Abstract
Background
The association of individual fatty acids with ischemic stroke has not been thoroughly studied, and results have been inconsistent. Few prospective studies have systematically explored the association of biomarkers of fatty acid intake with stroke. The aim of this study was to explore which individual plasma fatty acids would be associated with higher risk of ischemic stroke among whites.
Methods
We studied 3,870 white men and women from the Minneapolis field center of the Atherosclerosis Risk in Communities (ARIC) Study, aged 45–64 at baseline (1987–89) who had plasma cholesterol ester (CE) and phospholipid (PL) fatty acids measured. Participants were followed through 2008 for incident ischemic stroke. Hazard ratios (HRs) with 95% confidence intervals (CIs) across quartiles of each fatty acid, measured as the percentage of total fatty acids, were calculated using Cox proportional hazards model.
Results
During a maximum of 22-years of follow-up, we identified 168 cases of ischemic stroke. After adjustment for age and sex, plasma levels of saturated fatty acids were associated positively: HR (95%CI) of the highest quartile vs the lowest quartile for CE fraction was 1.93 (1.23–3.04), p for trend =0.01 and that for PL fraction was 1.64 (1.05–2.57), p for trend =0.03. There was also a positive linear association with monounsaturated fatty acids, especially with palmitoleic acid: HR (95%CI) of the highest quartile vs the lowest quartile for CE fraction was 1.86 (1.20–2.87), p for trend =0.003 for CE; and those for PL fraction was 1.52 (0.99–2.34), p for trend =0.005. No associations of ω-3 and ω-6 polyunsaturated fatty acids with ischemic stroke were observed, but linoleic acid was inversely and nonlinearly associated with ischemic stroke: HR (95%CI) of the highest quartile vs the lowest quartile for CE fraction was 0.64 (0.43–0.97), p for trend =0.13 and that for PL fraction was 0.69 (0.45–1.05), p for trend =0.24. These associations were generally unchanged after adjustment for cardiovascular risk factors.
Conclusions
In this US cohort of whites, we found significant positive associations of plasma saturated and monounsaturated fatty acids, especially of palmitoleic acid, with ischemic stroke. We also found an inverse nonlinear association between linoleic acid and ischemic stroke.
Keywords: longitudinal study, epidemiology, fat, biomarkers, risk factors
Introduction
It is well established that dietary intakes of fatty acids have a substantial impact on coronary disease; ie, saturated fatty acids (SFA) increase and polyunsaturated fatty acids (PUFA) decrease serum cholesterol levels and risk of coronary disease [1]. However, the association of fatty acid intake with ischemic stroke has been studied less and results have been inconsistent [2–4]. Furthermore, few prospective studies have systematically explored the association of biomarkers of fatty acid intake with stroke [5–8].
In this study, we examined the association between plasma fatty acid composition and incident ischemic stroke in participants recruited from the Minneapolis ARIC field center, where plasma cholesterol ester (CE) and phospholipid (PL) fatty acids were measured. Generally, plasma fatty acids are considered good biomarkers of dietary fatty acid intake over the recent weeks [9], but are also affected by endogenous synthesis influenced by genetic variation, intrauterine programming, and other lifestyle factors [10,11]. Several desaturases, Δ9, Δ6 and Δ5-desaturases, are known to be involved in endogenous fatty acid synthesis and affected by both diet and genetics [10]. They have been reported to be associated with several cardiovascular risk factors, such as insulin resistance and the metabolic syndrome [12]. Therefore, it would also be of interest whether these enzyme activities are associated with risk of stroke. In the present study, we sought to explore which individual fatty acids in plasma, and desaturase indices, are associated with risk of ischemic stroke in the US white population.
Methods
Study cohort
The ARIC Study selected a population-based sample of 15,792 persons from 4 communities: Forsyth County, North Carolina; the city of Jackson, Mississippi; north-western suburbs of Minneapolis, Minnesota; and Washington County, Maryland. Participants were 45–64 years of age during the baseline period (1987–1989). The ARIC study protocol was approved by each field center’s institutional review board. After written informed consent was obtained, participants underwent a baseline clinical examination, as detailed elsewhere [13]. In the Minneapolis field center only (n=4,009), baseline plasma was analyzed for fatty acids. We excluded persons who reported a self-reported history of stroke and/or transient ischemic attack at the baseline survey or those without plasma fatty acid data. We also excluded non-white subjects due to their small number. Our final sample included 1,859 men and 2,011 women.
Plasma fatty acids measurement
Twelve-hour fasting blood was collected into 10-ml vacuum tubes containing EDTA. Plasma was separated and dispensed into two 1.5ml aliquots and frozen at −70°C for approximately 2 years before analysis for individual fatty acid composition by a single technician, who was blinded to stroke outcome status. The samples were only thawed once for the fatty acid measurements themselves. A previous study has shown that there were no significant changes in most fatty acids, regardless of lipid fraction, after 8–10 years of −80°C storage [14].
Details of plasma fatty acid measurement have been described elsewhere [15]. After separation of PL and CE fractions, the identity of 28 fatty acid peaks was ascertained by comparison of each peak’s retention time shown by the gas-liquid chromatogram relative to the retention times of fatty acids in synthetic standards of known fatty acid composition. The percentage of each fatty acid among total fatty acids (% total fatty acids) was quantified by the total area for all fatty acids. Absolute values of each fatty acid were not estimated. The data were electronically transferred from the gas chromatogram to a computer. Short-term test-retest reliability coefficients have been reported previously [16]; the values for SFA were r=0.70 for CE, 0.57 for PL; for monounsaturated fatty acids (MUFA) were r=0.71 for CE, 0.46 for PL; and for PUFA were r=0.71 for CE, 0.32 for PL. That report also provided long-term reliabilities [16], which were somewhat lower than short-term reliabilities. The correlations between plasma and dietary fatty acids measured by food frequency questionnaire have also been reported [15]: SFA (r=0.23 for CE, 0.15 for PL), PUFA (r=0.31 for CE, 0.25 for PL), and MUFA (r=0.01 for CE, 0.05 for PL); p<0.01 for r >0.05.
