Abstract
Hyperoxia exposure can inhibit alveolar growth in the neonatal lung through induction of p21/ p53 pathways and is a risk factor for the development of bronchopulmonary dysplasia (BPD) in preterm infants. We previously found that activation of nuclear factor erythroid 2 p45-related factor (Nrf2) improved survival in neonatal mice exposed to hyperoxia likely due to increased expression of anti-oxidant response genes. It is not known however, whether hyperoxic induced Nrf2 activation attenuates the growth impairment caused by hyperoxia in neonatal lung. To determine if Nrf2 activation modulates cell cycle regulatory pathway genes associated with growth arrest we examined the gene expression in the lungs of Nrf2−/− and Nrf2+/+ neonatal mice at one and three days of hyperoxia exposure.
Methods
Microarray analysis was performed in neonatal Nrf2+/+ and Nrf2−/− lungs exposed to one and three days of hyperoxia. Sulforaphane, an inducer of Nrf2 was given to timed pregnant mice to determine if in utero exposure attenuated p21 and IL-6 gene expression in wildtype neonatal mice exposed to hyperoxia.
Results
Cell cycle regulatory genes were induced in Nrf2−/− lung at one day of hyperoxia. At 3 days of hyperoxia, induction of cell cycle regulatory genes was similar in Nrf2+/+ and Nrf2−/− lungs, despite higher inflammatory gene expression in Nrf2−/− lung.
Conclusion
p21/ p53 pathways gene expression was not attenuated by Nrf2 activation in neonatal lung. In utero SUL did not attenuate p21 expression in wildtype neonatal lung exposed to hyperoxia. These findings suggest that although Nrf2 activation induces expression of antioxidant genes, it does not attenuate alveolar growth arrest caused by exposure to hyperoxia.
Keywords: Alveolar growth inhibition, cell cycle regulatory genes, inflammation, hyperoxia, chronic lung disease of prematurity, bronchopulmonary dysplasia neonatal lung, nuclear factor erythroid 2 p45-related factor
Introduction
Hyperoxia is commonly used to improve oxygenation in neonates with acute lung injury. However, exposure to hyperoxia can impair alveolar growth during a critical period of postnatal lung development and lead to structural and functional changes in the mature lung. 1,2,3 Exposure to hyperoxia has also been shown to be major risk factor for the development of chronic lung disease of prematurity also known as bronchopulmonary dysplasia (BPD). 4
Recent multicenter analyses have found that lower target oxygen saturations can reduce the incidence of retinopathy of prematurity and BPD. However lower target oxygen saturations have been shown to be associated with higher mortality, in very low birth weight infants. 5 This study and others, suggest that supplemental oxygen will continue to be used as an intervention for premature infants, despite it being a major risk factor for the development of chronic lung disease in this population. 6
Neonatal mice are commonly used to model BPD. Similar to the human infant, neonatal mice undergo significant postnatal alveolar growth following delivery.7 Hyperoxic-exposure in the neonatal mouse can cause inhibition of alveolar growth similar to that found with BPD, through induction of p21 and p53 cell cycle regulatory genes.2,3,8,9–11 We previously have shown that neonatal mice with Nrf2 null mutations have increased lung inflammation and decreased survival when exposed to hyperoxia. 12 Since alveolar growth inhibition and airway inflammation are characteristic features of BPD, we were also interested in understanding the temporal impact of Nrf2 activation on cell cycle regulatory gene expression. Recently, Cho and colleagues, used microarray gene profiling and computerized algorithms in Nrf2 mutant null mice to identify Nrf2-mediated mechanisms that may influence alveolar development and hyperoxia-induced lung injury in neonatal mice.13 Along those lines, strategies to induce activation of Nrf2 have been proposed as potential adjuvant therapies for the treatment of chronic obstructive pulmonary disease (COPD) and possibly BPD. Indeed it has been shown that sulforaphane (SUL), an inducer of Nrf2, can reduce inflammation in wildtype Nrf2+/+ mice exposed to chronic cigarette smoke. 14 However, the utility of Nrf2 inducers in BPD is less clear particularly since it is unknown whether hyperoxic growth inhibition can be attenuated by Nrf2 activation in the neonatal lung, independent of its potential modulatory effect on lung inflammation.
In this study we hypothesized that Nrf2 activation will not attenuate hyperoxic induced alveolar growth arrest in the lungs of neonatal mice and will not attenuate the induction of cell cycle regulatory genes despite an increased expression of Nrf2 inducible anti-oxidant genes. To test this hypothesis we examined gene expression in lungs of neonatal Nrf2+/+ and Nrf2−/− mice exposed to one or three days of 85–90% hyperoxia and analyzed results using ingenuity pathway software. We also measured the expression of p21 and IL-6 in Nrf2+/+ neonatal mice exposed to prenatal SUL and 3 days of postnatal hyperoxia using RT-PCR to assess the impact of prenatal SUL on markers of lung inflammation and growth arrest.
Methods
Nrf2−/− transgenic mice were generated as previously described15 and bred into a CD-1 background (in the laboratory of S. Biswal). Timed pregnant wildtype CD-1 mice for the SUL experiments were obtained from Charles River Laboratories International, Inc. (251 Ballardvale Street, Wilmington, MA). All experiments were conducted in accordance with the standards established by the United States Animal Welfare Acts, set forth in NIH guidelines, and the Policy and Procedures Manual of the Johns Hopkins University Animal Care and Use Committee.
