Abstract
The purpose of this study was to address the hypothesis that childhood apraxia of speech (CAS) is influenced by an underlying deficit in sequential processing that is also expressed in other modalities. In a sample of 21 adults from five multigenerational families, 11 with histories of various familial speech sound disorders, 3 biologically related adults from a family with familial CAS showed motor sequencing deficits in an alternating motor speech task. Compared with the other adults, these three participants showed deficits in tasks requiring high loads of sequential processing, including nonword imitation, nonword reading and spelling. Qualitative error analyses in real word and nonword imitations revealed group differences in phoneme sequencing errors. Motor sequencing ability was correlated with phoneme sequencing errors during real word and nonword imitation, reading and spelling. Correlations were characterized by extremely high scores in one family and extremely low scores in another. Results are consistent with a central deficit in sequential processing in CAS of familial origin.
Keywords: sequential processing deficit, motor speech deficit, nonword imitation, nonword decoding, spelling
Speech sound disorder (SSD) is a childhood disorder interfering with the ability to develop speech that is readily understood at the expected age. One proposed subtype of SSD is childhood apraxia of speech (CAS). At present, there is no validated list of unique diagnostic criteria for CAS, although several lists of characteristics have been proposed (Shriberg, Aram, & Kwiatkowski, 1997a, 1997b, 1997c; Stack-house, 1992; Thoonen, Maassen, Gabreels, Schreuder, & de Swart, 1997). Frequently cited speech characteristics of CAS include vowel distortions, difficulty initiating or transitioning between articulatory gestures, lack of differentiation between stressed and unstressed syllables or mis-stressing syllables, distorted substitutions, syllable segregation, schwa insertions, voicing errors, slow rate, slow diadochokinetic (DDK) rates and increased difficulty with multisyllabic words (MSWs; Shriberg, Potter, & Strand, 2011). According to a technical report published in 2007 by the American Speech-Language-Hearing Association (ASHA; www.asha.org/docs/pdf/TR2007-00278.pdf), children with CAS exhibit deficits across several domains including nonspeech oral motor, limb motor, motor speech, articulation, prosody, speech perception, processing of linguistic units larger than phonemes, metalinguistic skills and written language. There is debate whether deficits beyond the level of motor programming for speech production are primary traits of CAS (Marquardt, Jacks, & Davis, 2004) or a secondary effect of the motor programming deficit caused by CAS (McNeil, 1997, 2009). Here, we investigate the hypothesis that the speech traits of CAS, along with the traits in other modalities, are the result of an underlying general deficit in sequential processing.
There is evidence to suggest that individuals with CAS have characteristic difficulty with the temporospatial integration of multiple and complex motor activities and that this deficit resides at a central locus of impairment, affecting motor systems besides the speech system. In two multigenerational families with children who had a CAS diagnosis, slowed alternating, but not repetitive movements were observed in participants with a positive history of speech difficulties, not only in the oral system but also during hand tasks (Peter & Raskind, 2011). This suggests that CAS represents a multimodal motor-based disorder in which complex motor processes requiring the integration of multiple muscle groups are impaired. The fact that this deficit was observed not only in children with a CAS diagnosis but also in biologically related adults with a history of SSD and normalized speech was interpreted as evidence that slowed alternating motor speed is a potential endophenotype of CAS that persists into adulthood. We performed genome-wide parametric and nonparametric linkage analyses for a phenotype defined by a discrepancy greater than 1 standard deviation (SD) between monosyllabic and multisyllabic DDK rates in a family with familial SSD that included two diagnosed cases of CAS (Peter, Matsushita, & Raskind, 2012). Four genomic areas of interest were detected, including one region on chromosome 6p that was recently implicated in rapid naming in families with dyslexia (Konig et al., 2011). Given the limited power provided by the small number of subjects, the emergence of regions of interest is consistent with the notion that CAS represents a biologically based deficit in sequential motor processing in this family.
In a companion paper (Peter, Button, Stoel-Gammon, Chapman, & Raskind, in press), a multi-generational family with familial CAS is described. During articulation testing based on single-word responses, two 3-year-old cousins produced errors that fit many of the proposed characteristics of CAS, including vowel errors, simple syllable shapes and low intelligibility. A qualitative error analysis of these words showed a large number of errors that affected the sequence of sounds in the target words. Most prominent were omissions, followed by insertions, assimilations, migrations and metatheses. Among the other family members with current or past SSD in this family, a similar profile of error types emerged during nonword and MSW imitation tasks, where the frequency of these errors was substantially higher, compared to the family members without a history of SSD. In four standardized tasks involving low loads of sequential processing (repetitive keyboard tapping, monosyllable repetition, nonverbal processing and sight word reading), average scores in the family members with current or past SSD differed to a small or moderate extent from the average scores in the unaffected family members. In nine tasks involving high loads of sequential processing (alternating syllable repetition, verbal processing, rapid alternating naming with and without category switches, three measures of nonword imitation, nonword reading and spelling), the average scores in the affected family members were substantially lower, compared with those in the unaffected family members. This was true even when the motor programming loads were low, suggesting that the locus of impairment in the affected family members included not only motor programming for sequentially complex oral movements but also sequential processing during encoding, storage and/or maintenance in working and long-term memory. The sequential processing deficit hypothesis proposed in the companion study thus models CAS in a broader context than the traditional motor programming framework. Similar to Shriberg, Lohmeier, Strand, and Jakielski (2012), it includes cognitive tiers upstream from motor programming, i.e. encoding and memory processes, but it emphasizes the sequential nature of the processes at each tier. Similar to Klapp’s two-stage model of motor programming (Klapp, 1995, 2003), it predicts higher motor programming loads with more complex motor processes, but it extends the concept of sequential processing beyond the motor system to also encompass linguistic and cognitive processes.
The study of this family provided valuable data including the unremediated speech productions of two preschoolers and performance on a wide variety of tasks by children and adults. The conclusions regarding deficits in sequential processing must be corroborated in other families. In addition, sufficient numbers of individuals within given age ranges were not available to allow correlational analyses among measures incorporating sequential processing. Consequently, the purpose of the present study was to replicate the results from the family study in a sample of adults and to investigate the hypothesis that sequential processing ability is a continuous trait that underlies performance in a variety of modalities. The following research questions are addressed:
Do individuals with sequencing deficits in motor speech tasks also show sequencing deficits in other modalities? If so, these results would replicate the findings in the companion paper (Peter et al., in press).
Are measures that incorporate elements of sequential processing ability across different modalities mutually correlated? If so, this would suggest that sequential processing ability is a continuous trait that affects functions in various modalities.
Method
Participants
This study is part of a larger project to investigate the molecular genetics in multigenerational families with SSD. It was conducted with the approval of the University of Washington’s Human Subject Division. All participants gave written consent.
Families were ascertained through a proband child who met the following requirements: 1) age 5–9 years, 2) positive SSD history, 3) absence of overt neurologic impairments, cognitive impairments or impairments in oral structures and 4) positive family history of SSD as defined by at least two additional biological relatives with a history of SSD. Both biological parents of each proband child participated, as well as additional relatives such as siblings, grandparents, great-grandparents, aunts and uncles.
Data from five families (total number of participants = 57) were queried for this study. Of these, 20 were children and 37 adults. A subset of 25 adults participated in the full test protocol, whereas the remainder only gave DNA samples and questionnaire information. Four of the 25 adults were excluded for the following reasons: non-native speaker of English, oral appliance interfering with DDK testing, history of jaw surgery interfering with DDK testing and history of broken wrist interfering with keyboard tapping performance. The final sample size, hence, was 21. Of these, 11 reported a history of SSD, 9 reported no history of SSD and 1 participant had an uncertain SSD status. Children were excluded to minimize developmental or age effects on qualitative error counts during nonword and MSW imitations, as age-adjusted published norms for qualitative error categories are not available. Participants were assigned a four-digit code, representing family code, generation number and a two-digit individual number.
As previously reported (Peter et al., 2012; Peter & Raskind, 2011), members of two families, 002 and 005, demonstrated evidence of a motor sequencing deficit in the oral and hand motor domains. In both families, the proband had a CAS diagnosis. In Family 002, one of the proband’s three siblings had a CAS diagnosis. Neither his mother, code 2405 (codes are only listed for participants selected for the present study) nor father, code 2404, reported a history of speech problems, but the maternal grandmother, code 2303, reported severe childhood speech difficulties. Her mother, code 2201, the proband’s great-grandmother, did not report a history of childhood speech difficulties. The maternal great-aunt of the proband, code 2203, reported a history of difficulties with speech. The maternal grandmother’s brother had a history of childhood speech difficulties. His adult son, code 2401, reported a childhood history of speech and language delays.
