Abstract
Objective
To evaluate the effect of neuromuscular electrical stimulation on spasticity in patients with spinal cord injury.
Methods
The study included eleven subjects with spinal cord injuries (C4 to T5). The modified Ashworth scale and pendulum test, which is accomplished through the Pendular Test Device - PTD (equipment which has a quartz crystal transducer accelerometer and optic fiber flexible electrogoniometer measuring the tensions and angular displacements). Patients underwent neuromuscular electrical stimulation (NMES) to the quadriceps muscle from fibular nerve, and the tests were administered before and after therapy.
Results
The data show a decrease in spasticity after NMES, with features such as increased variation between maximum and minimum peaks, i.e. increased amplitude of the curves. Furthermore, data from the subjective scale, and modified Ashworth scale after neuromuscular electrical stimulation also showed a reduction in the values of spasticity.
Conclusion
The data suggest that NMES is effective in reducing spasticity immediately after completion. Level of Evidence II, Therapeutic Studies-Investigating the Results of Treatment.
Keywords: Spinal cord injuries, Spasticity, Neuromuscular electrical stimulation
INTRODUCTION
Spinal cord injury, according to available estimates for the global population, affects about 20 to 40 people per million inhabitants, causing a great impact on patients' and their families' lives. 1 It is a condition that, depending on the degree of impairment, generates motor and sensitivity alterations. 2
A spinal cord injury brings many complications to patients, and these are generated by the injury itself, such as recurrent urinary infections, osteoporosis by reducing the mechanical stress on the bones, cardiovascular deficiencies, muscle atrophy, and spasticity, among others. 3 , 4
One of the characteristics of the patient with spinal cord injury is spasticity. It occurs when there is an upper motor neuron lesion causing an increased stretch reflex, abnormal muscle tone, and especially greater resistance to passive movement, among others. 5 , 6
There are some ways of measuring spasticity, for example, the scales (of which the most used is the modified Ashworth scale) and the pendulum test. Another technique often used is the Wartenberg's pendulum test, which consists in measuring spasticity and rigidity through the passive movement of the knee joint. 7 , 8
Drug treatment of spasticity is used when there is a change in musculoskeletal function or deformities. They work by decreasing the excitability of spinal reflexes. Among the drugs used, there are: baclofen, diazepam, clorazepate, clonazepam, pirazepam, tizanidine, and many others. 9
The objective of this study was to determine, using the pendulum test and applying the modified Ashworth scale in patients with spinal cord injury, whether after neuromuscular electrical stimulation there is a significant reduction in the intensity of spasticity.
MATERIALS AND METHODS
Subjects
Participated in the study eleven males with spinal cord injury (level of injury C4 to T5, of these, nine were ASIA A, 1 ASIA B, and 1 ASIA D), with a mean age of 34 ± 11.57 years old, mean body mass of 68.5 ± 13.13 kg, mean height 1.74 ± 0.05 meters. Five patients were not taking any medications for spasticity, and six did, five were taking baclofen and one used clonazepam. All volunteers are outpatients of the Laboratory of Biomechanics and Rehabilitation of the Locomotor System, Hospital das Clinicas da UNICAMP, and signed a consent form. This paper was submitted to and approved by the institution's Ethics Committee. Table 1 shows some of the physical characteristics of the patients involved in the research. As inclusion criteria, patients with spinal cord injury should be adults with a diagnosis of spinal cord injury for more than three years, and do not present any pathology that contraindicate his participation in the study.
Table 1. Anthropometric characteristics of volunteers studied.
| Patients | Age (years old) | Body mass (kg) | Height (m) |
|---|---|---|---|
| 1 | 22 | 50 | 1.70 |
| 2 | 33 | 78 | 1.72 |
| 3 | 30 | 60 | 1.73 |
| 4 | 29 | 65 | 1.70 |
| 5 | 22 | 56 | 1.80 |
| 6 | 40 | 67.8 | 1.81 |
| 7 | 41 | 65 | 1.65 |
| 8 | 39 | 72 | 1.70 |
| 9 | 24 | 64 | 1.79 |
| 10 | 62 | 80 | 1.80 |
| 11 | 37 | 95 | 1.75 |
| Mean (± S.D.) | 34.54 (±11.57) | 68.43 (±13.13) | 1.74 (±0.05) |
EXPERIMENTAL PROTOCOL
The experimental protocol was divided into three steps:
1st step
Pendulum test:
Prior to the neuromuscular electrical stimulation (NMES), the pendulum test was conducted through the device, designed and built specifically to assess spasticity. 10 (Figure 1)
Figure 1. DTP equipment connected to computer.
