Abstract
Rotator cuff injuries are a common clinical problem either as a result of overuse or aging. Biological approaches to tendon repair that involve use of scaffolding materials or cell-based approaches are currently being investigated. The cell-based approaches are focused on applying multipotent mesenchymal stem cells (MSCs) mostly harvested from bone marrow. In the present study, we focused on characterizing cells harvested from tissues associated with rotator cuff tendons based on an assumption that these cells would be more appropriate for tendon repair. We isolated MSCs from bursa tissue associated with rotator cuff tendons and characterized them for multilineage differentiation in vitro and in vivo. Human bursa was obtained from patients undergoing rotator cuff surgery and cells within were isolated using collagenase and dispase digestion. The cells isolated from the tissues were characterized for osteoblastic, adipogenic, chondrogenic, and tenogenic differentiation in vitro and in vivo. The results showed that the cells isolated from bursa tissue exhibited MSCs characteristics as evidenced by the expression of putative cell surface markers attributed to MSCs. The cells exhibited high proliferative capacity and differentiated toward cells of mesenchymal lineages with high efficiency. Bursa-derived cells expressed markers of tenocytes when treated with bone morphogenetic protein-12 (BMP-12) and assumed aligned morphology in culture. Bursa cells pretreated with BMP-12 and seeded in ceramic scaffolds formed extensive bone, as well as tendon-like tissue in vivo. Bone formation was demonstrated by histological analysis and immunofluorescence for DMP-1 in tissue sections made from the scaffolds seeded with the cells. Tendon-like tissue formed in vivo consisted of parallel collagen fibres typical of tendon tissues. Bursa-derived cells also formed a fibrocartilagenous tissue in the ceramic scaffolds. Taken together, the results demonstrate a new source of MSCs with a high potential for application in tendon repair.
Introduction
Tendon injuries are a common clinical problem due to overuse or aging. There are over 300,000 rotator cuff surgical repairs a year in the United States alone.1 Surgical repair or replacements of tendons include the use of autografts, allografts, prosthetic devices and xenografts. Tendons heal poorly and slowly, mostly with a scar tissue.2–6 Present treatment regimens are inefficient in augmenting tendon healing; thus, alternative approaches are being sought. One of the alternatives is the application of stem cells.7–9 There are two types of stem cells, embryonic stem cells (ESC) and adult derived stem cells. Because of ethical concerns, use of ESC is not a viable option. induced pluripotent stem cells derived by reprogramming somatic cells, possess all characteristics of ESC but are still under intense investigation.10,11 Adult derived stem cells, specifically mesenchymal stem cells or multipotent mesenchymal stromal cells (MSCs) have generated excitement because of their ability to differentiate into mature cells of mesenchymal tissues. The major source of MSCs is bone marrow but there are several other sources, which have been found to contain these cells, adipose tissue being one of them.12–14 MSCs found in various tissues may all originate from a similar source. Currently, it is believed that these cells are associated with blood vessels; MSCs found in different tissues may all originate from this source.15 Subtle differences, however, have been noted between MSCs harvested from different tissues, for example, MSCs isolated from synovium are more proliferative, have higher potential for differentiation toward osteogenic, chondrogenic, adipogenic, and myogenic cell lineages than MSCs harvested from bone marrow or adipose tissue.16 Cells with MSC characteristics have also been isolated from mouse and human tendons.17 These cells referred to as tendon stem cells were demonstrated to exist within inches of the tendon extracellular matrix.17–19 Tendon derived stem cells were shown to give rise to differentiated cells of mesenchymal lineages that include osteoblasts, adipocytes, chondrocytes, and tenocytes under appropriate conditions or when supplemented with specific factors.20 Isolation of MSCs from tendons for cell-based tissue engineering is however, a challenge because of the difficulty in tissue accessibility and low cell yield. Adipose tissue is a major source of MSCs and is easily accessible. Cells harvested from this tissue have been shown to exhibit low ability to differentiate into chondrocytes and osteocytes when compared to MSCs isolated from other sources, for example bone marrow.21,22 Although bone marrow is a good source for MSCs, the cell yield is low, MSCs comprise only about 0.01% of the cells in bone marrow.12
In the present investigation, we searched for new sources of MSCs that could be applied in augmenting tendon repair with a focus on tissues associated with tendons. Our hypothesis was that MSCs harvested from tissues associated with tendons may contain stem cells with a higher potential for augmenting tendon healing than MSCs from other sources. We focused on the subacromial bursa, a fluid filled sac or sac-like cavity in the shoulder where friction might occur. Although there are some reports indicating that subacromial bursa contains MSCs, full characterization of the cells including in vivo differentiation, response to growth factors, as well as tendon differentiation were not carried out.23,24 The present report has carried out extensive characterization of bursa-derived cells including assessment of their differentiation in vivo.
We report here that bursa-derived MSCs possess high proliferative capacity and ability to differentiate into cells of mesenchymal lineages in vitro, as well as form bone, fibrocartilage and tendon-like tissues in vivo. The results suggest that bursa tissue is an excellent source of stem cells for cell-based tendon tissue engineering.
Materials and Methods
Cell isolation and culture
Human subacromial bursa and tendon tissues were obtained from five patients undergoing arthroscopic shoulder surgery for repair of rotator cuff tears, (ages 50–70 years) under approved exempt protocol. The bursal tissue was isolated into a cloth sleeve that captured the tissue specimen at the end of the suction unit at the time of arthroscopic bursectomy. The samples were then immediately transported to the laboratory for analysis. The tissues were cut into small pieces and washed several times in phosphate-buffered saline (PBS) supplemented with antibiotics. Tissue fragments were digested in PBS for 1 h with a mixture of 3 mg/mL collagenase type I and 4 mg/mL dispase at 37°C.18 Tissue digests were centrifuged, washed in PBS several times, and single-cell suspensions were plated in 100 mm Petri dishes and incubated in Dulbecco's modified Eagle's medium (DMEM, low glucose; Gibco by Life Technologies) for 8–10 days in 5% CO2 at 37°C. The medium was supplemented with 20% fetal bovine serum (FBS) and 1% penicillin–streptomycin (P/S) v/v (Gibco by Life Technologies), and 60 mM 2-mercaptoethanol (Gibco by Life Technologies). Medium changes were performed thereafter every 3 days; cells were expanded in culture with passaging.
Colony forming units and proliferation assays
For assays of colony-forming efficiency, 50 single-cell suspensions of bursa-derived cells were cultured in 60 mm Petri dishes. After 10 days in culture, bursa-derived MSCs formed colonies, which were stained with methyl violet. Colony numbers were quantified by counting colonies that contained more than 25 cells observed under a light microscope.
To assess cell proliferation, an equal number of bursa-derived MSCs (3000 cells/well) were seeded into 96-well plates in triplicates for each group of cells. Cell proliferation was assessed by counting cell numbers every day at the same time for 7 days.
