Abstract
Although aging is associated with changes in brain structure and cognition it remains unclear which specific structural changes mediate individual cognitive changes. Several studies have reported that white matter (WM) integrity, as assessed by diffusion tensor imaging (DTI), mediates, in part, age-related differences in processing speed (PS). There is less evidence for WM integrity mediating age-related differences in higher order abilities (e.g., memory and executive functions). In 165 typically aging adults (age range 54–89) we show that WM integrity in select cerebral regions is associated with higher cognitive abilities and accounts variance not accounted for by PS or age. Specifically, voxel-wise analyses using tract-based spatial statistics (TBSS) revealed that WM integrity was associated with reasoning, cognitive flexibility and PS, but not memory or word fluency, after accounting for age and gender. While cerebral fractional anisotropy (FA) was only associated with PS; mean (MD), axial (AD) and radial (RD) diffusivity were associated with reasoning and flexibility. Reasoning was selectively associated with left prefrontal AD, while cognitive flexibility was associated with MD, AD and RD throughout the cerebrum. Average WM metrics within select WM regions of interest accounted for 18% and 29% of the variance in reasoning and flexibility, respectively, similar to the amount of variance accounted for by age. WM metrics mediated ~50% of the age-related variance in reasoning and flexibility and different proportions, 11% for reasoning and 44% for flexibility, of the variance accounted for by PS. In sum, i) WM integrity is significantly, but variably, related to specific higher cognitive abilities and can account for a similar proportion of variance as age, and ii) while FA is selectively associated with PS; while MD, AD and RD are associated with reasoning, flexibility and PS. This illustrates both the anatomical and cognitive selectivity of structure-cognition relationships in the aging brain.
Keywords: DTI, aging, cognition, MRI, processing speed, executive function, TBSS
1. INTRODUCTION
The neurobiologic underpinnings of age-related cognitive decline remain unclear. Although cerebral atrophy and neuronal death occur in select brain regions during specific diseases (e.g., hippocampal cell loss in Alzheimer’s disease), it appears that a majority of nonspecific age-related cerebral volume loss is the result of reduced neuronal complexity and loss of connections (Fjell and Walhovd, 2010). Since the coordinated activity of both local and global neural networks relies on cortical-cortical connections, white matter (WM) integrity, i.e., the “intactness” of cortical connections, must be essential for normal cognitive function and loss of WM integrity likely contributes to age-related cognitive decline (Bartzokis et al., 2004; Deary et al., 2010).
Age-related cognitive decline is observed in many domains important in the maintenance of everyday function including memory, executive function and processing speed (PS) (Hedden and Gabrieli, 2004). While reliance of higher order abilities on PS is complex (Park and Reuter-Lorenz, 2009), it has been hypothesized that reductions in PS may account for much of the age-related cognitive change in higher order abilities (Finkel et al., 2009; Salthouse, 1996, 2010). Thus we sought to determine if WM integrity is related to higher order cognitive abilities after accounting for age or PS, and whether WM integrity mediates the associations between higher order cognitive abilities, age and PS.
Both higher order abilities (e.g., executive function and episodic memory) and PS have been variably associated with WM integrity (Gold et al., 2010; Kennedy and Raz, 2009; O’Sullivan et al., 2001; Turken et al., 2008; Vernooij et al., 2009). However, few studies have tried to determine if reduced WM integrity accounts for the association between higher order abilities and PS. Bucur et al (2008) reported that WM integrity in prefrontal regions partially mediates the association between PS and episodic memory while Salami et al (2011) reported that although WM integrity partially mediates the association between age and PS, it is not strongly related to age-related changes in higher cognitive functions (Bucur et al., 2008; Salami et al., 2011). Thus it remains unclear if WM integrity mediates the association between age-related changes in higher order abilities and declining PS (Madden et al., 2009a).
Age-related changes in WM integrity (i.e., microstructure) can be assessed with diffusion tensor imaging (DTI). DTI allows for the in situ assessment of WM integrity since water diffuses more freely parallel to myelinated axons (i.e. axial diffusivity (AD)) than perpendicular to them (i.e., radial diffusivity (RD)) (Le Bihan, 2003). Fractional anisotropy (FA) is essentially the ratio of AD to RD while mean diffusivity (MD) is the average diffusion across all directions. Major WM tracts have high FA. Loss of WM integrity is typically defined by reduced FA, perhaps reflecting the loss/degeneration of axons, and increased MD, perhaps reflecting demyelination and loss of parenchymal complexity (Jones, 2008). Aging is associated with decreasing FA and increasing MD throughout much of the cerebral WM (Bennett et al., 2010; Burzynska et al., 2010; Damoiseaux et al., 2009; Madden et al., 2009a; Sullivan and Pfefferbaum, 2003) that is linearly related to average cortical thickness (P Kochunov et al., 2011). While AD and RD have also been shown to increase with age (Michielse et al., 2010) it is less clear how they are related to cognitive changes during typical aging (Madden et al., 2012). Some evidence points to greater RD changes with aging than AD (Burzynska et al., 2010) while others report equal or greater AD changes (Sala et al., 2010).
Herein we examine where higher order cognitive abilities and PS are associated with WM integrity and whether regional WM integrity mediates the associations between higher order abilities, PS and age. This is important because if changes in WM integrity can explain the association between PS and age-related cognitive decline, it would suggest that WM pathology, and not cell loss, account for cognitive changes during typical aging. To determine where higher order abilities and PS are associated with WM integrity we used tract based spatial statistics (TBSS), a 3D voxel-wise approach that assesses local maximum DTI values (the presumed core of cortical WM tracts (Smith et al., 2006)). We hypothesize that PS will be more strongly and globally related to WM integrity than higher order abilities (Wen et al., 2011) and that executive abilities will be most related to WM integrity in the frontal lobes. In addition, given that flexibility is more associated with PS than other higher order abilities (Schaie et al., 1991) we predict that flexibility may be more related to WM integrity than other higher order abilities. To determine if WM integrity can account for the associations between higher order abilities, PS and age, mediation analysis using average regional WM metrics is used. In mediation analysis three conditions must be met (Baron and Kenny, 1986); i) the cognitive abilities must be correlated with age and PS, ii) WM integrity must be correlated with age and PS and iii) WM integrity should account for significant variance in multiple regressions that include the “mediated” variable. This approach has been used in prior studies (Bucur et al., 2008; Madden et al., 2009b; Salami et al., 2011) although the mediation effects of WM integrity on age were not explored (Bucur et al., 2008) or only found for PS and not higher order abilities (Madden et al., 2009b; Salami et al., 2011). Finally, we hypothesize that WM integrity will account for unique variance in cognitive abilities beyond that explained by either age or PS.
2. MATERIALS AND METHODS
2.1 Participants
The present study involved 188 subjects who were selected to undergo MRI in 2006–2007 from the Seattle Longitudinal Study (SLS), a cohort-sequential longitudinal study of the relationship between aging, health, cognition and life-style (Schaie, 2005). Imaging data from 165 subjects was used secondary quality control measures (see Image Analysis below). SLS members at recruitment represent a stratified-by-age and gender sample from the Group Health Cooperative of Puget Sound, a large HMO in western Washington State. This study has been approved by the University of Washington Medical Center and the Group Health Cooperative of Puget Sound Institutional Review Boards. Participants were selected from the larger group sample (n=572) of SLS subjects who had been cognitively assessed in middle age; selection criteria were; i) had undergone 2 or more cognitive assessments in midlife and/or old age, ii) participated in the 2005 SLS data collection, and iii) were willing and capable of undergoing MRI. A vascular risk score (0 to 3) was assigned to each subject based on self-reported diagnoses of hypertension, diabetes or hyperlipidemia (each risk factor being given an equal weight of 1 point). APOE genotyping was performed at Northwest Lipid Research Laboratories (Seattle WA, USA).
2.2 Cognitive assessment
SLS participants were assessed on a broad battery of psychometric abilities in two, 2.5-hour sessions (Schaie, 2005; Schaie et al., 2004). Cognitive testing was performed independently of the MRI and occurred on average 24 weeks prior to scanning (s.d. 34 weeks, range 2 years prior to 1.25 years after). The testing-MRI interval was not correlated with age (r = −0.01), any DTI metric (all p values > 0.05, uncorrected for multiple comparisons) or any other demographic factor. However, the testing-MRI interval was correlated with immediate recall (partial correlation ρ = −0.28 p < 0.01), delayed recall (ρ = −0.23 p < 0.01), word fluency (ρ = −0.23 p < 0.01), cognitive flexibility (ρ = −0.16 p < 0.05) and PS (ρ = −0.28 p < 0.01) but not reasoning, after accounting for age and gender. This suggests that at any given age at scanning, those taking the test earlier (i.e., prior to the MRI) had slightly better scores than those scanned later (i.e., after the MRI) as expected if scores decline with age.
All subjects were cognitively normal based on neuropsychological assessment and consensus review (MMSE ranged from 24–30 with 2 individuals scoring 24 and one individual scoring 25). Two measures of episodic memory (immediate and delayed recall), word fluency, two measures of executive function (reasoning and cognitive flexibility), and a measure of PS were used in this analysis. All cognitive measures were converted into t-scores using first occasion data from the entire SLS sample for standardization to permit quantitative, longitudinal comparisons across measures with different metrics (Schaie et al., 2005).