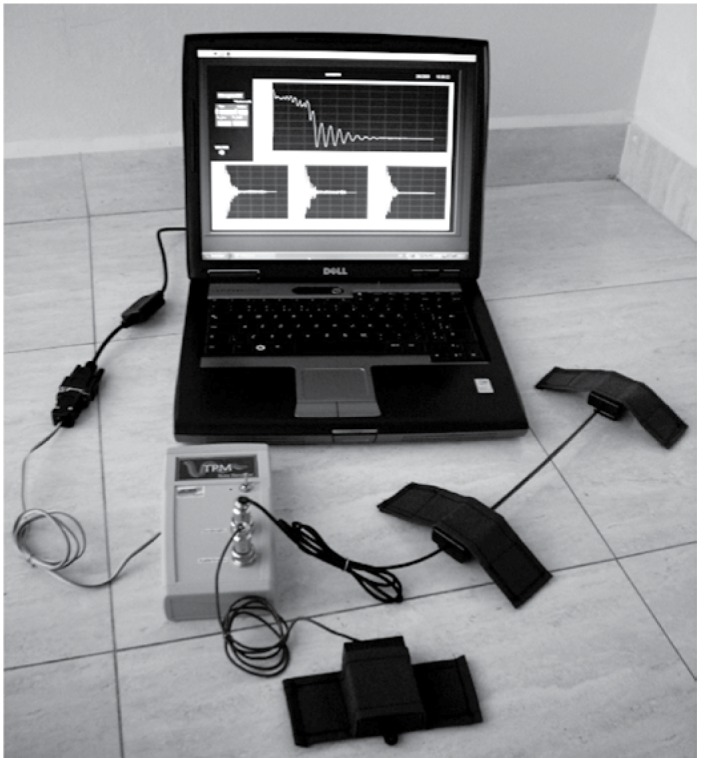
The equipment (DTP) comprises two types of sensors: accelerometer and electrogoniometer. The accelerometer consists in quartz crystals transducers that are sensitive to the piezoelectric effect due to the pressure caused by muscle tremors. The signal is amplified through a specially designed circuit to measure the oscillating frequencies of the electrical signal in three perpendicular axes (X, Y and Z-axis).
The electrogoniometer measures angular displacement. It is a device consisting of a flexible optical fiber. The changes in curvature of the fiber cause attenuation of the light propagation. The attenuation observed is related to the angular displacement. For the collection of patient data the accelerometer and the eletrogoniometer devices are applied, fixed into the patient through a surface that adheres to the sensor's Velcro tape. The values of angular displacements and tensions, which are sent to a microcontroller, are converted from analog signals into digital signals. Specific software was developed for data analysis and the final result of the variation of the pendulum is shown on a computer screen. (Figure 1)
For fixing the goniometer and accelerometer into the patients, we used the sensors' Velcro tapes, one on the thigh and the other in the leg. The accelerometer was fixed in the tight 8 cm above the top edge of the patella and the goniometer in the center of the knee joint. (Figure 2)
Figura 2. Positioning of patient for pendulum test.
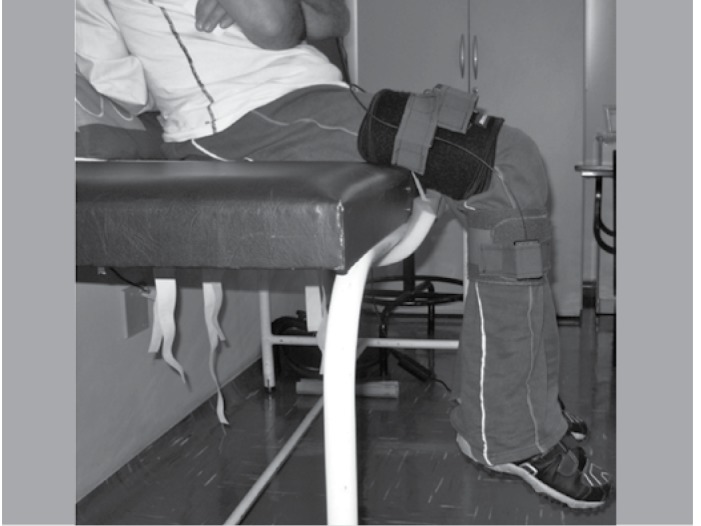
The measurements were performed in the right leg of the patient and repeated three times. Prior to each experiment, another researcher evaluated spasticity through the modified Ashworth scale.
