Abstract
Synthetic seeds were formed from shoot tips of two in vitro grown Begonia cultivars using 3% sodium alginate in Murashige and Skoog medium (MS) salt solution as the gel matrix and 100 mM calcium chloride for complexation. Synthetic seed formation was achieved by releasing the sodium alginate/explant combination into 100 mM calcium chloride (CaCl2 ·H2O) solution for 30 or 45 min. Both control and encapsulated shoots were transferred into sterile Petri dishes and stored at 4°C or 22°C for 0, 2, 4, 6, or 8 weeks. Conversion of synthetic seeds into plantlets for both storage environments was assessed in MS medium or peat-based substrate. No significant difference was found between the 30 and 45 min CaCl2 ·H2O treatments or the two cultivars. Encapsulation of explants improved survival rate over time irrespective of the medium type or storage environment. Survival rates of 88, 53, 28, and 11% for encapsulated microshoots versus 73, 13, 0, and 0% for control explants were achieved in microshoots stored for 2, 4, 6, and 8 weeks, respectively. The best results were obtained when synthetic seeds were stored at 4°C and germinated on MS medium. Regenerated plantlets were successfully established in potting soil.
1. Introduction
Recently, the use of alginate encapsulation of in vitro cultured shoot tips as an alternative to somatic embryos to develop synthetic seeds has increased [1, 2]. This increase in the use of microshoots in synthetic seed development is due to the fact that encapsulation of vegetative propagules offers an efficient and cost-effective system for clonal propagation of plant species [3, 4]. Moreover, most plant species are more readily amenable to in vitro shoot culture than to regeneration via somatic embryogenesis, which is, for the most part, highly species or genotype dependent. Synthetic seed technology can be used for several purposes. For example, it can be used in conjunction with micropropagation for in vitro conservation, germplasm storage, and reduction of the need for transferring and subculturing during off-season periods [5]. Cold storage of encapsulated or synthetic seeds has the potential to reduce the cost of maintaining germplasm cultures as well as to reduce the possibility of genetic instability that could result from frequent subculturing [5, 6]. The technology is particularly useful for the propagation of rare hybrids, elite genotypes, and genetically engineered plants whose seeds are either too expensive or not readily available [7].
Begonias are among the most popular ornamental plants in the world thanks to their large, showy, and long-lasting multicolor flowers, ranging from white to pink, red, and yellow. They are used as garden plants and potted plants, in hanging baskets, and as greenhouse flowers [8]. Begonias have also been used as potherbs or leaf vegetables in many parts of the world, and the roots and tubers of some species have been reported to possess antimicrobial activities and are used to treat various ailments [9–11]. Begonias are divided into three categories based on rootstocks: tuberous, fibrous, and rhizomatous. Tuberous and fibrous begonias are grown mostly for their flowers, whereas rhizomatous begonias are cultivated for their large, attractive foliage. As one the largest angiosperm genera containing about 2000 species [8] and with new species continuing to be discovered in various parts of the world [12–16], Begonia is a major component of the floriculture industry with a wholesale value of over $4 billion in 2011 statistics reported for 15 US states [17]. The main purpose of the current study was to establish an encapsulation method for Begonia microshoots as explants for short-term storage and germplasm exchange. Two Begonia semperflorens cultivars, Sweetheart Mix and BabyWing White, were used.
2. Materials and Methods
2.1. Plant Materials
Explants were excised from in vitro cultures of Begonia “Sweetheart Mix” and “BabyWing White” maintained on Murashige and Skoog (MS [18]) medium with 2% sucrose, 0.75 g/L MgCl2, and pH 5.8. All media were sterilized by autoclaving at 121°C and 1.1 kg/cm2 for 15 minutes after addition of 2 g/L Gelrite (Sigma-Aldrich, St. Louis, MO). Explants consisted of microshoots 4–8 mm long.