We examined several individual fatty acids in both CE and PL: myristic (14:0), pentadecanoic (15:0), palmitic (16:0), stearic (18:0), palmitoleic (16:1,ω7), oleic (18:1,ω9), linoleic (18:2,ω6), γ-linoleic (18:3,ω6), dihomo-γ-linoleic (20:3,ω6), arachidonic (20:4,ω6), α-linoleic (18:3,ω3), eicosapentaenoic (20:5,ω3) and docosahexaenoic (22:6,ω3) acids. For the major fatty acid groups, we defined SFA as the sum of (12:0), (14:0), (15:0), (16:0), (18:0), (20:0), (22:0), (23:0) and (24:0); MUFA as the sum of (14:1 ,ω5), (16:1,ω7), (18:1,ω9), (20:1,ω9), (22:1,ω9) and (24:1,ω9); ω-6 (n-6) PUFA as the sum of (18:2,ω6), (18:3,ω6), (20:2,ω6), (20:3,ω6), (20:4,ω6), (22:4,ω6) and (22:5,ω6); ω-3 PUFA as the sum of (18:3,ω3), (20:3,ω3), (20:5,ω3), (22:5,ω3) and (22:6,ω3); and long-chain ω-3 PUFA as the sum of (20:5,ω3), (22:5,ω3) and (22:6,ω3).
Ascertainment of incident ischemic stroke events
Ischemic strokes were identified through annual telephone calls to participants to ascertain all hospitalizations in the past year, through review of local hospital discharge lists, and via death certificates. Incident stroke was classified by a computer algorithm and physician review based on signs, symptoms, neuroimaging (CT/MRI) and other diagnostic reports, according to criteria adapted from the National Survey of Stroke [17]. A stroke was classified into definite or probable hospitalized ischemic (cardioembolic or thrombotic) or hemorrhagic stroke on the basis of neuroimaging studies and autopsy, when available. Definite and probable ischemic stroke, where a brain neuroimaging revealed acute infarction or showed no evidence of hemorrhage, was considered ischemic stroke. A definite thrombotic infarction was further classified into large-artery occlusive or lacunar, based on neuroimaging results. More details on the methods for ascertainment of incident stroke in ARIC have been given elsewhere [18].
Statistical Analysis
For each participant, we calculated the person-years of follow-up from baseline in 1987–1989 to the first stroke endpoint, death, loss to follow-up, or the end of 2008. The median and maximum years of follow-up were 19.9 and 22.1, respectively. Hazard ratios (HRs) with 95% confidence intervals (CIs) across quartiles of each fatty acid, measured as the percentage of total fatty acids, were calculated after adjustment in Cox proportional hazards models for age and sex, and for multivariable adjustment models, smoking status (never, ex- or current smoker), cigarette-years and alcohol intake. We did not adjust for blood pressure, diabetes, plasma triglycerides, total or HDL-cholesterol levels, because we considered them possible mediators of any association between fatty acids and ischemic stroke. Factors that were tested but did not confound the associations included body mass index, education levels, sports index, hormone replacement therapy, energy intake and fish oil supplement use. The linear trend of HRs across the quartiles was tested using a variable with −3, −1, 1, 3 assigned to successive quartiles. Since there was no significant multiplicative interaction between sex and plasma FA in relation to ischemic stroke, men and women were pooled. The proportional hazards assumption was tested using time by fatty acid interaction terms and was not violated except for myristic acid in the PL fraction and pentadecanoic acid in both fractions, for which the Cox analyses were further stratified by follow-up time (≤10 and >10 years). Since no significant associations with ischemic stroke were found for either stratum, we only present pooled results.
In secondary analyses, we defined desaturase and elongase activity indices [12,19] to examine the activity of each enzyme with incident ischemic stroke: the ratios of (16:1,ω7)/(16:0) as the Δ9-desaturase index; (18:3,ω6)/(18:2,ω6) as the Δ-6 desaturase index for the CE fraction; (20:3,ω6)/(18:2,ω6) as the Δ-6 desaturase index for the PL fraction; (20:4,ω6)/(20:3,ω6) as the Δ-5 desaturase index; and (18:0)/(16:0) as the elongase index. Quartiles of each index were used in the analyses.
We used SAS version 9.1.3 Service Pack 4 (SAS Institute Inc., Cary, NC) for the analyses. All p-values for statistical tests were two-tailed and values of p<0.05 were regarded as statistically significant.
Results
During 70,735 person-years of follow-up of 3,870 persons (1,859 men and 2,011 women), we documented 168 incident ischemic stroke events (103 in men and 65 in women). Of these, there were 35 cardioembolic, 60 definite large-artery occlusive, 34 definite lacunar, and 39 unclassified/possible thrombotic infarctions.
Compared with those who had no ischemic stroke (Table 1), ischemic stroke cases had higher mean age and triglycerides, and lower HDL-cholesterol at baseline, and for women, higher total cholesterol. Those who developed ischemic stroke were more often hypertensive and diabetic (men and women), and smokers (men only).
Table 1.