2.2.1 Episodic memory
Both immediate and delayed recall measures were used (Zelinski et al., 1993); (Zelinski and Kennison, 2007). For immediate recall, participants studied a 20-word list for 3.5 minutes followed by 3.5 minutes of free recall. Delayed recall involved free recall after 1-hour of interim activities (test-retest reliability r = 0.7) (Zelinski and Lewis, 2003). Words correctly recalled are scored. Immediate and delayed recall are highly correlated (r = 0.92, p < 0.001) and generated qualitatively identical results in all analyses. Combining immediate and delayed recall into a factor score resulted in qualitatively identical results to each individual score, e.g., DTI parameters were not associated with the factor score.
2.2.2 Word Fluency
The Primary Mental Abilities (PMA) word fluency test (Thurstone and Thurstone, 1949) requires participants to recall as many words as possible according to a lexical rule in a 5-minute period (e.g., words starting with the letter “S”). In factor analytic work it has been shown to load on verbal memory and ability (Schaie, Dutta, & Willis, 1991). No factor score for word fluency is available as factor analysis has shown that word fluency is a complex ability with loading on memory, verbal and speed factors (Willis, unpublished results).
2.2.2 Reasoning
The PMA reasoning test (Thurstone and Thurstone, 1949) requires participants to view a series of letters (e.g., abXcdXefXghX…) that are arranged according to one or more rules. Participants are asked to discover the rule(s) and mark the letter that should come next in the series. In the preceding example, normal alphabetical progression is interrupted with an “x” after every second letter and the solution would be the letter “i”. Scores represent the number of items (out of 30) that are answered correctly within a time limit of 6 minutes. The PMA reasoning test is highly correlated with a reasoning factor score (r = 0.88) that is composed of PMA reasoning, the ADEPT Letter Series (Blieszner et al., 1981) and number and word series measures (Schaie, 2005).
2.2.3 Cognitive flexibility
A factor score was derived from two timed measures in the Test of Behavioral Rigidity (Schaie, 2005): i) the Capitals test (Cap-R score): in which the number of words copied from a paragraph is compared to when subjects copy the same paragraph substituting capitals for lower case letters (and vise versa) and, ii), the Opposites test (Opp scores): in which subjects respond to three lists of simple words (list 1–3 (L1–3), 40 words/list) by first giving antonyms (L1), then synonyms (L2), and finally antonyms or synonyms depending on whether the stimulus word is printed in upper or lower case letters (L3). The Opp-R1 score represents the ratio of correct to total responses during L3 while, the Opp-R2 score is the ratio of correct responses in L3 compared to the correct responses in L1 and L2. Cognitive flexibility is calculated with the following equation {flexibility = 0.25*Cap-R + 0.35*Opp-R1 + 0.40*Opp-R2}.
2.2.4 Processing speed (PS)
A factor score was derived from three timed measures (Ekstrom et al., 1976; Schaie, 2005): i) Identical Pictures requires the subject to identify which of five numbered shapes or pictures in a row is identical to the model at the left of the row (number correct out of 50 items in 1.5 min); ii) Finding A’s requires the subject to identify the 5 words in a column of 40 words that contain the letter “a” (number correct out of 50 items in 1.5 min); and iii) Number Comparison requires the subject to inspect pairs of multi-digit numbers and indicate whether the numbers in each pair are the same or different (number correct out of 40 items in 1.5 min). PS is calculated by averaging the normalized values for each of the three tests.
2.3 Image acquisition
All scanning was performed on a Philips 3T Achieva scanner. The DTI scan consisted of a single-shot echo-planar sequence with the following parameters: TR/TE/flip angle: 10.5 s/63 ms/90°, a matrix size of 128 × 128, a FoV of 240 × 240 with a 2 mm slice thickness. Diffusion weighting consisted of 32 non-colinear gradient directions, a non-diffusion weighted b0 map and a b-factor set at 1000 s/mm2. Magnetization prepared rapid gradient echo (MPRAGE) imaging was performed using the following parameters; TR/TE/flip = 7 ms/3.2 ms/8°; a matrix size of 240 × 240 and with 160 sagittally collected slices and a slice thickness of 1 mm. FLAIR images were performed with a TR/TE/flip of 11 sec/125 ms/90°, respectively; with full coverage of the cerebrum using 0.86 × 0.86 × 3 mm transverse slices.
2.4 Image Analysis
DTI
For quality control DTIPrep (Liu et al., 2010) was used to correct for eddy-currents and head motion artifacts and to perform slice-wise intensity related artifact and gradient-wise checking. DTI scalar indices are estimated using the DTIProcess toolkit (www.niral.unc.edu). After this process 33 of the 188 data sets were rejected for having more than 20% bad directions resulting in data analysis being done on a total of 165 subjects. There were no significant differences in age, gender, or measures of health between subjects who were excluded from DTI processing and those included. FMRIB’s Diffusion Toolbox (FDT version 2.0) and Tract-Based Spatial Statistics (TBSS) were used to perform voxel-wise analysis of the DTI data (Smith et al., 2006). Preprocessing included the creation of 3D fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD) and radial diffusivity (RD) maps for each subject. For TBSS, each subject’s FA map was brought into a common space using FMRIB’s nonlinear registration tool (FNIRT), an average FA skeleton was created and thresholded at 0.2 to exclude large inter-subject variability, and each subject’s aligned FA data was projected onto this skeleton by searching perpendicular to the skeleton to find local maxima. Rather than using a standardized template, because of the older age of our study population, transformation matrices were calculated for each subject pair (162 × 161 calculations) and the most anatomically representative subject from our dataset was mathematically identified and used as a template. MD, AD and RD values were mapped onto the template by using the projection vectors from each individual’s FA-to-skeleton transformation (Smith et al., 2006).
White matter hyperintensities (WMHs)
Visual ratings of WMHs were performed in FLAIR images by a blinded fashion by a trained clinician (B.R.S.) using the 10-point Cardiovascular Health Study (CHS) scale (Yue et al., 1997). B.R.S. was trained by Dr. Dean K. Shibata who has considerable experience using the CHS scale (Fornage et al., 2011; Knopman et al., 2011). The CHS scale reflects actual volume of WMH and has good inter-rater reliability (Kapeller et al., 2003). Automated segmentation of WMH was performed with the open-source Fuzzy Lesion Extractor (FLEX, using default parameters) (Gibson et al., 2010), a semi-unsupervised method that employs clustering followed by template based spatial analysis. Each step of the FLEX protocol including skull stripping, WMH assignment and volume calculation was manually reviewed for quality assurance. Each subject’s WMH map was transformed into standard space and mapped onto the TBSS skeleton using the same transformation matrices as applied to DTI metrics. This allows us to determine the location of WMH within the skeleton and the proportion of the skeleton and each sub-region that is affected in each individual by WMH.
Cortical thickness
Using MPRAGE images, cortical reconstruction and volumetric segmentation was performed with the FreeSurfer image analysis suite version 5.1.0 (http://surfer.nmr.mgh.harvard.edu/). The technical details of these procedures are fully described elsewhere (Dale et al., 1999; Fischl et al., 1999). After automatic analysis, the grey/white matter surfaces were checked, edited, and reprocessed to ensure that tissue classification was as accurate as possible. For this manuscript, the average cortical thickness was obtained by calculating a weighted average of the 68 parcels defined by the Desikan atlas (Desikan et al., 2006). All processing was performed blinded on de-identified data.
Brain volumes
Using MPRAGE images, brain volumes were calculated using SIENAX which is part of the FSL toolbox (Smith et al., 2004, 2002). Briefly, brain tissue volume, normalized for subject head size, was estimated by extracting brain and skull images. The brain image was then affine-registered to MNI152 space (Jenkinson et al., 2002) in order to obtain the volumetric scaling factor used in head size correction. Next, tissue-type segmentation with partial volume estimation was carried out (Zhang et al., 2001) in order to calculate the total volume of brain tissue. In a similar fashion, gray and white matter volumes were calculated using FAST which is part of the FSL toolbox (Smith et al., 2004; Zhang et al., 2001).
2.5 Statistical analysis
Descriptive statistics, pairwise and partial correlations and hierarchical analysis were calculated with STATA 10. Average values for each DTI metric were calculated within the TBSS skeleton (99,336 voxels = 99cm3) or selected regions of interest (ROIs). For ROI analysis, the group average TBSS skeleton was overlaid with tracts from the JHU WM tractography atlas (Mori et al., 2005) provided in the FSL package; thus the ROIs represent TBSS aligned skeletal regions, not simple untransformed WM regions. These skeletal regions were edited to make them non-overlapping and chosen specifically for their anatomical connectivity (Figure 1A). ROIs are inclusive regions and do not represent individual WM tracts but rather anatomic WM regions. Given the non-Gausian distribution of WMH burden in the left anterior thalamic radiation (ATR) across subjects (73% of subjects with no WMH volume, 13% of subjects with < 1% WMH volume, 9% of subjects with < 10% WMH volume, 2% of subjects with < 20% WMH volume, and a single subject with 38% WMH volume), these values were transformed by taking the square root of the percentage prior to using them as an independent variable. Qualitatively similar results were obtained using non-transformed data.
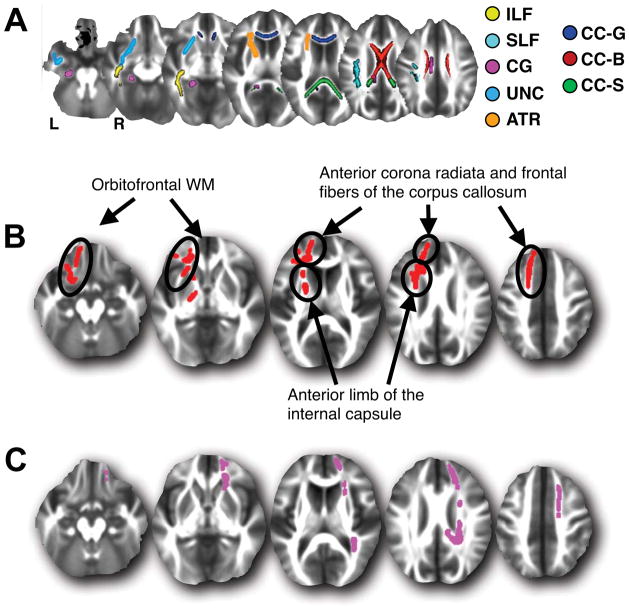
Figure 1.