2.2. Encapsulation Procedure and Duration of Exposure to Calcium Chloride
The steps involved in the scheme used for encapsulation, germination, and maturation of Begonia microshoots are shown in Figure 1. Synthetic seeds were formed using 3% sodium alginate in MS salt solution as the gel matrix and 100 mM calcium chloride (CaCl2·H2O) for complexation. Both the sodium alginate and calcium chloride solutions were sterilized by autoclaving at 121°C and 1.1 kg/cm2 for 15 minutes. Explants were submerged in sodium alginate solution for ~2 minutes. Explants, along with enough sodium alginate solution to encapsulate each one, were individually suctioned into a sterile, disposable pipette that had been modified by cutting the tip so that its diameter was ~7 mm.
Figure 1.
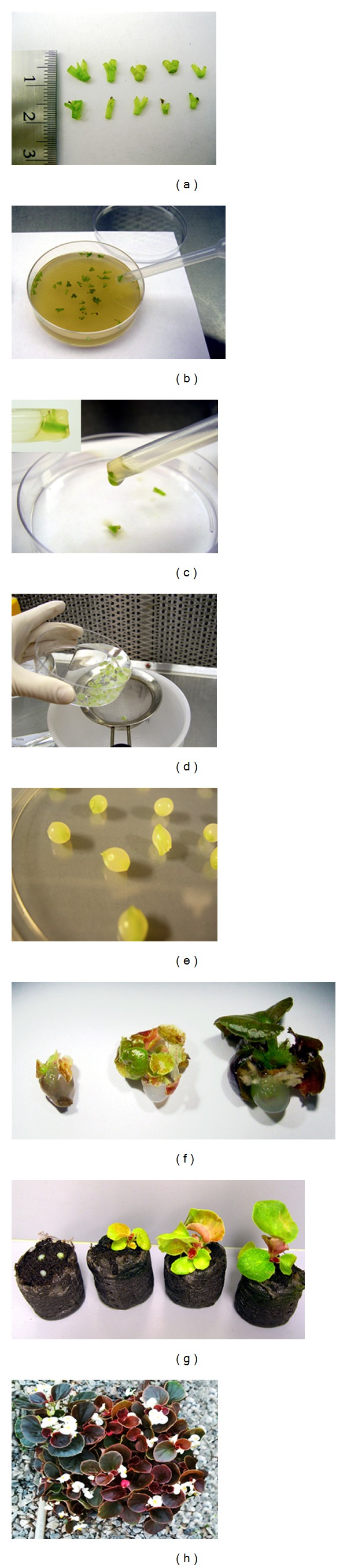
Encapsulation, germination, and maturation of Begonia microshoots. (a) Microshoot explants (4–8 mm long) were excised from in vitro cultures of Begonia cv. Sweetheart Mix and cv. BabyWing White. (b) Explants were submerged in sodium alginate solution for ~2 minutes and then individually suctioned into a sterile, disposable pipette with enough sodium alginate solution to encapsulate. (c) Each explant/sodium alginate combination was then released into a container of CaCl2·H2O and left for 30 or 45 minutes. (d) After the allotted time, the calcium chloride was drained from the newly formed synthetic seeds (encapsulated explants) by pouring the mixture into a sterile strainer. The synthetic seeds were rinsed at least three times with sterilized water to remove any remaining CaCl2·H2O. (e) Encapsulated explants or synthetic seeds were then placed in one of three container types: empty, sterile Petri dish (for storage), MS media, or peat-based substrate (PBS; Jiffy 7 peat pellets). (f) Typical growth pattern of synthetic seeds in MS media. (g) Typical growth pattern of synthetic seeds in soil. (h) Both MS media and PBS grown synthetic seeds developed into mature, thriving plants.
During synthetic seed formation, the sodium alginate/explant combination was released into CaCl2·H2O and maintained for either 30 or 45 minutes to examine how duration in CaCl2·H2O might affect germination percentage. After the allotted time, the calcium chloride was drained from the newly formed synthetic seeds (encapsulated explants) by pouring the mixture into a sterile strainer. The synthetic seeds were rinsed at least three times with sterilized distilled water to remove any remaining CaCl2·H2O.