In Family 005, the proband’s father, code 5403, and mother, code 5402, had childhood speech difficulties, and on both sides of the family, biological relatives of the proband reported histories of speech difficulties. The proband’s father’s sister had childhood histories of speech difficulties, as did the proband’s paternal grandmother, code 5308. The proband’s mother reported childhood speech difficulties that had resolved with therapy. Her mother, the proband’s maternal grandmother, reported no speech difficulties and neither did her father, the proband’s maternal grandfather, although his brother had received speech therapy as a child for a brief period. For a more detailed description of all participants, see the previous reports on these five families (Peter et al., 2012; Peter & Raskind, 2011). Note that only families 002 and 005 had children with a CAS diagnosis.
Of the 21 adult participants, 10 were biologically related to a child with CAS; the remaining ones either had married into families 2 and 5 or were members of families 001, 003 and 004 where none of the children had a CAS diagnosis. According to information gathered from questionnaires and interviews, there was strong evidence that at least one of the ten adults biologically related to children with CAS, code 2303, also had a childhood history of CAS. She reported having extreme difficulty being understood by others throughout her childhood and struggling with learning to read. She never received professional interventions and attempted to correct her speech on her own, reporting that even as an adult, she practiced words like “spaghetti” and “pajamas.” Two individuals trained in phonetics rated her conversational regarding intelligibility using a 7-point scale (1 = no noticeable differences from normal, 2 = intelligible though some differences occasionally noticeable, 3 = intelligible although noticeably different, 4 = intelligible with careful listening although some words unintelligible, 5 = speech is difficult to understand with many words unintelligible, 6 = usually is unintelligible, 7 = unintelligible). On this scale, her speech was rated by the two listeners as 2 and 3, respectively. During conversation and responses to test items, she occasionally omitted consonants from consonant sequences (e.g. [lkækəleɾɚ] for “calculator”; [lsɪfəni] for “symphony”) and syllables in MSWs (e.g. [lɹisli] for “recently”; [ldʌktɚ] for “conductor”). Sound reversals ([lkɹapəntɚ] for “carpenter”) and insertions ([skwɝl] for “swirl”) were also noted. Occasional disfluencies in the form of false starts were observed. Intonation and speech rhythm were judged to be within normal limits.
Protocol and data reduction
Study sessions took place in a quiet laboratory room or in a room in a clinic or library facility in cases where participants were willing to participate in the full study protocol but were not able to travel to the University of Washington. All sessions were video and audio-recorded. For the purposes of replication, the measures described in this study are identical or similar to those in the companion paper (Peter et al., in press). To test the hypothesis that global sequencing deficits are associated with CAS, several tasks with high sequential processing loads representing various modalities were administered, including rapid multisyllable repetition, nonword imitation, MSW imitation, nonword reading and spelling. Tasks with low sequential processing loads were administered as well, including rapid monosyllable repetition, repetitive keyboard tapping and untimed sight word reading.
As described in two prior studies reporting on this project (Peter et al., 2012; Peter & Raskind, 2011), participants tapped the spacebar of a laptop computer as many times as possible during a 10-second interval, following published protocols of this activity (Gualtieri & Johnson, 2006). Tap intervals were recorded with a program designed with LabView (National Instruments, Austin, TX, USA). For each hand, five trials were administered, where fatigue effects were minimized by switching hands after each trial. Raw tap interval durations were converted to z scores using norms for ages 5–7 years (Gray, Livingston, Marshall, & Haak, 2000) and 8–83 years (Gualtieri & Johnson, 2006). Participants also tapped two keys with two fingers in alternating manner, but published norms are not available and results are not reported here. All 21 participants completed the keyboard tapping task.
Also as previously described, repetitive and alternating DDK tasks were administered to assess the motor speech ability. Following the methods in Fletcher (1972), participants were instructed to produce series of monosyllables (/pa/, /ta/, /ka/), disyllables (/pata/, /taka/) and trisyllables (/pataka/) as fast as possible. Each DDK trial was preceded by a model and a practice run. At least 20 productions of the monosyllables, 15 of the disyllables and 10 of the trisyllables were collected. In the case of inaccurate production in the disyllable task, the inaccurately produced syllables were included in the calculation of average syllable duration. The syllable durations from the motor speech tasks were measured using the software Praat (version 5.1.25; Boersma, 2001). Both the first token in a series and the last token prior to an inhalation were excluded to minimize nonlinear initiation effects, final lengthening effects and the unreliability of vowel endpoints in open syllables due to variations in the acoustic environment. Inhalations were also excluded, although most participants completed the target set of syllables in one breath. In terms of sequential processing, the multi-syllabic task requires a higher load, compared with the monosyllabic task, at the level of motor programming. DDK data were available for all 21 participants.
Norms for mono- and disyllabic repetitions are available for 6–13 years (Fletcher, 1972). Due to the unavailability of norms for adults, norms from 13-year-olds were used for all participants, even though it is possible that these norms slightly underestimate age-adjusted oral motor speeds (Peter, Matsushita, & Raskind, 2011). These norms were used to calculate z scores for each participant. To observe relative deficits, the z score from the multisyllable durations was subtracted from the z score from the monosyllable durations. A positive discrepancy indicated that monosyllable rates were faster than multisyllable rates, which was interpreted as a relative deficit in motor sequencing. This variable was of particular interest, not only because the focus of this study was on sequencing ability but also because DDK speeds during multisyllable repetitions differed to a much greater extent between the affected and unaffected family members in the companion study than did DDK speeds during monosyllable repetition. A z score difference >1 was used to assign positive affectation status for a motor sequencing deficit (“motor affected”, MA), whereas all others were classified as unaffected with respect to the motor sequencing deficit (“motor unaffected”, MU). In sum, data from 3 MA and 18 MU adults were available. The three MA participants, codes 2201, 2303 and 2401, were from family 002 and biologically related to each other; 2201 was the mother of 2303 and the grandmother of 2401.
The MSW imitation task (Catts, 1986) was administered to observe imitations of multisyllabic real words. Administration and scoring followed the procedures described by Catts. Participants were asked to imitate 20 multisyllabic words with a total sum of 181 phonemes. Stimuli consisted of audio files previously recorded, in this case by a male adult speaker. Imitating multisyllabic real words requires sequential processing at the level of motor programming but less so during encoding and storage in memory, because the words are recognized and retrieved from long-term memory prior to assembling the motor program. Data were available for 16 MU and 3 MA participants. Published norms for this test are not available.
Nonword imitation ability was measured with three word lists representing different phoneme inventories and prosodic characteristics. The Nonword Repetition (NWR) subtest from the Comprehensive Test of Phonological Processing (CTOPP; Wagner, Torgesen, & Rashotte, 1999) is complex in terms of phoneme inventory and word shape. It contains 18 nonwords with a variety of lexical stress patterns and a phoneme inventory of 20 consonants (Cs) including late-developing Cs such as / ɹ, l, ʃ, ʧ, ʥ/ and 13 vowels (Vs), of which 4 were diphthongs. The phonemes in the NWR sum to 147, with an average nonword length of 8.2 phonemes. Word shapes range from simple structures such as single CVC syllables to complex structures such as 6 stressed and unstressed syllables with and without consonant clusters. The NWR responses were scored according to the test guidelines and standard scores were derived from the published norms. The Nonword Repetition Task (NRT; Dollaghan & Campbell, 1998) is less complex than the NWR regarding its phoneme inventory and word shapes. It consists of 16 nonwords where all syllables carry equal stress. Only 11 consonants, 4 monophthongs and 5 diphthongs are arranged into simple syllable shapes (CV or CVC) without C clusters or sequences. Some of the Cs such as /ʃ, ʧ, ʥ/ are late-developing. The sum of NRT target phonemes is 96, and the average nonword length is 6 phonemes. The NRT target words were presented to the participants using a custom audio recording. Z scores were calculated using the normative information in a technical report published online (http://www.waisman.wisc.edu/phonology/BIB/tech.htm). Data for both nonword imitation tasks were available for all 21 participants. The Syllable Repetition Test (SRT; Shriberg et al., 2009) is less complex in terms of phonology. The phoneme set is limited to the vowel /ɑ/, the stops /b, d/ and the nasals /m, n/, which are all early-developing phonemes and seldom produced in error by children with SSD. In sum, 50 target consonants arranged in C + /ɑ/ sequences are sampled, with an average nonword length of 5.6 phonemes. Z scores for the NRT and SRT were calculated using the normative information in a technical report published online (http://www.waisman.wisc.edu/phonology/BIB/tech.htm). The three nonword imitation tasks all require high sequential processing loads during encoding and storage in working memory. The SRT, however, requires lower sequential programming loads when converting the phoneme strings to strings of speech sounds, compared with the NWR and NRT, because of the small phoneme inventory, the invariant vowel and the use of early-developing, unmarked phonemes. NWR, NRT and SRT scores were available for 3, 3 and 2 MA participants, respectively, and 18, 18 and 17 MU participants, respectively.
