10.1128/JVI.00200-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman RL, Sher A, Seder RA. Vaccine adjuvants: putting innate immunity to work. Immunity. 2010;33:492–503. doi: 10.1016/j.immuni.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBuysscher BL, de Wit E, Munster VJ, Scott D, Feldmann H, Prescott J. Comparison of the pathogenicity of Nipah virus isolates from Bangladesh and Malaysia in the Syrian hamster. PLoS neglected tropical diseases. 2013;7:e2024. doi: 10.1371/journal.pntd.0002024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillaume V, Contamin H, Loth P, Georges-Courbot MC, Lefeuvre A, Marianneau P, Chua KB, Lam SK, Buckland R, Deubel V, Wild TF. Nipah virus: vaccination and passive protection studies in a hamster model. Journal of virology. 2004;78:834–840. doi: 10.1128/JVI.78.2.834-840.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harcourt BH, Tamin A, Ksiazek TG, Rollin PE, Anderson LJ, Bellini WJ, Rota PA. Molecular characterization of Nipah virus, a newly emergent paramyxovirus. Virology. 2000;271:334–349. doi: 10.1006/viro.2000.0340. [DOI] [PubMed] [Google Scholar]
- Jones SM, Feldmann H, Stroher U, Geisbert JB, Fernando L, Grolla A, Klenk HD, Sullivan NJ, Volchkov VE, Fritz EA, Daddario KM, Hensley LE, Jahrling PB, Geisbert TW. Live attenuated recombinant vaccine protects nonhuman primates against Ebola and Marburg viruses. Nature medicine. 2005;11:786–790. doi: 10.1038/nm1258. [DOI] [PubMed] [Google Scholar]
- Kahn JS, Roberts A, Weibel C, Buonocore L, Rose JK. Replication-competent or attenuated, nonpropagating vesicular stomatitis viruses expressing respiratory syncytial virus (RSV) antigens protect mice against RSV challenge. Journal of virology. 2001;75:11079–11087. doi: 10.1128/JVI.75.22.11079-11087.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapadia SU, Rose JK, Lamirande E, Vogel L, Subbarao K, Roberts A. Long-term protection from SARS coronavirus infection conferred by a single immunization with an attenuated VSV-based vaccine. Virology. 2005;340:174–182. doi: 10.1016/j.virol.2005.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapadia SU, Simon ID, Rose JK. SARS vaccine based on a replication-defective recombinant vesicular stomatitis virus is more potent than one based on a replication-competent vector. Virology. 2008;376:165–172. doi: 10.1016/j.virol.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao JB, Publicover J, Rose JK, DiMaio D. Single-dose, therapeutic vaccination of mice with vesicular stomatitis virus expressing human papillomavirus type 16 E7 protein. Clinical and vaccine immunology : CVI. 2008;15:817–824. doi: 10.1128/CVI.00343-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo MK, Lowe L, Hummel KB, Sazzad HM, Gurley ES, Hossain MJ, Luby SP, Miller DM, Comer JA, Rollin PE, Bellini WJ, Rota PA. Characterization of Nipah virus from outbreaks in Bangladesh, 2008–2010. Emerging infectious diseases. 2012;18:248–255. doi: 10.3201/eid1802.111492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo MK, Rota PA. The emergence of Nipah virus, a highly pathogenic paramyxovirus. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2008;43:396–400. doi: 10.1016/j.jcv.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Luby SP, Gurley ES. Epidemiology of henipavirus disease in humans. Current topics in microbiology and immunology. 2012;359:25–40. doi: 10.1007/82_2012_207. [DOI] [PubMed] [Google Scholar]
- McEachern JA, Bingham J, Crameri G, Green DJ, Hancock TJ, Middleton D, Feng YR, Broder CC, Wang LF, Bossart KN. A recombinant subunit vaccine formulation protects against lethal Nipah virus challenge in cats. Vaccine. 2008;26:3842–3852. doi: 10.1016/j.vaccine.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mungall BA, Middleton D, Crameri G, Bingham J, Halpin K, Russell G, Green D, McEachern J, Pritchard LI, Eaton BT, Wang LF, Bossart KN, Broder CC. Feline model of acute nipah virus infection and protection with a soluble glycoprotein-based subunit vaccine. Journal of virology. 2006;80:12293–12302. doi: 10.1128/JVI.01619-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palin A, Chattopadhyay A, Park S, Delmas G, Suresh R, Senina S, Perlin DS, Rose JK. An optimized vaccine vector based on recombinant vesicular stomatitis virus gives high-level, long-term protection against Yersinia pestis challenge. Vaccine. 2007;25:741–750. doi: 10.1016/j.vaccine.