2.3. Storage Temperature and Duration
Two storage environments, 4°C refrigeration and room temperature (~22°C), and five storage durations (0, 2, 4, 6, and 8 weeks) were evaluated. Synthetic seeds as well as nonencapsulated explants (control) were placed in empty, sterile Petri dishes and stored under 4°C refrigeration or at room temperature (~22°C) for selected durations (0, 2, 4, 6, or 8 weeks). After the allotted storage period, the synthetic seeds and the control explants were placed in either MS media or peat-based substrate as described below. Germination percentages were monitored for all groups beginning with week 0 and extending through week 8.
2.4. In Vitro and Ex Vitro Germination
Two types of growth media, an in vitro germination medium and an ex vitro nonsterile peat-based substrate (PBS; Jiffy-7 Pellets, Shippegan, NB, Canada), were used. The culture medium consisted of Murashige and Skoog (MS) [18] medium supplemented with 2% sucrose, 0.75 g/L MgCl2 and adjusted to pH 5.8. All media were sterilized by autoclaving at 121°C for 15 minutes after addition of 2 g/L Gelrite. Synthetic seeds were planted in both MS medium and nonsterile PBS as parallel studies. The synthetic seeds that were plated on MS media were maintained at 22°C, 50% humidity, and 16-hour days under fluorescent lights with a photon flux averaging 154 μmoL m−2 s−1. Those in PBS were placed under grow lights and maintained at 23–25°C, 47–50% relative humidity, and 16-hour days under fluorescent lights with a photon flux of 71 μmol m−2 s−1. A set of nonencapsulated explants served as a control and was maintained on the media described above and under the same environmental conditions. Germination, also referred to as conversion or regrowth by various authors [19–22], was defined as the point at which plant growth was observed outside of the synthetic seed matrix.
2.5. Experimental Design and Statistical Analysis
The experiment was conducted in a five-factorial randomized complete block design, with two media (MS medium and Jiffy 7 peat pellets), two cultivars (BabyWing White and Sweetheart Mix), three CaCl2·H2O exposure durations (0, 30, and 45 min), two storage temperatures (4 and 22°C), and five storage durations (0, 2, 4, 6, and 8 weeks). There were five replications for each treatment, with three microshoots per replication. The experiment was repeated twice. Data were collected as the count of shoots that “germinated” (developed successfully) in each experimental unit. Data were analyzed using generalized linear mixed models with the GLIMMIX procedure of SAS (version 9.3; SAS Institute Inc., Cary, NC) and the Poisson distribution. Models were evaluated beginning with a full model containing main factors and all interactions. Models were reduced by sequentially eliminating nonsignificant (P ≤ 0.05) interaction terms. The Schaffer-Simulated method was used for multiple mean comparisons.
Initial analyses determined that two factors, medium and storage duration, were highly influential in significant interaction terms; therefore, subsequent analyses were conducted individually by combination of medium and storage duration. Further analyses using cultivar, CaCl2·H2O exposure duration, and storage temperature determined that there were no significant interactions; therefore, final models contained only the three main effects. Means are presented as percentages.
3. Results and Discussion
Germination percentage of encapsulated as well as nonencapsulated (control) Begonia microshoots stored in two environments, namely, at 4°C and room temperature (~22°C) for various durations (0, 2, 4, 6, and 8 weeks) was evaluated. In both environments, Begonia shoot tips were dipped into 3% sodium alginate in MS salt solution as the gel matrix developed into nicely formed beads (hydrogels) and subsequently mature plants, irrespective of time of exposure (30 or 45 min) to the complexing agent, 100 mM CaCl2·H2O, or the growing medium, MS or PBS medium (Figure 1).