To observe deficits on the level of phoneme sequences, a qualitative analysis of the imitations during MSW, NWR and NRT was completed. For this purpose, only the first full imitation in each task was considered, even if a participant self-corrected an incorrect response. In analogy to the companion paper (Peter et al., in press), the following error types were tabulated for each participant: 1) assimilation (A), altering a sound so that it is the same as another sound in the target, 2) migration (Mig), shifting a sound from one position to another, 3) metathesis (Met), switching two sounds so that each occupies the position of the other, 4) omission (O), deleting a sound, 5) insertion from within the target (IW), adding a sound that is found elsewhere in the target, 6) insertion from outside the target (IO), adding a sound that is not found elsewhere in the target, 7) substitution (S), replacing a sound with one not found in the target, 8) false start (FS), partially producing a word prior to production of the entire word, 9) hesitation (H), either interrupting production the word by a pause or prolonging a sound in the word and 10) syllable repetition (SR), partially or completely revising a non-initial syllable in the target. Whereas all of these error types altered the phoneme sequence of the target, the assimilations, migrations, metatheses, omissions and insertions were interpreted as most consistent with a sequential rearrangement of the target phonemes. Substitution errors, especially in the nonword imitations, were interpreted as possibly resulting from an incorrect perception of the target. False starts, hesitations and syllable repetitions were interpreted as fluency disruptions reflecting the speaker’s attempt to respond to a perceived error. Additional details and examples for each error type are presented in the companion paper (Peter et al., in press).
Four tests of word reading were administered. Sight words do not consistently follow standard rules of English orthography and reading them requires whole-chunk recognition of the word shape. The Word Identification (WID) subtest of the Woodcock Reading Mastery Tests – Revised (WRMT-R; Woodcock, 1998) is a measure of sight word reading ability under untimed conditions. The Sight Word Efficiency (SWE) subtest of the Test of Word Reading Efficiency (Torgesen, Wagner, & Rashotte, 1999) is designed to evaluate word recognition ability under timed conditions, where participants are instructed to read lists of sight words as rapidly and accurately as possible. The raw score is the number of correctly read words in 45 seconds. Reading sight words under time pressure introduces an element of parallel and simultaneous processing, where speech production for one word overlaps in time with analysis and storage of one or more subsequent words, similar to rapid automatized naming of objects, making temporal integration of multiple perceptual, cognitive, linguistic and motor processes a challenge in this task. Decoding nonwords requires sequentially converting strings of graphemes into phonemes. Graphemes must be processed individually or as sets of digraphs (e.g. “ph” = /f/) or trigraphs (e.g. “ide” = /aɪd/). To observe the performance on a reading task requiring substantial amounts of sequential processing, participants were asked to read nonwords, using the Word Attack (WATT) subtest of the WRMT-R (Woodcock, 1998). The Phonemic Decoding Efficiency subtest (PDE) of the Test of Word Reading Efficiency (Torgesen et al., 1999) evaluates the ability to sound out nonwords that follow standard orthographic rules of English rapidly and accurately. Like the SWE subtest, the PDE subtest measures the number of accurately produced words in 45 seconds. Adding time pressure to this highly sequential task increases the challenge of temporally integrating multiple sensory and memory-related processes. Data were available for 17 MU and 3 MA participants for the WRMT-R and 16 MU and 3 MA participants for the TOWRE.
The spelling subtest from the Wechsler Individual Achievement Test (Wechsler Individual Achievement Test, 1992) measures the ability to spell sight words to dictation. This task requires high loads of sequential processing, as the letter sequence must be stored in long-term memory and retrieved from there, and then converted to a written sequence of letters. The test contains many words that do not follow standard English orthography due to silent letters (“knight”) and ambiguous letter/sound associations (“patients” vs. “patience”), and the letter sequences, hence, must be memorized correctly. Data were available for 17 MU and 3 MA participants.
To include measures of verbal and nonverbal processing ability, two verbal and two nonverbal tasks from the Reynolds Intellectual Assessment Scales (RIAS; Reynolds & Kamphaus, 2003) were administered. During the verbal tasks, participants name terms that fit a verbally presented, multi-part definition (Guess What, GW) and complete verbal analogies (Verbal Reasoning, VRZ). During GW, the order of the listed features frequently proceeds from the more general to the more specific characteristics and, hence, evokes a larger set of possible responses, from which subsets are selected with each additional characteristic. The VRZ requires sequential processing in that the order of the two model items is crucial for finding the analogous term for the test item where only one term is given. The Verbal Intelligence Index (VIX) is a composite standard score capturing both verbal tasks. During the nonverbal tasks, participants identify one pictured item in an array of several that differs from the rest (Odd Item Out, OIO) and identify a missing object in a pictured scene (What’s Missing, WHM). Neither task has high sequential processing loads. The Nonverbal Intelligence Index (NIX) is a composite standard score incorporating both nonverbal tasks. VIX and NIX scores were available for 15 MU and 3 MA participants.
Reliability
The second author collected all data and completed the initial data reduction and standard analysis. Approximately 15% of the data were checked for reliability by a team of undergraduate and graduate students in the Department of Speech and Hearing Sciences at the University of Washington. The mean syllable durations from the mono- and disyllabic production task differed by <1 ms. There were no differences regarding the spelling measure. For the measures of reading and verbal and non-verbal processing, any discrepancies >2 raw score points were resolved by consensus. The error counts for the three imitation measures were tabulated jointly and by consensus by the first and second authors.
Statistical analysis
All standard scores were expressed in units of z scores. To replicate the results in the companion study (Peter et al., in press), group differences between the participants with positive affectation regarding motor sequencing deficits (MA) and the unaffected participants (MU) regarding standardized measures of interest with low sequencing demands (repetitive key tapping, WID, NIX) and high sequencing demands (NWR, NRT, WATT, SWE, PDE, WIAT Spelling, VIX) were evaluated for significance using rank-sum tests. Due to the small sample size in the MA group and the difference in sample sizes between the two groups, parametric testing such as t tests or effect size using Cohen’s d were not feasible.
The qualitative error types within the three error classes (sequencing, substitution, fluency) during the three imitation tasks (MSW, NWR, NRT) were compared descriptively. To create a measure that allowed comparison among the two participant groups and the three imitation tasks, error types were expressed in terms of percent phonemes in error. For instance, the NWR task represents 147 phonemes. A participant who produced four migration errors would obtain a percent sequencing error rate of (4/147) × 100 = 2.7%; in other words, 2.7% of the target phonemes in the NWR task were altered by migration errors. These percentages were summed into the aggregate percentages for each error class, so that a participant whose assimilation, migration, metathesis, omission and insertion errors summed to 14 would obtain a percent phonemes in error score of (14/147) × 100 = 9.5. Furthermore, for each error type and class, per-group averages were calculated. Similar to the standardized measures, the qualitative error types were evaluated for group differences between the MA and MU groups, using rank-sum testing.
For pairwise correlational analyses, the difference score between mono- and multisyllabic DDK ability was selected because it was the variable on which the participants were classified into affected and unaffected groups. The measure of repetitive keyboard tapping was selected as the measure deemed to require the least amount of sequential processing and, hence, would be expected to show the least amount of cross-correlation with measures that incorporate sequential processing. Of those measures deemed to require high levels of sequential processing, two standardized measures with the strongest evidence of MA/MU group differences and two qualitative sequencing measures (MSW, NWR) were selected. The pairwise correlation matrix thus comprised 15 pairwise correlations. These were calculated and described as Pearson correlation coefficients and corresponding p values.
All p values from testing for group differences and correlations are reported as nominal statistics. Bonferroni adjustments for multiple testing, for instance for the group differences in nine standardized tasks, would lead to an adjusted α of 0.0056; however, the assumption of independence is not fulfilled because several of the variables are expected to be mutually correlated. Similarly, the three qualitative error types in the three imitation tasks are based on the same word productions and adjusting for nine tests, leading to an adjusted α of 0.0056, would be overly conservative as well, due to the lack of independence among the measures.
Results
Individual results from the standardized testing are shown in Appendix 1. For individual results from the qualitative error analyses in the two nonword imitation tasks and the MSW imitation task, see Appendix 2.
Appendix 1.