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Pallister J, Middleton D, Wang LF, Klein R, Haining J, Robinson R, Yamada M, White J, Payne J, Feng YR, Chan YP, Broder CC. A recombinant Hendra virus G glycoprotein-based subunit vaccine protects ferrets from lethal Hendra virus challenge. Vaccine. 2011;29:5623–5630. doi: 10.1016/j.vaccine.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallister JA, Klein R, Arkinstall R, Haining J, Long F, White JR, Payne J, Feng YR, Wang LF, Broder CC, Middleton D. Vaccination of ferrets with a recombinant G glycoprotein subunit vaccine provides protection against Nipah virus disease for over 12 months. Virology journal. 2013;10:237. doi: 10.1186/1743-422X-10-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploquin A, Szecsi J, Mathieu C, Guillaume V, Barateau V, Ong KC, Wong KT, Cosset FL, Horvat B, Salvetti A. Protection against henipavirus infection by use of recombinant adeno-associated virus-vector vaccines. The Journal of infectious diseases. 2013;207:469–478. doi: 10.1093/infdis/jis699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Publicover J, Ramsburg E, Rose JK. A single-cycle vaccine vector based on vesicular stomatitis virus can induce immune responses comparable to those generated by a replication-competent vector. Journal of virology. 2005;79:13231–13238. doi: 10.1128/JVI.79.21.13231-13238.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A, Kretzschmar E, Perkins AS, Forman J, Price R, Buonocore L, Kawaoka Y, Rose JK. Vaccination with a recombinant vesicular stomatitis virus expressing an influenza virus hemagglutinin provides complete protection from influenza virus challenge. Journal of virology. 1998;72:4704–4711. doi: 10.1128/jvi.72.6.4704-4711.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A, Reuter JD, Wilson JH, Baldwin S, Rose JK. Complete protection from papillomavirus challenge after a single vaccination with a vesicular stomatitis virus vector expressing high levels of L1 protein. Journal of virology. 2004;78:3196–3199. doi: 10.1128/JVI.78.6.3196-3199.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockx B, Brining D, Kramer J, Callison J, Ebihara H, Mansfield K, Feldmann H. Clinical outcome of henipavirus infection in hamsters is determined by the route and dose of infection. Journal of virology. 2011;85:7658–7671. doi: 10.1128/JVI.00473-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose NF, Marx PA, Luckay A, Nixon DF, Moretto WJ, Donahoe SM, Montefiori D, Roberts A, Buonocore L, Rose JK. An effective AIDS vaccine based on live attenuated vesicular stomatitis virus recombinants. Cell. 2001;106:539–549. doi: 10.1016/s0092-8674(01)00482-2. [DOI] [PubMed] [Google Scholar]
- Steinhagen F, Kinjo T, Bode C, Klinman DM. TLR-based immune adjuvants. Vaccine. 2011;29:3341–3355. doi: 10.1016/j.vaccine.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeratna RD, Brazolot Millan CL, McCluskie MJ, Davis HL. CpG ODN can re-direct the Th bias of established Th2 immune responses in adult and young mice. FEMS immunology and medical microbiology. 2001;32:65–71. doi: 10.1111/j.1574-695X.2001.tb00535.x. [DOI] [PubMed] [Google Scholar]
- Weingartl HM, Berhane Y, Caswell JL, Loosmore S, Audonnet JC, Roth JA, Czub M. Recombinant nipah virus vaccines protect pigs against challenge. Journal of virology. 2006;80:7929–7938. doi: 10.1128/JVI.00263-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witko SE, Johnson JE, Kalyan NK, Felber BK, Pavlakis GN, Sidhu MK, Hendry RM, Udem SA, Parks CL. Refined methods for propagating vesicular stomatitis virus vectors that are defective for G protein expression. Journal of virological methods. 2010;164:43–50. doi: 10.1016/j.jviromet.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong KT, Grosjean I, Brisson C, Blanquier B, Fevre-Montange M, Bernard A, Loth P, Georges-Courbot MC, Chevallier M, Akaoka H, Marianneau P, Lam SK, Wild TF, Deubel V. A golden hamster model for human acute Nipah virus infection. The American journal of pathology. 2003;163:2127–2137. doi: 10.1016/S0002-9440(10)63569-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda M, Georges-Courbot MC, Ikeda F, Ishii M, Nagata N, Jacquot F, Raoul H, Sato H, Kai C. Recombinant measles virus vaccine expressing the Nipah virus glycoprotein protects against lethal Nipah virus challenge. PloS one. 2013;8:e58414. doi: 10.1371/journal.pone.0058414. [DOI] [PMC free article] [PubMed] [Google Scholar]