(A) Skeletal WM regions of interest (ROI). Areas are shown in the left hemisphere only except for regions of the corpus callosum. Inferior and superior longitudinal fasciculi (ILF and SLF), cingulate gyrus (CG), uncinate fasciculus (UNC), anterior thalamic radiation (ATR), corpus callosum genu (CC-G), body (CC-B) and splenium (CC-S). (B) Areas where lower reasoning scores are associated with higher skeletal AD after accounting for the effects of age and gender. (C) Areas where lower flexibility scores are associated with higher skeletal AD after accounting for the effects of PS, age and gender. Depicted slices in (B) and (C) are the same and correspond to MNI z-values of -16mm, -2mm, 12mm, 26mm and 40mm.
Hierarchical regressions were performed to determine i) what proportion of the association between age and each cognitive ability was mediated by WM integrity, ii) what proportion of the association between PS and each cognitive ability was mediated by WM integrity, and iii) whether WM integrity continued to be significantly associated with higher order abilities if both age and PS were accounted for (Table 5). Two modeling approaches were used. Model 1 (Table 5): In partial correlations accounting for age and gender, each WM metric from the 13 ROIs (thus 13 ROIs × 4 WM metrics = 52 values) was screened for a significant association with cognitive measures. Subsequently, each WM metric identified as significantly correlated was included in the model as a IV regardless of the significance of its β values in the final model. Model 2 (Supplementary Table 3): Forward stepwise regressions using cognition as the dependent variable with each of the 52 WM metrics with significant β values (p < 0.05) were added stepwise as independent variables after accounting for age and gender. This resulted in a single WM metric associated with reasoning and flexibility.
Table 5.
Hierarchical regressions evaluating the mediation effects of DTI metrics on the association between PS and cognitive measures
| DV | IVs | β | R2 | F-value | Δ R2 | %MED | AF | |
|---|---|---|---|---|---|---|---|---|
| Reasoning | SR | Regions I | n.a. | 0.183 | 9.0 | |||
| Age | −0.398** | 0.160 | 15.4 | |||||
| PS | 0.620** | 0.389 | 64.1 | |||||
| HR | Regions I | n.a. | ||||||
| Age | −0.210* | 0.210 | 8.4 | 0.027 | 47% | 83% | ||
| Regions I | n.a. | |||||||
| PS | 0.553** | 0.435 | 24.2 | 0.252 | 11% | 35% | ||
| MR | Regions I | n.a. | ||||||
| PS | 0.539** | |||||||
| Age | −0.110n.s. | 0.442 | 20.6 | |||||
|
| ||||||||
| Flexibility | SR | Regions II | n.a. | 0.294 | 4.8 | |||
| Age | −0.416** | 0.205 | 20.9 | |||||
| PS | 0.318** | 0.133 | 12.3 | |||||
| HR | Regions II | n.a. | ||||||
| Age | −0.210* | 0.321 | 5.1 | 0.027 | 50% | 87% | ||
| Regions II | n.a. | |||||||
| PS | 0.178* | 0.310 | 5.0 | 0.016 | 44% | 88% | ||
| MR | Regions II | n.a. | ||||||
| PS | 0.153* | |||||||
| Age | −0.200* | 0.340 | 5.1 | |||||
ΔR2 represents the change in R2 when age (or PS) is added to the model after the WM metric. %MED is the % of the age (or PS) effect mediated by the WM metric. The attenuation factor (AF) represents the % reduction in the amount of variance explained by age (or PS) when WM metrics are added to the model first. Single regression (SR); hierarchical regression (HR); multiple regression (MR); processing speed (PS). See table 4 for anatomical abreviations. All F-values have p < 0.01.
p < 0.05,
p < 0.005. All models include gender as a CV of no interest
The proportion of a variable (e.g., age) mediated by another (e.g., a WM metric) was determined by calculating the difference between the β value of the variable alone as a predictor (e.g., βage) versus when the other variable is also in the model (e.g., βage_WM) in the equation (%MED = (βage − βage_WM)/βage × 100). Likewise, the attenuation factor (AF) is determined by calculating variance accounted for when the variable alone is a predictor (e.g., R2age) versus the amount of additional variance explained by the variable in a hierarchal regression (e.g., ΔR2age_WM) in the equation (AF = (R2age − ΔR2age_WM)/R2age × 100) (Salthouse, 1993). Mediation analysis requires that all variables are significantly correlated in pair-wise comparisons (Baron & Kenny, 1986). Pairwise correlations for age, PS and higher order abilities were all significant (Table 2). Likewise the pairwise correlations between regional DTI parameters used in our hierarchical regressions and age, PS and the higher order ability were highly significant with uncorrected p-values < 0.005 (not shown).
Table 2.
Pearson’s correlations (r) between age, processing speed (PS) and higher order cognitive abilities
| Age | PS | Immediate recall | Delayed recall | Fluency | Reasoning | |
|---|---|---|---|---|---|---|
| PS | −0.36** | -- | ||||
| Immediate recall | −0.26** | 0.51** | -- | |||
| Delayed recall | −0.24** | 0.47** | 0.92** | -- | ||
| Word fluency | −0.15* | 0.62** | 0.38** | 0.38** | -- | |
| Reasoning | −0.43** | 0.64** | 0.33** | 0.35** | 0.44** | -- |
| Flexibility | −0.40** | 0.34** | 0.29** | 0.22** | 0.26** | 0.44** |
Uncorrected
p < 0.05,
p < 0.005. All p-values significant using a Benjamini and Hochberg (1995) approach to controlling for multiple comparisons (n=21 tests).
Voxel-wise analysis was performed using FSL’s ‘Randomise’ method, a permutation-based method developed for applications where the null distribution is unknown (Nichols and Holmes, 2002). For all TBSS, 5000 permutations were performed and only voxels with p values < 0.05 (one-sided tests) fully corrected for multiple comparisons are reported unless noted. 3D maps of the DTI metric were used as the dependent variables. Independent predictor variables included cognitive scores, PS, age and gender. Randomise performs a type 1 partial sum of squares analysis and only reports the orthogonal significance level. Thus significant voxels reported for covariates represent areas where the covariate accounts for significant variance after accounting for all other variables in the model. Significant portions of the WM skeleton were thickened in Figure 1 for easy visualization.
Multiple comparisons were controlled for using a Benjamini-Hochberg (1995) procedure (Benjamini and Hochberg, 1995). In this method, pi values from a given series of statistical tests are ordered from smallest to largest: p1 < p2 < p3 < … < pm where m is the # of tests. Pi is considered significant if pi < (i/m) * α, where i the place of pi in the ordered list and α = 0.05. The largest p value (pm, i.e., the least significant) is tested first and if this test is significant all other tests with lower p values are considered significant. If pm is not significant, then pm−1 followed by pm−2 and continuing with p values of decreasing value (increasing significance) are tested per the prior equation until one is found significant or p1 is reached.
3. RESULTS
3.1 Study participants and the association between cognition, PS and age
Study participants (n=165, Table 1) represent a well educated, largely Caucasian, typically aging population who had no clinically diagnosed cognitive disorder at the time of imaging (e.g. MCI or dementia). Visual WMH ratings were associated with age (ordered logistic regression, pseudo R2 = 0.10, F = 52.8, p < 0.001) but were generally low with 80% having CHS ratings of 2 or less on a 10-point scale indicative of mild disease (Yue et al., 1997). 73% had a single or no vascular risk factors (hypertension, diabetes or hyperlipidemia). The frequency of APOE 4 carriers was 31% (heterozygous or homozygous), similar to that reported for Northern European communities from which many of our participants derive (Saunders et al., 1993).
Table 1.
Study participants (n=165)
| Age (s.d.) | 66.9 (7.8) | |
| (range) | (54–89) | |
| % Female | 60% | |
| Years education (s.d.) | 16.2 (2.5) | |
| % Caucasian | 96% | |
| % APOE 4 carriers | 31% | |
| MMSE (s.d.) | 29.2 (1.2) | |
|
| ||
| CHS WMH scorea | 0 – 2 | 80% |
| > 2 | 20% | |
|
| ||
| # Vascular risk factorsb | 0 or 1 | 73% |
| 2 or more | 27% | |
|
| ||
| WMH Burden in cm3 (s.d.) | 3.86 cm3 (5.04) | |
| (range) | 0.03 – 34.08 | |
Cardiovascular Health Study (CHS) WMH scores can range from 0 to 9 with scores between 0 and 2 considered mild (Yue et al, 1997).
Vascular risk factors include hypertension, hyperlipidemia, diabetes and smoking.
Mini Mental Status Exam (MMSE); Apolipoprotein (APOE)
Typical patterns of age-related cognitive decline were apparent for each measure (recall, word fluency, reasoning and flexibility) and PS (Table 2). Older age was associated with lower cognitive scores and slower PS (negative r-values) while faster PS was associated with higher cognitive scores (positive r-values). Although significantly correlated, individual higher order cognitive abilities other than immediate and delayed recall (r = 0.92) accounted for < 20% of the variance in any other ability (r-values 0.22 to 0.44), leaving a majority of the variability unexplained. Word fluency was less highly correlated with age (r = −0.15, p < 0.05) than other cognitive measures and PS.