Sex-specific unadjusted baseline characteristics for those who did or did not develop ischemic stroke; ARIC Study, Minneapolis field center, 1987–1989
| Men | Women | |||||
|---|---|---|---|---|---|---|
| incident stroke | no stroke | p value | incident stroke | no stroke | p value | |
| Number | 103 | 1,756 | 65 | 1,946 | ||
| Age, years | 57.7 | 54.4 | <0.001 | 56.8 | 53.2 | <0.001 |
| Body mass index | 27.9 | 27.8 | 0.74 | 27.1 | 26.3 | 0.23 |
| Systolic blood pressure, mm Hg | 125 | 120 | 0.002 | 126 | 117 | <0.001 |
| Diastolic blood pressure, mm Hg | 77 | 75 | 0.11 | 74 | 72 | 0.17 |
| Antihypertensive medication, % | 33 | 21 | 0.005 | 37 | 23 | 0.008 |
| Plasma total cholesterol, mg/dl | 218 | 212 | 0.15 | 237 | 215 | <0.001 |
| Plasma HDL cholesterol, mg/dl | 42 | 44 | 0.05 | 54 | 60 | 0.01 |
| Plasma triglyceride1, mg/dl | 138 | 117 | 0.01 | 123 | 98 | <0.001 |
| Diabetes, % | 20 | 8 | <0.001 | 18 | 6 | |
| Plasma creatinine, mg/dl | 1.2 | 1.2 | 0.64 | 1.0 | 1.0 | 0.84 |
| Sports index1 (range 1–5) | 2.8 | 2.8 | 0.22 | 2.5 | 2.5 | 0.28 |
| Education (>high school graduate), % | 66 | 69 | 0.59 | 41 | 51 | 0.13 |
| Current smoking, % | 31 | 20 | 0.01 | 25 | 24 | 0.86 |
| Cigarette-years1 | 320 | 320 | 0.63 | 68 | 26 | 0.16 |
| Alcohol intake1, g/week | 32.4 | 39.6 | 0.98 | 0.0 | 0.0 | 0.06 |
| Total energy intake, kcal/day | 1,738 | 1,805 | 0.26 | 1,505 | 1,486 | 0.77 |
| Fish oil supplement use, % | 2 | 3 | 0.60 | 3 | 3 | 0.83 |
| Prevalence of coronary heart disease, % | 14 | 8 | 0.03 | 2 | 1 | 0.73 |
| Hormone therapy use, % | – | – | – | 20 | 23 | 0.69 |
Median values.
Since mean fatty acid composition at baseline did not differ much by sex [20], and no sex-interaction with any fatty acids in relation to ischemic stroke was observed, analyses were performed for men and women together. Table 2 presents age and sex-adjusted hazard ratios of ischemic stroke for the highest vs lowest quartiles of each fatty acid. Greater plasma total SFA were associated with higher risk of ischemic stroke (HR=1.93 for the CE fraction and 1.64 for the PL fraction). An increased risk was also observed for MUFA, particularly for palmitoleic acid (HR=1.86 for the CE fraction and 1.52 for the PL fraction). Higher plasma levels of ω-6 PUFA were not significantly associated with reduced risks of ischemic stroke, but linoleic acid was associated with 36% lower risk of ischemic stroke in the highest vs lowest quartiles for the CE fraction, although p-values for trend were not statistically significant. Lower risks of ischemic stroke were not observed for greater total ω-3 PUFA, α-linolenic, or eicosapentaenoic acids. However, being in the highest vs lowest quartile of docosahexaenoic acid was associated approximately 30% less risk of ischemic stroke with marginal statistical significance (p for trend =0.07 for CE and 0.08 for PL fractions). The Δ9-desaturase index was associated positively with ischemic stroke incidence. There was also a positive, but a marginal, association for Δ6-desaturase index. No significant associations were found for Δ5-desaturase and elongase indices.
Table 2.
Age and sex-adjusted HRs and 95% CIs of incident ischemic stroke for the highest and lowest quartiles of each fatty acid; ARIC Study, Minneapolis field center, 1987–2008
| Ischemic stroke | ||||
|---|---|---|---|---|
| CE fraction | PL fraction | |||
| HR (95% CI) | p for trend | HR (95% CI) | p for trend | |
| SFA | 1.93(1.23–3.04) | 0.01 | 1.64 (1.05–2.57) | 0.03 |
| Myristic (14:0) | 1.43 (0.92–2.22) | 0.14 | 1.29 (0.83–2.01) | 0.26 |
| Pentadecanoic (15:0) | 0.83 (0.55–1.25) | 0.59 | 0.89 (0.60–1.33) | 0.95 |
| Palmitic (16:0) | 1.44 (0.93–2.34) | 0.09 | 1.30 (0.84–2.02) | 0.17 |
| Stearic (18:0) | 0.92 (0.60–1.42) | 0.71 | 1.17 (0.74–1.84) | 0.42 |
| MUFA | 1.49 (0.97–2.28) | 0.03 | 1.42 (0.92–2.18) | 0.15 |
| Palmitoleic (16:1,ω7) | 1.86 (1.20–2.87) | 0.003 | 1.52 (0.99–2.34) | 0.005 |
| Oleic (18:1,ω9) | 1.58 (1.04–2.41) | 0.08 | 1.38 (0.88–2.15) | 0.20 |
| ω–6 PUFA | 0.74 (0.49–1.12) | 0.09 | 0.93 (0.60–1.45) | 0.40 |
| Linoleic (18:2,ω6) | 0.64 (0.43–0.97) | 0.13 | 0.69 (0.45–1.05) | 0.24 |
| γ-Linolenic (18:3,ω6) | 1.00 (0.66–1.53) | 0.48 | 1.26 (0.80–1.99) | 0.26 |
| Dihomo-γ-linolenic (20:3,ω6) | 0.99 (0.67–1.47) | 0.99 | 1.17 (0.78–1.78) | 0.31 |
| Arachidonic (20:4,ω6) | 1.03 (0.68–1.55) | 0.78 | 0.87 (0.58–1.30) | 0.82 |
| ω–3 PUFA | 1.16 (0.75–1.79) | 0.65 | 0.86 (0.56–1.32) | 0.30 |
| α-Linolenic (18:3,ω3) | 1.14 (0.76–1.72) | 0.61 | 1.29 (0.82–2.02) | 0.16 |
| Long-chain ω–3 PUFA | 1.08 (0.70–1.66) | 0.88 | 0.85 (0.55–1.29) | 0.23 |
| Eicosapentaenoic (20:5,ω3) | 1.16 (0.76–1.76) | 0.39 | 1.18 (0.78–1.78) | 0.37 |
| Docosahexaenoic (22:6,ω3) | 0.70 (0.45–1.08) | 0.07 | 0.69 (0.46–1.06) | 0.08 |
| Δ-9-desaturase index (16:1,ω7/16:0) | 1.72 (1.10–2.70) | 0.004 | 1.62 (1.04–2.53) | 0.007 |
| Δ-6-desaturase index1 | 1.37 (0.89–2.10) | 0.15 | 1.19 (0.78–1.81) | 0.35 |
| Δ-5-desaturase index (20:4,ω6/20:3,ω6) | 0.85 (0.54–1.32) | 0.37 | 0.84 (0.55–1.29) | 0.49 |
| Elongase index (18:0/16:0) | 0.85 (0.54–1.36) | 0.47 | 0.86 (0.55–1.36) | 0.44 |
Δ-6-desaturase indices were defined as 18:3,ω6/18:2,ω6 for CE and as 20:3,ω6/18:2,ω6 for PL.