Flow activated cell sorting analysis
Bursa-derived MSCs were lifted off the plates by trypsinization with 0.25% trypsin/EDTA and washed with cold PBS. Cell aliquots (5×105) were incubated for 1 h at 4°C in a buffer containing monoclonal antibodies specific for markers to be assessed. Antibodies used for flow activated cell sorting (FACS) analysis were phycoerythrin-conjugated anti-CD44, anti-CD45, anti-CD73, anti-CD105, and anti-CD146 (all from BD Bioscience). The flow cytometry instrument is housed in Penn State College of Medicine Core Facility. CD44, CD73, CD105, and CD146 are putative markers attributed to MSCs, CD45 is associated with hematopoietic stem cells.
Alkaline phosphatase activity assay
Bursa-derived MSCs were seeded onto 24-well plates and cultured in osteogenic induction medium, 10 mM β-glycerol phosphate, 0.1 mM dexamethasone, and 50 μg/mL ascorbic acid in DMEM-high glucose supplemented with 10% FBS and 1% P/S. Medium was replaced every 2 days, and the cells were maintained in culture for 7 or 14 days. Alkaline phosphatase activity (ALP) activity was determined in cell lysates using an ALP activity assay kit (Sigma) at 7 and 14 days after induction of differentiation. Cell lysates were analyzed for protein concentration using a Bio-Rad protein assay kit and ALP activity was normalized for total protein concentration.25,26
Osteogenic differentiation
Bursa-derived MSCs were assessed for multilineage by determining their ability to give rise to osteoblasts, adipocytes, chondrocytes, and tenocytes cell lineages in vitro. For osteogenic differentiation, bursa MSCs were seeded at low density (1–5×103 cells/cm2) into 12-well plates and incubated in DMEM containing high glucose supplemented with 10% FBS, 1% P/S, 10 mM β-glycerol phosphate, 0.1 mM dexamethasone, and 50 μg/mL ascorbic acid. The medium was replaced every 3 days for 21 days. Mineral deposition was assessed by Alizarin Red staining using methods described previously.25,26 Genes associated with osteoblast differentiation were assessed by PCR (see below).
Adipogenic differentiation
For adipogenic differentiation, bursa MSCs were cultured to near confluence followed by incubation in DMEM containing high glucose and supplemented with 10% FBS, 1% P/S, 10−2 μM dexamethasone, 10 μM insulin, 200 μM indomethacin, and 0.5 mM isobutyl-methylxanthine (all from Sigma). Medium was replaced every 3 days for 28 days, after 28 days, cells were incubated in 0.3% Oil Red O to stain for cells that differentiated into adipocytes.27 Genes associated with adipogenic differentiation were assessed by PCR (see below).
Chondrogenic differentiation
For chondrogenic induction, bursa MSCs (2×105) in DMEM were suspended in 15 mL conical tubes in DMEM and centrifuged. The DMEM was removed and replaced with a defined medium, which consisted of DMEM with high glucose supplemented with 10 ng/mL transforming growth factor (TGF)-β3 and 200 μM 2-ascorbate, ITS premix, and 10−7 M dexamethasone. The pellets were maintained in this chondrogenic medium for 21 days with medium changes every 3 days as described previously.28,29 After 21 days of incubation, the pellets were fixed in 4% paraformaldhyde for 24 h, washed and embedded in OCT. Ten micron tissue sections were cut and stained in haematoxylin and eosin (H&E) to assess for presence of cartilage matrix.27 Genes associated with chondrogenic differentiation, type II collagen, and aggrecan were assessed by PCR.
Tenogenic differentiation
To assess the potential of MSCs for tenogenic differentiation, bursa MSCs were cultured to 90% confluence in DMEM supplemented with 10% FBS. The cells were then starved for 12 h in DMEM supplemented with 1% FBS followed by treatment with 50 ng/mL recombinant bone morphogenetic protein-12 (BMP-12) (Biovision) in the same low-serum medium for 12 h. After 12 h, the cells were transferred into the growth medium supplemented with 50 ng/mL of BMP-12, cells were maintained in this medium with changes every 3 days for 7days.20 Expression of tenocyte markers was assessed by RT-PCR using specific primers. The tendon markers assessed were tenomodulin, scleraxis, tenacin C, and collagen type I a1.
Gene expression analysis
Total RNA was isolated by lysing cells in TRIzol (invitrogen). Isolated RNA was reverse transcribed with SuperScript™ First-Strand Synthesis System for RT-PCR (Invitrogen) according to the manufacturer's instructions. The cDNA was amplified by PCR with Taq DNA polymerase or RT-PCR with SYBR Green PCR Master Mix (Quanta Biosciences). The osteogenic genes assessed were Runx2, osterix, osteopontin (OPN), and osteocalcin (OCN). Adipogenic related differentiation genes assessed were PPAR-γ2, adipsin and LPL. Chondrogenic differentiation genes assessed were collagen type II and aggrecan. Genes related to tenogenic differentiation, Scleraxis, Tenomodulin, Collagen type I, and Tenacin C were assessed by RT-PCR. The primers used for PCR are indicated in Table 1. Beta -actin levels were used as internal controls. The PCR cycling consisted of 40 cycles of amplification of the template cDNA with primer annealing at 60°C. The relative level of expression of each target gene was calculated using the 2−ΔΔCt method.30
Table 1.