We conducted a subjective scale before and after the NMES session. A scale from 0 to 10 where 0 represents no spasticity and 10 high spasticity was shown to the patients. They were asked to rank their perception of spasticity before performing pre -training evaluation with neuromuscular electrical stimulation and before the post-training assessment.
2nd Step
Neuromuscular electrical stimulation (NMES):
At this stage the patients performed NMES through a 4-channel electrical stimulator that features a 25Hz signal with 300µs duration and maximum intensity 100mV (1KΩ load) monophasic rectangular pulses. NMES was held in the quadriceps muscles and fibular nerve for 20 and 15 min, respectively.
3rd Step
Pendulum test:
Reassessment was performed after NMES session. Once again, another researcher evaluated spasticity using the Asworth's scale, and then the pendulum test was performed through the PST device.
DATA ANALYSIS
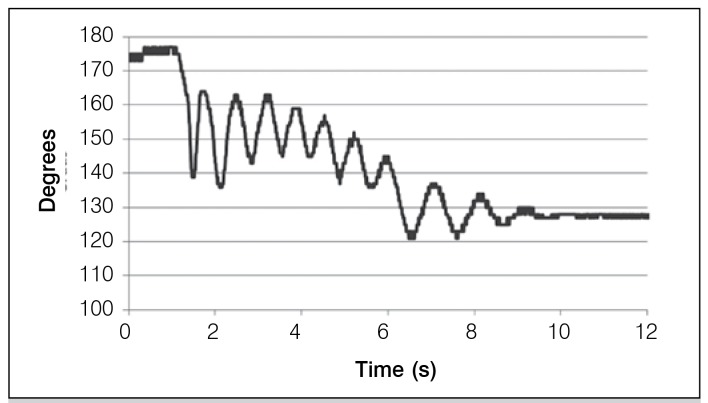
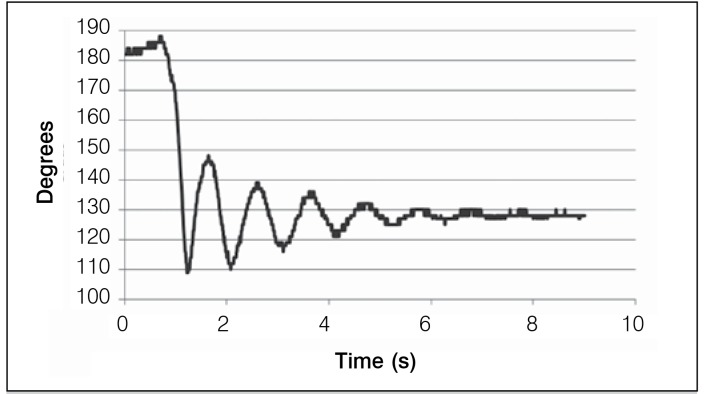
The software was developed for the operation of the program, which consists in the analysis of the pendulum test, data collection and projection of graphs. In the data analysis and projection graphs it was possible to check the values of the variation between the peak maximum and minimum of each oscillation peak of the graph. The differences between these peaks were measured curves obtained from each patient before and after the session of NMES. Figures 3 and 4 show the graphs of the pendulum test of a patient with spinal cord injury. Measurements were made before and after NMES.
Figure 3. Curve of the pendulum test before NMES (Right leg).
Figure 4. Curve of the pendulum test after NMES (Right leg).
In addition to these data, and adopting Stillman's et al. nomenclature, 11 we calculated the onset angle of the test (onset angle=On Ang ), final angle of the response test (Rest end angle=Rest Ang), the angle at the end of the first flexion movement (F1 Ang), the angle at the end of the first extension movement (Ang E1), the initial flexion amplitude (F1Amp=F1 Ang-On Ang), initial extension amplitude (E1 Amp=F1 ang- E1 Ang), total movement amplitude (Plat Amp=Rest Ang-On Ang), relaxation index (RI=F1 Amp/Plat Amp), and relaxation extension index (REI=E1 Amp/Plat Amp).