Hyperoxia
Newborn mice were kept in room air until 24 hours of age and then placed in a hyperoxia chamber (85–90%). Nursing mothers were rotated every 24 hours to prevent injury from acute oxygen toxicity. Excess CO2 was absorbed using anhydrous calcium sulfate (Drierite # 23001). Newborn mice were exposed to one or three days of hyperoxia and then sacrificed immediately on removal from the hyperoxia chamber.
Sulforaphane
Pregnant Nrf2+/+ mice were treated with Sulforaphane (LKT Laboratories, Inc. 545 Phalen Blvd., St. Paul, MN) (SUL), based on a previous published protocol studying the effects of Nrf2 activation in neonatal mice with epidermolysis bullosa simplex.16 SUL was administered by intra-peritoneal injection to pregnant-timed mice at gestational days of 13, 15, and 17. Mice received 5 micromoles/dose of SUL diluted in 300 microliters of sterile PBS as previously described.16 Control mice were given 300 microliters of sterile PBS.
Lung Fixation and Morphometry
Lungs were infused through the trachea with 0.5% lowmelting agarose, fixed overnight in 4% paraformaldehyde, paraffin-embedded and cut into 5 µm sections. Lung sections were stained with hematoxylin and eosin. Ten randomly chosen areas from each lung section were photographed with the 10x objective of a Nikon Eclipse 80i microscope (Nikon Instruments Inc., Melville, NY) as previously described.3 Mean airspace chord length (MCL) was measured from each image using NIS-Elements AR (Nikon Instruments Inc., Melville, NY). The software allowed for manual identification and exclusion of large airways and vessels prior to MCL calculations.
Western
25ug of lung protein was loaded, transferred and blocked in 5% milk in TBS-T. Primary antibodies were incubated overnight (p21, BD Pharmingen, catalog # 556431, 1:2000 dilution, Xedar, Sigma, catalog# WH0060401M2, 1:3000 dilution, β-actin, Abcam, catalog#:ab8227 1:10,000 dilution). Secondary antibody (horse anti-mouse IgG,HRP-linked antibody, Cell signaling, catalog #,7076, 1:10,000 concentration) was incubated for 1 hour in 5% milk in TBS-T, washed and developed.
Microarray data analysis
Lung was isolated from Nrf2+/+ and Nrf2−/− mice and used for gene expression profiling. Total RNA was isolated from lung tissue using the RNeasy kit (Qiagen). Total lung RNA (n=3 for each group, for a total of 24 specimens) was applied to mouse gene 1.0 ST arrays from Affymetrix. Microarray data was imported as CEL files into Genomic Suite Software (Partek, St. Louis, MO), Robust Multichip Analysis (RMA) background was corrected for GC content and quantile normalization and median polish were used for probe summarization. Principal component analysis was performed on the analyzed gene expression data. Array data was analyzed using Ingenuity Pathway analysis (IPA; Ingenuity Systems Inc.) Differentially expressed genes were considered significant if p-value (P) ≤ 0.05 at a fold change (FC) ≥ 1.5 with a FDR<0.05.
Quantitative RT-PCR (QRT-PCR) Analysis
Reverse transcription was performed using total RNA and processed with the SuperScript first-strand synthesis system for RT-PCR according to the manufacturer’s protocol (Invitrogen). QRT-PCR was performed using the Applied Biosystems (Foster City, CA) TaqMan assay system, as previously described. 3 Probes and primers were designed and synthesized by Applied Biosystems (cyclin-dependent kinase inhibitor 1A and interleukin 6). The GADPH gene was used an internal endogenous control. Differences in measured variables between treated and control groups were determined using Student’s t test (two-tailed, equal variance). Statistical significance was accepted at p<0.05, error bars reflect standard error of the mean.
Results
Nrf2 status and cell cycle regulatory and inflammatory pathway gene expression in neonatal lung after 3 days of hyperoxia
Since alveolar growth inhibition and lung inflammation are characteristic of BPD 17,18 we were interested in determining if Nrf2 status influenced the expression of cell cycle regulatory and inflammatory pathway genes in neonatal mice exposed to hyperoxia. To address this question we exposed neonatal Nrf2−/− and Nrf2+/+ mice to one or three days of hyperoxia starting at 24 hours of age. All mice were immediately sacrificed following removal from hyperoxia to assess differential gene expression by microarray. Assessment was done immediately after removal from hyperoxia, based on our previous finding that neonatal CD-1 Nrf2−/−mice had a high mortality during the first 24 hours of room air recovery after 3 days of hyperoxia.12 Control mice were kept in room air and were matched for age, background and genotype.
As expected, hyperoxia caused a marked induction of Nrf2 inducible genes in the lungs of wildtype mice, in contrast to that of Nrf2−/− mice. After 3 days of hyperoxia, expression of glutathione S-transferase alpha was 5.3 fold greater, glutathione peroxidase 2 was 5.1 fold greater and NAD(P)H dehydrogenase, quinine 1 was 3.0 fold greater in the Nrf2+/+ lung above that of Nrf2−/− lung (Table 1). Glutathione peroxidase 3, a gene not regulated by Nrf2 19 was found to be equally induced in the lungs of Nrf2−/− and Nrf2+/+ hyperoxia exposed mice (2.16 and 2.26 fold respectively above room air controls).
Table 1.