Primer Sequences Used for PCR Gene Analysis
| Genes | 5′-3′ | Primers | Product size (bp) |
|---|---|---|---|
| Beta-actin |
Forward |
CGTCTTCCCCTCCATCG |
94 |
| |
Reverse |
CTCGTTAATGTCACGCAC |
|
| OPN |
Forward |
GGTGATAGTGTGGTTTATGG |
91 |
| |
Reverse |
TGATGTCCTCGTCTGTAG |
|
| OSX |
Forward |
GCCAGAAGCTGTGAAACCTC |
160 |
| |
Reverse |
GCTGCAAGCTCTCCATAACC |
|
| OCN |
Forward |
CGGTGCAGAGTCCAGCAAA |
100 |
| |
Reverse |
GGCTCCCAGCCATTGATACA |
|
| RUNX2 |
Forward |
TGGGCTTCCTGCCATCAC |
120 |
| |
Reverse |
TTGGAGAAGCGGCTCTCAGT |
|
| LPL |
Forward |
GTCCGTGGCTACCTGTCAT |
717 |
| |
Reverse |
AGCCCTTTCTCAAAGGCTTC |
|
| Adipsin |
Forward |
GGTCACCCAAGCAACAAAGT |
271 |
| |
Reverse |
CCTCCTGCGTTCAAGTCATC |
|
| PPAR-r2 |
Forward |
GCTGTTATGGGTGAAACTCT |
351 |
| |
Reverse |
ATAAGGTGGAGATGCAGGCT |
|
| Collagen II |
Forward |
CTATCTGGACGAAGCAGCTGGCA |
209 |
| |
Reverse |
ATGGGTGCAATGTCAATGATGG |
|
| Aggrecan |
Forward |
CACTGTTACCGCCACTTCCC |
184 |
| |
Reverse |
ACCAGCGGAAGTCCCCTTCG |
|
| Tenascin C |
Forward |
GGTACAGTGGGACAGCAGGTG |
230 |
| |
Reverse |
AACTGGATTGAGTGTTCGTGG |
|
| Scleraxis |
Forward |
CCACTCCGGGCCCGCCTTCTTCC |
153 |
| |
Reverse |
TCGCGGTCCTTGCTCAACTTTCTC |
|
| Tenomodulin |
Forward |
CCCGTCACGCCAGACAAGCAA |
150 |
| |
Reverse |
GTTCACAGACGCGGCGGCAA |
|
| Collagen I a1 |
Forward |
GCTTCACCTACAGCGTCACT |
154 |
| Reverse | AAGCCGAATTCCTGGTCTGG |
In vivo transplantation
Bursa-derived MSCs were assessed for in vivo differentiation by seeding cells onto ceramic scaffolds followed by implantation into the backs of SCID mice. Briefly, bursa MSCs were seeded in 100 mm Petri dishes and incubated in the growth medium supplemented with or without 50 ng/mL of BMP-12 for 24 h. About 5×106 cells of each population (bursa MSCs exposed to BMP-12 or not exposed to BMP-12) were seeded onto 40 mg of hydroxyapatite/tricalcium phosphate (HA/TCP) ceramic powder particles ranging from 0.3 to 0.5 μm (Zimmer). The cells were allowed to attach onto the ceramic particles by incubation in the growth medium for 3–4 h at 37°C in 5% CO2. The cell-seeded scaffolds were centrifuged at 200 g for 1 min, and the supernatants were discarded. Cell-seeded scaffolds were mixed with mouse fibrinogen and thrombin to form a pellet shaped cohesive mixture of cells and particles. The cell-seeded scaffolds were then implanted subcutaneously into the backs of 8–10 week-old female immunocompromised mice. Four mice were used, three mice received two implants each, and the implants were seeded with bursa MSCs either pre-exposed or not exposed to BMP-12. One mouse received two implants that were not seeded with cells. The implants were harvested 8 weeks after implantation and processed as described below.18,31,32
Histology and immunohistochemistry
Scaffolds were harvested from the recipient mice at 8 weeks after implantation; they were fixed in 4% paraformaldehyde in the dark for 7 days at 4°C. The implants were then demineralised in 0.5 M EDTA in the dark for 7 days. After demineralization, scaffolds were embedded in paraffin and 10 μm tissue sections were cut. Tissue sections were stained in H&E or toluidine blue or Sirus red to assess bone, cartilage, and tendon formation in vivo.
Immunohistochemistry
Tissue sections were deparaffinized and antigens were retrieved by incubation in 0.04% pepsin solution at 37°C for 30 min. To confirm bone formation in vivo by bursa MSCs, differentiation of the implanted cells into osteocytes was assessed by immunofluorescence staining for DMP-1 in tissue sections made from scaffolds seeded with the cells. After antigen unmasking, tissue sections were incubated in anti- DMP-1 antibodies (1:100; R&D Systems) at 4°C for 24 h. Control tissue sections were incubated in 1% bovine serum albumin in 0.05% Tween 20 in TBS. To demonstrate that the human donor bursa-derived cells made the new bone in the implants, immunofluorescence staining for human lamin A/C was performed using specific antibodies. Lamin A/C is a nuclear protein found in vertebrates. After antigen retrieval, tissue sections were incubated in acetone for 10 min to allow antibodies to penetrate the cell membrane and localize in the nuclei. This was followed by incubation of the sections in TBS containing the primary antibody to human Lamin A/C (1:50; Santa Cruz) for 24 h. For visualization, sections were incubated in secondary antibodies conjugated to Rhodamine in TBS for 1 h. Sections were observed under fluorescence and photographed. To determine if the cells differentiated into chondrocytes in vivo, antibodies to type II collagen (Hybridoma Bank) were used for immunofluorescence staining of type II collagen in the tissue sections made from the implants seeded with the cells. For visualization, sections were incubated in secondary antibodies conjugated with Rhodamine (Santa Cruz) for 1 h. The nuclei were stained with DAPI as described.33 For presence of tendon-like tissue in vivo, sections were stained with Sirus red for 24 h and examined under a light microscope.
Statistical analysis
For statistical evaluations of the calculated relative expression variations, data were analyzed for significance by one-way analysis of variance (ANOVA). A level of p<0.05 was considered statistically significant.
Results
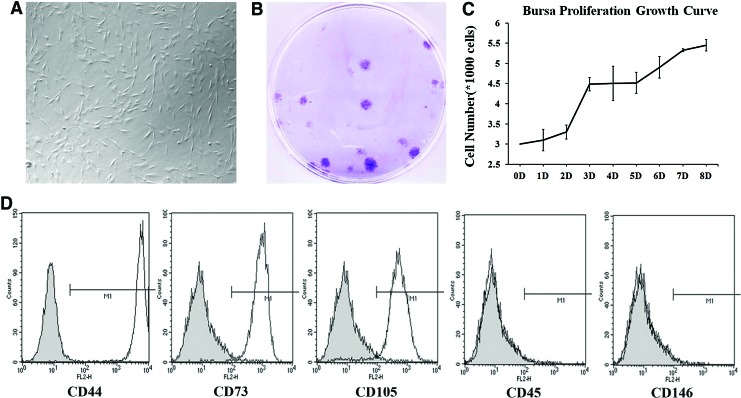
Bursa tissue was separated from tendon to avoid contamination with tendon stem cells. Initially, cells were isolated from both tendon and bursa tissues but further analyses revealed that the yield of stem cells from tendon was extremely low. The present studies were focused on bursa tissue which provided a higher yield of the cells for analysis than tendon tissue. Bursa-derived cells, morphologically exhibited spindle shaped morphology (Fig. 1A), and they grew in colonies (Fig. 1B) and proliferated rapidly (Fig. 1C). FACS analysis was used to determine whether bursa-derived cells expressed putative MSCs surface markers. The surface markers examined were CD44, CD45, CD105, CD73 and CD146. CD44, CD73, CD105, and 146 have been shown to be associated with MSCs harvested from various sources, while CD45 is associated with hematopoietic stem cells. Results showed that the cells were positive for CD44, CD73, and CD105 and were negative for CD45 and CD146. CD146 has been shown to be expressed by MSCs harvested from bone marrow and tendon previously; however, bursa-derived cells were negative for this antigen. Absence of CD45 expression indicated that the bursa cell preparation was devoid of hematopoetic stem cells (Fig. 1D). Absence of CD146 expression suggests that bursa-derived MSCs may represent a unique population.
FIG. 1.