Results from standardized testing, sorted by DDK mono-/multisyllable z score difference.
| Code | Sex | Age | Aff. | DDK monos. | DDK multis. | DDK diff. monos. – multis. | Rep. key tap | NWR | NRT | WID | WATT | SWE | PDE | WIAT Sp. | VIX | NIX |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1203 | M | 39 | N | 1.02 | 1.76 | −0.74 | 0.70 | 0.67 | 1.60 | 0.53 | 1.13 | 0.87 | 1.00 | 1.87 | 1.67 | 1.00 |
| 2404 | M | 33 | N | 0.22 | 0.41 | −0.18 | 0.97 | −0.33 | 2.14 | −0.27 | 0.67 | 0.60 | 0.00 | 0.73 | 0.67 | 1.87 |
| 3204 | M | 18 | N | −0.69 | −0.69 | 0.00 | −0.27 | −0.67 | −2.41 | 0.47 | −0.20 | −0.60 | 1.33 | 0.53 | 1.20 | 1.53 |
| 3203 | F | 21 | N | 1.17 | 1.16 | 0.01 | 0.55 | 0.00 | −0.01 | 0.60 | 1.47 | 0.87 | 1.33 | 1.13 | 0.93 | 1.13 |
| 5402 | F | 28 | N | 1.37 | 1.29 | 0.08 | 1.18 | 0.67 | 2.50 | 0.40 | 0.07 | 0.87 | 1.00 | 1.60 | 1.93 | 1.73 |
| 5407 | M | 47 | N | 1.38 | 1.30 | 0.08 | 0.56 | −1.33 | −3.61 | −0.07 | −0.60 | −0.60 | −1.27 | −0.67 | −0.27 | 0.67 |
| 1204 | M | 45 | N | 0.97 | 0.83 | 0.14 | −0.03 | 0.33 | 0.40 | 0.80 | 0.87 | 0.87 | 0.80 | 1.87 | 1.80 | −1.00 |
| 3101 | M | 57 | N | 0.98 | 0.78 | 0.19 | 0.61 | −0.67 | 1.60 | 0.60 | 0.60 | NA | NA | 1.87 | 1.47 | 1.33 |
| 3202 | F | 27 | N | 1.20 | 0.98 | 0.22 | −0.92 | 0.00 | 1.78 | NA | NA | 0.87 | 1.33 | 1.60 | 1.47 | 0.40 |
| 5406 | F | 47 | N | 0.61 | 0.23 | 0.37 | 0.89 | −1.00 | 2.14 | −0.87 | −1.13 | −1.40 | −1.93 | −1.47 | 0.60 | 0.33 |
| 2405 | F | 34 | N | 0.97 | 0.45 | 0.52 | 0.22 | −0.67 | 2.50 | −0.13 | 1.93 | −0.13 | 0.00 | 1.33 | 1.00 | 0.73 |
| 5308 | F | 65 | N | −0.36 | −0.93 | 0.57 | −0.08 | −1.00 | −1.09 | −0.20 | −0.53 | −0.67 | −1.20 | NA | NA | NA |
| 1205 | F | 41 | N | 0.22 | −0.42 | 0.64 | −0.11 | 1.00 | 1.78 | 0.27 | 0.73 | −1.33 | −0.47 | 1.60 | 1.13 | 1.07 |
| 5403 | M | 31 | N | 0.50 | −0.15 | 0.65 | 0.19 | −0.67 | 2.00 | 0.27 | 0.33 | −0.87 | −0.40 | 0.93 | 1.27 | 0.20 |
| 2203 | F | 69 | N | −1.16 | −1.86 | 0.70 | −0.20 | −0.33 | −0.01 | −0.27 | 0.40 | NA | NA | −0.47 | NA | 0.47 |
| 4204 | F | 31 | N | 0.57 | −0.18 | 0.75 | −0.84 | −0.33 | −0.37 | −0.20 | 0.07 | −0.27 | −1.13 | 0.73 | 0.80 | NA |
| 3102 | F | 56 | N | 1.77 | 0.85 | 0.91 | 0.65 | −0.33 | −0.01 | 1.20 | 0.80 | 0.60 | 0.60 | 1.33 | 1.40 | 0.80 |
| 4203 | M | 35 | N | 0.87 | −0.06 | 0.94 | 0.53 | 0.00 | 0.40 | −0.13 | 0.80 | 0.40 | −0.13 | 1.33 | NA | NA |
| 2201 | F | 69 | Y | −0.29 | −1.63 | 1.35 | −0.12 | −1.00 | 0.35 | −1.00 | −0.73 | −0.80 | −1.67 | −1.47 | −0.53 | 0.93 |
| 2303 | F | 51 | Y | 0.43 | −1.66 | 2.09 | 0.19 | −1.67 | −2.17 | −1.33 | −0.73 | −1.40 | −1.93 | −2.47 | −0.33 | 0.13 |
| 2401 | M | 29 | Y | 0.61 | −1.57 | 2.18 | 1.41 | −1.00 | −7.21 | −0.80 | 0.07 | 0.40 | −0.87 | 0.13 | −0.13 | −0.67 |
Note. All units in z scores; Aff. = affectation status for the motor sequencing deficit; Y = yes; N = no.
Appendix 2.
Qualitative errors during nonword and multisyllabic word imitation.
| Code | Aff. | DDK monos. – multis. z | % Sequ. err. (MSW) | % Subst. err. (MSW) | % Fl. err. (MSW) | % Sequ. err. (NWR) | % Subst. err. (NWR) | % Fl. err. (NWR) | % Sequ. err. (NRT) | % Subst. err. (NRT) | % Fl. err. (NRT) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1203 | N | −0.74 | 0.00 | 0.00 | 0.55 | 3.40 | 2.72 | 0.00 | 0.00 | 1.04 | 1.04 |
| 2404 | N | −0.18 | 1.66 | 0.55 | 0.55 | 6.80 | 0.68 | 0.68 | 1.36 | 0.00 | 1.04 |
| 3204 | N | 0.00 | 0.55 | 0.55 | 0.55 | 10.20 | 6.12 | 0.00 | 2.04 | 5.21 | 2.08 |
| 3203 | N | 0.01 | 0.00 | 0.00 | 0.55 | 6.80 | 3.40 | 0.68 | 2.04 | 3.13 | 5.21 |
| 5402 | N | 0.08 | 0.00 | 0.00 | 0.55 | 5.44 | 3.40 | 0.00 | 1.04 | 3.13 | 0.00 |
| 5407 | N | 0.08 | 0.55 | 0.00 | 0.00 | 8.84 | 4.76 | 0.68 | 9.38 | 13.54 | 0.00 |
| 1204 | N | 0.14 | 0.00 | 0.00 | 0.00 | 4.08 | 0.68 | 0.68 | 1.04 | 0.00 | 2.08 |
| 3101 | N | 0.19 | 0.00 | 1.10 | 1.66 | 5.44 | 4.76 | 0.68 | 0.00 | 0.00 | 1.04 |
| 3202 | N | 0.22 | NA | NA | NA | 5.44 | 3.40 | 0.00 | 1.36 | 1.04 | 0.00 |
| 5406 | N | 0.37 | 1.66 | 0.00 | 0.00 | 5.44 | 5.44 | 1.36 | 8.33 | 0.00 | 0.00 |
| 2405 | N | 0.52 | 1.10 | 0.00 | 0.55 | 4.76 | 3.40 | 1.36 | 0.00 | 3.13 | 0.00 |
| 5308 | N | 0.57 | 1.66 | 0.55 | 0.55 | 7.48 | 10.20 | 1.36 | 6.25 | 11.46 | 0.00 |
| 1205 | N | 0.64 | 0.00 | 0.00 | 0.00 | 2.04 | 2.04 | 0.68 | 0.00 | 4.17 | 0.00 |
| 5403 | N | 0.65 | 0.55 | 0.00 | 0.55 | 6.80 | 4.76 | 0.68 | 1.04 | 1.04 | 0.00 |
| 2203 | N | 0.70 | 1.10 | 0.00 | 0.00 | 6.80 | 4.76 | 4.08 | 6.25 | 1.04 | 4.17 |
| 4204 | N | 0.75 | 0.55 | 0.55 | 0.00 | 4.08 | 0.68 | 1.36 | 6.25 | 5.21 | 1.04 |
| 3102 | N | 0.91 | 0.00 | 0.00 | 0.00 | 3.40 | 4.08 | 0.68 | 3.40 | 3.13 | 0.00 |
| 4203 | N | 0.94 | NA | NA | NA | 3.40 | 3.40 | 1.36 | 1.04 | 1.04 | 0.00 |
| 2201 | Y | 1.35 | 3.31 | 1.10 | 0.00 | 12.93 | 4.76 | 0.00 | 4.08 | 4.17 | 0.00 |
| 2303 | Y | 2.09 | 8.29 | 1.10 | 0.00 | 13.61 | 5.44 | 2.72 | 7.48 | 5.21 | 1.04 |
| 2401 | Y | 2.18 | 7.73 | 2.76 | 0.55 | 25.85 | 6.80 | 0.00 | 11.46 | 9.38 | 1.04 |
Note. Aff. = affectation status for the motor sequencing deficit; Y = yes; N = no.