Fold change of Nrf2 inducible genes after 3 days of hyperoxia
| Nrf2 inducible genes | Gene Symbol | FC of Nrf2+/+(3dO2) above Nrf2−/− (3dO2) |
|---|---|---|
| Glutathione S-transferase, alpha 3 | Gsta3 | 5.33 |
| Glutathione peroxidase 2 | Gpx2 | 5.16 |
| Aldo-keto reductase family 1, member B1 | AKR1B1 | 3.61 |
| NAD(P)H dehydrogenase, quinone 1 | Nqo1 | 3.04 |
| Aldehyde oxidase 1 | Aox1 | 2.80 |
| Aldehyde dehydrogenase family 1, subfamily A1 | Aldh1a1 | 2.65 |
| Aldehyde oxidase 3 | Aox3 | 2.59 |
| Glutathione S-transferase, mu 5 | Gstm5 | 2.49 |
| Pirin | Pir | 2.39 |
| Carboxylesterase 1g | Ces1g | 2.15 |
| Carboxylesterase 1e | Ces1e | 2.10 |
| Carbonyl Reductase 2 | Cbr2 | 2.07 |
| Microsomal glutathione S-transferase 1 | Mgst1 | 1.88 |
| Aldo-keto reductase family 1, member B10 | AKR1B10 | 1.75 |
| Glutathione S-transferase, alpha 4 | Gsta4 | 1.62 |
Numbers indicate fold changes (FC) found in lungs of Nrf2+/+ neonatal mice exposed to 3 days (3d) of hyperoxia (O2) above that of lungs of Nrf2−/− mice exposed to 3dO2.
After one day of hyperoxia, X-linked ectodermal dysplasia receptor (Xedar) and p21, were the most highly induced genes in the lungs of Nrf2+/+ and Nrf2−/− mice (Supplemental Table 1). These genes have been shown to regulate cell cycle progression and are p53 mediated. Interestingly, 3 days of hyperoxia caused similar induction of cell cycle regulatory genes in both Nrf2+/+ and Nrf2−/− neonatal mice, despite higher induction of inflammatory genes in the Nrf2−/− mice (Table 2 and supplemental Table 2). We then examined protein expression of p21 and Xedar in the lungs of Nrf2+/+ and Nrf2−/− mice. In the Nrf2+/+ mice exposed to hyperoxia, expression of lung p21 and Xedar protein was significantly increased above room air controls. In the lungs of Nrf2−/− mice exposed to hyperoxia, p21 but not Xedar was significantly increased above room air controls. There was no significant difference in lung p21 and Xedar protein expression between Nrf2+/+ and Nrf2−/− neonatal mice exposed to hyperoxia (Figure 1).
Table 2.
Fold change of cell cycle regulatory genes mediated by p53 after three days of hyperoxia
| Cell cycle regulatory and apoptosis genes |
Gene symbol |
FC of Nrf2−/−3dO2 lung above Nrf2−/−3dRA |
FC of Nrf2+/+3dO2 lung above Nrf2+/+3dRA |
Description |
|---|---|---|---|---|
| X-linked ectodermal dysplasia receptor | Xedar/ TNFRSF27 |
19.03 | 19.52 | Inhibits cell growth/apoptosis |
| Cyclin-dependent kinase inhibitor 1A | p21 | 8.67 | 10.11 | G1 checkpoint regulator |
| Cyclin G1 | Ccng1 | 4.64 | 4.73 | Cell cycle inhibitor |
| Zinc finger matrin type 3 | Zmat3/Wig1 | 4.48 | 4.86 | Inhibits tumor growth |
| Proline/serine-rich coiled-coil 1 | Psrc1/Dda3 | 5.13 | 4.67 | Promotes cell growth |
| Pleckstrin homology-like domain, family A, member 3 |
PHLDA3 | 3.66 | 3.05 | Regulates AKT |
| Adenylate kinase 1 | AK1 | 3.79 | 3.57 | Growth regulation |
| Sestrin 2 | Sesn2 | 3.35 | 2.99 | Inhibits mTOR |
| Ribosomal protein S27-like | Rps27L | 2.71 | 2.31 | Positively regulates p21 |
| Growth differentiation factor 15 | Gdf15 | 3.48 | 2.79 | Induced in response to stress |
| Glycoprotein (transmembrane) nmb | GPNMB | 3.64 | 2.23 | Growth delay |
| G2 S phase expressed protein 1 | Gtse1 | 2.49 | 2.08 | Regulates p21 stability |
| Transformation related protein 53 inducible nuclear protein 1 |
Trp53inp1 | 2.42 | 2.04 | Regulates cell progression |
| B-cell translocation gene 2, anti- proliferative |
BTG2 | 2.31 | 1.57 | Inhibits cyclin D1 |
| Tumor necrosis factor receptor superfamily, member 10b |
Tnfrsf10b | 3.05 | 3.22 | Apoptosis |
| Apoptosis enhancing nuclease | Aen | 3.84 | 3.16 | Exonuclease involved in apoptosis induction |
| Nuclear protein 1 | Nupr1/p8 | 2.30 | 2.63 | Regulates cell cycle/ apoptosis |
| BCL2-associated x protein | Bax | 2.80 | 2.69 | Apoptosis |
| Prostaglandin-endoperoxide synthase 2 | Ptgs2 | 4.71 | 2.20 | Apoptosis |
| Solute Carrier Family 19, Member 2 | SLC19A2 | 4.81 | 3.77 | Thiamin transporter protein |
| Sulfatase 2 | SULF2 | 4.56 | 2.30 | Regulates signaling pathways |
Numbers indicate fold changes in lungs of Nrf2−/−mice and Nrf2+/+ neonatal mice exposed to 3dO2 above that of Nrf2−/− and Nrf2+/+ RA controls. Fold changes of 1.5 and above/below are significant, p<0.05.