Morphological appearance, proliferation, and surface marker expression of Bursa-derived MSCs. (A) Bursa MSCs display spindle shaped morphology. (B) The cells were efficient in forming colonies suggesting stem cell properties. (C) Bursa MSCs displayed rapid growth in culture. (D) Flow cytometry analysis for the expression of putative MSCs surface markers demonstrated expression of CD44, CD73, and CD105 with absence of CD45; thus, the cells are devoid of hematopoetic stem cells. The cells were also negative for CD146 expression, indicating uniqueness of the cell population. MSCs, mesenchymal stem cells. Color images available online at www.liebertpub.com/tea
Multilineage differentiation
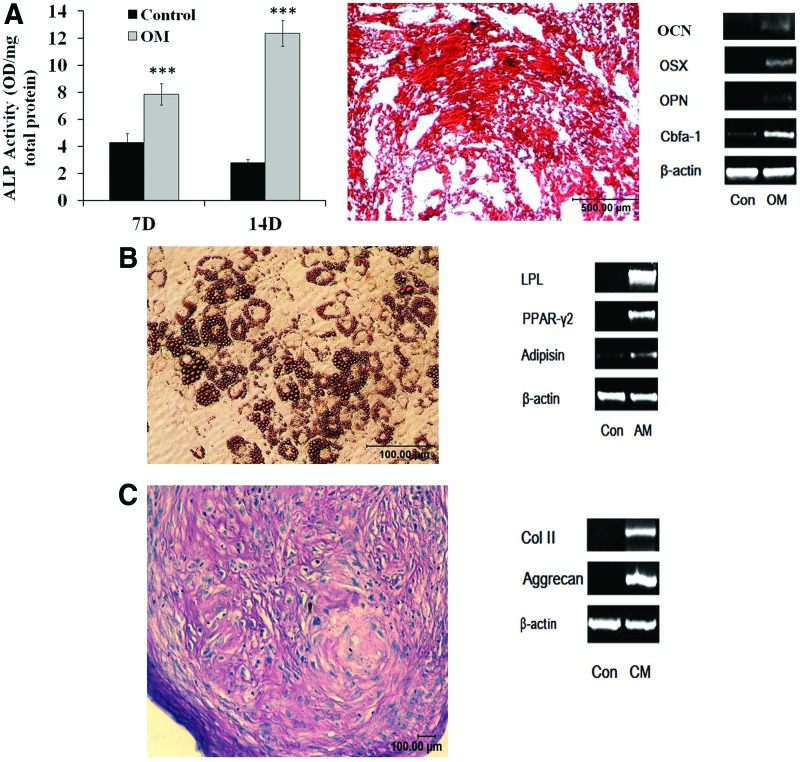
To further determine the characteristics of bursa-derived cells, cells were assessed for differentiation into various cell lineages in vitro. The cells exhibited ability to give rise to osteoblasts as demonstrated by ALP activity after incubation of the cells in osteogenic medium (Fig. 2A). Bursa-derived cells incubated in osteogenic medium deposited high levels of mineral as assessed by Alizarin Red staining after 21 days of culture (Fig. 2A). The mineralized matrix demonstrated heavy staining for Alizarin Red indicating that the cells have ability to differentiate into osteoblasts/osteocytes (Fig. 2A). Osteoblasts differentiation by the bursa MSCs was further confirmed by analysis of osteoblast-specific gene expression. The cells expressed OCN, osterix, OPN, and Runx2 when maintained in osteogenic medium (Fig. 2A).
FIG. 2.
Multilineage differentiation of Bursa MSCs in vitro. (A) Osteogenic differentiation: The cells exhibited ALP activity when cultured in osteogenic medium and this expression was highest at day 14 compared to other time points. Alizarin Red staining of osteogenic cultures at 21 days demonstrated extensive mineral deposition. Analysis of osteoblasts related genes of differentiating bursa MSCs demonstrated expression of OCN, OSX, OPN, and Runx2 indicating that the cells possess ability to differentiate into osteoblasts. (B) Bursa MSCs differentiated into adipocytes as demonstrated by extensive Oil Red O staining; analysis of adipogenic related genes showed expression of LPL, PPAR-γ2, and adipsin. (C) Chondrogenic differentiation indicated by H&E staining of pellet cultures and expression of type II and aggrecan genes. The results demonstrate multilineage differentiation of bursa cells. Con, cells cultured in maintenance medium; OM, osteogenic medium; AM, adipogenic medium; CM, chondrogenic medium; H&E, haematoxylin and eosin; ALP, alkaline phosphatase activity; OCN, osteocalcin; OPN, osteopontin; OSX, osterix. Color images available online at www.liebertpub.com/tea
Adipogenic differentiation was assessed by Oil Red O staining. The data demonstrated Oil Red O deposits in adipogenic cultures of bursa-derived MSCs indicating that the cells exhibited ability to differentiate into adipocytes (Fig. 2B). Adipogenic differentiation was further confirmed by analysis for expression of adipocyte related genes by the cells incubated in adipogenic medium; the cells expressed LPL, PPAR-γ2, and adipsin genes (Fig. 2B).
Ability of bursa-derived MSCs to differentiate into chondrocytes was assessed after incubation of the cells in chondrogenic medium in pellet cultures. Chondrogenic differentiation was assessed by H&E staining for cartilage matrix and expression of type II collagen gene, results are shown in Figure 2C. Taken together, the results demonstrated that bursa-derived cells exhibit all the characteristics of MSCs and that the cells are highly efficient in differentiating into cells of mesenchymal lineages.
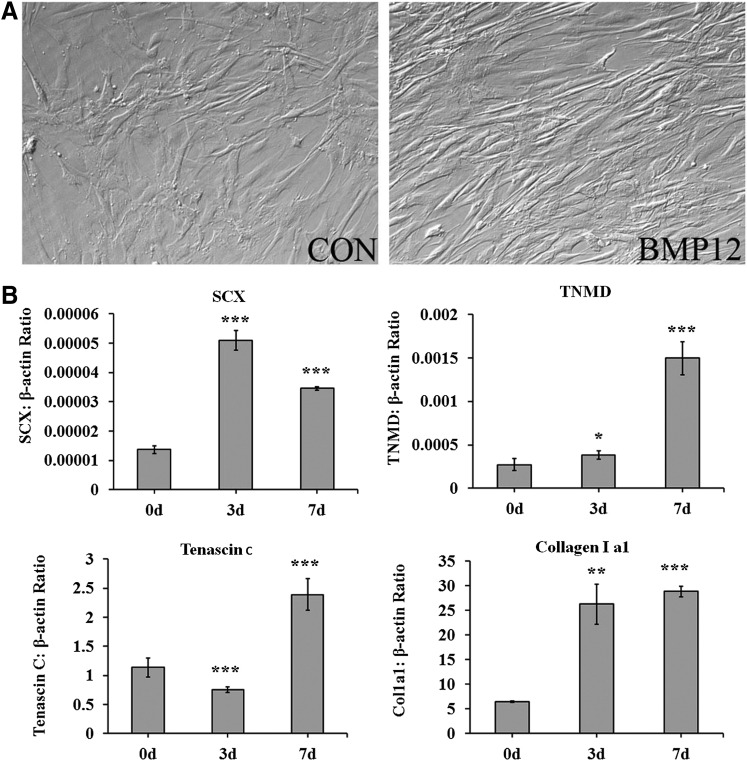
We examined whether bursa MSCs exhibited potential for differentiation into tenocytes. Previous reports showed that BMP-12, BMP-13 and BMP-14 induce tendon differentiation;34–38 thus, potential of bursa-derived MSCs to give rise to tenocytes after exposure to BMP-12 was assessed. Bursa-derived MSCs were incubated in a medium supplemented with 50 ng/mL of BMP-12 and assessed for morphological changes and expression of tenocyte related genes. After incubation of the cells in a medium supplemented with BMP-12 for 7 days, bursa-derived MSCs adapted aligned and elongated morphological appearances (Fig. 3A). Analysis for expression of tendon related genes, revealed expression of Scleraxis (SCX), Tenomodulin (TNMD), Tenacin C and increased in type I collagen expression (Fig. 3B). These data showed that cells isolated from bursa tissue contain MSCs with ability to differentiate into multiple mature cell types including tenocytes.