3.1. Effect of Cultivar on Germination
There was no significant difference (P = 0.05) in germination percentages between synthetic seeds of the two cultivars, BabyWing White and Sweetheart Mix, grown in MS medium in vitro (Figure 2(a)). The results obtained under ex vitro conditions mirrored those achieved with MS medium, except that germination percentage for BabyWing White synthetic seeds in peat-based substrate was significantly higher (P = 0.05) than that obtained for Sweetheart Mix at week 2, with 66 and 45% germination percentage for BabyWing White and Sweetheart Mix, respectively (Figure 2(b)). In fact, germination percentage for BabyWing White was consistently higher, albeit not significantly, than that for Sweetheart Mix even after 8 weeks. For BabyWing White, germination percentages of 39% and 29% were obtained after 4 weeks when encapsulated shoots were grown in MS medium and peat-based substrate, respectively (Figures 2(a) and 2(b)). Similarly, for Sweetheart Mix, germination percentages of 36% and 21% were obtained after 4 weeks in MS medium and peat-based substrate, respectively. However, germination capacity for both cultivars decreased substantially with extended storage duration. Similar results have been reported by other workers [7]. In short, although low germination (3.3% for BabyWing White and 13.3% for Sweetheart Mix) could be achieved for synthetic seeds of both genotypes in an in vitro environment even at 8 weeks of storage duration (Figures 2(a) and 2(b)), similarly, encapsulation of explants improved germination under ex vitro conditions when Jiffy-7 pellets were used as nonsterile substrate (Figures 2(a) and 2(b)).
Figure 2.
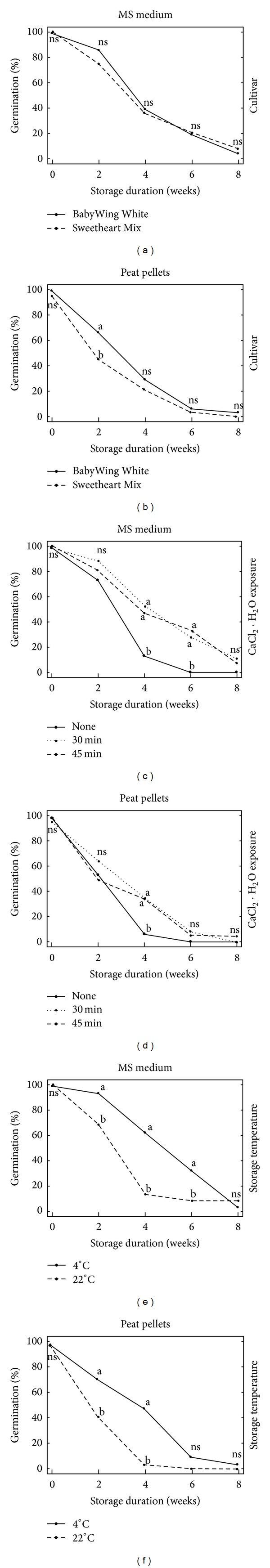
Percentages of encapsulated microshoots (synthetic seeds) and nonencapsulated microshoots of two Begonia cultivars “germinating” (developing successfully) after storage and subsequent transfer to substrate (MS medium (in vitro) or peat pellets (ex vitro)). Encapsulated microshoots were exposed to CaCl2·H2O after encapsulation, whereas nonencapsulated microshoots served as a control for this factor. Medium and storage duration were highly influential in significant interaction terms; therefore, analyses of cultivar ((a) and (b)), CaCl2·H2O exposure duration ((c) and (d)), and storage temperature ((e) and (f)) were conducted for each combination of medium and storage duration. Cultivar, CaCl2·H2O exposure duration, and storage temperature factors showed no significant interactions; therefore, means shown (dots) in each subfigure are averages over the other two factors. Means for a specific storage duration (vertical pairs or trios) within each figure are significantly different (P = 0.05) when labeled with “a” and “b,” or not significantly different when labeled with “ns.” The Schaffer-Simulated method was used for multiple mean comparisons.