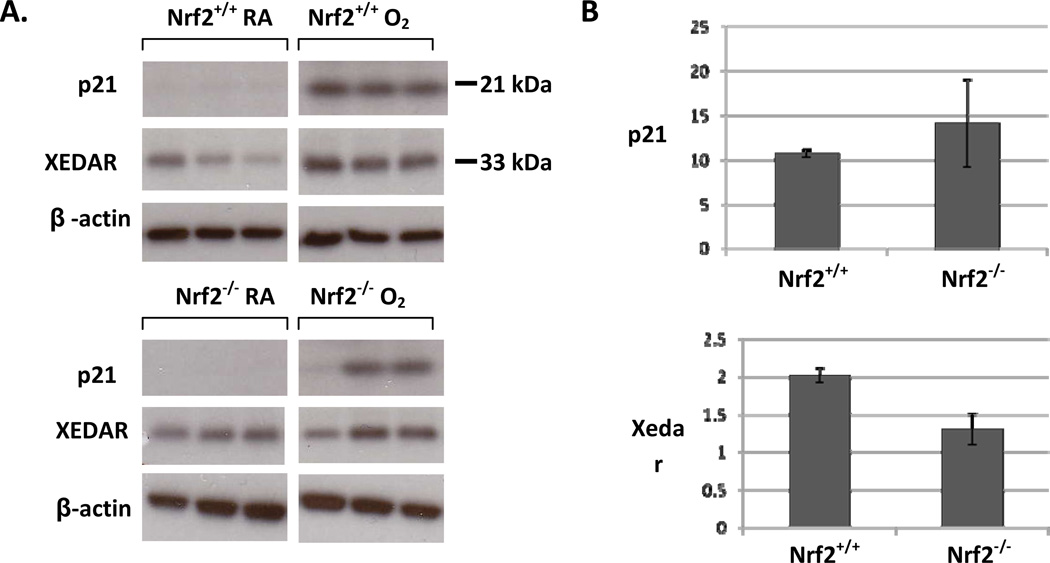
Figure 1. Induction of p21 and Xedar in the lungs of neonatal Nrf2+/+ and Nrf2−/−mice exposed to 3 days of hyperoxia.
A. Representative examples of western blots using lung homogenate from Nrf2+/+ and Nrf2−/− neonatal mice. Increased protein expression of p21 and Xedar found in lung from Nrf2 +/+ and Nrf2−/− neonatal mice exposed to 3 days of hyperoxia. B. Quantification of protein expression normalized to room air controls by densitometry. Significant differences were found between Nrf2 +/+ O2 and Nrf2+/+ RA for p21 and Xedar respectively (p<0.0001 and p<0.0001) and Nrf2 −/− O2 and Nrf2 −/− RA for p21 (p<0.03) but not Xedar. No differences in p21 or Xedar expression were found between Nrf2+/+ O2 and Nrf2−/− O2, n=5–6
Since induction of cell cycle regulatory genes, including p21 have been associated with alveolar growth inhibition in neonatal mice, 11 we then measured mean chord lengths (MCLs) in Nrf2+/+ and Nrf2−/− neonatal mice exposed to hyperoxia and room air to assess the effect of hyperoxia on alveolar growth with regard to Nrf2 status. We found that Nrf2+/+ and Nrf2−/− neonatal mice exposed to 3 days of hyperoxia had larger and more simplified appearing alveoli compared to room air controls and there was no difference in MCL measurements between Nrf2+/+ and Nrf2−/− neonatal mice exposed to 3 days of hyperoxia, (Figure 2).
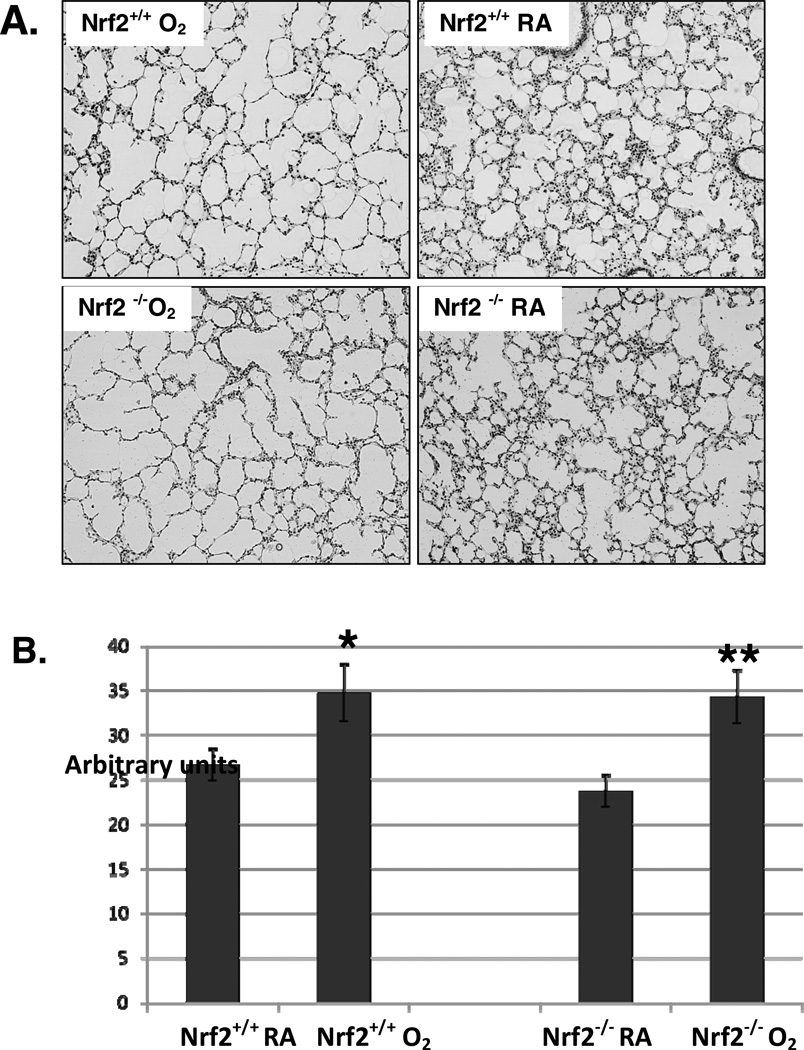
Figure 2. Enlarged and fewer alveoli in four day old Nrf2+/+ and Nrf2−/−mice exposed 3 days of hyperoxia.