FIG. 3.
Tenocyte differentiation in vitro. (A) Morphological appearance of bursa MSCs that were not incubated with BMP-12 (CON) and incubated with BMP-12 for 7 days. Bursa MSCs incubated with BMP-12 assumed an aligned and elongated morphology. (B) Expression of tendon markers by Bursa MSCs incubated in a medium supplemented with BMP-12. Tendon markers expressed by the cells were SCX, TNMD, Tenacin C, and Col1a1 at 3 and 7 days following exposure to BMP-12. (C). Tendon marker expression was assessed by RT-PCR. BMP-12, bone morphogenetic protein-12; SCX, Scleraxis; TNMD, tenomodulin. ±SD, *p<0.05, **p<0.01, ***p<0.001. N=4, triplicate experiments.
In vivo tissue formation by bursa MSCs
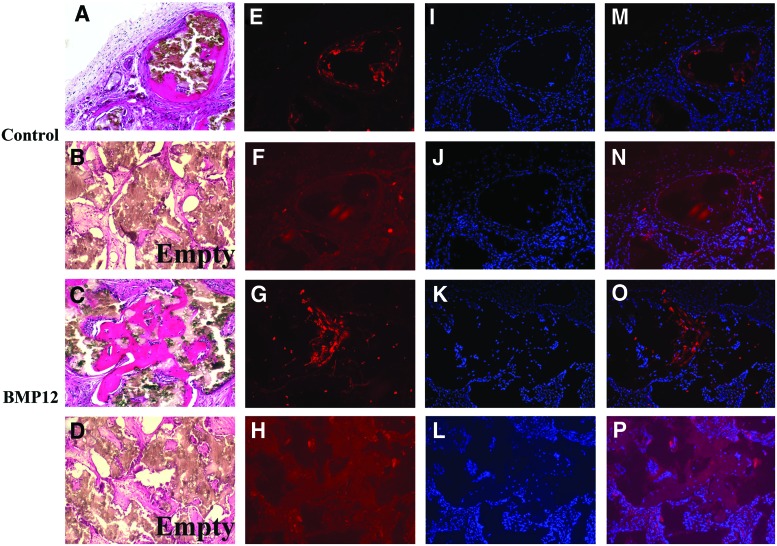
Formation of tissues in vivo by bursa-derived MSCs has never been demonstrated. We assessed potential of bursa MSCs to make bone, cartilage, and tendon in vivo. Bursa-derived MSCs that were pre-exposed or not to BMP-12 were seeded onto HA/TCP ceramic scaffolds and implanted into the backs of SCID mice. Tissue sections made from the implants harvested at 8 weeks after implantation, revealed bone formation by both cell populations (Fig. 4A, C). The cells pre-exposed to BMP-12, however, appeared to deposit more bone than the cells not treated with BMP-12 based on observation of tissue sections made from the scaffolds seeded with the cells (Fig. 4C). In addition, tendon-like tissue was seen surrounding the newly made bone (Fig. 4C). To further confirm bone formation by the bursa MSCs, immunofluorescence staining for DMP-1, a marker of osteocytes revealed presence of this protein in the newly made bone (Fig. 4E, G). DAPI staining showed that the newly formed bone tissue contained cells representing osteoblastic and osteocytic cells derived from bursa MSCs (Fig. 4J, K), overlay images of the immunofluorescence staining for DMP-1 and DAPI staining are shown (Fig. 4M, O). Control tissues that did not receive primary antibodies to DMP-1 were negative for DMP-1 staining (Fig. 4F, H). Overlay images with DAPI are shown (Fig. 4J, L, N, P) did not show any staining. These data confirm that bursa MSCs form bone in vivo. Scaffolds implanted without cells did not contain any bone (Fig. 4B, D).
FIG. 4.
Bone formation in vivo by Bursa MSCs. (A) Bursa MSCs that were not pretreated with BMP-12 showed bone formation in the ceramic scaffolds. (B) Scaffold implanted in mice without cells demonstrated absence of bone formation. (C) Scaffold seeded with bursa MSCs pretreated with BMP-12 showed extensive bone formation in vivo. (D) Empty scaffold implanted in mice did not show any bone formation. (E, G) Immunofluorescence showed DMP-1 staining within the newly made bone in scaffolds seeded with either of the bursa cell populations. (F, H) Immunofluorescence staining for DMP-1 in scaffolds in which primary antibodies were omitted. (I–L) DAPI staining of respective scaffolds. (M–P) Overlay images of DMP-1 immunofluorescence staining and DAPI staining of respective tissue sections. The data demonstrate that bursa MSCs differentiate into osteoblasts in vivo and deposit bone. Magnification 100×. Color images available online at www.liebertpub.com/tea
Cartilage formation in vivo
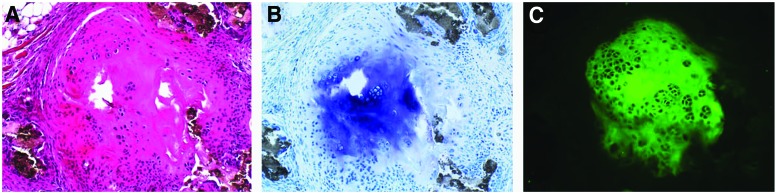
Next, we examined tissue sections made from the ceramic scaffolds seeded with bursa MSCs for the presence of cartilage tissue. Tissue sections made from implants seeded with bursa MSCs that were not pretreated with BMP-12, demonstrated areas containing cartilage like tissue as revealed by presence of cells with chondrocytic morphology (Fig. 5A). To confirm presence of cartilage tissue, sections were stained in toluidine blue for aggrecan and immunofluorescence for type II collagen. The results showed regions of toluidine blue staining within the ceramic tissue sections and these areas showed immunofluorescence staining for type II collagen (Fig. 5B, C). These data confirm that bursa MSCs that were not exposed to BMP-12 gave rise to a fibrocartilagenous tissue in vivo.
FIG. 5.