3.2. Effect of Exposure Duration of CaCl2·H2O and Encapsulation Matrix on Germination
The germination percentage was not significantly (P = 0.05) affected by the duration of exposure to calcium chloride whether the encapsulated seeds were maintained for 30 or 45 min in 100 mM CaCl2·H2O and whether MS medium or PBS was used (Figures 2(c) and 2(d)). Daud et al. [23] found that 30 min was the optimal exposure time for best germination percentage and that both shorter (10 min) and longer (90 min) exposure durations resulted in reduced germination of African violet (Saintpaulia ionantha Wendl.). On the other hand, Castillo et al. [24] reported that a relatively short (10 min) duration of exposure to CaCl2·H2O and only 2.5% alginate provided uniform encapsulation of embryos and the highest germination percentage (77.5%) of Carica papaya L. Successful production of synthetic seeds depends on several factors, including the concentration and type of gel needed for encapsulation of microshoots or somatic embryos, the duration of exposure of encapsulated seeds to CaCl2·H2O, and plant species [23, 25–28]. Several gel types are used for encapsulation, but the most commonly used matrix is sodium alginate because of low cost, gelling properties, and nontoxic nature [29]. The integrity or hardness of the hydrogels depends for the most part on the number of Na+ ions (in sodium alginate solution) exchanged with Ca++ ions (in CaCl2·H2O solution) resulting in the formation of insoluble calcium alginate [23]. In the current study, nicely formed beads were easily obtained with 100 mM CaCl2·H2O and 3% sodium alginate. Our results were in agreement with those of Daud et al. [23] who reported that an encapsulation matrix of 3% alginate with 100 mM CaCl2·H2O was ideal for the formation of Saintpaulia ionantha Wendl. microshoot beads as higher concentration of sodium alginate (4-5%) resulted in beads that were too hard, causing a germination delay, while lower concentration of sodium alginate (1-2%) produced beads that readily burst because they were too fragile and difficult to handle. Indeed, a 3% sodium alginate appears to be the optimum concentration for a great number of species as low concentrations (1-2%) result in beads too soft to handle and higher concentrations (≥4%) in beads too hard, preventing the emergence of shoots and roots [29–33]. On the other hand, germination percentage was significantly affected by encapsulation as marked improvement was achieved in germination percentage for encapsulated microshoots compared with nonencapsulated explants in both in vitro and ex vitro environments (Figures 2(c) and 2(d)). The germination percentage of encapsulated microshoots, whether exposed to CaCl2·H2O for 30 or 45 min, was consistently higher than that of nonencapsulated microshoots up to 6 and 4 weeks in MS medium and PBS, respectively. These improved germination percentages using synthetic Begonia seeds are in agreement with other findings from other workers for several other species including Camellia spp., Zingiber officinale, and Ruta graveolens [20, 21, 34].
3.3. Effect of Storage Temperature and Substrate on Germination
Storage temperature significantly (P = 0.05) affected germination frequency in both in vitro and ex vitro environments as germination percentage was significantly higher for encapsulated microshoots stored at 4°C (up to 6 weeks) than for those grown at 22°C (up to 4 weeks). Germination percentage of encapsulated microshoots in both growing environments declined over time, but this decrease was more pronounced when explants were stored at room temperature than under refrigeration (Figures 2(e) and 2(f)). For example, in MS medium, germination percentage was still relatively high at 32% after 6 weeks, whereas only 8% germination was obtained at room temperature at the same time point (Figure 2(e)). Similarly, in PBS, germination percentages after 4 weeks were 47% and 3% for 4 and 22°C, respectively (Figure 2(f)). In other words, germination was almost 16-fold higher when encapsulated explants were stored at low temperature. It is noteworthy that, even at room temperature and under nonsterile conditions, 41% of synthetic seeds still germinated after 2 weeks (Figure 2(f)).
Overall, significantly better germination percentages were obtained over time when the encapsulated seeds were grown in MS medium in vitro than in peat pellets ex vitro (Figure 3). Furthermore, the improved germination percentage of encapsulated microshoots over control explants, even after a considerable amount of storage time, can be attributed to the inclusion of MS salts in encapsulation matrix, which serves as an artificial endosperm to the encapsulated microshoots for conversion to plantlets [35, 36]. Hung and Trueman [37] reported that direct transfer of synthetic seeds of African mahogany (Khaya senegalensis) to nonsterile substrates was ineffective unless the seeds were allowed to preconvert (i.e., to form roots in vitro with the aid of growth regulators) prior to transfer as most of the seeds were contaminated with fungi within a week. However, in the current study, such an extra step was not required as synthetic Begonia seeds readily germinated, formed roots, and were easily acclimated when transferred to nonsterile peat-based media (Figure 1(g)).
Figure 3.