A. Representative examples of lung histology from Nrf2+/+ and Nrf2−/− neonatal mice exposed to 3 days of hyperoxia or room air. The Nrf2+/+ and Nrf2−/− exposed to hyperoxia had fewer and more simplified appearing alveoli compared to room air controls (20X magnification). B. Mean chord lengths (MCLs) of Nrf2+/+ and Nrf2−/− neonatal mice in room air and after 3 days of hyperoxia. Nrf2+/+ O2 mice had significantly larger MCLs compared to room air Nrf2+/+controls (*p<0.046) and Nrf2−/− O2 mice had significantly larger MCLs compared to room air Nrf2−/− controls (**p<0.028). There was no difference between Nrf2+/+ O2 and Nrf2−/− O2 mice, n=3–6, .
In utero exposure to SUL in Nrf2+/+ neonatal mice after 3 days of hyperoxia
Induction of pro-inflammatory cytokine, IL6 has been shown to be associated with increased levels of reactive oxygen species.20 Although IL6 was only minimally induced by microarray in Nrf2+/+ lung exposed to 3 days of hyperoxia, we previously found a modest but significant increase in IL6 expression in neonatal wildtype lung exposed to 3 days of hyperoxia using RT-PCR.12 To this end we treated pregnant wildtype mice with SUL, an inducer of Nrf2, to determine if in utero exposure to SUL would attenuate lung IL-6 expression in offspring exposed to hyperoxia (O2). By real-time PCR, we found significantly greater expression of lung IL6 in PBS-treated Nrf2+/+ mice exposed to postnatal O2 compared to SUL-treated Nrf2+/+ mice exposed to postnatal O2 (p<0.01). SUL treatment however, had no effect in attenuating the expression of p21 in the lungs of either PBS or SUL neonatal mice exposed to hyperoxia. Lung p21 was significantly induced in both PBS and SUL-treated O2 exposed mice with no significant difference between the two groups (p<0.15) (Figure 3). Although total cell counts in the BAL of the PBS-treated O2 mice trended towards higher numbers, we found no significant difference in BAL cell counts between the PBS and SUL-treated O2 exposed mice. Also we did not find significant differences between PBS and SUL treated O2 exposed neonatal mice with regard to IL6 protein expression in the BAL or lung homogenate (data not shown).
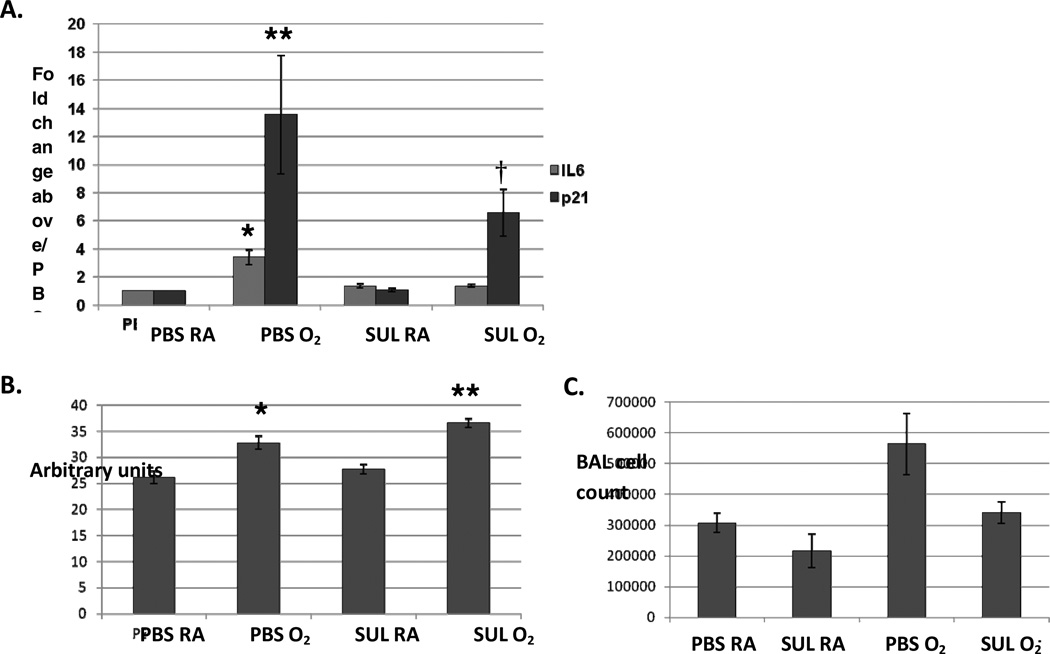
Figure 3. In uteroSUL exposure attenuated induction of lung IL6 but not p21 expression in neonatal wildtype (Nrf2+/+) mice exposed to 3 days of postnatal hyperoxia.