Fibrocartilage formation by the bursa MSCs. (A) H&E staining of the fibrocartilagenous area within the tissue section from the scaffold that was seeded with bursa MSCs not pretreated with BMP-12. (B) Fibrocartilagenous area stained with toluidine blue showed staining of cartilage matrix. (C) Immunofluorescence staining for type II collagen in the fibrocartilagenous tissue confirmed that bursa MSCs form cartilage in vivo. Magnification 100×. Color images available online at www.liebertpub.com/tea
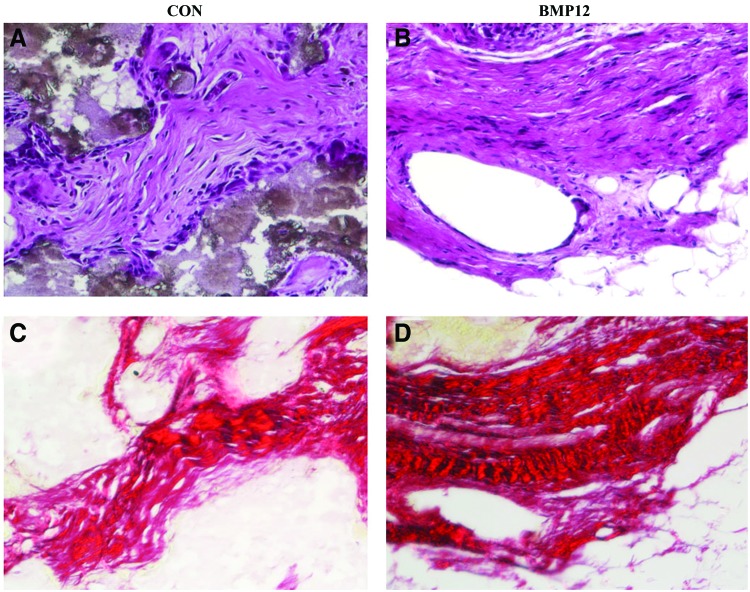
Tendon-like tissue formation
Tendon-like tissues surrounding the newly made bone were seen within the scaffolds seeded with the cells. Tissues were identified based on histological appearance and collagen staining with Sirus red. In either BMP-12 pretreated or not, bursa MSCs seeded scaffolds demonstrated presence of tendon-like tissues (Fig. 6). Because there are no specific markers available for identifying tendon cells, we used Sirus red to stain for collagen fibrils. The results showed parallel collagen fibres typical of tendon tissues indicating that the fibrous tissues present within the scaffolds exhibit tendon characteristics (Fig. 6). Although no quantitation was done, scaffolds seeded with bursa MSCs pretreated with BMP-12 appeared to contain more tendon-like tissues than bursa MSCs that were not exposed to BMP-12. These data demonstrate that bursa MSCs make tendon in vivo but BMP-12 may enhance this process. Although there are no specific markers to identify tendon-like tissue, the present findings are consistent with previous observation that tendon derived stem cells seeded on ceramic scaffolds and implanted into SCID mice made tendon-like tissue.18
FIG. 6.
Formation of tendon-like tissue in vivo by the bursa MSCs. Bursa MSCs formed tendon-like tissues in vivo. (A) Bursa MSCs not pretreated with BMP-12 formed tendon-like tissue in vivo. (B) Bursa MSCs pretreated with BMP-12 appeared to form more tendon-like tissues. (C) Sirus red staining for collagen in a scaffold seeded with bursa MSCs not pretreated with BMP-12. (D) Sirus red staining in a scaffold seeded with bursa MSCs pretreated with BMP-12. Sirus Red staining demonstrated bundles of parallel collagen fibres typical of tendon tissues for both cell populations. Because there are no specific markers available for tendon cells, the tissues were identified based on their morphological appearance. The data are consistent with data reported previously on tendon-like tissue formation by tendon stem cells in vivo.18 Magnification 100×. Color images available online at www.liebertpub.com/tea
To demonstrate that bursa-derived MSCs fabricated the newly made bone seen in the implants, immunofluorescence staining for human Lamin A/C was performed. The data showed staining for this antigen in the newly made bone (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/tea). These data confirmed that donor bursa-derived cells were present in the newly made tissues and were responsible for the tissues seen in the implants. It is unlikely that the donor cells were trapped in the newly made bone because their presence on the bone surface suggests that they differentiated into osteoblasts in vivo. In addition, donor cells were embedded in the newly made bone suggesting that these cells were osteocytes.
Taken together, in vivo studies demonstrated that bursa-derived MSCs are efficient in giving rise to multiple mesenchymal tissues in vivo. The tissues formed in vivo were bone, fibrocartilage and tendon-like tissue. Thus, bursa tissue represents an abundant source of MSCs with potential for application in tendon or ligament repair.
Discussion
The present report has shown that subacromial bursa tissue, a tissue usually discarded during rotator cuff tear repairs, contains a unique population of multipotent MSCs with high efficiency for differentiation into multiple cell lineages and tissues. This tissue represents a new source of MSCs for application in tendon or ligament repair including fibrocartilagenous zones at the bony attachments. There are several reports in literature on isolation of MSCs from tendons and ligaments.18,20,39,40 Although, these tissue sources contain MSCs that could be applied in tendon or ligament repair, they are not easily accessible and they contain low levels of MSCs. The present findings that bursa tissue contains MSCs with high proliferative capacity and differentiation ability to multiple cell lineages, as well as easy accessibility makes this tissue a rich source of MSCs for cell-based tendon repair. Tendon formation in vivo including a fibrocartilagenous tissue has been reported but the studies were based on the use of a MSC lines and transduction of the cells with vectors carrying genes, for example Smad8 gene.41 The unique aspect of the bursa-derived cells is their ability to make a fibrocartilagenous tissue without supplementation with any growth factors. This is the major finding of the data reported here.
Most of the tendon tears occur at the bony junction sites, methods to repair these junction sites are therefore, crucial to achieving tendon healing. Most approaches to repair soft tissue bony attachment sites involve surgical reattachments.42 These approaches often fail because regeneration of the entheses is difficult to achieve, in most cases it results in a fibrovascular disorganized scar tissue. Many studies in animal models to evaluate efficacy of MSCs in improving healing of tendons and ligaments have been carried out. Some studies examined efficacy of the cells to regenerate ligament or tendon insertion sites, while others looked at use of the cells in repairing tendon or ligament defects.8,19,43 Use of MSCs as vehicles to deliver therapeutic growth factor genes into models of ligament and tendon repair has also been examined.20 These experimental models have generated promising results but many obstacles remain to be addressed before clinical application of stem cells for tendon or ligament repair can be achieved. Some of the obstacles to be addressed include sources of MSCs, mode of application and concern for potential immunological response if allogenic cells are to be used. In the present communication, we have shown that bursa is an abundant source of MSCs; MSCs in this tissue exhibit high proliferation rate, and ability to differentiate efficiently into cells of mesenchymal lineages including tenocytes. Although presence of MSCs in bursa tissue has been described previously, characterization of the cells for differentiation into various cell lineages,23,24 response to growth factors, and differentiation into tenocytes, as well as fibrocatilagenous tissue formation in vivo were not reported. Because bursa tissue is usually discarded during rotator cuff surgery, the tissue can be harvested by arthroscopy, stem cells can then be isolated ex vivo and used to augment tendon repair during surgery. Bursa-derived MSCs could be delivered to tendon defects using appropriate scaffolds or combined with specific carriers. Some of the carriers that have been examined include collagen gels or fibrin.18,19
Growth factors of interest for enhancing tendon repair include basic fibroblast growth factor (bFGF), BMP-12, BMP-13, BMP-14, cartilage oligomeric matrix protein (COMP), connective tissue growth factor (CTGF), platelet-derived growth factor beta (PDGF-B), and TGF-B1.35–38,44–49 Because these factors were demonstrated to be present during the healing process of rotator cuff, it is hypothesized that exogenous additions of these factors can further augment the healing process. Growth differentiation factors, BMP-12 (growth and differentiation factor 7) and BMP-14 (growth differentiation factor 5) and BMP-13 (growth and differentiation factor 6) are expressed during embryonic development of tendons and their insertion sites. We showed that bursa-derived MSCs exposed to BMP-12 induced expression of tendon related genes in bursa MSCs and formed tendon-like tissue with parallel collagen fibres when seeded onto HA/TCP scaffolds. The fibrocartilagenous tissue was detected only in scaffolds that were seeded with cells not pre-exposed to BMP-12 before implantation. Because of limited sample size, three scaffolds of each cell population was implanted, we cannot conclude with certainty whether bursa MSCs exposed to BMP-12 are not able to give rise to a fibrocartilagenous tissue in vivo. Because growth factors have short half-lives, for sustained delivery, bursa-derived MSCs transduced with the growth factor genes can be used as delivery vehicles to tendon or ligament defects.