3.2 Voxel-wise associations between WM metrics and cognitive measures after accounting for age or PS
To determine if and where in the brain higher cognitive abilities and PS were associated with WM integrity, 3D voxel-wise analysis was performed on the WM skeleton using DTI metrics (FA, MD, AD and RD) as dependent variables and cognitive abilities and/or PS as predictors, with/without age and gender as covariates of no interest. As expected, older age was associated with lower FA and higher MD in 84% and 95% of the WM skeleton, respectively. Age was not associated with higher FA or lower MD in any region. Within regions of age-related changes in FA and MD, both AD (~95% of the voxels) and RD (~98% of the voxels) were increased, with no areas of decreased AD or RD. Thus older individuals had greater AD and RD values that resulted in lower FA and higher MD throughout a vast majority of the WM skeleton. When uncorrected t-scores are mapped, higher MD, AD and RD appear to be more significant (i.e., greater uncorrected t-values) in a greater proportion of the WM skeleton than decreasing FA values; specifically in regions of the anterior/superior corona radiata (Supplementary Figure 1). Likewise, prior to covarying for age and gender, higher cognitive ability scores (except word fluency) and faster PS were associated with higher FA and lower MD, AD and RD in a large proportion of the WM skeleton (data not shown). Given the large proportion of voxels significantly associated with age, higher order cognitive abilities and PS when they were modeled alone, no overt localization (e.g., parietal vs. frontal) could be discerned for any variable and maps of these associations are not presented.
To determined if cognitive abilities and PS continued to be associated DTI metrics after accounting for age, models were tested that included age and gender as covariates (Table 3, single regressions). Faster PS continued to be associated with higher FA, and lower MD, AD and RD in overlapping, diffuse regions of the WM skeleton after accounting for age (Supplementary Figure 2). Neither immediate or delayed recall, nor word fluency, were associated with skeletal WM integrity after covarying for age and gender. For reasoning, select regions containing ~5% of the voxels remained associated with AD. Areas where reasoning was associated with AD included the left uncinate fasciculus and orbitofrontal areas, and the anterior thalamic radiation and frontal fibers of the corpus callosum (Figure 1B). Akin to PS, higher flexibility scores were related to lower MD, AD and RD in a large percentage (12–36%) of the WM skeleton after accounting for age and gender (Table 3, single regressions). In contrast to reasoning (Figure 1B), Supplementary Figure 2 shows that the association of flexibility with WM metrics is not anatomically restricted to the frontal lobe, but dispersed throughout the entire brain. This demonstrates that reasoning and flexibility account for variance in WM integrity beyond that explained by age.
Table 3.
Voxel-wise associations between WM integrity, higher cognitive abilities, PS and age
| Higher FA |
Lower MD |
Lower AD |
Lower RD |
|
|---|---|---|---|---|
| SRs with age and gender as CVs | ||||
| PS | 35 | 32 | 14 | 32 |
| Immediate recall | 0 | 0 | 0 | 0 |
| Delayed recall | 0 | 0 | 0 | 0 |
| Word fluency | 0 | 0 | 0 | 0 |
| Reasoning | 0 | 0 | 5 | 0 |
| Flexibility | 0 | 28 | 36 | 12 |
|
| ||||
| MRs with age and gender as CVs | ||||
| Reasoning | 0 | 0 | 0 | 0 |
| PS | 38 | 19 | 0 | 29 |
| Flexibility | 0 | 0 | 3 | 0 |
| PS | 30 | 16 | 1 | 21 |
| Reasoning | 0 | 0 | 0 | 0 |
| Flexibility | 0 | 0 | 0 | 0 |
| PS | 37 | 16 | 0 | 29 |
Summary of voxel-wise TBSS analysis. Values represent the % of voxels within the WM skeleton significantly associated with the cognitive measure (one tailed test p < 0.05, fully corrected for multiple comparisons). Single regression (SR), multiple regression (MR), covariates (CVs), processing speed (PS).
We next determined if WM metrics remained associated with either reasoning or flexibility after accounting for PS in addition to age and gender. When reasoning and PS were modeled together, reasoning no longer accounted for significant WM variability while PS remained associated with WM integrity throughout the brain (Table 3, multiple regressions). In contrast, when flexibility and PS were modeled together, flexibility remained associated with select regions of AD in the right frontal and parietal lobes including areas of the right uncinate fasciculus, anterior thalamic radiation and anterior corpus radiata (Figure 1C), while PS remained associated with diffuse regions of FA, MD and RD (data not shown). Thus, for reasoning and flexibility, AD appears more closely associated with the ability than the other WM parameters. When all three cognitive parameters (reasoning, flexibility and PS) are modeled together as independent variables, only PS continues to explain unique variance in FA, MD and RD after controlling for age and gender (Table 3).
3.3 Correlations between regional DTI parameters age, cognitive abilities and PS
Average skeletal WM metrics were associated with PS and flexibility after accounting for age and gender (Supplementary Table 1) directly mirroring our voxel-wise analysis. To determine if regional WM integrity mediated the associations between reasoning, flexibility, age and PS, average WM metrics were extracted from skeletal areas corresponding to the major white matter tracts of the frontal lobes and the corpus callosum. These regions were chosen a priori given the association of the prefrontal cortex with executive function (Salthouse, 2011) and included the i) cingulum (CG) ii) the inferior and superior longitudinal fasciculi (ILF and SLF), iii) the uncinate fasciculus (UNC), iv) the anterior thalamic radiation (ATR) and v) the genu, body and splenium of the corpus callosum (CC-G, CC-B and CC-S, see Figure 1A).
Older age was associated with lower FA and higher MD, AD and RD in every WM ROI (uncorrected p-values of Pearson’s r < 0.005, data not shown). After accounting for age and gender, PS was associated with WM in multiple regions (Supplementary Table 2) with ventral areas (e.g., UNC, ILF, and ATR) appearing to be more highly associated dorsal areas (e.g., SLF and CG). After correcting for multiple comparisons, only WM parameters in the right ILF and left ATR remained significantly associated with PS. Given our findings with voxel-wise TBSS we opted to restrict our analysis with reasoning to ROI AD values since other parameters were not found to be associated with reasoning (n = 13 correlations). After accounting for age and gender, reasoning was associated with AD in the left UNC, left ATR and right ILF (Table 4, Regions I). Analogously, we restricted our analysis with flexibility to average MD, AD and RD (n = 13 × 3 correlations). After accounting for age and gender, flexibility was found to be associated with multiple areas of MD, AD and RD in no obvious pattern (Table 4, Regions II). The association between regional AD values and reasoning did not survive correction for multiple comparisons while several regional MD and AD values did remain associated with flexibility using a HB procedure to correct for type 1 error.
Table 4.
Partial correlations (ρ) of regional DTI parameters with higher order cognitive abilities
| Region | Partial Correlation | p value | |
|---|---|---|---|
| Reasoning (Regions I) | L.ATR AD | −0.20 | 0.012 |
| R.ILF AD | −0.19 | 0.013 | |
| L.UNC AD | −0.18 | 0.025 | |
|
| |||
| Flexibility (Regions II) | L.SLF MD | −0.19 | 0.013* |
| R.CG MD | −0.20 | 0.013* | |
| R.ATR MD | −0.19 | 0.013* | |
| R.ILF MD | −0.19 | 0.015* | |
| L.UNC MD | −0.17 | 0.026 | |
| L.ILF MD | −0.16 | 0.044 | |
| R.ILF AD | −0.25 | 0.001* | |
| R.ATR AD | −0.21 | 0.008* | |
| L.SLF AD | −0.21 | 0.008* | |
| L.ILF AD | −0.19 | 0.014* | |
| L.UNC AD | −0.19 | 0.015* | |
| R.CG AD | −0.17 | 0.030 | |
| R.CG RD | −0.20 | 0.011 | |
| R.ATR RD | −0.18 | 0.020 | |
| L.SLF RD | −0.18 | 0.024 | |
| L.UNC RD | −0.16 | 0.044 | |
Partial correlations of reasoning and flexibility with regional MD, AD and RD after controlling for the effects of age and gender. Only regions with p < 0.05, uncorrected for multiple comparisons, are listed. See Figure 1 for anatomical abbreviations.
significant after BH procedure for controlling for multiple comparisons.
3.4 The ability of WM integrity to mediate the effects of PS and age on reasoning and flexibility
To determine if WM parameters identified in our correlation analysis (section 3.3) could mediate variance in reasoning and flexibility explained by age and PS, we performed a series of hierarchical regressions. The maximum amount of variance in reasoning and flexibility that could be accounted for by WM metrics was determined by using WM integrity values from all the regions identified in the correlational analysis with an uncorrected p < 0.05. Thus for reasoning, 3 AD regions (Regions I, Table 4), and for flexibility, 16 WM parameters (Regions II, Table 4) were used in the models. We found that WM regions II accounted for more of the variance in flexibility (29%) than WM regions I did for reasoning (18%, single regressions in Table 5). However, for both abilities, WM regions mediated ~50% of the variance attributed to age but a different proportion of the variance attributed to PS, 11% for reasoning and 44% for flexibility (hierarchical regressions in Table 5). Finally, when WM regions, PS, age and gender are all included in the model, 44% of the variance in reasoning and 34% of the variance in flexibility can be explained (multiple regression in Table 5). For WM metrics included in regions II, we explored if regional MD (n=6), AD (n=6) and RD (n=4) explained differing amounts of variance in flexibility. No dramatic differences were found; with R2 = 0.225 F=6.5 for MD values; R2 = 0.249 F=7.4 for AD values; and R2 = 0.211 and F = 8.5 for RD values.