A. Lung IL6 expression by real-time PCR was significantly greater in neonatal wildtype mice treated with in utero PBS and 3 days of postnatal O2 (PBS O2) compared to neonatal wildtype mice treated with in utero SUL and 3 days of postnatal O2 (SUL O2) (* p<0.01). Expression of p21 was significantly higher in PBS O2 and SUL O2 mice compared to PBS RA and SUL RA controls (**, † p<0.03). No significant difference, in p21 expression was found between PBS O2 and SUL O2 mice, n=5–6. B. Mean chord lengths (MCLs) of PBS RA, PBS O2, SUL RA and SUL O2 were measured in neonatal mice. MCLs of PBS O2 and SUL O2 mice were significantly larger compared to PBS RA and SUL RA mice (p<0.02 and p<0.001, respectively). There were no significant differences between PBS RA and SUL RA (p<0.37) or PBS O2 and SUL O2 mice, (p<0.06), n=3. C. There was a trend towards higher number of total cell counts in the bronchoalveolar lavage of PBS-treated O2 compared to SUL-treated O2 mice (p<0.10), n=3.
Discussion
Alveolar growth inhibition and lung inflammation are common features of BPD. Interventions that minimize the impact of hyperoxia on growth inhibition and inflammation in neonatal lung may prevent long-term respiratory sequelae. Gene profiling was performed on Nrf2−/− and Nrf2+/+ lung to examine the influence of Nrf2 status on cell cycle regulatory and proflammatory gene expression in neonatal mice exposed to hyperoxia. At one day of hyperoxia, greater expression of the cell cycle regulatory genes Xedar 21 and p2110 were found in the lungs of neonatal Nrf2−/− mice. However, at 3 days of hyperoxia, expression of cell cycle regulatory genes, including p21 and Xedar were equally induced in both Nrf2−/− and Nrf2+/+ lung despite higher expression of inflammatory pathway genes in Nrf2−/− lung. These findings suggest that Nrf2 induction can attenuate hyperoxia-induced lung inflammation but may be less effective in attenuating alveolar growth inhibition, particularly with longer exposures to hyperoxia.
In response to an oxidative stress such as hyperoxia, the induction of p21 and other p53-mediated cell cycle regulatory genes can help preserve the integrity of the genome by limiting progression of the cell into S phase and mitosis.11,22 The p21senescence pathway may also be activated by unrepaired double stranded DNA breaks from reactive oxygen species and p53 accumulation. 23 Therefore, growth inhibition and senescence through induction of cell cycle regulatory genes may help preserve genomic integrity in neonatal lung exposed to hyperoxia but may also impair alveolar growth during a critical period of development. Indeed, although we found that genes involved in cell cycle regulation were induced at one day of hyperoxia in the lungs of Nrf2−/− mice, by 3 days of hyperoxia the lungs of Nrf2−/− and Nrf2+/+ mice had similar induction of these genes. Supporting this was an earlier study in which we found that p21 was similarly induced in neonatal Nrf2+/+ and Nrf2−/− lung exposed to 3 days of hyperoxia. 12
We previously reported that neonatal O2 exposed Nrf2+/+ mice had better survival compared to neonatal O2 exposed Nrf2−/− mice. In our present study we found that lung IL-6 mRNA expression was significantly higher in neonatal O2 exposed Nrf2−/− mice compared to neonatal O2 exposed Nrf2+/+ mice, but we were unable to find differences at the protein level. Nevertheless Choo-Wing and colleagues reported that neonatal IL6 transgenic mice had markedly worse survival in hyperoxia 24 similar to what we found in neonatal Nrf2−/− O2 exposed mice. In their study they also reported that preterm infants with respiratory distress syndrome had higher levels of tracheal IL6 levels compared to controls. Another study reported that the Nrf2 activator CDDO-IM decreased IL-6 levels in LPS-treated peritoneal neutrophils. 25 We speculate that the induction of Nrf2 responsive anti-oxidant genes attenuates the induction of inflammatory pathway genes such as IL6 in the Nrf2+/+ mice exposed to hyperoxia and that this in turn may help to improve survival. Similarly Cho and colleagues noted augmented lung injury and decreased survival of Nrf2−/− mice with prolonged hyperoxia.13 Taken together these studies suggest that Nrf2 activation may limit injury by attenuating hyperoxia-induced inflammation in the lungs of neonates exposed to hyperoxia.
A recent study in neonatal mice found that hyperoxia caused epigenetic changes which induced p21 expression, alveolar growth inhibition and cell senescence.26 Interestingly in this study azithromycin a drug that has both anti-inflammatory and antimicrobial properties was not effective in attenuating the alveolar hypoplasia caused by hyperoxia. In addition, clinical studies in which preterm infants at risk for BPD were treated with the anti-oxidant n-acetylcysteine failed to show a decrease in incidence of BPD in those treated with n-acetylcysteine. 27 28 It is possible that these studies 26,27,28 were not beneficial because n-acetylcysteine and/or azithromycin does not attenuate the induction of regulatory genes associated with impaired alveolar growth in BPD caused by hyperoxia. Indeed the lack of efficacy of these animal and human studies suggests that anti-oxidant and/or anti-inflammatory interventions alone cannot attenuate alveolar growth inhibition in the BPD lung. When we gave SUL to pregnant mice we found that in utero exposure attenuated IL-6 expression in the lungs of neonatal mice exposed to hyperoxia, but SUL did not decrease p21 expression or mitigate the effect of hyperoxia on alveolar growth inhibition. Our findings indicate that SUL has other complex biological effects that may have influenced the results of our study. For example SUL has been reported to have growth inhibitory effects.29 While this may be potentially beneficial as an anti-carcinogenic therapy, in the neonate SUL may potentially interfere with critical postnatal alveolar growth in the developing lung. Nevertheless, SUL or other selective Nrf2 inducers could potentially be useful as adjuvant therapies in preventing or modulating the severity of BPD by decreasing lung inflammation. Studies examining the long term effects of in utero Nrf2 inducers on alveolar growth, lung inflammation, lung function and structure and overall survival would be helpful in determining the utility of these agents.