Group differences in measures with high and low sequential processing loads
Two of the three measures with low sequential processing loads, repetitive keyboard tapping and NIX, did not differentiate between the MA and MU group. In three measures of nonword imitation (NWR, NRT, SRT), four measures of word reading (WID, WATT, SWE, PDE), one measure of spelling (WIAT spelling) and one composite measure of verbal processing (VIX), the three participants with sequential movement deficits during DDK testing (affected regarding the motor sequencing deficit, MA) produced average group scores that were below the population mean, ranging from −0.33 (VIX) to −3.01 (NRT). The group without sequential DDK deficits (i.e. unaffected regarding the motor sequencing deficit, MU) produced group scores in these tasks that fell within 1 SD of the population mean, ranging from −0.40 (SRT) to 1.14 (VIX). For WID, NWR, WATT, PDE, WIAT spelling and VIX, the group differences were nominally statistically significant. Note that WID involves more whole-chunk processing and less sequential processing, compared with the other tasks described here. Table 1 summarizes these results.
Table 1.
Group means (number of participants), standard deviations and test statistics from rank-sum testing in measures with low and high sequential processing loads.
| Sequencing load | Variable (acronym) | Variable (full name) | Mean MA | SD | Mean MU | SD | Z score | p value |
|---|---|---|---|---|---|---|---|---|
| Low | Rep. Keyboard Tap. | Repetitive Keyboard Tapping | 0.49 (3) | 0.81 | 0.26 (18) | 0.59 | −0.20 | 0.8407 |
| WID | Word Identification | −1.04 (3) | 0.27 | 0.18 (17) | 0.51 | 2.60 | *0.0094 | |
| NIX | Nonverbal Intelligence Index | 0.13 (3) | 0.8 | 0.82 (15) | 0.72 | 1.48 | 0.1386 | |
| AVG | Average | −0.14 | 0.63 | 0.42 | 0.60 | 1.29 | 0.3296 | |
| SD | Standard Deviation | 0.80 | 0.31 | 0.35 | 0.11 | 1.41 | 0.4473 | |
| High | NWR | Nonword Repetition | −1.22 (3) | 0.38 | −0.26 (18) | 0.63 | 2.34 | *0.0194 |
| NRT | Nonword Repetition Task | −3.01 (3) | 3.85 | 0.63 (18) | 1.72 | 1.81 | 0.0696 | |
| SRT | Syllable Repetition Test | −2.15 (2) | 0.73 | −0.40 (17) | 2.53 | 1.90 | 0.0576 | |
| WATT | Word Attack | −0.47 (3) | 0.46 | 0.44 (17) | 0.77 | 1.96 | *0.0497 | |
| SWE | Sight Word Efficiency | −0.60 (3) | 0.92 | 0.00 (16) | 0.83 | 1.36 | 0.1751 | |
| PDE | Phonemic Decoding Efficiency | −1.49 (3) | 0.56 | 0.05 (16) | 1.06 | 2.08 | *0.0380 | |
| WIAT Sp. | Wechsler Individual Achievement Test – Spelling | −1.27 (3) | 1.31 | 0.93 (17) | 0.97 | 2.34 | *0.0192 | |
| VIX | Verbal Intelligence Index | −0.33 | 0.20 | 1.14 | 0.56 | 2.55 | *0.0108 | |
| AVG | Average | −1.32 | 1.05 | 0.32 | 1.13 | 2.04 | 0.0549 | |
| SD | Standard Deviation | 0.91 | 1.18 | 0.56 | 0.67 | 0.38 | 0.0527 |
Note. All units in z scores. RS = rank-sum;
nominal statistical significance; negative z values indicate lower scores in the MA group, compared with the MU group.
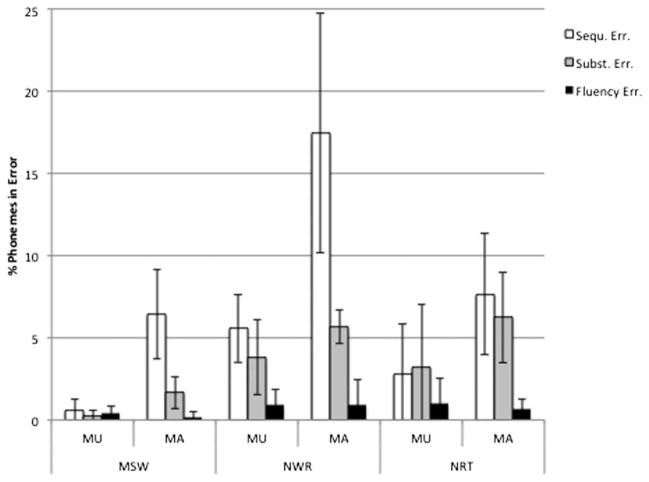
A qualitative error analysis was applied to the imitations from MSW, NWR and NRT to quantify percent phonemes in error for each of the three error classes, sequencing (assimilation, omission, migration, metatheses and insertions of sounds from within and outside the target word), substitution and fluency (false start, hesitation and syllable repetition). Individual results are shown in Appendix 2. The three participants with sequential movement deficits during DDK testing (i.e. affected regarding the motor sequencing deficit, MA) produced substantially more phoneme sequencing and substitution errors during MSW, NWR and NRT than the 18 participants without this deficit (unaffected regarding the motor sequencing deficit, MU). Except for the MU group during NRT testing, sequencing errors were more frequent than substitution errors in both groups and all tasks. Fluency errors were rare in general and the MA group produced slightly more fluency errors than the MU group during MSW, an equivalent number during NWR and slightly fewer fluency errors during NRT. Both groups showed the highest frequency of sequencing errors during NWR, followed by NRT and MSW. Figure 1 shows the group averages for the three error types across the three tasks, separately for the MU and MA groups. Table 2 shows the results from rank-sum testing for group differences for these error types and tasks.
Figure 1.
Average error percentages by task, participant group and error class.
Note: MU = unaffected regarding motor sequencing deficit; MA = affected regarding motor sequencing deficit. Error bars indicate ±1 SD.
Table 2.
Group means, standard deviations and test statistics from rank-sum testing for three error classes (sequencing, substitution, fluency) and three imitation tasks (MSW, NWR, NRT).
| Task | Error class | Mean MA | SD | Mean MU | SD | RS z | RS p value |
|---|---|---|---|---|---|---|---|
| MSW | % Sequencing err. | 6.45 | 2.73 | 0.59 | 0.65 | −2.77 | 0.0056** |
| % Substitution err. | 1.66 | 0.96 | 0.21 | 0.34 | −2.89 | 0.0039** | |
| % Fluency err. | 0.18 | 0.32 | 0.38 | 0.44 | 0.76 | 0.4503 | |
| NWR | % Sequencing err. | 17.46 | 7.27 | 5.59 | 2.08 | −2.74 | 0.0062* |
| % Substitution err. | 6.25 | 2.76 | 3.82 | 2.26 | −1.89 | 0.0591 | |
| % Fluency err. | 0.69 | 0.60 | 0.91 | 0.93 | 0.63 | 0.5272 | |
| NRT | % Sequencing err. | 7.67 | 3.69 | 2.82 | 3.06 | −2.03 | 0.0426* |
| % Substitution err. | 6.25 | 2.76 | 3.18 | 3.82 | −1.79 | 0.0742 | |
| % Fluency err. | 0.69 | 0.60 | 0.98 | 1.54 | −0.22 | 0.8258 |
Note. MU = unaffected regarding motor sequencing deficit; MA = affected regarding motor sequencing deficit; RS = rank-sum;
nominal statistical significance;
adjusted statistical significance. Negative z values indicate lower scores in the MA group, compared with the MU group.
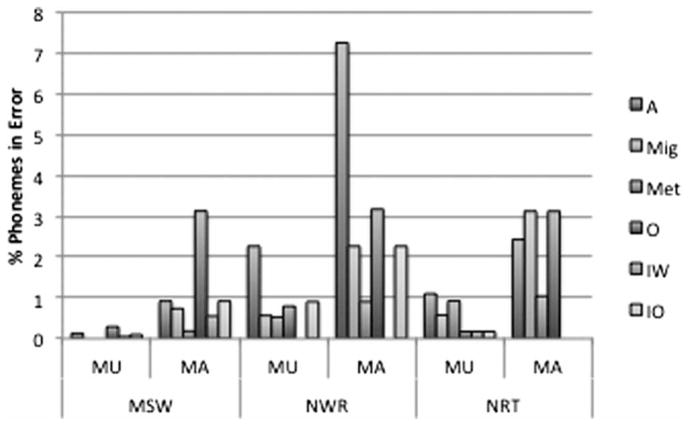
Within the sequencing error class, by far the most frequently observed error type in the MA group during MSW was omission, followed by insertions if the two insertion types, sounds from within and outside the word, were collapsed. During NWR, the most frequently observed error type was assimilation, followed by omission. During NRT, migrations and omissions were equally frequent in the MA group. In the MU group, the most frequently observed error type during MSW was omission, followed by assimilation. During NWR, assimilation was the most frequently observed error type, followed by insertion. During NRT, assimilations were most frequently observed, followed by metathesis. Figure 2 summarizes the sequencing error types by task and group in units of percent phonemes in error.
Figure 2.
Average percent phonemes in error by task, participant group and sequencing error type.