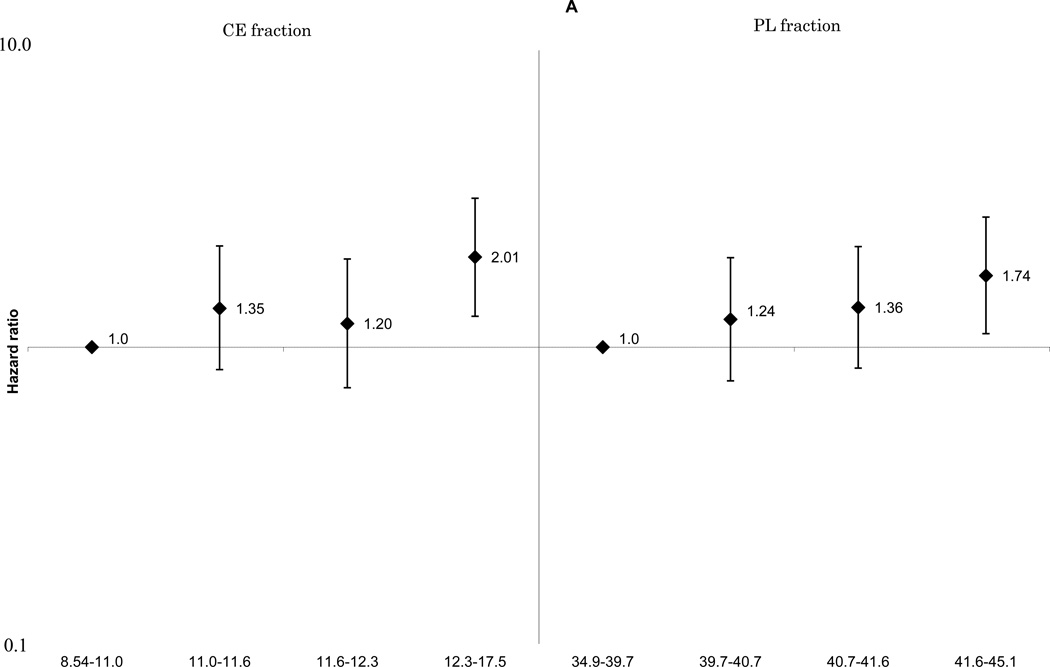
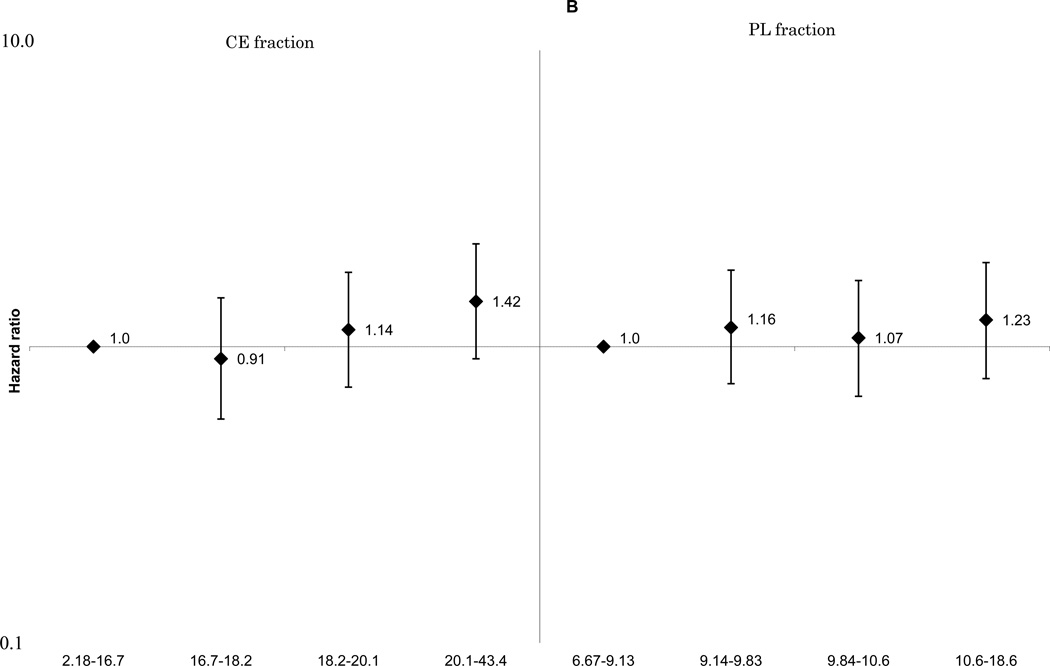
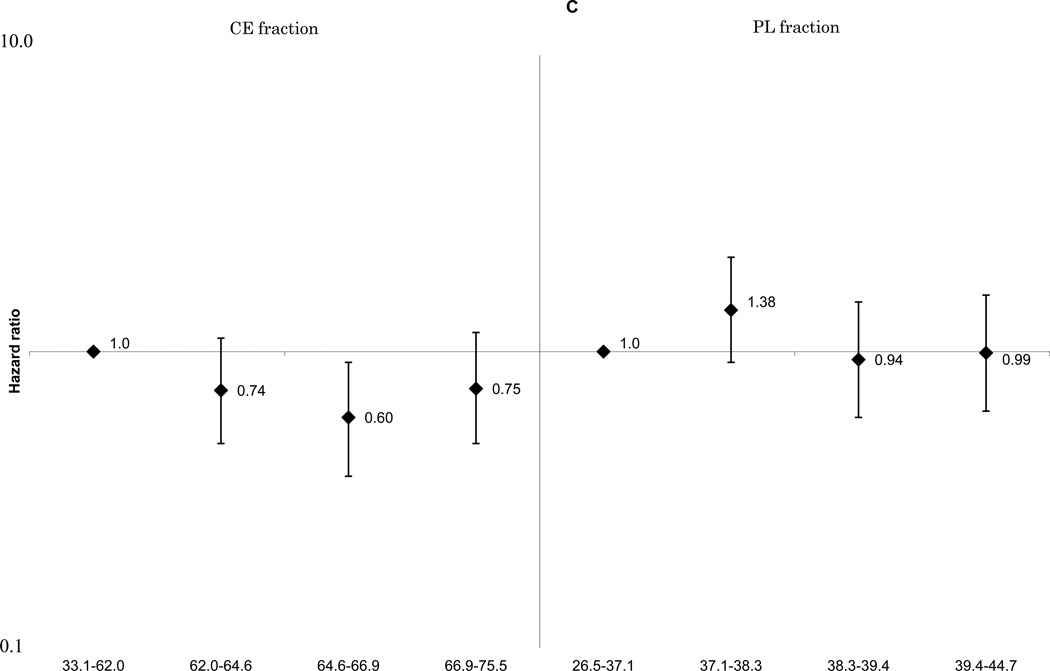
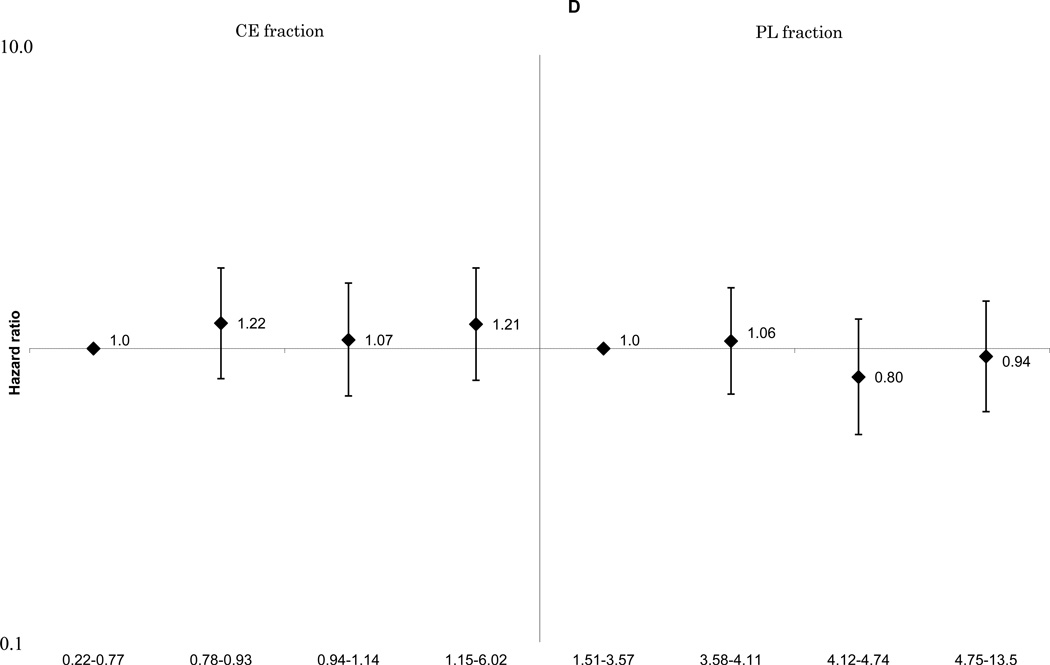
These associations did not change materially with further adjustment for cardiovascular risk factors including smoking, cigarette-years and alcohol consumption. The positive linear associations between ischemic stroke and total SFA (Figure 1-A) and palmitoleic acid (Supplemental Figure 1-E), remained statistically significant for both the CE and PL fractions. The associations of MUFA (Figure 1-B), ω-6 PUFA (Figure 1-C), and docosahexaenoic acid (Supplemental Figure 1-G) with ischemic stroke were attenuated. A non-linear inverse association between linoleic acid and ischemic stroke was still observed (Supplemental Figure 1-F). No association was found for total long-chain ω-3 PUFA (Figure 1-D). For enzyme indices, the ischemic stroke associations were generally unchanged, and that for the Δ9-desaturation index for CE fraction was still statistically significant: HRs were 1.0 for quartile 4, 1.09(0.67–1.76) for quartile 3, 1.75(1.11–2.74) for quartile 2 and 1.93(1.20–3.10) for quartile 1, p for linear trend =0.002 (not shown in Figures).
Figure 1.
A. Multivariable adjusted hazard ratios and 95% confidence interval of ischemic stroke according to quartiles of plasma saturated fatty acids. Left: cholesterol ester fraction (p for linear trend across the quartiles=0.008), right: phospholipid fraction (p for linear trend across the quartiles=0.02).
B. Multivariable adjusted hazard ratios and 95% confidence interval of ischemic stroke according to quartiles of plasma monounsaturated fatty acids. Left: cholesterol ester fraction (p for linear trend across the quartiles=0.08), right: phospholipid fraction (p for linear trend across the quartiles=0.47).