In summary, the present findings that bursa is an abundant source of MSCs and that the cells exhibit proliferative ability and efficient differentiation into osteoblasts, tenocytes and fibrochondrocytes provide an ideal plat form to evaluate application of these cells for tendon repair. The major contributions of the present report are that we have clearly demonstrated that bursa tissue is an abundant source of MSCs, the cells exhibit high ability to differentiate into cells of mesenchymal lineages in vitro and in vivo and most importantly that they have ability to differentiate into a fibrocartilagenous tissue in vivo without any growth factor supplementation. The cells could be utilized in combination with specific growth factors to enhance repair of tendon-bone attachment sites or tendon defects. Bursa tissue is easily accessible during rotator cuff surgery and is a rich source of MSCs than tendon proper. Taken together, the present findings have revealed a new source of MSCs, these findings provide opportunities for investigating application of these cells in tendon repair in animal models.
Supplementary Material
Acknowledgments
This work was supported by Department of Orthopaedics and Rehabilitation Research Initiation Grant and a research fellowship to N.S. by China Scholarship Council, No. 2011617158.
Disclosure Statement
Authors have no disclosures to declare.
References
- 1.Pennnisi E.Tending tender tendons. Science 295,1011, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Goh J., Ouyang H.W., Teoh S.H., Chan C.K., and Lee E.H.Tissue-engineering approach to the repair and regeneration of tendons and ligaments. Tissue Eng 9,S31, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Vunjak-Novakovic G., Altman G., Horan R., and Kaplan D.L.Tissue engineering of ligaments. Annu Rev Biomed Eng 6,131, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Coons D.A., and Alan Barber F.Tendon graft substitutes-rotator cuff patches. Sports Med Arthrosc 14,185, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Sclamberg S.G., Tibone J.E., Itamura J.M., and Kasraeian S.Six-month magnetic resonance imaging follow-up of large and massive rotator cuff repairs reinforced with porcine small intestinal submucosa. J Shoulder Elbow Surg 13,538, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Iannotti J.P., Zlatkin M., Esterhai J.L, Kressel H.Y., Dalinka M.K., and Spindler K.P.Magnetic resonance imaging of the shoulder. Sensitivity, specificity, and predictive value. J Bone Joint Surg Am 73,17, 1991 [PubMed] [Google Scholar]
- 7.Gulotta L.V., Kovacevic D., Montgomery S., Ehteshami J.R., Packer J.D., and Rodeo S.A.Stem cells genetically modified with the developmental gene Mt1-Mmp improve regeneration of the supraspinatus tendon-to-bone insertion site. Am J Sports Med 38,1429, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Gulotta L.V., Kovacevic D., Packer J.D., Deng X.H., and Rodeo S.A.Bone marrow-derived mesenchymal stem cells transduced with scleraxis improve rotator cuff healing in a rat model. Am J Sports Med 39,1282, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Randelli P., Conforti E., Piccoli M., Ragone V., Creo P., Cirillo F., Masuzzo P., Tringali C., Cabitza P., Tettamanti G., Gagliano N., and Anastasia L.Isolation and characterization of 2 new human rotator cuff and long head of biceps tendon cells possessing stem cell-like self-renewal and multipotential differentiation capacity. Am J Sports Med 41, 1653, 2013 [DOI] [PubMed] [Google Scholar]
- 10.Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., and Yamanaka S.Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131,861, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Li F., Bronson S., and Niyibizi C.Derivation of murine induced pluripotent stem cells (Ips) and assessment of their differentiation toward osteogenic lineage. J Cell Biochem 109,643, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Pittenger M.F.Multilineage potential of adult human mesenchymal stem cells. Science 284,143, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Huang A.H., Farrell M.J., and Mauck R.L.Mechanics and mechanobiology of mesenchymal stem cell-based engineered cartilage. J Biomech 43,128, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hass R., Kasper C., Bohm S., and Jacobs R.Different populations and sources of human mesenchymal stem cells (MSC): a comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal 9,12, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crisan M., Yap S., Casteilla L., Chen C.W., Corselli M., Park T.S., Andriolo G., Sun B., Zheng B., Zhang L., Norotte C., Teng P.N., Traas J., Schugar R., Deasy B.M., Badylak S., Buhring H.J., Giacobino J.P., Lazzari L., Huard J., and Peault B.A.Perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3,301, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Jones E.Synovial mesenchymal stem cells in vivo: potential key players for joint regeneration. World J Rheumatol 1,4, 2011 [Google Scholar]
- 17.Zhang J., and Wang J.H.Characterization of differential properties of rabbit tendon stem cells and tenocytes. BMC Musculoskele Disord 11,10, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bi Y., Ehirchiou D., Kilts T.M., Inkson C.A., Embree M.C., Sonoyama W., Li L., Leet A.I., Seo B.M., Zhang L., Shi S., and Young M.F.Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med 13,1219, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Gulotta L.V., Chaudhury S., and Wiznia D.Stem cells for augmenting tendon repair. Stem Cells Int 2012,291431, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee J.Y., Zuping Z., Taub P.J., Ramcharan M., Li Y., Akinbiyi T., Maharam E.R., Leong D.J., Laudier D.M., Ruike T., Torina P.J., Zaidi M., Majeska R.J., Schaffler M.B., Flatow E.L., and Sun H.B.BMP-12 treatment of adult mesenchymal stem cells in vitro augments tendon-like tissue formation and defect repair in vivo. PLoS One 6,e17531, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith R.K., and Wwbbon P.M.Harnessing the stem cell for the treatment of tendon injuries: heralding a new dawn? Br J Sports Med 39,582, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pacini S., Spinabella S., Trombi L., Fazzi R., Galimberti S., Dini F., Carlucci F., and Petrini M.Suspension of bone marrow-derived undifferentiated mesenchymal stromal cells for repair of superficial digital flexor tendon in race horses. Tissue Eng 13,2949, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Moon Y.L., Song C.H., Noh K.H., Lee S.J., Lee H.J., and Kim S.H.Isolation and phenotypic characterization of multipotent mesenchymal-like stem cells from human subacromial bursa. Tissue Eng Regen Med 6,527, 2009 [Google Scholar]