Note: MU = unaffected regarding motor sequencing deficit; MA = affected regarding motor sequencing deficit; A = assimilation; Mig = migration; Met = metathesis; O = omission; IW = insertion of a sound within the word; IO = insertion of a sound outside the target word.
Associations among measures with high and low sequential processing loads
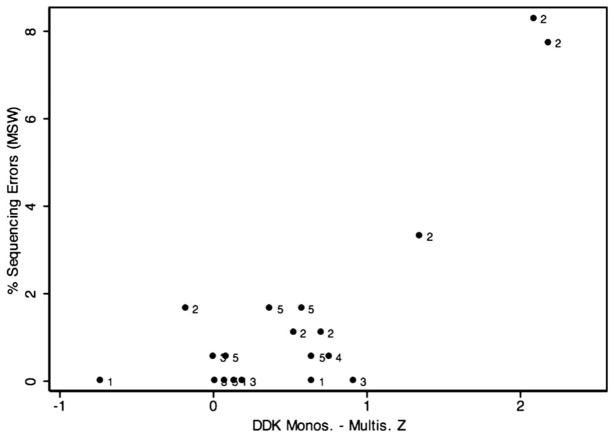
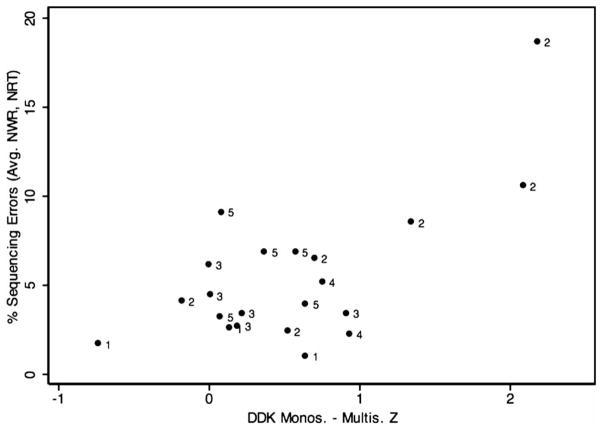
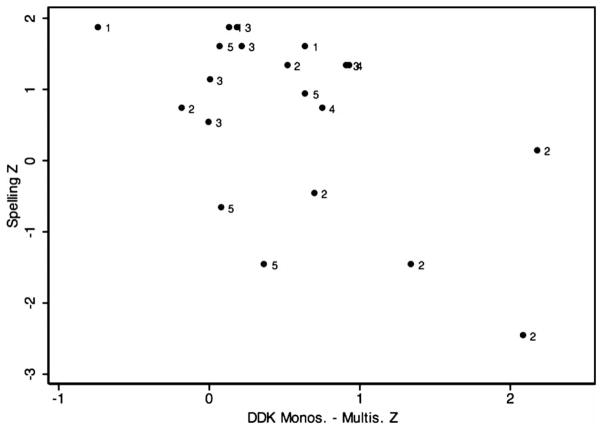
Results from pairwise correlation calculations showed no statistically significant correlation between repetitive keyboard tapping and the selected five measures incorporating an element of sequential processing; p values ranged from 0.3198 (SRT) to 0.9667 (WIAT Sp.). By contrast, strong associations among these measures themselves were observed. For instance, the difference score between the mono- and multisyllabic DDK z score was correlated with the percent sequencing error scores from MSW and NWR at highly statistically significant levels and with PDE and the spelling measure at a nominally statistically significance. The only tested pairwise association not to reach even nominal statistical significance was between percent sequencing errors in the NWR and PDE. Table 3 summarizes correlation coefficients and p values for pairwise correlations among the selected variables, all of which except the measure of repetitive keyboard tapping have high loads of sequential processing. Figures 3, 4 and 5 show the distribution of percent sequencing errors during MSW, percent sequencing errors averaged for NWR and NRT, and z scores for WIAT Spelling, respectively, as a function of the DDK z score difference. The three highest DDK z score differences were produced by participants 2401, 2303 and 2201, respectively. Two brothers from family 001, codes 1203 and 1204, occupied ranks 1 and 7 in the smallest DDK z score difference, respectively, indicating intact sequential motor processing.
Table 3.
Pairwise correlations among variables of interest.
| DDK mono–multi Z | Rep. keyb. tap. | VIX | WIAT Sp. | % Sequ. err. (MSW) | % Sequ. err. (NWR) | |
|---|---|---|---|---|---|---|
| DDK mono-multi Z | 1 | |||||
| Rep. Keyb. Tap. | 0.01 | 1 | ||||
| 0.9493 | ||||||
| VIX | −0.64 | −0.08 | 1 | |||
| 0.0044* | 0.7568 | |||||
| WIAT Sp. | −0.54 | −0.01 | 0.86 | 1 | ||
| 0.0131* | 0.9667 | <0.0001** | ||||
| % Sequ. err. (MSW) | 0.83 | 0.20 | −0.72 | −0.67 | 1 | |
| <0.0001** | 0.4063 | 0.0011** | 0.0024** | |||
| % Sequ. err. (NWR) | 0.66 | 0.29 | −0.68 | −0.51 | 0.84 | 1 |
| 0.0013** | 0.2033 | 0.0021** | 0.0207* | <0.0001** |
Note.
Nominal statistical significance;
adjusted statistical significance.
Figure 3.
Percent sequencing errors during MSW as a function of the DDK z score difference for mono- and multisyllables. Marker labels represent family codes.
Figure 4.
Percent sequencing errors averaged for NWR and NRT as a function of the DDK z score difference for mono- and multisyllables. Marker labels represent family codes.
Figure 5.
Z scores from the WIAT spelling test as a function of the DDK z score difference for mono- and multisyllables. Marker labels represent family codes.
Case study
One of the participants with a motor sequencing deficit during DDK testing, code 2201, who also had one of the lowest test scores during timed nonword decoding with the PDE, produced a large number of errors that merited analysis in terms of error type. Of 34 incorrectly pronounced nonwords, 15 were produced as a real word with spelling similar to the target nonword, 12 showed mis-sequencing of the graphemes (migration, metathesis, omission, insertion), 2 showed substitution of a grapheme of similar shape (b/d, l/r), 1 was a vowel error ([ɪ]/i) and 5 reflected multiple and complex errors. When a real word instead of a nonword was produced, the initial grapheme was typically preserved, as was the number of syllables, but syllable shape was often altered by inserting or omitting a grapheme. Table 4 lists the errors from PDE testing.
Table 4.
Case study of participant 2201 (PDE, Form A).
| Target | Production | Gloss | Error type |
|---|---|---|---|
| ga | gæp | “Gap” | Real word: insertion |
| ta | kæm | Multiple errors | |
| lat | æt | “At” | Real word: insertion |
| bave | breɪ̯v | “Brave” | Real word: insertion |
| pate | pæt | “Pat” | Real word: omission |
| herm | hʌm | “Hum” | Real word: substitution |
| dess | drεs | “Dress” | Real word: insertion |
| chur | ʧɝn | “Churn” | Real word: insertion |
| barp | bɑrb | “Barb” | Real word: letter confusion |
| stip | strɪp | “Strip” | Real word: insertion |
| poth | prɑθ | Insertion | |
| meest | mɪst | Real word: Vowel error | |
| shlee | ʃɪli | Insertion | |
| guddy | kʌbi | Real word: Letter confusion | |
| skree | sri | Omission | |
| dreef | drɪf | Vowel error | |
| trisk | tɪsk | Omission | |
| kelm | klεm | Metathesis | |
| strone | stoʊ̯n | “Stone” | Real word: omission |
| lunaf | llufə | Migration | |
| cratty | lkræfti | “Crafty” | Real word: letter confusion |
| sploosh | glus | Multiple errors | |
| dreker | drεk | Omission | |
| hedfert | lhɑrtfεlt | “Heartfelt” | Real word: substitution |
| bremick | lblεmɪk | Letter confusion | |
| nifplate | lnaɪ̯plɪ̯et | Omission | |
| brinbert | lbræŋtɪn | Multiple errors | |
| clabom | lkæmbo | Omission, migration | |
| drepnort | ldəpɔrtnər | Multiple errors | |
| shratted | lsætəri | Multiple errors | |
| plofent | lproʊ̯flεnt | Insertion, migration | |
| smuncritt | lskrʌmfɪnt | Multiple errors | |
| pelnador | pənldɔrə | “Pandora” | Real word: substitution |
| fornalask | fɔrlnæsk | Omission |
Discussion
Evidence for the sequential deficit hypothesis in CAS described in the companion paper (Peter et al., in press) was found in the present study of 21 adults with and without SSD histories. Participants whose alternating DDK speeds were >1 SD lower than their repetitive DDK speeds resembled those described in the companion study who had a familial form of CAS with respect to performance on linguistic measures that require high loads of sequential processing.