C. Multivariable adjusted hazard ratios and 95% confidence interval of ischemic stroke according to quartiles of plasma ω-6 polyunsaturated fatty acids. Left: cholesterol ester fraction (p for linear trend across the quartiles=0.13), right: phospholipid fraction (p for linear trend across the quartiles=0.55).
D. Multivariable adjusted hazard ratios and 95% confidence interval of ischemic stroke according to quartiles of plasma long-chain ω-3 polyunsaturated fatty acids. Left: cholesterol ester fraction (p for linear trend across the quartiles=0.52), right: phospholipid fraction (p for linear trend across the quartiles=0.51).
Discussion
By and large, the results were consistent with previous prospective studies of ischemic stroke and fatty acid biomarkers [5–8], which reported positive associations of SFA and palmitoleic acid, and an inverse association of linoleic acid, with ischemic or total stroke , although some of these associations were attenuated after adjustment for other risk factors. Some inconsistencies among studies could be partly explained by (1) whether studies adjusted or not for factors potentially on the causal pathway (eg, hypertension, lipids) or (2) different proportions of stroke subtypes (large-artery occulusive, lacunar and hemorrhagic) across the studies. For example, 78% of ischemic strokes were lacunar in a study of Japanese [6], while definite lacunar infarction was only 20% among these ARIC white Minnesotans. Some other fatty acid studies of whites did not classify stroke into ischemic and hemorrhagic types [5,8], but would be expected to involve mostly ischemic. The tissues studied, fatty acid assay methods, distribution of fatty acids among populations, and endpoint determinations also were not equivalent across the studies.