Similar results were found using forward stepwise regressions to identify WM regions accounting for significant variance in reasoning and flexibility. Herein, reasoning or flexibility was used as the dependent variable and all 52 DTI parameters were screened as possible predictors in a stepwise fashion (4 DTI metrics X 13 regions) after inclusion of age and gender as covariates. We found that AD in single ROIs, the left ATR for reasoning and the R. ILF for flexibility could account for 14% and 21% of the variance in reasoning and flexibility, respectively (SRs in Supplementary Table 3). Similar to our results using multiple regions above, AD accounted for a similar amount of variance in reasoning (30%) and flexibility (38%) attributed to age, but less of the variance in reasoning (7%) than flexibility (40%) attributed to PS. Importantly, and in contrast to prior reports (Salami et al., 2011), the β values for these individual DTI parameters remained significant in the models after including PS. When individual WM metrics, PS, age and gender were all included as predictors, the WM metric continued to account for significant variance in flexibility but not reasoning as illustrated by their significant β values in the final multiple regression (MRs in Supplementary Table 3).
3.5 White matter hyperintensities and cognition
As discussed, our typically aging sample is relatively healthy with a low incidence of cardiovascular risk factors and few WMH (Table 1). To assess the role of WMH in cognition, WMH were mapped in each individual using automated segmentation (Gibson et al., 2010). WMH burden (i.e., volume) was significantly associated with age (F1,185 = 39.2, p < 0.001, adjR2=0.17) with ventricular end-capping found in ~15% of subjects and periventricular hyperintensities in ~10% (Supplementary Figure 3). Total WMH burden was not significantly associated with any cognitive variable after accounting for age and gender (F3,161 and β values for the WMH term were never significant with p > 0.1). However, multiple regressions revealed that greater WMH burden was associated with lower skeletal FA and greater skeletal MD, AD and RD after accounting for age and gender (Supplementary Table 4). Individual subject’s WMH maps were then transformed onto the standard space WM skeleton (see methods). In voxel-wise analysis, age was associated with WMH throughout the WM skeleton (data not shown) while none of our higher cognitive abilities or PS was significantly associated with WMH after accounting for age.
To control for WMH burden in the mediation analysis, we calculated the percent of each ROI affected by WMHs. Only the bilateral ATRs, which lie anterior-lateral to the frontal horns of the lateral ventricles, i.e., the regions were periventricular end capping occurs, had > 1% WMH in more than 10 subjects. Given this, regional WMH burden could not be accurately modeled in our regressions since most subjects had a trivial burden. Excluding subjects with > 1% WMH burden in any individual ROI in the reasoning and flexibility regressions reported in Table 5 and Supplementary Table 3 had no meaningful impact on the significance or interpretation of the models (data not shown). In the left ATR WMH burden ranged from 0 to 37% with 119 subjects having no WMHs and the average burden within 44 subjects with WMH being ~4% (s.d. 6%) of the ROI. Including left ATR WMH burden in regressions with reasoning as the dependent variable as done in Supplementary Table 3 increased the significance of left ATR AD in predicting reasoning. In MRs with reasoning as dependent variable, βL.ATR AD=−0.074, p=0.32 (see Table 5); while after inclusion of WMH burden as a covariate, βL.ATR AD =−0.217, p < 0.05 (Fmodel=25.8, R2=0.45).
Grey matter volume, cortical thickness and cognitive abilities
The relationship between brain volumetric measures, cognition and DTI metrics was explored. Flexibility was the only cognitive variable significantly correlated with either raw or intracranial volume (ICV) corrected cerebral volumes (Supplementary Table 5). The partial correlation of flexibility, after accounting for age and gender, with ICV adjusted GM volume was ρ = 0.16 (p < 0.05) and given that 36 correlations were performed we believe this may reflect type I error. In contrast, GM thickness, volume and brain volume, but not WM volume (excluding FA), were all highly associated with DTI metrics. After controlling for age and gender, greater brain or GM volume is associated with higher FA and lower MD, AD and RD (Supplementary Table 5). Thus larger brain and GM volumes are not directly related to higher cognitive abilities but are related to DTI metrics, suggesting that DTI metrics are more tightly linked to cognitive abilities.
4. DISCUSSION
Cognitive decline during typical aging is highly variable with substantial inter-individual differences. Herein, we examined where WM integrity is related to higher order cognitive abilities and whether it mediates the associations between higher order abilities, age and PS. Overall, we found that after accounting for the effects of age and gender, WM integrity is associated with reasoning, flexibility, and PS but not memory or word fluency. This suggests that WM integrity is important for coordinating function of distant cortical regions during higher cognitive processing. Voxel-wise analysis revealed that the spatial localization of the associations between WM integrity and cognition is variable. Some abilities (e.g., immediate and delayed recall, word fluency) have no significant association with WM integrity, others (e.g., reasoning) are selectively associated with individual DTI metrics in specific regions, while others (e.g. cognitive flexibility and PS) are related to WM integrity throughout the cerebrum. Finally, WM metrics mediated a significant proportion of age-associated variance in reasoning and flexibility but less of the variance associated with PS. In addition to these major findings, several other results are of interest. First, wide spread age-related reductions in FA and increases in MD are both associated with increases in AD and RD. Second, FA was more associated with PS than other cognitive measures. Third, and in agreement with prior reports (Salami et al., 2011), PS appears to be more tightly associated with WM integrity than other cognitive measures, but the exact relationship differs for each cognitive domain. Finally, whole brain volumetric measures and WMHs were related to DTI metrics but not cognition illustrating the centrality of WM integrity in higher cortical function.
4.1 Localization of WM integrity related to higher order cognitive abilities and PS
Voxel-wise analysis revealed that frontal WM integrity was associated with reasoning and flexibility after accounting for age and/or PS. As anticipated by prior work (reviewed in (Gunning-Dixon et al., 2009; Madden et al., 2012; Sullivan and Pfefferbaum, 2006)) age is a strong predictor of WM integrity and we found that a majority of the WM skeleton demonstrated lower FA and higher MD, AD and RD in older individuals. After accounting for age, select regions of left frontal AD were associated with reasoning while AD, RD and MD throughout the cerebrum were associated with flexibility. Areas of AD associated with reasoning included regions of commissural fibers (forceps minor), projection fibers (corona radiata), thalamicocortical fibers (anterior thalamic radiations) and fibers connecting the left ventral prefrontal regions to medial temporal regions (uncinate fasciculus) and thus could underlie many aspects of executive function (see (Schmahmann and Pandya, 2009)). This is consistent with substantial literature localizing executive function to the prefrontal lobes (Stuss and Knight, 2002) and reports relating prefrontal WM integrity to executive function (Davis et al., 2009; Kennedy and Raz, 2009). The global association of WM integrity with flexibility may reflect the close association of flexibility with PS (Schaie et al., 1991).
After accounting for age, PS remained associated with WM integrity throughout the cerebrum. This is consistent with prior reports demonstrating the strong associations between PS and WM integrity (Kennedy and Raz, 2009; Madden et al., 2009b). Furthermore, when age and individual higher order abilities were included as predictors, PS remained variably, but more robustly, associated with DTI metrics than cognitive abilities did (again, with the exception of AD and flexibility). Thus, while accounting for PS and age reduced or entirely accounted for the associations between DTI metrics and higher cognitive abilities, PS continued to variably predict DTI metrics, demonstrating that PS is more tightly linked to WM structure than most other abilities.
Finally, immediate and delayed recall and word fluency were not found to be associated with WM integrity which is consistent with some (Salami et al., 2011; Vernooij et al., 2009), but not other reports (Deary et al., 2006; O’Sullivan et al., 2001). In our sample word fluency was assessed by the number of “S” words generated in 5 minutes and it was only weakly associated with age, although it was highly associated with PS (Deary et al., 2006; O’Sullivan et al., 2001). In contrast both immediate and delayed recall were moderately correlated with age (r = 0.24 and 0.26, respectively) showing that the lack of association with DTI metrics cannot be solely accounted for by differing age-related changes.
4.2 Regional WM integrity partially mediates the association between age and higher order abilities
Regional WM integrity (i.e., average DTI metrics from within ROIs) accounted for a small proportion of variance in reasoning and flexibility and could mediate ~50% of the variance attributed to age. WM metrics appear to mediate a greater proportion of variance in flexibility than reasoning (R2 = 0.29 and 0.20, respectively). Areas of AD found to be associated with reasoning include, amongst others, fibers of frontal-striatal circuits of the dorsolateral prefrontal cortex and are likely central to executive function (Saint-Cyr, 2003). That WM metrics in the ILF (both sides) were associated with reasoning and/or flexibility may be due to the centrality of visual processing (e.g., occipitotemporal connections) on our cognitive paradigm.
Of note, after performing voxel-wise analysis with TBSS we did not extract average DTI metrics from the significant regions to perform mediation or other statistical tests, given the multitude of problems interpreting post-hoc statistics from statistically defined regions, especially given the non-independence of our covariates (Kriegeskorte et al., 2009; Vul et al., 2009). Thus, our ROIs of the aforementioned areas were drawn from a priori from an anatomical atlas.