Group differences between participants with, and without, sequential motor processing deficits
The three participants who were identified as affected with a motor sequencing deficit during DDK testing (MA) showed lower standard scores during nonword imitation as measured with the NWR task, nonword decoding (WATT and PDE), spelling (WIAT Spelling) and verbal processing (VIX) to a nominally statistically significant extent. Group differences approached nominal significance for the other two measures of nonword imitation (NRT, SRT). All these measures require a high sequential processing load, and lower scores in the MA were expected under the hypothesis that the motor sequencing deficit is influenced by the same underlying sequencing deficit that also influences sequential processing in other domains. The group difference for WID was nominally statistically significant as well, despite the expectation of no group difference, as WID involves less sequential processing and more whole-chunk processing, compared with WATT. PDE incorporates elements of sequential processing due to the nature of the decoding task, combined with an element of time pressure, leading to simultaneous processing of consecutive words, and the low scores in the MA group may reflect this compounded challenge. Consistent with the sequential deficit hypothesis, the two groups did not differ in performance on the repetitive keyboard tapping task, a task that carries low sequential sequencing loads.
Group differences in tasks that carry a high sequential processing load are consistent with the results from the companion study reporting on a multigenerational family with familial CAS (Peter et al., in press). In that sample, the family members with present or past histories of the familial speech disorder differed significantly from those without such a history in their DDK speeds during alternating syllable repetition. To capture this indicator of sequential processing in the present study, participants were grouped by relative motor speeds during mono- and multisyllable repetition, where a z score difference >1 was used to assign the affectation status. The three participants who were labeled as affected with respect to motor sequencing were biologically related to each other, and two children in the family had a CAS diagnosis. In both studies, the affected groups obtained lower scores than the unaffected group in tasks that involved high loads of sequential processing but not in tasks that involve low loads of sequential processing. The results from the present study replicate the findings in the companion study, and they are consistent with the hypothesis that the speech traits in CAS are influenced by an underlying deficit in sequential processing that also affects the performance in other modalities such as written language and cognitive domains such as sequence maintenance in long-term and working memory.
Phoneme sequencing errors during real word and nonword imitation
The qualitative error analyses from the real word and nonword imitation tasks replicate the patterns described in the companion study (Peter et al., in press) to a very close extent. In both studies, participants who were identified as affected (familial SSD in the multigenerational family; motor sequencing deficits during DDK testing in the present sample of five families) produced substantially more sequencing errors, compared with the unaffected participants, when imitating nonwords and multisyllabic real words. These convergent results strengthen the interpretation that sequential motor processing deficits are associated with sequencing deficits upstream from motor programming and involve linguistic units in the form of phonemes, whether during encoding, storage in working memory, or storage in long-term memory.
As in the companion study, the affected group also produced more substitution errors in all imitation tasks, compared with the unaffected group, a finding that may indicate difficulty with auditory processing when encoding and storing words and nonwords. As in the companion study, fluency errors were rare in both groups and no clear group differences emerged. False starts, hesitations and syllable repetitions may indicate awareness of an error and attempt at correction. In both studies, fluency errors occurred on approximately 1% of phonemes or less. Overall, sequencing errors occurred even more frequently in the affected group in the present study, compared with the affected group in the companion study, whereas the frequency profile of all other error classes in both groups and all three tasks was comparable between the two studies.
In both studies and both participant groups, the highest sequencing error proportions were seen during the NWR task, followed by the NRT and the MSW tasks. The two nonword tasks require high loads of sequential processing during encoding and storage in working memory, whereas the MSW imitation task requires recognizing the word as stored in long-term memory. Of the two nonword imitation tasks, the NWR is more complex in terms of syllable shapes and phoneme inventory, which may explain the high error frequency in general.
Associations among measures with high sequential processing loads
Correlational analyses showed that the measure of sequential motor processing during DDK testing was associated with the performance of other tasks involving high loads of sequential processing. The three participants labeled as affected with regard to sequential motor processing, all from family 002, generally provided the scores at the low end of the score distribution, whereas two brothers in family 001 provided some of the highest scores in the variables of interest. These results are consistent with the interpretation that sequential processing ability is distributed as a continuous trait in this sample and that extreme values may have a genetic component.
As would be expected, measures of verbal processing and spelling were cross-correlated. The fact that they also were correlated with the measures of alternating DDK speeds and accurate phoneme sequences during nonword and multisyllabic real word imitation is consistent with the view that sequential processing underlies performance across many modalities.
Case study of timed nonword decoding
One participant, code 2201, with a deficit in sequential motor processing, as determined by a z score difference >1 between mono- and multisyllable repetition, also obtained a low score on the PDE subtest that evaluates nonword decoding under timed conditions. Nonword decoding requires converting graphemes into phonemes sequentially and the added time pressure inherent in the PDE may increase error rates. The error analysis in participant 2201’s nonword reading (Table 4) showed several sequencing error types also observed during nonword imitation at high frequencies in the MA group, including omissions, insertions, migrations and metatheses. Unlike during nonword imitations, assimilations were not observed in this case study of nonword decoding. One possible explanation for this difference is that during nonword imitation, the target is presented auditorily and fleetingly in time, whereas during nonword reading, the target is presented visually and remains accessible to the participant. Imitating a nonword, hence, requires rapid analysis of the phoneme sequence when encoding and storing it in working memory. It is possible that assimilation errors occur during the storage and/or retrieval processes regarding short-term memory, where a sound may get overwritten by another salient sound in the phoneme sequence.
Participant code 2201 also produced many real words with similar word shapes as the target non-words. It is possible that she bypassed the sequential decoding process in these cases altogether, defaulting instead to whole-chunk identification. A comparison of the target letter sequence and the phoneme sequence in the production showed that the first letter and the general word shape were generally preserved. The middle and end parts of the nonword were altered by inserted, deleted or substituted graphemes, which indicates that the participant did not closely analyze and decode the graphemes beyond the first letter.
Integrating the findings of this study with prior findings
Three participants were labeled “affected” with respect to a motor sequencing deficit (MA), as quantified with a z score difference between alternating and repetitive DDK motor speeds of ≥1.0: participant codes 2201, 2303 and 2401. These three individuals were biologically related. Participant code 2201 was the mother of 2303 and the grandmother of 2401. Two children in this family had a CAS diagnosis. Participant 2201 had not reported a childhood history of speech difficulties. It is possible that she carried the endophenotype without full expression of the disorder trait. Alternatively, she may have been unaware of speech struggles she experienced as a very young child. The three MA individuals demonstrated a similar profile across administered tasks, especially with respect to sequencing errors on MSW and nonword imitation tasks and difficulty with reading. Findings are consistent with an underlying deficit of genetic etiology that manifests mainly in tasks requiring substantial amounts of sequential processing. In this respect, these three relatives resemble the affected individuals in the family with familial CAS described in the companion study (Peter et al., in press).
Only adults were included in the present study. All, including those with a history of speech disorders, had normalized speech, yet those with evidence of motor sequencing deficits during DDK testing also showed evidence of deficits in a variety of other tasks requiring high loads of sequential processing. This finding replicates the results from the companion study, and it is consistent with an endophenotype in the area of sequential processing that affects speech development in childhood and can be observed in other modalities even when conversational speech has normalized.
Future studies
Results from this study are consistent with a deficit in sequential processing as an endophenotype of genetic etiology in CAS. Future studies should investigate the causal genes in individuals with CAS in light of sequential processing deficits. As mentioned in the companion study, the cerebellum may be implicated in CAS, as it plays a role in sequential processing across various modalities. Future studies, hence, should investigate cerebellar structures and functions in CAS.
Future studies should address the clinical implications of the role of sequencing ability in CAS. Especially of interest is whether therapy targeting sequential processing in speech is more effective at ameliorating the speech deficits in individuals with CAS than other forms of therapy. It may also be of interest to investigate whether therapy targeting sequential processing across multiple modalities has a beneficial effect on the speech of individuals with CAS. Conversely, it is possible that therapy targeting sequential processing in speech could have an ameliorating effect on reading ability, especially reading of unfamiliar words. Because the relationship between sequencing ability and disorders of speech and language is not yet clear, this type of therapy should not be implemented until evidence for the efficacy of such interventions becomes available.
Because sequential processing plays an important role in reading and spelling, the results from this study may have implications for dyslexia. The framework presented here should be translated into studies of dyslexia. Questions regarding clinical management analogous to those raised for CAS may apply to dyslexia as well, and targeted therapeutic focus on sequential processing ability in dyslexia should be addressed in rigorous trials.
As in the companion study, this study does not necessarily prove the sequential deficit hypothesis. It is theoretically possible that the deficits in sequential processing are not so much an integral corollary of CAS (Marquardt et al., 2004) but rather a secondary effect of the motor programming deficit caused by CAS (McNeil, 1997, 2009). For instance, deficits in reading and spelling could be the result of inadequate feedback loops from speech production in early childhood, also causing impairments in phonemic processing ability. To further evaluate the evidence for each of these two views, nonverbal measures, e.g. number sequences, visual-spatial stimuli and elements of sequential logic, should be evaluated for association with CAS. Because of its role in sequential processing during motor, linguistic and cognitive tasks, the cerebellum should be evaluated for functional deficits in CAS.