Our study is similar to Cho and colleagues and our microarray analysis revealed similar results with regard to the hyperoxia experiments. Some differences however exist in our methodology and focus. We used a different background of mice and we exposed mice to 85–92% for one or three days of exposure rather than 100% hyperoxia for up to 3 days. We limited our interpretation of the data to pathways known to be dysregulated in BPD, specifically pathways involved in cell cycle regulation and inflammation. Cho and colleagues, in turn reported on many cellular pathways that were differentially expressed in lungs of neonatal Nrf2−/−mice exposed to 100% hyperoxia compared to wildtype lung. Redox homeostasis and ARE-anti-oxidant pathways were among some of the pathways they found to be suppressed, while endocytosis, transport and developmental pathways were noted to be induced in Nrf2−/− lung. Although informative, further interpretation of the relationships and clinical significance of this data with regard to the multiple pathways found to be differentially regulated is required to determine if these pathways are relevant with regard to BPD development or severity.
In summary using transgenic mice, we found that Nrf2 activation appears to attenuate the induction of inflammatory gene expression, but not cell cycle regulatory gene expression in a model of BPD in neonatal mice. We also found that in utero exposure to SUL helped attenuate lung inflammation in neonatal mice exposed to hyperoxia but not alveolar growth arrest. These findings suggest that caution should be exercised in using Nrf2 inducers such as SUL as a therapy for chronic lung disease in the neonate. For instance, although inflammation in the neonatal lung may be attenuated, growth inhibition during a period of rapid postnatal alveolar growth may be exacerbated or not improved by induction of Nrf2. Further study evaluating the efficacy of Nrf2 inducers is warranted in infants with chronic lung disease before clinical use of this strategy should be recommended.
Supplementary Material
Highlights.
Nrf2 activation attenuates inflammatory gene expression in hyperoxia exposed neonatal lung
Induction of cell cycle regulatory genes in chronic hyperoxia is Nrf2 independent in neonatal lung
Nrf2 inducers may limit neonatal lung injury in hyperoxia by mediating lung inflammation
Acknowledgments
This work was funded by Flight Attendant Medical Research Institute Clinical Innovator Award (SM), COPD SCCOR grant, P50HL084945 (S.B and SM) and the Grace Anne Dorney Fund
Abbreviation List
- CLDP
Chronic lung disease of prematurity
- Nrf2
Nuclear factor erythroid 2 p45-related factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Bagchi A, Viscardi RM, Taciak V, Ensor JE, McCrea KA, Hasday JD. Increased activity of interleukin-6 but not tumor necrosis factor-alpha in lung lavage of premature infants is associated with the development of bronchopulmonary dysplasia. Pediatr Res. 1994;36:244–252. doi: 10.1203/00006450-199408000-00017. [DOI] [PubMed] [Google Scholar]
- 2.Warner BB, Stuart LA, Papes RA, Wispe JR. Functional and pathological effects of prolonged hyperoxia in neonatal mice. Am J Physiol. 1998;275:L110–L117. doi: 10.1152/ajplung.1998.275.1.L110. [DOI] [PubMed] [Google Scholar]
- 3.McGrath-Morrow SA, Lauer T, Collaco JM, Yee M, O’Reilly M, Mitzner W, et al. Neonatal Hyperoxia Contributes Additively to Cigarette Smoke-induced COPD Changes in Adult Mice. Am J Respir Cell Mol Biol. 2011 doi: 10.1165/rcmb.2010-0259OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chess PR, D’Angio CT, Pryhuber GS, Maniscalco WM. Pathogenesis of bronchopulmonary dysplasia. Semin Perinatol. 2006;30:171–178. doi: 10.1053/j.semperi.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Saugstad OD, Aune D. In search of the optimal oxygen saturation for extremely low birth weight infants: a systematic review and meta-analysis. Neonatology. 2011;100:1–8. doi: 10.1159/000322001. [DOI] [PubMed] [Google Scholar]
- 6.Stenson B, Brocklehurst P, Tarnow-Mordi W. Increased 36-week survival with high oxygen saturation target in extremely preterm infants. N Engl J Med. 2011;364:1680–1682. doi: 10.1056/NEJMc1101319. [DOI] [PubMed] [Google Scholar]
- 7.Dauger S, Ferkdadji L, Saumon G, Vardon G, Peuchmaur M, Gaultier C, et al. Neonatal exposure to 65% oxygen durably impairs lung architecture and breathing pattern in adult mice. Chest. 2003;123:530–538. doi: 10.1378/chest.123.2.530. [DOI] [PubMed] [Google Scholar]
- 8.Yee M, White RJ, Awad HA, Bates WA, McGrath-Morrow SA, O’Reilly MA. Neonatal hyperoxia causes pulmonary vascular disease and shortens life span in aging mice. Am J Pathol. 2011;178:2601–2610. doi: 10.1016/j.ajpath.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corroyer S, Maitre B, Cazals V, Clement A. Altered regulation of G1 cyclins in oxidant-induced growth arrest of lung alveolar epithelial cells. Accumulation of inactive cyclin E-DCK2 complexes. J Biol Chem. 1996;271:25117–25125. doi: 10.1074/jbc.271.41.25117. [DOI] [PubMed] [Google Scholar]