We found a relatively strong significant positive association of both CE and PL SFA with ischemic stroke. Although there have been no dietary studies showing positive associations between SFA intake and stroke [21–26], and some reported even inverse associations with ischemic stroke [21,24,26,27], a different finding for biomarkers is not a surprise. Plasma SFA is affected by endogenous synthesis; but self-report dietary intake is known for its measurement error [28]. A study in Scotland [8] reported an inverse association between SFA in adipose tissue and stroke, but they included in the stroke endpoint hemorrhagic stroke, which has been reported to be inversely associated with SFA in dietary studies [27,29,30]. A Swedish study reported positive associations of myristic and palmitic acids with ischemic stroke/transient ischemic attack [7]. A Japanese nested case-control study found a positive association of serum SFA with incident ischemic stroke (predominantly lacunar stroke), but they noted the association was confounded by linoleic acid, and only linoleic acid showed a significant inverse association after multiple adjustment [6]. We speculate that SFA may have an adverse impact on non-lacunar thrombotic stroke more strongly than lacunar stroke, because an adverse impact of total or LDL-cholesterol has been established only for non-lacunar thrombotic infarction and not lacunar infarction [31,32]. This could partly explain the relatively strong association of SFA with ischemic stroke in the present study involving low proportion of lacunar strokes. Some inconsistency with Japanese dietary studies, which showed inverse associations between dietary SFA and ischemic stroke [26,27], could also be explained by a large difference in distribution of SFA intake between the US and Japanese populations [33,34].
We observed a strong positive association of MUFA, especially palmitoleic acid, as well as that of Δ9-desaturase index, with ischemic stroke. It is unlikely that plasma MUFA is well-reflected dietary MUFA intake since it did not correlate with diet (r=0.01 for CE, 0.05 for PL) [15]. Rather, plasma MUFA may reflect both SFA intake and Δ9-desaturase activity, since much of plasma MUFA is derived from SFA desaturated by Δ9-desaturase [35].
Some previous studies reported inverse associations of linoleic acid with incident ischemic stroke [6,7]. This was also replicated here, although the association was non-linear. On the other hand, no association of ischemic stroke with ω-3 PUFA, either α-linolenic or eicosapentaenoic acid, was observed in this cohort of white subjects, although a weak inverse association was detected for docosahexaenoic acid. One of the reasons for no associations for ω-3 PUFA could be the very low proportion of these fatty acids in this Minnesotan population. Mean proportions of α-linolenic, eicosapentaenoic and docosahexaenoic acids at baseline were 0.4%, 0.5% and 0.4%, respectively for the CE fraction, and 0.1%, 0.6% and 2.8%, respectively for the PL fraction.
Previous studies showed that high Δ9-desaturase index, an estimate of Δ9-desaturase (stearoyl-coenzyme A desaturase) activity, has been associated with obesity and mortality from cardiovascular disease.[36,37] Increased Δ9-desaturase activity has been demonstrated with higher SFA intake with dietary intervention [38]. In the present study, risk of ischemic stroke was greater with higher Δ9-desaturase index defined by 16:1/16: 0 ratio. Potential mediating mechanisms between fatty acids intake and risk of stroke include high total cholesterol, obesity, inflammation, and endothelial dysfunction [39,40].
A strength of the present study was the use of a biomarker, which is a more objective indicator of dietary fatty acid intake than a self-report dietary questionnaire. On the other hand, plasma fatty acids do not fully reflect dietary intake, since they are also influenced by preferential incorporation, endogenous synthesis, genetics, or lifestyle [11]. Plasma ω-3 PUFA, especially docosahexaenoic acid, correlated well with dietary intake (r=0.42 for CE and 0.42 for PL docosahexaenoic acid), whereas SFA correlated moderately with diet, but MUFA did not [15].
We did not adjust for blood pressure, diabetes or lipids, because we considered them possible mediators of any association between fatty acids and ischemic stroke. However, these are time-varying covariates and as such they may be confounders as well as mediators on the causal pathway. When we further adjusted for systolic blood pressure, antihypertensive medication use, diabetes, total and HDL-cholesterol, as well as age, smoking, cigarette-years and alcohol consumption, the associations with stroke were generally attenuated for SFA, palmitoleic, and linoleic acids. Other potential residual confounders, such as socioeconomic status, might have also affected the results. Another limitation is that the short- and long-term repeatability of some plasma fatty acids was modest, especially those composing low proportions of total fatty acids, including α-linolenic (r=0.32–0.50 for short-term, 0.35–0.41 for long-term) and docosahexaenoic (r=0.53–0.58 for short-term, 0.46–0.48 for long-term) acids, although the repeatability of most major fatty acids (e.g. palmitic, stearic, palmitoleic, linoleic, and arachidonic acids) were fair (r>0.65) [16].
In conclusion, we found significant positive associations of SFAs and MUFAs, especially palmitoleic acid in plasma CE and PL with incident ischemic stroke, and an inverse nonlinear association between plasma linoleic acid and ischemic stroke among whites in Minnesota. As for clinical and public health implications, high dietary SFA intake and low linoleic acid intake could be risk factors for ischemic stroke, given at least modest correlations with diet and fair repeatability for these plasma fatty acids. Further investigation, including meta-analyses and clinical trials, would be needed to confirm this finding.
Supplementary Material
Acknowledgment
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). This study was also supported by National Institute of Health Research Project Grant Program (R01 HL40848). Kazumasa Yamagishi was supported by the Institutional Program for Young Researcher Overseas Visits, Japan Society for the Promotion of Sciences, Japan.
The authors thank the staff and participants of the ARIC study for their important contributions. The authors also thank Linda Lewis for analyzing the plasma fatty acids.
Footnotes
None of the authors had a personal or financial conflict of interest.
