Abstract
Radical antegrade modular pancreatosplenectomy (RAMPS) is regarded as a reasonable approach for margin-negative and systemic lymph node clearance in left-sided pancreatic cancer. We present a patient with more than 5 years disease-free survival after robotic anterior RAMPS for pancreatic ductal adenocarcinoma in the body of the pancreas. The distal part of pancreas, soft tissue around the celiac trunk, and the origin of splenic vessels was dissected with the underlying fascia between the pancreas and adrenal gland. Resected specimen was removed through small vertical abdominal incision. Robot working time was about 8 hours, and blood loss was about 700 mL without blood transfusion. He returned to an oral diet on the postoperative first day and recovered without any clinically relevant complications. There was no lymph node metastasis, perineural or lymphovascular invasion. Both the pancreatic resection margin and the tangential posterior margin were free of carcinoma. The patient received only postoperative adjuvant radiotherapy around the tumor bed. The patient has survived for more than 5 years without evidence of cancer recurrence. Minimally invasive radical left-sided pancreatectomy with splenectomy may be oncologically feasible in well-selected pancreatic cancer.
Keywords: Robotic surgery, pancreatosplenectomy, pancreatic cancer
INTRODUCTION
In the last few decades, laparoscopic surgery has replaced conventional open surgery in the treatment of most elective gastrointestinal diseases, even malignancies such as gastric and colorectal cancers, with sound oncologic outomes.1,2 In addition, robotic surgical systems have been developed as a means of overcoming the limitations of conventional laparoscopic surgery and enhancing its efficacy.3
However, it was only recent movement that minimally invasive approaches began to be used in the treatment of pancreatic cancer. Radical antegrade modular pancreatosplenectomy (RAMPS) is regarded as a reasonable approach for margin-negative and systemic lymph node clearance in left-sided pancreatic cancer,4 and operarecent literature has reported the long-term oncologic outcomes of RAMPS.5 Based on our past experiences with distal pancreatosplenectomy (DPS) for pancreatic cancer,6 we have selectively applied minimally invasive modified anterior RAMPS since 2007. In this report, we present a patient who underwent first robotic anterior RAMPS and survived for more than five years without cancer recurrence.
CASE REPORT
Patient
A 72 year-old man (hight-172 cm, weight-78 kg, body mass index-26.37) with a three-month history of weight loss of 5 kg was admitted to the Department of Surgery in Severance Hospital, Seoul, Korea. On preoperative axial imaging, he had a suspicious mass-like lesion confined to the pancreas, which was suggestive of pancreatic cancer (Fig. 1A and B). The tumor seemed not to invade retroperitoneal space and it was apart from celiac axis. The patient's CA 19-9 was elevated to 60 IU/mL. After careful consideration, a decision to perform minimally invasive pancreatectomy was made based on the concept of anterior RAMPS, and a robotic surgical system was subsequently used.
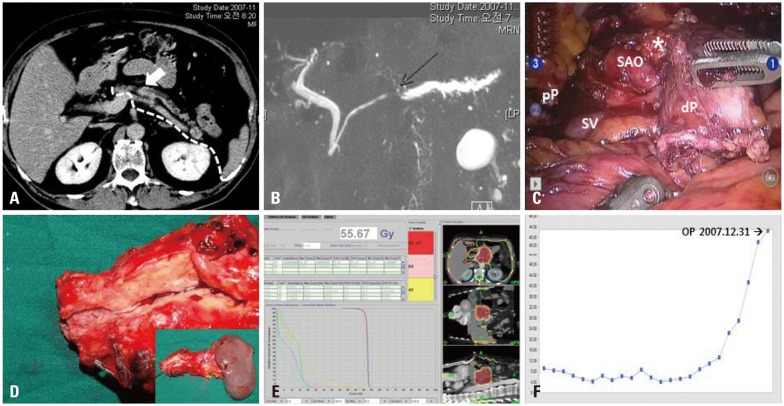
Fig. 1.
Perioperative surgical outcomes. Low attenuated pancreatic tumor located to the pancreatic body (A) with obstructive distal pancreatic duct dilatation (B) was noted on pre-operative radiologic images. The mass was confined to the pancreatic body with intact fascia layer between the pancreas and left adrenal gland. In addition, tumor was apart from celiac axis more than 2 cm. Therefore, it was possible to be removed safely by modified anterior RAMPS [note the dissection plane, white dotted line, (A)]. Robot-assisted anterior RAMPS was performed, and pathology examination revealed that the tumor was a pancreatic ductal adenocarcinoma with pT3N0M0, stage IIA (C and D). Postoperative adjuvant radiotherapy was given (E). Postoperative CA 19-9 declined and remained within the normal range (F). *Soft tissue around celiac axis. pP, proximal pancreas; dP, distal pancreas; SAO, the origin of the splenic artery; SV, the junction of splenic vein to superior mesenteric vein; RAMPS, radical antegrade modular pancreatosplenectomy.