RESULTS
In Figure 3 it can be seen that the range of motion is reduced and disorganized oscillatory motions are present. This is because there is neither muscle control nor constant oscillation frequency. The start of the movement is marked by an angular position which is generally lower than expected. Typically, the end of the movement is a result of energy loss due to friction caused by the oscillation of the leg with the air as well as muscle relaxation.
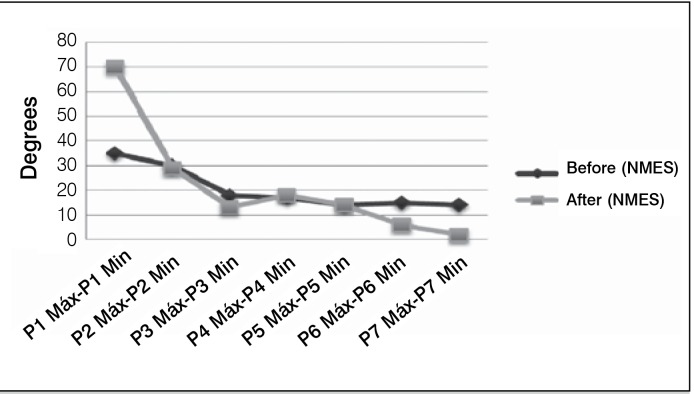
Figure 4, after NMEE, shows a decrease in spasticity intensity, which is characterized in the graph with the largest variation between the maximum and minimum peak, as well as for a more organized oscillatory motion showing more harmonic curves. It is observed in this graph a mild decay of the intensity of the curve. Figure 5 shows the results of intensity measurements of the curve before and after NMEE of a patient, showing that there was a decrease in spasticity.
Figure 5. Angular variation between maximum and minimum peaks - Patient 1.
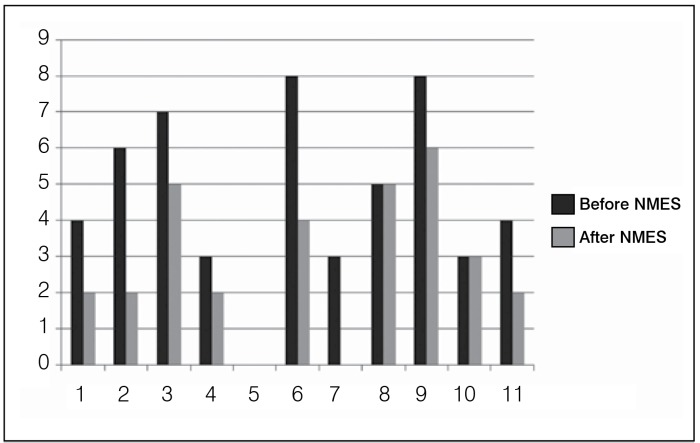
Table 2 shows the results of the evaluations performed with the pendulum test of eleven patients that were evaluated before and after neuromuscular electrical stimulation. The mean and standard deviation of the amplitude of the curve, the magnitude of initial bending, the amplitude of the initial extent, the relaxation index, and the extension index were measured according to the above definitions. 11 The values calculated before and after neuromuscular electrical stimulation show an increase in the rate of relaxation, indicating that stimulation helps to reduce the severity of spasticity. (Table 2) In Tables 3 and 4, the results were calculated using the assessments in patients using medications and those that do not. Moreover, the pendulum test was applied before and after neuromuscular electrical stimulation (NMES). The results of the modified Ashworth scale and subjective scale of spasticity before and after NMEE are shown respectively in Table 5 and in Figure 6. It was also observed that there was a reduction of spasticity.