References
- 1.Erkkilä A, de Mello VD, Riserus U, Laaksonen DE. Dietary fatty acids and cardiovascular disease: An epidemiological approach. Prog Lipid Res. 2008;47:172–187. doi: 10.1016/j.plipres.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Wang C, Harris WS, Chung M, Lichtenstein AH, Balk EM, Kupelnick B, Jordan HS, Lau J. n-3 fatty acids from fish or fish-oil supplements, but not alpha-linolenic acid, benefit cardiovascular disease outcomes in primary- and secondary-prevention studies: A systematic review. Am J Clin Nutr. 2006;84:5–17. doi: 10.1093/ajcn/84.1.5. [DOI] [PubMed] [Google Scholar]
- 3.Siri-Tarino P, Sun Q, Hu F, Krauss R. Meta-analysis of prospective cohort studies evaluating the association of saturated fat with cardiovascular disease. Am J Clin Nutr. 2010;91:535–546. doi: 10.3945/ajcn.2009.27725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feigin VL, Anderson CS, Mhurchu CN. Systemic inflammation, endothelial dysfunction, dietary fatty acids and micronutrients as risk factors for stroke: A selective review. Cerebrovasc Dis. 2002;13:219–224. doi: 10.1159/000057846. [DOI] [PubMed] [Google Scholar]
- 5.Simon JA, Fong J, Bernert JT, Jr, Browner WS. Serum fatty acids and the risk of stroke. Stroke. 1995;26:778–782. doi: 10.1161/01.str.26.5.778. [DOI] [PubMed] [Google Scholar]
- 6.Iso H, Sato S, Umemura U, Kudo M, Koike K, Kitamura A, Imano H, Okamura T, Naito Y, Shimamoto T. Linoleic acid, other fatty acids, and the risk of stroke. Stroke. 2002;33:2086–2093. doi: 10.1161/01.str.0000023890.25066.50. [DOI] [PubMed] [Google Scholar]
- 7.Wiberg B, Sundström J, Árnlöv J, Terént A, Vessby B, Zethelius B, Lind L. Metabolic risk factors for stroke and transient ischemic attacks in middle-aged men: A community-based study with long-term follow-up. Stroke. 2006;37:2898–2903. doi: 10.1161/01.STR.0000249056.24657.8b. [DOI] [PubMed] [Google Scholar]
- 8.Woodward M, Tunstall-Pedoe H, Batty GD, Tavendale R, Hu FB, Czernichow S. The prognostic value of adipose tissue fatty acids for incident cardiovascular disease: Results from 3944 subjects in the scottish heart health extended cohort study. Eur Heart J. 2011;32:1416–1423. doi: 10.1093/eurheartj/ehr036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riboli E, Ronnholm H, Saracci R. Biological markers of diet. Cancer Surv. 1987;6:685–718. [PubMed] [Google Scholar]
- 10.Vessby B, Gustafsson IB, Tengblad S, Boberg M, Andersson A. Desaturation and elongation of fatty acids and insulin action. Ann N Y Acad Sci. 2002;967:183–195. doi: 10.1111/j.1749-6632.2002.tb04275.x. [DOI] [PubMed] [Google Scholar]
- 11.Hodson L, Skeaff CM, Fielding BA. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog Lipid Res. 2008;47:348–380. doi: 10.1016/j.plipres.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Warensjö E, Risérus U, Vessby B. Fatty acid composition of serum lipids predicts the development of the metabolic syndrome in men. Diabetologia. 2005;48:1999–2005. doi: 10.1007/s00125-005-1897-x. [DOI] [PubMed] [Google Scholar]
- 13.The ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) Study: Design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 14.Matthan NR, Ip B, Resteghini N, Ausman LM, Lichtenstein AH. Long-term fatty acid stability in human serum cholesteryl ester, triglyceride, and phospholipid fractions. J Lipid Res. 2010;51:2826–2832. doi: 10.1194/jlr.D007534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma J, Folsom AR, Shahar E, Eckfeldt JH. The Atherosclerosis Risk in Communities (ARIC) Study Investigators: Plasma fatty acid composition as an indicator of habitual dietary fat intake in middle-aged adults. Am J Clin Nutr. 1995;62:564–571. doi: 10.1093/ajcn/62.3.564. [DOI] [PubMed] [Google Scholar]
- 16.Ma J, Folsom AR, Eckfeldt JH, Lewis L, Chambless LE. The Atherosclerosis Risk in Communities (ARIC) Study Investigators: Short- and long-term repeatability of fatty acid composition of human plasma phospholipids and cholesterol esters. Am J Clin Nutr. 1995;62:572–578. doi: 10.1093/ajcn/62.3.572. [DOI] [PubMed] [Google Scholar]
- 17.Walker AE, Robins M, Weinfeld FD. The National Survey of Stroke. Clinical findings. Stroke. 1981;12:I13–I44. [PubMed] [Google Scholar]
- 18.Rosamond WD, Folsom AR, Chambless LE, Wang CH, McGovern PG, Howard G, Copper LS, Shahar E. Stroke incidence and survival among middle-aged adults: 9-year follow-up of the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke. 1999;30:736–743. doi: 10.1161/01.str.30.4.736. [DOI] [PubMed] [Google Scholar]
- 19.Okada T, Furuhashi N, Kuromori Y, Miyashita M, Iwata F, Harada K. Plasma palmitoleic acid content and obesity in children. Am J Clin Nutr. 2005;82:747–750. doi: 10.1093/ajcn/82.4.747. [DOI] [PubMed] [Google Scholar]
- 20.Yamagishi K, Nettleton JA, Folsom AR. Plasma fatty acid composition and incident heart failure in middle-aged adults: The Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J. 2008;156:965–974. doi: 10.1016/j.ahj.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gillman MW, Cupples LA, Millen BE, Ellison RC, Wolf PA. Inverse association of dietary fat with development of ischemic stroke in men. JAMA. 1997;278:2145–2150. [PubMed] [Google Scholar]