Surgery
The complete procedure is described in a multimedia article published previously.7,8 In brief, surgery was performed with the patient placed in the supine position. After division of both the gastrocolic and gastrosplenic ligaments, the whole pancreas was exposed. Following the main outline of the original RAMPS, a complete window could be made via the avascular plane between the pancreatic neck and superior mesenteric vein-portal vein-splenic vein (SMV-PV-SV) confluence. This facilitated dissection of the lymph nodes around the common hepatic artery. Division of the pancreas was performed by endo-GIA at the pancreatic neck portion. The left gastric vein with soft tissue around celiac trunk and common hepatic artery were dissected (Fig. 1C). The splenic artery and vein were controlled at the origin of the celiac trunk and t the junction of the SV and SMV, respectively. The robotic surgical system is thought to be helpful for these dissectional processes. The superior mesenteric artery and left renal vessels were not aggressively dissected. The distal part of the pancreas was dissected with soft tissue around the celiac trun and the fascia layer covering the adrenal gland and perinephric soft tissue in a right-to-left fashion. An endo-pouch was introduced and surgical specimen was retrieved through a small vertical abdominal wound. The total operation time was eight hours and the intraoperative blood loss was 700 mL.
Postoperative course
The patient recovered without any complications. He returned to an oral diet on the first day and was discharged on the tenth day after operation. Pathologic examination revealed a pancreatic ductal adenocarcinoma with extension into the peripancreatic soft tissue (pT3). Regional lymph node metastasis was not identified (pN0, 0/2). Both the pancreatic resection margin and the tangential posterior margin were free of carcinoma (Fig. 1D). No evidence of perineural and lymphovascular invasion was noted. The patient received only postoperative adjuvant radiotherapy around the tumor bed (total 55.67 Gy/19 fractions, daily 2.93 Gy) (Fig. 1E). His CA 19-9 remained within normal limits after surgery (Fig. 1F), and the patient survived for more than five years without cancer recurrence.
DISCUSSION
Based on our surgical experiences,6 bloodless and margin-negative resection is an important factor in treating left-sided pancreatic cancer. In general, the use of posterior RAMPS for pancreatic cancer that has invaded the retroperitoneal space is technically challenging. Thus, in applying minimally invasive approaches to left-sided pancreatic cancer, it would be the best to consider anterior RMAPS, in which the posterior dissection plane is placed anterior to the left adrenal gland. Potential tumor conditions that can be completely removed by minimally invasive (MI) anterior RAMPS include those (Yonsei-Criteria for-MI RMAPS): 1) confined to the pancreas, 2) with the intact fascia layer between the pancreas and left adrenal gland/kidney, and 3) more than 1 to 2 cm from the celiac axis.7,8 In the past, several studies9-11 have been published demonstrating the technical feasibility and clinical benefit of laparoscopic distal pancreatectomy over open distal pancreatectomy; however, most cases of pancreatic cancer (ductal adenocarcinoma) were included in these studies only incidentally. As a result, we cannot fully assess the surgical quality of these procedures in the treatment of cancer based on the literature. In addition, the lack of information on tumor biologic characteristics, such as pT stage, pN stage, margin status, perineural/lymphovascular invasion, and survival outcomes, makes it difficult to determine the oncologic feasibility of minimally invasive approach for left-sided pancreatic cancer. However, recent clinical study suggest the technical feasibility and sound short-term perioperative oncologic outcomes.7,8,12-14 Particularly, Daoudai, et al.15 evaluated a total of 27 cases of robotic distal pancreatectomy for pancreatic ductal adenocarcinoma and showed excellent perioperative oncologic outcomes. It is true that current robot surgical system has unique characteristics to compensate the limitation of conventional laparoscopic surgery. In this case, we could take great advantages of three dimensional stable operative field provided by robotic surgical system and wrist-like movement of laparoscopic instrument in dissection of soft tissue around celiac axis, effective vascular control at the origin of the splenic artery/vein, and making window between pancreas and SMV-SV-PV confluence. However, it may be difficult to conclude that robot surgery will be superior in oncologic surgery for left sided pancreatic cancer, because 1) both robotic and laparoscopic approaches belong to the same category of minimally invasive surgery, therefore, the outcome may be similar. 2) Daoudai, et al.'s data should be criticized because of critical selection bias according to the time of surgical approach.15 3) Moreover, conventional laparoscopic technique has been far advanced these days. Therefore, it is thought that the concept of minimally invasive radical pancreatectomy using either robotic or laparoscopic approach seems more necessary rather than the view of which procedure would be superior in this moment. In the near future, not only the technical feasibility but also oncologic concerns of minimally invasive radical pancreatectomy for left-sided pancreatectomy should be properly evaluated, based on long-term survival outcomes.