4.3 Regional WM integrity partially mediates the association between PS and higher order abilities
Regional WM integrity mediated a variable proportion of the association between PS, reasoning and flexibility. Declining PS has been proposed to account for a significant proportion of cognitive decline during typical aging (Finkel et al., 2007; Salthouse, 1996). Mechanisms underlying this are unknown but have been hypothesized to involve changes in the “scaffolding” or the coordination of large neural systems secondary to multiple insults associated with aging and disease (Park and Reuter-Lorenz, 2009). As expected, PS can account for 62% or 32% of the association between age and reasoning or flexibility, respectively (statistics not shown). Thus WM integrity was unable to explain most of the association between PS and higher order abilities, and accounting for PS did not fully explain the association between reasoning, flexibility and WM integrity.
4.4 Relationship of WM metrics to cerebral volumes and WMH burden
The primary aim of this paper was to explore the relationship of WM metrics with cognition. In the process brain volumes and WMH burden was assessed and we found that neither cerebral volumetric parameters (GM volume, WM volume, and GM thickness) nor total or regional WMH burden was associated with cognitive measures while most of these parameters were associated with WM integrity. There are few reports that examine the relationship of WM integrity, WMH burden and cognition during aging. While cortical thickness and WM integrity is highly correlated across the lifespan (Kochunov et al., 2011a) and share genetic underpinnings (Kochunov et al., 2011b) there are mixed reports of how these parameters are related to cognition. In a mixed sample of healthy controls and individuals with cognitive impairment, WMH burden was related to cognition while FA summary scores were not (Meier et al., 2012) while GM and WMH volume were related to regional FA (and cognitive status) to a greater extent than FA was related to cognitive status (Lee et al., 2010). In non-demented samples WM metrics appear more strongly associated with cognition than WMH burden (Schiavone et al., 2009). Our findings suggest that while volumetric measures and WMH burden are highly related to WM integrity, WM integrity may actually be more proximal to cognitive function.
4.5 Interpreting WM metrics
In voxel-wise analysis, we found that after accounting for age and gender, FA was less associated with higher order abilities than PS, and in contrast with Davis et al (2009), we found MD, AD and RD to be variably associated with other cognitive abilities and PS. Finally, right cerebral AD was selectively associated with cognitive flexibility after accounting for PS, age and gender. Although anisotropy of water diffusion is known to reflect WM microstructure, with AD more related to axonal properties and RD more related to myelination (Concha et al., 2010), the exact histological correlates of FA, MD, AD and RD remain obscure. Axonal loss in SOD1 mice is associated with reduced AD and FA (Underwood et al., 2011) and in Wallerian degeneration reductions in AD coincide with axonal injury and increasing RD with demyelination (Sun et al., 2008). However, in other disease models results are less definitive. For instance, after trauma AD accurately reflected axonal damage but RD was not associated with demyelination (Budde et al., 2007) and in hypoxia AD reflected axonal damage whereas MD was not associated with axonal pathology (Holland et al., 2010). Likewise, in one of the few studies of fresh tissue in humans (Concha et al., 2010), FA accurately reflected density of axonal membranes whereas MD, AD and RD were not clearly associated with any specific histologic measure of WM structure, although there was a trend for RD to be associated with myelination and intra vs. extra-axonal space. Beyond this, the interpretation AD and RD is further complicated by the density of crossing fibers, WM pathology and partial voluming, all which may impact the biophysical interpretation of the eigen-vectors (Wheeler-Kingshott and Cercignani, 2009).
With this in mind, it is unclear why select regions of AD are more associated with flexibility than PS, whereas diffuse regions of FA and RD are more associated with PS than flexibility. Expansion of the extracellular matrix or loss of WM complexity (Norris, 2001), via reduction of axon collaterals or crossing fibers (Counsell et al., 2006) could lead to higher AD, and this process could be more fundamental for flexibility than PS. Using a similar approach with MD as a dependent variable, Kantarci et al (2011) have shown that regional cortical MD values are selectively associated with various cognitive domains. In contrast to our results, they report that memory and language are associated with temporal lobe cortical MD whereas no specific cortical associations could be found for executive function or visual-spatial PS (Kantarci et al., 2011).
Declining FA during aging has been largely attributed to increases in RD, more so than decreases in AD (Bhagat and Beaulieu, 2004; Sullivan and Pfefferbaum, 2006) although prior reports using age stratified samples revealed increases in both AD and RD during aging (Bennett et al., 2010; Burzynska et al., 2010). These WM changes are suggested to reflect changes in myelination rather than loss of axons (Madden et al., 2009b). In our sample, aging was accompanied by higher, not lower, AD values although proportionally greater increases in RD resulted in the well established decline in FA throughout the cerebral white matter. This pattern of changes may reflect axonal loss, myelin degradation and expansion of the extracellular component (Sen and Basser, 2005). Regions of declining AD in the anterior/superior corona radiata and paricallosal regions of the frontal cortex have also been reported in aging (Bennett et al., 2010). Although we did not find any regions of decreasing AD it is of interest that AD in the anterior/superior corona radiata and pericallosal fibers was specifically associated with reasoning and flexibility. This suggests that while increasing AD in individuals > 65 may be associated with aging and loss of executive function, across the lifespan, when executive functions are maturing (i.e., between age 20 and 65, (Schaie, 2005)) AD is actually diminishing in these same areas. One interpretation of these findings is that reductions in AD prior age 65 may reflect functionally beneficial changes while increases in AD after age 65 reflects degeneration. However, as we have noted, the interpretation of DTI WM integrity parameters is tenuous at this time.
4.5 Study limitations
Limitations to this study are its cross-sectional design, reliance on a single technique (TBSS) to evaluate DTI metrics and the use of valid but atypical measures of cognitive abilities. Cross sectional studies are confounded by cohort effects and thus we cannot unambiguously conclude that the age/cognition associations we have observed truly represent age-related changes. Longitudinal imaging is underway to evaluate this more completely and determine the rate at which FA/MD declines during aging. Our cognitive testing-MRI interval was variable, occurring on average 24 weeks prior to scanning. As would be predicted, at any given MRI age, those tested earlier (who would be younger at testing), scored better than those tested later. However, the testing-MRI interval was not associated with any DTI or volumetric parameter and thus does not change the interpretation of our results. The testing-MRI interval was variable secondary to the complexities of the longitudinal design and MRI scheduling.
We chose to use TBSS for our analysis given the difficulties of appropriately aligning WM structures necessary for group level voxel-wise analysis. Careful visual inspection of the alignment steps confirmed that it was not possible to “warp” a group level region of interest into subject specific space whereas the TBSS skeleton appeared to accurately reflect individual subjects WM structure in all cases. The Seattle Longitudinal Study has been ongoing since 1956 necessitating the consistency of cognitive metrics employed over the decades and the sample is largely comprised of middle-class Caucasian subjects (Schaie, 2005). In addition, we chose to use average GM and WM volumes rather than regional volumeteric parameters because simultaneous analysis of DTI metrics with GM (or WM) variations was felt to be beyond the scope of this paper. Furthermore, although anatomically meaningful, TBSS does not provide connectivity information and thus WM integrity changes found within a region cannot be attributed to specific regions of the cortex. Future work will undoubtedly untangle these complicated regional volumetric-DTI relationships.
Accounting for genetic, demographic and biological factors in this study is difficult. For voxel-wise analysis, gender was included as a covariate of no interest to ensure that reported associations did not reflect gender effects. We did not include gender in our mediation analyses given that it would change reported R2 and F values, and thus the calculated mediation effects. Controlling for either cortical atrophy or the extent of WMH raises other issues. Both factors are associated with aging and WM metrics. Given this, and similar to other authors (Bucur et al., 2008; Salami et al., 2011), we did not feel it was appropriate to control for either factor in mediation or voxel-wise analysis. Although > 50% of the general population has a least one WMH at age 50 (Wen et al., 2009) how they are associated with cognitive decline during typical aging remains unclear (Debette and Markus, 2010). Fortunately, 80% of our subjects have CHS WMH rating of 2 or less, reflecting rather minimal white matter disease (Yue et al., 1997).
4.5 Summary
These data show that WM integrity is variably associated with higher order cognitive abilities above and beyond that explained by age or PS. Episodic memory, reasoning and flexibility were all significantly related to WM integrity after accounting for age or PS. Voxel-wise analysis confirmed that the associations between reasoning and WM integrity are the strongest in the frontal lobe, whereas flexibility was associated with WM integrity throughout the cerebrum. Moreover, AD appeared more strongly related to higher order abilities than FA or RD and regional AD values could mediate a significant proportion of variance attributed to age or PS. These data demonstrate that loss of WM integrity contributes to cognitive change during typical aging in ways other than its effects on PS and suggests that coordination of distant brain regions via axonal connections is essential for higher order cognitive abilities.
Supplementary Material
HIGHLIGHTS.