Table 2. Results from eleven patients with spinal cord lesion in pendulum test before and after NMES Data expressed as Mean ± SD.
| Measurement | Before NMES | After NMES |
|---|---|---|
| Plat Amp(º) | 41.53 ± 4.99 | 52.81 ± 10.54 |
| F1 Amp(º) | 75.92 ± 25.1 | 95.27 ± 29.40 |
| E1 Amp(º) | 67.73 ± 26.94 | 81.22 ± 32.64 |
| RI | 1.79 ± 0.56 | 1.86 ± 0.46 |
| REI | 1.59 ± 0.62 | 1.62 ± 0.55 |
Amplitude of the curve (Plat Amp), initial flexion amplitude (F1Amp), initial extension amplitude (E1Amp) e relaxation extension index (REI) and relaxation index (RI).
Table 3. Results from six patients that performed treatment with medication and five without medication before NMES.
| Data expressed as Mean ± SD | ||
|---|---|---|
| Measurement | With medication | Without medication |
| Plat Amp(º) | 43.20 ± 3.55 | 39.85 ± 6.38 |
| F1 Amp(º) | 81.12 ± 22.84 | 71.55 ± 30.38 |
| E1 Amp(º) | 72.20 ± 23.00 | 63.55 ± 33.77 |
| RI | 1.89 ± 0.57 | 1.72 ± 0.62 |
| REI | 1.69 ± 0.59 | 1.50 ± 0.72 |
Amplitude of the curve (Plat Amp), initial flexion amplitude (F1Amp), initial extension amplitude (E1Amp) e relaxation extension index (REI) and relaxation index (RI)
Table 4. Results from six patients that performed treatment with medication and five without medication after NMES.
| Data expressed as Mean ± SD | ||
|---|---|---|
| Measurement | With medication | Without medication |
| Plat Amp(º) | 45.33 ± 12.32 | 48.8 ± 11.94 |
| F1 Amp(º) | 103.16 ± 28.04 | 84.6 ± 31.48 |
| E1 Amp(º) | 89.5 ± 33.33 | 73.3 ± 32.21 |
| RI | 1.96 ± 0.43 | 1.75 ± 0.52 |
| REI | 1.70 ± 0.58 | 1.53 ± 0.57 |
Amplitude of the curve (Plat Amp), initial flexion amplitude (F1Amp), initial extension amplitude (E1Amp) e relaxation extension index (REI) and relaxation index (RI)
Table 5. Scales before and after NMES.
| Patients | Level of the Lesion and ASIA | Time of Lesion (years) | Ashworth scale before and after NMES |
|---|---|---|---|
| 1 | C6 A | 3 | 3 / 1 |
| 2 | C5 A | 14 | 1 / 0 |
| 3 | T4 A | 12 | +1 / 0 |
| 4 | C4 A | 5 | 0 / 0 |
| 5 | C6 A | 3 | + 1 / 1 |
| 6 | C6 A | 8 | 1 / 0 |
| 7 | T3 A | 8 | 3 / 0 |
| 8 | T3 A | 7 | 1 / 0 |
| 9 | C6 B | 3 | 3 / 3 |
| 10 | C3-C4 A | 4 | +1 / 1 |
| 11 | T5 D | 23 | 2 / 2 |
Obs: Patients on medication: 1, 2, 4, 5, 7, and 10. Patients without medication: 3, 6, 8, 9, and 11.
Figure 6. Subjective scale before and after spasticity.
DISCUSSION
Spasticity can be beneficial or detrimental. It is beneficial when it diminishes the loss of muscle mass, improves blood circulation and facilitates the transfer processes, among others. Moreover, it involves slow movements and promotes involuntary contraction of agonist and antagonist muscles. It is detrimental for both hindering activities of daily living as to be painful in some cases. 12 - 15 In general, it is assumed that spasticity is more severe the higher the level of injury and loss of function (ASIA A and B). However, it was found that levels B and D present spasticity greater than in complete lesion. 16 Corroborating these results, we verified by the Ashworth scale that in two patients (one ASIA B and another ASIA D), that NMES did not significantly decrease spasticity.