In summary, we report a case of a patient selected for robotic anterior RAMPS in the treatment of his left-sided pancreatic cancer. The patient may be the first long-term survivor following robotic radical pancreatectomy for pancreatic ductal adenocarcinoma. Our findings underscore the rationale of Yonsei selection criteria for margin-negative anterior RAMPS and may also suggest the oncologic feasibility of minimally invasive DPS in well-selected patients with pancreatic cancer.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Colon Cancer Laparoscopic or Open Resection Study Group, authors. Buunen M, Veldkamp R, Hop WC, Kuhry E, Jeekel J, et al. Survival after laparoscopic surgery versus open surgery for colon cancer: long-term outcome of a randomised clinical trial. Lancet Oncol. 2009;10:44–52. doi: 10.1016/S1470-2045(08)70310-3. [DOI] [PubMed] [Google Scholar]
- 2.Huscher CG, Mingoli A, Sgarzini G, Sansonetti A, Di Paola M, Recher A, et al. Laparoscopic versus open subtotal gastrectomy for distal gastric cancer: five-year results of a randomized prospective trial. Ann Surg. 2005;241:232–237. doi: 10.1097/01.sla.0000151892.35922.f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jacob BP, Gagner M. Robotics and general surgery. Surg Clin North Am. 2003;83:1405–1419. doi: 10.1016/S0039-6109(03)00159-2. [DOI] [PubMed] [Google Scholar]
- 4.Strasberg SM, Linehan DC, Hawkins WG. Radical antegrade modular pancreatosplenectomy procedure for adenocarcinoma of the body and tail of the pancreas: ability to obtain negative tangential margins. J Am Coll Surg. 2007;204:244–249. doi: 10.1016/j.jamcollsurg.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Mitchem JB, Hamilton N, Gao F, Hawkins WG, Linehan DC, Strasberg SM. Long-term results of resection of adenocarcinoma of the body and tail of the pancreas using radical antegrade modular pancreatosplenectomy procedure. J Am Coll Surg. 2012;214:46–52. doi: 10.1016/j.jamcollsurg.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 6.Kang CM, Kim DH, Lee WJ. Ten years of experience with resection of left-sided pancreatic ductal adenocarcinoma: evolution and initial experience to a laparoscopic approach. Surg Endosc. 2010;24:1533–1541. doi: 10.1007/s00464-009-0806-7. [DOI] [PubMed] [Google Scholar]
- 7.Choi SH, Kang CM, Hwang HK, Lee WJ, Chi HS. Robotic anterior RAMPS in well-selected left-sided pancreatic cancer. J Gastrointest Surg. 2012;16:868–869. doi: 10.1007/s11605-012-1825-6. [DOI] [PubMed] [Google Scholar]
- 8.Choi SH, Kang CM, Lee WJ, Chi HS. Multimedia article. Laparoscopic modified anterior RAMPS in well-selected left-sided pancreatic cancer: technical feasibility and interim results. Surg Endosc. 2011;25:2360–2361. doi: 10.1007/s00464-010-1556-2. [DOI] [PubMed] [Google Scholar]
- 9.Taylor C, O'Rourke N, Nathanson L, Martin I, Hopkins G, Layani L, et al. Laparoscopic distal pancreatectomy: the Brisbane experience of forty-six cases. HPB (Oxford) 2008;10:38–42. doi: 10.1080/13651820701802312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kooby DA, Gillespie T, Bentrem D, Nakeeb A, Schmidt MC, Merchant NB, et al. Left-sided pancreatectomy: a multicenter comparison of laparoscopic and open approaches. Ann Surg. 2008;248:438–446. doi: 10.1097/SLA.0b013e318185a990. [DOI] [PubMed] [Google Scholar]
- 11.Jayaraman S, Gonen M, Brennan MF, D'Angelica MI, DeMatteo RP, Fong Y, et al. Laparoscopic distal pancreatectomy: evolution of a technique at a single institution. J Am Coll Surg. 2010;211:503–509. doi: 10.1016/j.jamcollsurg.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 12.Fernández-Cruz L, Cosa R, Blanco L, Levi S, López-Boado MA, Navarro S. Curative laparoscopic resection for pancreatic neoplasms: a critical analysis from a single institution. J Gastrointest Surg. 2007;11:1607–1621. doi: 10.1007/s11605-007-0266-0. [DOI] [PubMed] [Google Scholar]
- 13.Marangos IP, Buanes T, Røsok BI, Kazaryan AM, Rosseland AR, Grzyb K, et al. Laparoscopic resection of exocrine carcinoma in central and distal pancreas results in a high rate of radical resections and long postoperative survival. Surgery. 2012;151:717–723. doi: 10.1016/j.surg.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 14.Song KB, Kim SC, Park JB, Kim YH, Jung YS, Kim MH, et al. Single-center experience of laparoscopic left pancreatic resection in 359 consecutive patients: changing the surgical paradigm of left pancreatic resection. Surg Endosc. 2011;25:3364–3372. doi: 10.1007/s00464-011-1727-9. [DOI] [PubMed] [Google Scholar]
- 15.Daouadi M, Zureikat AH, Zenati MS, Choudry H, Tsung A, Bartlett DL, et al. Robot-assisted minimally invasive distal pancreatectomy is superior to the laparoscopic technique. Ann Surg. 2013;257:128–132. doi: 10.1097/SLA.0b013e31825fff08. [DOI] [PubMed] [Google Scholar]