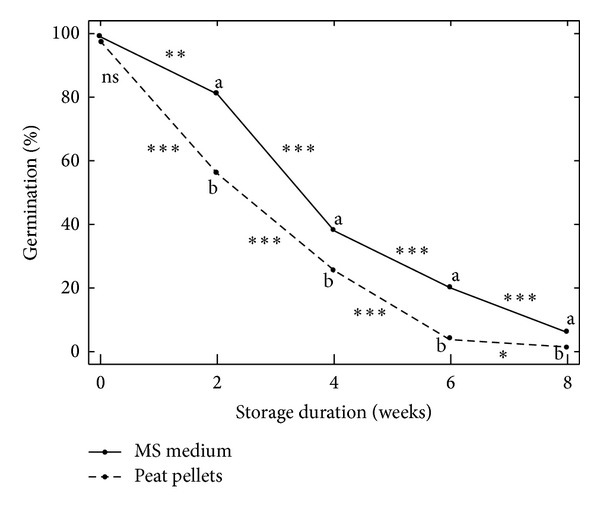
Percentages of encapsulated microshoots (synthetic seeds) and nonencapsulated microshoots of two Begonia cultivars “germinating” (developing successfully) after storage and subsequent transfer to MS medium (in vitro) or peat pellets (ex vitro). Medium and storage duration were highly influential in significant interaction terms among five treatment factors; therefore, analyses were run at each combination of these two factor levels. Means shown (dots) are averages among the three additional treatment factors (cultivar, CaCl2·H2O exposure duration, and storage temperature), which showed no significant interaction effects when analyzed by medium and storage duration. Means for the two media at a specific storage duration (vertical pairs) are significantly different (P = 0.05) when labeled with “a” and “b” or not significantly different when labeled with “ns.” Means for storage durations connected by a line segment are significantly different at P = 0.05 (*), 0.01 (**), or 0.001 (***). The Schaffer-Simulated method was used for multiple mean comparisons.
4. Conclusions
Production of synthetic seeds of both Begonia cultivars, BabyWing White and Sweetheart Mix, using the protocol developed in this study was relatively easy. Encapsulated seeds kept at low temperature (4°C) could be stored for a longer period of time and had a higher germination percentage than those stored at room temperature (~22°C). Also, better germination percentages were obtained over time when the encapsulated seeds were grown in MS medium than in PBS, irrespective of the storage environment (4°C or 22°C). However, storage at room temperature has the advantage of avoiding costs associated with refrigeration equipment. Seeds of Sweetheart Mix are actually a mixture of seeds from different genotypes, so the fact that synthetic seeds of this cultivar could be easily formed and germinated using our protocol suggests that this procedure could be applied for an efficient production of synthetic seeds from microshoots of other Begonia species and cultivars.
Disclosure
Mention of a trademark or proprietary product does not constitute a guarantee or warranty of the product by the U. S. Department of Agriculture and does not imply its approval to the exclusion of other products that may also be suitable.
Acknowledgment
The authors are very grateful to Carrie Lee Witcher for technical support.
Abbreviations
- cv.:
Cultivar
- MS:
Murashige and Skoog medium
- PBS:
Peat-based substrate.