Acknowledgments
The authors thank the families whose participation made this study possible. Many thanks to the following undergraduate and graduate students for their assistance with the data collection and analyses: Yayin Chen, Alice Cho, Erica Gonzales, Mariya Legesse, Jonathan Mahaffie, Kyle Middleton, Nancy Nguyen, David Ramm, Kate Sailor and Nancy Yuan. Many thanks to Elias Peter for technical assistance.
Footnotes
Declaration of Interest: The authors gratefully acknowledge the following funding sources: American Speech-Language-Hearing Foundation New Century Scholars Research Grant (B. Peter), NIDCD T32DC00033 (B. Peter), NIDCD 1R03DC010886 (B. Peter) and R01HD054562 (W. H. Raskind). The authors report no conflict of interest.
References
- Boersma P. Praat, a system for doing phonetics by computer. Glot International. 2001;5(9/10):341–345. [Google Scholar]
- Catts HW. Speech production/phonological deficits in reading-disordered children [Research Support, Non-U.S. Gov’t] Journal of Learning Disabilities. 1986;19(8):504–508. doi: 10.1177/002221948601900813. [DOI] [PubMed] [Google Scholar]
- Dollaghan C, Campbell TF. Nonword repetition and child language impairment. [Research Support, U.S. Gov’t, P.H.S.] Journal of Speech Language and Hearing Research. 1998;41(5):1136–1146. doi: 10.1044/jslhr.4105.1136. [DOI] [PubMed] [Google Scholar]
- Fletcher SG. Time-by-count measurement of diadochokinetic syllable rate. Journal of Speech and Hearing Research. 1972;15(4):763–770. doi: 10.1044/jshr.1504.763. [DOI] [PubMed] [Google Scholar]
- Gray RM, Livingston RB, Marshall RM, Haak RA. Reference group data for the Reitan-Indiana Neuropsychological Test Battery for young children. Perceptual and Motor Skills. 2000;91(2):675–682. doi: 10.2466/pms.2000.91.2.675. [DOI] [PubMed] [Google Scholar]
- Gualtieri CT, Johnson LG. Reliability and validity of a computerized neurocognitive test battery, CNS vital signs. Archives of Clinical Neuropsychology. 2006;21(7):623–643. doi: 10.1016/j.acn.2006.05.007. S0887-6177(06)00083-7 [pii] doi:10.1016/j. acn.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Klapp ST. Motor response programming during simple and choice-reaction time – The role of practice. Journal of Experimental Psychology-Human Perception and Performance. 1995;21(5):1015–1027. [Google Scholar]
- Klapp ST. Reaction time analysis of two types of motor preparation for speech articulation: Action as a sequence of chunks [Research Support, Non-U.S. Gov’t] Journal of Motor Behavior. 2003;35(2):135–150. doi: 10.1080/00222890309602129. [DOI] [PubMed] [Google Scholar]
- Konig IR, Schumacher J, Hoffmann P, Kleensang A, Ludwig KU, Grimm T, Schulte-Korne G. Mapping for dyslexia and related cognitive trait loci provides strong evidence for further risk genes on chromosome 6p21. American Journal of Medical Genetics, Part B, Neuropsychiatric Genetics. 2011;156B(1):36–43. doi: 10.1002/ajmg.b.31135. [DOI] [PubMed] [Google Scholar]
- Marquardt TP, Jacks A, Davis BL. Token-to-token variability in developmental apraxia of speech: Three longitudinal case studies [Case Reports] Clinical Linguistics and Phonetics. 2004;18(2):127–144. doi: 10.1080/02699200310001615050. [DOI] [PubMed] [Google Scholar]
- McNeil MR. Clinical management of sensorimotor speech disorders. New York: Thieme; 1997. [Google Scholar]
- McNeil MR. Clinical management of sensorimotor speech disorders. 2. New York: Thieme; 2009. [Google Scholar]
- Peter B, Button LA, Stoel-Gammon C, Chapman K, Raskind WH. Deficits in sequential processing manifest in motor and linguistic tasks in a multigenerational family with childhood apraxia of speech. Clinical Linguistics and Phonetics. doi: 10.3109/02699206.2012.736011. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter B, Matsushita M, Raskind WH. Global processing speed in children with low reading ability and in children and adults with typical reading ability: Exploratory factor analytic models [Research Support, N.I.H., Extramural] Journal of Speech Language and Hearing Research. 2011;54(3):885–899. doi: 10.1044/1092-4388(2010/10-0135). doi:10.1044/1092-4388(2010/10-0135) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter B, Matsushita M, Raskind WH. Motor sequencing deficit as an endophenotype of speech sound disorder: A genome-wide linkage analysis in a multigenerational family. Psychiatric Genetics. 2012;22(5):226–234. doi: 10.1097/YPG.0b013e328353ae92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter B, Raskind WH. A multigenerational family study of oral and hand motor sequencing ability provides evidence for a familial speech sound disorder subtype. Topics in Language Disorders. 2011;31(2):145–167. doi: 10.1097/TLD.0b013e318217b855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds CR, Kamphaus RW. RIAS, Reynolds Intellectual Assessment Scales. Lutz, FL: Psychological Assessment Resources; 2003. [Google Scholar]
- Shriberg LD, Aram DM, Kwiatkowski J. Developmental apraxia of speech: I. Descriptive and theoretical perspectives [Research Support, U.S. Gov’t, P.H.S.] Journal of Speech Language and Hearing Research. 1997a;40(2):273–285. doi: 10.1044/jslhr.4002.273. [DOI] [PubMed] [Google Scholar]
- Shriberg LD, Aram DM, Kwiatkowski J. Developmental apraxia of speech: II. Toward a diagnostic marker [Comparative Study Research Support, U.S. Gov’t, P.H.S.] Journal of Speech Language and Hearing Research. 1997b;40(2):286–312. doi: 10.1044/jslhr.4002.286. [DOI] [PubMed] [Google Scholar]
- Shriberg LD, Aram DM, Kwiatkowski J. Developmental apraxia of speech: III. A subtype marked by inappropriate stress [Comparative Study Research Support, U.S. Gov’t, P.H.S.] Journal of Speech Language and Hearing Research. 1997c;40(2):313–337. doi: 10.1044/jslhr.4002.313. [DOI] [PubMed] [Google Scholar]
- Shriberg LD, Lohmeier HL, Campbell TF, Dollaghan CA, Green JR, Moore CA. A nonword repetition task for speakers with misarticulations: The Syllable Repetition Task (SRT) [Research Support, N.I.H., Extramural Validation Studies] Journal of Speech Language and Hearing Research. 2009;52(5):1189–1212. doi: 10.1044/1092-4388(2009/08-0047). DOI: 10.1044/1092-4388 (2009/08-0047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shriberg LD, Lohmeier HL, Strand EA, Jakielski KJ. Encoding, memory, and transcoding deficits in Childhood Apraxia of Speech [Research Support, N.I.H., Extramural] Clinical Linguistics and Phonetics. 2012;26(5):445–482. doi: 10.3109/02699206.2012.655841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shriberg LD, Potter NL, Strand EA. Prevalence and phenotype of childhood apraxia of speech in youth with galactosemia [Research Support, N.I.H., Extramural] Journal of Speech Language and Hearing Research. 2011;54(2):487–519. doi: 10.1044/1092-4388(2010/10-0068). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stackhouse J. Developmental verbal dyspraxia. I: A review and critique [Review] European Journal of Disorders of Communication. 1992;27(1):19–34. doi: 10.3109/13682829209012027. [DOI] [PubMed] [Google Scholar]
- Thoonen G, Maassen B, Gabreels F, Schreuder R, de Swart B. Towards a standardised assessment procedure for developmental apraxia of speech [Research Support, Non-U.S. Gov’t] European Journal of Disorders of Communication. 1997;32(1):37–60. doi: 10.3109/13682829709021455. [DOI] [PubMed] [Google Scholar]
- Torgesen JK, Wagner RK, Rashotte CA. Test of word reading efficiency. Austin, TX: PRO-ED; 1999. [Google Scholar]
- Wagner RK, Torgesen JK, Rashotte CA. CTOPP, Comprehensive Test of Phonological Processing. Austin, TX: PRO-ED; 1999. [Google Scholar]
- Wechsler Individual Achievement Test. Wechsler Individual Achievement Test. San Antonio, TX: The Psychological Corporation; 1992. [Google Scholar]
- Woodcock RW. Woodcock Reading Mastery Tests – Revised. Minneapolis, MN: Pearson; 1998. [Google Scholar]