- 24.Utsunomiya H., Uchida S., Sekiya I., Sakai A., Moridera K., and Nakamura T.Isolation and characterization of human mesenchymal stem cells derived from shoulder tissues involved in rotator cuff tears. Am J Sports Med 41,657, 2013 [DOI] [PubMed] [Google Scholar]
- 25.Li F., Wang X., and Niyibizi C.Distribution of single-cell expanded marrow derived progenitors in a developing mouse model of osteogenesis imperfecta following systemic transplantation. Stem Cells 25,3138, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Liao X., Li F., Wang X., Yanoso J., and Niyibizi C.Distribution of murine adipose-derived mesenchymal stem cells in vivo following transplantation in developing mice. Stem Cells Dev 17,303, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Ogawa R., Mizuno H., Watanabe A., Migita M., Hyakusoku H., and Shimada T.Adipogenic differentiation by adipose-derived stem cells harvested from GFP transgenic mice-including relationship of sex differences. Biochem Biophys Res Commun 319,511, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Mackay A.M., Beck S.C., Murphy J.M., Barry F.P., Chichester C.O., and Pittenger M.F.Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng 4,415, 1998 [DOI] [PubMed] [Google Scholar]
- 29.Solchaga L.A., Penick K.J., and Welter J.F.Chondrogenic differentiation of bone marrow-derived mesenchymal stem cells: tips and tricks. Methods Mol Biol 698,253, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Livak K.J., and Schmittgen T.D.Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 25,402, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Krebsbach P.H., Kuznetsov S.A., Satomura K., Emmons R.V., Rowe D.W., and Robey P.G.Bone formation in vivo: comparison of osteogenesis by transplanted mouse and human marrow stromal fibroblasts. Transplantation 63,1059, 1997 [DOI] [PubMed] [Google Scholar]
- 32.Kuznetsov S.A., Mankani M.H., and Robey P.G.Effect of serum on human bone marrow stromal cells: ex vivo expansion and in vivo bone formation. Transplantation 70,1780, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Wu S.Y., Yu Y.R., Cai Y., Jia L.X., Wang X., Xiao C.S., Tang C.S., and Qi Y.F.Endogenous aldosterone is involved in vascular calcification in rat. Exp Biol Med 237,31, 2012 [DOI] [PubMed] [Google Scholar]
- 34.Wolfman N.M., Hattersley G., Cox K., Celeste A.J., Nelson R., Yamaji N., et al. Ectopic induction of tendon and ligament in rats by growth and differentiation factors 5, 6, and 7, members of the TGF-B gene family. J Clin Invest 100,321, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haddad-Weber M., Prager P., Kunz M., Seefried L., Jakob F., Murray M.M., Evans C.H., Noth U., and Steinert A.F.BMP12 and BMP13 gene transfer induce ligamentogenic differentiation in mesenchymal progenitor and anterior cruciate ligament cells. Cytotherapy 12,505, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berasi S.P., Varadarajan U., Archambault J., Cain M., Souza T.A., Abouzeid A., Li J., Brown C.T., Dorner A.J., Seeherman H.J., and Jelinsky S.A.Divergent activities of osteogenic Bmp2, and tenogenic Bmp12 and Bmp13 independent of receptor binding affinities. Growth Factors 29,128, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park A., Hogan M.V., Kesturu G.S., James R., Balian G., and Chhabra A.B.Adipose-derived mesenchymal stem cells treated with growth differentiation factor-5 express tendon-specific markers. Tissue Eng Part A 16,2941, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hogan M., Girish K., James R., Balian G., Hurwitz S., and Chhabra A.B.Growth differentiation factor-5 regulation of extracellular matrix gene expression in murine tendon fibroblasts. J Tissue Eng Regen Med 5,191, 2011 [DOI] [PubMed] [Google Scholar]
- 39.Yin Z., Chen X., Chen J.L., Shen W.L., Hieu Nguyen T.M., Gao L., and Ouyang H.W.The regulation of tendon stem cell differentiation by the alignment of nanofibers. Biomaterials 31,2163, 2010 [DOI] [PubMed] [Google Scholar]
- 40.Kishore V., Bullock W., Sun X., Van Dyke W.S., and Akkus O.Tenogenic differentiation of human mscs induced by the topography of electrochemically aligned collagen threads. Biomaterials 33,2137, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shahab-Osterloh S., Witte F., Hoffmann A., Winkel A., Laggies S., Neumann B., Seiffart V., Lindenmaier W., Gruber A.D., Ringe J., Haupl T., Thorey F., Willbold E., Corbeau P., and Gross G.Mesenchymal stem cell-dependent formation of heterotopic tendon-bone insertions (osteotendinous junctions). Stem Cells 28,1590, 2010 [DOI] [PubMed] [Google Scholar]
- 42.Benjamin M., Toumi H., Ralphs J.R., Bydder G., Best T.M., and Milz S.Where tendons and ligaments meet bone: attachment sites (‘Entheses’) in relation to exercise and/or mechanical load. J Anat 208,471, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gulotta L.V., Kovacevic D., Packer J.D., Ehteshami J.R., and Rodeo S.A.Adenoviral-mediated gene transfer of human bone morphogenetic protein-13 does not improve rotator cuff healing in a rat model. Am J Sports Med 39,180, 2011 [DOI] [PubMed] [Google Scholar]
- 44.Wolfman N.M., Karen Cox G.H., Celeste A.J., Nelson R., Yamaji N., Dube J.L., DiBlasio-Smith E., Nove J., Song J.J., Wozney J.M., and Rosen V.Ectopic induction of tendon and ligament in rats by growth and differentiation factors 5, 6, and 7, members of the TGF-beta gene family. J Clin Invest 100,321, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brent A.E., Schweitzer R., and Tabin C.J.A Somitic compartment of tendon progenitors. Cell 113,235, 2003 [DOI] [PubMed] [Google Scholar]
- 46.Hankemeier S., Keus M., Zeichen J., Jagodzinski M., Barkhausen T., Bosch U., Krettek C., and Van Griensven M.Modulation of proliferation and differentiation of human bone marrow stromal cells by fibroblast growth factor 2: potential implications for tissue engineering of tendons and ligaments. Tissue Eng 11,41, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Wurgler-Hauri C.C., Dourte L.M., Baradet T.C., Williams G.R., and Soslowsky L.J.Temporal expression of 8 growth factors in tendon-to-bone healing in a rat supraspinatus model. J Shoulder Elbow Surg 16,198, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mazzocca A.D., McCarthy M.B., Chowaniec D., Cote M.P., Judson C.H., Apostolakos J., Solovyova O., Beitzel K., and Arciero R.A.Bone marrow-derived mesenchymal stem cells obtained during arthroscopic rotator cuff repair surgery show potential for tendon cell differentiation after treatment with insulin. Arthroscopy 27,1459, 2011 [DOI] [PubMed] [Google Scholar]
- 49.Majewski M., Porter R.M., Betz O.B., Betz V.M., Clahsen H., Flückiger R., and Evans C.H.Improvement of tendon repair using muscle grafts transduced with TGF-B1 CDNA. Eur Cell Mater 16,94, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.