- 10.O’Reilly MA, Staversky RJ, Watkins RH, Maniscalco WM. Accumulation of p21(Cip1/WAF1) during hyperoxic lung injury in mice. Am J Respir Cell Mol Biol. 1998;19:777–785. doi: 10.1165/ajrcmb.19.5.3200. [DOI] [PubMed] [Google Scholar]
- 11.McGrath SA. Induction of p21WAF/CIP1 during hyperoxia. Am J Respir Cell Mol Biol. 1998;18:179–187. doi: 10.1165/ajrcmb.18.2.2964m. [DOI] [PubMed] [Google Scholar]
- 12.McGrath-Morrow S, Lauer T, Yee M, Neptune E, Podowski M, Thimmulappa RK, et al. Nrf2 increases survival and attenuates alveolar growth inhibition in neonatal mice exposed to hyperoxia. Am J Physiol Lung Cell Mol Physiol. 2009;296:L565–L573. doi: 10.1152/ajplung.90487.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho HY, van HB, Wang X, Miller-Degraff L, Fostel J, Gladwell W, et al. Targeted Deletion of Nrf2 Impairs Lung Development and Oxidant Injury in Neonatal Mice. Antioxid Redox Signal. 2012 doi: 10.1089/ars.2011.4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harvey CJ, Thimmulappa RK, Sethi S, Kong X, Yarmus L, Brown RH, et al. Targeting Nrf2 signaling improves bacterial clearance by alveolar macrophages in patients with COPD and in a mouse model. Sci Transl Med. 2011;3:78ra32. doi: 10.1126/scitranslmed.3002042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 16.Kerns ML, DePianto D, Dinkova-Kostova AT, Talalay P, Coulombe PA. Reprogramming of keratin biosynthesis by sulforaphane restores skin integrity in epidermolysis bullosa simplex. Proc Natl Acad Sci U S A. 2007;104:14460–14465. doi: 10.1073/pnas.0706486104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wright CJ, Kirpalani H. Targeting inflammation to prevent bronchopulmonary dysplasia: can new insights be translated into therapies? Pediatrics. 2011;128:111–126. doi: 10.1542/peds.2010-3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thebaud B, Abman SH. Bronchopulmonary dysplasia: where have all the vessels gone? Roles of angiogenic growth factors in chronic lung disease. Am J Respir Crit Care Med. 2007;175:978–985. doi: 10.1164/rccm.200611-1660PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Westphal K, Stangl V, Fahling M, Dreger H, Weller A, Baumann G, et al. Human-specific induction of glutathione peroxidase-3 by proteasome inhibition in cardiovascular cells. Free Radic Biol Med. 2009;47:1652–1660. doi: 10.1016/j.freeradbiomed.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 20.Michaeloudes C, Sukkar MB, Khorasani NM, Bhavsar PK, Chung KF. TGF-beta regulates Nox4, MnSOD and catalase expression, and IL-6 release in airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2011;300:L295–L304. doi: 10.1152/ajplung.00134.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanikawa C, Ri C, Kumar V, Nakamura Y, Matsuda K. Crosstalk of EDA-A2/XEDAR in the p53 signaling pathway. Mol Cancer Res. 2010;8:855–863. doi: 10.1158/1541-7786.MCR-09-0484. [DOI] [PubMed] [Google Scholar]
- 22.Morgan SE, Kastan MB. p53 and ATM: cell cycle, cell death, and cancer. Adv Cancer Res. 1997;71:1–25. doi: 10.1016/s0065-230x(08)60095-0. [DOI] [PubMed] [Google Scholar]
- 23.Azad A, Jackson S, Cullinane C, Natoli A, Neilsen PM, Callen DF, et al. Inhibition of DNA-dependent protein kinase induces accelerated senescence in irradiated human cancer cells. Mol Cancer Res. 2011;9:1696–1707. doi: 10.1158/1541-7786.MCR-11-0312. [DOI] [PubMed] [Google Scholar]
- 24.Choo-Wing R, Nedrelow JH, Homer RJ, Elias JA, Bhandari V. Developmental differences in the responses of IL-6 and IL-13 transgenic mice exposed to hyperoxia. Am J Physiol Lung Cell Mol Physiol. 2007;293:L142–L150. doi: 10.1152/ajplung.00434.2006. [DOI] [PubMed] [Google Scholar]
- 25.Thimmulappa RK, Scollick C, Traore K, Yates M, Trush MA, Liby KT, et al. Nrf2-dependent protection from LPS induced inflammatory response and mortality by CDDO-Imidazolide. Biochem Biophys Res Commun. 2006;351:883–889. doi: 10.1016/j.bbrc.2006.10.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Londhe VA, Sundar IK, Lopez B, Maisonet TM, Yu Y, Aghai ZH, et al. Hyperoxia impairs alveolar formation and induces senescence through decreased histone deacetylase activity and up-regulation of p21 in neonatal mouse lung. Pediatr Res. 2011;69:371–377. doi: 10.1203/PDR.0b013e318211c917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soghier LM, Brion LP. Cysteine, cystine or N-acetylcysteine supplementation in parenterally fed neonates. Cochrane Database Syst Rev. 2006:CD004869. doi: 10.1002/14651858.CD004869.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahola T, Lapatto R, Raivio KO, Selander B, Stigson L, Jonsson B, et al. N-acetylcysteine does not prevent bronchopulmonary dysplasia in immature infants: a randomized controlled trial. J Pediatr. 2003;143:713–719. doi: 10.1067/S0022-3476(03)00419-0. [DOI] [PubMed] [Google Scholar]
- 29.Chiao JW, Chung FL, Kancherla R, Ahmed T, Mittelman A, Conaway CC. Sulforaphane and its metabolite mediate growth arrest and apoptosis in human prostate cancer cells. Int J Oncol. 2002;20:631–636. doi: 10.3892/ijo.20.3.631. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.