It is noted that the assessment of muscle tone to quantify the intensity of spasticity is a difficult task only if subjective interpretation based on passive movements is used. Thus, due to the lack of methods to assess spasticity, the pendulum test, as described above, provides a simple process with high reliability. Thus, the pendulum test used to assess quadriceps muscle tone in normal and abnormal movements became effective in the clinical evaluation of spasticity. A clinical procedure to reduce spasticity is neuromuscular electrical stimulation, which has local action and can be used at the desired intensity. This is important to preserve patients to perform some essential functions in daily life. It is reported that electrical stimulation has a short span. 17 In addition, electrical stimulation in tetraplegic patients also contributes to reduce and prevent heterotopic ossification. 18 Robinson et al. 19 conducted a study to assess the initial and long term effects of electrical stimulation in hemiplegic with spasticity. However, little has been reported on the long term effects of stimulation of spinal cord injured patients with spasticity. In this work, the graphics obtained through the pendulum test clearly show that after neuromuscular electrical stimulation in spinal cord injured individuals there is a reduction of spasticity. This stems from two important observations: first, that after NMES the graphs resulting from the pendulum test are more harmonics and organized, consisting in a qualitative result, the second is quantitative, since the variation between the maximum and minimum peaks after electrical stimulation increases, i.e., the curves present a higher intensity, thus increasing the relaxation index while decreasing spasticity.
The motor neuron lesion weakens and paralyzes the muscle contraction as can be clearly observed in Figure 3. With the application of Neuromuscular Electrical Stimulation (NMES) inhibition of the spastic muscle relaxation and stimulation of afferent pathways is initially observed. Subsequently, actions occur in neuroplasticity, which enable modifying the viscoelastic properties of muscles. Thus, the motor units are developed, what causes rapid and ordered muscle contractions.
Figure 4 shows that neuromuscular electrical stimulation provides a harmonic motion, and periodic intensity curves decreasing over time. These observations suggest that there may occur some restructuring of the motor act provoked by electrical stimulation. This response corresponds to a rearrangement in the activation sequence between synergist muscles, agonists and antagonists, resulting in a coordinated movement, with the consequent reduction of spasticity.
The pharmacological treatment is otherwise widely used to reduce spasticity. This process can lead to a greater improvement when there is a multimodal approach. The drugs baclofen, diazepam, tizanidine are the most frequently used. The most effective and that present less side effects is intrathecal Baclofen. 20 Rekand, 21 in his research, reports that the best therapy for spasticity is the combination of physical therapy with oral medications like baclofen and tizanidine, but this type of clinical procedure (medication) causes the patient many side effects, and the best option is the botulinum toxin along with physiotherapy.
Tables 3 and 4 show the results of pendulum test measurements of patients using medication. Measurements were made before and after NMES. It is observed that there is a reduction of spasticity when the combination medication with electrical stimulation was used. This is evidenced by the increase in the amplitude of the curve, the amplitude of the initial flexion, amplitude of the initial extension, the relaxation index and the extension index.
CONCLUSION
The data suggest that NMES is effective in reducing spasticity immediately after its completion. This was demonstrated through the scales applied and the pendulum test. However, more studies are needed to evaluate the long-term effects of NMES in reducing spasticity and its relationship with oral medications.
Footnotes
All the authors declare that there is no potential conflict of interest referring to this article.
Citation: Tancredo JR, Maria RM, Azevedo ERF, Alonso KC, Varoto R, Cliquet Junior A. Clinical assessment of spasticity in individuals with spinal cord injury. Acta Ortop Bras. [online]. 2013;21(6):310-4. Available from URL: http://www.scielo.br/aob.
Work performed at the Laboratory of Biomechanics and Rehabilitation of the Locomotor System, Department of Orthopedics and Traumatology, Faculdade de Ciências Médicas, Universidade Estadual de Campinas
REFERENCES
- 1.Pereira ME, Araujo TC. Enfrentamento e reabilitação de portadores de lesão medular e seus cuidadores. Psico PUCRS, Porto Alegre. 2006;37(1):37–45. [Google Scholar]
- 2.Lim PA, Tow AM. Recovery and regeneration after spinal cord injury: a review and summary of recent literature. Ann Acad Med Singapore. 2007;36(1):49–57. [PubMed] [Google Scholar]
- 3.Carvalho DC, Garlipp CR, Bottini PV, Afaz SH, Moda MA, Cliquet A Jr. Effect of treadmill gait on bone markers and bone mineral density of quadriplegic subjects. Braz J Med Biol Res. 2006;39(10):1357–1363. doi: 10.1590/s0100-879x2006001000012. [DOI] [PubMed] [Google Scholar]
- 4.Carvalho DCL, Carvalho MM, Cliquet A. Osteoporose por desuso: aplicação na reabilitação do lesado medular. Acta Ortop Bras. 2001;9(3):34–43. [Google Scholar]
- 5.Leitao AV, Muss CAI, Granero LHM, Rossetto R, Pavan K, Lianza S. Spasticity: Clinical Evaluation. Braz Assoc Phy Med Rehabil. 2006;87(12):1551–1558. [Google Scholar]
- 6.Baldo MVC. [Acessado em: 05 January 2010];Physiology of Human Movement. Department of Physiology and Biophysics- USP, 2009. Text Support. a Disponível em: www.fisio.icb.usp.br/aulasfisio/cursos/mdidatico/movimento.pdf.