References
- 1.Sarkar D, Naik PS. Nutrient-encapsulation of potato nodal segments for germplasm exchange and distribution. Biologia Plantarum. 1998;40(2):285–290. [Google Scholar]
- 2.Singh SK, Rai MK, Asthana P, Pandey S, Jaiswal VS, Jaiswal U. Plant regeneration from alginate-encapsulated shoot tips of Spilanthes acmella (L.) Murr., a medicinally important and herbal pesticidal plant species. Acta Physiologiae Plantarum. 2009;31(3):649–653. [Google Scholar]
- 3.Sarkar D, Naik PS. Synseeds in potato: an investigation using nutrient-encapsulated in vitro nodal segments. Scientia Horticulturae. 1998;73(2-3):179–184. [Google Scholar]
- 4.Singh AK, Sharma M, Varshney R, Agarwal SS, Bansal KC. Plant regeneration from alginate-encapsulated shoot tips of Phyllanthus amarus Schum and Thonn, a medicinally important plant species. In Vitro Cellular and Developmental Biology—Plant. 2006;42(2):109–113. [Google Scholar]
- 5.West TP, Ravindra MB, Preece JE. Encapsulation, cold storage, and growth of Hibiscus moscheutos nodal segments. Plant Cell, Tissue and Organ Culture. 2006;87(3):223–231. [Google Scholar]
- 6.Standardi A, Piccioni E. Recent perspectives on synthetic seed technology using nonembryogenic in vitro-derived explants. International Journal of Plant Sciences. 1998;159(6):968–978. [Google Scholar]
- 7.Mandal J, Pattnaik S, Chand PK. Alginate encapsulation of axillary buds of Ocimum americanum L. (hoary basil), O. basilicum L. (sweet basil), O. gratissimum L. (shrubby basil), and O. sanctum L. (sacred basil) In Vitro Cellular and Developmental Biology—Plant. 2000;36(4):287–292. [Google Scholar]
- 8.Nada S, Chennareddy S, Goldman S, et al. Direct shoot bud differentiation and plantlet regeneration from leaf and petiole explants of Begonia tuberhybrida . HortScience. 2011;46(5):759–764. [Google Scholar]
- 9.Wong W. Some folk medicinal plants from Trinidad. Economic Botany. 1976;30(2):103–142. [Google Scholar]
- 10.Roia F, Smith R. The antibacterial screening of some common ornamental plants. Economic Botany. 1977;31(1):28–37. [Google Scholar]
- 11.Basurto-Peña F, Castro-Lara D, Martinez-Alfaro MA. Edible begonias from the north of puebla, Mexico. Economic Botany. 2003;48(1):48–53. [Google Scholar]
- 12.Shui Y-M. Begonia tetralobata (Begoniaceae), a new species from China. Annals Botany Fennici. 2007;44(1):76–79. [Google Scholar]
- 13.Phutthai T, Sridith K. Begonia pteridiformis (Begoniaceae), a new species from Thailand. Thai Forest Bulletin. 2010;38:37–41. [Google Scholar]
- 14.Sosef MSM, Miyono N. A new species of Begonia section Loasibegonia (Begoniaceae) from the Monte Alen region, Equatorial Guinea. Blumea. 2010;55(1):91–93. [Google Scholar]
- 15.Burt-Utley K, Utley JF. New species and notes on Begonia (Begoniaceae) from Middle America, I. Novon. 2011;21(4):393–401. [Google Scholar]
- 16.Hughes M. A new species of fleshy-fruited Begonia (Begoniaceae) from the Masoala Peninsula, Madagascar. Adansonia. 2011;33(1):81–85. [Google Scholar]
- 17.USDA-NASS. Floriculture crops, 2011 summary. 2012, http://usda01.library.cornell.edu/usda/nass/FlorCrop/2010s/2012/FlorCrop-05-31-2012.pdf.
- 18.Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiologiae Plantarum. 1962;15(3):473–497. [Google Scholar]
- 19.Singh SK, Rai MK, Asthana P, Sahoo L. Alginate-encapsulation of nodal segments for propagation, short-term conservation and germplasm exchange and distribution of Eclipta alba (L.) Acta Physiologiae Plantarum. 2010;32(3):607–610. [Google Scholar]
- 20.Sundararaj SG, Agrawal A, Tyagi RK. Encapsulation for in vitro short-term storage and exchange of ginger (Zingiber officinale Rosc.) germplasm. Scientia Horticulturae. 2010;125(4):761–766. [Google Scholar]