- 22.Seino F, Date C, Nakayama T, Yoshiike N, Yokoyama T, Yamaguchi M, Tanaka H. Dietary lipids and incidence of cerebral infarction in a japanese rural community. J Nutr Sci Vitaminol. 1997;43:83–99. doi: 10.3177/jnsv.43.83. [DOI] [PubMed] [Google Scholar]
- 23.He K, Merchant A, Rimm EB, Rosner BA, Stampfer MJ, Willett WC, Ascherio A. Dietary fat intake and risk of stroke in male us healthcare professionals: 14 year prospective cohort study. BMJ. 2003;327:777–782. doi: 10.1136/bmj.327.7418.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sauvaget C, Nagano J, Hayashi M, Yamada M. Animal protein, animal fat, cholesterol intakes and risk of cerebral infarction mortality in the adult health study. Stroke. 2004;35:1531–1537. doi: 10.1161/01.STR.0000130426.52064.09. [DOI] [PubMed] [Google Scholar]
- 25.Leosdottir M, Nilsson PM, Nilsson JÅ, Berglund G. Cardiovascular event risk in relation to dietary fat intake in middle-aged individuals: Data from the malmo diet and cancer study. Eur J Cardiovasc Prev Rehabil. 2007;14:701–706. doi: 10.1097/HJR.0b013e3282a56c45. [DOI] [PubMed] [Google Scholar]
- 26.Yamagishi K, Iso H, Yatsuya H, Tanabe N, Date C, Kikuchi S, Yamamoto A, Inaba Y, Tamakoshi A for the JACC Study Group. Dietary intake of saturated fatty acids and mortality from cardiovascular disease among Japanese: The JACC Study. Am J Clin Nutr. 2010;92:759–765. doi: 10.3945/ajcn.2009.29146. [DOI] [PubMed] [Google Scholar]
- 27.Yamagishi K, Iso H, Kokubo Y, Saito I, Yatsuya H, Ishihara J, Inoue A, Tsugane S for the JPHC Study Group. Dietary intake of saturated fatty acids and incident stroke and coronary heart disease in Japanese communities: The JPHC Study. Eur Heart J. doi: 10.1093/eurheartj/eht043. (in press) [DOI] [PubMed] [Google Scholar]
- 28.Poppitt S, Swann D, Black A, Prentice A. Assessment of selective under-reporting of food intake by both obese and non-obese women in a metabolic facility. Int J Obes Relat Metab Disord. 1998;22:303–311. doi: 10.1038/sj.ijo.0800584. [DOI] [PubMed] [Google Scholar]
- 29.Iso H, Stampfer MJ, Manson JE, Rexrode K, Hu FB, Hennekens CH, Colditz GA, Speizer FE, Willett WC. Prospective study of fat and protein intake and risk of intraparenchymal hemorrhage in women. Circulation. 2001;103:856–863. doi: 10.1161/01.cir.103.6.856. [DOI] [PubMed] [Google Scholar]
- 30.Iso H, Sato S, Kitamura A, Naito Y, Shimamoto T, Komachi Y. Fat and protein intakes and risk of intraparenchymal hemorrhage among middle-aged Japanese. Am J Epidemiol. 2003;157:32–39. doi: 10.1093/aje/kwf166. [DOI] [PubMed] [Google Scholar]
- 31.Ohira T, Shahar E, Chambless LE, Rosamond WD, Mosley TH, Jr, Folsom AR. Risk factors for ischemic stroke subtypes: The Atherosclerosis Risk in Communities Study. Stroke. 2006;37:2493–2498. doi: 10.1161/01.STR.0000239694.19359.88. [DOI] [PubMed] [Google Scholar]
- 32.Imamura T, Doi Y, Arima H, Yonemoto K, Hata J, Kubo M, Tanizaki Y, Ibayashi S, Iida M, Kiyohara Y. LDL cholesterol and the development of stroke subtypes and coronary heart disease in a general Japanese population: The Hisayama Study. Stroke. 2009;40:382–388. doi: 10.1161/STROKEAHA.108.529537. [DOI] [PubMed] [Google Scholar]
- 33.Muramatsu K, Tsuchihashi N, Tanaka E, Yamaguchi M, Suzuki A, Ishii K, Watanabe T. Estimated intake of cholesterol and fatty acids in Japanese. Bull Chiba Coll Health Sci. 2004;23:1–25. [Google Scholar]
- 34.Kennedy ET, Bowman SA, Powell R. Dietary-fat intake in the US population. J Am Coll Nutr. 1999;18:207–212. doi: 10.1080/07315724.1999.10718853. [DOI] [PubMed] [Google Scholar]
- 35.Vessby B. Dietary fat, fatty acid composition in plasma and the metabolic syndrome. Curr Opin Lipidol. 2003;14:15–19. doi: 10.1097/00041433-200302000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Warensjö E, Ohrvall M, Vessby B. Fatty acid composition and estimated desaturase activities are associated with obesity and lifestyle variables in men and women. Nutr Metab Cardiovasc Dis. 2006;16:128–136. doi: 10.1016/j.numecd.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 37.Warensjö E, Sundström J, Vessby B, Cederholm T, Risérus U. Markers of dietary fat quality and fatty acid desaturation as predictors of total and cardiovascular mortality: A population-based prospective study. Am J Clin Nutr. 2008;88:203–209. doi: 10.1093/ajcn/88.1.203. [DOI] [PubMed] [Google Scholar]
- 38.Warensjö E, Risérus U, Gustafsson I, Mohsen R, Cederholm T, Vessby B. Effects of saturated and unsaturated fatty acids on estimated desaturase activities during a controlled dietary intervention. Nutr Metab Cardiovasc Dis. 2008;18:683–690. doi: 10.1016/j.numecd.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 39.Sacks F, Katan M. Randomized clinical trials on the effects of dietary fat and carbohydrate on plasma lipoproteins and cardiovascular disease. Am J Med. 2002;113:13S–24S. doi: 10.1016/s0002-9343(01)00987-1. [DOI] [PubMed] [Google Scholar]
- 40.De Caterina R, Zampolli A, Del Turco S, Madonna R, Massaro M. Nutritional mechanisms that influence cardiovascular disease. Am J Clin Nutr. 2006;83:421S–426S. doi: 10.1093/ajcn/83.2.421S. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.