- 7.Bajd T, Vodovnik L. Pendulum testing of spasticity. J Biomed Eng. 1984;6(1):9–16. doi: 10.1016/0141-5425(84)90003-7. [DOI] [PubMed] [Google Scholar]
- 8.Valle MS, Casabona A, Sgarlata R, Garozzo R, Vinci M, Cioni M. The pendulum test as a tool to evaluate passive knee stiffness and viscosity of patients with rheumatoid arthritis. BMC Musculoskelet Disord. 2006;7:89–89. doi: 10.1186/1471-2474-7-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lianza S, Quagliato E, Bang G, Botelho LA, Gianini MAC, Spósito MMM. Espasticidade: tratamento medicamentoso. São Paulo: Associação Médica Brasileira e Conselho Federal de Medicina; 2006. [Google Scholar]
- 10.Maria RM, Cliquet A., Júnior . Development of a system for evaluation of spasticity in spinal cord. São Carlos, SP: Universidade de São Carlos; 2009. [Google Scholar]
- 11.Stillman B, McMeeken J. A video-based version of the pendulum test: technique and normal response. Arch Phys Med Rehabil. 1995;76(2):166–176. doi: 10.1016/s0003-9993(95)80026-3. [DOI] [PubMed] [Google Scholar]
- 12.Kirshblum S. Treatment alternatives for spinal cord injury related spasticity. J Spinal Cord Med. 1999;22(3):199–217. doi: 10.1080/10790268.1999.11719570. [DOI] [PubMed] [Google Scholar]
- 13.Hsieh JT, Wolfe DL, Miller WC, Curt A. Spasticity outcome measures in spinal cord injury: psychometric properties and clinical utility. Spinal Cord. 2008;46(2):86–95. doi: 10.1038/sj.sc.3102125. [DOI] [PubMed] [Google Scholar]
- 14.Kita M, Goodkin DE. Drugs used to treat spasticity. Drugs. 2000;59(3):487–495. doi: 10.2165/00003495-200059030-00006. [DOI] [PubMed] [Google Scholar]
- 15.Jozefczyk PB. The management of focal spasticity. Clin Neuropharmacol. 2002;25(3):158–173. doi: 10.1097/00002826-200205000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Kinh GAB. On spasticity in spinal cord injury. The challenge of measurement and the role of novel intervention (segway) Columbia: The University of British Columbia; 2009. [Google Scholar]
- 17.van der Salm A, Veltink PH, Ijzerman MJ, Groothuis-Oudshoorn KC, Nene AV, Hermens HJ. Comparison of electric stimulation methods for reduction of triceps surae spasticity in spinal cord injury. Arch Phys Med Rehabil. 2006;87(2):222–228. doi: 10.1016/j.apmr.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 18.Ferreira DBJ, Lippelt HC, Cliquet A., Junior Estimulação elétrica neuromuscular na reversão da ossifação heterotópica. Acta Ortop Bras. 2006;14(2):72–74. [Google Scholar]
- 19.Robinson CJ, Kett NA, Bolam JM. Spasticity in spinal cord injured patients: 2. Initial measures and long-term effects of surface electrical stimulation. Arch Phys Med Rehabil. 1988;69(10):862–868. [PubMed] [Google Scholar]
- 20.Sternberg TL. Treatment of Spasticity Associated with Spinal Cord Injury and Other Central Nervous System Damage. Northeast Florida Med. 2009;60(3):19–22. [Google Scholar]
- 21.Rekand T. Clinical assessment and management of spasticity: a review. Acta Neurol Scand; [DOI] [PubMed] [Google Scholar]