Cognitive abilities are differentially associated with white matter integrity
Reasoning is associated with axial diffusivity in the left prefrontal cortex
Flexibility is associated with white matter integrity throughout the brain
White matter integrity can partially account for variance attributed to speed
Acknowledgments
We would like to thank the staff of the Seattle Longitudinal Study for coordinating the imaging sessions and neuropsychological assessments, and Paul Choi MD and UW Radiology staff for MRI acquisition. As always, we are indebted to our study participants. This work was supported by National Institute of Aging (grant no. R37-AG024102) and PRB is a KL2 scholar supported by National Center for Research Resources (grant no. 5KL2RR025015-04).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Sultzer D, Lu PH, Nuechterlein KH, Mintz J, Cummings JL. Heterogeneous age-related breakdown of white matter structural integrity: implications for cortical “disconnection” in aging and Alzheimer’s disease. Neurobiol Aging. 2004;25:843–851. doi: 10.1016/j.neurobiolaging.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society Series B (Methodological) 1995;57:289–300. [Google Scholar]
- Bennett IJ, Madden DJ, Vaidya CJ, Howard DV, Howard JH., Jr Age-related differences in multiple measures of white matter integrity: A diffusion tensor imaging study of healthy aging. Hum Brain Mapp. 2010;31:378–390. doi: 10.1002/hbm.20872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagat YA, Beaulieu C. Diffusion anisotropy in subcortical white matter and cortical gray matter: changes with aging and the role of CSF-suppression. J Magn Reson Imaging. 2004;20:216–227. doi: 10.1002/jmri.20102. [DOI] [PubMed] [Google Scholar]
- Blieszner R, Willis SL, Baltes PB. Training research in aging on the fluid ability of inductive reasoning. Journals of Applied Developmental Psychology. 1981;2:247–265. [Google Scholar]
- Bucur B, Madden DJ, Spaniol J, Provenzale JM, Cabeza R, White LE, Huettel SA. Age-related slowing of memory retrieval: contributions of perceptual speed and cerebral white matter integrity. Neurobiol Aging. 2008;29:1070–1079. doi: 10.1016/j.neurobiolaging.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budde MD, Kim JH, Liang HF, Schmidt RE, Russell JH, Cross AH, Song SK. Toward accurate diagnosis of white matter pathology using diffusion tensor imaging. Magnetic Resonance in Medicine. 2007;57:688–95. doi: 10.1002/mrm.21200. [DOI] [PubMed] [Google Scholar]
- Burzynska AZ, Preuschhof C, Bäckman L, Nyberg L, Li SC, Lindenberger U, Heekeren HR. Age-related differences in white matter microstructure: Region-specific patterns of diffusivity. NeuroImage. 2010;49:2104–2112. doi: 10.1016/j.neuroimage.2009.09.041. [DOI] [PubMed] [Google Scholar]
- Concha L, Livy DJ, Beaulieu C, Wheatley BM, Gross DW. In vivo diffusion tensor imaging and histopathology of the fimbria-fornix in temporal lobe epilepsy. J Neurosci. 2010;30:996–1002. doi: 10.1523/JNEUROSCI.1619-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counsell SJ, Shen Y, Boardman JP, Larkman DJ, Kapellou O, Ward P, Allsop JM, Cowan FM, Hajnal JV, Edwards AD, Rutherford MA. Axial and radial diffusivity in preterm infants who have diffuse white matter changes on magnetic resonance imaging at term-equivalent age. Pediatrics. 2006;117:376–386. doi: 10.1542/peds.2005-0820. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical Surface-Based Analysis: I. Segmentation and Surface Reconstruction. NeuroImage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Smith SM, Witter MP, Sanz-Arigita EJ, Barkhof F, Scheltens P, Stam CJ, Zarei M, Rombouts SARB. White matter tract integrity in aging and Alzheimer’s disease. Hum Brain Mapp. 2009;30:1051–1059. doi: 10.1002/hbm.20563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Buchler NG, White LE, Madden DJ, Cabeza R. Assessing the effects of age on long white matter tracts using diffusion tensor tractography. Neuroimage. 2009;46:530–541. doi: 10.1016/j.neuroimage.2009.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deary IJ, Bastin ME, Pattie A, Clayden JD, Whalley LJ, Starr JM, Wardlaw JM. White matter integrity and cognition in childhood and old age. Neurology. 2006;66:505–512. doi: 10.1212/01.wnl.0000199954.81900.e2. [DOI] [PubMed] [Google Scholar]
- Deary IJ, Penke L, Johnson W. The neuroscience of human intelligence differences. Nat Rev Neurosci. 2010;11:201–211. doi: 10.1038/nrn2793. [DOI] [PubMed] [Google Scholar]
- Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2010;341:c3666. doi: 10.1136/bmj.c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RSR, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31:968– 980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Ekstrom RB, French JW, Harman HH, Dermam D. Kit of factor-referenced cognitive tests. Education Testing Service; Princeton, NJ: 1976. [Google Scholar]
- Finkel D, Reynolds CA, McArdle JJ, Hamagami F, Pedersen NL. Genetic variance in processing speed drives variation in aging of spatial and memory abilities. Developmental Psychology. 2009;45:820–834. doi: 10.1037/a0015332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel D, Reynolds CA, McArdle JJ, Pedersen NL. Age changes in processing speed as a leading indicator of cognitive aging. Psychol Aging. 2007;22:558–568. doi: 10.1037/0882-7974.22.3.558. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical Surface-Based Analysis: II: Inflation, Flattening, and a Surface-Based Coordinate System. NeuroImage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB. Structural brain changes in aging: courses, causes and cognitive consequences. Rev Neurosci. 2010;21:187–221. doi: 10.1515/revneuro.2010.21.3.187. [DOI] [PubMed] [Google Scholar]
- Fornage M, Debette S, Bis JC, Schmidt H, Ikram MA, et al. Genome-wide association studies of cerebral white matter lesion burden: the CHARGE consortium. Ann Neurol. 2011;69:928–939. doi: 10.1002/ana.22403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson E, Gao F, Black SE, Lobaugh NJ. Automatic segmentation of white matter hyperintensities in the elderly using FLAIR images at 3T. Journal of Magnetic Resonance Imaging. 2010;31:1311–1322. doi: 10.1002/jmri.22004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold BT, Powell DK, Xuan L, Jicha GA, Smith CD. Age-related slowing of task switching is associated with decreased integrity of frontoparietal white matter. Neurobiol Aging. 2010;31:512–522. doi: 10.1016/j.neurobiolaging.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Brickman AM, Cheng JC, Alexopoulos GS. Aging of cerebral white matter: a review of MRI findings. Int J Geriatr Psychiatry. 2009;24:109–117. doi: 10.1002/gps.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden T, Gabrieli JD. Insights into the ageing mind: a view from cognitive neuroscience. Nat Rev Neurosci. 2004;5:87–96. doi: 10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]
- Holland PR, Bastin ME, Jansen MA, Merrifield GD, Coltman RB, Scott F, Nowers H, Khallout K, Marshall I, Wardlaw JM, Deary IJ, McCulloch J, Horsburgh K. MRI is a sensitive marker of subtle white matter pathology in hypoperfused mice. Neurobiol Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.11.009. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–41. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jones DK. Studying connections in the living human brain with diffusion MRI. Cortex. 2008;44:936–952. doi: 10.1016/j.cortex.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Kantarci K, Senjem ML, Avula R, Zhang B, Samikoglu AR, Weigand SD, Przybelski SA, Edmonson HA, Vemuri P, Knopman DS, Boeve BF, Ivnik RJ, Smith GE, Petersen RC, Jack CR., Jr Diffusion tensor imaging and cognitive function in older adults with no dementia. Neurology. 2011;77:26–34. doi: 10.1212/WNL.0b013e31822313dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapeller P, Barber R, Vermeulen RJ, Adèr H, Scheltens P, Freidl W, Almkvist O, Moretti M, Del Ser T, Vaghfeldt P, Enzinger C, Barkhof F, Inzitari D, Erkinjunti T, Schmidt R, Fazekas F. Visual rating of age-related white matter changes on magnetic resonance imaging: scale comparison, interrater agreement, and correlations with quantitative measurements. Stroke. 2003;34:441–445. doi: 10.1161/01.str.0000049766.26453.e9. [DOI] [PubMed] [Google Scholar]
- Kennedy KM, Raz N. Aging white matter and cognition: Differential effects of regional variations in diffusion properties on memory, executive functions, and speed. Neuropsychologia. 2009;47:916–927. doi: 10.1016/j.neuropsychologia.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopman DS, Penman AD, Catellier DJ, Coker LH, Shibata DK, Sharrett AR, Mosley TH., Jr Vascular risk factors and longitudinal changes on brain MRI: the ARIC study. Neurology. 2011;76:1879–1885. doi: 10.1212/WNL.0b013e31821d753f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochunov P, Glahn DC, Lancaster J, Thompson PM, Kochunov V, Rogers B, Fox P, Blangero J, Williamson DE. Fractional anisotropy of cerebral white matter and thickness of cortical gray matter across the lifespan. Neuroimage. 2011a;58:41–49. doi: 10.1016/j.neuroimage.2011.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochunov P, Glahn DC, Nichols TE, Winkler AM, Hong EL, Holcomb HH, Stein JL, Thompson PM, Curran JE, Carless MA, Olvera RL, Johnson MP, Cole SA, Kochunov V, Kent J, Blangero J. Genetic analysis of cortical thickness and fractional anisotropy of water diffusion in the brain. Front Neurosci. 2011b;5:120. doi: 10.3389/fnins.2011.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegeskorte N, Simmons WK, Bellgowan PSF, Baker CI. Circular analysis in systems neuroscience: the dangers of double dipping. Nat Neurosci. 2009;12:535–540. doi: 10.1038/nn.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bihan D. Looking into the functional architecture of the brain with diffusion MRI. Nat Rev Neurosci. 2003;4:469–480. doi: 10.1038/nrn1119. [DOI] [PubMed] [Google Scholar]