- 21.Ahmad N, Faisal M, Fatima N, Anis M. Encapsulation of microcuttings for propagation and short-term preservation in Ruta graveolens L.: a plant with high medical value. Acta Physiologiae Plantarum. 2012;34(6):2303–2310. [Google Scholar]
- 22.Hung CD, Trueman SJ. Alginate encapsulation of shoot tips and nodal segments for short-term storage and distribution of the eucalypt Corymbia torelliana × C. citriodora . Acta Physiologiae Plantarum. 2012;34(1):117–128. [Google Scholar]
- 23.Daud N, Taha RM, Hasbullah NA. Artificial seed production from encapsulated micro shoots of Saintpaulia ionantha Wendl. (African Violet) Journal of Applied Sciences. 2008;8(24):4662–4667. [Google Scholar]
- 24.Castillo B, Smith MAL, Yadava UL. Plant regeneration from encapsulated somatic embryos of Carica papaya L. Plant Cell Reports. 1998;17(3):172–176. doi: 10.1007/s002990050373. [DOI] [PubMed] [Google Scholar]
- 25.Redenbaugh K, Paasch BD, Nichol JW, Kossler ME, Viss PR, Walker KA. Somatic seeds: encapsulation of asexual plant embryos. Nature Biotechnology. 1986;4(9):797–801. [Google Scholar]
- 26.Redenbaugh K, Slade D, Viss P, Fujii JA. Encapsulation of somatic embryos in synthetic seed coats. HortScience. 1987;22(5):803–809. [Google Scholar]
- 27.Redenbaugh K, Fujii JA, Slade D. Synthetic seed technology. In: Vasil KI, editor. Scale-Up and Automation in Plant Propagation. Cell Culture and Somatic Cell Genetics of Plants. New York, NY, USA: Academic Press; 1991. pp. 35–74. [Google Scholar]
- 28.Redenbaugh K, Fujii JA, Slade D. Hydrated coatings form synthetic seeds. In: Redenbaugh K, editor. Synseeds: Application of Synthetic Seeds to Crop Improvement. Boca Raton, Fla, USA: CRC Press; 1993. pp. 305–327. [Google Scholar]
- 29.Cheruvathur MK, Najeeb N, Tomas TD. In vitro propagation and conservation of Indian sarsaparilla, Hemidesmus indicus L. R. Br. through somatic embryogenesis and synthetic seed production. Acta Physiologiae Plantarum. 2013;35(3):771–779. [Google Scholar]
- 30.Ganapathi TR, Suprasanna P, Bapat VA, Rao PS. Propagation of banana through encapsulated shoot tips. Plant Cell Reports. 1992;11(11):571–575. doi: 10.1007/BF00233095. [DOI] [PubMed] [Google Scholar]
- 31.Manjkhola S, Dhar U, Joshi M. Organogenesis, embryogenesis, and synthetic seed production in Arnebia euchroma —a critically endangered medicinal plant of the Himalaya. In Vitro Cellular and Developmental Biology. 2005;41(3):244–248. [Google Scholar]
- 32.Sarmah DK, Borthakur M, Borua PK. Artificial seed production from encapsulated PLBs regenerated from leaf base of Vanda coerulea Grifft. ex. Lindl. - an endangered orchid. Current Science. 2010;98(5):686–690. [Google Scholar]
- 33.Tabassum B, Nasir IA, Farooq AM, Rehman Z, Latif Z, Husnain T. Viability assessment of in vitro produced synthetic seeds of cucumber. African Journal of Biotechnology. 2010;9(42):7026–7032. [Google Scholar]
- 34.Ballester A, Janeiro LV, Vieitez AM. Cold storage of shoot cultures and alginate encapsulation of shoot tips of Camellia japonica L. and Camellia reticulata Lindley. Scientia Horticulturae. 1997;71(1-2):67–78. [Google Scholar]
- 35.Bapat VA, Rao PS. Plantlet regeneration from encapsulated and non-encapsulated desiccated somatic embryos of forest trees: sandalwood (Santalum album L.) Journal of Plant Biochemistry and Biotechnology. 1992;1(2):109–113. [Google Scholar]
- 36.Ganapathi TR, Srinivas L, Suprasanna P, Bapat VA. Regeneration of plants from alginate-encapsulated somatic embryos of banana cv. Rasthali (Musa spp. AAB group) In Vitro Cellular and Developmental Biology. 2001;37(2):178–181. [Google Scholar]
- 37.Hung CD, Trueman SJ. Encapsulation technology for short-term preservation and germplasm distribution of the African mahogany Khaya senegalensis . Plant Cell, Tissue and Organ Culture. 2011;107(3):397–405. [Google Scholar]