- Lee DY, Fletcher E, Martinez O, Zozulya N, Kim J, Tran J, Buonocore M, Carmichael O, DeCarli C. Vascular and degenerative processes differentially affect regional interhemispheric connections in normal aging, mild cognitive impairment, and Alzheimer disease. Stroke. 2010;41:1791–1797. doi: 10.1161/STROKEAHA.110.582163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Wang Y, Gerig G, Gouttard S, Tao R, Fletcher T, Styner M. Quality control of diffusion weighted images. 2010:76280J, 76280J–9. doi: 10.1117/12.844748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Bennett IJ, Burzynska A, Potter GG, Chen NK, Song AW. Diffusion tensor imaging of cerebral white matter integrity in cognitive aging. Biochim Biophys Acta. 2012;1822:386–400. doi: 10.1016/j.bbadis.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Bennett IJ, Song AW. Cerebral white matter integrity and cognitive aging: contributions from diffusion tensor imaging. Neuropsychol Rev. 2009a;19:415–435. doi: 10.1007/s11065-009-9113-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Spaniol J, Costello MC, Bucur B, White LE, Cabeza R, Davis SW, Dennis NA, Provenzale JM, Huettel SA. Cerebral white matter integrity mediates adult age differences in cognitive performance. J Cogn Neurosci. 2009b;21:289–302. doi: 10.1162/jocn.2009.21047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier IB, Manly JJ, Provenzano FA, Louie KS, Wasserman BT, Griffith EY, Hector JT, Allocco E, Brickman AM. White matter predictors of cognitive functioning in older adults. J Int Neuropsychol Soc. 2012;18:414–427. doi: 10.1017/S1355617712000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michielse S, Coupland N, Camicioli R, Carter R, Seres P, Sabino J, Malykhin N. Selective effects of aging on brain white matter microstructure: A diffusion tensor imaging tractography study. NeuroImage. 2010;52:1190–1201. doi: 10.1016/j.neuroimage.2010.05.019. [DOI] [PubMed] [Google Scholar]
- Mori S, Wakana S, Van Zijl PC. MRI atlas of human white matter. Elsevier; 2005. [Google Scholar]
- Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Human Brain Mapping. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris DG. The effects of microscopic tissue parameters on the diffusion weighted magnetic resonance imaging experiment. NMR Biomed. 2001;14:77–93. doi: 10.1002/nbm.682. [DOI] [PubMed] [Google Scholar]
- O’Sullivan M, Jones DK, Summers PE, Morris RG, Williams SC, Markus HS. Evidence for cortical “disconnection” as a mechanism of age-related cognitive decline. Neurology. 2001;57:632–638. doi: 10.1212/wnl.57.4.632. [DOI] [PubMed] [Google Scholar]
- Park DC, Reuter-Lorenz P. The Adaptive Brain: Aging and Neurocognitive Scaffolding. Annual Review of Psychology. 2009;60:173–196. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Cyr JA. Frontal-striatal circuit functions: context, sequence, and consequence. J Int Neuropsychol Soc. 2003;9:103–127. doi: 10.1017/s1355617703910125. [DOI] [PubMed] [Google Scholar]
- Sala S, Agosta F, Pagani E, Copetti M, Comi G, Filippi M. Microstructural changes and atrophy in brain white matter tracts with aging. Neurobiol Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.04.027. [DOI] [PubMed] [Google Scholar]
- Salami A, Eriksson J, Nilsson L-G, Nyberg L. Age-related white matter microstructural differences partly mediate age-related decline in processing speed but not cognition. Biochim Biophys Acta. 2011 doi: 10.1016/j.bbadis.2011.09.001. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Speed mediation of adult age differences in cognition. Dev Psychol. 1993;29:722–738. [Google Scholar]
- Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychol Rev. 1996;103:403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Neuroanatomical substrates of age-related cognitive decline. Psychol Bull. 2011;137:753–784. doi: 10.1037/a0023262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. Selective review of cognitive aging. J Int Neuropsychol Soc. 2010;16:754–760. doi: 10.1017/S1355617710000706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders AM, Strittmatter WJ, Schmechel D, George-Hyslop PH, Pericak-Vance MA, Joo SH, Rosi BL, Gusella JF, Crapper-MacLachlan DR, Alberts MJ, et al. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer’s disease. Neurology. 1993;43:1467–72. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- Schaie KW. Developmental influences on adult intelligence: the Seattle longitudinal study. Oxford University Press; Oxford; New York: 2005. [Google Scholar]
- Schaie KW, Dutta R, Willis SL. Relationship between rigidity-flexibility and cognitive abilities in adulthood. Psychol Aging. 1991;6:371–383. doi: 10.1037//0882-7974.6.3.371. [DOI] [PubMed] [Google Scholar]
- Schaie KW, Willis SL, Caskie GI. The Seattle longitudinal study: relationship between personality and cognition. Neuropsychol Dev Cogn B Aging Neuopsychol Cogn. 2004;11:304–24. doi: 10.1080/13825580490511134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaie KW, Willis SL, Pennak S. An historical framework for cohort differences in intelligence. Research in human development. 2005;2:43. doi: 10.1080/15427609.2005.9683344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavone F, Charlton RA, Barrick TR, Morris RG, Markus HS. Imaging age-related cognitive decline: A comparison of diffusion tensor and magnetization transfer MRI. J Magn Reson Imaging. 2009;29:23–30. doi: 10.1002/jmri.21572. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN. Fiber Pathways of the Brain. Oxford University Press; 2009. [Google Scholar]
- Sen PN, Basser PJ. A model for diffusion in white matter in the brain. Biophys J. 2005;89:2927–2938. doi: 10.1529/biophysj.105.063016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TEJ. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Smith SM, Zhang Y, Jenkinson M, Chen J, Matthews PM, Federico A, De Stefano N. Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage. 2002;17:479–489. doi: 10.1006/nimg.2002.1040. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Knight RT. Principles of Frontal Lobe Function. 1. Oxford University Press; USA: 2002. [Google Scholar]
- Sullivan EV, Pfefferbaum A. Diffusion tensor imaging in normal aging and neuropsychiatric disorders. Eur J Radiol. 2003;45:244–55. doi: 10.1016/s0720-048x(02)00313-3. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Diffusion tensor imaging and aging. Neurosci Biobehav Rev. 2006;30:749–761. doi: 10.1016/j.neubiorev.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Sun SW, Liang HF, Cross AH, Song SK. Evolving Wallerian degeneration after transient retinal ischemia in mice characterized by diffusion tensor imaging. Neuroimage. 2008;40:1–10. doi: 10.1016/j.neuroimage.2007.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurstone LL, Thurstone TG. Examiner manual for the SRA Primary Mental Abilities Test (Form 10-14) Science Research Associates; Chicago: 1949. [Google Scholar]
- Turken A, Whitfield-Gabrieli S, Bammer R, Baldo JV, Dronkers NF, Gabrieli JDE. Cognitive processing speed and the structure of white matter pathways: convergent evidence from normal variation and lesion studies. Neuroimage. 2008;42:1032–1044. doi: 10.1016/j.neuroimage.2008.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood CK, Kurniawan ND, Butler TJ, Cowin GJ, Wallace RH. Non-invasive diffusion tensor imaging detects white matter degeneration in the spinal cord of a mouse model of amyotrophic lateral sclerosis. Neuroimage. 2011;55:455–461. doi: 10.1016/j.neuroimage.2010.12.044. [DOI] [PubMed] [Google Scholar]
- Vernooij MW, Ikram MA, Vrooman HA, Wielopolski PA, Krestin GP, Hofman A, Niessen WJ, Van der Lugt A, Breteler MMB. White matter microstructural integrity and cognitive function in a general elderly population. Arch Gen Psychiatry. 2009;66:545–553. doi: 10.1001/archgenpsychiatry.2009.5. [DOI] [PubMed] [Google Scholar]
- Vul E, Harris C, Winkielman P, Pashler H. Puzzlingly High Correlations in fMRI Studies of Emotion, Personality, and Social Cognition. Perspectives on Psychological Science. 2009;4:274–290. doi: 10.1111/j.1745-6924.2009.01125.x. [DOI] [PubMed] [Google Scholar]
- Wen W, Sachdev PS, Li JJ, Chen X, Anstey KJ. White matter hyperintensities in the forties: their prevalence and topography in an epidemiological sample aged 44–48. Hum Brain Mapp. 2009;30:1155–1167. doi: 10.1002/hbm.20586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen W, Zhu W, He Y, Kochan NA, Reppermund S, Slavin MJ, Brodaty H, Crawford J, Xia A, Sachdev P. Discrete neuroanatomical networks are associated with specific cognitive abilities in old age. J Neurosci. 2011;31:1204–1212. doi: 10.1523/JNEUROSCI.4085-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler-Kingshott CAM, Cercignani M. About “axial” and “radial” diffusivities. Magn Reson Med. 2009;61:1255–1260. doi: 10.1002/mrm.21965. [DOI] [PubMed] [Google Scholar]
- Yue NC, Arnold AM, Longstreth WT, Jr, Elster AD, Jungreis CA, O’Leary DH, Poirier VC, Bryan RN. Sulcal, ventricular, and white matter changes at MR imaging in the aging brain: data from the cardiovascular health study. Radiology. 1997;202:33–39. doi: 10.1148/radiology.202.1.8988189. [DOI] [PubMed] [Google Scholar]
- Zelinski EM, Gilewski MJ, Schaie KW. Individual differences in cross-sectional and 3-year longitudinal memory performance across the adult life span. Psychology and Aging. 1993;8:176–86. doi: 10.1037//0882-7974.8.2.176. [DOI] [PubMed] [Google Scholar]
- Zelinski EM, Kennison RF. Not your parents’ test scores: cohort reduces psychometric aging effects. Psychology and Aging. 2007;22:546–57. doi: 10.1037/0882-7974.22.3.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelinski EM, Lewis KL. Adult age differences in multiple cognitive functions: differentiation, dedifferentiation, or process-specific change? Psychology and Aging. 2003;18:727–45. doi: 10.1037/0882-7974.18.4.727. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. Medical Imaging, IEEE Transactions on. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.